We have found that HCMV infection promotes the elimination of the developmentally important basement membrane protein nidogen 1 (NID1) from its host. The virus both decreased transcription and induced degradation of expressed protein.
KEYWORDS: basement membrane, chromosome mapping, congenital infections, human cytomegalovirus, nidogen 1, transmigration, virus/host interaction
ABSTRACT
In 2000, we reported that human cytomegalovirus (HCMV) induced specific damage on chromosome 1. The capacity of the virus to induce DNA breaks indicated potent interaction between viral proteins and these loci. We have fine mapped the 1q42 breaksite. Transcriptional analysis of genes encoded in close proximity revealed virus-induced downregulation of a single gene, nidogen 1 (NID1). Beginning between 12 and 24 hours postinfection (hpi) and continuing throughout infection, steady-state (ss) NID1 protein levels were decreased in whole-cell lysates and secreted supernatants of human foreskin fibroblasts. Addition of the proteasomal inhibitor MG132 to culture medium stabilized NID1 in virus-infected cells, implicating infection-activated proteasomal degradation of NID1. Targeting of NID1 via two separate pathways highlighted the virus’ emphasis on NID1 elimination. NID1 is an important basement membrane protein secreted by many cell types, including the endothelial cells (ECs) lining the vasculature. We found that ss NID1 was also reduced in infected ECs and hypothesized that virus-induced removal of NID1 might offer HCMV a means of increased distribution throughout the host. Supporting this idea, transmigration assays of THP-1 cells seeded onto NID1-knockout (KO) EC monolayers demonstrated increased transmigration. NID1 is expressed widely in the developing fetal central and peripheral nervous systems (CNS and PNS) and is important for neuronal migration and neural network excitability and plasticity and regulates Schwann cell proliferation, migration, and myelin production. We found that NID1 expression was dramatically decreased in clinical samples of infected temporal bones. While potentially beneficial for virus dissemination, HCMV-induced elimination of NID1 may underlie negative ramifications to the infected fetus.
IMPORTANCE We have found that HCMV infection promotes the elimination of the developmentally important basement membrane protein nidogen 1 (NID1) from its host. The virus both decreased transcription and induced degradation of expressed protein. Endothelial cell (EC) secretion of basement membrane proteins is critical for vascular wall integrity, and infection equivalently affected NID1 protein levels in these cells. We found that the absence of NID1 in an EC monolayer allowed increased transmigration of monocytes equivalent to that observed after infection of ECs. The importance of NID1 in development has been well documented. We found that NID1 protein was dramatically reduced in infected inner ear clinical samples. We believe that HCMV’s attack on host NID1 favors viral dissemination at the cost of negative developmental ramifications in the infected fetus.
INTRODUCTION
The cellular mechanisms responsible for the broad array of human cytomegalovirus (HCMV)-induced birth defects are ill defined. Epidemiological study has found that HCMV congenital infections occur during ∼40,000 pregnancies annually in the United States (1). These infections are responsible for birth defects in ∼8,000 children per year, 25 to 50% of which present at birth, while the remainder are manifested at later times. The induced sequelae display a broad spectrum of central and peripheral nervous system (CNS and PNS) disorders, including microcephaly, mental retardation, vision loss, and, in ∼30 to 50% of all cases, sensorineural hearing loss (SNHL). SNHL is the most prevalent manifestation in late-onset cases. The focus of our work is deciphering the molecular mechanism(s) responsible for inducing these birth defects.
HCMV infection induces a wide array of perturbations in the host cellular protein complement, including changes to cell cycle and DNA repair proteins, proteins central to cellular differentiation and signaling, and developmentally important proteins. Interactions between any viral protein(s) in a given cell type could lead to perturbations in host cell proteins that are consequential to the fetus. What then is sufficient evidence to pursue study of an individual protein? In our case, earlier observation of specifically induced damage on chromosome 1 at 1q42 and 1q23 (2, 3) identified these sites as regions of potent interactions between viral proteins and the cellular DNA. An extensive array of HCMV strains induced both DNA breaks in a broad array of cell types (reference 2 and our unpublished results). The potency of the interactions suggested that the expression of genes encoded nearby these sites might be affected. Our earlier work mapping the 1q23 breaksite locus found that nearby gene loci were linked to negative developmental ramifications (3). Here, we focused on the consequences of HCMV infection at the 1q42 locus and found that, although several genes were encoded in this region, expression of only one, nidogen 1 (NID1), was specifically regulated at the transcriptional and posttranslational levels.
NID1 is an essential basement membrane (BM) protein that acts as a bridge between collagen IV, the scaffold for all basement membranes, and laminin (4). These protein complexes bind to proteoglycans within the extracellular matrix (ECM), creating the support structure for developing organs. Laminin-NID1 complexes play a major role in regional assembly and remodeling of BMs in developing tissues (reviewed in reference 5). In human embryos, NID1 underlies the BM of all developing organs (6). Cells are connected to the underlying BMs via transmembrane receptors of the integrin family (4), β1 and β3 integrins in particular (7). Vascular wall BM integrity is maintained through secretion of NID1, not only by the endothelial cells (ECs) that line all vasculature within the body, but also by the smooth muscle cells (SMCs) and fibroblasts that underly these cells (5, 6, 8). Timely and sufficient NID1 expression is clearly essential for structural development.
The literature also demonstrates the importance of NID1 expression for proper neural development. Studies in NID1-knockout (KO) mice point to NID1’s significance in neural network excitability and plasticity (9, 10). The absence of NID1 from the mouse cerebral cortex led to compromised cortical development (11). NID1 is secreted by both astrocytes and radial glia in the cortex, providing a substrate for proper neuronal migration during cortical development (12, 13). NID1 also regulates Schwann cell proliferation and migration in the PNS (14, 15). NID1’s presence in nerve sheaths is thought to promote Schwann cell myelin production (16). NID1 is expressed in the developing organ of Corti (OC), particularly in the BMs underlying nerve fibers that innervate the sensory cells and those that surround the interdental support cells of the spiral limbus (4, 17). NID1 is also thought to be essential for proper optic cup morphogenesis (18). The broad array of NID1’s neural functions indicates that disruption of NID1 expression could be expected to have negative consequences in the developing CNS and PNS.
We report here that HCMV infection of cultured fibroblasts and ECs dysregulates NID1 transcriptionally and posttranslationally, ultimately decreasing the steady-state (ss) levels of NID1. ECs that lacked NID1 due to CRISPR-mediated gene targeting promoted increased transmigration of monocytes through a confluent monolayer of these cells. Clinical samples of infected temporal bones displayed drastically reduced levels of NID1 in the OC and nerve tissue innervating the cochlea. Our results are discussed in the context of advantages this activity may offer to the virus and potential negative ramifications in the development of the infected fetus.
RESULTS
The 1q42 breaksite was mapped with fosmid clones.
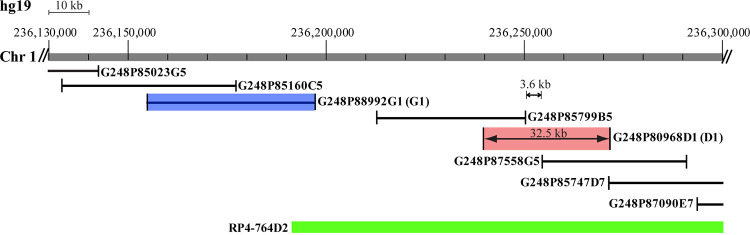
Originally, metaphases derived from cells infected in the S phase were used to map the 1q21 and 1q42 HCMV infection-induced breaksites via cytogenetic analysis (2). Refined fluorescence in situ hybridization (FISH) mapping of the former site defined its location at 1q23. A deafness-associated locus was located in its vicinity (3). In order to map the 1q42 breaksite and to determine if relevant chromosomal features were present at this location, we used bacterial artificial chromosome (BAC) clones mapped to the region for preliminary screening in infected human foreskin fibroblasts (HFFs). This screen identified a large clone that spanned the break (RP4-764D2 on the map in Fig. 1). However, we believed that more precisely mapping the break would aid in identifying potential viral protein interactions with this region. Therefore, we utilized a group of highly overlapping/tiled fosmid clones to more closely define the breaksite. The clones we tested are pictured in Fig. 1. Metaphase FISH analysis allowed us to identify one clone which consistently bridged the break, G248P80968D1 (here D1, shown in red in Fig. 1 and 2D and hybridized to a metaphase in red in Fig. 2A). We also identified two clones which consistently flanked the break; G248P85799B5 hybridized proximally and G248P87558G5 hybridized distally to the break site. Accounting for the size of the intervening distance between the mapped ends of the two flanking clones, and the full length of clone D1, we narrowed the breaksite to a 3.6- to 32.5-kb region of chromosome 1. Identifying clone D1 had the added benefit of its use for interphase FISH analysis, allowing detection of breaks during any stage of the cell cycle and all stages of infection.
FIG 1.
The 1q42 breaksite was mapped using fosmids. The fosmid clone marked in red (D1) bridges the breaksite. The G1 clone marked in blue was used as the proximal marker for interphase FISH analysis. Other clones tested are pictured. The RP4-764D2 bacterial artificial chromosome (BAC) preliminarily identified as containing the breaksite is shown for reference.
FIG 2.
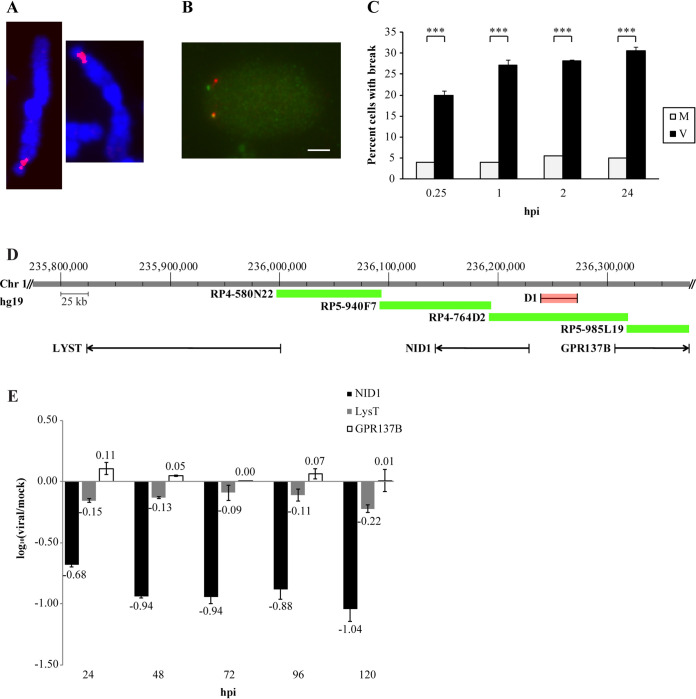
Site-specific DNA breaks occurred immediately after entry. (A) Metaphase chromosomes were used to define the bridging clone D1 to the 1q42 breaksite. (B) Interphase fluorescence in situ hybridization (FISH) using clones D1 (red) and G1 (green) allowed assessment of break frequency during G0 infection of human foreskin fibroblasts (HFFs). Bar, 5 μm. (C) Interphase FISH analysis of virus-infected (black) and mock-infected (light gray) cells in the first 24 h postinfection (hpi). Data represent an average of two experiments. ***, P < 0.001. Three genes were encoded in the breaksite vicinity. Only one, NID1, was significantly downregulated. (D) Genes encoded in the breaksite vicinity include GPR137B (74 kb distal to the break), a G-protein coupled receptor; LysT (181 kb proximal to the break), involved in lysosomal transport; and NID1 (35 kb proximal to the break), a basement membrane protein. Bridging clone D1 is shown for reference in red. (E) Real-time qPCR (RT-qPCR) analysis was performed in mock- and virus-infected cells at the indicated time points. Changes are represented as log10 (base 10) relative quantification (log RQ) of virus/mock transcript levels (normalized to G6PD as the internal reference gene).
