Abstract
Background: Given that residual congestion is a predictor of poor outcome in patients with heart failure (HF), a therapeutic strategy for decongestion is required.
Methods and Results: Eighteen HF patients with fluid retention despite oral furosemide >20 mg/day, with chronic kidney disease (CKD; estimated glomerular filtration rate [eGFR], <59 mL/min/1.73 m2) were enrolled. Patients were randomized into 2 groups: a tolvaptan group (tolvaptan, 7.5 mg/day, n=10) and a furosemide group (additional furosemide 20 mg/day, n=8), and followed up for 7 days. The urine volume significantly increased on day 3 in the tolvaptan group but not in the furosemide group. The body weight significantly decreased in the tolvaptan compared with the furosemide group on days 3 and 5. Although there was no difference in serum creatinine or eGFR in the 7 days between the 2 groups, serum cystatin C significantly decreased on day 7 in the tolvaptan group compared with the furosemide group. The residual congestion was more improved in the tolvaptan group than in the furosemide group.
Conclusions: Adding tolvaptan but not furosemide significantly increased urine volume, decreased body weight and improved residual congestion without affecting the renal function or electrolytes in patients with HF with CKD under furosemide treatment.
Key Words: Chronic kidney disease, Diuretic, Furosemide, Residual congestion, Tolvaptan
Heart failure (HF) is the end-stage pathophysiological condition of all cardiac diseases, and is one of the leading causes of morbidity and mortality.1 HF is accompanied by activation of the sympathetic nervous system2 and the renin-angiotensin-aldosterone system,3 and by fluid accumulation in the systemic and pulmonary circulation due to excessive sodium retention.4 Diuretic treatment is a first-line standard treatment for congestive HF.5 Diuretics are usually effective for HF but sometimes insufficient because of residual congestion despite their use. Residual congestion is usually caused by diuretic resistance, defined as a failure to achieve a therapeutic reduction in excessive fluid despite adequate dosing of a diuretic.6 Residual congestion at discharge is one of the risk factors for early re-admission and mortality.7 In recent studies on the relationship between diuretic efficacy and clinical outcome in HF, outcomes were poor in patients with diuretic resistance.8,9 Patients with diuretic resistance also had lower glomerular filtration rate (GFR).9 Therefore, it is important to resolve residual congestion in HF patients with diuretic resistance in order to achieve a better clinical outcome.
The vasopressin V2 receptor antagonist tolvaptan has been reported to reduce symptoms of HF by increasing water excretion, which is mediated through a reduction in the aquaporin water-transporting system in the distal portion of the nephron.10 The efficacy and safety of tolvaptan in the treatment for congestive HF has been evaluated mainly in the acute phase of HF.11–14 There are only a very few studies, however, on HF patients with diuretic resistance and residual congestion.15,16 The aim of the present study was therefore to compare the effect of tolvaptan with that of loop-diuretic dosing up in loop-diuretic-resistant HF patients with residual congestion and renal impairment who had been treated with a loop diuretic.
Methods
The protocol of the present study was approved by the ethics committee of Gifu University Graduate School of Medicine (approval number: 27-520). All of the patients gave written informed consent before the study commenced. The investigation conformed to the principles outlined in the Declaration of Helsinki.17 The public and trial registry number is UMIN 000014312.
Patients
Eighteen patients with congestive HF with renal impairment, who were admitted to Gifu University Hospital, were included in this study. The patients enrolled in this study were not in the acute phase of HF but the patients required treatment due to residual congestion. The study period was from July 2012 to December 2017. Enrollment was performed consecutively for only patients who provided written informed consent: the tolvaptan group (n=10), patients with HF who were being treated with a loop diuretic were additionally treated with 7.5 mg/day tolvaptan for 7 days; and the furosemide group (n=8), patients with HF who were being treated with a loop diuretic were additionally treated with furosemide for 7 days.
The inclusion criteria were: (1) admission to Gifu University Hospital with HF and fluid retention such as leg edema, pulmonary congestion, and jugular venous distension; (2) treatment with furosemide >20 mg/day; (3) chronic kidney disease (CKD) stage III (estimated GFR [eGFR], 30–59) or CKD stage IV (eGFR, 15–29); and (4) age, 20–85 years old. The exclusion criteria were: (1) diagnosis of acute myocardial infarction in the last 30 days; (2) active myocarditis or amyloid cardiomyopathy; (3) auxiliary circulating device; (4) decreased circulatory blood volume; (5) hypertrophic cardiomyopathy (excluding dilation phase); (6) clinically significant valvular disease; (7) hepatic coma; (8) poorly controlled diabetes; (9) anuria; (10) urinary tract excretion failure; and (11) high systolic blood pressure in the supine position.
