Abstract
Immune cells rely on a functional vascular network to enter tissues. In solid tumors, blood vessels are abnormal and dysfunctional, so immune effector cell infiltration is impaired. Although normalizing the tumor vasculature has been shown to improve the efficacy of cancer immunotherapies, recent studies suggest that enhanced immune stimulation also, in turn, improves tumor vascular normalization. This new paradigm of immune-vessel mutual reprogramming thus opens up the possibility of identifying new cancer treatment strategies that combine vascular targeting and immunotherapies. In this Opinion, we highlight the current evidence supporting immune-vascular cross-talk and outline how this relationship can provide new rationales for developing more effective combination immunotherapy strategies for treating human cancers.
Keywords: Cancer immunotherapy, vascular normalization, immune checkpoint blockade, tumor vasculature, tumor microenvironment
Cancer immunotherapies, including immune checkpoint blockers (ICBs), have significantly improved clinical outcomes for patients with cancer.1–8 However, despite the prolonged overall survival in some patients treated with ICBs, their response rates remain suboptimal, so efforts are now being increased to explore additional strategies that can overcome immunosuppression mediated by tumors.9–13 In addition to the intrinsic inhibitory signals generated by cancer cells, a growing body of evidence indicates that both structural and functional abnormalities within the tumor microenvironment also contribute greatly to its dysfunctional immune landscape.9,10,13–16
In this Opinion article, we highlight research showing that the morphologically and functionally abnormal tumor vasculatures produced from a dysregulated balance between pro- and anti-angiogenic signals contribute to the immune suppressive microenvironment within solid tumors. We also review recent evidence showing that, although tumor blood vessel normalization using anti-angiogenic therapy (AT) improves effector T cell infiltration and reduces immune inhibitory processes to augment the effect of cancer immunotherapies, cancer immunotherapies such as ICBs may also remodel the tumor vasculature, which in turn improves tissue perfusion. Therefore, this reciprocal interaction between tumor vascular normalization and immune activation provides opportunities for developing new combination strategies to improve the efficacy of cancer immunotherapies. Finally, we propose potential translational applications that would take advantage of ICB-induced vascular normalization effects to treat human cancers.
Tumor vasculature normalization
Tumor angiogenesis is a hallmark of cancer.17,18 New blood vessel formation provides tumor cells with nutrition and facilitates tumor progression and metastasis.19 It is logical, therefore, that AT would be an ideal strategy to disrupt the tumor vasculature with the goal of starving tumors to death.20,21 However, the initial excitement surrounding AT soon met with clinical reality.22,23 Yet, when anti-angiogenic agents such as the monoclonal antibody targeting the vascular endothelial growth factor (VEGF) were given with systemic chemotherapies, an improvement in overall survival was observed in patients;24,25 this improvement was also observed with radiation treatment.26 Since chemotherapies require functional blood vessels to enter the tumor parenchyma, and ionizing radiation relies on the presence of oxygen to generate free radicals to induce cytotoxic tumoricidal effects, it was unclear how destroying the very vascular network necessary for transporting these molecules would improve therapeutic efficacy. In answer to this seemingly paradoxical observation, it was hypothesized that perhaps certain ATs, instead of completely obliterating tumor vessels, induce a normalizing effect.23,27 As the normalized tumor blood vessels regain a certain degree of their perfusion capacity, they are more efficient in transporting chemotherapies and oxygen into tumors, thus increasing the antitumor efficacy of cytotoxic treatments.25
Anti-angiogenic therapy-induced tumor blood vessel normalization possesses several unique characteristics: structural normalization, functional normalization, a normalization window, and dose-dependence.28,29 Structural normalization due to anti-angiogenic treatments is characterized by morphological changes in the tumor vascular network, wherein tumor vessel diameters, density and tortuosity are drastically reduced. Functionally, vascular permeability is decreased with increased coverage of pericytes. This leads to improved vessel perfusion and reduced tissue hypoxia. Both structural and functional normalization of tumor vessels in the setting of AT are transient in nature and exhibit dose-dependent relationships. Administering a higher dose of anti-angiogenic agents often results in a brief normalization window. Conversely, low-dose AT tends to prolong the vascular normalization effect.25 In preclinical models of colorectal cancer, high-dose anti-VEGF antibody treatment decreased microvessel density and increased tumor hypoxia, findings that are consistent with vascular pruning, resulting in inadequate tissue perfusion.30 When used in lower dosages, anti-VEGFR2 antibody treatment was found to drastically improve tumor vessel perfusion and reduce tumor tissue hypoxia, compared to IgG control or high-dose anti-VEGFR2 antibody treatment in murine breast cancer models.31 Furthermore, the more durable normalization window produced by low-dose anti-VEGFR2 antibody treatment augmented cancer immunotherapies by improving intratumoral effector T cell infiltration as well as by relieving immunosuppressive signals within the tumor microenvironment, thus providing direct evidence of a potential synergism between vascular and immune targeted therapies in cancer.31 Although the precise dose of AT needed to generate the optimal vascular normalization effect is difficult to determine and is likely to vary across different tumor types, stages of tumor development, particular angiogenesis targets, and specific clones of antibodies used, the discovery of the vascular normalization window has resulted in the identification of therapeutic strategies that target tumor blood vessels to enhance conventional cancer treatments.
Strategies to normalize tumor vasculatures
One of the main strategies to remove the excess pro-angiogenic signals within tumors is to disrupt the signaling mediated by the interactions between angiogenic growth factors and their receptors. Genetic disruption of regulator of G-protein signaling 5 (Rgs5) expression in mice resulted in a shift in pericytes from an immature (PDGFR-β+) to a mature (α-smooth muscle actin, αSMA+; and neural/glial antigen 2, NG2+) phenotype.32 Despite a lack of changes in the overall vascular coverage by these pericytes, the phenotypic changes resulted in tumor vessel normalization and promoted an influx of immune effector cells into the tumor.32 However, the cellular component that mediates the Rgs5 knockout (KO)-induced vascular normalization effect remains unclear. Previous studies have found that deleting Rgs5 causes both endothelial cell and macrophage apoptosis.33 Therefore, it is possible that vascular normalization in the setting of Rgs5 deletion may be due to direct vascular regression through endothelial cell apoptosis, or it may be a secondary response to changes in perivascular cells such as pericytes or monocytes.
