It is well established that selective pressures in mosquito vectors impose population bottlenecks for arboviruses. Here, we used a CHIKV Caribbean lineage mutant carrying a deletion in the 3′ UTR to study host-virus interactions in vivo in the epidemic mosquito vector Aedes aegypti. We found that the mutant virus had a delayed replication rate in mosquitoes, which lengthened the extrinsic incubation period (EIP) and reduced fitness relative to the wild-type virus. As a result, the mutant virus displayed a reduced capacity to cross anatomical barriers during the infection cycle in mosquitoes, thus reducing the virus transmission rate. Our findings show how selective pressures act on CHIKV noncoding regions to select variants with shorter EIPs that are preferentially transmitted by the mosquito vector.
KEYWORDS: 3′ UTR, alphavirus, arthropod vectors, bottlenecks, extrinsic incubation period
ABSTRACT
Chikungunya virus (CHIKV) is a reemerging and rapidly spreading pathogen transmitted by mosquitoes. The emergence of new epidemic variants of the virus is associated with genetic evolutionary traits, including duplication of repeated RNA elements in the 3′ untranslated region (UTR) that seemingly favor transmission by mosquitoes. The transmission potential of a given variant results from a complex interplay between virus populations and anatomical tissue barriers in the mosquito. Here, we used the wild-type CHIKV Caribbean strain and an engineered mutant harboring a deletion in the 3′ UTR to dissect the interactions of virus variants with the anatomical barriers that impede transmission during the replication cycle of the virus in Aedes mosquitoes. Compared to the 3′-UTR mutant, we observed that the wild-type virus had a short extrinsic incubation period (EIP) after an infectious blood meal and was expectorated into mosquito saliva much more efficiently. We found that high viral titers in the midgut are not sufficient to escape the midgut escape barrier. Rather, viral replication kinetics play a crucial role in determining midgut escape and the transmission ability of CHIKV. Finally, competition tests in mosquitoes coinfected with wild-type and mutant viruses revealed that both viruses successfully colonized the midgut, but wild-type viruses effectively displaced mutant viruses during systemic infection due to their greater efficiency of escaping from the midgut into secondary tissues. Overall, our results uncover a link between CHIKV replication kinetics and the effect of bottlenecks on population diversity, as slowly replicating variants are less able to overcome the midgut escape barrier.
IMPORTANCE It is well established that selective pressures in mosquito vectors impose population bottlenecks for arboviruses. Here, we used a CHIKV Caribbean lineage mutant carrying a deletion in the 3′ UTR to study host-virus interactions in vivo in the epidemic mosquito vector Aedes aegypti. We found that the mutant virus had a delayed replication rate in mosquitoes, which lengthened the extrinsic incubation period (EIP) and reduced fitness relative to the wild-type virus. As a result, the mutant virus displayed a reduced capacity to cross anatomical barriers during the infection cycle in mosquitoes, thus reducing the virus transmission rate. Our findings show how selective pressures act on CHIKV noncoding regions to select variants with shorter EIPs that are preferentially transmitted by the mosquito vector.
INTRODUCTION
Chikungunya virus (CHIKV) is an arthropod-borne virus that after 60 years of exclusive circulation in Asia and Africa has recently spread into Europe and America, producing about 1.7 million infections (1–5). CHIKV infection has thus emerged as a major public health concern since it may affect a large proportion of the population within an outbreak area (6). CHIKV infections are usually nonfatal and resolve over time, but they cause considerable pain, distress, and anxiety as well as a significant economic burden due to severe clinical manifestations (7–9). There is no commercially available vaccine against CHIKV, and intervention efforts during outbreaks focus on preventing mosquito exposure and inhibiting local mosquito population growth (10, 11).
CHIKV cycles between mosquito and human hosts and has evolved strategies that allow maintenance of efficient replication in these two disparate host environments. Research efforts have focused on the identification of viral genome sequences that determine the virus host range (12). The CHIKV genome is a single-stranded positive-sense RNA of 11 to 12 kb that carries a 3′ untranslated region (UTR) containing 50- to 80-nucleotide (nt)-long sequence repetitions referred to as direct repeats (13, 14) that change in copy number among viral strains (15–17). Evidence shows that the 3′ UTR is subjected to conflicting selective pressures in mammalian and mosquito hosts and that duplicated direct repeats are maintained in nature due to positive selection in the mosquito host (17). The Caribbean strains bear the longest 3′ UTRs among CHIKV lineages and display 5 copies of direct repeats. Previous work from our group showed that virus replication in mammalian cells results in the emergence of variants carrying large 3′-UTR deletions that are cleared in mosquitoes (18). In addition, Chen et al. reported that for the Asian CHIKV strain, an intact 3′ UTR provides a selective advantage in mosquitoes over a virus with a shorter 3′ UTR, as viruses with intact 3′ UTRs prevailed in the heads of mosquitoes 10 days after mixed infections (16). While in vitro studies demonstrate delayed replication rates of 3′-UTR deletion mutants in C6/36 mosquito cells, a detailed investigation of the relevance of CHIKV replication kinetics in mosquitoes in vivo is still lacking. Moreover, consequences on transmission dynamics for viral variants with delayed growth have not yet been explored (19).
The transmission efficiency (TE) and the extrinsic incubation period (EIP) are two common indexes used to describe the interaction between viruses and their vectors. While the first one is related to the ability of the pathogen to be successfully transmitted to another susceptible host, the second one defines the interval of time for this infectious cycle to be completed (20, 21). Both parameters are highly dependent on four anatomical barriers or bottlenecks that viruses must cross within the mosquito in order to be transmitted (22–24). The first barrier is determined by the capacity of the virus to infect and replicate in midgut epithelial cells of the mosquito after a blood meal (midgut infection barrier). Once it has successfully established a midgut infection, escape from the midgut imposes a barrier for the virus to disseminate through the hemolymph to secondary organs and peripheral tissues such as the fat body and trachea. The inability to disseminate at this step could result from defects in the release of virions from midgut epithelial cells (midgut escape barrier). The next anatomical barrier to infection occurs at the end of the dissemination process when the virus has to reach the salivary glands (salivary gland infection barrier). Finally, in order to be successfully transmitted, viruses must replicate efficiently inside salivary glands to be released into the saliva, which is injected into a human host when the mosquito takes the next blood meal (salivary gland escape barrier). For CHIKV, the salivary gland escape barrier has a very strong impact on virus transmission efficiency (25–27).
In this work, we addressed the relationship between CHIKV replication kinetics and its capacity to overcome successive physiological barriers and complete a replication cycle in mosquitoes in order to be successfully transmitted. We gained insight into barriers to arbovirus transmission using an engineered variant of the Caribbean strain of CHIKV bearing a deletion of the first 500 nt of the 3′ UTR as a tool. Our data show that delayed growth kinetics in Aedes mosquitoes resulted in an extended EIP, which in turn compromised transmission efficiency. We found that this effect on transmission is associated with a severe bottleneck during escape from the midgut and, to a lesser extent, impaired secretion into saliva. In addition, virus competition assays in mosquitoes showing that small amounts of fast-replicating viral variants were able to displace slow-replicating viruses in disseminated tissues provide novel insight into how mosquito bottlenecks restrict arbovirus diversity.
RESULTS
Mosquito replication cycle of wild-type and 3′-UTR deletion mutant viruses.
