Abstract
Objective
Coronavirus Disease 2019 (COVID-19) initially thought to be confined to the respiratory system only, is now known to be a multisystem disease. It is critical to be aware of and timely recognize neurological and neuroradiological manifestations affecting patients with COVID-19, to minimize morbidity and mortality of affected patients.
Methods
We performed a retrospective chart review of patients admitted to our Level 1 trauma and stroke center during the peak of the COVID-19 outbreak in New York from March 1st to May 30, 2020, with a positive test for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) who presented mainly with neurological findings and had acute radiological brain changes on Computed Tomography (CT) scan. Patients with known chronic neurological disease processes were excluded from the study. We obtained and reviewed demographics, complete blood count, metabolic panel, D-dimer, inflammatory markers such as erythrocyte sedimentation rate (ESR), C reactive protein (CRP), imaging, and patient’s hospital course. We reviewed the current literature on neuroimaging, pathophysiology, and their clinical correlations on COVID-19. This case series study was approved by our institutional review board.
Result
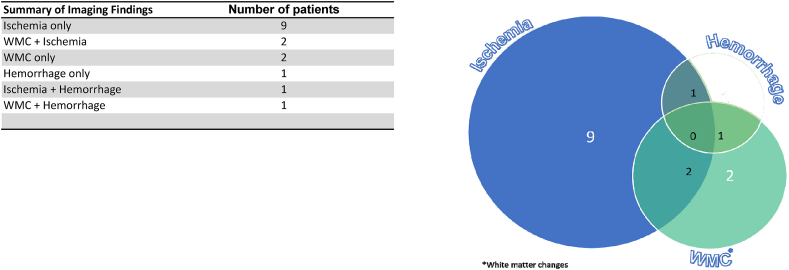
A total of 16 patients were selected for our case series. The most common neuroimaging features on CT, were territorial to multifocal ischemic infarcts, followed by a combination of ischemia and acute white matter encephalopathic changes, followed by temporal lobe predominant focal or more generalized encephalopathy with both confluent and non-confluent patterns, isolated cortical or more extensive intracranial hemorrhages and some combination of ischemia or hemorrhage and white matter changes. All our patients had severe acute respiratory distress syndrome (ARDS), most of them had elevated inflammatory markers, and D dimer.
Conclusion
COVID-19 infection is a multi-organ disease, which can manifest as rapidly progressive neurological disease beyond the more common pulmonary presentation. Early recognition of various neurological findings and neuroimaging patterns in these patients will enable timely diagnosis and rapid treatment to reduce morbidity and mortality. Our retrospective study is limited due to small non-representative sample size, strict selection criteria likely underestimating the true extent of neurological manifestations of COVID-19, mono-modality imaging technique limited to predominantly CT scans and lack of CSF analysis in all except one patient. Multi-institutional, multi-modality, largescale studies are needed with radio-pathological correlation to better understand the complete spectrum of neurologic presentations in COVID-19 patients and study the causal relationship between SARS-CoV-2 and CNS disease process.
Keywords: COVID-19, Severe Acute Respiratory Syndrome Coronavirus 2, Nervous System, Stroke, Encephalopathy, Computed tomography (CT)
Highlights
-
•
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-COV-2) is the causative virus for Coronavirus Disease 2019 (COVID-19).
-
•
COVID-19 most commonly affects the respiratory system but is now known to be a multisystem disease.
-
•
SARS-COV-2 has neurotropic/neuroinvasive abilities and may present with a multitude of neurological symptoms.
-
•
CT Brain patterns include ischemic infarcts, encephalopathy, hemorrhages isolated or in some combination.
-
•
Encephalopathy always involved at least one temporal lobe.
-
•
Larger studies are necessary to understand the complete spectrum of neurologic presentations in COVID-19 patients.
1. Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is a novel single-strand positive sense enveloped RNA virus which belongs to the family Coronaviridae, the causative agent for Coronavirus Disease 2019 (COVID-19). The earliest reported case of this infection was in December 2019 in the city of Wuhan, China. Shortly thereafter, the disease rapidly spread all over the world. World health organization (WHO) declared it a pandemic on March 11th, 2020 (Mousavizadeh and Ghasemi, 2020).
COVID-19 initially thought to affect only the respiratory system is now known to be a multisystem disease. Symptoms range from mild features such as fever, headache, myalgia, fatigue, cough, and shortness of breath to life-threatening complications such as acute respiratory distress syndrome, acute cardiac injury, and septic shock (Huang et al., 2019), as well as neurological symptoms from headaches to altered mental status, seizure, focal neurological deficits, neuropathy including hypogeusia and hyposmia as well as in some cases Guillain Barre syndrome (GBS) like ascending weakness (Filatov et al., 2020).
These can be better classified into broad clinical neurological syndromes inclusive of cerebrovascular event (ischemic/hemorrhagic/thrombotic event), encephalopathies (acute alteration in behavior, cognition or mental status), inflammatory CNS syndromes (encephalitis, acute disseminating encephalomyelitis, ADEM) and peripheral neuropathy (GBS like presentation) (Paterson et al., 2020). Common neuroimaging manifestations reported, include ischemic and hemorrhagic strokes, inflammatory syndromes as well as encephalitis, demyelinating disease including acute hemorrhagic leukoencephalopathy (AHLE), and postinfectious conditions (post-viral autoimmune disorders including Guillen barre syndrome) (Paterson et al., 2020; Fokke et al., 2014; Montalvan et al., 2020). Although, most early research has focused on the typical respiratory presentation of COVID-19, a thorough in-depth analysis of critical neurologic manifestations as well as the neuroradiological and pathological findings and their correlation is still to be fully explored.
