Optimal bNAb immunotherapeutics will need to mediate multiple antiviral functions against a broad range of HIV strains. Our systematic assessment of triple bNAb combinations against SHIVs will identify bNAbs with synergistic, polyfunctional antiviral activity that will inform the selection of candidate bNAbs for optimal combination designs. The identified combinations can be validated in vivo in future passive immunization studies using the SHIV challenge model.
KEYWORDS: ADCC, ADCP, SHIV, bNAbs, neutralization
ABSTRACT
Daily burden and clinical toxicities associated with antiretroviral therapy (ART) emphasize the need for alternative strategies to induce long-term human immunodeficiency virus (HIV) remission upon ART cessation. Broadly neutralizing antibodies (bNAbs) can both neutralize free virions and mediate effector functions against infected cells and therefore represent a leading immunotherapeutic approach. To increase potency and breadth, as well as to limit the development of resistant virus strains, it is likely that bNAbs will need to be administered in combination. It is therefore critical to identify bNAb combinations that can achieve robust polyfunctional antiviral activity against a high number of HIV strains. In this study, we systematically assessed the abilities of single bNAbs and triple bNAb combinations to mediate robust polyfunctional antiviral activity against a large panel of cross-clade simian-human immunodeficiency viruses (SHIVs), which are commonly used as tools for validation of therapeutic strategies targeting the HIV envelope in nonhuman primate models. We demonstrate that most bNAbs are capable of mediating both neutralizing and nonneutralizing effector functions against cross-clade SHIVs, although the susceptibility to V3 glycan-specific bNAbs is highly strain dependent. Moreover, we observe a strong correlation between the neutralization potencies and nonneutralizing effector functions of bNAbs against the transmitted/founder SHIV CH505. Finally, we identify several triple bNAb combinations comprising of CD4 binding site-, V2-glycan-, and gp120-gp41 interface-targeting bNAbs that are capable of mediating synergistic polyfunctional antiviral activities against multiple clade A, B, C, and D SHIVs.
IMPORTANCE Optimal bNAb immunotherapeutics will need to mediate multiple antiviral functions against a broad range of HIV strains. Our systematic assessment of triple bNAb combinations against SHIVs will identify bNAbs with synergistic, polyfunctional antiviral activity that will inform the selection of candidate bNAbs for optimal combination designs. The identified combinations can be validated in vivo in future passive immunization studies using the SHIV challenge model.
INTRODUCTION
Worldwide, approximately 37.9 million people are living with human immunodeficiency virus (HIV), and 1.7 million new infections were reported in 2019 (1). Antiretroviral therapy (ART) remains the gold standard of treatment for controlling the replication and spread of the virus. Unfortunately, only approximately 60% of infected individuals are currently on or have access to these lifesaving drugs (2). ART initiation commits an individual to a lifetime of adhering to strict drug regimens, which can incur not only a significant financial burden but also non-AIDS comorbidities (3, 4). Daily use is essential due to latently integrated proviral DNA encoded in the host CD4+ T cells that can become reactivated once ART is halted (5–8). Furthermore, prolonged use of ART in individuals with intermittent adherence can result in drug resistance, and thus viral load (VL) should be monitored to ensure that specific drugs remain active against the virus (9–11). In nonhuman primate (NHP) models of HIV, ART initiation within 24 h of infection was associated with decreased seeding of the viral reservoir and absence of viral rebound after ART interruption (12). However, ART intervention after 24 h of infection appears to be too late to halt the establishment of the viral reservoir; subsequently, rebound in viral replication is observed once treatment has been interrupted (13). Very early ART initiation has also been demonstrated to be successful in delaying viral rebound in one reported case of pediatric HIV. This infant, also known as the Mississippi baby, was started on ART at 30 h after birth until 18 months of age, at which time ART was interrupted. The toddler remained negative for HIV in the blood for 27 months post ART interruption (14, 15) but subsequently became viremic. Similarly, a small cohort of HIV-infected individuals, the VISCONTI cohort, who started ART within the first few weeks of HIV infection (1.6 months after initial exposure) were able to spontaneously control HIV replication for an extended period of time (median of 89 months) and remained free of disease progression in the absence of HIV treatment (16). Unfortunately, the majority of HIV infections cannot be diagnosed very early, and ART is rarely initiated a few days after infection. Therefore, there is a critical need for interventions that can be implemented in infected individuals with established HIV reservoirs to achieve viral remission and induce a functional cure.
The discovery of new-generation broadly neutralizing antibodies (bNAbs) that exhibit extraordinary breadth and potency has prompted the evaluation of this therapy in a variety of preclinical and clinical studies (17). Notably, the intravenous administration of N6-LS (CD4 binding site [CD4BS]) alone or in combination with PGT121 (V3-glycan) to chronically infected rhesus macaques (RMs) resulted in rapid clearance of viral RNA (18). Detection of virus was directly attributed to persistence of bNAbs in the serum. Interestingly, the addition of PGT121 to N6-LS did not increase the neutralization activity, highlighting the need for optimization of bNAb combinations in order to achieve a synergistic effect (18). In another study, the administration of 3BNC117 (CD4BS) in combination with 10-1074 (V3-glycan) to chronically HIV-infected patients led to decreased viral load when the bNAb concentrations remained above 10 μg/ml (19). Escape mutants, likely due to preexisting resistant virus populations, were observed in all participants when 3BNC117 levels in plasma dropped below effective concentrations (<10 μg/ml; mean, 1.9 μg/ml), essentially resulting in monotherapy of 10-1074. These findings demonstrate the importance of selecting potent, synergistic bNAb combinations to achieve optimal therapeutic efficiency against highly diverse virus populations.
The development of chimeric simian-human immunodeficiency viruses (SHIVs) that encode HIV-1 envelope (Env) from transmitted/founder (T/F) viruses have been instrumental in testing antibody-based vaccine strategies (20–23). These new-generation SHIVs have been shown to preserve the antigenic and replication properties of their parental T/F HIV Env variants (24). Additionally, SHIV infection models have been widely utilized as tools to assess the protective and curative effects of either single or combination bNAbs in NHP models (17, 20–23, 25–33). Despite promising findings of the ability of single and combinations of bNAbs to suppress viremia and mediate killing of virus-infected cells (17, 22, 28), a challenge study using a mixture of SHIVs showed that combination bNAbs failed to protect RMs against infections (34), likely due to greater frequency of viral diversity. This finding suggests that antigenic diversity of HIV-1 Env remains an importance challenge in the development of effective bNAb combinations against a broad range of HIV variants. Yet, few studies have compared the antiviral potencies of individual bNAbs against SHIVs that encode different HIV Env variants, which will facilitate testing in preclinical models of ART cessation. Moreover, the ability of bNAb combination to mediate synergistic polyfunctional antiviral activity against cross-clade SHIVs has not been thoroughly characterized.
In the present study, we address these gaps through systematic assessment of the neutralizing potencies and nonneutralizing effector functions of individual bNAbs against new-generation clade C SHIVs to identify candidate bNAbs with robust polyfunctional antiviral activities and synergistic effects for triple bNAb combinations testing against cross-clade SHIVs. We first evaluated a panel of 18 bNAbs targeting major HIV Env sites (CD4 binding site [CD4BS], V2-glycan, V3-glycan, membrane-proximal external region [MPER], and gp120-gp41 interface) for their neutralization potencies and breadth against 3 new-generation clade C SHIVs (CH505, CH848, and 1086c) and their parental T/F HIV-1 Env variants (CH505 T/F, CH848 T/F, and 1086c T/F). We then selected 7 candidate bNAbs based on their ability to mediate polyfunctional antiviral activities against SHIV CH505 to design a total of 9 triple bNAb combinations, each consisting of 1 bNAb each targeting the CD4BS, V2-glycan, and gp41-gp120 interface regions. We identified several triple bNAb combinations capable of mediating synergistic and robust polyfunctional antiviral activities against SHIV CH505 and a panel of other cross-clade tier 2 SHIVs. Together, these data will guide the selection of candidate triple bNAb combinations that will be tested in future passive immunization studies in SHIV challenge models, and in subsequent human clinical trials, to explore the potential of bNAb as immune-based therapy alone or in combination with ART.
RESULTS
The neutralization potency of bNAbs against new-generation clade C SHIVs and their parental transmitted/founder HIV Env variants is comparable.
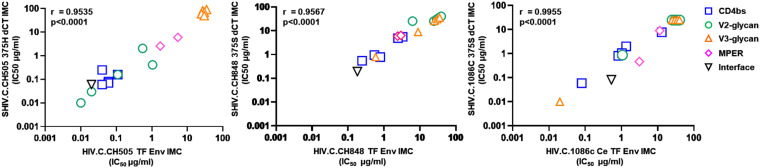
We first assessed the ability of 18 bNAbs targeting 5 distinct regions of the HIV Env (CD4 BS, MPER, V2, V3, and gp120-gp41interface) to neutralize 3 new-generation clade C SHIVs (CH505 375H dCT, [CH505], CH848 375S dCT [CH848], and Ce1086 375S dCT [1086c]) and their corresponding T/F HIV Env variants (CH505 T/F, CH848 T/F, and Ce1086c T/F) (see Table S2 in the supplemental material). The neutralization titers (50% inhibitory concentration [IC50] in μg/ml) of the individual bNAbs against the new generation SHIVs were strongly correlated with the neutralization titers against their corresponding T/F HIV Env variants (P < 0.001) (Fig. 1), indicating that these SHIVs retained the neutralization sensitivity of their parental T/F HIV Env variants to neutralization by bNAbs.
FIG 1.