Beyond the classical VEGF-VEGFR axis, the angiopoietin (Ang)/Tie2 signaling pathway also plays a critical role in tumor angiogenesis. Ang1, which is primarily expressed by perivascular cells, maintains the survival and quiescence of endothelial cells in mature blood vessels.34 Ang2, by contrast, competes with Ang1 for Tie2 binding and promotes the sprouting of tumor vessels, leading to vascular destabilization.35 Simultaneous activation of Tie2 and blockade of Ang2 induced tumor vessel normalization and enhanced the delivery and antitumor effect of chemotherapy.36 Targeting Tie2 and Ang2 simultaneously produced enhanced antitumor responses than Ang2 blockade alone, which was attributed to decreased expression of multiple pro-angiogenic factors that are often upregulated in response to Ang2 blockade-induced tissue hypoxia.36 The need for a multi-targeted approach to elicit tumor vascular normalization was further demonstrated by dual blockade of VEGFa and Ang2 signaling, which improved tumor vessel normalization compared to VEGFa inhibition alone.37,38 More importantly, the antitumor responses of this treatment strategy were further enhanced by adding anti-PD1 treatment, as endothelial cells release IFNγ to upregulate PD-L1 in order to promote adaptive resistance to the dual blockade.38
In addition to disrupting pro-angiogenic signaling, the local delivery of angiostatic factors can also induce tumor vascular normalization.39 Intratumoral delivery of low dose TNFα stabilized the tumor vascular network, improved vessel perfusion, and, as a result, substantially enhanced cancer vaccine and adoptive T cell therapies. This was markedly different from intratumoral injection of IFNγ, which caused rapid vessel loss and impeded antitumor immunity.39,40 The intratumoral delivery of TNF superfamily member LIGHT, using a tumor homing peptide (CGKRK), induced tumor vascular normalization.41 Furthermore, CGKRK-LIGHT treatment induced the formation of high endothelial venules (HEVs), which are specialized structures that facilitate T cell infiltration into solid tumors, resulting in improved cancer immunotherapy responses.41,42 These studies confirm that interventions to reduce pro-angiogenic signaling and/or to increase angiostatic factors within the tumor microenvironment can lead to vascular normalization responses that may improve the antitumor efficacies of cytotoxic or immunotherapies.
Immune-mediated tumor vessel normalization
Although immune cells are known to regulate endothelial cell functions and, thus, play an essential role in tumor angiogenesis, it was only recently found that immune cell stimulation can also promote the normalization of tumor vessels.43,44 In addition to eliciting immune-mediated tumor cell eradication, ICB was also shown to promote tumor vascular normalization in orthotopic breast and ectopic colon tumor models.43,44 In both studies, blockade of CTLA-4 or PD-1 reduced tumor vascular density, improved vessel perfusion, and decreased tumor tissue hypoxia, all of which are hallmarks of the vascular normalization effect.43,44 However, because the tumor microenvironment is strongly influenced by local cellular and molecular signals, it is unclear whether the ICB-mediated vessel normalization observed in these tumor models is indicative of a universal effect. Since the therapeutic responses to ICBs themselves differ across tumor types and even across similar tumors established at different organ sites,45 it is reasonable to suspect that the associated vascular normalization effect is also subject to such variation.
In CD4−/− and CD8−/− mouse models, it was found that blockade of CTLA-4 and PD-1 promoted tumor vessel normalization via the activation of Th1 CD4+ helper T cells.43 Another study found that CD4+ T cells alone were insufficient to induce tumor vasculature remodeling effects. Instead, the depletion of CD4+ T cells resulted in the accumulation of CD8+ T cells and increased production of IFNγ, as well as a tumor vessel normalization response. The authors thus suggest that the vascular normalizing effect of immune checkpoint therapy is probably mediated by the activation of CD8+ T cells via the IFNγ signaling pathway.44 At the present, the precise role of CD4+ and CD8+ T cell in promoting the tumor vascular normalization effect in the setting of ICB is unclear and likely depends on the tumor type and, perhaps more importantly, on the cross-talk between immune cell subtypes and other cellular components of the tumor microenvironment. On one hand, using monoclonal antibodies to pharmacologically deplete CD4+ or CD8+ T cells may lead to antibody-dependent cell-mediated cytotoxicity (ADCC), which might elicit inflammatory responses that affect the tumor vasculature. On the other hand, genetic knockout of CD4+ or CD8+ T cells completely removes the presence of lymphocytes from the outset, thus raising the question of whether the tumorigenesis or even the angiogenesis process would be affected in such a lympho-depleted environment. The latter effect may be especially important given that lymphocyte subpopulations are differentially associated with various stages of tumor development. For example, the number of Regulatory T cells (Treg) (CD4+CD25+) was found to correlate positively with increases in tumor size. In contrast, CD8+ effector T cell numbers decrease as tumors grow.46 Depleting Tregs using a monoclonal anti-CD25 antibody synergized with anti-PD-1 therapy to inhibit tumor growth and increased IFNγ production within both CD4+ and CD8+ effector T cells.47 Furthermore, using a Foxp3-LuciDTR-4 mouse model (which simultaneously expressed enhanced green fluorescent protein, luciferase, and diphtheria toxin receptor (DTR)), another study recently demonstrated that selective depletion of Tregs increases the tumor infiltration of CD11b+Gr1loF4/80+Siglec-F+ eosinophils, which prompts the activation of CD8+ T cells and the normalization of tumor blood vessels.48 Simultaneous depletion of eosinophils and Tregs abrogates CD8+ T cell activation and vessel normalization, suggesting that CD8+ T cells mediate vessel normalization upon Treg depletion.48 Therefore, how CD4+ and CD8+ T cells induce vascular normalization responses may depend on context and may rely on coordinating with immune cell types within the tumor microenvironment.