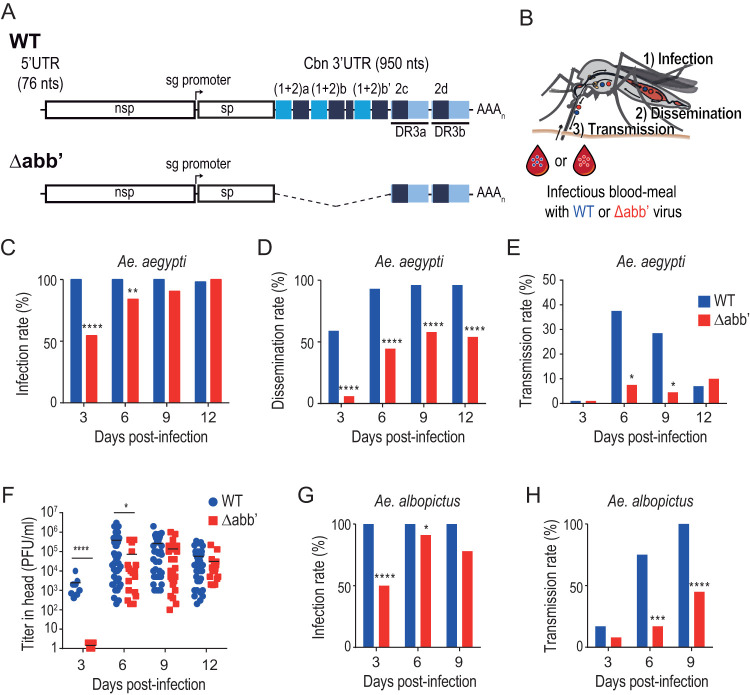
To gain insight into the mosquito cycle of the Caribbean CHIKV strain in its epidemic vector, we used Aedes aegypti mosquito infections to determine the EIPs of wild-type (WT) virus and an engineered 3′-UTR deletion mutant (referred to here as Δabb′) that has been previously described to show impaired growth rates in mosquito cells in vitro (18) (Fig. 1A). Laboratory colonies of Aedes aegypti mosquitoes were fed with an infectious blood meal containing 106 PFU/ml of wild-type or Δabb′ mutant virus. At 3, 6, 9, and 12 days after the blood meal, we analyzed the presence of each virus in the body (as a proxy of the infection rate [IR]), in the head (as a proxy of dissemination rate [DR] to salivary glands) (28–30), and in the saliva (indicative of the transmission rate [TR]) in individual mosquitoes (Fig. 1B). For each virus, the infection rate was estimated as the percentage of mosquitoes with infectious viruses in their bodies (Fig. 1C), measured by the development of cytopathic effect (CPE) on Vero cells inoculated with whole-body extracts. At day 3, we observed that 100% of the engorged mosquitoes were infected with the wild-type virus, while only 50% of the mosquitoes exposed to the mutant virus became infected. Eventually, infection with the mutant virus progressed, and the whole pool of mosquitoes was infected by day 12. This result indicates that the Δabb′ mutant has no impediment in crossing the midgut infection barrier. Therefore, differences in the infection rates at short times after blood feeding rather reflect the lower growth rate of the mutant than of the wild type, resulting in longer times to reach the threshold level to be detected by our method. Next, we determined the dissemination rate, i.e., the ratio between the number of mosquito heads with detectable virus and the number of infected mosquitoes (Fig. 1D). The results showed a 50% dissemination rate for the wild type at day 3 and a 100% dissemination rate by day 6. In contrast, the Δabb′ virus was detected in the heads of infected mosquitoes only after 6 days, and even at later time points, it reached the head in no more than 50% of the individuals, pointing to a defect at a stage between colonization of the midgut and arrival to salivary glands. Finally, we measured the transmission rate, i.e., the ratio between the number of mosquito saliva specimens with detectable virus and the number of mosquitoes with disseminated infection (Fig. 1E). The transmission rate peaked at almost 40% for the wild type at day 6 and decreased by day 9. In contrast, Δabb′ CHIKV reached maximum transmission at day 12, with a rate of only 10%. For both dissemination and transmission rates, we used the cytopathic effect assay to score infection as it is informative of the nature of the infectivity of the virus in the disseminated tissues and, importantly, of the virus expectorated into saliva, respectively. As noted above, it may be possible that dissemination and transmission rates are underestimated compared to those determined by molecular methods because of the limit of detection of the assay. However, as opposed to the increase observed in the infection rate of the mutant virus, dissemination rates did not increase over the course of the experiment (compare days 6, 9, and 12 in Fig. 1D), suggesting that the mutant virus likely encounters a midgut escape barrier to infection. The results obtained for the wild-type transmission rate are similar to those in previous reports and show that the salivary gland entry and exit barriers impose the greatest limiting effect for transmission in nature (25, 26, 31). Infection, dissemination, and transmission rates of wild-type and Δabb′ viruses are summarized in Table 1.
FIG 1.
Extrinsic incubation period of wild-type and Δabb′ mutant CHIKVs in Aedes mosquitoes. (A) Schematic representation of the genomes of wild-type (WT) and Δabb′ mutant viruses. The Δabb′ mutant bears a deletion of the first 500 nucleotides of the 3′ UTR. (B) Extrinsic incubation period of WT and Δabb′ CHIKVs. Mosquitoes were blood fed with 106 PFU/ml of WT or Δabb′ mutant viruses, and the presence of virus was analyzed in the body (as a proxy of the infection rate), the head (as a proxy of the rate of dissemination to salivary glands), and the saliva (indicative of the transmission rate) at different times postinfection. (C to E) Bar graphs showing infection, dissemination, and transmission rates of WT and Δabb′ viruses in infected Aedes aegypti mosquitoes. (C) The infection rate was calculated as the percentage of infected mosquito bodies at each time point. (D) The dissemination rate was scored as the number of infected mosquito heads over the number of infected bodies. (E) The transmission rate was measured as the ratio between the number of mosquito saliva samples with detectable virus and the number of mosquitoes in which dissemination was successful. Bars for infection, dissemination, and transmission rates represent cumulative data from two independent experiments (n = 48). Data were analyzed by Fisher’s exact test. (F) Dot plot showing mean viral titers and standard deviations (SD) of WT and Δabb′ viruses in the heads of infected mosquitoes. Infectious virus titers were measured in the heads of mosquitoes displaying positive CPE at each time point by plaque assays in Vero cells. Data represent the titers in individual mosquitoes. Statistics were performed by a Mann-Whitney U test. (G and H) Infection and dissemination rates in Aedes albopictus mosquitoes. Bar graphs for infection (G) and dissemination (H) rates are shown (n = 24). Data were analyzed by Fisher’s exact test.
TABLE 1.
Infection, dissemination, and transmission rates (percentages) estimated on different days after exposure of A. aegypti to the CHIKV wild-type or Δabb′ mutant straina
| Day postinfection | CHIKV wild type |
CHIKV Δabb′ |
||||
|---|---|---|---|---|---|---|
| IR [no. of mosquitoes (%)] | DR [no. of mosquitoes (%)] | TR [no. of mosquitoes (%)] | IR [no. of mosquitoes (%)] | DR [no. of mosquitoes (%)] | TR [no. of mosquitoes (%)] | |
| 3 | 48 (100) | 28 (58) | 0 (0) | 48 (55) | 2 (6) | 0 (0) |
| 6 | 48 (100) | 45 (93) | 17 (38) | 48 (84) | 18 (45) | 1 (7) |
| 9 | 48 (100) | 46 (96) | 13 (29) | 48 (91) | 25 (58) | 1 (4) |
| 12 | 48 (98) | 45 (96) | 3 (7) | 48 (100) | 26 (54) | 2 (10) |
Abbreviations: IR, infection rate; DR, dissemination rate; TR, transmission rate.
In order to determine whether the decreased dissemination rate of the mutant is accompanied by lower viral titers in disseminated tissues, we measured the viral titers of wild-type and Δabb′ viruses in mosquito heads at different times postinfection (Fig. 1F). Consistent with the estimates of dissemination rates, the wild-type virus reached an average titer of 2 × 103 PFU/ml at day 3, while at this time point, mutant viruses were not detectable. However, as soon as infection disseminated at 6 days postinfection, the mutant virus reached viral titers comparable to those of the wild type. Therefore, the defect in transmission is likely related to a growth delay rather than to a defect to reach high viral titers.