Study Design
Figure 1 shows the design of the comparative study on the effects between the addition of tolvaptan or furosemide on the urine volume in HF patients with CKD who were under treatment with furosemide. All 18 HF patients with CKD were assigned to either the tolvaptan group or furosemide group. The patients were followed up for 7 days.
Figure 1.
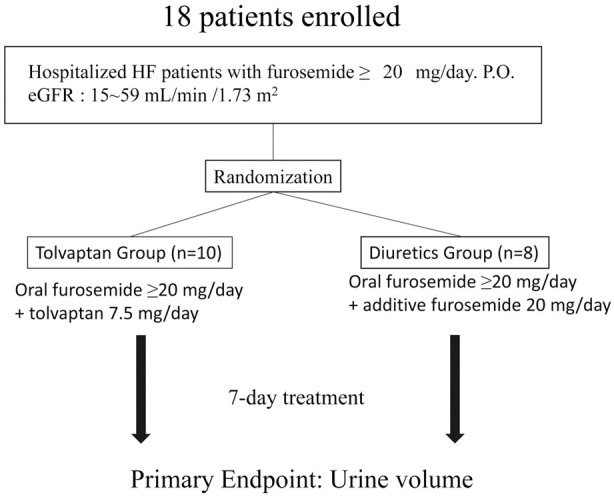
Study flowchart. eGFR, estimated glomerular filtration rate; HF, heart failure.
Endpoint
The primary endpoint of the present study was the average change in the urine volume during the 7-day treatment period compared with baseline. The secondary endpoints were change in body weight (BW), plasma brain natriuretic peptide (BNP), renal function such as eGFR, serum creatinine and cystatin C, urine N-acetyl-β-D-glucosaminidase (NAG) and β2-microglobulin (β2-MG), left ventricular ejection fraction (LVEF), left ventricular end-diastolic dimension (LVDd), tricuspid regurgitation pressure gradient (TRPG), inferior vena cava (IVC) diameter, and the parameters of residual congestion such as dyspnea on effort, leg edema, jugular vein dilatation, lung rales or congestion on chest X-ray.
LV Function on Echocardiography
The LVEF (%), LVDd (mm), TRPG (mmHg), and IVC diameter (mm) were obtained on echocardiography (iE33, PHILIPS, Tokyo, Japan). The same cardiologist performed the echocardiography, who was blinded to the protocol of the study.
Blood Biochemistry
Blood samples were taken from the antecubital veins. Peripheral blood cell count, hemogram, and blood biochemistry were analyzed for factors such as creatine kinase (CK), aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), creatinine, blood urea nitrogen (BUN), C-reactive protein (CRP), hemoglobin A1c (HbA1c), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), and BNP (Shionoria BNP RIA kit; Shionogi, Osaka, Japan).
Drugs Used and Complications
Drugs used and complications were examined.
Statistical Analysis
The data are given as mean±SD. Categorical data were converted to percentage and compared using chi-squared test or Fisher’s exact test. The normality of data distribution was tested using the Kolmogorov-Smirnov test. The significance of the differences between groups for normally distributed variables was determined using unpaired Student’s t-test. Otherwise, Mann-Whitney U-test was used to compare the differences between groups. Effects of drugs on echocardiographic parameters were assessed on univariate analysis. P<0.05 was considered significant. All statistical analyses were performed using Stat View version 5.0 (SAS Institution, Cary, NC, USA).
Results
Baseline Patient Characteristics
As shown in Table, there was no significant difference in patient characteristics between the tolvaptan and furosemide groups.
Table.