2. Methods
Our community hospital from March to May 2020 had a total of 1084 COVID-19 patients. In a retrospective observational case series, we identified a total of 16 patients with a SARS-CoV-2 positive test, who presented mainly with neurological findings and had proven radiological brain changes on Computed Tomography (CT). Neurologic symptoms and signs such as a change in mental status, motor or sensory deficits, seizure on admission were used to enroll the patients. The confirmation test was considered a positive reverse transcriptase polymerase chain reaction (rt-PCR) from a nasopharyngeal swab specimen, run by the Department of Health of New York City. We retrospectively obtained and reviewed demographics, complete blood count, metabolic panel, kidney and liver function, D-dimer, including inflammatory markers such as erythrocyte sedimentation rate (ESR), C reactive protein (CRP), imaging, and patient’s hospital course. Each case was reviewed by an experienced Neuroradiologist and patients who had a clear etiology of their neurologic deficits were excluded from the study. Our Institutional Review Board approved our study per our local policy.
3. Case presentations
3.1. Case 1: Isolated hemorrhage with non-confluent temporal lobe predominant white matter changes
A 78-year-old African American female presented with generalized weakness, poor appetite, and abdominal cramps. Physical exam showed an ill-appearing elderly lady, afebrile, vitally stable with scattered wheezing. Initial workup (Table 3 Supplement) showed elevated inflammatory markers with elevated troponins and Brain Natriuretic Peptide (BNP). The patient was started on dual antiplatelet therapy and anticoagulation for acute coronary syndrome. Empiric COVID-19 cocktail included ceftriaxone, azithromycin, and hydroxychloroquine for 5 days. During the hospital course her mental status progressively deteriorated and she also developed respiratory symptoms. On day 12th she was intubated due to hypoxic, hypercapnic respiratory failure with worsening infiltrates on chest imaging. During spontaneous awakening trials, the patient’s mental status was noted to be poor with a Glasgow Coma Scale (GCS) of 6 (open eyes to verbal stimuli, flexion response to pain, and no verbal response), despite the improvement on mechanical ventilation and decreased lung infiltrates on imaging. Initial head CT revealed cortical/subcortical isolated white matter hemorrhages involving the right parasagittal centrum semiovale (Fig. 3A). A follow-up head CT four days later showed extensive confluent and non-confluent deep white matter hypodensities with asymmetric involvement of the right temporal lobe more than the left, with development of new left cerebellar hemorrhage (Fig. 3B and C) suggestive of acute disseminated hemorrhagic leukoencephalopathy (ADHL).
Fig. 3.
A-C CT Pattern 3 depicts white matter changes only. Temporal lobe predominant confluent and more diffuse nonconfluent acute encephalopathy. Initial noncontrast head CT scan (A) axial image shows no significant white matter changes. Images B and C from non contrast CT done 4 days after the initial scan demonstrates remarkable interval development of both confluent and non confluent white matter hypodensities. Note the prominent asymmetric white matter changes involving the right temporal lobe (C).Findings are suggestive of COVID 19 virus related rapidly developing acute disseminated encephalomyelitis (ADEM).
The hospital course further deteriorated with development of acute kidney failure, septic shock secondary to urosepsis, and new pulmonary infiltrates growing methicillin-sensitive staphylococcus aureus. At this time, considering multiple organ failure and poor prognosis, the family decided to pursue comfort care only and palliative extubation. The patient passed away after 50 days of hospitalization.
3.2. Case 2: Guillain- Barre syndrome like features, multifocal supratentorial infarcts, temporal lobe predominant white matter changes
This is a 42-year-old African American male who presented with nonspecific gastrointestinal and neurological symptoms of nausea, non-bilious non-bloody vomiting, non-radiating burning epigastric pain, body aches, generalized weakness, and fever, progressing over the course of 2 weeks. Symptoms started after the patient was admitted into a rehabilitation facility for alcohol use disorder, with a possible COVID 19 sick contact. On physical examination, the patient was in distress, agitated, and tachypneic but otherwise vitally stable with an unremarkable neurologic exam. Initial work-up was remarkable only for lymphopenia with a negative COVID 19 point of care antigen test (quick Abbott BinaxNOW test) and normal Chest X-ray (CXR). The following day, the patient developed lower extremity weakness and gait instability with a witnessed fall. On physical exam, he had decreased strength of bilateral lower extremities 3/5 and absent deep tendon reflexes. Considering the lingering of the symptoms for 2 weeks and new findings on physical exam, Guillain Barre Syndrome was suspected, and Neurology was consulted. The patient underwent a lumbar puncture with cerebrospinal fluid studies showing normal protein and glucose level without pleocytosis. Initial head CT and Magnetic Resonance Imaging (MRI) were negative. At this point, the patient started to develop respiratory symptoms manifested by dyspnea, cough and was found to be hypoxic. Follow-up chest radiographs showed development of bilateral mixed ground-glass infiltrates, raising suspicion of COVID 19. An rt-PCR test was now positive with elevated inflammatory markers as depicted in Table 3 Supplement. Despite non-invasive O2 supplementation, the patient continued to be hypoxic, requiring intubation and transfer to the intensive care unit. The course was complicated by cardiac arrest, managed as per the Advanced Cardiovascular Life Support (ACLS) protocol with the return of spontaneous circulation in 20 min. EKG and troponins were negative. Bedside echocardiogram showed diffuse left ventricular wall hypokinesis. Hypothermia protocol was not started as the patient was vitally unstable. 24 h later, the patient had sluggish pupillary reflex with minimal corneal and gag reflexes suggesting brain stem damage. Follow-up repeat head CT done 9 days after the initial exam showed multiple infarcts throughout the supratentorial brain with focal isolated white matter encephalopathy at the left temporal lobe (Fig. 2). He continued to be on vasopressor, broad-spectrum antibiotics, and anticoagulation. On day 14th, the patient had a second cardiac arrest secondary to pulmonary embolism while in DNR status and passed away.
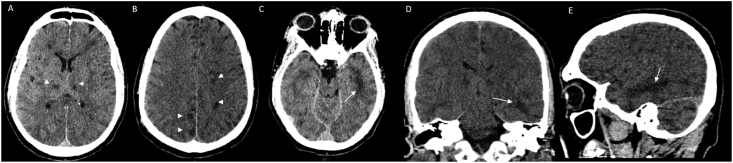
Fig. 2.