To evaluate whether this phenomenon extends to other vector species of CHIKV, the same experiment was performed by infecting Aedes albopictus mosquitoes. Estimates of infection and dissemination rates are presented in Fig. 1G and H. The results recapitulated our observations with A. aegypti mosquitoes, underscoring the role of viral replication kinetics in viral dissemination and subsequent transmission, regardless of the mosquito species.
Together, these data showed that similar to replication in cell culture, the mutant virus has a low replication rate at the site of colonization (i.e., mosquito midguts) that results in decreased abilities to disseminate as well as to be secreted into the mosquito saliva compared to the wild-type virus. This defect is also reflected in a longer EIP, defined as a quantitative trait of the mosquito population instead of a threshold time point at which the first mosquito becomes infectious (29).
Deficient dissemination of Δabb′ mutant virus is due to a defect to cross the midgut escape barrier.
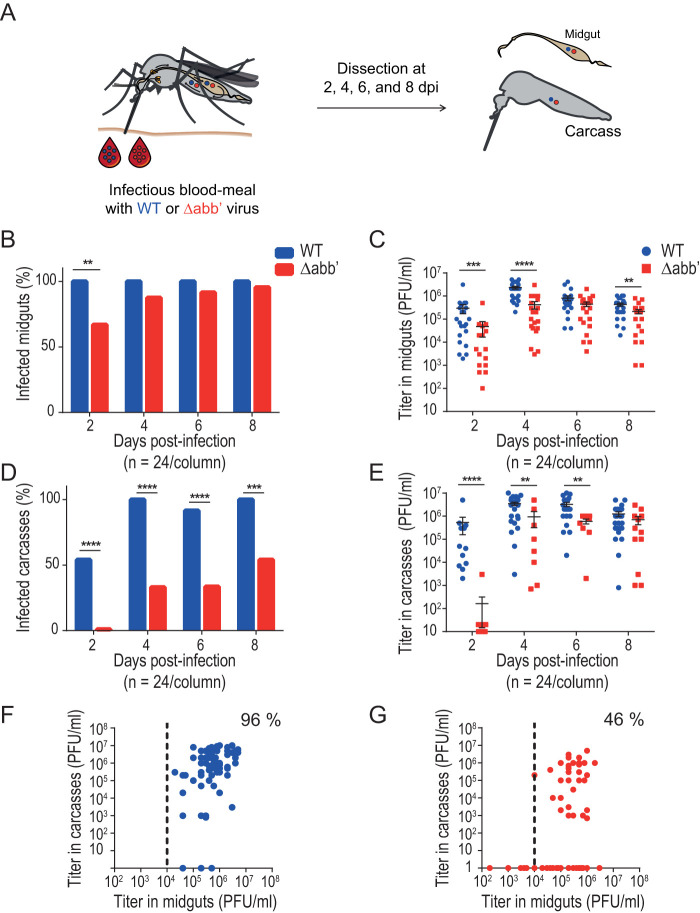
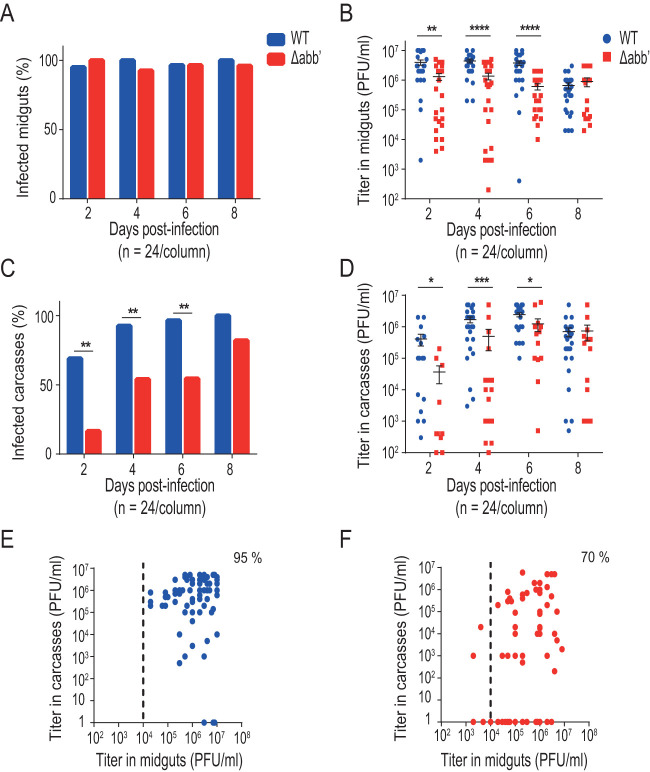
The delayed EIP of the Δabb′ mutant virus could reflect a problem of the virus either to leave midgut at the beginning of the infection or to spread through the hemolymph and reach secondary organs during dissemination. To differentiate between these two possibilities, we assessed infection rates and viral titers of wild-type or Δabb′ CHIKV in the midgut and carcass (i.e., the rest of the body after removing the midgut) of mosquitoes from days 2 to 8 after infectious blood feeding (Fig. 2A). Similar to the EIP, both viruses eventually reached almost a 100% rate of infection of midguts (day 2 versus day 6 for wild-type and mutant viruses, respectively), indicating efficient colonization of the midgut (Fig. 2B). Mean viral titers in the midgut were significantly lower for the mutant at early time points, and as of day 6, both viruses reached comparable titers (Fig. 2C), indicating delayed replication rates of Δabb′ compared to the wild type. The rate of carcass infection was used as a proxy for the ability to escape from the midgut and spread in the infected mosquito. The results showed that the mutant virus was detected in carcasses later than the wild type and failed to infect the carcass in half of the individuals (Fig. 2D), pointing to a defect in escape from the midgut. Similar to midgut viral titers, carcass titers were significantly lower for the mutant than for the wild-type virus at earlier times after infection. Despite the delayed replication kinetics, at day 8, both viruses reached comparable titers (Fig. 2E). Finally, we analyzed paired viral titers in the midgut and carcass of each individual as of the fourth day postinfection. Viral titers in the midgut were higher than 104 PFU/ml in 100% of mosquitoes infected with the wild-type virus, and in 96% of them, viral dissemination to the carcass was successful (Fig. 2F). In the case of mosquitoes infected with the mutant virus, although there was a slight drop in the number of individuals with midgut titers of >104 PFU/ml (89% of the analyzed mosquitoes), the virus was able to cross the midgut escape barrier in only 46% of these individuals (Fig. 2G). A possible interpretation of this result is that reaching a threshold value for viral titers in the midgut is necessary but not sufficient to guarantee successful dissemination. In addition to a threshold titer, a “window of opportunity” may define a timing effect that determines the ability to escape the midgut barrier (32). To test this hypothesis, we repeated the experiment using five-times-higher viral titers in the blood meal to increase the virus input in midgut cells (Fig. 3). We reasoned that increasing the viral titer in the input would allow the mutant to reach threshold titers earlier in the mosquito cycle and that it would favor escaping the midgut (33). Figure 3A shows that both viruses infected midguts at similar rates. In contrast to infections with a low input, infections with higher doses disseminated into the carcass as of day 2 for both viruses, and differences in dissemination rates disappeared at day 8 (Fig. 3C). Analysis of paired midgut and carcass viral titers further confirmed the effect of the input on the ability of the mutant virus to disseminate; we found that Δabb′ CHIKV achieved successful dissemination in 70% of mosquitoes with midgut titers of >104 PFU/ml (Fig. 3F). Thus, it appears that the delay to reach this threshold titer negatively impacted viral dissemination of the mutant, likely due to an impairment in overcoming the midgut escape barrier. In summary, these results indicate that the initial dose and viral replication kinetics have a strong effect on the ability of CHIKV to escape the midgut.