Patient Characteristics
| Furosemide (n=8) |
Tolvaptan (n=10) |
P-value | |
|---|---|---|---|
| Age (years) | 76.5±6.0 | 73.4±12.3 | 0.52 |
| Male | 6 (75.0) | 7 (70.0) | 0.62 |
| Body weight (kg) | 54.0±7.1 | 58.2±10.6 | 0.37 |
| BMI (kg/m2) | 21.3±3.5 | 23.2±3.2 | 0.28 |
| Clinical scenario (1/2/3/4/5) | 4/2/2/0/0 | 3/6/1/0/0 | 0.70 |
| Baseline (OMI/Myopathy/VHD) | 3/3/2 | 4/5/1 | 0.71 |
| DM | 2 (25.0) | 4 (40.0) | 0.87 |
| HTN | 3 (37.5) | 5 (50.0) | 0.48 |
| HL | 1 (12.5) | 5 (50.0) | 0.12 |
| UCG findings | |||
| LVEF (%) | 47.4±22.0 | 36.5±9.7 | 0.25 |
| LVDd (mm) | 59.3±13.4 | 63.5±8.0 | 0.47 |
| TRPG (mmHg) | 9.9±13.1 | 35.9±20.7 | 0.51 |
| Laboratory data | |||
| Hb | 11.6±2.0 | 11.6±2.6 | 0.98 |
| Hct | 35.8±5.6 | 35.5±7.7 | 0.92 |
| s-Na | 140±3.1 | 138±4.2 | 0.20 |
| s-CRE | 1.32±0.31 | 1.49±0.60 | 0.48 |
| eGFR | 38.2±11.3 | 38.6±12.9 | 0.94 |
| Cystatin C | 1.88±0.28 | 2.01±0.85 | 0.65 |
| BNP | 626.6±508.0 | 478.0±504.4 | 0.57 |
| PRA | 4.24±5.49 | 8.07±7.61 | 0.28 |
| Aldosterone | 26.4±17.9 | 41.4±20.7 | 0.19 |
| u-β2-MG | 529.5±830.3 | 176.0±232.6 | 0.31 |
| u-NAG | 10.7±6.2 | 9.5±5.1 | 0.66 |
| Premedication of dose and duration of furosemide | |||
| Furosemide (mg) | 45±20.0 | 70±27.2 | 0.050 |
| Duration (days) | 612.9±956.1 | 874.6±1,005.3 | 0.60 |
| Drugs | |||
| ACEI | 2 (25.0) | 3 (30.0) | 0.62 |
| ARB | 5 (62.5) | 5 (50.0) | 0.48 |
| β-blocker | 3 (37.5) | 1 (10.0) | 0.21 |
| Insulin | 1 (12.5) | 2 (20.0) | 0.59 |
| Digitalis | 1 (12.5) | 4 (40.0) | 0.23 |
| MRA | 5 (62.5) | 8 (80.0) | 0.38 |
Data given as mean±SD or n (%). ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; Hct, hematocrit; HL, hyperlipidemia; HTN, hypertension; LVDd, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; OMI, old myocardial infarction; PRA, plasma renin activity; s-CRE, serum creatinine; s-Na, serum sodium; TRPG, tricuspid regurgitation pressure gradient; u-β2-MG, urine β2-microglobulin; UCG, ultrasound cardiography; u-NAG, urine N-acetyl-β-D-glucosaminidase; VHD, valvular heart disease.
Urine Volume, Amount of Drinking and BW
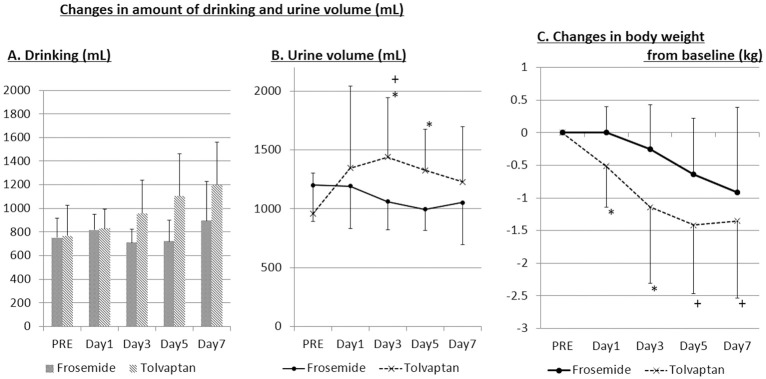
As compared with the baseline value on day 0 (PRE), the amount of drinking/day tended to increase in the tolvaptan group, while it did not change in the furosemide group during the 7 days (Figure 2A). As compared with the baseline value on day 0 (PRE), the urine volume/day was significantly increased on days 3 and 5 during the 7 days in the tolvaptan group (Figure 2B), while it was not significantly changed during the 7 days in the furosemide group, although there was a decreasing tendency (Figure 2A). BW decreased during the 7 days in the tolvaptan group, while it was not significantly changed during the 7 days in the furosemide group (Figure 2C). Decrease in BW, however, was significantly greater in the tolvaptan on days 1 and 3 compared with the furosemide group (Figure 2C).