Single bNAb neutralization profiles against new-generation clade C SHIVs showed strong correlations with their corresponding transmitted/founder (T/F) Env HIV variants. A panel of 18 bNAbs targeting the different regions of the HIV Env (CD4 binding site, V2-glycan, V3-glycan, membrane-proximal region [MPER], and gp120-gp41 interface) were tested for their neutralization potency against new-generation clade C SHIVs (CH505.375H dCT, CH848.375S dCT, and 1086c.375S dCT) and their corresponding transmitted founder (T/F) HIV Env variants (CH505 TF, CH848 TF, and 1086c.Ce TF). Neutralization titers (50 inhibitory concentration [IC50] in μg/ml) showed a strong correlation (P < 0.0001) of bNAbs against the new-generation clade C SHIVs and their corresponding T/F Env HIV variants, which indicated that these SHIVs conserved the neutralization profiles of their parental HIVs. Statistical analyses were done using Spearman’s rank correlation in GraphPad Prism. All viruses were produced by transfection of 293T cells.
bNAbs targeting different HIV Env regions have distinct potencies against the new-generation clade C SHIVs.
All 3 new-generation clade C SHIVs were sensitive to neutralization by the gp120-gp41 interface-targeting bNAb PGT151, with an IC50 range of 0.06 to 0.19 μg/ml (see Table S2 for IC80 values) and by most CD4BS-targeting bNAbs, with an IC50 range of 0.06 to 7.72 μg/ml (see Table S2 for IC80 values). 1086c was moderately susceptible to VRC01 (IC50/80, 1.05/2.49 μg/ml) and the VRC01-like bNAbs 3BNC117 and CH31 (IC50/80, 2.02/6.32 and 7.72/20.41 μg/ml, respectively). Meanwhile, CH848 was moderately susceptible to VRC01 (IC50/80, 4.98/12.40 μg/ml) and the VRC01-like bNAb CH235.15 (IC50/80, 5.64/14.64 μg/ml). In contrast, we found striking differences in the sensitivities of the new-generation clade C SHIVs to bNAbs targeting the V2-glycan and V3-glycan regions. CH848 and 1086c were resistant to all of theV2-glycan- and V3-glycan-specific bNAbs tested (IC50/80, >25 μg/ml). Meanwhile, CH505 was sensitive to neutralization by most V2-glycan-specific bNAbs, with an IC50 range of 0.01 to 2.04 μg/ml (see Table S2 for IC80 values), but was resistant to all V3-glycan-specific bNAbs tested (IC50/80 values of >25 μg/ml for all). Overall, CH505 was more sensitive to neutralization by bNAbs targeting different HIV Env regions than were the other tested SHIVs. We therefore selected CH505 for a comprehensive analysis of polyfunctional antiviral activities of the individual bNAbs.
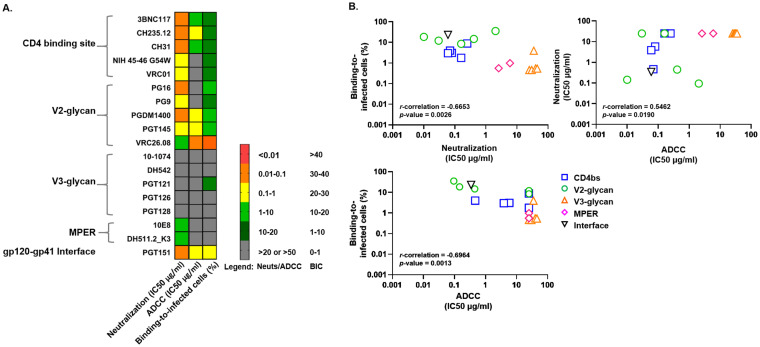
HIV Env-specific bNAbs mediate nonneutralizing antibody-mediated antiviral activities against CH505.
To further characterize the polyfunctional antiviral activities of individual bNAbs, we evaluated their ability to mediate antibody-dependent cell cytotoxicity (ADCC) and binding to infected cells (BIC) against CH505 (Fig. 2). PGT151, a bNAb targeting the gp120-gp41 interface region of the HIV Env, demonstrated not only high neutralization potencies against CH505 (IC50/80, 0.06/0.21 μg/ml), but also mediated robust BIC (23.1%) and ADCC (IC50, 0.3425 μg/ml) against CH505-infected cells (Fig. 2). Interestingly, even though most CD4BS-specific bNAbs demonstrated high neutralization and BIC activities against CH505-infected cells, only 3BNC117, CH235.12, and CH31 were capable of mediating robust ADCC activities (ADCC IC50 range, 0.47 to 5.81 μg/ml; Fig. 2). Similarly, even though most V2-glycan-specific bNAbs demonstrated high neutralization and BIC activities, only PGDM1400, PGT145, and VRC26.08 were capable of mediating robust ADCC (ADCC IC50 range, 0.09 to 0.44 μg/ml; Fig. 2). Neutralization potencies and antibody Fc-mediated effector functions of individuals bNAbs against CH505 were highly correlated (P value range, 0.0013 to 0.019; data not shown).
FIG 2.
Characterization of bNAb neutralization potencies and antibody-mediated effector functions against SHIV.C.CH505. SHIV.C.CH505.375H dCT IMC is sensitive to neutralization and antibody-mediated effector functions by most bNAbs but resistant to V3-glycan-specific bNAbs. A total of 7 bNAbs, including CD4bs-specific (3BNC117, CH235.12, and CH31), V2-glycan-specific (PGDM1400, PGT145, and VRC26.08), and gp120-gp41 interface-specific (PGT151) bNAbs were selected as candidates for designing polyfunctional combinations of triple bNAbs (A). Polyfunctional antiviral activities of bNAbs against SHIV.C.CH505 are highly correlated (B). Neutralization potencies of bNAbs (18 bNAbs) against SHIV.C.CH505 correlate with binding to infected cells and antibody-dependent cell cytotoxicity (ADCC) activities. All reported values are the mean of 2 to 3 independent experiments, each with two replicates. Statistical analyses were done using Spearman’s rank correlation. SHIV CH505 virus was produced by transfection of 293T cells.
Based on polyfunctional antiviral activities against CH505, the three most potent CD4BS-specific bNAbs (3BNC117, CH235.12, and CH31), the three most potent V2-glycan-specific bNAbs (PGDM1400, PGT145, and VRC26.08), and the gp120-gp41 interface-specific (PGT151) bNAb were selected for triple bNAb combination design and further evaluated for their ability to mediate various antiviral functions against cross-clade SHIVs in vitro. MPER-specific antibodies (10E8 and DH511.2_K3) were excluded from the bNAb combination design, as they did not mediate potent neutralization of CH505 (IC50/80 values, 5.99/17.54 μg/ml and 2.58/7.00 μg/ml, respectively) and had poor BIC (0.99% and 0.58% binding, respectively) and ADCC (both did not mediate 50% killing) activities against CH505-infected cells (Fig. 2). Moreover, 10E8-LS (a derivative of 10E8) was reported to exhibit possible safety concerns due to local reactogenicity at site of injection when used in the clinic (35), and it has been reported to be autoreactive to ubiquitous human protein (36) and lipid bilayers (37, 38). The ability of the bNAbs selected for triple bNAb combinations (7 bNAbs) to mediate neutralization, ADCC, and BIC against an additional clade C SHIV (SHIV.C.1157 ipd3N4) and against a clade A SHIV (SHIV.A.BG505) was also assessed (see Table S3 in the supplemental material). Most single bNAbs demonstrated robust neutralization activity with an IC50 range of <0.01 to 0.16 μg/ml (see Table S3 for IC80 values), but they only displayed moderate BIC and ADCC activity against SHIV BG505 (Table S3). Additionally, even though 3BNC117 and CH31 demonstrated robust polyfunctional antiviral potencies against CH505, these bNAbs were not capable of mediating potent neutralization and ADCC against the clade C SHIV.C.1157 ipd3N4 (Table S3). These data suggest that the ability of single bNAbs to mediate neutralizing and nonneutralizing effector functions varies between cross clades and that combination of bNAbs are needed to broaden coverage.
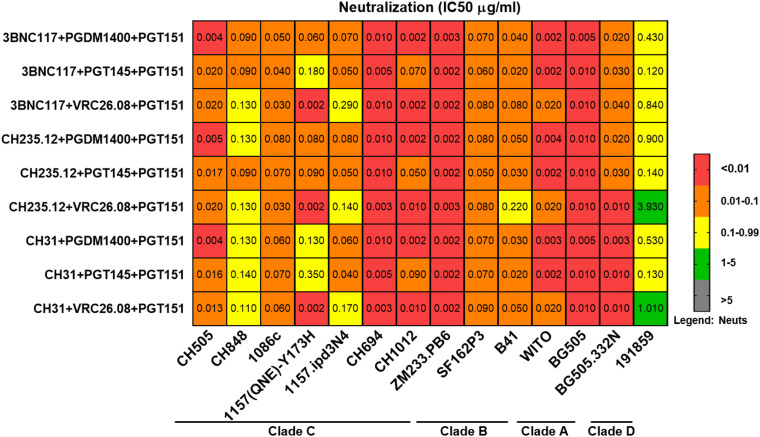
Triple bNAb combinations demonstrate robust neutralization potencies and breadth against clade A, B, C, and D SHIVs.