1q42-specific breaks occurred immediately after virus entry.
Our earlier results indicated relatively rapid and unrestricted cell cycle induction of breaks at the 1q23 site by 3 hpi (3). Having mapped a selection of the tiled fosmid clones, we used another more proximal, cleanly hybridizing and nonoverlapping, clone, G248P88992G1 (here G1) (shown in blue in Fig. 1), and the bridging clone, D1, to determine when breaks were initiated at 1q42 in HFFs. As described previously, the close proximity of these two fosmid clones in interphase nuclei in the G1 phase of the cell cycle was expected to produce two pairs of juxtaposed dual-color fluorescence cohybridization signals. Cells without breaks would present adjacent green and red signals, with a small region of yellow overlap. Cells damaged at 1q42 would display separated red and green FISH signals, lacking an overlapping yellow region (see Fig. 2B; the bottom signal pair is overlapping, and the top pair shows split signals).
HFFs were infected after release from G0 synchronization and rapidly harvested for analysis (Fig. 2C). While mock-infected cells consistently displayed the same baseline level of breaks in 3 to 5% of cells as we had observed previously (2, 3), virus-infected cells displayed breaks in more than 20% of the population by 15 min pi, reaching a maximum level of 30% by 24 hpi.
Three genes were encoded in close proximity to the defined 1q42 breaksite; one gene, NID1, was significantly regulated.
Interphase FISH analysis revealed that infection did not induce measurable DNA breaks in all cells, although it remained possible that smaller breaks might not be detectable by this methodology. Specific breaks in ∼30% of a population of cells indicated strong interaction between viral proteins and the host DNA, suggesting the possibility of transcriptional regulation. Three coding sequences were identified in proximity to the 1q42 break (Fig. 2D). GPR137B, a G-protein coupled receptor, was encoded 74 kb distal to the region. LysT, a protein involved in lysosomal transport, was encoded 181 kb proximal to the breaksite. The gene located closest to the breaksite (35 kb proximal) encodes NID1, a basement membrane protein. The large BAC (RP4-764D2) and fosmid D1 (shown in red) known to bridge the breaksite are indicated in the figure for reference. Three other BAC clones within the region are also indicated.
A time course of infection was performed in HFFs to determine whether expression of the three genes identified was influenced. Total RNA was extracted and reverse transcribed. The resultant cDNA was analyzed for changes in gene expression via real-time quantitative PCR (RT-qPCR). At each time point, relative concentrations of cDNA for each of the three genes of interest were compared in virus- versus mock-infected samples, using G6PD levels as the reference control. Figure 2E shows minimal regulation of GPR137B and LysT throughout the time course. NID1 was downregulated by ∼7-fold at 24 hpi and by ∼10-fold beginning at 48 hpi.
Infection decreased steady-state levels of NID1 in cellular lysates and secreted supernatants.
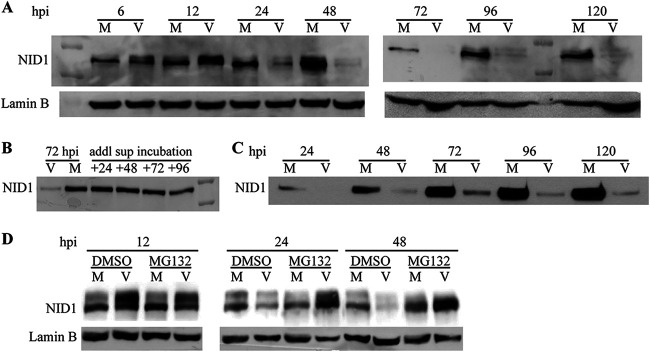
NID1 is a BM protein secreted by many cell types, including fibroblasts, ECs, and SMCs (5, 6, 8). Analysis of ss NID1 levels in whole-cell lysates by Western blotting revealed that substantial quantities of NID1 were present in HFFs soon after release from confluence arrest (Fig. 3A, “M” at 6 hpi), recommending these cells as an excellent model for assessing changes to NID1 protein synthesis and secretion. The ss level of protein in mock-infected cells was not constant over the time course, perhaps indicating natural processing within cells or increased secretion from cells. In contrast, while NID1 protein was present in virus-infected cells at equivalent, or higher, ss levels through 12 hpi, by 24 hpi the ss protein level declined sharply, and it was essentially undetectable after 48 hpi.
FIG 3.
Steady-state NID1 protein levels declined rapidly in lysates and supernatants. (A) HFFs were mock- or virus-infected at a multiplicity of infection (MOI) of 5 and harvested for Western blot analysis at the indicated times pi. Lamin B served as a loading control in all blots. (B) Supernatants were harvested from mock- or virus-infected HFFs at 72 hpi. An additional aliquot from mock-infected cells was placed in a fresh dish and sampled every 24 h for an additional 96 h. (C) Equivalent amounts of supernatant aliquots (100 μl) were harvested from a single plate (with 8 ml of medium) of either mock- or virus-infected HFFs at the indicated times pi and analyzed by Western blotting. (D) HFFs were mock- or virus-infected at an MOI of 5. At 3.5 hpi, virus was removed, and medium containing 0.5 μM MG132 (or dimethyl sulfoxide [DMSO] vehicle) was added to inhibit proteasomal activity. Cells were harvested for Western blot analysis at the indicated times pi.
It has been documented that HCMV infection substantially alters the cellular secretome (19–21). The postinfection decline in ss NID1 protein levels in cellular lysates could have resulted from an increase in NID1 secretion. To ascertain if this was the case, we first assessed the stability of secreted NID1. Mock-infected HFFs were seeded, and an aliquot of medium was harvested 3 days later. The aliquot was placed into a fresh dish and incubated for an additional 96 h, from which samples were collected every 24 h. The individual samples were analyzed for NID1 content via Western blot. NID1 protein secreted from mock-infected cells was stable for the 4 days after initial collection (Fig. 3B). Small aliquots (∼100 μl) of supernatant were collected from mock- and virus-infected cells over a 120-h time course (Fig. 3C). Medium was not changed during this period. NID1 accumulated continuously in the mock-infected supernatants. There was a very limited amount of protein secreted from the virus-infected cells, with little accumulation over the time course. The absence of NID1 in virus-infected cellular lysates after 24 hpi, combined with the stability of secreted NID1 in supernatants, determined that reduced levels of ss NID1 within the cell were not due to virus-induced increased secretion of NID1.
NID1 protein was targeted for degradation in infected cells.
Having ruled out increased secretion as the source of reduced NID1 in the cellular lysates, we more closely scrutinized the Western blot data over the time course of infection in Fig. 3A. Closer examination revealed that what appeared to be a protein doublet was frequently present in both the mock- and virus-infected samples. We reasoned that a slower migrating form might indicate a modification to the protein preceding degradation.
To test if proteasomal processing was involved in the observed decline of NID1 during infection, we performed parallel mock and virus infections in the presence of either the proteasome inhibitor MG132 or a dimethyl sulfoxide (DMSO) vehicle control. Gels were run for a substantially longer period to resolve the doublet. Gels were then probed for NID1 by Western blotting (Fig. 3D). Addition of DMSO to the medium did not alter NID1 expression in mock- or virus-infected cells compared to that in earlier experiments. Increased resolution allowed us to determine that in virus-infected cells the two forms were approximately equivalent at all time points. In these cells, both forms declined from 12 to 24 hpi and were absent by 48 hpi in cells treated with DMSO. MG132 inhibition of proteasomal activity did not substantively increase ss NID1 protein in mock-infected cells, indicating that proteasomal degradation was not a typical NID1 removal pathway for the cell. In the presence of MG132, NID1 protein was stabilized in the virus-infected cells (compare DMSO versus MG132 lanes at 24 and 48 hpi in Fig. 3D). Despite performing multiple iterations of the experiment, after 48 h of drug treatment we could not confidently resolve which band was present. The presence of MG132 always substantially increased the NID1 signal in virus-infected cells at 48 hpi. We concluded that virus infection induced proteasomal degradation of NID1.
Could the absence of NID1 increase dissemination of HCMV within the host?
Our data indicated that HCMV employed at least two pathways to remove NID1 from an infected cell, namely, transcriptional downregulation and proteasomal degradation. NID1 is secreted by several cell types, including fibroblasts, ECs, and SMCs. These cell types employ NID1 to support ECM/BM integrity (5, 6, 8). Vascular walls are lined with ECs that secrete ECM/BM proteins. Earlier work from the lab of Andrew Yurochko using transwell assays showed that HCMV infection of either migrating monocytes or underlying EC monolayers resulted in increased transmigration (22, 23). We reasoned that HCMV-targeted disruption of NID1 could aid in destabilizing vascular walls to promote virus dissemination within the host.
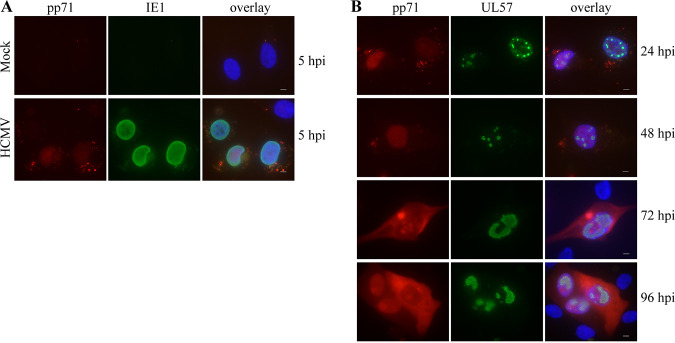
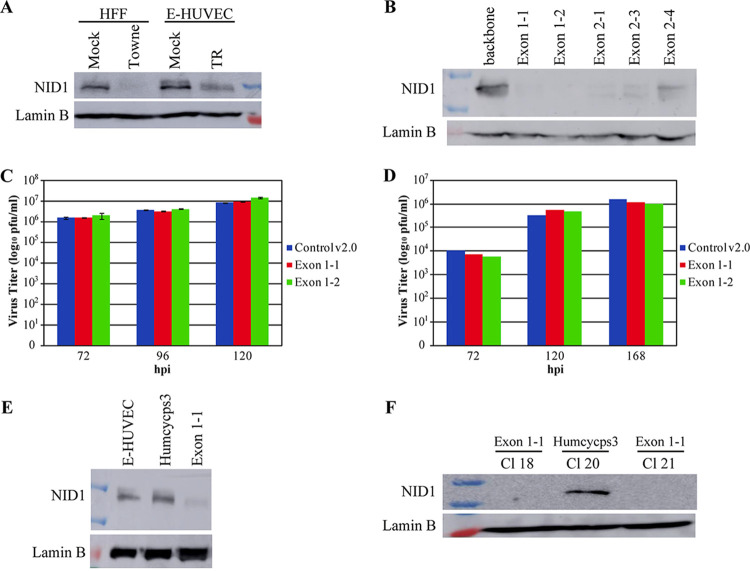
The monocytic cell line THP-1 has been utilized as a model for HCMV interaction with monocytes by several labs (see reference 24) and to model transmigration of monocytes through EC monolayers (25, 26). We utilized these cells to investigate any effects on their transmigration through NID1-downregulated virus-infected ECs. In order to rule out variations that might arise from using different populations of primary ECs, we utilized a human papillomavirus (HPV) E6/E7-immortalized HUVEC (E-HUVEC) cell line (27). First, we established that the E-HUVECs were susceptible to HCMV infection. Because the Towne strain (used for all fibroblast infections described thus far) has been lab-adapted to epithelial cells, we used the clinical isolate, TR, for all E-HUVEC infections. Figure 4A shows that the E-HUVECs were readily infected by TR (as evidenced by nuclear staining of the input tegument protein, pp71) and began expression of immediate early proteins (IE1) by 5 hpi. Second, we examined the progression of the infection between 24 and 96 hpi, and observed UL57 expression, confirming characteristic establishment of viral replication centers, and de novo expression of pp71, which progressed from nuclear to cytoplasmic localization over time (Fig. 4B). Third, we verified that the level of NID1 in the mock-infected E-HUVEC whole-cell lysates was roughly equivalent to that seen in HFFs (Fig. 5A). Fourth, we established that TR infection of E-HUVECs downregulated ss levels of NID1, as was observed in HFFs (Fig. 5A).