FIG 2.
Δabb′ mutant CHIKV is impaired in escaping the midgut. (A) Midgut escape barrier assay. Mosquitoes were blood fed with 106 PFU/ml of wild-type (WT) or Δabb′ mutant CHIKV and dissected from days 2 to 8 to separate midguts and carcasses. Infection rates and viral titers were measured in each sample. dpi, days postinfection. (B) Bar graph showing midgut infection rates. Data represent the percentages of infected mosquito midguts at each time point. (C) Dot plot showing mean viral titers and SD of WT and Δabb′ viruses in midguts of infected mosquitoes. Virus titers in midgut extracts scored positive by a CPE assay were measured by a plaque assay. Data represent titers of individual midguts. (D) Bar graph showing carcass infection rates. Data represent the percentages of infected carcasses at different times after blood feeding and reflect virus dissemination efficiencies. (E) Dot plot showing mean viral titers and SD of WT and Δabb′ viruses in carcasses of infected mosquitoes. Virus titers in carcass extracts were measured by plaque assays. Data represent titers in individual carcasses. (F and G) Scatterplots of viral titers in the midgut versus carcass for individual mosquitoes from the fourth to eighth days postinfection. The dotted line indicates the threshold titer needed to leave the midgut, which was set at 104 PFU/ml. The percentage of mosquitoes above this threshold with disseminated infection was measured for wild-type (F) and mutant (G) viruses. Statistics on infection rates were performed by Fisher’s exact test on cumulative data (n = 24) from two independent experiments. Statistics on viral titers were performed by a Mann-Whitney U test.
FIG 3.
Increasing the infectious dose decreases the midgut escape barrier effect. Mosquitoes were blood fed with 5 × 106 PFU/ml wild-type (WT) or Δabb′ mutant CHIKV and dissected from days 2 to 8 to separate midguts and carcasses. Infection rates and viral titers were measured in each sample. (A) Bar graph showing midgut infection rates. (B) Dot plot showing mean viral titers and SD of WT and Δabb′ viruses in midguts of infected mosquitoes. (C) Bar graph showing carcass infection rates. (D) Dot plot showing mean viral titers and SD of WT and Δabb′ viruses in carcasses of infected mosquitoes. (E and F) Scatterplot of viral titers in the midgut versus carcass for wild type (E) and mutant (F) viruses. Statistics on infection rates were performed by Fisher’s exact test on cumulative data (n = 24) from two independent experiments. Statistics on viral titers were performed by a Mann-Whitney U test.
Deficiency in viral replication capacity also occurs in secondary tissues during dissemination.
With the aim of assessing if the slow replication kinetics of the Δabb′ virus impacts barriers other than the midgut escape barrier during the mosquito replication cycle, we infected mosquitoes through the intrathoracic route to bypass the first two barriers that occur during infectious blood feeding (i.e., the midgut infection and escape barriers) (Fig. 4A). Mosquitoes were intrathoracically injected with 2,500 PFU of the wild-type or Δabb′ mutant virus so that the initial viral titers in the mosquito hemolymph were the same for both viruses. Next, infection and transmission rates as well as viral titers in the body of infected mosquitoes were measured every 2 days. Mosquito infection rates, estimated as the presence of viruses in the body at different times postinjection, were 100% for both viruses at all tested time points (Fig. 4B). Virus titration in the bodies showed ∼10-fold-higher viral titers for the wild type than for the mutant at days 2 and 4, and as of day 6, both viruses showed the same titers (Fig. 4C). In turn, the overall trend of the transmission rate, estimated as the presence of viruses in the saliva, was slightly lower for the mutant than for the wild type (Fig. 4D). These data indicate that the mutant virus growth rate is also affected in secondary tissues, impacting its ability to cross the salivary gland barriers and thus contributing to deficient transmission of the virus.
FIG 4.
Salivary glands impose a tight barrier to CHIKV transmission. (A) Intrathoracic injections of A. aegypti mosquitoes with wild-type (WT) and Δabb′ CHIKVs. In order to bypass the midgut barrier, A. aegypti mosquitoes were intrathoracically injected with 2,500 PFU of WT or mutant virus. (B) Bar graph showing infection rates in bodies after intrathoracic injection of viruses. The infection rate was calculated as the percentage of mosquitoes with virus presence in the body at different times postinjection. (C) Dot plot showing mean viral titers and SD in the bodies of intrathoracically injected mosquitoes. For the viral titers, statistics were performed by a Mann-Whitney U test. (D) Bar graph showing transmission rates after intrathoracic injection of viruses. The transmission rate was calculated as the percentage of mosquitoes with virus presence in the saliva at different times postinjection.
Wild-type CHIKV displays a fitness advantage to escape from the mosquito midgut.
To directly address the impact of the CHIKV growth rate on fitness, we performed competition experiments between wild-type and Δabb′ viruses. A. aegypti mosquitoes were fed with an infectious blood meal containing 106 PFU/ml of a mixture of wild-type and Δabb′ viruses in a 1:10 ratio in order to give a quantitative advantage to the virus with the impaired phenotype (Fig. 5A). At different times after the blood meal, total RNA was purified from individual mosquitoes and subjected to reverse transcription (RT) reactions with an oligo(dT) primer. The pool of viral cDNAs was used to amplify viral 3′ UTRs, which yielded fragments of different lengths for the wild-type and mutant viruses. The gel in Fig. 5B shows the amplification products of wild-type and mutant viruses in a 1:10 ratio in the input used for the blood meal (amplification products of the wild-type and the Δabb′ 3′ UTRs were used as a reference). The relative abundance of viruses with a full-length or Δabb′ 3′ UTR was assessed by agarose gel electrophoresis analysis of the RT-PCR products amplified from individual mosquitoes 2, 5, and 9 days after feeding (Fig. 5C). The gels show the fragments amplified from 12 individual mosquitoes at each time point. For each lane, we scored the ratio of the intensities of the bands corresponding to the wild-type and mutant 3′ UTRs and plotted the average ratio for each time point (Fig. 5D). The 1:10 ratio in the input was quickly reversed to a 1:1 ratio at the earliest time point evaluated. This rapid displacement of Δabb′ by wild-type virus in vivo indicates a fitness advantage of the wild-type virus during mosquito infections.
FIG 5.
Wild-type CHIKV has a fitness advantage over Δabb′ CHIKV to cross the midgut escape barrier. (A) Experimental setup of wild-type (WT) versus Δabb′ competitions in Aedes aegypti mosquitoes. Mosquitoes were offered an infectious blood meal containing a mixture of WT and Δabb′ viruses in a 1:10 ratio (106 PFU/ml). Total RNA was purified from individual mosquitoes at different time points postinfection, and the presence of WT and Δabb′ 3′ UTRs was assessed. (B) The RT-PCR product of the RNA extracted from the infectious blood meal containing wild-type and Δabb′ viruses in 1:1 and 1:10 ratios was resolved alongside fragments corresponding to wild-type and Δabb′ 3′ UTRs for reference. (C) Agarose gel electrophoresis of 3′-UTR amplification products from individual mosquitoes. The presence of WT and Δabb′ viruses was assessed by RT-PCR and agarose gel electrophoresis on 12 individual mosquitoes at three different times after the blood meal. (D) Bar graph showing the ratio of WT to Δabb′ 3′ UTRs in the input and in mosquito individuals during the time course of the experiment. Bars represent the average ratios of intensities for the bands corresponding to the products of amplification of WT and Δabb′ 3′ UTRs in individual mosquitoes at each time point. (E) Competition assays to assess the ability of WT and Δabb′ CHIKVs to cross the midgut escape barrier. Infectious blood feeding of A. aegypti mosquitoes was performed with blood containing a mixture of both viruses at a 1:1 ratio (106 PFU/ml). At different times postinfection, the midgut and carcass were dissected, total RNA was extracted, and the presence of virus was evaluated by RT-PCR as described above. (F) Representative agarose gels showing the products of amplification from midgut (top) and carcass (bottom) samples of 12 individual mosquitoes at 4 days postinfection. (G, top) Bar graph showing the presence of WT and Δabb′ viruses in the midgut as a function of time. Bars represent the percentages of midguts where WT and Δabb′ viruses were detected. (Bottom) Bar graph showing the presence of WT and/or Δabb′ viruses in carcasses as a function of time. Bars represent the percentages of carcasses where WT and/or Δabb′ viruses were detected.