A total of 9 triple bNAb combinations, each comprising of one CD4BS-specific bNAb (3BNC117, CH235.12, or CH31), one V2-glycan-specific bNAb (PGT145, PGDM1400, or VRC26.08), and the gp120-gp41 interface bNAb (PGT151) were tested for their ability to neutralize an expanded panel of 14 cross-clade SHIVs (Fig. 3; see also Fig. S1a and b in the supplemental material). All triple bNAb combinations demonstrated robust neutralization potencies against tested SHIVs with a median IC50 of 0.0332 μg/ml (see Table S5 in the supplemental material for IC80/90 values). As the selection of bNAb candidates was based on their potencies against CH505, we sought to determine if bNAb combinations improved the neutralizing efficiency against this virus using a previously published calculation model (39). We found that 7 of 9 triple bNAb combinations demonstrated synergistic neutralization potencies, while 2 of 9 triple bNAb combinations demonstrated additive neutralization potencies against CH505 (see Table S4 in the supplemental material) with an experimental IC50 range of 0.004 to 0.0200 μg/ml (Fig. S1a and b; see Table S5 for IC80/90 values). Interestingly, clade C SHIV CH505 was most sensitive to triple bNAb combinations that consisted of any of the tested CD4BS-specific bNAbs in combination with PGDM1400 (V2-glycan-specific bNAb) and PGT151 (gp120-gp41 interface-specific bNAb), with IC50/80 ranges of 0.004 to 0.005 and 0.01 to 3.82 μg/ml, respectively (see Table S5 for IC90 values). All 9 triple bNAb combinations demonstrated highest neutralization potencies against clade C SHIV ZM233, with IC50/80 ranges of 0.002 to 0.003 and 0.006 to 0.023 μg/ml, respectively (see Table S5 for IC90 values). Consequently, 4 of 8 triple bNAb combinations showed neutralization IC50 values of <0.002 μg/ml against clade C SHIV CH1012 and clade B SHIV WITO (see Table S5 for IC80/90 values). Interestingly, 7 of 9 triple bNAb combinations exhibited moderate neutralization efficiencies against clade C SHIV 1157 ipd3N4 with an IC50/80 range of 1.02 to 5.01 and 0.4 to >5 μg/ml, respectively (see Table S5 for IC90 values; see also Fig. S1a), which included triple bNAb combinations that included 3BNC117 and CH31. This is not surprising, as these two bNAbs demonstrated poor single neutralizing and ADCC potencies against this virus (Table S3). Additionally, 2 of 9 triple bNAb combinations showed moderate neutralization efficiencies against clade D SHIV 191859, with IC50/80 values of 1.01/3 μg/ml and 3.93/>5 μg/ml, and overall, SHIV 191859 was more resistant to neutralization by triple bNAb combinations (Fig. 3, Fig. S1b, and Table S5). Based on neutralization titers (Fig. S1a and b and Table S5), the triple bNAb combination that conferred the best neutralization breadth coverage against cross-clade SHIVs was determined to be 3BNC117 plus PGDM1400 plus PGT151. This triple bNAb combination was also among those that mediated synergistic effect based on neutralization profile against clade C SHIV CH505 (Table S4). Taken together, these data indicated that triple bNAb combinations targeting different HIV Env regions confer improved broad neutralization potencies and breadth against cross-clade SHIVs.
FIG 3.
Triple bNAb combinations demonstrated robust neutralization potencies and breadth against cross-clade SHIVs. A total of 9 triple bNAb combinations (each including 1 CD4bs, 1 V2-glycan, and 1 gp120-gp41 interface-specific bNAb) were tested against clade A, B, C, and D SHIVs. Neutralization titers (IC50 [μg/ml], shown above; for IC80/90 [μg/ml] values, see Table S3 in the supplemental material) demonstrated that these triple bNAb combinations were highly potent against tier 2 clade A, B, C, and D SHIVs (for details, see Fig. S1 and Table S1). SHIVs 1157.ipd3N4, 1157(QNE)-Y173H, and SF162P3 were produced by propagation in rhesus macaque peripheral blood mononuclear cells (PBMCs). All other SHIVs were produced by transfection of 293T cells.
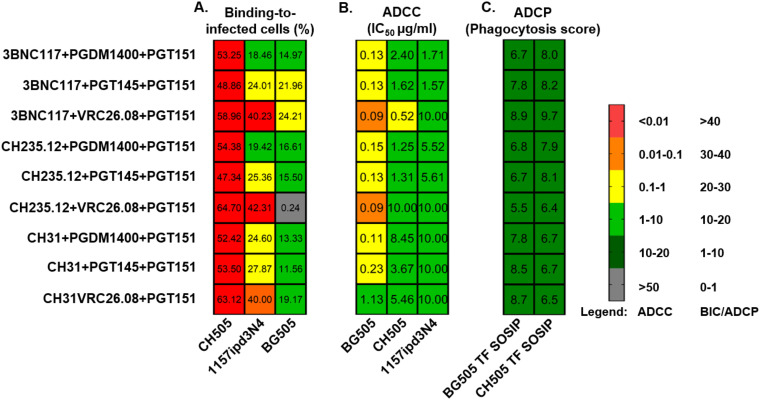
Triple bNAb combinations mediate robust nonneutralizing antibody-mediated effector functions against cross-clade SHIVs.
Next, we assessed the nonneutralizing antiviral activities of the triple bNAb combinations against cross-clade SHIVs. Due to limiting ability of SHIVs to infect target cells, we were able to assess the BIC and ADCC activities of the triple bNAb combinations against SHIV CH505, 1157ipd3N4, and BG505. Similar to their neutralization profiles, all 9 triple bNAb combinations were capable of mediating robust BIC (binding range, 48.9 to 64.7%) and ADCC (IC50 range, 0.09 to 0.23 μg/ml) activities against SHIV CH505-infected cells (Fig. 4). We found that 7 of 9 triple bNAb combinations exhibited predicted ADCC activities against CH505, while 2 of 9 triple bNAb combination demonstrated no additive or synergistic effect (Table S4). Interestingly, triple bNAb combinations consisting of any of the CD4BS-specific bNAbs in combination with VRC26.08 (V2-glycan) and PGT151 (gp120-gp41 interface) mediated robust binding to SHIV 1157ipd3N4-infected cells (binding range, 40.0 to 42.3%), but limited ADCC activities (IC50 range, 0.5 to 10.0 μg/ml) (Fig. 4). Similarly, the triple bNAb combinations of 3BNC117 plus PGT151 plus PGT145 or VRC26.08 were capable of mediating robust binding to SHIV BG505-infected cells (binding of 21.9 and 24.2%, respectively), but have limited ADCC activities (IC50 range, 1.7 and >10.0 μg/ml, respectively). Overall, the triple bNAb combinations of 3BNC117 plus PGT151 plus PGDM1400 or PGT145 were the top 2 combinations capable of mediating robust binding to infected cells (binding range, 15.9 to 53.3%) and ADCC (IC50 range, 0.13 to 2.40 μg/ml) activities against different clade SHIVs. The triple bNAb combination of 3BNC117 plus PGDM1400 plus PGT15 was among those that mediated the additive effect of ADCC activity against CH505-infected cells (Table S4).
FIG 4.
Triple bNAb combinations demonstrated robust nonneutralizing Fc-mediated effector functions against SHIVs. Triple bNAb combinations showed high capabilities to bind SHIV-infected cells (A), and to mediate ADCC of SHIV-infected cells (B) and antibody-dependent cell phagocytosis (ADCP) of beads coated with cross clade HIV SOSIP antigens (C). ADCC IC50 values and phagocytosis scores represent the average of 2 independent experiments. SHIV 1157.ipd3N4 was produced by propagation in rhesus PBMCs. SHIV CH505 and BG505 were produced by transfection of 293T cells.
To expand our assessment of the nonneutralizing effector functions of these triple bNAb combinations, we evaluated their capability to mediate antibody-dependent cell phagocytosis (ADCP) using beads coated with clade C HIV CH505 T/F (40) and clade A HIV BG505 T/F (40) SOSIP antigens (Fig. 4). All 9 triple bNAb combinations were capable of mediating modest phagocytosis of antigen-coated beads (phagocytosis score range, 6.4 to 9.7). These findings corroborated previous studies (34, 39) that indicated that combination of bNAbs improve neutralization potencies and simultaneously mediate different nonneutralizing antibody-mediated effectors functions against different cross-clade SHIVs. We next examined whether the polyfunctional antiviral activities of triple bNAb combinations against cross-clade SHIVs correlated with one another. Interestingly, the neutralizing potencies of triple bNAb combinations against clade C SHIV CH505 and SHIV 1157 ipd3N4, as well as against clade A SHIV BG505, did not correlate with nonneutralizing activities, ADCC, and BIC (data not shown).
V3-glycan targeting bNAbs demonstrate variable neutralization potency and breadth against cross-clade SHIVs.
