Abstract
INTRODUCTION:
Primary sclerosing cholangitis (PSC) is a progressive liver disease characterized by bile duct inflammation and fibrosis. The role of macrophages in PSC development and progression is less studied. Macrophage activation markers soluble (s)CD163 and mannose receptor (sMR) are associated with disease severity and outcome in other liver diseases, but not previously investigated in PSC. We evaluated sCD163 and sMR regarding disease severity and prognosis in patients with PSC.
METHODS:
We investigated 2 independent PSC cohorts from Oslo (n = 138) and Helsinki (n = 159) and analyzed blood sCD163 and sMR levels. The Mayo score, Enhanced Liver Fibrosis Test, and Amsterdam-Oxford model were assessed for comparison.
RESULTS:
Median (interquartile range) sCD163 was 3.32 (2.27–5.60) and 1.96 (1.47–2.70) mg/L in the Oslo and Helsinki cohorts, respectively, reflecting differences in disease severity between cohorts. Median sMR was similar in both cohorts, 0.28 (0.22–0.44) and 0.28 mg/L (0.20–0.36), respectively. In both cohorts, sCD163 and sMR levels raised with increasing disease severity (liver enzymes, Mayo score, and enhanced liver fibrosis test). Patients with high baseline levels of sCD163 had shorter transplant-free survival than patients with low baseline levels. Furthermore, sCD163 was associated with transplant-free survival in univariate cox-regression analyses. Both sCD163 and sMR performed better in the Oslo cohort of more severely diseased patients than those in the Helsinki cohort of more mildly diseased patients.
DISCUSSION:
Macrophage activation markers are elevated according to disease severity suggesting an important role of macrophages in PSC. Furthermore, sCD163 was identified as a prognostic marker and predictor of transplant-free survival in PSC (see Visual Abstract, Supplementary Digital Content 4, http://links.lww.com/CTG/A516).
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a progressive autoimmune liver disease with chronic inflammation and strictures of intra- and extra-hepatic bile ducts, ultimately leading to fibrosis and cirrhosis of the liver (1,2). There is no approved pharmacological treatment, and the only curative option is liver transplantation (LT) (3). A better understanding of disease pathology and progression and better biomarkers are unmet needs to improve use of surrogate endpoints in the design of clinical trials for assessment of disease severity, progression, and treatment response (4) because these trials suffer from difficulties with low event rates and hence a low ability to prove effect of a specific intervention (5).
Macrophages are generally activated in inflammatory liver diseases and may also play a role in PSC development and progression. Previous studies showed that the numbers of perisinusoidal macrophages, investigated in liver biopsies, were higher in patients with PSC than in patients with primary biliary cholangitis (PBC) and healthy controls (6,7). Furthermore, a recent study showed elevated frequencies of macrophages in the livers of patients with PSC compared with other liver diseases, concluding that macrophages may have a potential role in PSC pathophysiology (8). Recent data also suggest that soluble CD14, often regarded as a monocyte activation marker, is elevated in patients with PSC and predicts liver transplantation-free survival (9). However, others found similar levels of granulocyte-macrophage colony-stimulating factor in patients with PSC and age- and sex-matched controls (10).
The macrophage activation markers soluble (s)CD163 (11,12) and mannose receptor (sMR) (13) are associated with disease severity in multiple liver diseases including autoimmune hepatitis (AIH) and primary biliary cholangitis (14–24). Furthermore, sCD163 and sMR are associated with prognosis in patients with liver cirrhosis, acute alcoholic hepatitis, and acute-on chronic liver failure (ACLF) (17,18,25–27). However, macrophage activation markers have not yet been investigated in patients with PSC. We aimed to investigate the macrophage activation markers sCD163 and sMR in 2 independent cohorts of patients with PSC and hypothesized that high levels of sCD163 and sMR would be associated with more severe liver disease and worse prognosis.
MATERIALS AND METHODS
Patient population and data collection
The protocol was in accordance with the Declaration of Helsinki and approved by the regional committee for research ethics in South Eastern Norway (reference number 2011/2,572) and Helsinki. The patient panels included 138 patients with large-duct PSC from the Norwegian NoPSC biobank, collected during 2008–2012, and 159 patients with large-duct PSC from Helsinki, collected during 2006–2015. Patients were retrospectively included from 2 research registries (Oslo and Helsinki) into which patients were enrolled on referral to the 2 respective centers. Referrals in Oslo were mainly for complex or advanced cases, whereas in Finland, all patients were referred on diagnosis.
PSC was diagnosed based on typical cholangiographic findings by either magnetic resonance cholangiopancreatography (MRCP) or endoscopic retrograde cholangiopancreatography (ERCP) according to acknowledged standard criteria (28,29). The first pathological cholangiography defined the date of diagnosis of PSC. PSC duration was defined as the time from the date of diagnosis to the date of inclusion. Data on sCD163 and sMR and platelets, creatinine, total bilirubin, albumin, international normalized ratio (INR), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and gamma-glutaryltransferase (GGT) stem from blood samples taken on the date of inclusion. Data on UDCA use stems from the date of inclusion. Cases of PSC with features of AIH, fulfilling diagnostic criteria of AIH and PSC (30), were included. Cases of secondary cholangitis or small duct PSC were excluded.
Mayo risk scores were calculated (31). Serum samples were also analyzed by the commercially available enhanced liver fibrosis (ELF) test (Siemens Healthineers, Tarrytown, NY) using an ADVIA Centaur XP analyzer (Siemens Healthineers, Tarrytown, NY). The prognostic Amsterdam-Oxford Model (AOM) for PSC was calculated (32). Plasma concentrations of sCD163 and sMR were measured in duplicate by an in-house sandwich enzyme-linked immunosorbent assay, as previously described in samples frozen at −80°C (20,33). Reference intervals for sCD163 is 0.69–3.86 mg/L11 and for sMR 0.10–0.43 mg/L.20 Soluble CD163 and sMR are resistant to repeated freezing and thawing (20,33).
Patient records and the research database were queried for information on clinical and laboratory data. Unfortunately, all data were not available in the Helsinki cohort because of the retrospective design of this study. Inflammatory bowel disease (IBD) diagnosis was based on findings at colonoscopy and histology. Updated information on liver transplantation dates and indications by December 31, 2016, were retrieved from the Norwegian/Nordic Liver Transplant Registry, and data on all-cause death by the same date were retrieved from the Norwegian Death Registry. The Helsinki cohort was updated with events through August 2018.