FIG 4.
E-HUVECs are susceptible to HCMV infection and progress through late viral protein production. Cells were infected with TR at an MOI of 15 on coverslips and harvested at the indicated time points pi. (A) Coverslips harvested at 5 hpi were stained for tegument protein pp71 to analyze input virus and for immediate early protein IE1 to assess new viral protein synthesis. (B) Coverslips were stained from 24 to 96 hpi for de novo expression of pp71 and UL57 (HCMV polymerase) to monitor the progression of infection. Bar, 5 μm.
FIG 5.
E-HUVECs secrete NID1, which was downregulated by TR infection. Targeting NID1 with CRISPR constructs effectively eliminated expression. (A) E-HUVECs were mock- or virus-infected with TR at an MOI of 15 and harvested at 96 hpi for Western blot analysis. HFFs infected with Towne (MOI = 5) and harvested at 96 hpi were run as a control for virus-induced NID1 downregulation. (B) THFs were transduced with one of five NID1-KO CRISPR (or backbone control) lentiviruses, then pools were selected with puromycin, seeded at an equal density, and harvested 72 h postplating for Western blot analysis for NID1. (C and D) THF exon 1-1 and exon 1-2 pooled cells were assayed for virion production in comparison to the control v2.0 pooled cells, as described in Materials and Methods. Elimination of NID1 expression in these pools did not affect virus production at either (C) high- (MOI = 5) or (D) low- (MOI = 0.1) MOI infection. High-MOI infections were performed twice and low MOI infections once. No statistical significance between groups was observed. (E) E-HUVECs were transduced with the same exon 1-1 lentivirus or a control (HUMCYCPS3) lentivirus targeting a pseudogene. (F) Two single NID1-KO E-HUVEC clones derived from the exon 1-1 transduction and one from the control pseudogene transduction were assayed for NID1 expression. These clones were used for the subsequent transmigration assays.
After establishing that HCMV infection decreased ss levels of NID1 in E-HUVECs, we used transmigration assays to test our hypothesis that NID1 downregulation could increase virus dissemination within the host. As mentioned above, it has been established that monocyte transmigration through an HCMV-infected EC monolayer is increased (23, 28). Could any of this increase be caused by NID1 downregulation in that EC monolayer? To answer this question, we generated EC lines that lack NID1 using CRISPR-Cas9 gene editing. We designed five different CRISPR guide RNAs to target the NID1 locus (see Materials and Methods for sequences) and tested the efficiency of these guides to eliminate NID1 in telomerase-immortalized fibroblasts (THFs). THFs were transduced with lentivirus particles representing one of the five individual targeting vectors (or the control backbone plasmid). Pools of transfected cells were puromycin selected. Selected THF pools were expanded and harvested for Western blot analysis of NID1 expression. All five guide RNAs successfully produced cells that lacked NID1 expression (Fig. 5B). Virus titers were compared between two of these CRISPR-mediated NID1-KO THF pools (exon 1-1 and exon 1-2) and the backbone v2.0 pool. The absence of NID1 produced no statistically significant changes in these cells’ production of virus under either high or low multiplicity of infection (MOI) conditions (Fig. 5C, MOI = 5; Fig. 5D, MOI = 0.1). Therefore, HCMV targeting of NID1 did not increase the replicative capacity of infected cells. We then utilized the most consistent guide RNA, exon 1-1 (or a pseudogene targeting control construct HUMCYCPS3), to transduce the E-HUVECs (Fig. 5E). We selected single clones from the exon 1-1 guide RNA (or control HUMCYCPS3 guide RNA) E-HUVEC pools to maximize long-term elimination of NID1 expression. NID1 expression was strong in the control clone CL20, while in expanded populations from two exon 1-1 clones, CL18 and CL21, NID1 was absent (Fig. 5F). These three clones were used in parallel with mock- or virus-infected parental E-HUVECs for transmigration assays.
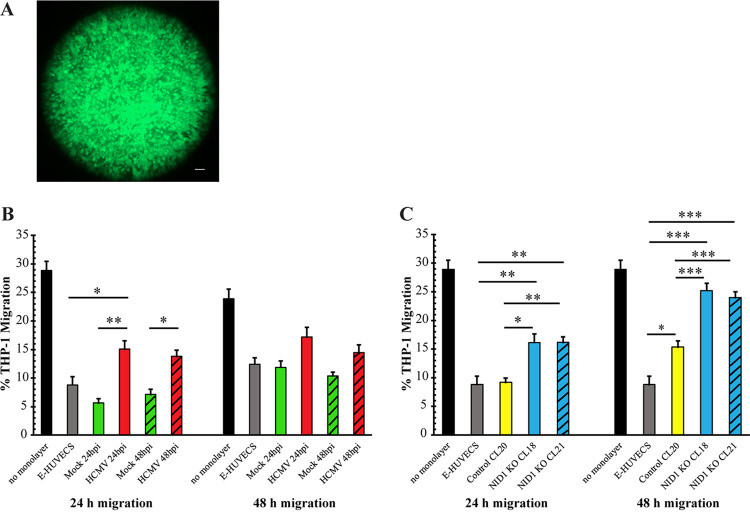
Parental E-HUVECs were seeded onto transwell inserts and allowed to grow to confluence (∼11 to 15 days). Monolayers seeded in parallel were used to confirm confluence via calcein dye staining (see Fig. 6A for an example). Confluent monolayers were either mock or HCMV-TR- infected at an MOI of 15 (Fig. 6B). THP-1 cells were seeded onto confluent monolayers at 24 hpi (Fig. 6B, solid green and red bars) or 48 hpi (Fig. 6B; hatched green and red bars). After 24 and 48 h, THP-1 cells were collected from the lower chamber and counted. Confirming previous reports (23, 28), after 24 h, significantly fewer THP-1 monocytes transmigrated through mock-infected E-HUVEC monolayers than virus-infected E-HUVEC monolayers (Fig. 6B, left side). In the subsequent 24 h, THP-1 cell transmigration through virus-infected monolayers was also increased, although not reaching statistical significance (Fig. 6B, right side). The same transmigration assays were performed using the two E-HUVEC NID1 CRISPR KO clones (CL18, solid blue bars, and CL21, hatched blue bars) and the control CRISPR-targeted clone (CL20, yellow bars) (Fig. 6C) to determine whether the lack of NID1 alone might recapitulate the increased transmigration. After 24 and 48 h, significantly more THP-1 cells transmigrated through both the NID1 CRISPR KO E-HUVEC clones than through either the E-HUVEC parental cells (gray bars) or the CRISPR-control clone. The lack of NID1 expression alone induced higher THP-1 transmigration in the second 24-h period than virus infection. These data support our hypothesis that infection-induced knockdown of NID1 could promote virus dissemination in its host.
FIG 6.
HCMV infection and CRISPR-mediated NID1 knockout increased transmigration of THP1 cells through E-HUVEC monolayers. (A) Examples of EC monolayers stained with calcein dye to show confluence. Bar, 250 μm. (B and C) Transwell assays were performed to assess THP-1 transmigration through E-HUVEC monolayers. Migration was compared to that in transwells seeded with control E-HUVECs (gray bars) or seeded with no cells at all (black bars). (B) THP-1 transmigration (measured after 24 and 48 h) through mock- (green bars) and virus-infected (MOI = 15) (red bars) E-HUVEC monolayers. The solid and hatched bars represent migration assays performed at either 24 or 48 h postinfection of the monolayer, respectively. (C) Control- (CL20, yellow bars) or NID1-targeted CRISPR clone monolayers (CL18, solid blue bars; CL21, hatched blue bars) were used for transmigration assays. Migration was assessed at 24 and 48 h post THP-1 seeding. For statistical analysis, *, P < 0.05; **, P < 0.01; and ***, P < 0.001. All comparisons against the no-monolayer control showed P values of <0.001 (not shown for clarity of graphs). If any other comparison is not shown, it did not rise to the level of significance (NS). No statistics were done to compare the 24-h to 48-h migration of a particular treatment or cell type. The error bars represent a standard error of the mean.
NID1 staining was dramatically reduced in infected tissue samples.