A-E CT Pattern 2 depicts ischemia with Temporal Lobe Predominant Encephalopathic Changes. Noncontrast Head CT obtained 9 days after an initial normal head CT and normal brain MRI showed bilateral thalamic and scattered subcortical white matter hypodensities (arrowhead) as well as nonconfluent scattered subcortical and patchy white matter hypodensities with asymmetric involvement of the left temporal lobe (arrows). Findings likely represent developing viral leukoencephalopathy reflective of the neurotropism of COVID 19 and mulifocal thromboembolic infarcts in a COVID 19 positive patient with GBS like ascending weakness.
3.3. Case 3: Large acute left middle cerebral artery (MCA) territorial ischemic infarct
This is a 77-year-old African American male with progressively worsening shortness of breath who was admitted for COVID-19 pneumonia. Chest radiograph showed extensive bilateral pulmonary consolidations and laboratory studies showed elevated inflammatory markers with elevated d dimer. The patient was started on anticoagulation, azithromycin, ceftriaxone, and hydroxychloroquine as part of the empiric treatment for COVID-19. The next day, the patient was found to have right hemiparesis which prompted a CT Stroke code. Non-contrast head CT demonstrated early ischemic changes in the left centrum semiovale and corona radiata. The CT angiogram of the head showed a patent left middle cerebral artery. However, a follow up CT a few days later showed progression to a large full blown left middle cerebral artery territory ischemic infarct. In the upcoming days, the patient became vitally unstable, secondary to worsening pneumonia, complicated by septic shock and cardiorespiratory arrest. The patient passed away on day 7th of hospitalization.
3.4. Case 4: Multifocal ischemic progressing to hemorrhagic infarcts
This is a 64-year-old African American male who presented to our facility for altered mental status and witnessed seizure at home. The patient had fever, headaches, generalized malaise and decreased appetite for the past 10 days. He was diagnosed with COVID-19 infection in another facility and was discharged home with supportive treatment. On presentation, the patient was obtunded, tachypneic and tachycardic. Work up showed bilateral patchy pulmonary infiltrates and elevated inflammatory markers including elevated d dimer. The lumbar puncture was negative. He was started on empiric antibiotics and anticoagulation. Initial head CT showed multiple hypodensities bilaterally in the supratentorial brain suggestive of multifocal multi-territorial acute ischemic infarcts. Follow up head CT scan on day 5th showed further progression into hemorrhagic and non-hemorrhagic infarcts involving both the supratentorial and infratentorial brain with involvement of the bilateral cerebellum. There were hemorrhagic infarcts in the right frontoparietal temporal lobes as well as in the left frontal lobe with ischemic infarcts involving the bilateral cerebellar hemispheres [Fig. 5 (A-C)]. He was transferred to the intensive care unit and anticoagulation was discontinued. His medical conditions further deteriorated, requiring mechanical ventilation, and needed multiple blood transfusions for gastrointestinal bleeding. The patient passed away on day 11th.
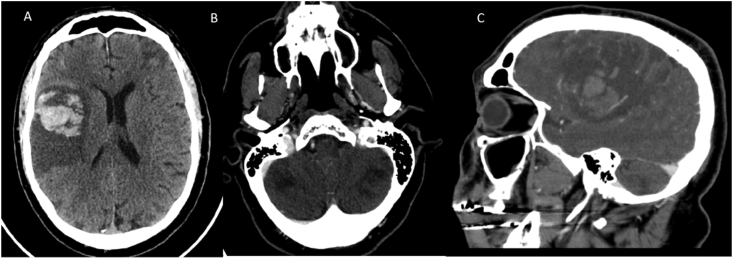
Fig. 5.
A–C CT pattern 5: Ischemia and Hemorrhage. Multiple hemorrhagic and non-hemorrhagic infarcts in varying vascular territories were identified involving both in the supratentorial and infratentorial brain with scattered subarachnoid hemorrhage in the right frontal lobe. A & B show hemorrhagic infarcts in the right frontoparietal temporal lobes in the MCA distribution, left frontal lobe in the left cortical (external) ACA/MCA border zone watershed territory and bilateral cerebellar infarcts in the vertebrobasilar distribution. CTA of the head was negative (C).
3.5. Case 5: Large acute right MCA ischemic infarct with occluded right MCA and right internal carotid artery (ICA)
A 64-year-old Hispanic female presented with right sided headache and neck pain, as well as acute left sided weakness. She has a past medical history of cerebrovascular accident, atrial fibrillation (not on anticoagulation), hypertension and diabetes mellitus. Initial head CT showed a large right middle cerebral territory ischemic infarct with occluded right internal carotid artery and middle cerebral artery on Computed Tomography Angiography (CTA). There was extensive edema with acute right sided uncal herniation. Her laboratory values showed increased inflammatory markers such as ferritin and Lactate dehydrogenase (LDH). Patient had a cardiac arrest in the emergency room with elevated cardiac troponins. Patient also had an atrial fibrillation with rapid ventricular response, treated with diltiazem drip. She was not a candidate for targeted temperature management because of hemodynamic instability and multiple comorbidities in the light of new large vessel stroke with herniation. The patient passed away later that same day.
3.6. Case 6: Left parietooccipital lobar ischemic infarct
This is a 32-year-old African American female with past medical history of CVA with right hemiparesis, diabetes mellitus and peripheric vascular disease presented with worsening shortness of breath on exertion, abdominal pain and decrease sensation on the right side of the body. She was found to have bilateral patchy opacity on chest X-ray, new onset heart failure with decrease ejection fraction and positive for SARS-CoV-2. CT brain showed subacute left parieto-occipital infarct, and encephalomalacia in the frontoparietal areas, outside of the treatment window. Elevated levels of ferritin, LDH, ESR, and D-dimer were noted. She was started on antibiotics, anticoagulants, and pain medications. The hospital course was complicated with an acute embolic arterial occlusion of the right leg, despite being on anticoagulation. She was seen by vascular surgery, deemed to be non-salvageable, underwent amputation. She was discharged in a rehab facility.