Statistical analysis
Statistical analyses were performed using SPSS version 24.0 software (SPSS, Chicago, IL) and Stata version 13 (StataCorp, Texas). Continuous variables were tested for normal distribution, and for comparison between groups, the Student t test, the Mann-Whitney U test, or the Kruskal-Wallis test was applied, as appropriate. Biochemical values showing a skewed distribution were transformed using natural logarithmic transformation. A composite endpoint composed by all-cause death and LT was defined. All patients who died in the Oslo cohort, died from PSC-related cancer. Three groups of disease severity (low, intermediate, and high) were defined by Mayo risk score ≤0, >0 to <2, and ≥2. Three groups of fibrosis severity (mild, moderate, and severe) were defined according to the ELF test level <7.7, ≥7.7 to <9.8, and ≥9.8, respectively, as recommended by the manufacturer (34). Follow-up started at the date of inclusion and ended at the date of reaching the composite endpoint, or, in case, no endpoint was reached, date of the last follow-up. To evaluate the prognosis, the patients were stratified in 2 groups according to established upper limit of normal of sCD163 (3.86 mg/L) and sMR (0.43 mg/L), respectively, and 8-year transplant-free survival were estimated while using the logrank test to compare crude risk. Correlations between sCD163 and sMR, respectively, and other laboratory variables were assessed by the Spearman rank correlation test. We used Cox regression to assess the potential association of sCD163 and sMR with the occurrence of the endpoint while adjusting for confounding by ELF test and AOM. We did not include the Mayo score in the regression analysis because of the large overlap of variables included in the Mayo score and AOM. We performed an additional Cox regression analysis including a ‘recruitment center’ variable after combining the 2 cohorts while adjusting for the ELF test and AOM. We used smoothing splines to determine the functional form of sCD163 and sMR and the logarithmic values of both variables and included the logarithmic values in our Cox regression analysis because this gave a more linear hazard ratio over the range of measured values (see Supplemental Figure 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A513). The ability of sCD163 and sMR to discriminate between patients who experienced an outcome and those who did not were determined by computing the area under the receiver operating characteristic curve. The optimal threshold to distinguish patients reaching an endpoint from those who did not was calculated by the Youden index.
RESULTS
Patients
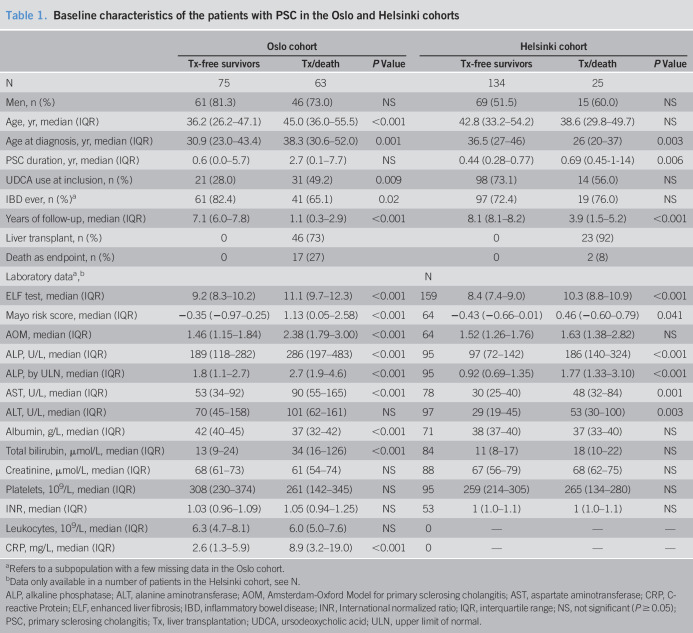
Patient characteristics from both cohorts are described in Table 1. We included 138 patients with PSC from a well-characterized Norwegian panel with a median Mayo score of 0.12 (median age 40 years, 78% men). The median follow-up time was 5.4 years (range, 0.0–8.3), during which 17 patients died and 46 underwent LT. Eleven patients had features of AIH.
Table 1.
Baseline characteristics of the patients with PSC in the Oslo and Helsinki cohorts
| Oslo cohort | Helsinki cohort | ||||||
| Tx-free survivors | Tx/death | P Value | Tx-free survivors | Tx/death | P Value | ||
| N | 75 | 63 | 134 | 25 | |||
| Men, n (%) | 61 (81.3) | 46 (73.0) | NS | 69 (51.5) | 15 (60.0) | NS | |
| Age, yr, median (IQR) | 36.2 (26.2–47.1) | 45.0 (36.0–55.5) | <0.001 | 42.8 (33.2–54.2) | 38.6 (29.8–49.7) | NS | |
| Age at diagnosis, yr, median (IQR) | 30.9 (23.0–43.4) | 38.3 (30.6–52.0) | 0.001 | 36.5 (27–46) | 26 (20–37) | 0.003 | |
| PSC duration, yr, median (IQR) | 0.6 (0.0–5.7) | 2.7 (0.1–7.7) | NS | 0.44 (0.28–0.77) | 0.69 (0.45-1-14) | 0.006 | |
| UDCA use at inclusion, n (%) | 21 (28.0) | 31 (49.2) | 0.009 | 98 (73.1) | 14 (56.0) | NS | |
| IBD ever, n (%)a | 61 (82.4) | 41 (65.1) | 0.02 | 97 (72.4) | 19 (76.0) | NS | |
| Years of follow-up, median (IQR) | 7.1 (6.0–7.8) | 1.1 (0.3–2.9) | <0.001 | 8.1 (8.1–8.2) | 3.9 (1.5–5.2) | <0.001 | |
| Liver transplant, n (%) | 0 | 46 (73) | 0 | 23 (92) | |||
| Death as endpoint, n (%) | 0 | 17 (27) | 0 | 2 (8) | |||
| Laboratory dataa,b | N | ||||||
| ELF test, median (IQR) | 9.2 (8.3–10.2) | 11.1 (9.7–12.3) | <0.001 | 159 | 8.4 (7.4–9.0) | 10.3 (8.8–10.9) | <0.001 |
| Mayo risk score, median (IQR) | −0.35 (−0.97–0.25) | 1.13 (0.05–2.58) | <0.001 | 64 | −0.43 (−0.66–0.01) | 0.46 (−0.60–0.79) | 0.041 |
| AOM, median (IQR) | 1.46 (1.15–1.84) | 2.38 (1.79–3.00) | <0.001 | 64 | 1.52 (1.26–1.76) | 1.63 (1.38–2.82) | NS |
| ALP, U/L, median (IQR) | 189 (118–282) | 286 (197–483) | <0.001 | 95 | 97 (72–142) | 186 (140–324) | <0.001 |
| ALP, by ULN, median (IQR) | 1.8 (1.1–2.7) | 2.7 (1.9–4.6) | <0.001 | 95 | 0.92 (0.69–1.35) | 1.77 (1.33–3.10) | <0.001 |
| AST, U/L, median (IQR) | 53 (34–92) | 90 (55–165) | <0.001 | 78 | 30 (25–40) | 48 (32–84) | 0.001 |
| ALT, U/L, median (IQR) | 70 (45–158) | 101 (62–161) | NS | 97 | 29 (19–45) | 53 (30–100) | 0.003 |
| Albumin, g/L, median (IQR) | 42 (40–45) | 37 (32–42) | <0.001 | 71 | 38 (37–40) | 37 (33–40) | NS |
| Total bilirubin, µmol/L, median (IQR) | 13 (9–24) | 34 (16–126) | <0.001 | 84 | 11 (8–17) | 18 (10–22) | NS |
| Creatinine, µmol/L, median (IQR) | 68 (61–73) | 61 (54–74) | NS | 88 | 67 (56–79) | 68 (62–75) | NS |
| Platelets, 109/L, median (IQR) | 308 (230–374) | 261 (142–345) | NS | 95 | 259 (214–305) | 265 (134–280) | NS |
| INR, median (IQR) | 1.03 (0.96–1.09) | 1.05 (0.94–1.25) | NS | 53 | 1 (1.0–1.1) | 1 (1.0–1.1) | NS |
| Leukocytes, 109/L, median (IQR) | 6.3 (4.7–8.1) | 6.0 (5.0–7.6) | NS | 0 | — | — | — |
| CRP, mg/L, median (IQR) | 2.6 (1.3–5.9) | 8.9 (3.2–19.0) | <0.001 | 0 | — | — | — |
Refers to a subpopulation with a few missing data in the Oslo cohort.