Figure 2.
Change in (A) amount of drinking; (B) urine volume; and (C) body weight in patients with heart failure and chronic kidney disease. PRE, baseline value on day 0. *P<0.05 vs. frosemide; +P<0.05 vs. PRE.
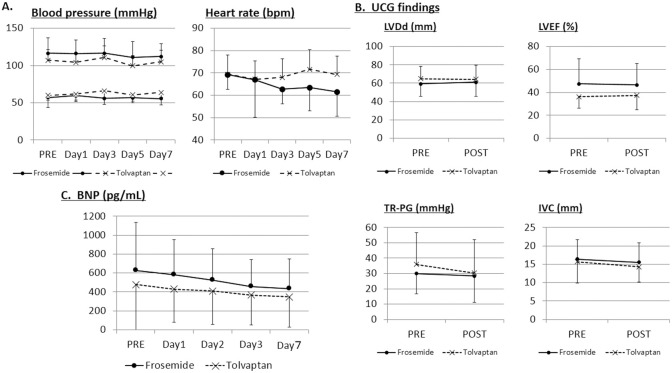
Hemodynamic Parameters, BNP, and Cardiac Function
There was no significant difference in changes in the pulse rate and systolic or diastolic blood pressure during the 7 days in the tolvaptan compared with the furosemide group (Figure 3A). Although IVC diameter tended to decrease with both tolvaptan and furosemide, LVEF, LVDd, and TRPG were not affected by either tolvaptan or furosemide (Figure 3B). BNP tended to decrease during the 7 days both in the tolvaptan and furosemide groups, but there was no significant difference in BNP change between the groups (Figure 3C).
Figure 3.
Change in (A) systolic, diastolic blood pressure, and pulse rate; (B) left ventricular end-diastolic dimension (LVDd), left ventricular ejection fraction (LVEF), tricuspid regurgitation pressure gradient (TRPG), and inferior vena cava (IVC) diameter; and (C) brain natriuretic peptide (BNP) in patients with heart failure and chronic kidney disease. PRE, baseline value on day 0; UCG, ultrasound cardiography.
Renal Function
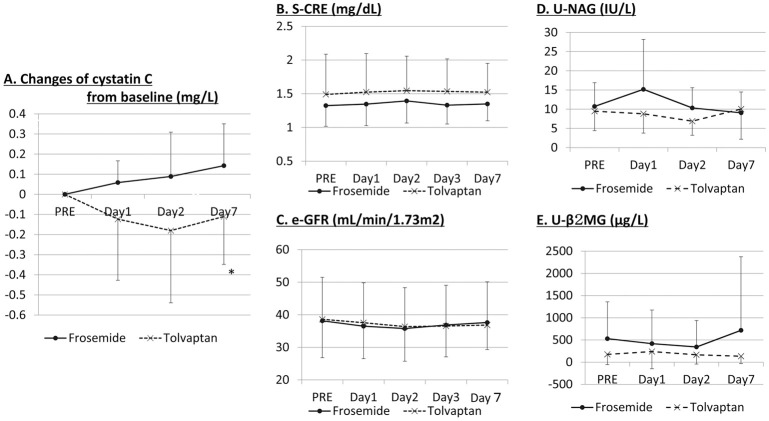
Serum creatinine and eGFR did not change significantly in either the tolvaptan or furosemide groups during the 7 days (Figure 4B,C). Plasma cystatin C, however, an early indicator of renal impairment, significantly decreased on day 7 in the tolvaptan group while it tended to increase in the furosemide group (Figure 4A). There was no significant change in urine NAG or urine β2-MG, a marker of renal tubule injury, in the tolvaptan or furosemide groups (Figure 4D,E). Urine β2-MG, however, tended to increase on day 7 in the furosemide group.
Figure 4.