The functional changes within the tumor vascular network from an immune-mediated normalization effect can be monitored by analyzing alterations in tumor blood vessel perfusion through noninvasive imaging techniques. Improved vessel perfusion (IVP) was found mainly in tumors sensitive to ICB, but not in resistant ones.44 More importantly, increased vessel perfusion appears to positively correlate with a tumor’s responsiveness to ICBs.44 This enables quantification of global IVP using noninvasive imaging to predict the potential therapeutic efficacy of immune checkpoint blockers, even before changes in tumor size become evident.44 Since noninvasive radiologic methods could be used to monitor vascular perfusion in real time, it is conceivable that IVP measurement could be incorporated into current cancer immunotherapy practice to achieve genuinely personalized cancer immunotherapies to benefit patients.9
These newly discovered functions of cancer immunotherapies, including ICBs, exert effects beyond immune cells and act on non-immune cells within the tumor microenvironment, thus providing a strong rationale to explore their combination with other treatment modalities that target these cell populations. For example, given that AT’s inhibition of vascular growth factors VEGF and ANG2 also induces adaptive resistance in endothelial cells by upregulating PD-L1,38 it makes logical sense to combine anti-angiogenic therapy with immunotherapy to achieve the optimal antitumor effect.49,50 In fact, multiple clinical trials have been initiated to investigate whether combining the two treatment strategies can improve clinical responses.
Combining vascular and cancer immunotherapy
The increasing evidence supporting a reciprocal interaction between immunotherapy and tumor vasculature suggests that combining anti-angiogenic agents with ICBs may improve clinical efficacy by creating positive feedback loops through which the therapies reinforce each other.9,51,52 When administered in the right doses,28 AT could reverse the immune suppressive tumor microenvironment by normalizing tumor blood vessels, which would reduce tissue hypoxia and acidosis and increase the infiltration of effector T cells.9 The increased infiltration and decreased immune suppressive signals would further augment ICBs to enhance effector T cell activation.53,54 These activated T cells not only kill tumor cells but also further contribute to modulating and normalizing tumor blood vessels, thus creating a positive reinforcement loop that leads to tumor regression.9 Meanwhile, anti-angiogenic agents may also directly or indirectly reduce immune suppressive cell populations within the tumor microenvironment. For example, low-dose anti-VEGFR2 therapy, but not high-dose therapy, promoted the polarization of tumor-associated macrophages toward the immune-stimulatory M1 phenotype and increased tumor infiltration of CD4+ and CD8+ T cells.31 In another study, inhibition of angiogenesis in a murine glioblastoma model promoted macrophage polarization and prolonged survival.37 In addition to polarizing tumor-associated macrophages, AT may also reduce Treg-mediated immune suppression. Treg cells have been found to express multiple receptors for VEGF, including Neurophilin-1, a co-receptor for VEGF with Flt-1 (VEGFR 1) and KDR (VEGFR2).55–58 Blockading VEGF using a monoclonal anti-VEGFa antibody significantly decreased the number of Treg cells in CT26 colorectal cancer.59 Therefore, whether through more efficient recruitment and activation of effector T cells or via decreased immune inhibition mediated by suppressor cells, anti-angiogenic treatment appears to promote immunologically favorable effects in the tumor microenvironment.
Recent preclinical studies have also demonstrated a potential synergy between anti-angiogenic agents and ICB.38,42 Currently, a number of ongoing clinical trials are testing the combination of anti-angiogenic agents and ICBs in solid tumors (Table 1). However, questions still remain regarding the best regimen for administering the two modalities together. Based on lessons learned from anti-angiogenic agents and chemotherapies, the dose and timing of these agents’ administration will probably be key to their potential success. Although AT may provide an initial normalization effect and decrease immune suppressive signals within the tumor microenvironment, excessive inhibition may lead to vessel regression, resulting in impeded drug delivery and lymphocyte infiltration into the tumor. As phase I trials are often designed to identify the dose limiting toxicities and maximal tolerated doses of the drug combination, they may not reflect the doses needed to generate optimal antitumor responses. Furthermore, effective dosages of anti-angiogenic agents for normalizing tumor vasculatures may depend on additional factors, such as tumor size, vascularity, and expression levels of pro-angiogenic growth factors. The complexities governing the interplay between anti-angiogenic therapy and immunotherapy thus highlight the importance of identifying potential biomarkers that can predict treatment responses. Serum-based biomarkers reflecting the functional status of tumor blood vessels have been used to monitor responses to anti-angiogenic agents.60,61 Some of these biomarkers have already demonstrated potential to predict cancer immunotherapy responses.62,63 Therefore, whether they can be combined with immune biomarkers to serve as predictors for combination anti-angiogenic and immunotherapy remains to be seen.
Table 1:
Ongoing clinical trials investigating ICB in combination with AT for cancer treatments.