Data only available in a number of patients in the Helsinki cohort, see N.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AOM, Amsterdam-Oxford Model for primary sclerosing cholangitis; AST, aspartate aminotransferase; CRP, C-reactive Protein; ELF, enhanced liver fibrosis; IBD, inflammatory bowel disease; INR, International normalized ratio; IQR, interquartile range; NS, not significant (P ≥ 0.05); PSC, primary sclerosing cholangitis; Tx, liver transplantation; UDCA, ursodeoxycholic acid; ULN, upper limit of normal.
In the Helsinki cohort including 159 patients with PSC, the median Mayo score was −0.37, median age was 42.2 years, and 53% were men. During a median follow-up time of 8.1 years (range, 0.1–8.3), 2 patients died and 23 were liver transplanted. Twelve patients had features of AIH.
Patients in the Oslo cohort had a longer duration of PSC before inclusion in the study than patients in the Helsinki cohort, were more often men, less often used ursodeoxycholic acid (UDCA), and had more severe liver disease at inclusion as defined by higher ELF test, Mayo score, AOM, ALP, and bilirubin levels (see Supplemental Table 1, Supplementary Digital Content 2, http://links.lww.com/CTG/A514).
sCD163 and sMR levels in patients with PSC
In the Oslo cohort, median levels (interquartile range) of sCD163 and sMR were 3.32 mg/L (2.27–5.60) and 0.28 mg/L (0.22–0.44), respectively. In the Helsinki cohort, median sCD163 was 1.96 mg/L (1.47–2.70) and median sMR was 0.28 mg/L (0.20–0.36).
For correlations between sCD163 and sMR with liver parameters, we combined the Oslo and Helsinki cohorts and sCD163 and sMR correlated with ALP, bilirubin, ALT, and albumin (see Supplemental Table 2, Supplementary Digital Content 3, http://links.lww.com/CTG/A515). Data for the individual cohorts are shown in supplemental Table 2 (see Supplementary Digital Content 3, http://links.lww.com/CTG/A515).
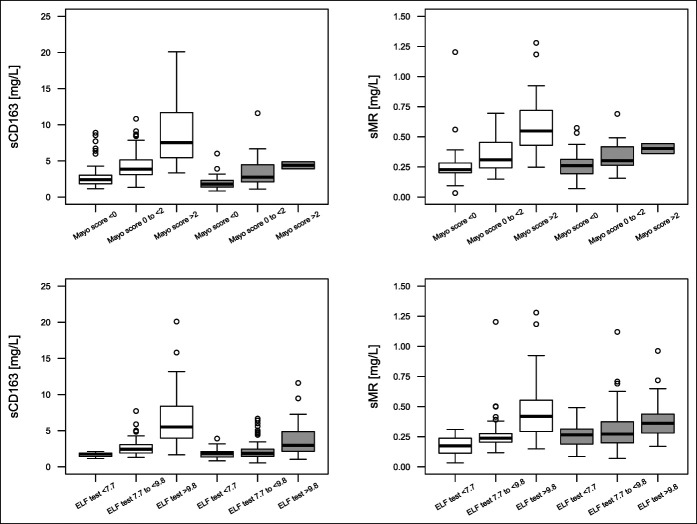
Furthermore, sCD163 and sMR increased with increasing Mayo score, ELF test, and AOM in the combined cohort (Figure 1, Supplemental Table 2 [see Supplementary Digital Content 3, http://links.lww.com/CTG/A515]). Data for the individual cohorts are shown in supplemental Table 2 (see Supplementary Digital Content 3, http://links.lww.com/CTG/A515).
Figure 1.
sCD163 and sMR distribution in 3 prespecified risk groups of Mayo score and ELF test. White boxes to the left are from the Oslo cohort, gray boxes to the right are from the Helsinki cohort. Mayo score is available in 64 patients in the Helsinki cohort, of whom only 2 had a Mayo score >2. ELF, enhanced liver fibrosis; sCD163, soluble CD163; sMR, soluble mannose receptor.
There was no difference in median sCD163 levels between patients with or without a diagnosis of IBD (data not shown). Furthermore, there were no differences in sCD163 levels between male and female patients with PSC or in patients with or without features of AIH. Similar results were found for sMR.
Association between sCD163 and sMR and long-term prognosis in patients with PSC
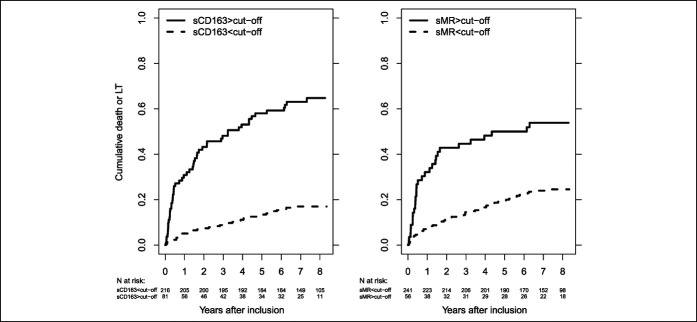
In the combined cohort, patients with high sCD163 had lower 8-year transplant-free survival (35.2%, 95% CI [24.9%–45.7%]) than those with low sCD163 (83.0%, 95% CI [77.2%–87.5%]). Similarly, those with high sMR had lower eight-year transplant-free survival (46.2%, 95% CI [32.7%–58.6%]) than those with low sMR (75.5%, 95% CI [69.4%–80.5%]) (Figure 2). We found similar results in the Oslo cohort alone, but in the Helsinki cohort, there was no difference in eight-year transplant-free survival between patients with low and high sMR. The transplant-free survival was in general lower in the Oslo cohort than in the Helsinki cohort (see Supplemental Figure 2, Supplementary Digital Content 1, http://links.lww.com/CTG/A513).