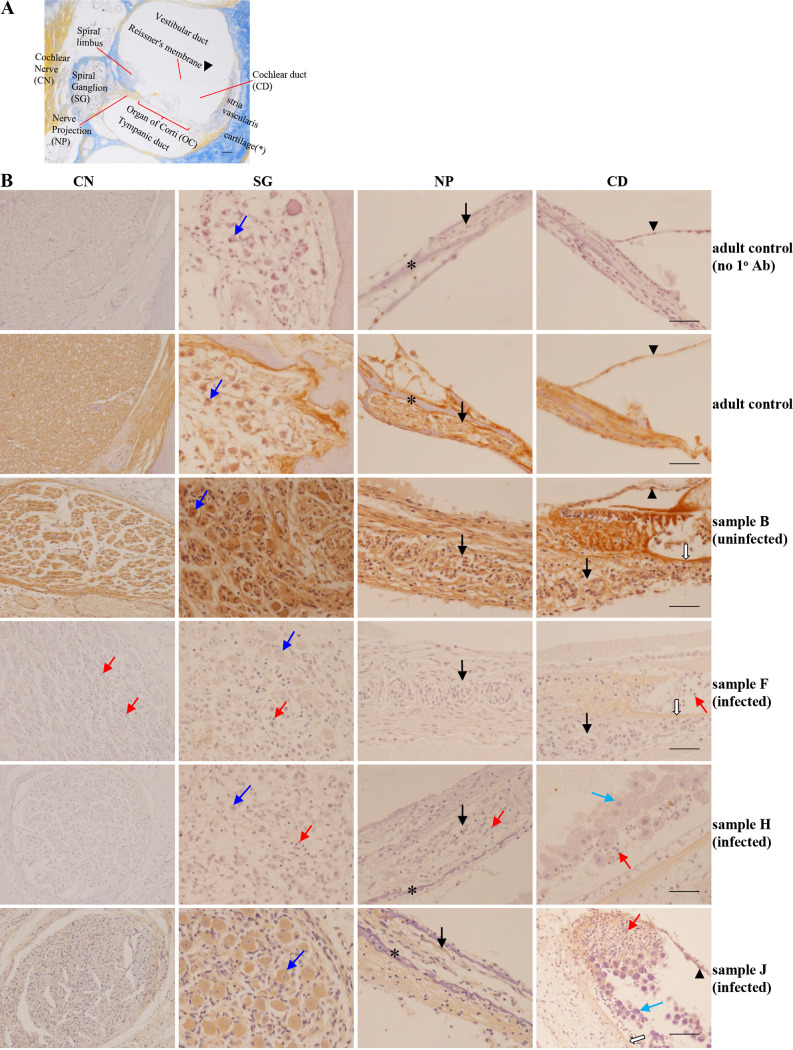
In culture, we observed that infection induced substantial downregulation of NID1. Would a similar effect be present in vivo? To answer this question, we reanalyzed our clinical temporal bone tissue samples (29, 30) for neuropathological affects and NID1 expression, in the context of our current in vitro findings. In brief, compared with temporal bones of uninfected samples, infected samples often showed inflammation in all regions of the nerve circuit, extending from the cochlear nerve (CN) in the modiolus to the structures within the cochlear duct (CD), including the OC itself (as pictured in Fig. 7A). This inflammation was typified by the presence of pyknotic cells and cellular fragments/debris, as well as neutrophil, lymphocyte, and macrophage infiltrates (see red arrows for examples in Fig. 7B). Some infected samples displayed enlarged, disorganized epithelial cells within the CD lining, including along the stria vascularis (see light blue arrows in Fig. 7B, rows 5 and 6), with classic “owl’s eye” nuclei and intranuclear and intracytoplasmic cytomegalic inclusions. In the spiral ganglion (SG) of some HCMV-infected samples, fewer bipolar neurons and supporting satellite glial cells were observed. Bipolar neurons in infected samples were often smaller in size and lacked a “complete crown” of satellite glial cells, and the neuropil was often less dense (compare dark blue arrows in infected and uninfected samples, Fig. 7B). Inflamed mesenchymal connective tissue in the spiral ligament, in the spiral limbus, and between the bone around the SG and the spiral ligament often had fewer fibroblasts, vascular congestion, and edema. Importantly, although not repeated here, our earlier analyses of these same tissues found only sporadic viral antigen-positive cells (for examples, see Fig. 1 in reference 29 and Fig. 1 and 2 in reference 30) amid widespread histopathological changes.
FIG 7.
HCMV infection markedly diminishes NID1 expression in clinical samples. (A) Image of the entire cochlear nerve circuit to provide context/orientation. Sample courtesy of Victor Eroschenko. (B) Images from sections of the inner ear stained for NID1 and lightly counterstained with hematoxylin. NID1 staining was analyzed in the cochlear nerve (CN), spiral ganglion (SG), nerve projections (NP, black arrows) from SG to the organ of Corti (OC) within the cochlear duct (CD), and basilar membrane (BM; white arrows). Top row shows control adult tissue stained with no primary (1o) antibody (Ab) for reference. Compared to adult (row 2) and uninfected age-matched (row 3) controls, the overall NID1 staining in infected samples was reduced in the CN, SG, NP, and structures in the CD. Intracellular and extracellular NID1 was reduced in supporting cells in the CN and SG, neuronal cytoplasm (dark blue arrows) in the SG, and cells constituting the NP and OC. In comparison with controls, NID1 staining in infected samples varied from almost no staining (rows 4 and 5) to moderate staining (row 6). CMV-induced inflammation in the CN, SG, NP, and CD caused pyknosis and infiltration by inflammatory cells (neutrophils, lymphocytes, and macrophages; red arrows). Light blue arrows show enlarged, infected cytomegalic cells sloughing off from the stria vascularis within the CD. The asterisks in the NP panel mark cartilage in the spiral lamina, and arrowheads in the CD panel mark Reissner’s membrane. Bar, 50 μm.
As outlined in Materials and Methods, the degree of NID1 staining in each sample was assigned a semiquantitative score from 0 (no staining) to 4 (intense staining). Scoring for each region of the cochlear circuit for each sample is presented in Table 1. Comparison of NID1 staining in infected samples and matched uninfected control samples found that expression of NID1 was decreased in the entire CN circuit analyzed in all infected samples. The intensity of NID1 staining varied from sample to sample in both control and infected groups. Infected samples ranged from an almost complete lack of NID1 staining (Fig. 7B, rows 4 and 5) to moderate staining (Fig. 7B, row 6), versus consistently robust staining in control adult tissue (Fig. 7B, row 2) and control uninfected samples (Fig. 7B, row 3). Notably, in infected samples, NID1 staining intensity and overall area were decreased both extracellularly and intracellularly in epithelial cells, fibroblasts, and bipolar neurons in all regions analyzed. Comparison of semiquantitative scores found significantly decreased staining in all regions of the CN circuit (Fig. 8). Although signs of inflammation were present in many of the infected samples (see red arrows in Fig. 7B), inflammation did not always correlate with decreased NID1 expression or distribution. This can be observed in sample J (Fig. 7B, row 6), which contains signs of inflammation in the CD yet still displays moderate NID1 staining.
TABLE 1.
Semiquantitative scoring of NID1 expression in control and CMV+ clinical samples
| Sample designationa | Infection status | Cochlear nerve (CN) | Spiral ganglion (SG) | Neuronal cytoplasm (NC) | Nerve projections (NP) | Basilar membrane (BM) |
|---|---|---|---|---|---|---|
| Adult control (no primary [1o] Ab control) | Uninfected | 0 | 0 | 0 | 0 | 0 |
| Adult control | Uninfected | 3 | 3 | 3 | 3 | 4 |
| A | Uninfected | 4 | 2 | 2 | 2 | 3 |
| B | Uninfected | 4 | 4 | 4 | 4 | 3 |
| C | Uninfected | 4 | 3 | 2 | 2 | 2 |
| D | Uninfected | 2 | 3 | 3 | 3 | 4 |
| E | Uninfected | 3 | 3 | 3 | 2 | 4 |
| F | Infected | 0 | 1 | 1 | 0 | 1 |
| G | Infected | 1 | 0 | 0 | 0 | 0 |
| H | Infected | 0 | 0 | 0 | 0 | 0 |
| I | Infected | 2 | 1 | 1 | 1 | 1 |
| J | Infected | 2 | 1 | 2 | 0 | 1 |
| K | Infected | 3 | 3 | 3 | 2 | 2 |
FIG 8.

HCMV infection significantly decreases NID1 expression in clinical samples. Summary data showing the comparison of NID1 staining between control (C) and infected (I) inner ear samples. All regions analyzed showed statistically significant decreases in staining. *, P < 0.05; **, P < 0.01.
DISCUSSION
Twenty years after making the remarkable discovery that HCMV was capable of inducing site-specific damage to the host chromatin (2), adenovirus (31) and HCMV remain the only two viruses known to possess this capacity. HCMV’s propensity to induce specific breaks led us to finely map the 1q23 breaksite (3) and examine a variety of virus-host interactions, including the host’s capacity to detect and repair DNA damage after the onset of infection (32–35). We found that the virus dramatically impaired a cell’s capacity to properly repair damage to its DNA. We reasoned that DNA damage left unrepaired could have negative ramifications for the host, particularly during development.
With the 1q23 stage set, we returned to the “theater” and defined the other, and what appeared to be more prevalent, breaksite at the 1q42 locus. We hoped to determine whether there were any direct ramifications from this interaction. We more finely mapped the 1q42 breaksite using fosmid clones (Fig. 1). Using these clones, we determined that damage occurred very rapidly after infection (as soon as 15 min pi) and could be inflicted regardless of the cell cycle phase (Fig. 2). More refined mapping also allowed us to survey the region for potentially regulated gene products. To our surprise, only a single nearby gene, NID1, was significantly transcriptionally regulated (Fig. 2), with declines commencing as early as 24 hpi.
The interphase FISH analyses shown in Fig. 2 illuminated several points and opened many avenues for further research. We found that virus interactions with the host DNA began almost immediately after entry into the host cell (our earlier work had defined the necessity for entry, but that breakage did not require de novo protein synthesis [2]). The rapidity of induction of breaks (∼15 min pi) suggested that a tegument protein(s) was responsible. Studies are under way to determine if this is the case. However, with infection inducing breaks in a maximum of 30% of cells, what was effecting such a large and rapid downregulation of NID1 transcription? The possibility of smaller, less quantifiable damage exists; however, we think it likely that the breaks themselves are not responsible for the downregulation. Rather, we believe the large decline in the transcript levels indicates a pervasive interaction between the virus and this region of the cellular DNA in the large majority of cells, an interaction which results in breaks in only a proportion of the population. Changes in the epigenetic profile surrounding the gene promoter may also contribute. Future experiments will further explore this interaction, looking for potential binding to the region and exploring the mechanism of break induction and transcriptional regulation.
Transcriptional downregulation could readily explain the overall decrease in protein within total cell lysates over the entire course of infection. However, the rapid decrease of ss protein by 24 hpi coupled with a lack of increased secretion suggested that targeted degradation could also be involved. Proteasomal inhibition with MG132 revealed that this was the case (Fig. 3). The stabilization of ss NID1 levels observed in virus-infected samples after addition of MG132 was not mirrored in mock-infected lysates, indicating that proteasomal degradation was abnormal for this protein. Future experiments will determine what modifications, e.g., ubiquitination/sumoylation, NID1 undergoes prior to removal via the proteasome. Data from two large-scale transcriptomic/proteomic analyses confirm decreased transcription, cell lysate ss protein levels, and secretion of NID1 (36–38; M. Weekes, personal communication).
We found that the virus employs two methods to downregulate NID1. There are numerous examples of HCMV-induced transcriptional and posttranslational changes to gene products; however, there are few reports of multipronged regulation of a single cellular protein. BclAF1 is targeted by pp71 and UL35 for proteasomal degradation and then later in infection by the miR-UL112-1 viral microRNA (39). The large-scale data analyses referred to above have identified several more cellular targets in this category (36, 40). The resources HCMV allocates to NID1’s downregulation underscore the import its removal must carry for the successful infection of a host. Our data show convincingly that HCMV’s aggressive removal of NID1 from the cellular environment serves some purpose other than increased viral replication efficiency (Fig. 5), and, in part, may contribute to increased virus dissemination throughout the host (Fig. 6). Future experiments will determine which virus proteins are responsible for NID1’s removal.
The level of effort the virus expends on the removal of NID1 from its host environment begs the question of why. As mentioned above, NID1 is an essential component of the BM. Its role is particularly important during organogenesis. NID1 is involved in BM assembly and remodeling and aids in maintaining BM integrity (4–6). Perhaps the most important BM HCMV encounters as it attempts to disseminate throughout its host is that of the vasculature. A significant proportion of the responsibility for preserving vessel integrity falls to ECs lining the vascular walls. These cells’ secretion of NID1 is important to this effort. The SMCs and adventitial fibroblasts that underly the ECs also secrete NID1 (5, 6, 8). We reconfirmed secretion of NID1 (5) and HCMV infection of ECs previously reported (22) in the E-HUVECs we used (Fig. 4 and 5).