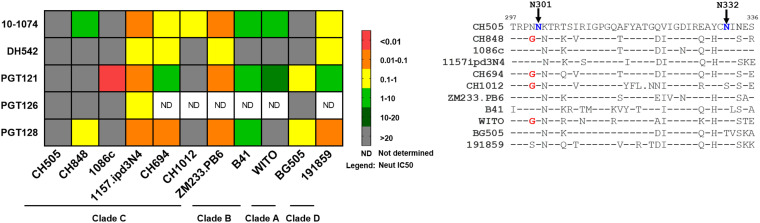
Because the first three clade C SHIVs (CH505, CH848, and Ce1086c) that we tested were resistant to neutralization by most V3-glycan targeting bNAbs evaluated (Table S2), we expanded the neutralization assessment of V3-glycan targeting bNAbs against additional clade C, B, A, and D SHIVs. Several SHIVs demonstrated resistance to at least one V3-glycan specific bNAb (Fig. 5). Overall, the neutralization potency of V3-glycan targeting bNAbs varied across clade C SHIVs (IC50/80 range, 0.01 to >25/0.05 to >25 μg/ml), with SHIV CH505 being the most resistant to 5 of 5 V3-glycan bNAbs tested (IC50/80, >25 μg/ml), followed by SHIV 1086c, which was resistant to 4 of 5 V3-glycan bNAbs tested (IC50/80, >25 μg/ml except for PGT121 with an IC50/80 of 0.01/0.05 μg/ml). Clade C SHIVs (CH848 and CH1012) and clade B SHIV (WITO) were moderately sensitive to 3 of 5 V3-glycan bNAbs tested (IC50/80 range, 13.67 to >25/>25 μg/ml). Meanwhile, SHIV 1157ipd3N4 and SHIV ZM233 were the most susceptible to neutralization by V3-glycan targeting bNAbs, with IC50 ranges of 0.04 to 0.33 μg/ml and 0.04 to 0.17 μg/ml, respectively (see Table S6 for IC80 values). Clade A SHIV (BG505) and clade B SHIV (B41) were moderately susceptible to neutralization by most V3-glycan targeting bNAbs but were resistant to neutralization by DH542 (IC50/80, >25 μg/ml for all SHIVs). Clade D SHIV (191859) was more susceptible to neutralization by different V3-glycan bNAbs compared to clade A and B SHIVs, with n IC50/80 range of 0.06 of 8.46/0.17 to >25 μg/ml. The V3-glycan bNAb 10-1074 exhibited the most neutralization breadth against cross-clade SHIVs (Fig. 5). Meanwhile, most cross-clade SHIVs showed resistance to V3-glycan targeting bNAb, DH542 (Fig. 5). Taken together, these data suggest that that the sensitivity to V3-dependent glycan is strain dependent, which may limit the use of bNAbs of this class in combination aiming at achieving both high potency and high coverage.
FIG 5.
V3-glycan specific bNAb neutralization potencies were variable against cross-clade SHIVs. A total of 5 V3-glycan specific bNAbs (10-1074, DH542, PGT121, PGT126, and PGT128) were evaluated for their ability to neutralize clade D, C, B, and A SHIVs. Based on neutralization titers (IC50 [μg/ml]), only SHIV.C.CH505 showed resistance to all 5 V3-specific bNAbs. Meanwhile, 4 other SHIVs (CH848, 1086c, CH1012, and BG505) were resistant to at least 3 of 5 V3-specific bNAbs, suggesting that V3-specific bNAb neutralization potencies differ between cross-clade SHIVs. SHIV 1157.ipd3N4 was produced by propagation in rhesus PBMCs. All other SHIVs were produced by transfection of 293T cells. Amino acid sequence alignment for V3 region for cross-clade SHIVs highlights the N301 and N332 V3-glycan signature sites on different SHIVs tested. Denoted in blue are V3 contact regions previously shown to mediate sensitivities to V3-glycan-dependent bNAbs. Denoted in red are V3 contact regions (G300) previously shown to mediate resistance to some V3-glycan-dependent bNAbs. V3 region sequence analyses showed lack of an N332 glycan hole in SHIV CH505 and 1086c.
DISCUSSION
While previous studies have demonstrated that combining bNAbs can improve their neutralization potency and breadth (34, 39, 41, 42), the impact of bNAb combination on nonneutralizing functions has been less studied. Therefore, systematic assessment of the ability of bNAbs to mediate diverse antiviral functions with broad coverage could inform the selection of candidates for optimal bNAb therapy. New-generation SHIVs that preserved the antigenic Env properties of their parental T/F variants (24) represent an important tool for the preclinical assessment of interventions directed against the HIV envelope. In accordance with previous reports (24, 43), in this study, we observed that the neutralization sensitivity of three new generation clade C SHIVs (CH505, CH848, and 1086c) to bNAbs was highly correlated with that of their parental T/F HIV-1 Env variants (Fig. 1; see also Table S2 in the supplemental material). This provides biological relevance to the use of SHIV challenge models for bNAb passive immunization studies. Nevertheless, an important limitation with the use of SHIV models for evaluation of bNAb therapies lies in the use of the specific strain of SHIV for challenge (20, 23, 32, 44). The neutralization breadth of bNAbs is influenced by the genetic diversity of HIV-1 virus subtypes (34, 45–48). Globally, subtype C HIV-1 viruses are responsible for approximately 50% of infections, followed by subtypes B and A with approximately 10% of infections each (49). Meanwhile, CRF strains (CRF02_AG and CRF01_AE) and subtype G each account for 5 to 8% of infections (49). HIV-1 viruses within the same clade differ in their viral Env regions by 8 to 17%, whereas differences in the Env region between subtypes can amount up to 35%, illustrating the outstanding variability of the HIV-1 viruses (50, 51). Thus, it is unclear if the results from a specific SHIV challenge model can be extend to other SHIVs from the same subtypes or across clades. In this study, we selected bNAb candidates for triple combinations based on their potencies against SHIV CH505, then assessed their antiviral activity against a large cross-clade SHIV panel. To our knowledge, this is the most comprehensive analysis of bNAb antiviral activity against SHIVs conducted to date.
The dual functionality of bNAbs via the antigen binding variable (Fab) and the constant (Fc) region (52–54) make them attractive as both prophylactic and immunotherapeutic adjunctive alternatives to ART (55). Unlike ART, bNAbs are not only able to neutralize (52) but also to mediate clearance of virus particles or infected cells (53, 54). Interestingly, we noted some important differences in the polyfunctional antiviral activity of bNAbs against different new-generation clade C SHIVs (see Tables S3 and S4 in the supplemental material). Specifically, we note that the sensitivity of the SHIV to neutralization by V2-glycan- and V3-glycan-dependent bNAbs was highly variable (Tables S3 and S4). In addition, V3-glycan dependent bNAbs showed poor nonneutralizing effector functions (ADCC and BIC) against SHIV CH505-infected cells (Fig. 2). The neutralization sensitivity to V3-glycan-dependent bNAbs is highly dependent on the presence of the potential N-linked glycosylation site (PNGS) at position N332, N301, and N295 (47, 56–58). Previous studies demonstrated that the loss of the PNGS from the HIV-1 Env position 332 resulted in resistance of HIV-1 subtype B viruses to the bNAbs PGT121 and PGT128 (59–62). Accordingly, an amino acid sequence alignment for the V3 region of SHIVs tested (Fig. 5) indicated that several of the SHIVs tested lacked the Env N332 glycosylation site, suggesting that this signature may contribute to explain the observed resistance of SHIVs CH505, 1086c, CH1012, and BG505 to V3-glycan-dependent bNAbs. In addition to the lack of the N332 glycosylation site, SHIVs CH505 and 1086c also demonstrated the presence of an N334 signature site previously reported to be associated with resistance to some V3-glycan-dependent bNAbs (58). Moreover, others SHIVs, such as the SHIVs CH848, CH1012, CH694, and WITO, demonstrated the presence of a G300 signature site that was also previously reported to confer resistance to some V3-glycan targeting bNAbs (58). Taken together, our data show the importance of carefully selecting the challenge SHIV strain in studies aiming at assessing V3-glycan-dependent bNAbs.
We identified several triple bNAb combinations with synergistic (7 of 9) and additive (2 of 9) neutralization potencies against SHIV CH505, as well as additive (7 of 9) ADCC activities against SHIV CH505-infected cells (Table S4). We also identified triple bNAb combinations that mediated broad neutralization against other tier 2 clade A, B, C, and D SHIVs (Fig. 3). In addition to neutralization, these triple bNAbs combinations exhibited robust BIC, ADCC, and ADCP activities against cross-clade SHIVs (Fig. 4). The triple bNAb combination of 3BNC117 plus PGDM1400 plus PGT151 conveyed the best overall in vitro polyfunctional antiviral potency and breadth coverage against cross-clade SHIVs. However, this observation needs further validation in vivo to determine the true potential for the use of this combination in passive immunization strategies in the clinic. 3BNC117 and PGDM1400 have been evaluated in human clinical trials and were reported to be safe and well tolerated (63, 64). Proof-of-concept preclinical studies provided evidence that early administration of 3BNC117 and PGDM1400 prevent virus acquisition, suppress viremia, and mediate clearance of virus-infected cells (23, 28, 33, 65). Together, our data and those collected by others support the concept that combination of bNAbs could maximize the polyfunctional antiviral efficacies and breadth of passively delivered bNAbs in preventing initial virus infection through virus neutralization and limiting the size of the virus reservoir through clearance of virus-infected cells, while potentially reducing the risk of emerging resistant strains.
In summary, our study demonstrates that potent polyfunctional antiviral activities against cross-clade SHIVs can be achieved using triple bNAb combinations that target distinct HIV-1 Env regions such as CD4BS, V2-glycan, and the gp120-gp41 interface. These data also demonstrate the importance of systematic assessment of neutralization potencies and nonneutralizing effector functions in developing bNAb cocktails with broad breadth coverage and optimal antiviral activities to cover high viral diversity within HIV-1 virus populations, as well as emerging resistant strains. This study will inform current and future passive immunization studies in preclinical animal models and in clinics in exploring the potential of combination bNAb therapy alone or with ART as preventative and therapeutic strategies to achieve functional HIV cure.
MATERIALS AND METHODS
Virus and antibody production.