Figure 2.
Cumulative death or LT in patients with low or high sCD163 (left) or sMR (right) in the combined cohort. Cutoffs for sCD163 (3.86 mg/L) and sMR (0.43 mg/L), respectively. The logrank test for equal survivor functions: sCD163, P < 0.001; sMR, P < 0.001. LT, liver transplantation; sCD163, soluble CD163; sMR, soluble mannose receptor.
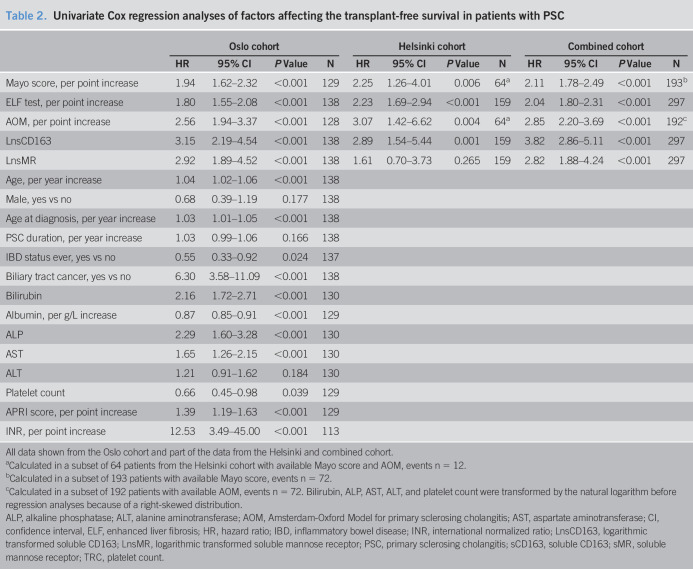
Results from the univariate Cox regressions are listed in Table 2. sCD163 and sMR showed association with transplant-free survival in the combined cohort, and so did the Mayo risk score, ELF test, and AOM (Table 2). We found similar results in the individual cohorts, except for sMR not being associated with transplant-free survival in the Helsinki cohort (Table 2).
Table 2.
Univariate Cox regression analyses of factors affecting the transplant-free survival in patients with PSC
| Oslo cohort | Helsinki cohort | Combined cohort | ||||||||||
| HR | 95% CI | P Value | N | HR | 95% CI | P Value | N | HR | 95% CI | P Value | N | |
| Mayo score, per point increase | 1.94 | 1.62–2.32 | <0.001 | 129 | 2.25 | 1.26–4.01 | 0.006 | 64a | 2.11 | 1.78–2.49 | <0.001 | 193b |
| ELF test, per point increase | 1.80 | 1.55–2.08 | <0.001 | 138 | 2.23 | 1.69–2.94 | <0.001 | 159 | 2.04 | 1.80–2.31 | <0.001 | 297 |
| AOM, per point increase | 2.56 | 1.94–3.37 | <0.001 | 128 | 3.07 | 1.42–6.62 | 0.004 | 64a | 2.85 | 2.20–3.69 | <0.001 | 192c |
| LnsCD163 | 3.15 | 2.19–4.54 | <0.001 | 138 | 2.89 | 1.54–5.44 | 0.001 | 159 | 3.82 | 2.86–5.11 | <0.001 | 297 |
| LnsMR | 2.92 | 1.89–4.52 | <0.001 | 138 | 1.61 | 0.70–3.73 | 0.265 | 159 | 2.82 | 1.88–4.24 | <0.001 | 297 |
| Age, per year increase | 1.04 | 1.02–1.06 | <0.001 | 138 | ||||||||
| Male, yes vs no | 0.68 | 0.39–1.19 | 0.177 | 138 | ||||||||
| Age at diagnosis, per year increase | 1.03 | 1.01–1.05 | <0.001 | 138 | ||||||||
| PSC duration, per year increase | 1.03 | 0.99–1.06 | 0.166 | 138 | ||||||||
| IBD status ever, yes vs no | 0.55 | 0.33–0.92 | 0.024 | 137 | ||||||||
| Biliary tract cancer, yes vs no | 6.30 | 3.58–11.09 | <0.001 | 138 | ||||||||
| Bilirubin | 2.16 | 1.72–2.71 | <0.001 | 130 | ||||||||
| Albumin, per g/L increase | 0.87 | 0.85–0.91 | <0.001 | 129 | ||||||||
| ALP | 2.29 | 1.60–3.28 | <0.001 | 130 | ||||||||
| AST | 1.65 | 1.26–2.15 | <0.001 | 130 | ||||||||
| ALT | 1.21 | 0.91–1.62 | 0.184 | 130 | ||||||||
| Platelet count | 0.66 | 0.45–0.98 | 0.039 | 129 | ||||||||
| APRI score, per point increase | 1.39 | 1.19–1.63 | <0.001 | 129 | ||||||||
| INR, per point increase | 12.53 | 3.49–45.00 | <0.001 | 113 | ||||||||
All data shown from the Oslo cohort and part of the data from the Helsinki and combined cohort.
Calculated in a subset of 64 patients from the Helsinki cohort with available Mayo score and AOM, events n = 12.
Calculated in a subset of 193 patients with available Mayo score, events n = 72.
Calculated in a subset of 192 patients with available AOM, events n = 72. Bilirubin, ALP, AST, ALT, and platelet count were transformed by the natural logarithm before regression analyses because of a right-skewed distribution.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AOM, Amsterdam-Oxford Model for primary sclerosing cholangitis; AST, aspartate aminotransferase; CI, confidence interval, ELF, enhanced liver fibrosis; HR, hazard ratio; IBD, inflammatory bowel disease; INR, international normalized ratio; LnsCD163, logarithmic transformed soluble CD163; LnsMR, logarithmic transformed soluble mannose receptor; PSC, primary sclerosing cholangitis; sCD163, soluble CD163; sMR, soluble mannose receptor; TRC, platelet count.
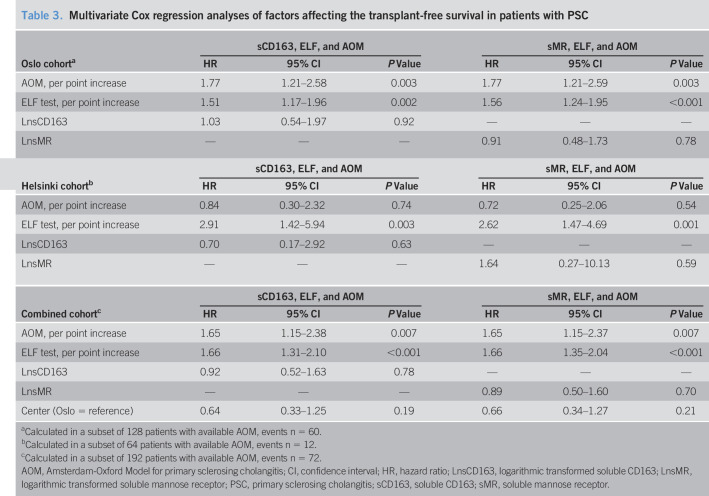
In multivariate analyses including the novel markers, ELF test and AOM, neither sCD163 nor sMR were associated with the outcome in the combined or any of the individual cohorts (Table 3). Recruitment center was not associated with outcome in the combined cohort (Table 3).