The lab of Andrew Yurochko used transwell assays to convincingly show that infection of either migrating monocytes or seeded EC monolayers resulted in increased transmigration (22, 23). Could a decrease in NID1 secretion contribute to this phenomenon? Voisin and colleagues found that transendothelial migration of leukocytes through the vascular wall occurred in regions of low ECM expression (41). Additionally, Dorosz and colleagues showed that inflammation specifically decreased the secretion of NID1 by HUVECs and postulated that this promoted leukocyte transmigration (42). These results suggested the virus-induced downregulation of NID1 might contribute to an increase in migration. To test this hypothesis, we created NID1-KO E-HUVECs via CRISPR-mediated constructs. These cells were almost completely absent NID1 expression. Assays using these cells increased transmigration as efficiently as the infection of EC monolayers. Regulation of numerous other factors likely contribute to increased migration (as reviewed in references 43 and 44); however, downregulation of NID1 in infected ECs may well promote migration of monocytes through vessel walls.
Augmented migration through the vessel walls may also be affected by infection of the underlying SMCs. Work by Reinhardt and colleagues in an organ culture model showed that vascular SMCs are a main target for HCMV infection (45, 46). Several ECM components were preferentially downregulated in SMCs, particularly the transcription of collagen and fibronectin. Similarly to our observations of NID1 degradation, collagen was also targeted for degradation. Downregulation of NID1 in SMCs could further destabilize the ECM. Downregulation of ECM components may also contribute to the increased migration of infected SMCs observed by Streblow et al. (47). Importantly, several labs have shown distinct changes to the secretome of infected cells; our study adds to that body of work (19–21).
Increased transmigration may also be promoted by cells’ loss of connection to their underlying BMs via transmembrane receptors of the integrin family (4). In particular, β1 and β3 integrins are important for NID1 contacts (7). Relevant to our studies, Warren and colleagues showed that HCMV induced downregulation of β1 integrins in infected fibroblasts (48). The interactions between integrins and the ECM are essential for the maintenance of vascular wall integrity. Shahgasempour and colleagues found downregulation of β1 integrins after infection of HUVECs (49), which, coupled with downregulation of NID1, could also lead to decreased integrity of the vascular walls. A loss of integrity could promote extravasation of viral particles and virus-infected monocytes and neutrophils. Other potential benefits that NID1 downregulation might offer the virus include an increase in the motility of infected cells. This is observed in colon cancer cells that become increasingly metastatic after downregulation of NID1 (50). Additionally, a decrease in the integrity of BMs underlying organs could promote more proficient cell-to-cell spread, which has been shown to play an important role with clinical isolates (see reference 51 and references therein).
Virus-induced downregulation of NID1 expression in BMs of the vasculature could be beneficial for the virus; however, it is easy to envision a variety of negative ramifications such a change could have for the developing fetus. The literature verifies NID1’s presence in the BM of the amnion, chorion, and trophoblast layers of the placenta and in maternal blood vessels of the decidua and the villus core (52). Infection-increased porosity of the BMs of vessels could provide HCMV direct access to the fetal bloodstream. β1 integrin downregulation may also play a role in the negative ramifications of infection on fetal development, as trophoblasts in the placenta rely upon β1 integrin expression for adherence to NID1 within the endometrium (7). Fisher and colleagues have shown that HCMV decreases expression of β1 integrin on the surface of cytotrophoblasts (53). The combination of decreased β1 integrin expression on the trophoblasts and decreased NID1 within the BM may very well contribute to the poor invasion capabilities of these cells and inhibit proper attachment of the fetus and development of the placenta.
Downregulation of NID1 during development could also have dramatic effects on the CNS and PNS. NID1-KO mouse studies found that NID1 was important in neural network excitability and plasticity, and its absence led to alterations in BMs of brain capillaries and the lens capsule of the eye (9, 10). Also, improper optic cup morphogenesis has been reported when NID1 was absent (18), and NID1’s absence from the pial BM led to greatly compromised cortical development in mice (11). NID1 has also been found to be directly involved in neural anatomy and physiology. NID1 is secreted by both astrocytes and radial glia in the cortex, providing a substrate for proper neuronal migration during cortical development (12, 13). NID1 also regulates Schwann cell proliferation and migration in the PNS (14, 15). NID1’s presence in nerve sheaths is thought to promote Schwann cell myelin production (16). It has also been suggested that NID1 is important for proper Schwann cell-mediated regenerative peripheral nerve outgrowth after injury (15). NID1 is expressed in the developing OC, particularly in the BMs underlying nerve fibers that innervate the sensory cells and those that surround the interdental support cells of the spiral limbus (4, 17). When combined with this wide array of developmental neurological processes that NID1 contributes to, our in vitro findings of NID1 downregulation and confirmatory analysis of radically reduced NID1 protein staining in cytomegalovirus-positive (CMV+) clinical tissue suggest that HCMV’s propensity to induce CNS and PNS birth defects is linked to virus-induced downregulation of NID1. The widespread histopathological changes and drastically reduced levels of NID1 observed in these clinical samples occurred amid only sporadic observation of actively infected, viral antigen-positive cells (for examples, see Fig. 1 in reference 29 and Fig. 1 and 2 in reference 30). Whether the observed effects are due to an amount of viral infection below the level of detection or to bystander effects in uninfected cells is a question we hope to answer in the future. Interestingly, we observed the same widespread pathological effects in the relatively sparse presence of antigen-positive cells in our previous cerebral organoid studies (54). Future studies will examine the effects of NID1 downregulation in this cerebral organoid model as well.
Our study has found that in HCMV-infected HFFs and ECs, the important BM protein, NID1, is targeted for removal. We have also demonstrated that the known increase in transmigration of THP-1 cells through infected ECs is recapitulated by NID1 KO in E-HUVECs. These results support our hypothesis that HCMV gains increased capacity to disseminate within the infected host via reduced levels of NID1 proteins. The plethora of literature citing negative ramifications to CNS and PNS development which are attributable to NID1 downregulation supports our hypothesis that the protein downregulation we observed in infected tissue samples contributes to HCMV-induced birth defects. Our future work will focus on characterization of the NID1 targeting mechanisms, further defining the benefits of NID1 downregulation to viral infection and the negative ramifications of NID1 downregulation to an infected fetus.
MATERIALS AND METHODS
Cell culture conditions.
Primary HFFs (a kind gift from Steven Spector, UCSD) and THFs (a kind gift from Victor DeFilippis, OHSU) (55, 56) were propagated in Earle’s minimal essential media (MEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), l-glutamine (2 mM), penicillin (200 U/ml), streptomycin (200 μg/ml), and amphotericin B (1.5 μg/ml). HPV E6/E7 immortalized E-HUVECs were a kind gift from Ashlee Moses (OHSU) and were immortalized as described previously (27). E-HUVECs were cultured in human EC growth medium (catalog no. 211-500; Cell Applications, Inc.) per the manufacturer’s instructions. The acute monocytic leukemia cell line THP-1 was obtained from ATCC (TIB-202) and grown in suspension in RPMI 1640 medium supplemented with 10% FBS, l-glutamine (2 mM), penicillin (200 U/ml), and streptomycin (200 μg/ml). 293T cells were obtained from ATCC (CRL 3216) and were cultured in Dulbecco’s MEM (DMEM) supplemented with 10% FBS, l-glutamine (2 mM), penicillin (200 U/ml), and streptomycin (200 μg/ml). All cells were grown in humidified incubators maintained at 37°C and 5% CO2.
Virus strains used.
The lab-adapted Towne strain (ATCC VR977) and the clinical isolate TR (for triple resistant; generously provided at passage 4 by Jay Nelson, OHSU [57]) were used in these studies. TR was propagated for fewer than 4 additional passages on HFFs as previously described (58). High-titer TR stocks were generated by high-speed ultracentrifugation of viral supernatants through a 20% sucrose cushion. All TR stocks were tested to ensure maintenance of the clinical cassette of gene products (57).
HCMV infection conditions.
(i) HFF infections. G0-synchronized HFFs were trypsinized, counted, reseeded at a lower density, and allowed to settle for approximately 2 h. With one exception, all experiments in fibroblasts (Fig. 1 to 3 and 5A to D) used HCMV Towne at an MOI of 5. The exception was the low-MOI titer experiment (MOI = 0.1) pictured in Fig. 5D. At 2 to 4 hpi, virus inoculum was removed, and cells were refed with fresh medium and allowed to incubate until harvesting at the indicated times pi. Medium was not changed again for the entire time course unless otherwise indicated. NID1 secretion was determined by harvesting small aliquots of supernatant from an individual plate (mock- or virus-infected) (∼100 μl at each time point from a total starting volume of 8 ml) at the indicated times pi for Western blot analysis.
(ii) E-HUVEC infections. All experiments in E-HUVECs (Fig. 4, 5A, E, and F, and 6) used HCMV TR at an MOI of 15. Prior to virus infection, E-HUVECs were seeded onto glass coverslips or tissue culture plates, allowed to settle overnight, and then incubated with virus. After 4 h, the inoculum was removed and fresh medium was added. Coverslips or cells were harvested at the indicated time points for analysis. Transwell infections were performed by seeding cells as described below and allowing cells to reach confluence prior to infection.
Proteasome inhibition.
G0-synchronized HFFs were mock- or virus-infected at an MOI of 5. At 3.5 hpi, virus was removed, and medium containing 0.5 μM MG132 (catalog no. 1748; Tocris Biosciences) (or DMSO vehicle) was added to inhibit proteasomal activity. Cells were harvested for Western blot analysis at the indicated times pi.
Fluorescence in situ hybridization (FISH) and mapping.
Fosmid clones used for the chromosome breakpoint mapping were selected from the physical maps provided by the genome browsers of University of California, Santa Cruz (UCSC) (http://www.genome.ucsc.edu), the National Centre for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/), and Ensembl (EMBL, WTSI) (http://www.ensembl.org/). The GRCh37/hg19 assembly was used for our analyses. Genes that mapped in the vicinity of the breaksite were also chosen using these genome browsers.
To map the virus-induced breakpoint, the following fosmid clones, encompassing ∼230 kb, were included in metaphase and interphase FISH analyses: ←cen 236.10 Mb–G248P85023G5, G248P85160C5, G248P88992G1, G248P85799B5, G248P80968D1 (bridging clone—pictured in red in Fig. 1 and 2D), G248P87558G5, G248P85747D7, G248P87090E7–236.33 Mb tel→.
FISH analyses were performed using standard protocols (59). Probes were labeled by nick translation with either digoxigenin-11-dUTP (catalog no. 11-558-706-910; Roche) or biotin-16-dUTP (catalog no. 40022; Biotium) and precipitated in the presence of sheared salmon sperm and human Hybloc DNA (HHB; Applied Genetics) to block nonspecific binding. After denaturation of both slides and probes at 76°C, hybridizations were performed overnight at 37°C in a humidified chamber. Slides were washed posthybridization, and signals were amplified using the following series of incubations: (i) Alexa 488 Avidin (catalog no. A21370; Molecular Probes), (ii) Mouse anti-digoxigenin monoclonal antibody (MAb) (catalog no. D8156; Sigma) diluted in biotinylated anti-avidin (catalog no. BA-0300; Vector), (iii) anti-mouse IgG-digoxigenin (catalog no. AQ300D; Chemicon), and (iv) anti-digoxigenin rhodamine antigen-binding fragment (Fab) (catalog no. 11207750910; Roche) diluted in Alexa 488 avidin. Nuclei/chromosomes were counterstained with 4′,6-diamidino-2-phenylindole (DAPI), and slides were mounted using glycerol containing paraphenylene diamine to block photobleaching.