We next assessed whether the fitness advantage of the wild type reflected the observed differences in the abilities of the wild-type and mutant viruses to cross the midgut escape barrier. To this end, A. aegypti mosquitoes were fed with a blood meal containing a mixture of both viruses at a 1:1 ratio (Fig. 5B). The midgut and carcass were dissected at different time points, total RNA was extracted, and the presence of virus was evaluated by RT-PCR (Fig. 5E). Representative agarose gels of the midgut and carcass from day 4 postinfection illustrate the differential mobilities of wild-type and Δabb′ 3′-UTR amplification products (Fig. 5F). When analyzing the presence of viruses as a function of time, we observed that both viruses were detected in all mosquito midguts even at 8 days postinfection (Fig. 5G, top). Based on previous reports, we reasoned that incoming viruses likely formed independent foci of infection within the midgut and thus coexisted independently of their growth rates (24, 26, 34, 35). Wild-type virus was readily detected as of 2 days postinfection in the carcasses, while the mutant virus was detected only after 8 days, indicating that the wild type had a higher dissemination rate than the mutant virus at all times postinfection (Fig. 5G, bottom). Altogether, our experiments demonstrate that wild-type CHIKV has a fitness advantage over Δabb′ CHIKV due to a higher replication rate that enhances its ability to escape the midgut.
DISCUSSION
The infection kinetics of arboviruses in their mosquito vectors have long been recognized as a powerful determinant of transmission and epidemiology (29). Viral genetic variations influence growth kinetics and their interaction with mosquito barriers, which together contribute to the overall phenotype of virus transmission (23, 24, 36, 37). For instance, comparisons between dengue virus (DENV) serotypes and even between strains from single serotypes showed differences in EIPs that are most likely due to differences in viral replication kinetics in mosquitoes (29, 38). For CHIKV, the emergence of new viral lineages has been linked to large variations in the 3′ UTR, which enhances replication in mosquito cells in vitro (15, 16, 18, 39, 40). Using an engineered 3′-UTR deletion mutant of the Caribbean lineage of CHIKV, we characterized the interaction of this mutant with mosquito barriers in vivo. We found that the replication rate of the 3′-UTR mutant is compromised in Aedes mosquitoes, and based on our results, we propose a model (Fig. 6) where the viral replication rate is intimately linked to the viral capacity to overcome barriers within mosquitoes. Viruses with high replication rates efficiently infect mosquitoes, disseminate to secondary tissues, and reach the mosquito saliva, resulting in a short EIP that ensures transmission. In contrast, viruses with low replication rates experience hurdles to overcoming the barriers imposed by the mosquitoes, resulting in a longer EIP and a lower transmission rate.
FIG 6.
Model for the effect of the viral growth rate on the ability to cross barriers during the infectious cycle in mosquitoes. The infection rate in Aedes mosquitoes (midgut infection barrier) is almost 100%, regardless of the virus growth rate. Within midgut cells, wild-type (WT) CHIKV replicates and reaches the necessary threshold (>10,000 PFU) to cross the midgut escape barrier and spread into secondary tissues. A slow-growing virus accomplishes leaving the midgut at later times, and it spreads to secondary tissues in only 50% of individuals. WT disseminated viruses colonize the salivary glands and are successfully secreted into the saliva in 40% of individuals. Secretion into the saliva of mutant viruses is achieved in only 10% of mosquitoes with disseminated infection. The outcome is a longer EIP and a lower transmission efficiency of mutant (5%) than of WT (35%) CHIKV. After peaking (between 4 and 8 days postinfection for the WT and between 9 and 12 days postinfection for Δabb′), the transmission efficiency drops to undetectable levels.
Important bottlenecks have been reported for arboviruses such as West Nile virus, Western equine encephalitis virus, Sindbis virus, and CHIKV during infection of their natural vectors (26, 33, 34, 41–43). These bottlenecks have been found at the midgut level and/or at the salivary gland level. By assessing viral infection rates in the midgut and carcass, we found that although there were no differences in the infectivity rates of both viral variants, the mutant virus had an impaired ability to leave the midgut, suggesting a strong midgut escape barrier effect. The outcome is a proportion of the mosquito population exhibiting dissemination and the rest exhibiting no dissemination. This scenario of mosquito subpopulation structure has already been reported for DENV (38). In turn, a dose-dependent effect has also been associated with escape from the midgut and occurred only when low doses of virus had been ingested (24). In agreement, in this work, we found that increasing blood meal viral titers reduced the midgut escape barrier effect.
Once midgut infection has been established, in order to disseminate, the virus must cross the basal lamina surrounding the midgut epithelium. It has been shown that after a blood meal, both an alteration of the expression of specific enzymes in the mosquito midgut as well as mechanical distention occur (32, 44–46). Several works have proposed that this results in transient degradation and increased permissibility of the basal lamina, promoting a “window of opportunity” of 48 h during which large quantities of CHIKV are allowed to disseminate (32, 44). In this sense, viruses with longer mosquito replication cycles, such as DENV or Zika virus (ZIKV), may not benefit as much from the early transient degradation of the basal lamina following a blood meal (23). Interestingly, recent work has demonstrated that the acquisition of a second noninfectious blood meal significantly shortens the EIP of all these viruses in infected Aedes mosquitoes by triggering mechanical distention in the basal lamina and thus enhancing virus dissemination from the mosquito midgut (46). Our results suggest that CHIKV may need to reach threshold viral titers within midgut cells that are necessary but not sufficient to cross the midgut escape barrier and spread into secondary tissues. We speculate that the Δabb′ CHIKV mutant may miss that window of opportunity because it does not reach the threshold titers required to disseminate at early times after infection. Whether the administration of a second blood meal with the mutant virus has a positive effect on dissemination as a consequence of the mechanical distention of the basal lamina remains to be tested. Altogether, our data indicate that the low replication rate of the 3′-UTR mutant has a strong effect on the ability of CHIKV to escape the midgut at the onset of infection.
It is well established that selective pressures in the mosquito vector impose important population bottlenecks to arboviruses (23, 36, 37, 47). Given that the viral infection cycle in mosquitoes moves in a stepwise fashion, selective pressures in an initial tissue might have effects on the viral kinetics in downstream tissues (38, 48). CHIKV replication in mammalian cells was previously shown to generate virus variants with shorter 3′ UTRs, including large deletions of direct repeat elements similar to the engineered mutation evaluated here (18). Furthermore, viruses with shorter 3′ UTRs seemingly display a replicative advantage in mammalian cells. Similar to previous work (16), by using virus competitions in mosquitoes coinfected with wild-type and mutant viruses, we observed a displacement of the mutant virus by the wild-type virus. In addition, we found that this fitness advantage is due to an increased capacity to escape from the midgut to secondary tissues, which results in a shift in the composition of the viral population. Interestingly, both viruses were simultaneously detected in the midguts of most of the mosquitoes even at 8 days postinfection. This suggests that coinfecting viruses formed independent foci of infection within the midgut, allowing both viruses to coexist independently of their replication rates (24, 26, 34, 35). These results widen the notion of how intrahost diversity plays a role in transmission, with variants with a fitness advantage spreading faster and eventually displacing those with lower fitness (38, 49). Epidemiological consequences might also be possible, like the 2008 large outbreak of dengue in Australia that was attributed to the very short EIP of the DENV3 strain in the mosquito (50). In nature, a significant proportion of mosquitoes are expected to die before they are capable of transmitting virus, and in this scenario, a virus variant with a shorter EIP would confer an evolutionary advantage by increasing its probability of transmission (5, 29, 51).