Table 3.
Multivariate Cox regression analyses of factors affecting the transplant-free survival in patients with PSC
| Oslo cohorta | sCD163, ELF, and AOM | sMR, ELF, and AOM | ||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| AOM, per point increase | 1.77 | 1.21–2.58 | 0.003 | 1.77 | 1.21–2.59 | 0.003 |
| ELF test, per point increase | 1.51 | 1.17–1.96 | 0.002 | 1.56 | 1.24–1.95 | <0.001 |
| LnsCD163 | 1.03 | 0.54–1.97 | 0.92 | — | — | — |
| LnsMR | — | — | — | 0.91 | 0.48–1.73 | 0.78 |
| Helsinki cohortb | sCD163, ELF, and AOM | sMR, ELF, and AOM | ||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| AOM, per point increase | 0.84 | 0.30–2.32 | 0.74 | 0.72 | 0.25–2.06 | 0.54 |
| ELF test, per point increase | 2.91 | 1.42–5.94 | 0.003 | 2.62 | 1.47–4.69 | 0.001 |
| LnsCD163 | 0.70 | 0.17–2.92 | 0.63 | — | — | — |
| LnsMR | — | — | — | 1.64 | 0.27–10.13 | 0.59 |
| Combined cohortc | sCD163, ELF, and AOM | sMR, ELF, and AOM | ||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| AOM, per point increase | 1.65 | 1.15–2.38 | 0.007 | 1.65 | 1.15–2.37 | 0.007 |
| ELF test, per point increase | 1.66 | 1.31–2.10 | <0.001 | 1.66 | 1.35–2.04 | <0.001 |
| LnsCD163 | 0.92 | 0.52–1.63 | 0.78 | — | — | — |
| LnsMR | — | — | — | 0.89 | 0.50–1.60 | 0.70 |
| Center (Oslo = reference) | 0.64 | 0.33–1.25 | 0.19 | 0.66 | 0.34–1.27 | 0.21 |
Calculated in a subset of 128 patients with available AOM, events n = 60.
Calculated in a subset of 64 patients with available AOM, events n = 12.
Calculated in a subset of 192 patients with available AOM, events n = 72.
AOM, Amsterdam-Oxford Model for primary sclerosing cholangitis; CI, confidence interval; HR, hazard ratio; LnsCD163, logarithmic transformed soluble CD163; LnsMR, logarithmic transformed soluble mannose receptor; PSC, primary sclerosing cholangitis; sCD163, soluble CD163; sMR, soluble mannose receptor.
Prediction of transplant-free survival by sCD163 and sMR in patients with PSC
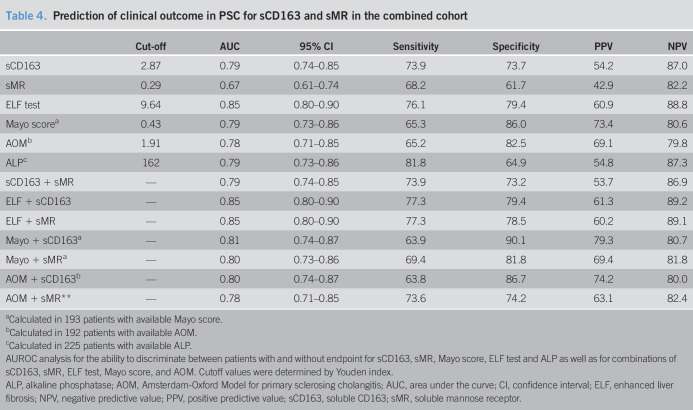
AUROC analysis showed good discrimination for sCD163 between patients with PSC with and without endpoints (death or LT) after 8-year follow-up in the combined cohort, with an area under the curve (AUC) of 0.79 (95% CI; 0.74–0.85), whereas sMR had an AUC of 0.67 (95% CI; 0.61–0.74) (Figure 3, Table 4). The AUC did not increase when combining sCD163 and sMR with either Mayo score, ELF test, or AOM (Table 4).
Figure 3.
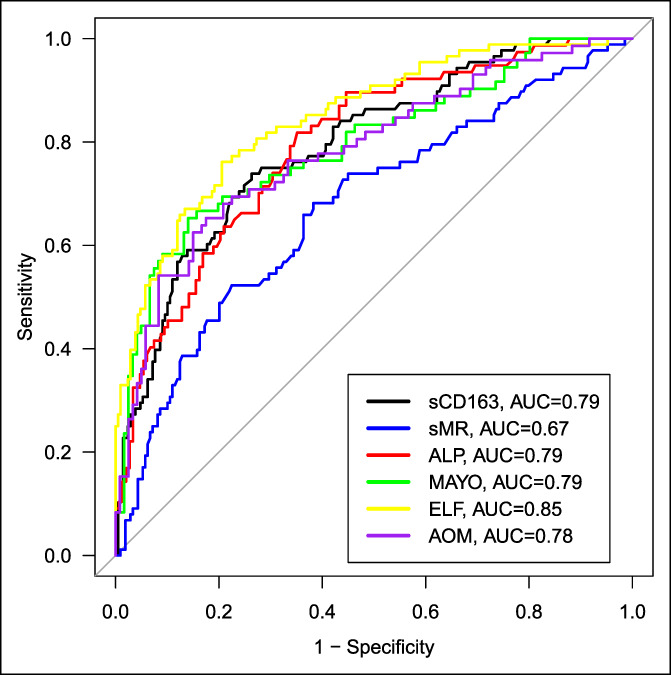
AUC's for sCD163, sMR, ALP, ELF test, Mayo score, and AOM in patients with PSC in discriminating patients experiencing the composite endpoint (LT or death) or not. ALP, alkaline phosphatase; AOM, Amsterdam-Oxford model for primary sclerosing cholangitis; AUC, area under the receiver operating curve; ELF, enhanced liver fibrosis; PSC, primary sclerosing cholangitis; sCD163, soluble CD163; sMR, soluble mannose receptor.
Table 4.