Metaphases present in S-phase infected cells were analyzed, as previously described (3), to identify fosmids bridging the virus-induced breaksite at 1q42. Interphase FISH analysis utilized cohybridization of nuclei with G248P80968D1 (labeled in red with rhodamine) and G248P88992G1 (labeled in green with Alexa 488). Analyses were scored as previously described (3). Briefly, dual-color fluorescence cohybridization of these fosmid clones, found in close proximity in interphase nuclei of cells in G1, produced two pairs of juxtaposed signals. Cells without breaks displayed overlapping red and green signals, producing a small region of yellow. Cells with DNA damage at this location displayed separate red and green signals.
Real-time quantitative PCR (RT-qPCR).
(i) RNA preparation and reverse transcription. Cell pellets were frozen in liquid nitrogen and stored at −80°C until RNA extraction. RNA was extracted using an SV total RNA isolation system kit (catalog no. Z3100; Promega) according to the manufacturer’s instructions. Total RNA (100 ng) was reverse transcribed into cDNA using 50 mM deoxynucleotide triphosphate (dNTPs), 250 ng of random primers, and 100 units of SuperScript II reverse transcriptase (catalog no. 18064; Invitrogen) according to the manufacturer’s instructions. cDNAs were diluted 1:100 just prior to RT-qPCR assays.
(ii) Standard curve preparation and RT-qPCR assays. Target gene transcripts were quantified using a relative standard curve (60). A mock-infected sample was harvested at 48 hpi and sequentially diluted at 1:50, 1:100, 1:500, and 1:1,000. These dilutions were used for baseline calibrations. Dilutions of the same cDNA stock were used to prepare standard curves for the target genes and the endogenous control in all RT-qPCR assays. All standard curves showed a coefficient of determination (R2) of ≥0.99. Quantification was conducted on a StepOnePlus real-time PCR system (Applied Biosystems, USA). Reactions were carried out in 20-μl volumes that contained 8 μl of diluted cDNA, 10 μl of PowerUp SYBR green mastermix (catalog no. A25742; Applied Biosystems), and a final concentration of 0.4 μM each primer for LysT, GPR137B, and G6PD or 0.5 μM for NID1. Specific forward and reverse primers, respectively, were as follows: NID1 (5′-GTGGATGCAGGCACCAATCGG-3′ and 5′-GGGCCGTGGTGATGCCATAC-3′), LysT (5′-CTCACACCCATGCCCCGAGAGG-3′ and 5′-GGTACATGATTGACCGCACTTTCTCCTG-3′), GPR137B (5′-ACAAAGGACCTTACCAACCCTG-3′ and 5′-TCTGGAGCAAAACCTCCCTGA-3′), and G6PD (5′-GAGGTGGGATGGGGTGCCCTTC-3′ and 5′-CGCTCGTAGGCGTCAGGGAGC-3′). One reaction plate was used for the standards and all test samples. All reactions were performed in triplicate for the target genes and the endogenous controls. No-template control (NTC) reactions with nuclease-free water instead of the template were included in each assay. Each RT-qPCR was performed with the following condition: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, followed by 62°C (NID1), 60°C (GPR137B), or 65°C (LysT) for 1 min. G6PD amplifications were performed at the same temperature as for the target gene in any given experiment. Melt curves were run at the end of each RT-qPCR from 68°C to 95°C to ensure generation of a single product. Product purity was also confirmed by gel inspection.
(iii) Data analysis. Raw data were exported from StepOne Real-Time PCR Software Version 2.3 (Life Technologies, USA) to Microsoft Excel 2007. For each time point-specific mock- or virus-infected sample, the expression of the target genes was first normalized to the endogenous control G6PD expression level. Each sample was then normalized to the calibrator (mock-infected 24 hpi) to obtain its relative quantification (RQ). To present the fold change of a target gene between mock- and virus-infected cells at a specific time point, we used the log10 of the values obtained from the RQ of virus-infected cells over the RQ of mock-infected cells. Error bars represent the minimum and maximum values derived from these comparisons. Data shown are derived from one experiment and are representative of two independent experiments.
Western blot analysis.
Cells were harvested for Western blot time courses at the indicated times pi, counted, pelleted, and snap-frozen in liquid nitrogen. Pellets were stored at −80°C until the time course was completed. Cell lysates were prepared as described previously (32). Lysates derived from an equivalent number of cells (2 to 3 × 105 cells) were run on 6% SDS-polyacrylamide gels, and then transferred to a Protran membrane (catalog no. 10600002; GE Healthcare Life Sciences). Lamin B was used as a loading control for all lysate analyses. Equivalent amounts of supernatant harvested on sequential days from the same plate of mock- or virus-infected cells were analyzed for NID1 secretion. Detection of proteins was as described previously (61) (catalog no. K-12042-D20; Advansta). Primary antibodies (Abs) used were mouse anti-NID1 (IgG1 clone 302117, catalog no. MAB2570-100; R&D Systems) and rabbit anti-Lamin B (catalog no. 12987-1-AP; Proteintech). Horseradish peroxidase (HRP)-coupled secondary Abs used were sheep-anti-mouse IgG (catalog no. 515-035-062; Jackson Immunoresearch Labs) and donkey anti-rabbit IgG (catalog no. NA934V; Amersham).
Immunofluorescence.
Coverslips were harvested, fixed, and stained as previously described (62). Vectashield antifade kit containing DAPI (catalog no. H1200; Vector Labs) was used to mount the slides (and counterstain nuclei). Images were obtained using a Nikon Eclipse E800 fluorescence microscope equipped with a Nikon DS-Ri1 high-resolution color camera and Nikon NIS Elements software. The primary Abs used were anti-IE1 (IgG2A; a kind gift from Bill Britt), anti-pp71 (IgG1, clone IE233; a kind gift from Rob Kalejta), and anti-UL57 (IgG2A clone CH167, catalog no. P1209; Virusys). The secondary Abs used were goat anti-mouse IgG2A Alexa Fluor 488-coupled Ab (catalog no. A21131; Molecular Probes) and goat anti-mouse IgG1 TRITC-coupled Ab (catalog no. 1070-03; Southern Biotech).
Production of NID1-KO cells by CRISPR-Cas9-directed genome editing.
(i) Targeting constructs. Design and construction of genome editing vectors for delivery of CRISPR-Cas9 components was performed following general protocols available from the Zheng Lab (63). Briefly, six different 20-nucleotide guide RNA (gRNA) sequences were designed to target NID1 protein-coding regions in exons one and two. The targeted NID1 sequences were as follows: NID1 exon 1-1, TGTCGGATCTGTCGTAGAAG; NID1 exon 1-2, CACGTAGACTGCGTCGATGT; NID1 exon 2-1, GGGGCGACTGCACCGAATGT; NID1 exon 2-3, TTTCCAGCCTAGTAGCGCGG; and NID1 exon 2-4, GTGACAACCACCGCGCTACT.
Guide RNAs were inserted into the lentiCRISPRv2 vector (catalog no. 52961; Addgene). The vector backbone, or a construct targeting a human pseudogene (HUMCYCPS3 [64]), were used as negative controls for targeting and selection.
(ii) Lentivirus production and transduction to create pooled populations. Lentiviruses were generated by transfection of 293T cells with a targeting plasmid (4 μg), a packaging plasmid (psPAX2, 4 μg, catalog no. 12260; Addgene), and a vesicular stomatitis virus G protein coding pseudotyping plasmid (pMD2.G, 4 μg, catalog no. 12259; Addgene) using Lipofectamine 2000 (catalog no. 11668; Invitrogen Life Technologies, Inc.). 293T cell supernatant was harvested at 48 h posttransfection, filtered through a 0.45-μm filter to remove cell debris, and then added to subconfluent THF or E-HUVEC cells with Polybrene at 8 μg/ml. Lentivirus supernatants were collected again at 72 h posttransfection, and the process was repeated. After 24 h, fresh medium was added, and transduced cells were allowed to recover for an additional 24 h. After recovery, medium was replaced with selection medium containing 1 to 2 μg/ml puromycin. Selection was continued until no viable cells remained on a control plate (generally 3 to 5 days). Puromycin-resistant cells were pooled, seeded at an equal density, and harvested 72 h post plating for Western blot analysis of NID1 protein expression.
(iii) Virus titration in THF-pooled CRISPR populations. THF cells (5 × 105 cells/10-cm plate) from the control v2.0, NID1 exon 1-1, and NID1 exon 1-2 pooled cell populations were seeded for titration of secreted virus. After cells adhered to the plates, they were infected with HCMV Towne at an MOI of 5 (high MOI) or 0.1 (low MOI) as described above. At the indicated times pi, a small aliquot (100 μl from a total of 8 ml) of supernatant was harvested from each dish and stored at −80°C (with 1% DMSO). Virus titer was then determined on HFFs using standard techniques. Plaques were counted at days 7 and 9 postplating, with multiple wells seeded for each dilution in the series, so that an average could be obtained for that dilution. High-MOI infections were performed twice. Low-MOI infections were performed once.
(iv) Selection of single clones in E-HUVECs. Pooled populations were analyzed for elimination of NID1 expression. The two pools exhibiting the most robust elimination were chosen for single-clone selection. Approximately 50 to 100 puromycin-selected E-HUVECs from the NID1 exon 1-1 or control HUMCYCPS3 pools were seeded onto 10-cm plates for single-colony isolation. The NID1 protein level was assessed in eight individual NID1 exon 1-1 and five individual control clones via Western blotting. Two of the NID1 exon 1-1 clones, CL18 and CL21, and one control HUMCYCPS3 clone, CL20, were used for transmigration assays. Continued NID1 elimination in clones was verified after every 5 passages or when new cell stocks were thawed.
Transmigration assays.
Transmigration assays were performed essentially as previously described (22, 23). Briefly, 2 × 104 E-HUVECs were seeded and grown in 200 μl of endothelial growth medium on 8-μm-pore-size filters in the upper chamber of transwell inserts (ThinCert, catalog no. 662638; Greiner-BioOne). Lower chambers of the transwells contained 600 μl of the same medium. Cells reached confluence in 11 to 15 days. Calcein (catalog no. 89203; Anaspec, Inc.) staining was used to verify monolayer confluence on a Zeiss Axiovert.A1 microscope fitted with an Axiocam ICm1 digital camera with Zen 3.1 software. After reaching confluence, migration assays commenced by removing medium from the upper chamber and seeding 5 × 105 THP-1 cells suspended in 200 μl of RPMI 1640 onto the E-HUVEC monolayers. Lower chamber medium was also changed to RPMI 1640. At 24 and 48 h post THP-1 seeding, lower chamber medium was exchanged, and migrated cells located in the removed medium were counted using a hemocytometer. Each experiment was performed in triplicate, and all experiments were repeated at least three times.
Analysis of clinical samples for NID1 expression.