Change in (A) cystatin C; (B) serum creatinine (S-CRE); (C) estimated glomerular filtration rate (eGFR); (D) urine N-acetyl glucosaminidase (U-NAG); and (E) urine microglobulin (U-β2-MG) in patients with heart failure and chronic kidney disease. PRE, baseline value on day 0. *P<0.05 vs. frosemide; +P<0.05 vs. PRE.
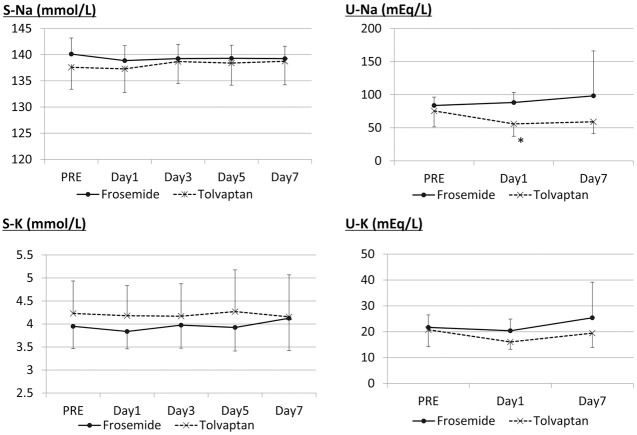
Plasma and Urine Electrolytes
There was no significant change in plasma sodium and potassium or urine potassium in either the tolvaptan or furosemide groups. Urine sodium, however, significantly increased on day 1 in the furosemide group (Figure 5).
Figure 5.
Change in plasma (P) and urine (U) sodium (Na) and potassium (K) in patients with heart failure and chronic kidney disease. PRE, baseline value on day 0. *P<0.05 vs. frosemide; +P<0.05 vs. PRE.
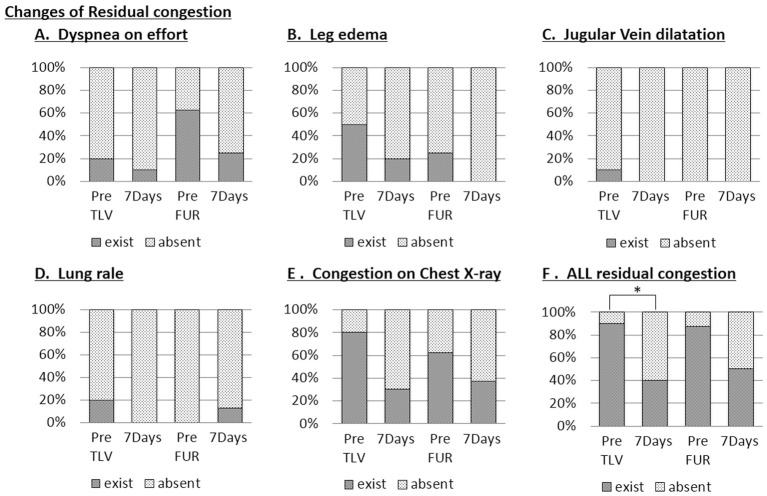
Residual Congestion
Figure 6 shows the parameters of residual congestion before and after the treatment for 7 days in the tolvaptan and furosemide groups. There was no significant difference in dyspnea on effort, leg edema, jugular vein dilatation, lung rales or congestion on chest X-ray (Figure 6A–E) after treatment in the tolvaptan or in the furosemide groups. When these parameters of residual congestion were assessed all together, however, the residual congestion was significantly improved in the tolvaptan group, but was not in the furosemide group (Figure 6F).
Figure 6.
Change in residual congestion parameters: (A) dyspnea on effort; (B) leg edema; (C) jugular vein dilatation; (D) lung rales; (E) congestion on chest X-ray; and (F) all residual congestion in patients with heart failure and chronic kidney disease. PRE, baseline value on day 0. FUR, frosemide; TLV, tolvaptan. *P<0.05.