3.7. Case 7: Multifocal supratentorial and infratentorial ischemic infarcts with anoxic encephalopathy
A 68-year-old African American female with multiple comorbidities presented with 6 days of cough, malaise, and shortness of breath at rest and was found to be in acute hypoxic respiratory failure secondary to COVID-19. Her oxygen saturation was 78% despite being on non-rebreather and required immediate intubation. Her hospital course was complicated by acute coronary syndrome (NSTEMI) for which she was placed on dual antiplatelet therapy and anticoagulation. Despite some improvement on her respiratory status, patient was minimally responsive during her daily sedation vacations. On day 14 of hospitalization, she was noted to have fixed and dilated pupils and underwent an emergent CT Brain which showed multiple bilateral infarcts involving both the supratentorial, and infratentorial brain with superimposed diffuse cerebral edema, left to right midline shift, trans-tentorial uncal herniation and secondary hypoxic ischemic encephalopathy (Fig. 1). CTA brain showed severe cerebral edema with midline shift and decreased flow. She passed away on day 23 of hospitalization.
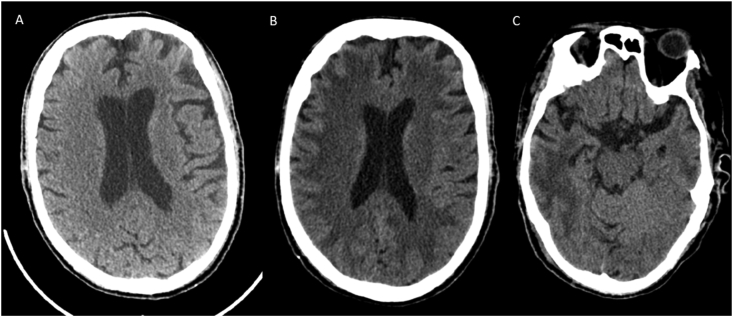
Fig. 1.
A-C CT Pattern 1 depicts multifocal ischemic infarcts. Multiple images from a non contrast CT in a patient with COVID 19 post cardiac arrest demonstrates multiterritorial ischemic infarcts both bilaterally involving the supratentorial and infratentorial compartments. There is diffuse cerebral edema with loss of sulcal pattern diffusely with effaced basilar cisterns suggestive of bilateral uncal and transtentorial herniation.
3.8. Case 8: Acute large left MCA territory ischemic infarct
74-years-old African American female who presented complaining of intermittent, progressively worsening shortness of breath, that started 3 weeks ago, associated with productive cough, malaise, and chills. Her past medical history was significant for hypertension, hyperlipidemia, moderate persistent asthma, on inhalers for which she showed to be compliant. She was found to have COVID-19 pneumonia. On day 8 of hospitalization, she developed weakness involving the right side of the body and slurred speech. Stroke alert was called, and CT head showed hyperdense left MCA suggestive of intravascular thrombosis with no early signs of infarct. She was emergently transferred to an advanced comprehensive stroke center with active thrombectomy capabilities for an urgent intra-arterial thrombolysis.
3.9. Case 9: Acute large left MCA territorial ischemic infarct with unilateral left medial temporal lobe white matter encephalopathy
A 53-year-old Caucasian male presented with cough, shortness of breath, fever, chills, malaise and myalgias for 1 week. His past medical history was significant for diabetes, hypertension, and hyperlipidemia. He was found to haveCOVID-19 pneumonia and acute renal failure. On day 2, he woke in the morning with new right sided motor deficits. CT brain showed hyperdense left MCA with early developing infarcts with hypodensity in the left medial temporal and occipital lobes. High oxygen requirement precluded the option of thrombectomy. Therefore, he was managed medically with aspirin, statins, and enoxaparin on therapeutic dose. The patient was discharged on day 10 with home physical therapy.
3.10. Case 10: Left frontal ischemic infarct
A 72-year-old male presented as a STROKE alert with right sided weakness and altered mental status. Immediate CT stroke showed old left occipital lobar infarct and subacute left frontal lobe infarct. Initial work up showed decreased oxygen saturation, which improved on nasal cannula, Chest X-rays showed extensive ground glass opacities bilaterally. His nasal swab was positive for SARS-CoV-2. Blood chemistry showed elevated inflammatory markers such as ferritin and LDH with elevated creatinine suggestive of acute renal failure. Rest of work up including echocardiography, ultrasound carotid, Holter monitor were unremarkable. Patient was treated conservatively, considering he was out of the window for tissue plasminogen activator (tPA) and he was discharged in a rehab facility after 6 days of hospitalization.
3.11. Case 11: Right centrum semiovale and periventricular corona radiata ischemic infarcts
A 55-year-old African American male with past medical history of hypertension presented with altered mental status, lethargy and hypoxia, and tachypnea requiring intubation for acute respiratory failure. Chest X-ray showed extensive bilateral opacities. He was found to be in acute renal failure and developed septic shock requiring 2 pressors plus a stress dose of steroids, broad spectrum antibiotics. He had elevated d-dimer, liver enzymes, ferritin, IL-6 and ESR. Considering his critical conditions, he was given Tocilizumab under compassionate use treatment. New left lower extremity weakness was found associated with pain and sensory loss. Initial head CT was negative. However, brain MRI on day 15th showed subacute right subcortical centrum semiovale and periventricular corona radiata lacunar infarcts. Patient was discharged after 2 weeks of hospitalization with physical therapy follow up.