Stocks of HIV and SHIV Env-pseudotyped viruses and infectious molecular clones (IMCs) were produced in-house by transfection of 293T cells as previously described (66, 67). Briefly, 293T cells (ATCC, Manassas, VA) were transfected with either 4 μg of Env plasmid DNA and 8 μg of Env-deficient HIV plasmid DNA or 12 μg of IMC plasmid DNA using the FuGene 6 transfection reagent (Roche Diagnostics). Viruses were harvested after 48 h, and titration of virus stocks (50% tissue culture infective dose [TCID50]) was performed as described previously (67). SHIV.B.SF162P3 (catalog no. 6526) and SHIV.C.1157ipd3N4 (catalog no. 11689) were obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. Details on the origin and clade of the viruses included in this study are provided in Table S1 in the supplemental material. bNAbs were either obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH (NIH-45-56 G54W, catalog no. 12174 and 10-1074, and catalog no. 12477) or produced in-house. In-house production was performed by transfecting 293T cells (ATCC, Manassas, VA) with plasmids containing the full-length heavy- and light-chain regions using previously described method (68). Briefly, 293T cells were transfected with plasmid DNA containing the full-length IgG1 (for heavy-chain) and kappa or lambda (for light chain) cassettes as previously described (68). Supernatants were harvested after 4 to 5 days of incubation at 37°C and 5% CO2, concentrated, and affinity purified by protein G chromatography per the manufacturer’s instructions (GE Healthcare). Antibody purity was evaluated by SDS-PAGE and Coomassie blue staining for appropriate sizes of heavy- and light-chain bands. PGDM1400 and PGT151 plasmids were generously provided by Dennis Burton (University of California, San Diego).
Cell line and culture condition.
TZM-bl cells (also called JC53BL-13) were a kind gift from John Kappes (University of Alabama at Birmingham) and were originally obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH (catalog no. 8129). The cell line was engineered to express CD4, CCR5, and CXCR4 (69) and to contain integrated reporter genes for firefly Luc and Escherichia coli β-galactosidase under the control of an HIV-1 long terminal repeat (70), which enable sensitive, highly permissible, and accurate measurements of infection by most strains of SHIV, HIV, and simian immunodeficiency virus (SIV), including primary or molecularly cloned viral isolates and molecularly cloned Env-pseudotyped viruses. TZM-bl cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with l-glutamine, sodium pyruvate, glucose, pyridoxine, and 25 mM HEPES [4-(2-hydroxyethyl)-l-piperazineethanesulfonic acid] (Gibco BRL Life Technologies) containing 10% heat-inactivated fetal bovine serum (FBS) and 50 μg gentamicin/ml in vented T-75 culture flasks (Corning Costar). Cell surface expression of key markers (CD4, CCR5, and CXCR4) were used to determine the authenticity of each new batch of TZM-bl cells and tested biannually for mycoplasma contamination. 293T cells (ATCC, Manassas, VA) were grown and maintained in DMEM with l-glutamine (Gibco BRL Life Technologies) containing 10% heat-inactivated FBS. Cells were used for transfection when the monolayer reached approximately 60 to 80% confluence.
TZM-bl cell-based neutralization assay.
Neutralization was measured as the ability to reduce virus infection of TZM-bl cells via reduction of luciferase reporter gene expression after a single round of infection (71–73). Briefly, single or combination bNAbs were incubated with a single strain of SHIV or HIV for 1 h at 37°C and 5% CO2. TZM-bl cells were then added and further incubated at 37°C and 5% CO2 for 48 h. Commercially available luciferase reagent (Bright-Glo; Promega, Madison, WI) was added into each well, and luminescence was measured using the Victor X3 multilabel plate reader (PerkinElmer, Waltham, MA) at 1 s/well. The bNAbs were either used as single-antibody suspensions starting at 25 μg and diluted out 3-fold or used in combinations starting at 5 μg/monoclonal antibody (MAb) and diluted out 3-fold. Results were reported as the 50%, 80%, and 90% inhibitory concentration (IC50/80/90), which are the concentrations of single or combination bNAbs resulting in 50, 80, and 90% reduction in relative luminescence units (RLU) compared to that in virus control wells.
Antibody-dependent cell cytotoxicity.
An antibody-dependent cell cytotoxicity (ADCC) assay was performed as previously described (74, 75). The CCR5+ CEM NKr cells with a Tat-inducible luciferase promoter were a kind gift from David Evans (University of Wisconsin at Madison). Briefly, CCR5+ CEM NKr cells with a Tat-inducible luciferase promoter were infected through spinoculation with 250 μl of the virus for 3 h at 1,500 × g, 25°C. Infection was allowed to proceed for 4 days at 37°C and 5% CO2. On day 3, infected cells were checked for infectivity by flow cytometry after intracellular staining for SIV Gag (2F12 antibody, catalog no. 1547; NIH AIDS Reagent Program). On day 4, infected targets were incubated with 15 to 50 μg of bNAbs. The bNAbs were either used as single-antibody suspensions starting at 50 μg and diluted out 3-fold or used in combinations starting at 15 μg/MAb and diluted out 3-fold. Rhesus CD16 expressing KHYG1 NK effector cells (also a kind gift from David Evans) and target cells were combined at a 10:1 effector to target ratio and incubated in the presence of antibody or antibodies for 8 h. A 150-μl volume of the cell mixture was then added to 50 μl of BriteLite Plus luciferase substrate reagent in a black 96-well plate (both from Perkin Elmer, Duluth, GA). Luciferase activity was measured 2 min later. ADCC activity was calculated as the percent reduction in luciferase compared to effector and target cells alone.
Binding to infected cells.
CCR5+ CEM NKr cells with a Tat-inducible luciferase promoter (kindly provided by David Evans, University of Wisconsin at Madison) were infected through spinoculation with 250 μl of the virus for 2 h at 1,200 × g at 25°C. Infection was allowed to proceed for 4 days. On day 4 postinfection, cells were washed twice with fluorescence-activated cell sorting (FACS) wash buffer (phosphate-buffered saline [PBS] with 2% FBS and 0.05% sodium azide) and stained extracellularly at 0.1 μg with either a single bNAb or triple bNAbs in combination at the same concentration for 20 min at 4°C. Cells were then washed with FACS wash buffer for 5 min at 1,500 rpm and then incubated with anti-human IgG Fc (Southern Biotech) diluted 1:1,000 in FACS wash buffer for 20 min at 4°C. Cells were washed and incubated with phycoerythrin (PE)-conjugated streptavidin (catalog no 554061; BD Bioscience) diluted 1:10,000 in FACS wash buffer for 20 min at 4°C. Cytofix/Cytoperm (catalog no. 554722; BD Bioscience) was used according to the manufacturer’s protocol to permeabilize cells following two washes with FACS wash buffer. Cells were again washed twice with Perm/Wash buffer (catalog no. 554723; BD Biosciences) and subsequently stained with anti-Gag 2F12 diluted 1:10,000 in Perm/Wash buffer for 25 min at 4°C. Lastly, cells were washed with Perm/Wash buffer followed by FACS wash buffer, then resuspended in FACS wash buffer. Cells were acquired on an LSR II flow cytometer (BD Immunocytometry Systems, San Jose, CA) and analyzed using FlowJo software (Tree Star, Ashland, OR). Percent binding to infected cells was determine as a percentage of SIV Gag-positive cells for all viruses tested. Infection rate varied for each virus, with that for SHIV CH505 being ∼15%, that for SHIV 1157 ipd3N4 being ∼12%, and that for SHIV BG505 being ∼10% (see Fig. S2 in the supplemental material).
Antibody-dependent cell phagocytosis.
An antibody-dependent cell phagocytosis (ADCP) assay was performed as previously described (76, 77). Briefly, clade C HIV CH505.TF\6R.SOSIP.664v4.1_avi.2-Bio\293F (40) and clade A HIV BG505\6R.SOSIP.664\T332N_avi\293F (40) antigens were covalently bound to fluorescent NeutrAvidin beads (Invitrogen). Single or triple bNAb combinations were diluted to a final concentration of 25 μg/ml (25 μg/ml of each bNAb in combination) and incubated for 2 h with antigen-conjugated beads to form immune complexes. Immune complexes were then subjected to spinoculation at 1,200 × g in the presence of a human-derived monocyte cell line, THP-1 cells (ATCC TIB-201), for 1 h at 4°C. Following spinoculation, antigens and cells were incubated at 37°C to allow phagocytosis to occur. After incubation, THP-1 cells were fixed with 2% paraformaldehyde (Sigma), and fluorescence of the cells was assessed using flow cytometry (LSRFortessa; BD). The CD4 binding site bNAb VRC01 was used as a positive control and the influenza-specific MAb CH65 as a negative control. A no-antibody control made of 0.1% phosphate-buffered saline supplemented with 0.1% bovine serum albumin (BSA) (1× PBS + 0.1% BSA) was used to determine the background phagocytosis activity. Phagocytosis scores were calculated by multiplying the mean fluorescence intensity (MFI) and frequency of bead-positive cells and dividing by the MFI and frequency of bead-positive cells in the antibody-negative control (PBS). All bNAbs were tested in two independent assays, and the average phagocytosis scores from these two independent assays is reported.
Statistical analysis.
Data were analyzed using Spearman’s rank correlation coefficient test and graphed with Prism Software (version 8; GraphPad, Inc., La Jolla, CA). The calculated exact r and P values are indicated in the figures. The combination effect was determined based on additive model by calculating the interaction score of predicted IC50 and experimental IC50 using previously a published formula (39). Briefly, predicted combination effects were based on the following criteria: synergistic effect = observed IC50 < predicted IC50 − 25%; additive effect = predicted IC50 − 25% < observed IC50 < predicted IC50 − 25%; and no effect = observed IC50 similar to that of more potent bNAbs.