| Trial ID | Phase | Immunotherapy | Anti-angiogenic therapy | Disease |
|---|---|---|---|---|
| NCT02982694 | II | Atezolizumab | Bevacizumab | Advanced CRC |
| NCT03074513 | II | Atezolizumab | Bevacizumab | Several solid tumors |
| NCT02724878 | II | Atezolizumab | Bevacizumab | Advanced non-clear-cell RCC |
| NCT02921269 | II | Atezolizumab | Bevacizumab | Cervical cancer |
| NCT02997228 | III | Atezolizumab | Bevacizumab | CRC |
| NCT01984242 | II | Atezolizumab | Bevacizumab | Advanced RCC |
| NCT02420821 | III | Atezolizumab | Bevacizumab | Advanced RCC |
| NCT03133390 | II | Atezolizumab | Bevacizumab | Metastatic urothelial carcinoma |
| NCT02659384 | II | Atezolizumab | Bevacizumab | Ovarian cancer |
| NCT01688206 | I | Atezolizumab | Vanucizumab | Epithelial ovarian, fallopian tube or primary peritoneal cancer |
| NCT03170960 | I/II | Atezolizumab | Cabozantinib | Several solid tumors |
| NCT02493751 | I | Avelumab | Axitinib | Advanced RCC |
| NCT02684006 | III | Avelumab | Axitinib | Advanced RCC |
| NCT02572687 | I | Durvalumab | Ramucirumab | Several solid tumors |
| NCT02336165 | II | Durvalumab | Bevacizumab | GBM |
| NCT02496208 | I | Ipilimumab + nivolumab | Cabozantinib | Metastatic genitourinary cancer |
| NCT00790010 | I | Ipilimumab | Bevacizumab | Melanoma |
| NCT01950390 | II | Ipilimumab | Bevacizumab | Melanoma |
| NCT02210117 | I | Nivolumab, ipilimumab | Bevacizumab | RCC |
| NCT02873962 | II | Nivolumab | Bevacizumab | Relapsed ovarian, fallopian tube or peritoneal cancer |
| NCT02999295 | I/II | Nivolumab | Ramucirumab | Gastric cancer |
| NCT02576509 | III | Nivolumab | Sorafenib | Advanced HCC |
| NCT03172754 | I/II | Nivolumab | Axitinib | Advanced RCC |
| NCT02681549 | II | Pembrolizumab | Bevacizumab | Melanoma or NSCLC brain metastasis |
| NCT02337491 | II | Pembrolizumab | Bevacizumab | GBM |
| NCT02348008 | I/II | Pembrolizumab | Bevacizumab | Clear cell RCC |
| NCT02853318 | II | Pembrolizumab | Bevacizumab | Recurrent ovarian, fallopian tube, or primary peritoneal cancer |
| NCT02856425 | I | Pembrolizumab | Nintedanib | Several solid tumors |
| NCT02133742 | I | Pembrolizumab | Axitinib | Metastatic RCC |
| NCT02853331 | III | Pembrolizumab | Axitinib | Metastatic RCC |
| NCT02636725 | II | Pembrolizumab | Axitinib | Sarcoma |
| NCT02443324 | I | Pembrolizumab | Ramucirumab | Several solid tumors |
| NCT02014636 | I/II | Pembrolizumab | Pazopanib | Advanced RCC |
| NCT02298959 | I | Pembrolizumab | Aflibercept | Advanced tumors |
| NCT02501096 | I/II | Pembrolizumab | Lenvatinib | Several solid tumors |
| NCT03006887 | I | Pembrolizumab | Lenvatinib | Several solid tumors |
| NCT02811861 | III | Pembrolizumab | Lenvatinib | Advanced RCC |
| NCT02722954 | I | Pembrolizumab | Demcizumab | Metastatic solid tumors |
| NCT03603756 | II | SHR-1210 | Apatinib | Esophageal squamous cell carcinoma |
| NCT02039674 | I/II | Pembrolizumab | Bevacizumab | NSCLC |
| NCT03424005 | I/II | Atezolizumab | Bevacizumab | TNBC |
| NCT03555149 | I/II | Atezolizumab | Bevacizumab | Metastatic CRC |
| NCT03280563 | I/II | Atezolizumab | Bevacizumab | Breast neoplasms |
| NCT03414983 | II/III | Nivolumab | Bevacizumab | Metastatic CRC |
| NCT03193190 | I/II | Atezolizumab | Bevacizumab | Pancreatic adenocarcinoma |
| NCT03424005 | I/II | Atezolizumab | Bevacizumab | TNBC |
| NCT03175432 | II | Atezolizumab | Bevacizumab | Melanoma and other malignant neoplasms of skin |
CRC, colorectal cancer; RCC, renal cell carcinoma; GBM, glioblastoma; NSCLC, non-small cell lung cancer; TNBC, triple negative breast cancer.
In addition to antitumor responses, combining AT with ICBs may increase the risk of adverse events over either treatment alone. Based on early clinical trial results, ICBs combined with AT appeared to be safe but are associated with higher incidences of acute Grade 3 or above toxicities in patients compared to either monotherapies (Table 2). The most common adverse reactions from the combination treatment include hematological and liver function abnormalities. Larger populations and longer follow-up are needed to better assess the potential long-term side effects associated with combined AT and ICB treatment in patients.
Table 2:
Incidence of Treatment-Related Adverse Events when combining AT with immunotherapy.
| Trial ID | Phase | Immunotherapy | Anti-angiogenic therapy | Disease | Toxicity | Adverse events64–67 |
|---|---|---|---|---|---|---|
| NCT02366143 | III | Atezolizumab | Bevacizumab | NSCLC | Combined Grade≥3 58.5% Bevacizumab Grade≥3 50% |
Hematological 41.8% Hypertension 6.4% Hematological 35.7% Hypertension 6.3% |
| NCT0308304 | Ia/Ib | Anti-PD1 antibody | Apatinib | HCC、EC/EGJC | Ia Grade 3 26.7% Ib Grade3 60.6% |
Biochemistry 6.97% Hypertension 20% Hypertension 15.2% Liver function 15.2% |
| NCT02853331 | III | Pembrolizumab | Axitinib/ sunitinib | Advanced RCC | Avelumab plus Axitinib Grade≥3 75.8% Sunitinib Grade≥3 70.6% |
Liver function 20.3% Hypertension 22.1% Diarrhea 9.1% Liver function 5.5% Hypertension 19.3% Fatigue 6.6% Hematological 29.4% |
| NCT02684006 | Ib | Avelumab | Axitinib/ sunitinib | Advanced RCC | Pembrolizumab–Axitinib Grade≥3 71.2% Sunitinib Grade≥3 71.5% |
Diarrhea 6.7% Hypertension 19.3% Liver function 9.9% Hypertension 17.1% Liver function 5.7% Hematological 33.1% |
NSCLC, non-small cell lung cancer; EC, esophageal cancer; EGJC, esophageal-gastric junction cancer; RCC, renal cell carcinoma.
Neoadjuvant cancer immunotherapy: A new path forward?