Taken together, our results show that a precisely timed replication rate is required for CHIKV to reach the necessary threshold titers to exit the midgut during the onset of the infection cycle, indicating that the viral replication rate is a determining factor in the ability to cross anatomical barriers and complete a successful replication cycle in mosquitoes. Understanding the factors that affect viral trajectories between mosquito infection and viral transmission will help to predict viral epidemic potential and design strategies to disrupt the viral transmission cycle.
MATERIALS AND METHODS
Cells and viruses.
Mammalian BHK and Vero cells were grown at 37°C in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% penicillin-streptomycin (Gibco). Mosquito C6/36 (Aedes albopictus) (ATCC CRL-1660) cells were grown at 28°C in Leibovitz L-15 medium supplemented with 10% FBS, 1% nonessential amino acids (Gibco), 2% tryptose phosphate broth (Sigma), and 1% penicillin-streptomycin. For RNA transfections, cell lines were grown to 60 to 70% confluence and transfected in 24-well plates using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s instructions. Caribbean wild-type and Δabb′ infectious clones were obtained as described previously (18). Viral stocks were obtained by transfection of 500 ng of in vitro-transcribed viral RNA and harvested from the cell culture supernatant at different times posttransfection. Viruses were quantified by plaque assays. To this end, 105 Vero cells per well were seeded into 24-well plates and allowed to attach overnight. Viral stocks were serially diluted, 0.1 ml was added to the cells, and the mixture was incubated for 1 h. Next, 1 ml of an overlay (1× DMEM, 2% fetal bovine serum, 1% penicillin-streptomycin, and 0.8% agarose) was added to each well. Cells were fixed 3 days after infection with 4% paraformaldehyde and stained with crystal violet.
Mosquito rearing.
Laboratory colonies of A. aegypti mosquitoes (17th generation; collected originally in Kamphaeng Phet Province, Thailand) and A. albopictus (19th generation; collected originally in Phu Hoa, Binh Duong Province, Vietnam) were used. The insectary conditions for mosquito maintenance were 28°C, 70% relative humidity, and a 12-h-light and 12-h-dark cycle. Adults were maintained with permanent access to a 10% sucrose solution. Adult females were offered commercial rabbit blood (BCL, Boisset-Saint-Priest, France) twice a week through a membrane feeding system (Hemotek Ltd.).
Experimental infections of mosquitoes. (i) Infectious blood meals.
Infection assays were performed with 7- to 10-day-old females starved 24 h prior to infection in a biosafety level 3 (BSL-3) laboratory. Mosquitoes were offered the infectious blood meal for 30 min through a membrane feeding system (Hemotek Ltd.) set at 37°C with a piece of desalted pig intestine as the membrane. The blood meal was composed of washed human erythrocytes resuspended in phosphate-buffered saline mixed 2:1 with a prediluted viral stock and supplemented with 10 mM ATP (Sigma-Aldrich). The viral stock was prediluted in Leibovitz L-15 medium with 0.1% sodium bicarbonate (Gibco) to reach an infectious titer ranging from 1 × 106 to 1 × 107 focus-forming units and back-titrated to ensure similar presented doses (the exact titer of each infectious blood meal is noted for each experiment). Following the blood meal, fully engorged females were selected and incubated at 28°C with 70% relative humidity and under a 12-h-light and 12-h-dark cycle with permanent access to 10% sucrose. At different times postinfection, mosquitoes were cold anesthetized for salivation and dissection. For saliva collection, wings and legs were removed from each individual, and its proboscis was inserted into a 20-μl tip containing 10 μl of FBS for 30 min at room temperature. Saliva-containing FBS was expelled in 90 μl of Leibovitz L-15 medium (Gibco) for amplification and titration. Following the collection of saliva, mosquitoes were dissected, and body parts were homogenized in microtubes containing steel beads (5-mm diameter) and 300 μl of DMEM supplemented with 2% FBS using a TissueLyser II instrument (Qiagen) at 30 shakes/s for 2 min. Homogenates were clarified by centrifugation and stored at 80°C until further processing. Viral titers in individual samples were determined by plaque assays. For the detection of 3′-UTR RNA from whole mosquitoes or mosquito parts, RNA TRIzol extracted from homogenates was used for reverse transcription using reverse oligonucleotide 5′-TTTTTTTTTTTTTTTTTTTGAAATAT-3′, complementary to the poly(A) tail plus the last 7 nucleotides of CHIKV genomes. PCRs were then carried out (DreamTaq; Thermo Fisher) using the same reverse oligonucleotide and forward oligonucleotide 5′-CTAATCGTGGTGCTATGC-3′. The length of the viral 3′ UTRs was estimated by resolving the product in 1% agarose gels. The intensity of the bands was measured with ImageJ software.
(ii) Intrathoracic inoculations of mosquitoes.
Seven- to ten-day-old female mosquitoes were cold anesthetized and injected with a transfection mix of CellFectin II reagent (Thermo Fisher) with 50 nl of Leibovitz L-15 medium containing 2.5 × 103 PFU of virus. The injection was performed intrathoracically using a nanoinjector (Nanoject III; Drummond Scientific) and a glass capillary needle. At 2, 4, 6, and 8 days postinjection, mosquitoes were cold anesthetized and dissected.
Virus titration and quantification.
The presence of infectious virus particles in mosquito bodies, midguts, carcasses, and head extracts was determined by plaque assays in homogenate samples following mosquito dissection. Briefly, 100 μl of the sample homogenates was serially diluted in cell culture medium and used to infect Vero cells in 24-well plates as described above for virus titration. Mosquito saliva samples were amplified in C6/36 cells for 5 days, and virus presence in amplified supernatants was assessed by cytopathic effect in Vero cells. The data were analyzed quantitatively for most of the samples (PFU per milliliter) and qualitatively for saliva samples and some body and head samples (i.e., the presence or absence of infectious virus in heads/bodies). The infection rate (IR) was calculated as the proportion of mosquitoes infected among all tested females. The dissemination rate (DR) was defined as the proportion of females with infected head tissues among those that were infected (i.e., in which the virus successfully disseminated from the midgut). The dissemination efficiency (DE) was calculated as the proportion of females with infected head tissues among all tested females. The transmission rate (TR) was defined as the proportion of females with infectious saliva among those that developed a disseminated infection. The transmission efficiency (TE) was calculated as the overall proportion of females that had infectious saliva (i.e., among all tested females with or without a disseminated infection).
Human blood and ethics statement.
Human blood used to feed mosquitoes was obtained from healthy volunteer donors. Healthy donor recruitment was organized by local investigator assessment using medical history, laboratory results, and clinical examinations. Biological samples were supplied through the participation of healthy volunteers at the ICAReB biobanking platform (BB-0033-00062/ICAReB platform/Institut Pasteur, Paris/BBMRI AO203 [Bioresource]) of the Institut Pasteur for the CoSImmGen and Diagmicoll protocols, which have been approved by the French Ethical Committee (CPP), Ile-de-France I. The Diagmicoll protocol was declared to the French Research Ministry under reference number DC 2008-68 COL 1.
Statistics.
All statistical analyses were performed in GraphPad Prism 6. Significant differences between virus infection, dissemination, and transmission rates were determined by Fisher’s exact test. For viral titers, where the data did not follow a Gaussian distribution, a Mann-Whitney U test was used to replace the t test. Statistical significance is represented in the figures (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001).