3.12. Case 12: Right basal ganglia subacute ischemic infarct
An 80-year-old African American female was found in the bathtub with altered mental status, left facial droop, and slurred speech. She had a past medical history of HTN, DM (diet controlled), CVA and hyperlipidemia. CT head showed extensive white matter microangiopathic ischemic changes suggestive of encephalopathy, age indeterminate right basal ganglia infarcts, multiple old lacunar infarcts in the left basal ganglia and bilateral cerebellar hemispheres. Patient was found to have SARS-CoV-2 test positive with elevated LDH, ferritin, ESR, CRP and creatinine. Hospital course was complicated with acute respiratory failure secondary to pneumonia requiring mechanical ventilation, and acute kidney failure. Unfortunately, despite the efforts, the patient expired on the 16th day of admission.
3.13. Case 13: Acute bilateral cerebellar ischemic infarcts
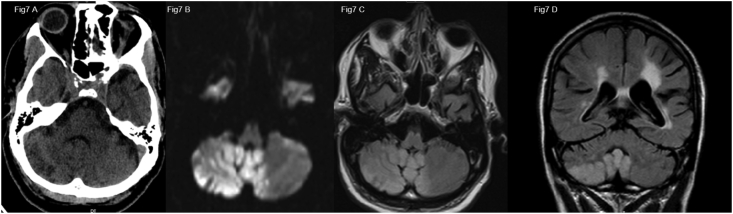
This is a 61-year-old African American male with past medical history of liver cirrhosis, diabetes and hypertension was brought in emergency department after being found unresponsive on the floor by his family. On initial evaluation he was in hypertensive crises with a blood pressure of >200 mmHg and encephalopathic, unable to follow commands, requiring emergent intubation due to low GCS. CT head showed old hemorrhagic infarcts in the left basal ganglia and corona radiata along with extensive acute ischemic infarcts in the bilateral cerebellar hemispheres and vermis as well as dorsal pons which were confirmed on MRI (Fig. 7). Complete blood count, basic metabolic panel, PT/PTT and age adjusted D-dimer were normal Electrocardiogram and echocardiogram were normal. He was found to have a positive. Patient was treated with supportive care, blood pressure control, and discharged after 2 weeks of hospitalization in a rehab facility.
Fig. 7.
A) Axial image from non-contrast head CT in a COVID-19 positive patient showing vague right cerebellar hypodensities confirmed to be acute ischemic infarcts with diffusion restriction (B) and increased T2 FLAIR signal (C, D).
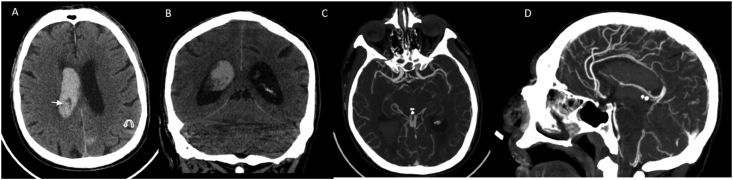
3.14. Case 14: Acute spontaneous intraventricular and subarachnoid hemorrhages
This is a 71-year-old Caucasian male with multiple comorbidities presented with altered mental status, Glasgow coma scale of 11. He was diagnosed with COVID 19 one week prior and discharged home with supportive treatment without any anticoagulation since his d dimer was <1. Considering his acute change in mental status a CT brain was ordered, showing spontaneous acute diffuse intraventricular hemorrhage and subarachnoid hemorrhages. CTA head showed no vascular abnormalities (Fig. 4). The rest of the workup were positive for SARS-CoV-2 rt-PCR positive, as well as antibodies and elevated inflammatory markers with a normal PT/PTT. Repeat CT brain showed stable but persistent intracranial bleeding. No history or signs of fall or head trauma were present or reported by family. Patient passed away after one week of hospitalization.
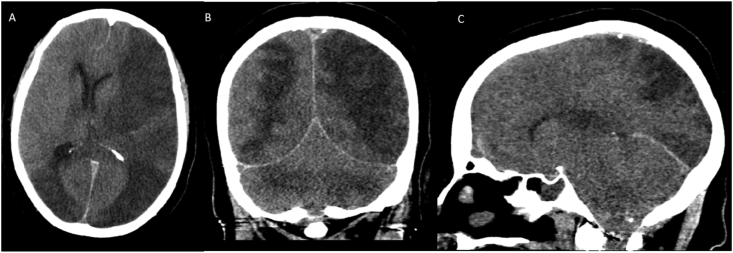
Fig. 4.
CT Pattern 4: Intracranial hemorrhage only. Noncontrast CT scan images (A,B) demonstrate extensive acute intraventricular hemorrhage (straight arrow) with mild ventricular prominence. Curvilinear acute subarachnoid hemorrhages (curved arrow) are also seen along the left parietal lobe. CTA (C,D) of the head is negative.
3.15. Cases 15 and 16: Encephalopathy patients
Two patients, a 68-year-old African American female and a 90-year-old Caucasian female, presented initially with altered mental status, with normal vitals and negative physical exam. On initial work up they tested positive for Sars-CoV-2 and had elevated inflammatory markers (Table 3 Supplement). A non-contrast CT showed confluent and non-confluent temporal lobe predominant white matter changes without hemorrhage suggestive of encephalopathy. They developed respiratory symptoms during their hospital stay, requiring oxygen supplementation but recovered completely after 11 and 8 days of hospitalization.
4. Discussion
In this article, we present a retrospective case series of sixteen cases of COVID-19 patients admitted to our Level 1 stroke center in Bronx, New York with distinct neurological symptoms during the peak of the first pandemic wave in New York. The study was conducted in the south Bronx community hospital primarily serving low socioeconomic community which includes predominantly African American and Latino population.
SARS-CoV-2 infected patients most frequently exhibited acute respiratory symptoms, but some patients presented with acute neurologic symptoms at the time of admission or during their hospital stay. They underwent further studies including brain imaging, with CT brain being the first and most of the times the only neuro-diagnostic technique.