Prediction of clinical outcome in PSC for sCD163 and sMR in the combined cohort
| Cut-off | AUC | 95% CI | Sensitivity | Specificity | PPV | NPV | |
| sCD163 | 2.87 | 0.79 | 0.74–0.85 | 73.9 | 73.7 | 54.2 | 87.0 |
| sMR | 0.29 | 0.67 | 0.61–0.74 | 68.2 | 61.7 | 42.9 | 82.2 |
| ELF test | 9.64 | 0.85 | 0.80–0.90 | 76.1 | 79.4 | 60.9 | 88.8 |
| Mayo scorea | 0.43 | 0.79 | 0.73–0.86 | 65.3 | 86.0 | 73.4 | 80.6 |
| AOMb | 1.91 | 0.78 | 0.71–0.85 | 65.2 | 82.5 | 69.1 | 79.8 |
| ALPc | 162 | 0.79 | 0.73–0.86 | 81.8 | 64.9 | 54.8 | 87.3 |
| sCD163 + sMR | — | 0.79 | 0.74–0.85 | 73.9 | 73.2 | 53.7 | 86.9 |
| ELF + sCD163 | — | 0.85 | 0.80–0.90 | 77.3 | 79.4 | 61.3 | 89.2 |
| ELF + sMR | — | 0.85 | 0.80–0.90 | 77.3 | 78.5 | 60.2 | 89.1 |
| Mayo + sCD163a | — | 0.81 | 0.74–0.87 | 63.9 | 90.1 | 79.3 | 80.7 |
| Mayo + sMRa | — | 0.80 | 0.73–0.86 | 69.4 | 81.8 | 69.4 | 81.8 |
| AOM + sCD163b | — | 0.80 | 0.74–0.87 | 63.8 | 86.7 | 74.2 | 80.0 |
| AOM + sMR** | — | 0.78 | 0.71–0.85 | 73.6 | 74.2 | 63.1 | 82.4 |
Calculated in 193 patients with available Mayo score.
Calculated in 192 patients with available AOM.
Calculated in 225 patients with available ALP.
AUROC analysis for the ability to discriminate between patients with and without endpoint for sCD163, sMR, Mayo score, ELF test and ALP as well as for combinations of sCD163, sMR, ELF test, Mayo score, and AOM. Cutoff values were determined by Youden index.
ALP, alkaline phosphatase; AOM, Amsterdam-Oxford Model for primary sclerosing cholangitis; AUC, area under the curve; CI, confidence interval; ELF, enhanced liver fibrosis; NPV, negative predictive value; PPV, positive predictive value; sCD163, soluble CD163; sMR, soluble mannose receptor.
Both sCD163 and sMR performed better in the Oslo cohort with AUCs of 0.80 and 0.73, respectively, than in the Helsinki cohort with AUCs of 0.70 and 0.60, respectively. The ELF test showed equally good discriminatory abilities in both cohorts with an AUC of 0.82. The Mayo score and AOM also performed better in the Oslo cohort (AUC ≥ 0.80) than in the Helsinki cohort (AUC < 0.70).
DISCUSSION
Here, we present, for the first time, analyses of the macrophage activation markers sCD163 and sMR in 2 independent cohorts of patients with PSC. The main findings were the increased degree of macrophage activation with increasing liver disease severity (liver parameters, Mayo score, ELF test, and AOM). Furthermore, the markers were associated with prognosis, and for sCD163, the discriminative performance was similar to that of the Mayo score, ELF test, and AOM in the Oslo cohort of more advanced disease.
The macrophage activation markers, sCD163 and sMR, have not previously been investigated in patients with PSC, but we contemplate that most of the measured sCD163 and sMR stem from infiltrating macrophages in the liver because the median level of sCD163 and sMR are similar to those previously presented in other chronic inflammatory liver diseases (14,16,22). We previously showed that sCD163 reflects CD163 expression by immunohistochemistry in liver biopsies (17,21), and levels of sCD163 decrease after treatment in chronic viral hepatitis B and C, AIH, and nonalcoholic steatohepatitis (15,16,21,35,36). Moreover, we previously demonstrated a gradient across the liver in patients with liver cirrhosis and nonalcoholic steatohepatitis suggesting intrahepatic excretion (16,18,37). Furthermore, a recent study found that the monocyte activation marker CD14 was elevated in patients with PSC and predicted liver-transplant-free survival (9). Thus, we believe that the measured sCD163 and sMR levels reflect liver disease severity and stage in PSC, also indicated by the relation found to disease pathophysiology (6–8).
PSC is a chronic liver disease primarily affecting the bile ducts, but as the disease progresses, liver fibrosis and cirrhosis may develop. In line with this, we observed elevated levels of markers of macrophage activation that correlated with disease progression as estimated by Mayo score, ELF test, AOM, ALP, and bilirubin. Thus, it may be suggested that macrophage activation markers are elevated in relation to the grade of fibrosis or cirrhosis, and hence a measure of unspecific liver disease.
On the other hand, PSC-specific characteristics, such as chronic inflammation in the biliary tree, may stimulate macrophages in the surrounding area, as supported by elevated numbers of perisinusoidal macrophages in liver biopsies from patients with PSC compared with patients with PBC and normal controls (6,7). Furthermore, genome-wide association studies have identified a single-nucleotide polymorphism in the gene coding the macrophage stimulating-1 (MST-1) protein as the strongest non-human leukocyte antigen risk locus in PSC (38). MST-1 is expressed throughout the liver and biliary epithelium and is associated with macrophage differentiation and skewing toward the anti-inflammatory M2 phenotype. The single-nucleotide polymorphism found in PSC makes the MST-1 protein hypofunctional and hence skews the macrophage pool toward the proinflammatory M1 phenotype (38), creating a more proinflammatory milieu. In such, a milieu more macrophages are activated and more CD163 cleaved and accompanied by increased sCD163 levels, which may suggest that macrophage activation is partly caused by PSC specific characteristics. This biological hypothesis, however, cannot be investigated in a study with a retrospective design, such as the present, and we cannot make any conclusions on whether the levels of macrophage activation markers reflect PSC-specific characteristics or unspecific measures of liver disease, such as fibrosis or cirrhosis. To make such conclusion, the markers needs to be further investigated in prospective studies including genetic testing and liver biopsies.
The Mayo score, the ELF test, and AOM are 3 of the best validated risk assessment tools used in patients with PSC (31,39). The macrophage activation markers investigated here closely correlated with both the Mayo score, ELF test, and AOM in the Oslo cohort, suggesting a good ability to discriminate between those patients with a good and a poor prognosis. This was supported by the AUROC analysis where sCD163 showed similar performance to the Mayo score, ELF test, and AOM in advanced disease, i.e. the Oslo cohort. sCD163 was also associated with outcome in the Helsinki cohort (primarily earlier stage disease), as were the Mayo score and AOM; however, here, the ELF test showed a better discriminatory ability. We cannot explain why sCD163 and AOM performed poorer in the Helsinki cohort of patients with earlier stage disease than in the Oslo cohort. We do, however, speculate that this may be due to a larger degree of fluctuation of the blood samples included in the scores in earlier stage disease, whereas the components reflective of fibrosis included in the ELF test are more stable. Although it is possible that the differing approaches to ERCP and endoscopic therapy between the 2 cohorts may have affected our results, it has not been established whether these differing approaches affect prognosis. Based on our data, we suggest that sCD163, as the best marker, should be included in future studies in PSC to further investigate the role of macrophage activation in PSC. We also encourage the inclusion of macrophage activation markers in prospective studies with repeated measurements to analyze the ability of these markers to reflect, e.g. treatment response of possible therapies affecting macrophage function.