ACKNOWLEDGMENTS
This work was supported by the European Research Council (FP7/2013-2019 ERC CoG 615220) and the French Government’s Investissement d’Avenir program, Laboratoire d’Excellence Integrative Biology of Emerging Infectious Diseases (grant ANR-10-LABX-62-IBEID), to M.C.-S. F.M. was supported by a fellowship of the Argentine National Council for Scientific and Technical Research (CONICET). D.E.A. and C.V.F. are members of the CONICET.
REFERENCES
- 1.Jones R, Kulkarni MA, Davidson TMV, RADAM-LAC Research Team, Talbot B. 2020. Arbovirus vectors of epidemiological concern in the Americas: a scoping review of entomological studies on Zika, dengue and chikungunya virus vectors. PLoS One 15:e0220753. doi: 10.1371/journal.pone.0220753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thaikruea L, Charearnsook O, Reanphumkarnkit S, Dissomboon P, Phonjan R, Ratchbud S, Kounsang Y, Buranapiyawong D. 1997. Chikungunya in Thailand: a re-emerging disease? Southeast Asian J Trop Med Public Health 28:359–364. [PubMed] [Google Scholar]
- 3.Lanciotti RS, Valadere AM. 2014. Transcontinental movement of Asian genotype chikungunya virus. Emerg Infect Dis 20:1400–1402. doi: 10.3201/eid2008.140268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver SC. 2014. Arrival of chikungunya virus in the New World: prospects for spread and impact on public health. PLoS Negl Trop Dis 8:e2921. doi: 10.1371/journal.pntd.0002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredericks AC, Fernandez-Sesma A. 2014. The burden of dengue and chikungunya worldwide: implications for the southern United States and California. Ann Glob Health 80:466–475. doi: 10.1016/j.aogh.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariconti M, Obadia T, Mousson L, Malacrida A, Gasperi G, Failloux A-B, Yen P-S. 2019. Estimating the risk of arbovirus transmission in Southern Europe using vector competence data. Sci Rep 9:17852. doi: 10.1038/s41598-019-54395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsinga J, Grobusch MP, Tami A, Gerstenbluth I, Bailey A. 2017. Health-related impact on quality of life and coping strategies for chikungunya: a qualitative study in Curaçao. PLoS Negl Trop Dis 11:e0005987. doi: 10.1371/journal.pntd.0005987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hossain MS, Hasan MM, Islam MS, Islam S, Mozaffor M, Khan MAS, Ahmed N, Akhtar W, Chowdhury S, Arafat SMY, Khaleque MA, Khan ZJ, Dipta TF, Asna SMZH, Hossain MA, Aziz KS, Al Mosabbir A, Raheem E. 2018. Chikungunya outbreak (2017) in Bangladesh: clinical profile, economic impact and quality of life during the acute phase of the disease. PLoS Negl Trop Dis 12:e0006561. doi: 10.1371/journal.pntd.0006561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soumahoro M-K, Boelle P-Y, Gaüzere B-A, Atsou K, Pelat C, Lambert B, La Ruche G, Gastellu-Etchegorry M, Renault P, Sarazin M, Yazdanpanah Y, Flahault A, Malvy D, Hanslik T. 2011. The chikungunya epidemic on La Réunion Island in 2005-2006: a cost-of-illness study. PLoS Negl Trop Dis 5:e1197. doi: 10.1371/journal.pntd.0001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rezza G, Weaver SC. 2019. Chikungunya as a paradigm for emerging viral diseases: evaluating disease impact and hurdles to vaccine development. PLoS Negl Trop Dis 13:e0006919. doi: 10.1371/journal.pntd.0006919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Achee NL, Grieco JP, Vatandoost H, Seixas G, Pinto J, Ching-Ng L, Martins AJ, Juntarajumnong W, Corbel V, Gouagna C, David J-P, Logan JG, Orsborne J, Marois E, Devine GJ, Vontas J. 2019. Alternative strategies for mosquito-borne arbovirus control. PLoS Negl Trop Dis 13:e0006822. doi: 10.1371/journal.pntd.0006822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsetsarkin KA, Chen R, Weaver SC. 2016. Interspecies transmission and chikungunya virus emergence. Curr Opin Virol 16:143–150. doi: 10.1016/j.coviro.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfeffer M, Kinney RM, Kaaden OR. 1998. The alphavirus 3′-nontranslated region: size heterogeneity and arrangement of repeated sequence elements. Virology 240:100–108. doi: 10.1006/viro.1997.8907. [DOI] [PubMed] [Google Scholar]
- 14.Ou JH, Trent DW, Strauss JH. 1982. The 3′-non-coding regions of alphavirus RNAs contain repeating sequences. J Mol Biol 156:719–730. doi: 10.1016/0022-2836(82)90138-3. [DOI] [PubMed] [Google Scholar]
- 15.Stapleford KA, Moratorio G, Henningsson R, Chen R, Matheus S, Enfissi A, Weissglas-Volkov D, Isakov O, Blanc H, Mounce BC, Dupont-Rouzeyrol M, Shomron N, Weaver S, Fontes M, Rousset D, Vignuzzi M. 2016. Whole-genome sequencing analysis from the chikungunya virus Caribbean outbreak reveals novel evolutionary genomic elements. PLoS Negl Trop Dis 10:e0004402. doi: 10.1371/journal.pntd.0004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen R, Wang E, Tsetsarkin KA, Weaver SC. 2013. Chikungunya virus 3′ untranslated region: adaptation to mosquitoes and a population bottleneck as major evolutionary forces. PLoS Pathog 9:e1003591. doi: 10.1371/journal.ppat.1003591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filomatori CV, Merwaiss F, Bardossy ES, Alvarez DE. 11 July 2020. Impact of alphavirus 3′UTR plasticity on mosquito transmission. Semin Cell Dev Biol doi: 10.1016/j.semcdb.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Filomatori CV, Bardossy ES, Merwaiss F, Suzuki Y, Henrion A, Saleh MC, Alvarez DE. 2019. RNA recombination at chikungunya virus 3′UTR as an evolutionary mechanism that provides adaptability. PLoS Pathog 15:e1007706. doi: 10.1371/journal.ppat.1007706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pezzi L, Diallo M, Rosa-Freitas MG, Vega-Rua A, Ng LFP, Boyer S, Drexler JF, Vasilakis N, Lourenco-de-Oliveira R, Weaver SC, Kohl A, de Lamballerie X, Failloux A-B, GloPID-R Chikungunya, O’nyong-nyong and Mayaro Virus Working Group. 2020. GloPID-R report on chikungunya, o’nyong-nyong and Mayaro virus, part 5: entomological aspects. Antiviral Res 174:104670. doi: 10.1016/j.antiviral.2019.104670. [DOI] [PubMed] [Google Scholar]
- 20.Schule PA. 1928. Dengue fever: transmission by Aedes aegypti. Am J Trop Med Hyg S1–S8:203–213. doi: 10.4269/ajtmh.1928.s1-8.203. [DOI] [Google Scholar]