The tumor vascular normalization effect of ICBs also provides a strong rationale for its use in the neoadjuvant setting, where it can prime the tumor microenvironment to improve the efficacy of subsequent definitive treatment. Encouraging results have already been reported on the use of neoadjuvant immune checkpoint inhibitors for resectable melanoma and non-small cell lung cancers,68–71 as well as in more traditionally immune-resistant tumors such as glioblastoma.72,73 However, most of these studies investigated whether immunotherapy can serve as a more effective alternative to chemotherapy in the neoadjuvant setting to improve surgical resection and pathological complete response rate. Through normalization of the tumor vasculature, the improved tissue perfusion is often accompanied by reductions in hypoxia, acidosis, and interstitial fluid pressure within the tumor microenvironment, all of which help to improve oxygenation and drug delivery into tumors (Figure 2).9 These pathophysiological changes in the setting of tumor microenvironmental normalization thus make neoadjuvant cancer immunotherapy a potential option to complement existing treatment strategies, such as chemotherapy, targeted therapy and radiation, or emerging modalities like photodynamic therapy, which relies on generating reactive oxygen species to mediate its tumoricidal effect.74 While the prospect of these combination therapies seems exciting, many questions need to be addressed before clinical translation can occur. The first question pertains to the kinetics of the immune-mediated vascular normalization effect and its duration. The immune-mediated response seems to be more durable than AT-induced transient vascular normalization and, thus, may provide more flexibility in terms of administering combination therapy with other agents. Second, whether vasculature normalization occurs in all tumors that respond to ICBs remains unclear. Finally, the optimum dosing of ICBs to induce tumor vascular normalization is unknown. In the setting of AT, a lower but more persistent dosing regimen of anti-VEGFR agent was found to be more potent in promoting the tumor vascular normalization effect.23 However, whether this holds true for immune-mediated tumor vascular normalization is unclear, since AT induces vascular changes by directly interacting with blood vessel endothelial cells, while the immune-mediated vascular effect is secondary to IFNγ activities in the setting of T cell activation.43,44 Regardless, since cancer immunotherapies such as ICBs are increasingly being used in combination with existing treatment modalities, both concurrently or as neoadjuvants, addressing these questions would undoubtedly help to identify the best combination regimen and delivery strategies to elicit the most potent antitumor responses.
Figure 2: Immune or AT-mediated tumor vascular normalization improves tissue perfusion, leading to a decrease in tumor hypoxia and acidosis.
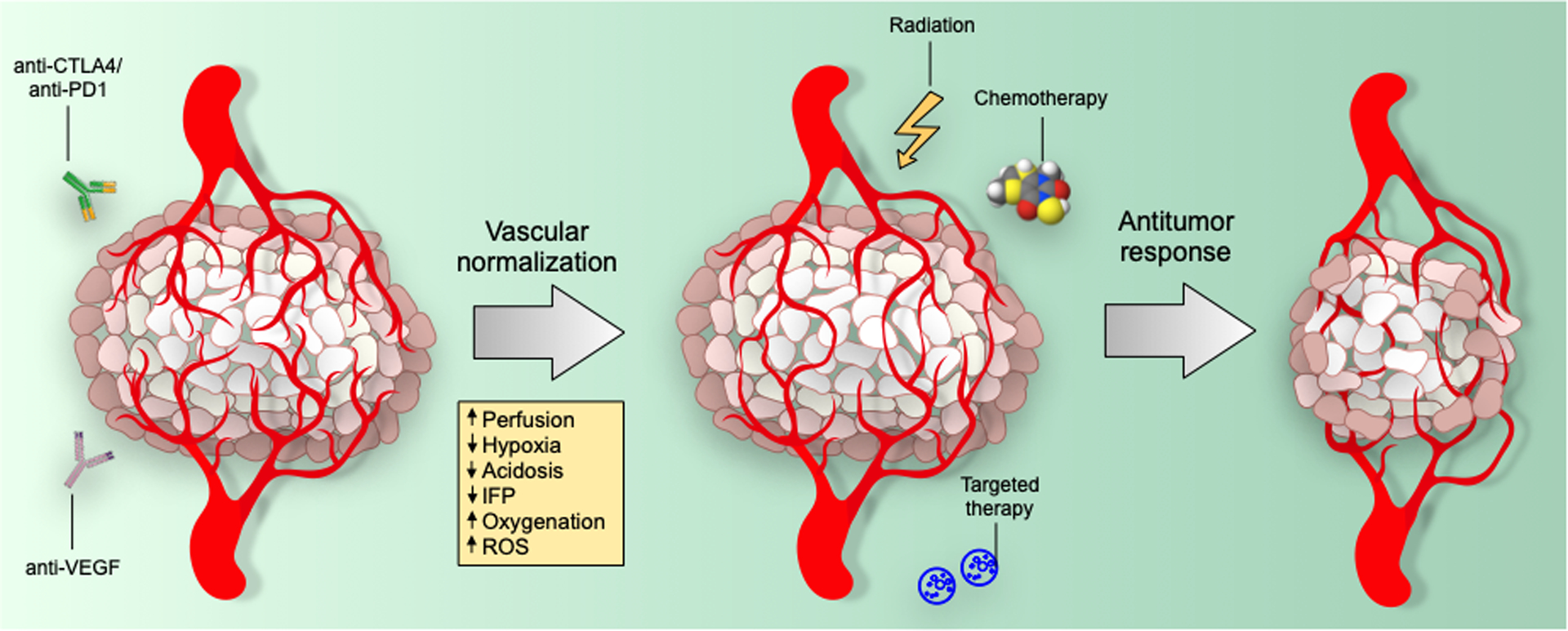
The reduced vascular permeability and leakiness also decreases interstitial fluid pressure (IFP) and thus helps to facilitate intratumoral delivery of chemo or targeted therapeutic agents. The increased oxygenation also enhances the production of reactive oxygen species (ROS), which further sensitizes solid tumors to definitive local treatments, such as targeted therapy or chemotherapy, as well as to ionizing radiation.