The data presented here stems from 2 well-characterized cohorts of patients with PSC with complete follow-up of all patients. A large number of events during the follow-up period provided important data for analyzing the discriminatory and prognostic values of variables. A weakness of the study is the difference in disease severity between the 2 cohorts. The Oslo cohort represents patients at a referral transplant center, and therefore, more patients presented with advanced liver disease and evaluation for liver transplants than in the Helsinki cohort. However, recruitment center itself, was not associated with outcome in multivariate Cox regression. Despite stage differences, we were able to reproduce findings in the 2 independent cohorts and in the combined cohort. Both cohorts included patients at different stages of disease progression as indicated by the variable disease duration, Mayo risk scores, and fibrosis severity as assessed by the ELF test. Hence, we believe that the measured levels of sCD163 and sMR are valid and representative for patients with PSC. Unfortunately, we only have one measurement of the macrophage activation markers at inclusion to the study. Thus, we do not know whether sCD163 and sMR fluctuates like ALP and other markers in PSC and whether continuous measurements would have improved their prognostic and discriminatory capabilities, and we cannot conclude anything related to their monitoring abilities.
In conclusion, we showed that macrophage activation markers are closely correlated with liver disease severity in patients with PSC. Moreover, sCD163 showed discriminatory abilities on the same level as the Mayo score and ELF test in advanced stage disease, and sCD163 predicted transplant-free survival in univariate analyses. This suggests an important role of macrophages in the pathophysiology of PSC, especially in advanced disease, and we suggest sCD163 to be explored in interventional studies of patients with PSC as marker of treatment response.
CONFLICTS OF INTEREST
Guarantor of the article: Henning Grønbæk, MD, PhD.
Specific author contributions: Lars Bossen, MD and Mette Vesterhus, MD, PhD, contributed equally to this work. M.V., T.H.K. and H.G. conceived and designed the study. K.M.B., M.V., and J.R.H. collected the biological samples and clinical data. H.J.M. performed the laboratory analyses. W.R. contributed to the ELF test analyses. M.V. and L.B. performed statistical analyses. L.B., M.V., T.H.K., J.R.H., and H.G. contributed to the interpretation of the data. L.B. and M.V. drafted the manuscript. All authors reviewed the manuscript for critical content and approved the final version of the manuscript.
Financial support: None to report.
Potential competing interests: M.V. has received advisory board fees from Intercept. L.B. and H.G. have received an investigator-initiated research grant from Intercept. H.G. also received research grants from AbbVie, ADS AIPHIA Development Services AG, (Switzerland), ARLA Food for Health, and the NOVO Nordisk Foundation. W.M.R. is an inventor of the ELF test and has received research support and speaking fees from Siemens Healthineers. W.M.R. is a NIHR Senior Investigator and is supported by the UCLH NIHR BRC. J.R.H. has received advisory board fees from Novartis and Orkla Health, and research support from Biogen.
Study Highlights.
WHAT IS KNOWN
✓ Macrophage activation markers are associated with disease severity and prognosis in multiple liver diseases.
✓ In primary sclerosing cholangitis (PSC), markers of prognosis are highly warranted.
✓ Macrophage activation markers, sCD163 and mannose receptor (sMR), have never been investigated in patients with PSC.
WHAT IS NEW HERE
✓ Macrophage activation markers correlate with the Enhanced Liver Fibrosis test, Mayo score, and AOM in PSC.
✓ sCD163 is associated with risk of death or liver transplantation in patients with PSC.
TRANSLATIONAL IMPACT
✓ Macrophage activation should be studied further in PSC.
✓ sCD163 should be included in future PSC trials with repeated measurements.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Liv Wenche Thorbjørnsen for assistance in the collection of serum samples.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A513, http://links.lww.com/CTG/A514, http://links.lww.com/CTG/A515, http://links.lww.com/CTG/A516
Contributor Information
Lars Bossen, Email: larsbossen@clin.au.dk.
Mette Vesterhus, Email: mette.namdal.vesterhus@haraldsplass.no.
Johannes R. Hov, Email: j.e.r.hov@medisin.uio.no.
Martti Färkkilä, Email: Martti.Farkkila@hus.fi.
William M. Rosenberg, Email: w.rosenberg@ucl.ac.uk.
Kirsten M. Boberg, Email: kboberg@ous-hf.no.
Tom H. Karlsen, Email: t.h.karlsen@medisin.uio.no.
References
- 1.Hirschfield GM, Karlsen TH, Lindor KD, et al. Primary sclerosing cholangitis. The Lancet 2013;382(9904):1587–99. [DOI] [PubMed] [Google Scholar]
- 2.Karlsen TH, Folseraas T, Thorburn D, et al. Primary sclerosing cholangitis: a comprehensive review. J Hepatol 2017;67(6):1298–323. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol 2017;67(1):145–72. [DOI] [PubMed] [Google Scholar]
- 4.Ponsioen CY, Chapman RW, Chazouilleres O, et al. Surrogate endpoints for clinical trials in primary sclerosing cholangitis: Review and results from an International PSC Study Group consensus process. Hepatology 2016;63(4):1357–67. [DOI] [PubMed] [Google Scholar]
- 5.Vesterhus M, Melum E. Prognostic biomarkers and surrogate end points in PSC. Liver Int 2016;36(12):1748–51. [DOI] [PubMed] [Google Scholar]
- 6.Cameron RG, Blendis LM, Neuman MG. Accumulation of macrophages in primary sclerosing cholangitis. Clin Biochem 2001;34(3):195–201. [DOI] [PubMed] [Google Scholar]
- 7.Guicciardi ME, Trussoni CE, Krishnan A, et al. Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice. J Hepatol 2018;69(3):676–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y-Y, Arndtz K, Webb G, et al. Intrahepatic macrophage populations in the pathophysiology of primary sclerosing cholangitis. JHEP Rep 2019;1:369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhillon AK, Kummen M, Troseid M, et al. Circulating markers of gut barrier function associated with disease severity in primary sclerosing cholangitis. Liver Int 2019;39(2):371–81. [DOI] [PubMed] [Google Scholar]