- 21.Hardy JL, Houk EJ, Kramer LD, Reeves WC. 1983. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol 28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- 22.Coffey LL, Failloux A-B, Weaver SC. 2014. Chikungunya virus-vector interactions. Viruses 6:4628–4663. doi: 10.3390/v6114628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rückert C, Ebel GD. 2018. How do virus-mosquito interactions lead to viral emergence? Trends Parasitol 34:310–321. doi: 10.1016/j.pt.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franz AWE, Kantor AM, Passarelli AL, Clem RJ. 2015. Tissue barriers to arbovirus infection in mosquitoes. Viruses 7:3741–3767. doi: 10.3390/v7072795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robison A, Young MC, Byas AD, Rückert C, Ebel GD. 2020. Comparison of chikungunya virus and Zika virus replication and transmission dynamics in Aedes aegypti mosquitoes. Am J Trop Med Hyg 103:869–875. doi: 10.4269/ajtmh.20-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong S, Kantor AM, Lin J, Passarelli AL, Clem RJ, Franz AWE. 2016. Infection pattern and transmission potential of chikungunya virus in two New World laboratory-adapted Aedes aegypti strains. Sci Rep 6:24729. doi: 10.1038/srep24729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vega-Rúa A, Zouache K, Girod R, Failloux A-B, Lourenço-de-Oliveira R. 2014. High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of chikungunya virus. J Virol 88:6294–6306. doi: 10.1128/JVI.00370-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delang L, Yen P-S, Vallet T, Vazeille M, Vignuzzi M, Failloux A-B. 2018. Differential transmission of antiviral drug-resistant chikungunya viruses by Aedes mosquitoes. mSphere 3:e00230-18. doi: 10.1128/mSphere.00230-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fontaine A, Lequime S, Moltini-Conclois I, Jiolle D, Leparc-Goffart I, Reiner RCJ, Lambrechts L. 2018. Epidemiological significance of dengue virus genetic variation in mosquito infection dynamics. PLoS Pathog 14:e1007187. doi: 10.1371/journal.ppat.1007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salazar MI, Richardson JH, Sánchez-Vargas I, Olson KE, Beaty BJ. 2007. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol 7:9. doi: 10.1186/1471-2180-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alto BW, Wiggins K, Eastmond B, Velez D, Lounibos LP, Lord CC. 2017. Transmission risk of two chikungunya lineages by invasive mosquito vectors from Florida and the Dominican Republic. PLoS Negl Trop Dis 11:e0005724. doi: 10.1371/journal.pntd.0005724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong S, Balaraman V, Kantor AM, Lin J, Grant DG, Held NL, Franz AWE. 2017. Chikungunya virus dissemination from the midgut of Aedes aegypti is associated with temporal basal lamina degradation during bloodmeal digestion. PLoS Negl Trop Dis 11:e0005976. doi: 10.1371/journal.pntd.0005976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramer LD, Hardy JL, Presser SB, Houk EJ. 1981. Dissemination barriers for Western equine encephalomyelitis virus in Culex tarsalis infected after ingestion of low viral doses. Am J Trop Med Hyg 30:190–197. doi: 10.4269/ajtmh.1981.30.190. [DOI] [PubMed] [Google Scholar]
- 34.Myles KM, Pierro DJ, Olson KE. 2004. Comparison of the transmission potential of two genetically distinct Sindbis viruses after oral infection of Aedes aegypti (Diptera: Culicidae). J Med Entomol 41:95–106. doi: 10.1603/0022-2585-41.1.95. [DOI] [PubMed] [Google Scholar]
- 35.Smith DR, Arrigo NC, Leal G, Muehlberger LE, Weaver SC. 2007. Infection and dissemination of Venezuelan equine encephalitis virus in the epidemic mosquito vector, Aedes taeniorhynchus. Am J Trop Med Hyg 77:176–187. doi: 10.4269/ajtmh.2007.77.176. [DOI] [PubMed] [Google Scholar]
- 36.Forrester NL, Coffey LL, Weaver SC. 2014. Arboviral bottlenecks and challenges to maintaining diversity and fitness during mosquito transmission. Viruses 6:3991–4004. doi: 10.3390/v6103991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forrester NL, Guerbois M, Seymour RL, Spratt H, Weaver SC. 2012. Vector-borne transmission imposes a severe bottleneck on an RNA virus population. PLoS Pathog 8:e1002897. doi: 10.1371/journal.ppat.1002897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novelo M, Hall MD, Pak D, Young PR, Holmes EC, McGraw EA. 2019. Intra-host growth kinetics of dengue virus in the mosquito Aedes aegypti. PLoS Pathog 15:e1008218. doi: 10.1371/journal.ppat.1008218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morley VJ, Noval MG, Chen R, Weaver SC, Vignuzzi M, Stapleford KA, Turner PE. 2018. Chikungunya virus evolution following a large 3′UTR deletion results in host-specific molecular changes in protein-coding regions. Virus Evol 4:vey012. doi: 10.1093/ve/vey012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyde JL, Chen R, Trobaugh DW, Diamond MS, Weaver SC, Klimstra WB, Wilusz J. 2015. The 5′ and 3′ ends of alphavirus RNAs—non-coding is not non-functional. Virus Res 206:99–107. doi: 10.1016/j.virusres.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khoo CCH, Piper J, Sanchez-Vargas I, Olson KE, Franz AWE. 2010. The RNA interference pathway affects midgut infection- and escape barriers for Sindbis virus in Aedes aegypti. BMC Microbiol 10:130. doi: 10.1186/1471-2180-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Göertz GP, Fros JJ, Miesen P, Vogels CBF, van der Bent ML, Geertsema C, Koenraadt CJM, van Rij RP, van Oers MM, Pijlman GP. 2016. Noncoding subgenomic flavivirus RNA is processed by the mosquito RNA interference machinery and determines West Nile virus transmission by Culex pipiens mosquitoes. J Virol 90:10145–10159. doi: 10.1128/JVI.00930-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grubaugh ND, Fauver JR, Rückert C, Weger-Lucarelli J, Garcia-Luna S, Murrieta RA, Gendernalik A, Smith DR, Brackney DE, Ebel GD. 2017. Mosquitoes transmit unique West Nile virus populations during each feeding episode. Cell Rep 19:709–718. doi: 10.1016/j.celrep.2017.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kantor AM, Grant DG, Balaraman V, White TA, Franz AWE. 2018. Ultrastructural analysis of chikungunya virus dissemination from the midgut of the yellow fever mosquito, Aedes aegypti. Viruses 10:571. doi: 10.3390/v10100571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui Y, Grant DG, Lin J, Yu X, Franz AWE. 2019. Zika virus dissemination from the midgut of Aedes aegypti is facilitated by bloodmeal-mediated structural modification of the midgut basal lamina. Viruses 11:1056. doi: 10.3390/v11111056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armstrong PM, Ehrlich HY, Magalhaes T, Miller MR, Conway PJ, Bransfield A, Misencik MJ, Gloria-Soria A, Warren JL, Andreadis TG, Shepard JJ, Foy BD, Pitzer VE, Brackney DE. 2020. Successive blood meals enhance virus dissemination within mosquitoes and increase transmission potential. Nat Microbiol 5:239–247. doi: 10.1038/s41564-019-0619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lequime S, Fontaine A, Ar Gouilh M, Moltini-Conclois I, Lambrechts L. 2016. Genetic drift, purifying selection and vector genotype shape dengue virus intra-host genetic diversity in mosquitoes. PLoS Genet 12:e1006111. doi: 10.1371/journal.pgen.1006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall MD, Bento G, Ebert D. 2017. The evolutionary consequences of stepwise infection processes. Trends Ecol Evol 32:612–623. doi: 10.1016/j.tree.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Lambrechts L, Fansiri T, Pongsiri A, Thaisomboonsuk B, Klungthong C, Richardson JH, Ponlawat A, Jarman RG, Scott TW. 2012. Dengue-1 virus clade replacement in Thailand associated with enhanced mosquito transmission. J Virol 86:1853–1861. doi: 10.1128/JVI.06458-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ritchie SA, Pyke AT, Hall-Mendelin S, Day A, Mores CN, Christofferson RC, Gubler DJ, Bennett SN, van den Hurk AF. 2013. An explosive epidemic of DENV-3 in Cairns, Australia. PLoS One 8:e68137. doi: 10.1371/journal.pone.0068137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson JR, Rico-Hesse R. 2006. Aedes aegypti vectorial capacity is determined by the infecting genotype of dengue virus. Am J Trop Med Hyg 75:886–892. doi: 10.4269/ajtmh.2006.75.886. [DOI] [PMC free article] [PubMed] [Google Scholar]