Future outlook and concluding remarks
The aberrant tumor vascular network has long been established as a major contributor to the immune suppressive microenvironment in solid tumors.9,75 Therefore, it was not surprising to observe that the normalization of tumor blood vessels can improve cancer immunotherapies. However, the fact that cancer immunotherapy, including ICBs, can also normalize tumor vasculatures through IFNγ produced by activated T cells suggests that immune-vascular cross-talk, at least in the context of tumor immunology, plays a critical role in tumor stromal invasion, immune evasion, and perhaps metastatic spread. At this point, it remains incompletely understood how IFNγ induces tumor vascular normalization, although previous evidence suggests that it is probably mediated via interactions with its receptor expressed on endothelial cells.40 Similarly, whether this immune-induced vascular normalization effect can also be observed in other IFNγ-producing immunotherapies, such as CAR T cells or oncolytic viruses, is unclear. Moreover, simultaneous blockade of two or more immune checkpoint molecules has been tested in the clinic.76,77 Although in some instances, targeting multiple immune checkpoints may improve antitumor effects, it is unclear whether this enhances tumor vessel normalization. Furthermore, the heightened T cell responses due to blockade of multiple immune checkpoints may in fact cause vessel regression, as seen in high-dose AT.31 Therefore, how to optimally induce tumor vessel normalization using cancer immunotherapy warrants further research. Nevertheless, the vascular remodeling effect of immunotherapy further broadens its potential oncological applications and provides new rationale for developing combination immunotherapy strategies with conventional cytotoxic treatment for cancer patients.
Figure 1. Potential mechanism of immunotherapy-mediated tumor vascular normalization.
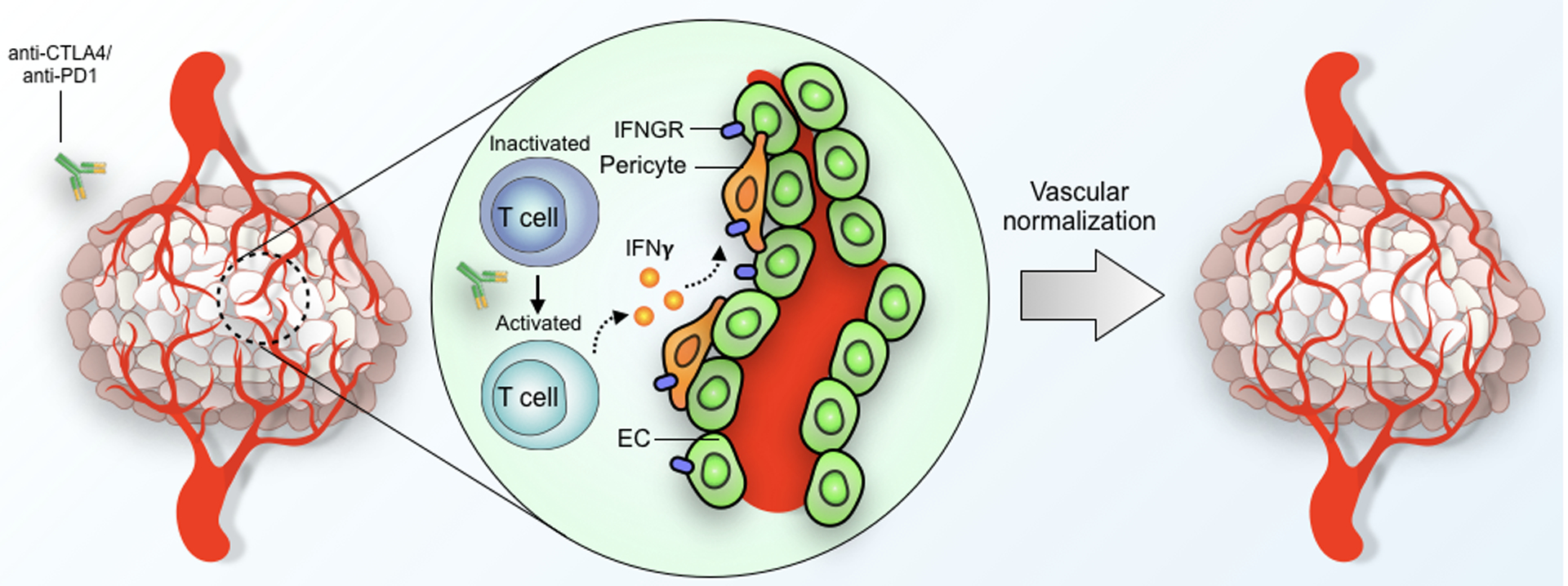
Activation of effector T cells in the setting of ICB results in production and secretion of IFNγ, which, through interaction with IFNγ receptors (IFNGR) expressed on vascular cells, results in normalization of the tumor vasculature. EC = endothelial cell.
Highlights:
Tumor vascular normalization via angiogenic blockade enhances immune effector cell infiltration and decreases immune suppressive signals within the tumor microenvironment, improving the efficacy of cancer immunotherapies.
Cancer immunotherapies, such as immune checkpoint inhibitors, can also promote tumor vascular normalization via stimulating γ-interferon release from immune effector cells.
This immune-vascular cross-talk offers unique opportunities to develop new combination strategies to improve the effectiveness of cancer immunotherapies.
A deeper insight into the underlying mechanisms governing immune-mediated tumor vessel normalization may guide future clinical trials of combined vascular and immunotherapies for patients with cancer.
Outstanding questions:
Does the tumor vascular normalization effect in the setting of ICB extend to other cancer immunotherapies such as CAR T cell or tumor vaccines?
What is the precise mechanism of γ-interferon–mediated tumor vascular normalization? Is this a direct (via endothelial cells) or indirect (via other cells) effect?
What are the differences in the nature of immunotherapy-mediated and AT-mediated tumor vasculature normalization effects?
Is there an immune-mediated mechanism in the development of AT resistance in solid tumors?
Can immunotherapy-mediated vessel normalization serve as a reliable biomarker for cancer immunotherapy response?
How will the effect of immunotherapy-mediated tumor vascular normalization differ for different tumors or tumor stages?
Acknowledgements
This work is supported by the National Natural Science Foundation of China (81572500 to Z.L.; 81673004 to Y.H.; 81430041 to H.S.), National Program on Key Basic Research Project of China (No. 2018YFC0910600 to H.S.), the Cancer Prevention and Research Institute of Texas (RR180017 to W.J.), the National Cancer Institute (K08CA241070 to W.J.) and the National Institute of Neurological Disorders and Stroke (R01 NS104315 to B.Y.S.K.). We also would like to thank Jonathan Feinberg of the Department of Radiation Oncology at The University of Texas Southwestern Medical Center for assistance with editing the manuscript.