- 10.Bansal AS, Thomson A, Steadman C, et al. Serum levels of interleukins 8 and 10, interferon gamma, granulocyte-macrophage colony stimulating factor and soluble CD23 in patients with primary sclerosing cholangitis. Autoimmunity 1997;26(4):223–9. [DOI] [PubMed] [Google Scholar]
- 11.Møller HJ. Soluble CD163. Scand J Clin Lab Invest 2012;72(1):1–13. [DOI] [PubMed] [Google Scholar]
- 12.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the haemoglobin scavenger receptor. Nature 2001;409(6817):198–201. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Pomares L. The mannose receptor. J Leukoc Biol 2012;92(6):1177–86. [DOI] [PubMed] [Google Scholar]
- 14.Kazankov K, Barrera F, Møller HJ, et al. Soluble CD163, a macrophage activation marker, is independently associated with fibrosis in patients with chronic viral hepatitis B and C. Hepatology 2014;60(2):521–30. [DOI] [PubMed] [Google Scholar]
- 15.Kazankov K, Møller HJ, Lange A, et al. The macrophage activation marker sCD163 is associated with changes in NAFLD and metabolic profile during lifestyle intervention in obese children. Pediatr Obes 2015;10(3):226–33. [DOI] [PubMed] [Google Scholar]
- 16.Kazankov K, Tordjman J, Møller HJ, et al. Macrophage activation marker soluble CD163 and non-alcoholic fatty liver disease in morbidly obese patients undergoing bariatric surgery. J Gastroenterol Hepatol 2015;30(8):1293–300. [DOI] [PubMed] [Google Scholar]
- 17.Sandahl TD, Grønbæk H, Møller HJ, et al. Hepatic macrophage activation and the LPS pathway in patients with alcoholic hepatitis: A prospective cohort study. Am J Gastroenterol 2014;109(11):1749–56. [DOI] [PubMed] [Google Scholar]
- 18.Holland-Fischer P, Grønbæk H, Sandahl TD, et al. Kupffer cells are activated in cirrhotic portal hypertension and not normalised by TIPS. Gut 2011;60(10):1389–93. [DOI] [PubMed] [Google Scholar]
- 19.Grønbæk H, Sandahl TD, Mortensen C, et al. Soluble CD163, a marker of Kupffer cell activation, is related to portal hypertension in patients with liver cirrhosis. Aliment Pharmacol Ther 2012;36(2):173–80. [DOI] [PubMed] [Google Scholar]
- 20.Rodgaard-Hansen S, Rafique A, Christensen PA, et al. A soluble form of the macrophage-related mannose receptor (MR/CD206) is present in human serum and elevated in critical illness. Clin Chem Lab Med 2014;52(3):453–61. [DOI] [PubMed] [Google Scholar]
- 21.Gronbaek H, Kreutzfeldt M, Kazankov K, et al. Single-centre experience of the macrophage activation marker soluble (s)CD163 - associations with disease activity and treatment response in patients with autoimmune hepatitis. Aliment Pharmacol Ther 2016;44(10):1062–70. [DOI] [PubMed] [Google Scholar]
- 22.Sandahl TD, Støy SH, Laursen TL, et al. The soluble mannose receptor (sMR) is elevated in alcoholic liver disease and associated with disease severity, portal hypertension, and mortality in cirrhosis patients. PLoS One 2017;12(12):e0189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bossen L, Rebora P, Bernuzzi F, et al. Soluble CD163 and mannose receptor as markers of liver disease severity and prognosis in patients with primary biliary cholangitis. Liver Int 2020;40(6):1408–14. [DOI] [PubMed] [Google Scholar]
- 24.Grønbæk H, Gantzel RH, Laursen TL, et al. Macrophage markers and innate immunity in cirrhosis. J Hepatol 2020;73:1586–8. [DOI] [PubMed] [Google Scholar]
- 25.Grønbæk H, Rodgaard-Hansen S, Aagaard NK, et al. Macrophage activation markers predict mortality in patients with liver cirrhosis without or with acute-on-chronic liver failure (ACLF). J Hepatol 2016;64(4):813–22. [DOI] [PubMed] [Google Scholar]
- 26.Sandahl TD, McGrail R, Møller HJ, et al. The macrophage activation marker sCD163 combined with markers of the Enhanced Liver Fibrosis (ELF) score predicts clinically significant portal hypertension in patients with cirrhosis. Aliment Pharmacol Ther 2016;43(11):1222–31. [DOI] [PubMed] [Google Scholar]
- 27.Rode A, Nicoll A, Møller HJ, et al. Hepatic macrophage activation predicts clinical decompensation in chronic liver disease. Gut 2013;62(8):1231–2. [DOI] [PubMed] [Google Scholar]
- 28.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010;51(2):660–78. [DOI] [PubMed] [Google Scholar]
- 29.European Association for the Study of the Liver. EASL clinical practice guidelines: Management of cholestatic liver diseases. J Hepatol 2009;51(2):237–67. [DOI] [PubMed] [Google Scholar]
- 30.Boberg KM, Chapman RW, Hirschfield GM, et al. Overlap syndromes: The international autoimmune hepatitis group (IAIHG) position statement on a controversial issue. J Hepatol 2011;54(2):374–85. [DOI] [PubMed] [Google Scholar]
- 31.Kim WR, Therneau TM, Wiesner RH, et al. A revised natural history model for primary sclerosing cholangitis. Mayo Clin Proc 2000;75(7):688–94. [DOI] [PubMed] [Google Scholar]
- 32.de Vries EM, Wang J, Williamson KD, et al. A novel prognostic model for transplant-free survival in primary sclerosing cholangitis. Gut 2018;67(10):1864–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Møller HJ, Hald K, Moestrup SK. Characterization of an enzyme-linked immunosorbent assay for soluble CD163. Scand J Clin Lab Invest 2002;62(4):293–9. [DOI] [PubMed] [Google Scholar]
- 34.Lichtinghagen R, Pietsch D, Bantel H, et al. The enhanced liver fibrosis (ELF) score: Normal values, influence factors and proposed cut-off values. J Hepatol 2013;59(2):236–42. [DOI] [PubMed] [Google Scholar]
- 35.Laursen TL, Wong GL, Kazankov K, et al. Soluble CD163 and mannose receptor associate with chronic hepatitis B activity and fibrosis and decline with treatment. J Gastroenterol Hepatol 2018;33(2):484–91. [DOI] [PubMed] [Google Scholar]
- 36.Lund Laursen T, Brockner Siggard C, Kazankov K, et al. Rapid and persistent decline in soluble CD163 with successful direct-acting antiviral therapy and associations with chronic hepatitis C histology. Scand J Gastroenterol 2018;53:986–93. [DOI] [PubMed] [Google Scholar]
- 37.Laursen TL, Rodgaard-Hansen S, Møller HJ, et al. The soluble mannose receptor is released from the liver in cirrhotic patients, but is not associated with bacterial translocation. Liver Int 2017;37(4):569–75. [DOI] [PubMed] [Google Scholar]
- 38.Eksteen B. Advances and controversies in the pathogenesis and management of primary sclerosing cholangitis. Br Med Bull 2014;110(1):89–98. [DOI] [PubMed] [Google Scholar]
- 39.Vesterhus M, Hov JR, Holm A, et al. Enhanced liver fibrosis score predicts transplant-free survival in primary sclerosing cholangitis. Hepatology 2015;62(1):188–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.