Supplementary Material
ACKNOWLEDGMENTS
We thank George M. Shaw for providing us with plasmids and virus stocks for new-generation SHIVs. We acknowledge the Duke CFAR Immunology Core for their service in performing the ADCP assays with SOSIP antigens.
This study was supported by the National Institutes of Health under awards 5P01 AI131276 to S.R.P. and U19AI109633 to R.R.A. This project was funded in part by the Yerkes National Primate Research Center (grant ORIP/OD P51OD011132), which is supported by the NIH, Office of Research Infrastructure Programs.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.UNAIDS. Global HIV & AIDS statistics—2020 fact sheet. UNAIDS, Geneva, Switzerland. https://www.unaids.org/en/resources/fact-sheet. [Google Scholar]
- 2.UNAIDS. The global HIV/AIDS epidemic. 2019. UNAIDS, Geneva, Switzerland. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. [Google Scholar]
- 3.Palella FJ, Hart R, Armon C, Tedaldi E, Yangco B, Novak R, Battalora L, Ward D, Li J, Buchacz K, Study HIVO. 2019. Non-AIDS comorbidity burden differs by sex, race, and insurance type in aging adults in HIV care. AIDS 33:2327–2335. doi: 10.1097/QAD.0000000000002349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakaranurack C, Manosuthi W. 2018. Prevalence of non-AIDS comorbidities and factors associated with metabolic complications among HIV-infected patients at a Thai referral hospital. J Int Assoc Provid AIDS Care 17:232595741775225. 2325957417752256. doi: 10.1177/2325957417752256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenbloom DIS, Hill AL, Laskey SB, Siliciano RF. 2017. Re-evaluating evolution in the HIV reservoir. Nature 551:E6–E9. doi: 10.1038/nature24634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. 1998. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A 95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. 1995. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med 1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 9.Hamers RL, Sigaloff KC, Wensing AM, Wallis CL, Kityo C, Siwale M, Mandaliya K, Ive P, Botes ME, Wellington M, Osibogun A, Stevens WS, Rinke de Wit TF, Schuurman R, PharmAccess African Studies to Evaluate Resistance. 2012. Patterns of HIV-1 drug resistance after first-line antiretroviral therapy (ART) failure in 6 sub-Saharan African countries: implications for second-line ART strategies. Clin Infect Dis 54:1660–1669. doi: 10.1093/cid/cis254. [DOI] [PubMed] [Google Scholar]
- 10.Fogel JM, Hudelson SE, Ou SS, Hart S, Wallis C, Morgado MG, Saravanan S, Tripathy S, Hovind L, Piwowar-Manning E, Sabin D, McCauley M, Gamble T, Zhang XC, Eron JJ, Gallant JE, Kumwenda J, Makhema J, Kumarasamy N, Chariyalertsak S, Hakim J, Badal-Faesen S, Akelo V, Hosseinipour MC, Santos BR, Godbole SV, Pilotto JH, Grinsztejn B, Panchia R, Mayer KH, Chen YQ, Cohen MS, Eshleman SH. 2016. Brief report: HIV drug resistance in adults failing early antiretroviral treatment: results from the HIV Prevention Trials Network 052 trial. J Acquir Immune Defic Syndr 72:304–309. doi: 10.1097/QAI.0000000000000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandera A, Gori A, Clerici M, Sironi M. 2019. Phylogenies in ART: HIV reservoirs, HIV latency and drug resistance. Curr Opin Pharmacol 48:24–32. doi: 10.1016/j.coph.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Whitney JB, Lim SY, Osuna CE, Kublin JL, Chen E, Yoon G, Liu PT, Abbink P, Borducci EN, Hill A, Lewis MG, Geleziunas R, Robb ML, Michael NL, Barouch DH. 2018. Prevention of SIVmac251 reservoir seeding in rhesus monkeys by early antiretroviral therapy. Nat Commun 9:5429. doi: 10.1038/s41467-018-07881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, Smith JY, Brinkman AL, Peter LE, Mathew SI, Smith KM, Borducchi EN, Rosenbloom DI, Lewis MG, Hattersley J, Li B, Hesselgesser J, Geleziunas R, Robb ML, Kim JH, Michael NL, Barouch DH. 2014. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 512:74–77. doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M, Jr, Chun TW, Strain M, Richman D, Luzuriaga K. 2013. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med 369:1828–1835. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ananworanich J, Robb ML. 2014. The transient HIV remission in the Mississippi baby: why is this good news? J Int AIDS Soc 17:19859. doi: 10.7448/IAS.17.1.19859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Descours B, Guergnon J, Viard JP, Boufassa F, Lambotte O, Goujard C, Meyer L, Costagliola D, Venet A, Pancino G, Autran B, Rouzioux C, ANRS VISCONTI Study Group. 2013. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro MB, Cheever T, Malherbe DC, Pandey S, Reed J, Yang ES, Wang K, Pegu A, Chen X, Siess D, Burke D, Henderson H, Lewinsohn R, Fischer M, Stanton JJ, Axthelm MK, Kahl C, Park B, Lewis AD, Sacha JB, Mascola JR, Hessell AJ, Haigwood NL. 2020. Single-dose bNAb cocktail or abbreviated ART post-exposure regimens achieve tight SHIV control without adaptive immunity. Nat Commun 11:70. doi: 10.1038/s41467-019-13972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Julg B, Pegu A, Abbink P, Liu J, Brinkman A, Molloy K, Mojta S, Chandrashekar A, Callow K, Wang K, Chen X, Schmidt SD, Huang J, Koup RA, Seaman MS, Keele BF, Mascola JR, Connors M, Barouch DH. 2017. Virological control by the CD4-binding site antibody N6 in simian-human immunodeficiency virus-infected rhesus monkeys. J Virol 91:e00498-17. doi: 10.1128/JVI.00498-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, Lehmann C, Suarez I, Oliveira TY, Lorenzi JCC, Cohen YZ, Wyen C, Kummerle T, Karagounis T, Lu CL, Handl L, Unson-O'Brien C, Patel R, Ruping C, Schlotz M, Witmer-Pack M, Shimeliovich I, Kremer G, Thomas E, Seaton KE, Horowitz J, West AP, Jr, Bjorkman PJ, Tomaras GD, Gulick RM, Pfeifer N, Fatkenheuer G, Seaman MS, Klein F, Caskey M, Nussenzweig MC. 2018. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561:479–484. doi: 10.1038/s41586-018-0531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura Y, Gautam R, Chun TW, Sadjadpour R, Foulds KE, Shingai M, Klein F, Gazumyan A, Golijanin J, Donaldson M, Donau OK, Plishka RJ, Buckler-White A, Seaman MS, Lifson JD, Koup RA, Fauci AS, Nussenzweig MC, Martin MA. 2017. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 543:559–563. doi: 10.1038/nature21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shingai M, Donau OK, Schmidt SD, Gautam R, Plishka RJ, Buckler-White A, Sadjadpour R, Lee WR, LaBranche CC, Montefiori DC, Mascola JR, Nishimura Y, Martin MA. 2012. Most rhesus macaques infected with the CCR5-tropic SHIV(AD8) generate cross-reactive antibodies that neutralize multiple HIV-1 strains. Proc Natl Acad Sci U S A 109:19769–19774. doi: 10.1073/pnas.1217443109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borducchi EN, Liu J, Nkolola JP, Cadena AM, Yu WH, Fischinger S, Broge T, Abbink P, Mercado NB, Chandrashekar A, Jetton D, Peter L, McMahan K, Moseley ET, Bekerman E, Hesselgesser J, Li W, Lewis MG, Alter G, Geleziunas R, Barouch DH. 2018. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 563:360–364. doi: 10.1038/s41586-018-0600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang HW, Shekhar K, Gupta S, Nkolola JP, Seaman MS, Smith KM, Borducchi EN, Cabral C, Smith JY, Blackmore S, Sanisetty S, Perry JR, Beck M, Lewis MG, Rinaldi W, Chakraborty AK, Poignard P, Nussenzweig MC, Burton DR. 2013. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Wang S, Kong R, Ding W, Lee FH, Parker Z, Kim E, Learn GH, Hahn P, Policicchio B, Brocca-Cofano E, Deleage C, Hao X, Chuang GY, Gorman J, Gardner M, Lewis MG, Hatziioannou T, Santra S, Apetrei C, Pandrea I, Alam SM, Liao HX, Shen X, Tomaras GD, Farzan M, Chertova E, Keele BF, Estes JD, Lifson JD, Doms RW, Montefiori DC, Haynes BF, Sodroski JG, Kwong PD, Hahn BH, Shaw GM. 2016. Envelope residue 375 substitutions in simian-human immunodeficiency viruses enhance CD4 binding and replication in rhesus macaques. Proc Natl Acad Sci U S A 113:E3413–22. doi: 10.1073/pnas.1606636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, Forthal DN, Koff WC, Poignard P, Watkins DI, Burton DR. 2010. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol 84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hessell AJ, Jaworski JP, Epson E, Matsuda K, Pandey S, Kahl C, Reed J, Sutton WF, Hammond KB, Cheever TA, Barnette PT, Legasse AW, Planer S, Stanton JJ, Pegu A, Chen X, Wang K, Siess D, Burke D, Park BS, Axthelm MK, Lewis A, Hirsch VM, Graham BS, Mascola JR, Sacha JB, Haigwood NL. 2016. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat Med 22:362–368. doi: 10.1038/nm.