Common neurological presentations in our case series, besides obvious hemiparesis/hemiplegia or stroke-like features, included altered mental status, neuropathy, and seizures. Most of these patients were found to have ischemic strokes with different infarct patterns, from single vascular territory to multiple embolic type infarcts diffusely followed by white matter encephalopathic changes with both confluent as well as non-confluent pattern with most of them involving medial temporal lobes, isolated hemorrhages and the remaining with both white matter changes and ischemia or hemorrhage (Table 1, Table 2). None of 16 patients in our case series had positive standard diagnostic cardiac and vascular work up for atherosclerotic disease or cardiogenic embolism to account for new onset CNS symptomatology.
Table 1.
CT imaging patterns seen in patients with COVID-19.
| Ischemic Stroke Territorial |
|---|
| Ischemic Stroke Multifocal |
| Non confluent (temporal lobe predominant) white matter hypodenisities±ischemia |
| Confluent and non confluent (temporal lobe predominant) white matter hypodenisities±ischemia |
| Isolated or non confluent temporal lobe predominant white matter hypodensities only |
| Confluent and non confluent (temporal lobe) white matter hypodensities only rapidly progressive (ADEM) |
| Isolated intracranial intraventricular hemorrhage |
| Multifocal infarcts with isolated cortical hemorrhages |
| Hemorrhagic territorial infarcts with isolated hemorrhages |
| Non confluent (temporal lobe predominant) white matter hypodenisities±microhemorrhage |
| Confluent and non confluent white matter hypodensities±microhemorrhage (Acute hemorrhagic leukoencephalopathy) |
| Multifocal ischemic infarcts and anoxic encephalopathy |
Table 2.
Summary of Imaging Findings and Pie chart representation.
The reported incidence of cerebrovascular accidents is variable ranging from around 0.9%–8.0% (Yaghi et al., 2020; Mao et al., 2019; Radmanesh et al., 2020). In a study conducted in New York city by Yaghi et al. on the etiology of the stroke, 65.6% of the patient had cryptogenic stroke compared to 34.4% who had embolic stroke. In another study, the main neuroimaging hallmark was acute ischemic infarct in 31%, mostly large and small vessel disease with only 3% of cardioembolic and 6% intracranial hemorrhages (Radmanesh et al., 2020). These data are markedly variable across the world between different countries, where studies in the Chinese population have reported higher incidence. The latter can be dependent on differences between various populations, criteria used for requesting brain imaging, and the number of critically ill patients who were able to undergo imaging. The etiology of acute cerebrovascular accidents in COVID-19, probably is multifactorial, with preexisting conditions, like hypertension, diabetes, cardiovascular disease, and their severity of disease playing the main part. The exact role of SARS-CoV-2 remains unclear, but it has been documented in autopsy reports that COVID-19 is associated with an increased incidence of thromboembolic events (Wichmann et al., 2020).
Some isolated patients had more unique neurological presentation ranging from rapid development of inflammatory CNS syndrome with acute disseminating encephalomyelitis (ADEM) showing progressive white matter changes with worsening Glasgow coma scale over a matter of days, to development of confluent and nonconfluent white matter encephalopathic changes, to involvement of the peripheral nervous system presenting with GBS like clinical features, to development of spontaneous intraventricular and subarachnoid hemorrhages in the absence of other factors to justify such changes.
One patient presented with progressively worsening mental status with later development of episodes of seizures. He was found to have rapidly progressive deep white matter changes in a matter of days with interspersed trace hemorrhages, compatible with acute hemorrhagic leukoencephalitis (AHL). The hemorrhagic changes were presumed to be the cause of the convulsions noted in this patient, due to the irritant effect of the blood.
Patient in case 2 who developed acute progressive symmetric ascending weakness and areflexia of lower extremities, which was suggestive of GBS had a normal CSF study without pleocytosis or cyto-albuminogenic dissociation. However, these findings are positive only in 64% of the GBS cases at the time of initial presentation. Patient was scheduled for electromyography study which, was not obtained due to the clinical deterioration of the patient. Case series reported from 13 European countries showed 42 cases of GBS in the first six months of the COVID-19 pandemic (Uncini et al., 2020).
There was one patient who presented with spontaneous intraventricular and subarachnoid hemorrhage with normal coagulation profile, platelets and no history of trauma or fall, arterial venous malformation, or aneurysm. The etiology remains unclear. The incidence of ICH reported so far is around 0.7%, where most of these patients had multiple risk factors, preexisting conditions, or being on anticoagulation. In a literature review about intracranial hemorrhage in COVID-19 patients, published by Cheruiyot et al. intraparenchymal hemorrhage was the most common variety (62.6%), followed by subarachnoid hemorrhage (15.0%), subdural hemorrhage (11.6%), and intraventricular hemorrhage (1.4%) (Cheruiyot et al., 2021).
A multitude of previous studies on the Corona-virus family and now SARS CoV-2 have suggested their neurotropic and neuro-invasive capabilities (Fokke et al., 2014; Montalvan et al., 2020). Although still under investigation, there are several different mechanisms proposed by which the virus can enter the central nervous system (CNS). SARS-CoV-2 can affect the CNS through direct routes—hematogenous and trans-neuronal pathways and by indirect mechanisms which include cytokine dysregulation, peripheral immune cell transmigration, neuroinflammation, postinfectious autoimmunity and, hypercoagulability.
The first one is hematogenous, through a direct invasion of capillary endothelium binding to ACE2 receptors and disrupting the blood-brain-barrier, further supported by recent electron microscopic studies that have demonstrated the presence of viral inclusion particles within the endothelium, and viral RNA detection in cerebrospinal fluid (Cuervo and Grandvaux, 2020; Zhou et al., 2020; Paniz-Mondolfi et al., 2020). The role of co-receptors, such as NRP1, HS, or sialic acids, and a two-step attachment mechanism should be further addressed in the future.
The second suggested mechanism is via retrograde axonal transport, using several cranial nerves, mostly cranial nerve I (Olfactory) and V (Trigeminal). The retrograde dissemination is reinforced from studies done on SARS COV and MERS COV where the virus was given intranasally and shown to invade the brain through the olfactory nerves with rapid spread to other intracranial structures such as thalamus and brainstem (Katal et al., 2020; von Weyhern et al., 2020).