Glossary
- Angiopoietin (ANG)
A family of vascular growth factors that play critical roles in angiogenesis.
- Antigens
Short sequences of peptides produced from digested proteins that are presented on the cell surface by major histocompatibility complex (MHC).
- CTLA-4 (cytotoxic T-lymphocyte-associated protein 4)
Also known as CD152, is a protein receptor that functions as an immune checkpoint. It binds to CD80 or CD86 to downregulate T cell responses.
- IFNγ
Also called type II interferon, is a cytokine that is critical for mediating immune responses against a number of pathogens as well as transformed cells.
- Interstitial fluid pressure (IFP)
The pressure exerted by the free interstitial fluid, which helps to determine transcapillary flow. IFP is often elevated in tumors.
- Immune checkpoints
A collection of inhibitory pathways within the immune system that maintain self-tolerance by modulating the duration and amplitude of physiological immune responses.
- Neoadjuvant therapy
The administration of therapeutic agents before a main treatment, which is usually a more definitive local therapy such as surgery, radiation, or chemoradiation.
- PD-1 (Programmed cell death protein 1)
Also known as CD279, is a cell surface protein that suppresses T cell inflammatory activity when binding to its ligands, such as PD-L1.
- Vascular normalization
Structural and functional changes within abnormal and dysfunctional tumor blood vessels that make them more closely resemble normal vasculatures.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
REFERENCES
- 1.Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribas A and Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P and Allison JP (2015) The future of immune checkpoint therapy. Science 348, 56–61 [DOI] [PubMed] [Google Scholar]
- 4.Hodi FS et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med 363, 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst RS et al. (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced nonsmall-cell lung cancer (KEYNOTE‑010): a randomized controlled trial. Lancet 387, 1540–1550 [DOI] [PubMed] [Google Scholar]
- 6.Reck M et al. (2016) Pembrolizumab versus chemotherapy for PD‑L1‑positive non-small-cell lung cancer. N. Engl. J. Med 375, 1823–1833 [DOI] [PubMed] [Google Scholar]
- 7.Nghiem PT et al. (2016) PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N. Engl. J. Med 374, 2542–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motzer RJ et al. (2015) Nivolumab versus everolimus in advanced renal cell carcinoma. N. Engl. J. Med 373, 1803–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y et al. (2018) Improving immune–vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol 18, 195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukumura D et al. (2018) Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol 15, 325–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borst J et al. (2018) CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol 18, 635–647 [DOI] [PubMed] [Google Scholar]
- 12.Zitvogel L et al. (2018) The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 359, 1366–1370 [DOI] [PubMed] [Google Scholar]
- 13.Khan KA and Kerbel RS (2018) Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat. Rev. Clin. Oncol 15, 310–324 [DOI] [PubMed] [Google Scholar]
- 14.Jiang W et al. (2016) Immune Priming of the Tumor Microenvironment by Radiation. Trends Cancer 2, 638–645 [DOI] [PubMed] [Google Scholar]
- 15.Nagarsheth N et al. (2017) Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol 17, 559–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyce JA and Fearon DT (2015) T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 [DOI] [PubMed] [Google Scholar]
- 17.Hanahan D and Weinberg RA (2000) The Hallmarks of Cancer. Cell 100, 57–70 [DOI] [PubMed] [Google Scholar]
- 18.Hanahan D and Weinberg RA (2011) Hallmarks of Cancer: The Next Generation. Cell 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 19.Kerbel RS (2008) Tumor Angiogenesis. N. Eng. J. Med 358, 2039–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folkman J (1971) Tumor angiogenesis: therapeutic implications. N. Eng. J. Med 285, 1182–1186 [DOI] [PubMed] [Google Scholar]
- 21.Jain RK et al. (2006) Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat. Clin. Prac. Oncol 3, 24–40 [DOI] [PubMed] [Google Scholar]
- 22.Raphael J et al. (2017) Antiangiogenic Therapy in Advanced Non-small-cell Lung Cancer: A Meta-analysis of Phase III Randomized Trials. Clin. Lung Cancer 18, 345–353 [DOI] [PubMed] [Google Scholar]
- 23.Jain RK (2005) Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 307, 58–62 [DOI] [PubMed] [Google Scholar]
- 24.Hurwitz H (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Eng. J. Med 350, 2335–42. [DOI] [PubMed] [Google Scholar]
- 25.Jain RK (2013) Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J. Clin. Oncol 31, 2205–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee CG et al. (2000) Anti-Vascular Endothelial Growth Factor Treatment Augments Tumor Radiation Response under Normoxic or Hypoxic Conditions. Cancer Res. 60, 5565–5570 [PubMed] [Google Scholar]
- 27.Kerbel RS (2006) Antiangiogenic Therapy: A Universal Chemosensitization Strategy for Cancer? Science 312, 1171–1175 [DOI] [PubMed] [Google Scholar]
- 28.Huang Y et al. (2013) Benefits of vascular normalization are dose and time dependent. Cancer Res. 73, 7144–7146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goel S et al. (2011) Normalization of the Vasculature for Treatment of Cancer and Other Diseases. Physiol. Rev 91, 1071–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahbari NN et al. (2016) Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci. Transl. Med 8, 360ra135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y et al. (2012) Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. USA 109, 17561–17566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamzah J et al. (2008) Vascular normalization in Rgs5‑deficient tumours promotes immune destruction. Nature 453, 410–414 [DOI] [PubMed] [Google Scholar]
- 33.Cheng WL et al. (2015) Regulator of G-protein signalling 5 protects against atherosclerosis in apolipoprotein E‐deficient mice. Br. J. Pharmacol 172, 5676–5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Palma M and Naldini L (2011) Angiopoietin-2 TIEs Up Macrophages in Tumor Angiogenesis. Clin. Cancer Res 17, 1–7 [DOI] [PubMed] [Google Scholar]
- 35.Hansen TM et al. (2010) Effects of angiopoietins-1 and −2 on the receptor tyrosine kinase Tie2 are differentially regulated at the endothelial cell surface. Cell Signal. 22, 527–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park JS et al. (2016) Normalization of tumor vessels by Tie2 activation and Ang2 inhibition enhances drug delivery and produces a favorable tumour microenvironment. Cancer Cell 30, 953–967 [DOI] [PubMed] [Google Scholar]