(i) Clinical samples. Serial sections of previously analyzed paraffin-embedded CMV+ and control temporal bone specimens were obtained from two institutions, in accordance with ethics committee parameters and with informed consent (as described in detail in reference 29 and 30). Slides were processed for immunohistochemistry following standard procedures. Briefly, slides were deparaffinized in Histo-Clear II (catalog no. HS2021GLL; National Diagnostics), followed by descending ethanol (EtOH) washes, ending in phosphate-buffered saline (PBS). Slides were blocked for 1 h in 10% human IgG (catalog no. 55836; MP Biomedicals), then incubated overnight at room temperature with rat anti-NID1 Ab (clone ELM1, catalog no. MAB1946; Chemicon International) diluted in PBS in a humidified chamber. After extensive washes in PBS, slides were incubated for 1 h with biotinylated donkey anti-rat Ab (catalog no.712-065-153; Jackson Immunoresearch Labs) diluted in PBS. After extensive rinsing in PBS, signal was enhanced using a Vectastain ABC kit (catalog no. PK4000; Vector Labs) following the manufacturer’s instructions. Slides were developed using diaminobenzidine (catalog no. D5637; Sigma), followed by a light counterstain in hematoxylin solution to identify cellular structures. Slides were then dehydrated and mounted with Permount (catalog no. SP15-100; Fisher).
(ii) Sample analysis and scoring. After NID1 immunohistochemical staining and hematoxylin counterstaining, sections were imaged on a Nikon Eclipse E800 microscope equipped with a Nikon DS-Ri1 camera and Nikon Elements software. Analyses were performed in a blind manner with regard to infection status. Histopathological analysis of the extent of inflammation and tissue damage, as well as NID1 staining, was conducted on the OC and its innervating nervous tissue, including evaluation of the cochlear nerve, the spiral ganglion, radial nerve fibers projecting from the spiral ganglion to the organ of Corti, the organ of Corti and the associated structures of the cochlear duct, and the surrounding temporal bone. Semiquantitative scoring was used to assess NID1 staining intensity and distribution pattern in the above regions. Score categories were as follows: no staining (0), very weak staining (1), weak staining (2), moderate staining (3), and intense staining (4) (Table 1).
Statistical analysis.
All analyses were performed using Prism software (GraphPad Software, Inc., La Jolla, CA). Statistical significance between groups was as follows for all analyses: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
For FISH analysis, a one-way analysis of variance (ANOVA) with Tukey’s post hoc test for multiple comparisons was performed. Bars represent the mean of the data, with error bars representing one standard error.
For THF titer analysis, Welch’s t test was used for comparing means between each NID1 targeted pool and the control v2.0 pool. Bars represent the mean of the data, with error bars representing one standard error.
For transmigration assays, a one-way ANOVA with Sidak’s post hoc test to account for multiple comparisons done separately for 24-h and 48-h THP-1 migrations was performed. Bars represent the mean of the data, with error bars representing one standard error.
For histopathological comparisons, a one-way ANOVA with Tukey’s post hoc test was used for multiple comparisons of data. Bars represent the mean of the data, with error bars representing one standard error.
ACKNOWLEDGMENTS
We thank Joe Adams, Joseph Siebert, and Jennifer O’Malley for their advice and technical expertise regarding analysis and staining of clinical samples, Carol Casavant for initial characterization of 1q42 breaksite bridging clones, Lisa Shaffer and Caron Glotzbach for expert technical assistance with FISH mapping, Maria Nagel for helpful discussions, Victor DeFilippis and Ashlee Moses for providing reagents and for helpful discussions, and Jim Deringer for design of guide RNAs for NID1 CRISPR constructs.
This study was funded by NIH RO1 AI051463 and AI139503 to E.A.F., NIH P20 RR016454, and NIH P20 GM104420 (imaging equipment usage).
REFERENCES
- 1.Cannon MJ, Davis KF. 2005. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health 5:70. doi: 10.1186/1471-2458-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fortunato EA, Dell’Aquila ML, Spector DH. 2000. Specific chromosome 1 breaks induced by human cytomegalovirus. Proc Natl Acad Sci U S A 97:853–858. doi: 10.1073/pnas.97.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nystad M, Fagerheim T, Brox V, Fortunato EA, Nilssen O. 2008. Human cytomegalovirus (HCMV) and hearing impairment: infection of fibroblast cells with HCMV induces chromosome breaks at 1q23.3, between loci DFNA7 and DFNA49—both involved in dominantly inherited, sensorineural, hearing impairment. Mutat Res 637:56–65. doi: 10.1016/j.mrfmmm.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuprun V, Santi P. 2001. Proteoglycan arrays in the cochlear basement membrane. Hear Res 157:65–76. doi: 10.1016/s0378-5955(01)00278-7. [DOI] [PubMed] [Google Scholar]
- 5.Dziadek M. 1995. Role of laminin-nidogen complexes in basement membrane formation during embryonic development. Experientia 51:901–913. doi: 10.1007/BF01921740. [DOI] [PubMed] [Google Scholar]
- 6.Miosge N, Holzhausen S, Zelent C, Sprysch P, Herken R. 2001. Nidogen-1 and nidogen-2 are found in basement membranes during human embryonic development. Histochem J 33:523–530. doi: 10.1023/a:1014995523521. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Todt JC, Svinarich DM, Qureshi F, Jacques SM, Graham CH, Chung AE, Gonik B, Yelian FD. 1996. Human trophoblast cell adhesion to extracellular matrix protein, entactin. Am J Reprod Immunol 36:25–32. doi: 10.1111/j.1600-0897.1996.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 8.Stiemer B, Springmeier G, el-Jarad L, Schroter-Kermani C. 1993. Matrix production of smooth muscle cells from rat aorta in vitro. Histol Histopathol 8:63–72. [PubMed] [Google Scholar]
- 9.Dong L, Chen Y, Lewis M, Hsieh JC, Reing J, Chaillet JR, Howell CY, Melhem M, Inoue S, Kuszak JR, DeGeest K, Chung AE. 2002. Neurologic defects and selective disruption of basement membranes in mice lacking entactin-1/nidogen-1. Lab Invest 82:1617–1630. doi: 10.1097/01.lab.0000042240.52093.0f. [DOI] [PubMed] [Google Scholar]
- 10.Vasudevan A, Ho MS, Weiergraber M, Nischt R, Schneider T, Lie A, Smyth N, Kohling R. 2010. Basement membrane protein nidogen-1 shapes hippocampal synaptic plasticity and excitability. Hippocampus 20:608–620. doi: 10.1002/hipo.20660. [DOI] [PubMed] [Google Scholar]
- 11.Halfter W, Dong S, Yip YP, Willem M, Mayer U. 2002. A critical function of the pial basement membrane in cortical histogenesis. J Neurosci 22:6029–6040. doi: 10.1523/JNEUROSCI.22-14-06029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimpe B, Probst JC, Hager G. 1999. Suppression of nidogen-1 translation by antisense targeting affects the adhesive properties of cultured astrocytes. Glia 28:138–149. doi:. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Chang YW, Mohan K, Su HW, Ricupero CL, Baridi A, Hart RP, Grumet M. 2008. Activated Notch1 maintains the phenotype of radial glial cells and promotes their adhesion to laminin by upregulating nidogen. Glia 56:646–658. doi: 10.1002/glia.20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HK, Seo IA, Park HK, Park YM, Ahn KJ, Yoo YH, Park HT. 2007. Nidogen is a prosurvival and promigratory factor for adult Schwann cells. J Neurochem 102:686–698. doi: 10.1111/j.1471-4159.2007.04580.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee HK, Seo IA, Suh DJ, Park HT. 2009. Nidogen plays a role in the regenerative axon growth of adult sensory neurons through Schwann cells. J Korean Med Sci 24:654–659. doi: 10.3346/jkms.2009.24.4.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey DJ, Todd MS, Rafferty CM. 1986. Schwann cell myelination: induction by exogenous basement membrane-like extracellular matrix. J Cell Biol 102:2254–2263. doi: 10.1083/jcb.102.6.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishiyama A, Mowry SE, Lopez IA, Ishiyama G. 2009. Immunohistochemical distribution of basement membrane proteins in the human inner ear from older subjects. Hear Res 254:1–14. doi: 10.1016/j.heares.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryan CD, Casey MA, Pfeiffer RL, Jones BW, Kwan KM. 2020. Optic cup morphogenesis requires neural crest-mediated basement membrane assembly. Development 147:dev181420. doi: 10.1242/dev.181420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botto S, Streblow DN, DeFilippis V, White L, Kreklywich CN, Smith PP, Caposio P. 2011. IL-6 in human cytomegalovirus secretome promotes angiogenesis and survival of endothelial cells through the stimulation of survivin. Blood 117:352–361. doi: 10.1182/blood-2010-06-291245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumortier J, Streblow DN, Moses AV, Jacobs JM, Kreklywich CN, Camp D, Smith RD, Orloff SL, Nelson JA. 2008. Human cytomegalovirus secretome contains factors that induce angiogenesis and wound healing. J Virol 82:6524–6535. doi: 10.1128/JVI.00502-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiorentini S, Luganini A, Dell'Oste V, Lorusso B, Cervi E, Caccuri F, Bonardelli S, Landolfo S, Caruso A, Gribaudo G. 2011. Human cytomegalovirus productively infects lymphatic endothelial cells and induces a secretome that promotes angiogenesis and lymphangiogenesis through interleukin-6 and granulocyte-macrophage colony-stimulating factor. J Gen Virol 92:650–660. doi: 10.1099/vir.0.025395-0. [DOI] [PubMed] [Google Scholar]
- 22.Bentz GL, Jarquin-Pardo M, Chan G, Smith MS, Sinzger C, Yurochko AD. 2006. Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV. J Virol 80:11539–11555. doi: 10.1128/JVI.01016-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith MS, Bentz GL, Alexander JS, Yurochko AD. 2004. Human cytomegalovirus induces monocyte differentiation and migration as a strategy for dissemination and persistence. J Virol 78:4444–4453. doi: 10.1128/jvi.78.9.4444-4453.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez V, Dong JJ, Battley J, Jackson KN, Dykes BC. 2012. Human cytomegalovirus infection of THP-1 derived macrophages reveals strain-specific regulation of actin dynamics. Virology 433:64–72. doi: 10.1016/j.virol.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Umehara H, Goda S, Imai T, Nagano Y, Minami Y, Tanaka Y, Okazaki T, Bloom ET, Domae N. 2001. Fractalkine, a CX3C-chemokine, functions predominantly as an adhesion molecule in monocytic cell line THP-1. Immunol Cell Biol 79:298–302. doi: 10.1046/j.1440-1711.2001.01004.x. [DOI] [PubMed] [Google Scholar]