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Julg B, Sok D, Schmidt SD, Abbink P, Newman RM, Broge T, Linde C, Nkolola J, Le K, Su D, Torabi J, Pack M, Pegu A, Allen TM, Mascola JR, Burton DR, Barouch DH. 2017. Protective efficacy of broadly neutralizing antibodies with incomplete neutralization activity against simian-human immunodeficiency virus in rhesus monkeys. J Virol 91:e01187-17. doi: 10.1128/JVI.01187-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Julg B, Tartaglia LJ, Keele BF, Wagh K, Pegu A, Sok D, Abbink P, Schmidt SD, Wang K, Chen X, Joyce MG, Georgiev IS, Choe M, Kwong PD, Doria-Rose NA, Le K, Louder MK, Bailer RT, Moore PL, Korber B, Seaman MS, Abdool Karim SS, Morris L, Koup RA, Mascola JR, Burton DR, Barouch DH. 2017. Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci Transl Med 9:eaal1321. doi: 10.1126/scitranslmed.aal1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moldt B, Le KM, Carnathan DG, Whitney JB, Schultz N, Lewis MG, Borducchi EN, Smith KM, Mackel JJ, Sweat SL, Hodges AP, Godzik A, Parren PW, Silvestri G, Barouch DH, Burton DR. 2016. Neutralizing antibody affords comparable protection against vaginal and rectal simian/human immunodeficiency virus challenge in macaques. AIDS 30:1543–1551. doi: 10.1097/QAD.0000000000001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moldt B, Rakasz EG, Schultz N, Chan-Hui PY, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, Burton DR. 2012. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A 109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho MW, Martin MA. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med 5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 32.Shingai M, Donau OK, Plishka RJ, Buckler-White A, Mascola JR, Nabel GJ, Nason MC, Montefiori D, Moldt B, Poignard P, Diskin R, Bjorkman PJ, Eckhaus MA, Klein F, Mouquet H, Cetrulo Lorenzi JC, Gazumyan A, Burton DR, Nussenzweig MC, Martin MA, Nishimura Y. 2014. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med 211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M, Jr, Lifson JD, Dimitrov DS, Nussenzweig MC, Martin MA. 2013. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Julg B, Liu PT, Wagh K, Fischer WM, Abbink P, Mercado NB, Whitney JB, Nkolola JP, McMahan K, Tartaglia LJ, Borducchi EN, Khatiwada S, Kamath M, LeSuer JA, Seaman MS, Schmidt SD, Mascola JR, Burton DR, Korber BT, Barouch DH. 2017. Protection against a mixed SHIV challenge by a broadly neutralizing antibody cocktail. Sci Transl Med 9:eaao4235. doi: 10.1126/scitranslmed.aao4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ClinicalTrials.gov. 2020. VRC 610: phase I safety and pharmacokinetics study to evaluate a human monoclonal antibody (MAb) VRC-HIVMAB095-00-AB (10E8VLS) administered alone or concurrently with MAb VRC-HIVMAB075-00-AB (VRC07-523LS) via subcutaneous injection in healthy adults. NCT03565315. https://clinicaltrials.gov/ct2/show/NCT03565315.
- 36.Liu M, Yang G, Wiehe K, Nicely NI, Vandergrift NA, Rountree W, Bonsignori M, Alam SM, Gao J, Haynes BF, Kelsoe G. 2015. Polyreactivity and autoreactivity among HIV-1 antibodies. J Virol 89:784–798. doi: 10.1128/JVI.02378-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Frey G, Peng H, Rits-Volloch S, Garrity J, Seaman MS, Chen B. 2014. Mechanism of HIV-1 neutralization by antibodies targeting a membrane-proximal region of gp41. J Virol 88:1249–1258. doi: 10.1128/JVI.02664-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verkoczy L, Diaz M. 2014. Autoreactivity in HIV-1 broadly neutralizing antibodies: implications for their function and induction by vaccination. Curr Opin HIV AIDS 9:224–234. doi: 10.1097/COH.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong R, Louder MK, Wagh K, Bailer RT, deCamp A, Greene K, Gao H, Taft JD, Gazumyan A, Liu C, Nussenzweig MC, Korber B, Montefiori DC, Mascola JR. 2015. Improving neutralization potency and breadth by combining broadly reactive HIV-1 antibodies targeting major neutralization epitopes. J Virol 89:2659–2671. doi: 10.1128/JVI.03136-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saunders KO, Verkoczy LK, Jiang C, Zhang J, Parks R, Chen H, Housman M, Bouton-Verville H, Shen X, Trama AM, Scearce R, Sutherland L, Santra S, Newman A, Eaton A, Xu K, Georgiev IS, Joyce MG, Tomaras GD, Bonsignori M, Reed SG, Salazar A, Mascola JR, Moody MA, Cain DW, Centlivre M, Zurawski S, Zurawski G, Erickson HP, Kwong PD, Alam SM, Levy Y, Montefiori DC, Haynes BF. 2017. Vaccine induction of heterologous tier 2 HIV-1 neutralizing antibodies in animal models. Cell Rep 21:3681–3690. doi: 10.1016/j.celrep.2017.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caskey M. 2020. Broadly neutralizing antibodies for the treatment and prevention of HIV infection. Curr Opin HIV AIDS 15:49–55. doi: 10.1097/COH.0000000000000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen YZ, Caskey M. 2018. Broadly neutralizing antibodies for treatment and prevention of HIV-1 infection. Curr Opin HIV AIDS 13:366–373. doi: 10.1097/COH.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 43.Bauer AM, Ziani W, Lindemuth E, Kuri-Cervantes L, Li H, Lee FH, Watkins M, Ding W, Xu H, Veazey R, Bar KJ. 2020. Novel transmitted/founder simian-human immunodeficiency viruses for human immunodeficiency virus latency and cure research. J Virol 94:e01659-19. doi: 10.1128/JVI.01659-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolton DL, Pegu A, Wang K, McGinnis K, Nason M, Foulds K, Letukas V, Schmidt SD, Chen X, Todd JP, Lifson JD, Rao S, Michael NL, Robb ML, Mascola JR, Koup RA. 2016. Human immunodeficiency virus type 1 monoclonal antibodies suppress acute simian-human immunodeficiency virus viremia and limit seeding of cell-associated viral reservoirs. J Virol 90:1321–1332. doi: 10.1128/JVI.02454-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hraber P, Korber BT, Lapedes AS, Bailer RT, Seaman MS, Gao H, Greene KM, McCutchan F, Williamson C, Kim JH, Tovanabutra S, Hahn BH, Swanstrom R, Thomson MM, Gao F, Harris L, Giorgi E, Hengartner N, Bhattacharya T, Mascola JR, Montefiori DC. 2014. Impact of clade, geography, and age of the epidemic on HIV-1 neutralization by antibodies. J Virol 88:12623–12643. doi: 10.1128/JVI.01705-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bricault CA, Yusim K, Seaman MS, Yoon H, Theiler J, Giorgi EE, Wagh K, Theiler M, Hraber P, Macke JP, Kreider EF, Learn GH, Hahn BH, Scheid JF, Kovacs JM, Shields JL, Lavine CL, Ghantous F, Rist M, Bayne MG, Neubauer GH, McMahan K, Peng H, Cheneau C, Jones JJ, Zeng J, Ochsenbauer C, Nkolola JP, Stephenson KE, Chen B, Gnanakaran S, Bonsignori M, Williams LD, Haynes BF, Doria-Rose N, Mascola JR, Montefiori DC, Barouch DH, Korber B. 2019. HIV-1 neutralizing antibody signatures and application to epitope-targeted vaccine design. Cell Host Microbe 25:59–72.e8. doi: 10.1016/j.chom.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stefic K, Bouvin-Pley M, Essat A, Visdeloup C, Moreau A, Goujard C, Chaix ML, Braibant M, Meyer L, Barin F. 2018. Sensitivity to broadly neutralizing antibodies of recently transmitted HIV-1 clade CRF02_AG viruses with a focus on evolution over time. J Virol 93 doi: 10.1128/JVI.01492-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stefic K, Bouvin-Pley M, Braibant M, Barin F. 2019. Impact of HIV-1 diversity on its sensitivity to neutralization. Vaccines (Basel) 7:74. doi: 10.3390/vaccines7030074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hemelaar J, Elangovan R, Yun J, Dickson-Tetteh L, Fleminger I, Kirtley S, Williams B, Gouws-Williams E, Ghys PD, on behalf of the WHO-UNAIDS Network for HIV Isolation Characterisation, et al. 2019. Global and regional molecular epidemiology of HIV-1, 1990–2015: a systematic review, global survey, and trend analysis. Lancet Infect Dis 19:143–155. doi: 10.1016/S1473-3099(18)30647-9. [DOI] [PubMed] [Google Scholar]
- 50.Taylor BS, Hammer SM. 2008. The challenge of HIV-1 subtype diversity. N Engl J Med 359:1965–1966. doi: 10.1056/NEJMc086373. [DOI] [PubMed] [Google Scholar]