- 37.Peterson TE et al. (2016) Dual inhibition of Ang‑2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc. Natl Acad. Sci. USA 113, 4470–4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmittnaegel M et al. (2017) Dual angiopoietin‑2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD‑1 checkpoint blockade. Sci. Transl. Med 9, eaak9670. [DOI] [PubMed] [Google Scholar]
- 39.Johansson A et al. (2012) Tumor-targeted TNFα stabilizes tumor vessels and enhances active immunotherapy. Proc. Natl. Acad. Sci. USA 109, 7841–7846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kammertoens T et al. (2017) Tumour ischaemia by interferon-gamma resembles physiological blood vessel regression. Nature 545, 98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He B et al. (2018) Vascular targeting of LIGHT normalizes blood vessels in primary brain cancer and induces intratumoural high endothelial venules. J. Pathol 245, 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen E et al. (2017) Combined antiangiogenic and anti– PD‑L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med 9, eaak9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian L et al. (2017) Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544, 250–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng X et al. (2018) Increased vessel perfusion predicts the efficacy of immune checkpoint blockade. J. Clin. Invest 128, 2104–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang C et al. (2017) Ipilimumab with stereotactic ablative radiation therapy: Phase I results and immunologic correlates from peripheral T-cells. Clin Cancer Res. 23, 1388–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elpek KG et al. (2007) CD4+CD25+ T Regulatory Cells Dominate Multiple Immune Evasion Mechanisms in Early but Not Late Phases of Tumor Development in a B Cell Lymphoma Model. J Immunol. 178, 6840–8 [DOI] [PubMed] [Google Scholar]
- 47.Vargas FA et al. (2017) Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes with PD-1 Blockade to Eradicate Established Tumors. Immunity 46, 577–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carretero R et al. (2015) Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat. Immunol 16, 609–617 [DOI] [PubMed] [Google Scholar]
- 49.Liu XD. et al. (2015) Resistance to antiangiogenic therapy is associated with an immunosuppressive tumor microenvironment in metastatic renal cell carcinoma. Cancer Immunol. Res 3, 1017–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu X et al. (2016) VEGF neutralization plus CTLA-4 blockade alters soluble and cellular factors associated with enhancing lymphocyte infiltration and humoral recognition in melanoma. Cancer Immunol. Res 4, 858–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang Y et al. (2013) Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Research 73, 2943–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Palma M and Jain RK (2017) CD4+ T Cell Activation and Vascular Normalization: Two Sides of the Same Coin? Immunity 46, 773–775 [DOI] [PubMed] [Google Scholar]
- 53.Trujillo JA et al. (2018) T Cell–Inflamed versus Non-T Cell–Inflamed Tumors: A Conceptual Framework for Cancer Immunotherapy Drug Development and Combination Therapy Selection. Cancer Immunol. Res 6, 990–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang H et al. (2016) Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD-L1 blockade. Cancer Cell 29, 285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kruger RP et al. (2005) Semaphorins command cells to move. Nat. Rev. Mol. Cell Biol 6, 789–800 [DOI] [PubMed] [Google Scholar]
- 56.Staton CA et al. (2007) Neuropilins in physiological and pathological angiogenesis. J. Pathol 212, 237–248 [DOI] [PubMed] [Google Scholar]
- 57.Bruder D et al. (2004) Neuropilin-1: A surface marker of regulatory T cells. Eur. J. Immunol 34, 623–630 [DOI] [PubMed] [Google Scholar]
- 58.Suzuki H et al. (2010) VEGFR2 is selectively expressed by FOXP3high CD4+ Treg. Eur. J. Immunol 40,197–203 [DOI] [PubMed] [Google Scholar]
- 59.Terme M et al. (2013) VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 73, 539–49 [DOI] [PubMed] [Google Scholar]
- 60.Rigamonti N et al. (2014) Role of angiopoietin‑2 in adaptive tumor resistance to VEGF signaling blockade. Cell Rep. 8, 696–706 [DOI] [PubMed] [Google Scholar]
- 61.Goede V et al. (2010) Identification of serum angiopoietin‑2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. Br. J. Can 103, 1407–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu W et al. (2017) Angiopoietin‑2 as a biomarker and target for immune checkpoint therapy. Can. Immunol. Res 5, 17–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schoenfeld J et al. (2010) Active immunotherapy induces antibody responses that target tumor angiogenesis. Cancer Res. 70, 10150–10160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. The New England journal of medicine. June 14 2018;378(24):2288–2301. [DOI] [PubMed] [Google Scholar]
- 65.Xu J, Zhang Y, Jia R, et al. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin Cancer Res. January 15 2019;25(2):515–523. [DOI] [PubMed] [Google Scholar]
- 66.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. The New England journal of medicine. February 16 2019. [DOI] [PubMed] [Google Scholar]
- 67.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. The New England journal of medicine. February 16 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Forde PM et al. (2018) Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N. Engl. J. Med 378, 1976–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amaria RN et al. (2018) Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma, Nat. Med 24, 1649–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blank CU et al. (2018) Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med 24, 1655–1661 [DOI] [PubMed] [Google Scholar]
- 71.Huang AC et al. (2019) A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat. Med 25, 454–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schalper KA et al. (2019) Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat. Med 25, 470–476 [DOI] [PubMed] [Google Scholar]
- 73.Cloughesy TF et al. (2019) Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med 25, 477–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown SB et al. (2004) The present and future role of photodynamic therapy in cancer treatment. Lancet 5, 497–508 [DOI] [PubMed] [Google Scholar]
- 75.Schmittnaegel M and De Palma M (2017) Reprogramming tumor blood vessels for enhancing immunotherapy. Trend Cancer 3, 809–812 [DOI] [PubMed] [Google Scholar]
- 76.Hodi SF et al. (2018) Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 19, 1480–1492 [DOI] [PubMed] [Google Scholar]
- 77.Wolchok JD et al. (2017) verall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 377, 1345–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]