The proposed indirect pathway can be through infection of the leukocytes which can, in turn, reach the CNS directly through the bloodstream. This has been widely explored in HIV infections where the virus accumulates in the monocytes, which then can traverse the brain blood barrier. Similar characteristics have been described in coronavirus infection like HCoV-229E which can constitute a reservoir in leukocytes and use them as a spread vector.
It has also been reported that COVID-19 is associated with a hyperimmune inflammatory response which incites activation of the coagulation cascade with release of cytokines and chemokines causing vasodilation, edema, and endothelial injury. Notably, a hypercoagulation state is known to be present due to elevated factor VIII, elevated fibrinogen, and hyperviscosity (Maier et al., 2020). This is called COVID-associated coagulopathy (CAC) or thromboinflammation (Connors and Levy, 2020). This is manifested also on real-time vascular sonogram as intravascular hyper viscosity with vein compressibility, ruling out intravascular clot or thrombus (Dugar et al., 2020).
Some of the above mechanisms are being validated by pathology studies. Von Weyhern CH et al. noted diffuse petechial hemorrhage affecting the entire brain, most commonly in younger patients. Considering the hypothesis of cranial nerve involvement, their evaluation of the brainstem showed perivascular and interstitial encephalitis with cell damage and axon degeneration.
Reichard et al. in their autopsy studies reported extensive white matter involvement from hemorrhagic lesions to perivascular acute disseminated encephalomyelitis (ADEM)-like appearance with microscopic areas of ischemia/necrosis of white matter, neocortex, and axonal injury [ (Reichard et al., 2020). They hypothesized that white matter lesions representing axonal injury can be vascular in origin. Paterson et al. reported a case with acute hemorrhagic leucoencephalitis confirmed by a brain biopsy at the time, which showed evidence of perivenular inflammation supporting aggressive hyper-acute ADEM.
Hence, the possible mechanisms of neurological manifestations might be a synergistic combination of multiple factors including cardiocirculatory, direct viral damage, and immune system activation (Reichard et al., 2020). Literature review suggests possible long term neurologic and immunologic complications, in particular, the development of various neurodegenerative diseases for which further studies are recommended.
At many institutions, due to the rapid deterioration of these patients and logistics involved around safely performing MRI in all patients due to COVID 19, CT remained the main workhorse of the diagnostic tools during the pandemic. Specific neuroimaging imaging findings on non-contrast head CT scans of COVID-19 patients in our case series are summarized in Table 3 Supplement. Key imaging findings included territorial or multifocal predominantly ischemic infarcts, non-confluent white matter hypodensities with temporal lobe predominance with or without microhemorrhages, isolated intracranial microhemorrhages which may be as subtle as isolated cortical/subcortical microhemorrhages to macrohemorrhages, acute necrotizing hemorrhagic encephalopathy; spontaneous intraventricular and subarachnoid hemorrhage and hemorrhagic infarcts.
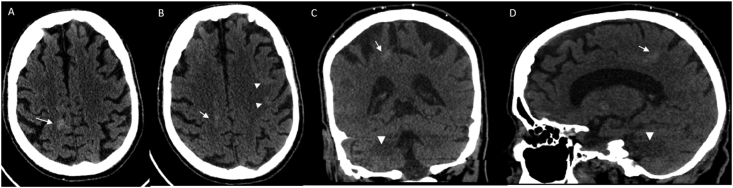
The frequency of various imaging patterns are summarized in Table 2 and accompanied pie chart, showing isolated acute ischemic changes only (56%), shown in Fig. 1 (A-C), pattern 1, combination of both ischemia and non-confluent white matter changes with all of them involving at least unilateral temporal lobe (12.5%) Fig. 2 (A-D), pattern 2, only white matter changes (12.5%) Fig. 3 (A-C), pattern 3, followed by intracranial hemorrhage only (6%), Fig. 4 (A-D), pattern 4, combination of ischemia and hemorrhage (6%), Fig. 5 (A-C), pattern 5 and combination of white matter encephalopathy and hemorrhage (6%) Fig. 6 (A-D), pattern 6. Overall white matter encephalopathic changes seen in 37.5% of the cases in some combination. Territorial ischemic infarcts (MCA and/or ICA occlusions) were as common as multifocal infarcts. Hemorrhage alone or with hemorrhagic infarcts were seen in 6.25%.
Fig. 6.
CT Pattern 6: White matter changes and Hemorrhage. Initial non contrast head CT axial (A), Coronal (B) and Sagittal (C) shows isolated right centrum semiovale hemorrhage (arrows) as well as cortical hyperdensities involving the left frontal centrum semiovale (arrowhead B) suggestive of acute cortical/subcortical hemorrhages. Asymmetrical right temporal lobe white matter encephalopathic changes are seen (arrowhead in C and D).
There are several limitations to this study. First, this study is limited due to its retrospective model, small number of patients, and single institution location. It is possible due to strict inclusion criteria of the patients, early minor neurological symptoms may have been overshadowed by other more life-threatening symptoms such as of the respiratory, cardiac, or vascular systems, so the true prevalence of neurological manifestations with COVID 19 is likely underestimated. Also, our serving community is mostly composed of African American population, who are shown to have a significantly higher mortality rate, not fully explained by other cofactors as age, comorbidities, and or social demographics disparity (Golestaneh et al., 2020). This will need to be addressed in the future with larger studies, with a multiethnic population. Second, most of our radiology exams were limited to progressive CT scans only rather than MRI which limits conclusive assessment. MRI is much more sensitive than CT in detecting early ischemic, encephalopathic, and inflammatory changes. Third, our patients had multiple comorbidities and a complicated hospital course. Despite the work up done to eliminate confounders, the complex clinical course and the long ICU stay of COVID-19 patients, a clear cause-effect relationship between SARS-CoV-2 infection and neuroimaging findings is difficult to establish. Fourth, cerebrospinal fluid and EEG studies were not routinely performed, limiting the diagnostic work up.