Discussion
For many HF patients, loop diuretics such as furosemide and azosemide are usually prescribed. The problem that sometimes occurs in such cases, however, is residual congestion because of resistance to diuretics.18 Resistance to diuretics is sometimes caused by hypoperfusion of renal circulation due to low cardiac output.19 Because residual congestion is one of the risk factors for mortality in HF patients,7 it is important to resolve residual congestion as much as possible by increasing the urine volume. One possible candidate drug to resolve residual congestion in HF patients with resistance to furosemide is tolvaptan.15,16 We, therefore, compared the effects of tolvaptan with additional furosemide on the urine volume in HF patients with CKD who were already being treated with furosemide >20 mg/day. We chose day 7 for the endpoint assessment because this duration has been used in many clinical trials on the effect of tolvaptan.11–14,16
In the present study, compared with the addition of furosemide, tolvaptan significantly increased the urine volume during the 7 days, and significantly decreased BW (Figure 2), although tolvaptan tended to increase the amount of drinking, suggesting that the addition of tolvaptan is more effective than that of furosemide in increasing the urine volume, despite the tendency in the amount of drinking to increase in HF patients with CKD who were being treated with furosemide. An important finding in the present study is that the addition of tolvaptan or furosemide did not change the plasma sodium and potassium, suggesting that the additional use of tolvaptan or furosemide in HF patients under treatment with furosemide is safe in terms of the changes in plasma electrolytes. Generally speaking, diuretic use for the treatment of HF may worsen renal function. In the present study, however, there was no change in serum creatinine or eGFR during the 7 days either in the tolvaptan or furosemide group, suggesting that neither tolvaptan nor furosemide caused the renal function to deteriorate. When the renal function was assessed on plasma cystatin C level, which is considered to be a more reliable marker of GFR20 or an early indicator of renal impairment,21 cystatin C significantly decreased on day 7 in the tolvaptan group as compared with the furosemide group, suggesting that the additional tolvaptan improved the renal function as compared with the additional furosemide, and that tolvaptan may be superior to furosemide regarding the effect on GFR or renal impairment. Although there was a significant difference in cystatin C between the 2 groups, it is not clear whether this difference has a clinical meaning (Figure 4A).
There was no significant change in urine NAG or urine β2-MG, a marker of renal tubule injury, during the 7 days either in the tolvaptan or furosemide group, suggesting that additional tolvaptan or furosemide did not damage the renal tubules. Collectively, additional treatment with tolvaptan or furosemide did not cause the renal function to deteriorate during the 7 days.
On cardiac echocardiography there was no significant change in LVEF, LVDd, TRPG, or IVC diameter during the 7 days in either the tolvaptan or furosemide groups, suggesting that additional treatment with tolvaptan and furosemide for HF patients under diuretic treatment did not affect the cardiac function in such a short period of 7 days. Given that residual congestion at discharge is one of the risk factors for early readmission and mortality,7 it is particularly important to assess the effect of adding tolvaptan or furosemide on residual congestion. When parameters of residual congestion such as dyspnea on effort, leg edema, jugular vein dilatation, lung rales or congestion on chest X-ray were assessed separately, there was no significant difference after treatment in either the tolvaptan or in the furosemide groups (Figure 6A–E). When these parameters of residual congestion were assessed together, however, the residual congestion was significantly improved in the tolvaptan group, while it was not in the furosemide group (Figure 6F), suggesting that adding tolvaptan is more beneficial than increasing furosemide in HF patients with CKD under furosemide treatment.
Study Limitations
First, we used the urine volume to estimate the possible resolution of residual congestion in HF patients. It is not clear, however, whether tolvaptan fully resolved the residual congestion in the present study. Second, this was a single-center study and a relatively small sample size, thus possible selection bias could not be excluded. Therefore, a multi-center study with a greater number of patients is warranted.
Conclusions
Additional treatment with tolvaptan but not furosemide significantly increased the urine volume, decreased BW and improved the residual congestion without affecting renal function, electrolytes or cardiac function in patients with HF and CKD under furosemide treatment. Treatment with tolvaptan in addition to furosemide may resolve residual congestion and improve the prognosis of HF patients.
Disclosures
The authors declare no conflict of interest.