- 51.Lynch RM, Shen T, Gnanakaran S, Derdeyn CA. 2009. Appreciating HIV type 1 diversity: subtype differences in Env. AIDS Res Hum Retroviruses 25:237–248. doi: 10.1089/aid.2008.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chun TW, Murray D, Justement JS, Blazkova J, Hallahan CW, Fankuchen O, Gittens K, Benko E, Kovacs C, Moir S, Fauci AS. 2014. Broadly neutralizing antibodies suppress HIV in the persistent viral reservoir. Proc Natl Acad Sci U S A 111:13151–13156. doi: 10.1073/pnas.1414148111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K, Donahue DA, Lorin V, Casartelli N, Noel N, Lambotte O, Mouquet H, Schwartz O. 2016. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun 7:10844. doi: 10.1038/ncomms10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu CL, Murakowski DK, Bournazos S, Schoofs T, Sarkar D, Halper-Stromberg A, Horwitz JA, Nogueira L, Golijanin J, Gazumyan A, Ravetch JV, Caskey M, Chakraborty AK, Nussenzweig MC. 2016. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science 352:1001–1004. doi: 10.1126/science.aaf1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parsons MS, Chung AW, Kent SJ. 2018. Importance of Fc-mediated functions of anti-HIV-1 broadly neutralizing antibodies. Retrovirology 15:58. doi: 10.1186/s12977-018-0438-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kong L, Lee JH, Doores KJ, Murin CD, Julien JP, McBride R, Liu Y, Marozsan A, Cupo A, Klasse PJ, Hoffenberg S, Caulfield M, King CR, Hua Y, Le KM, Khayat R, Deller MC, Clayton T, Tien H, Feizi T, Sanders RW, Paulson JC, Moore JP, Stanfield RL, Burton DR, Ward AB, Wilson IA. 2013. Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat Struct Mol Biol 20:796–803. doi: 10.1038/nsmb.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rademeyer C, Korber B, Seaman MS, Giorgi EE, Thebus R, Robles A, Sheward DJ, Wagh K, Garrity J, Carey BR, Gao H, Greene KM, Tang H, Bandawe GP, Marais JC, Diphoko TE, Hraber P, Tumba N, Moore PL, Gray GE, Kublin J, McElrath MJ, Vermeulen M, Middelkoop K, Bekker LG, Hoelscher M, Maboko L, Makhema J, Robb ML, Abdool Karim S, Abdool Karim Q, Kim JH, Hahn BH, Gao F, Swanstrom R, Morris L, Montefiori DC, Williamson C. 2016. Features of recently transmitted HIV-1 clade C viruses that impact antibody recognition: implications for active and passive immunization. PLoS Pathog 12:e1005742. doi: 10.1371/journal.ppat.1005742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bricault CA, Yusim K, Seaman MS, Yoon H, Theiler J, Giorgi EE, Wagh K, Theiler M, Hraber P, Macke JP, Kreider EF, Learn GH, Hahn BH, Scheid JF, Kovacs JM, Shields JL, Lavine CL, Ghantous F, Rist M, Bayne MG, Neubauer GH, McMahan K, Peng H, Cheneau C, Jones JJ, Zeng J, Ochsenbauer C, Nkolola JP, Stephenson KE, Chen B, Gnanakaran S, Bonsignori M, Williams LD, Haynes BF, Doria-Rose N, Mascola JR, Montefiori DC, Barouch DH, Korber B. 2019. HIV-1 neutralizing antibody signatures and application to epitope-targeted vaccine design. Cell Host Microbe 26:296. doi: 10.1016/j.chom.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouvin-Pley M, Morgand M, Moreau A, Jestin P, Simonnet C, Tran L, Goujard C, Meyer L, Barin F, Braibant M. 2013. Evidence for a continuous drift of the HIV-1 species towards higher resistance to neutralizing antibodies over the course of the epidemic. PLoS Pathog 9:e1003477. doi: 10.1371/journal.ppat.1003477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Protocol GPI, Koff WC, Wilson IA, Burton DR, Poignard P, Protocol G Principal Investigators. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doria-Rose NA, Georgiev I, O'Dell S, Chuang GY, Staupe RP, McLellan JS, Gorman J, Pancera M, Bonsignori M, Haynes BF, Burton DR, Koff WC, Kwong PD, Mascola JR. 2012. A short segment of the HIV-1 gp120 V1/V2 region is a major determinant of resistance to V1/V2 neutralizing antibodies. J Virol 86:8319–8323. doi: 10.1128/JVI.00696-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moore PL, Gray ES, Wibmer CK, Bhiman JN, Nonyane M, Sheward DJ, Hermanus T, Bajimaya S, Tumba NL, Abrahams MR, Lambson BE, Ranchobe N, Ping L, Ngandu N, Abdool Karim Q, Abdool Karim SS, Swanstrom RI, Seaman MS, Williamson C, Morris L. 2012. Evolution of an HIV glycan-dependent broadly neutralizing antibody epitope through immune escape. Nat Med 18:1688–1692. doi: 10.1038/nm.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bar-On Y, Gruell H, Schoofs T, Pai JA, Nogueira L, Butler AL, Millard K, Lehmann C, Suarez I, Oliveira TY, Karagounis T, Cohen YZ, Wyen C, Scholten S, Handl L, Belblidia S, Dizon JP, Vehreschild JJ, Witmer-Pack M, Shimeliovich I, Jain K, Fiddike K, Seaton KE, Yates NL, Horowitz J, Gulick RM, Pfeifer N, Tomaras GD, Seaman MS, Fatkenheuer G, Caskey M, Klein F, Nussenzweig MC. 2018. Safety and antiviral activity of combination HIV-1 broadly neutralizing antibodies in viremic individuals. Nat Med 24:1701–1707. doi: 10.1038/s41591-018-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.ClinicalTrials.gov. 2019. A phase 1/2a study of PGT121, VRC07-523LS and PGDM1400 monoclonal antibodies in HIV-uninfected and HIV-infected adults. NCT03721510. https://clinicaltrials.gov/ct2/show/NCT03721510.
- 65.van der Velden YU, Villaudy J, Siteur-van Rijnstra E, van der Linden CA, Frankin E, Weijer K, Schermer E, Vink MA, Berkhout B, Sanders RW, van Gils MJ. 2018. Short Communication: protective Efficacy of broadly neutralizing antibody PGDM1400 against HIV-1 challenge in humanized mice. AIDS Res Hum Retroviruses 34:790–793. doi: 10.1089/AID.2018.0114. [DOI] [PubMed] [Google Scholar]
- 66.Louder MK, Sambor A, Chertova E, Hunte T, Barrett S, Ojong F, Sanders-Buell E, Zolla-Pazner S, McCutchan FE, Roser JD, Gabuzda D, Lifson JD, Mascola JR. 2005. HIV-1 envelope pseudotyped viral vectors and infectious molecular clones expressing the same envelope glycoprotein have a similar neutralization phenotype, but culture in peripheral blood mononuclear cells is associated with decreased neutralization sensitivity. Virology 339:226–238. doi: 10.1016/j.virol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Martinez DR, Tu JJ, Kumar A, Mangold JF, Mangan RJ, Goswami R, Giorgi EE, Chen J, Mengual M, Douglas AO, Heimsath H, Saunders KO, Nicely NI, Eudailey J, Hernandez G, Morgan-Asiedu PK, Wiehe K, Haynes BF, Moody MA, LaBranche C, Montefiori DC, Gao F, Permar SR. 2020. Maternal broadly neutralizing antibodies can select for neutralization-resistant, infant-transmitted/founder HIV variants. mBio 11:e00176-20. doi: 10.1128/mBio.00176-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liao HX, Levesque MC, Nagel A, Dixon A, Zhang R, Walter E, Parks R, Whitesides J, Marshall DJ, Hwang KK, Yang Y, Chen X, Gao F, Munshaw S, Kepler TB, Denny T, Moody MA, Haynes BF. 2009. High-throughput isolation of immunoglobulin genes from single human B cells and expression as monoclonal antibodies. J Virol Methods 158:171–179. doi: 10.1016/j.jviromet.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol 72:2855–2864. doi: 10.1128/JVI.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother 46:1896–1905. doi: 10.1128/aac.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sarzotti-Kelsoe M, Bailer RT, Turk E, Lin CL, Bilska M, Greene KM, Gao H, Todd CA, Ozaki DA, Seaman MS, Mascola JR, Montefiori DC. 2014. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods 409:131–146. doi: 10.1016/j.jim.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Montefiori DC. 2005. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol Chapter 12:Unit 12.11. doi: 10.1002/0471142735.im1211s64. [DOI] [PubMed] [Google Scholar]
- 74.Alpert MD, Heyer LN, Williams DE, Harvey JD, Greenough T, Allhorn M, Evans DT. 2012. A novel assay for antibody-dependent cell-mediated cytotoxicity against HIV-1- or SIV-infected cells reveals incomplete overlap with antibodies measured by neutralization and binding assays. J Virol 86:12039–12052. doi: 10.1128/JVI.01650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Styles TM, Gangadhara S, Reddy PBJ, Hicks S, LaBranche CC, Montefiori DC, Derdeyn CA, Kozlowski PA, Velu V, Amara RR. 2019. Human immunodeficiency virus C.1086 envelope gp140 protein boosts following DNA/modified vaccinia virus Ankara vaccination fail to enhance heterologous anti-V1V2 antibody response and protection against clade C simian-human immunodeficiency virus challenge. J Virol 93:e00934-19. doi: 10.1128/JVI.00934-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tay MZ, Liu P, Williams LD, McRaven MD, Sawant S, Gurley TC, Xu TT, Dennison SM, Liao HX, Chenine AL, Alam SM, Moody MA, Hope TJ, Haynes BF, Tomaras GD. 2016. Antibody-mediated internalization of infectious HIV-1 virions differs among antibody isotypes and subclasses. PLoS Pathog 12:e1005817. doi: 10.1371/journal.ppat.1005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ackerman ME, Moldt B, Wyatt RT, Dugast AS, McAndrew E, Tsoukas S, Jost S, Berger CT, Sciaranghella G, Liu Q, Irvine DJ, Burton DR, Alter G. 2011. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods 366:8–19. doi: 10.1016/j.jim.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.