- 26.Masedunskas A, King JA, Tan F, Cochran R, Stevens T, Sviridov D, Ofori-Acquah SF. 2006. Activated leukocyte cell adhesion molecule is a component of the endothelial junction involved in transendothelial monocyte migration. FEBS Lett 580:2637–2645. doi: 10.1016/j.febslet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Moses AV, Fish KN, Ruhl R, Smith PP, Strussenberg JG, Zhu L, Chandran B, Nelson JA. 1999. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J Virol 73:6892–6902. doi: 10.1128/JVI.73.8.6892-6902.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan G, Nogalski MT, Yurochko AD. 2009. Activation of EGFR on monocytes is required for human cytomegalovirus entry and mediates cellular motility. Proc Natl Acad Sci U S A 106:22369–22374. doi: 10.1073/pnas.0908787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabrielli L, Bonasoni MP, Santini D, Piccirilli G, Chiereghin A, Guerra B, Landini MP, Capretti MG, Lanari M, Lazzarotto T. 2013. Human fetal inner ear involvement in congenital cytomegalovirus infection. Acta Neuropathol Commun 1:63. doi: 10.1186/2051-5960-1-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teissier N, Delezoide AL, Mas AE, Khung-Savatovsky S, Bessieres B, Nardelli J, Vauloup-Fellous C, Picone O, Houhou N, Oury JF, Van Den Abbeele T, Gressens P, Adle-Biassette H. 2011. Inner ear lesions in congenital cytomegalovirus infection of human fetuses. Acta Neuropathol 122:763–774. doi: 10.1007/s00401-011-0895-y. [DOI] [PubMed] [Google Scholar]
- 31.Fortunato EA, Spector DH. 2003. Viral induction of site-specific chromosome damage. Rev Med Virol 13:21–37. doi: 10.1002/rmv.368. [DOI] [PubMed] [Google Scholar]
- 32.Luo MH, Rosenke K, Czornak K, Fortunato EA. 2007. Human cytomegalovirus disrupts both ataxia telangiectasia mutated protein (ATM)- and ATM-Rad3-related kinase-mediated DNA damage responses during lytic infection. J Virol 81:1934–1950. doi: 10.1128/JVI.01670-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulkarni AS, Fortunato EA. 2011. Stimulation of homology-directed repair at I-SceI-induced DNA breaks during the permissive life cycle of human cytomegalovirus. J Virol 85:6049–6054. doi: 10.1128/JVI.02514-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulkarni AS, Fortunato EA. 2014. Modulation of homology-directed repair in T98G glioblastoma cells due to interactions between wildtype p53, Rad51 and HCMV IE1-72. Viruses 6:968–985. doi: 10.3390/v6030968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Dowd JM, Zavala AG, Brown CJ, Mori T, Fortunato EA. 2012. HCMV-infected cells maintain efficient nucleotide excision repair of the viral genome while abrogating repair of the host genome. PLoS Pathog 8:e1003038. doi: 10.1371/journal.ppat.1003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nightingale K, Lin KM, Ravenhill BJ, Davies C, Nobre L, Fielding CA, Ruckova E, Fletcher-Etherington A, Soday L, Nichols H, Sugrue D, Wang ECY, Moreno P, Umrania Y, Huttlin EL, Antrobus R, Davison AJ, Wilkinson GWG, Stanton RJ, Tomasec P, Weekes MP. 2018. High-definition analysis of host protein stability during human cytomegalovirus infection reveals antiviral factors and viral evasion mechanisms. Cell Host Microbe 24:447–460.e411. doi: 10.1016/j.chom.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tirosh O, Cohen Y, Shitrit A, Shani O, Le-Trilling VT, Trilling M, Friedlander G, Tanenbaum M, Stern-Ginossar N. 2015. The transcription and translation landscapes during human cytomegalovirus infection reveal novel host-pathogen interactions. PLoS Pathog 11:e1005288. doi: 10.1371/journal.ppat.1005288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weekes MP, Tomasec P, Huttlin EL, Fielding CA, Nusinow D, Stanton RJ, Wang ECY, Aicheler R, Murrell I, Wilkinson GWG, Lehner PJ, Gygi SP. 2014. Quantitative temporal viromics: an approach to investigate host-pathogen interaction. Cell 157:1460–1472. doi: 10.1016/j.cell.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SH, Kalejta RF, Kerry J, Semmes OJ, O'Connor CM, Khan Z, Garcia BA, Shenk T, Murphy E. 2012. BclAF1 restriction factor is neutralized by proteasomal degradation and microRNA repression during human cytomegalovirus infection. Proc Natl Acad Sci U S A 109:9575–9580. doi: 10.1073/pnas.1207496109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nobre LV, Nightingale K, Ravenhill BJ, Antrobus R, Soday L, Nichols J, Davies JA, Seirafian S, Wang EC, Davison AJ, Wilkinson GW, Stanton RJ, Huttlin EL, Weekes MP. 2019. Human cytomegalovirus interactome analysis identifies degradation hubs, domain associations and viral protein functions. Elife 8:e49894. doi: 10.7554/eLife.49894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voisin MB, Woodfin A, Nourshargh S. 2009. Monocytes and neutrophils exhibit both distinct and common mechanisms in penetrating the vascular basement membrane in vivo. Arterioscler Thromb Vasc Biol 29:1193–1199. doi: 10.1161/ATVBAHA.109.187450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dorosz SA, Ginolhac A, Kahne T, Naumann M, Sauter T, Salsmann A, Bueb JL. 2015. Role of calprotectin as a modulator of the IL27-mediated proinflammatory effect on endothelial cells. Mediators Inflamm 2015:737310. doi: 10.1155/2015/737310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins-McMillen D, Stevenson EV, Kim JH, Lee BJ, Cieply SJ, Nogalski MT, Chan GC, Frost RW, 3rd, Spohn CR, Yurochko AD. 2017. Human cytomegalovirus utilizes a nontraditional signal transducer and activator of transcription 1 activation cascade via signaling through epidermal growth factor receptor and integrins to efficiently promote the motility, differentiation, and polarization of infected monocytes. J Virol 91:e00622-17. doi: 10.1128/JVI.00622-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevenson EV, Collins-McMillen D, Kim JH, Cieply SJ, Bentz GL, Yurochko AD. 2014. HCMV reprogramming of infected monocyte survival and differentiation: a Goldilocks phenomenon. Viruses 6:782–807. doi: 10.3390/v6020782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reinhardt B, Vaida B, Voisard R, Keller L, Breul J, Metzger H, Herter T, Baur R, Luske A, Mertens T. 2003. Human cytomegalovirus infection in human renal arteries in vitro. J Virol Methods 109:1–9. doi: 10.1016/s0166-0934(03)00035-1. [DOI] [PubMed] [Google Scholar]
- 46.Reinhardt B, Winkler M, Schaarschmidt P, Pretsch R, Zhou S, Vaida B, Schmid-Kotsas A, Michel D, Walther P, Bachem M, Mertens T. 2006. Human cytomegalovirus-induced reduction of extracellular matrix proteins in vascular smooth muscle cell cultures: a pathomechanism in vasculopathies? J Gen Virol 87:2849–2858. doi: 10.1099/vir.0.81955-0. [DOI] [PubMed] [Google Scholar]
- 47.Streblow DN, Soderberg-Naucler C, Vieira J, Smith P, Wakabayashi E, Ruchti F, Mattison K, Altschuler Y, Nelson JA. 1999. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 99:511–520. doi: 10.1016/s0092-8674(00)81539-1. [DOI] [PubMed] [Google Scholar]
- 48.Warren AP, Owens CN, Borysiewicz LK, Patel K. 1994. Down-regulation of integrin alpha 1/beta 1 expression and association with cell rounding in human cytomegalovirus-infected fibroblasts. J Gen Virol 75:3319–3325. doi: 10.1099/0022-1317-75-12-3319. [DOI] [PubMed] [Google Scholar]
- 49.Shahgasempour S, Woodroffe SB, Sullivan-Tailyour G, Garnett HM. 1997. Alteration in the expression of endothelial cell integrin receptors alpha 5 beta 1 and alpha 2 beta 1 and alpha 6 beta 1 after in vitro infection with a clinical isolate of human cytomegalovirus. Arch Virol 142:125–138. doi: 10.1007/s007050050063. [DOI] [PubMed] [Google Scholar]
- 50.Ulazzi L, Sabbioni S, Miotto E, Veronese A, Angusti A, Gafa R, Manfredini S, Farinati F, Sasaki T, Lanza G, Negrini M. 2007. Nidogen 1 and 2 gene promoters are aberrantly methylated in human gastrointestinal cancer. Mol Cancer 6:17. doi: 10.1186/1476-4598-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schultz EP, Lanchy JM, Day LZ, Yu Q, Peterson C, Preece J, Ryckman BJ. 2020. Specialization for cell-free or cell-to-cell spread of BAC-cloned human cytomegalovirus strains is determined by factors beyond the UL128-131 and RL13 loci. J Virol 94. doi: 10.1128/JVI.00034-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith J, Ockleford CD. 1994. Laser scanning confocal examination and comparison of nidogen (entactin) with laminin in term human amniochorion. Placenta 15:95–106. doi: 10.1016/s0143-4004(05)80240-1. [DOI] [PubMed] [Google Scholar]
- 53.Fisher S, Genbacev O, Maidji E, Pereira L. 2000. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J Virol 74:6808–6820. doi: 10.1128/jvi.74.15.6808-6820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown RM, Rana PSJB, Jaeger HK, O’Dowd JM, Balemba OB, Fortunato EA. 2019. Human cytomegalovirus compromises development of cerebral organoids. J Virol 93:e00957-19. doi: 10.1128/JVI.00957-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bresnahan WA, Hultman GE, Shenk T. 2000. Replication of wild-type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J Virol 74:10816–10818. doi: 10.1128/jvi.74.22.10816-10818.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeFilippis VR, Alvarado D, Sali T, Rothenburg S, Fruh K. 2010. Human cytomegalovirus induces the interferon response via the DNA sensor ZBP1. J Virol 84:585–598. doi: 10.1128/JVI.01748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy E, Yu D, Grimwood J, Schmutz J, Dickson M, Jarvis MA, Hahn G, Nelson JA, Myers RM, Shenk TE. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc Natl Acad Sci U S A 100:14976–14981. doi: 10.1073/pnas.2136652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamashiro JC, Hock LJ, Spector DH. 1982. Construction of a cloned library of the EcoRI fragments from the human cytomegalovirus genome (strain AD169). J Virol 42:547–557. doi: 10.1128/JVI.42.2.547-557.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pole JC, Courtay-Cahen C, Garcia MJ, Blood KA, Cooke SL, Alsop AE, Tse DM, Caldas C, Edwards PA. 2006. High-resolution analysis of chromosome rearrangements on 8p in breast, colon and pancreatic cancer reveals a complex pattern of loss, gain and translocation. Oncogene 25:5693–5706. doi: 10.1038/sj.onc.1209570. [DOI] [PubMed] [Google Scholar]
- 60.Larionov A, Krause A, Miller W. 2005. A standard curve based method for relative real time PCR data processing. BMC Bioinformatics 6:62. doi: 10.1186/1471-2105-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuan MI, O’Dowd JM, Fortunato EA. 2016. The absence of p53 during human cytomegalovirus infection leads to decreased UL53 expression, disrupting UL50 localization to the inner nuclear membrane, and thereby inhibiting capsid nuclear egress. Virology 497:262–278. doi: 10.1016/j.virol.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenke K, Samuel MA, McDowell ET, Toerne MA, Fortunato EA. 2006. An intact sequence-specific DNA-binding domain is required for human cytomegalovirus-mediated sequestration of p53 and may promote in vivo binding to the viral genome during infection. Virology 348:19–34. doi: 10.1016/j.virol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 63.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. 2013. Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Botto S, Abraham J, Mizuno N, Pryke K, Gall B, Landais I, Streblow DN, Fruh KJ, DeFilippis VR. 2019. Human cytomegalovirus immediate early 86-kDa protein blocks transcription and induces degradation of the immature interleukin-1β protein during virion-mediated activation of the AIM2 inflammasome. mBio 10:e02510-18. doi: 10.1128/mBio.02510-18. [DOI] [PMC free article] [PubMed] [Google Scholar]