References
- 1. Roger VL.. Epidemiology of heart failure. Circ Res 2013; 113: 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Florea VG, Cohn JN.. The autonomic nervous system and heart failure. Circ Res 2014; 114: 1815–1826. [DOI] [PubMed] [Google Scholar]
- 3. Mentz RJ, Stevens SR, DeVore AD, Lala A, Vader JM, AbouEzzeddine OF, et al.. Decongestion strategies and renin-angiotensin aldosterone system activation in acute heart failure. JACC Heart Fail 2015; 3: 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eiskjaer H, Bagger JP, Danielsen H, Jensen JD, Jespersen B, Thomsen K, et al.. Mechanisms of sodium retention in heart failure: Relation to the renin-angiotensin-aldosterone system. Am J Physiol 1991; 260: F883–F889. [DOI] [PubMed] [Google Scholar]
- 5. Davies MK, Gibbs CR, Lip GYH.. Management: Diuretics, ACE inhibitors, and nitrates. BMJ 2000; 320: 428–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ellison DH.. Diuretic therapy and resistance in congestive heart failure. Cardiology 2001; 96: 132–143. [DOI] [PubMed] [Google Scholar]
- 7. Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, et al.. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur Heart J 2013; 34: 835–843. [DOI] [PubMed] [Google Scholar]
- 8. Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR, et al.. Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail 2014; 7: 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh D, Shrestha K, Testani JM, Verbrugge FH, Dupont M, Mullens W, et al.. Insufficient natriuretic response to continuous intravenous furosemide is associated with poor long-term outcomes in acute decompensated heart failure. J Card Fail 2014; 20: 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamamura Y, Nakamura S, Itoh S, Hirano T, Onogawa T, Yamashita T, et al.. OPC-41061, a highly potent human vasopressin V2-receptor antagonist: Pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther 1998; 287: 860–867. [PubMed] [Google Scholar]
- 11. Gheorghiade M, Konstam MA, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, et al.. Short term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The EVEREST Clinical Status Trials. JAMA 2007; 297: 1332–1343. [DOI] [PubMed] [Google Scholar]
- 12. Matsue Y, Suzuki M, Torii S, Yamaguchi S, Fukamizu S, Ono Y, et al.. Clinical effectiveness of tolvaptan in patients with acute heart failure and renal dysfunction. J Card Fail 2016; 22: 423–432. [DOI] [PubMed] [Google Scholar]
- 13. Jujo K, Saito K, Ishida I, Furuki Y, Kim A, Suzuki Y, et al.. Randomized pilot trial comparing tolvaptan with furosemide on renal and neurohumoral effects in acute heart failure. ESC Heart Fail 2016; 3: 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kimura K, Momose T, Hasegawa T, Morita T, Misawa T, Motoki H, et al.. Early administration of tolvaptan preserves renal function in elderly patients with acute decompensated heart failure. J Cardiol 2016; 67: 399–405. [DOI] [PubMed] [Google Scholar]
- 15. Imamura T, Kinugawa K, Ohtani T, Sakata Y, Higo T, Kinugawa S, et al.. Assessment of quality of life during long-term treatment of tolvaptan in refractory heart failure: Design and rationale of the AQUA-TLV Study. Int Heart J 2014; 55: 264–267. [DOI] [PubMed] [Google Scholar]
- 16. Inomata T, Ikeda Y, Kida K, Shibagaki Y, Sato N, Kumagai Y, et al.. Effects of additive tolvaptan vs. increased furosemide on heart failure with diuretic resistance and renal impairment: Results from the K-STAR Study. Circ J 2017; 82: 159–167. [DOI] [PubMed] [Google Scholar]
- 17. Rickham PP.. Human experimentation: Code of ethics of the world medical association. Declaration of Helsinki. Br Med J 1964; 2: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verbrugge FH, Dupont M, Steels P, Grieten L, Swennen Q, Tang WH, et al.. The kidney in congestive heart failure: Are natriuresis, sodium, and diuretics really the good, the bad and the ugly? Eur J Heart Fail 2014; 16: 133–142. [DOI] [PubMed] [Google Scholar]
- 19. Hoorn EJ, Wilcox CS, Ellison DH. Diuretics. In: Skorecki K, Chertow G, Marsden P, Taal M, Yu A, editors. Brenner and Rector’s the kidney, Vol. 2, 10th edn. Philadelphia: Elsevier, 2015; 1702–1734. [Google Scholar]
- 20. Kar S, Paglialunga S, Islam R.. Cystatin C is a more reliable biomarker for determining eGFR to support drug development studies. J Clin Pharmacol 2018; 58: 1239–1247. [DOI] [PubMed] [Google Scholar]
- 21. McNamara NV, Chen R, Janu MR, Bwititi P, Car G, Seibel M.. Early renal failure detection by cystatin C in type 2 diabetes mellitus: Varying patterns of renal analyte expression. Pathology 2009; 41: 269–275. [DOI] [PubMed] [Google Scholar]