5. Conclusion
COVID-19 can present with a constellation of various neurological symptoms like the one reported with the previous two SARS and MERS epidemics, in addition to the most common pulmonary complications. Being aware of these neurological findings and key neuroimaging patterns on readily available CT scans will enable early recognition and institution of new preventive as well as treatment strategies. A multi-institutional, multimodality, multiethnic, large scale study with thorough radiopathological correlation will be necessary to better understand the complete spectrum of neurologic presentations in COVID-19 patients to study the direct causal relationship between SARS-CoV-2 and CNS disease process.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2021.100238.
Contributor Information
Razia Rehmani, Email: razia.rehmani@gmail.com.
Scott Segan, Email: scott_segan@sbhny.org.
Srikanth Reddy Maddika, Email: smaddika@sbhny.org.
Yadanar Win Lei, Email: ylei@sbhny.org.
Andrea Broka, Email: andreabroka@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Cheruiyot I., Sehmi P., Ominde B., Bundi P., Mislani M., Ngure B. Intracranial hemorrhage in coronavirus disease 2019 (COVID-19) patients. Neurol. Sci. 2021;42:25–33. doi: 10.1007/s10072-020-04870-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID-19. J. Thromb. Haemostasis. 2020;18(7):1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo N.Z., Grandvaux N. Ace2: evidence of role as entry receptor for sars-cov-2 and implications in comorbidities. Elife. 2020 Oct 1;9:1–25. doi: 10.7554/eLife.61390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugar S., Duggal A., Bassel A., Soliman M., Moghekar A. vol. 46. Springer; 2020. Spontaneous echo contrast in venous ultrasound of severe covid-19 patients.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7251216/ (Intensive Care Medicine). [cited 2020 Nov 12]. p. 1637–9. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov A., Sharma P., Hindi F., Espinosa P.S. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3) doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fokke C., Van Den Berg B., Drenthen J., Walgaard C., Van Doorn P.A., Jacobs B.C. Diagnosis of Guillain-Barré Syndrome and Validation of Brighton Criteria. Brain. 2014 Jan 1;137(1):33–43. doi: 10.1093/brain/awt285. https://academic.oup.com/brain/article/137/1/33/358755 [cited 2020 Aug 27] Available from. [DOI] [PubMed] [Google Scholar]
- Golestaneh L., Neugarten J., Fisher M., Billett H.H., Gil R., Johns T. The Association of Race and COVID-19 mortality-NC-ND License. EClinicalMedicine. 2020;25:100455. doi: 10.1016/j.eclinm.2020.100455. http://creativecommons.org/licenses/by-nc-nd/4.0/ [cited 2021 Feb 14] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. https://isaric.tghn.org/protocols/ [cited 2020 Sep 28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katal S., Balakrishnan S., Gholamrezanezhad A. Journal of Neuroradiology. Elsevier Masson SAS; 2020. Neuroimaging and neurologic findings in COVID-19 and other coronavirus infections: a systematic review in 116 patients [Internet] [cited 2020 Aug 20]. Available from:/pmc/articles/PMC7320684/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier C.L., Truong A.D., Auld S.C., Polly D.M., Tanksley C.L., Duncan A. vol. 395. Lancet Publishing Group; 2020. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? (The Lancet). [cited 2020 Nov 11]. p. 1758–9. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6) doi: 10.1001/jamaneurol.2020.1127. https://jamanetwork.com/ [cited 2020 Aug 27] 683–90. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvan V., Lee J., Bueso T., De Toledo J., Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin. Neurol. Neurosurg. 2020;194 doi: 10.1016/j.clineuro.2020.105921. [cited 2020 Aug 10] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavizadeh L., Ghasemi S. Journal of Microbiology, Immunology and Infection. Elsevier Ltd; 2020. Genotype and phenotype of COVID-19: their roles in pathogenesis [Internet] [cited 2020 Sep 28]. Available from:/pmc/articles/PMC7138183/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniz-Mondolfi A., Bryce C., Grimes Z., Gordon R.E., Reidy J., Lednicky J. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J. Med. Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T. The Emerging Spectrum of COVID-19 Neurology: Clinical, Radiological and Laboratory Findings. Brain. 2020 Jul 8 doi: 10.1093/brain/awaa240. https://academic.oup.com/brain/advance-article/doi/10.1093/brain/awaa240/5868408 [cited 2020 Oct 20]; Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmanesh A., Raz E., Zan E., Derman A., Kaminetzky M. Brain imaging utilization and findings in COVID-19: a single academic center experience in the epicenter of disease in the United States. Am. J. Neuroradiol. 2020 May 1;41(7):1179–1183. doi: 10.3174/ajnr.A6610. [cited 2020 Aug 20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncini A., Vallat J.M., Jacobs B.C. vol. 91. BMJ Publishing Group; 2020. Guillain-Barré syndrome in SARS-CoV-2 infection: an instant systematic review of the first six months of pandemic [Internet]http://jnnp.bmj.com/ (Journal of Neurology, Neurosurgery and Psychiatry). [cited 2020 Nov 16]. p. 1105–10. Available from: [DOI] [PubMed] [Google Scholar]
- von Weyhern C.H., Kaufmann I., Neff F., Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395(10241):e109. doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann D., Sperhake J.-P., Lütgehetmann M., Steurer S., Edler C., Heinemann A. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann. Intern. Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghi S., Ishida K., Torres J., Mac Grory B., Raz E., Humbert K. SARS-CoV-2 and Stroke in a New York Healthcare System. Stroke. 2020 Jul;51(7) doi: 10.1161/STROKEAHA.120.030335. https://www.ahajournals.org/doi/10.1161/STROKEAHA.120.030335 [cited 2020 Aug 27]; 2002–11. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Zhang M., Wang J., Gao J. Sars-Cov-2: underestimated damage to nervous system. Travel. Med. Infect. Dis. 2020;36 doi: 10.1016/j.tmaid.2020.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.