Abstract
A 54-year-old woman underwent chemotherapy including rituximab and autologous peripheral blood stem cell transplantation (auto-PBSCT) for diffuse large B-cell lymphoma. Before the treatment, she exhibited a resolved hepatitis B virus (HBV) infection. She was diagnosed with HBV reactivation based on positive serum HBV-DNA test results, 55 months after her last treatment. Subsequently, he was treated with tenofovir alafenamide fumarate (TAF) therapy and her liver function improved. Patients undergoing chemotherapy including rituximab and auto-PBSCT are at a high risk of HBV reactivation. In such cases, careful and long-term observations may be required to detect HBV reactivation.
Keywords: hepatitis B virus reactivation, de novo hepatitis, rituximab, peripheral blood stem cell transplantation
Introduction
Hepatitis B virus (HBV) reactivation has emerged as a major complication induced by long-term chemotherapy or immunosuppressive therapy (1). De novo hepatitis B, a form of HBV reactivation, can occur as a result of the reactivation of latent HBV infection in subjects who are negative for hepatitis B surface antigen (HBs-Ag), but positive for anti-hepatitis B core antibody (HBc-Ab), anti-HBs antibody (HBs-Ab) or both (2,3). It is well known that patients receiving chemotherapy, including rituximab and hematopoietic stem cell transplantation (HSCT), are at high risk of HBV reactivation (4-6). Among HSCT recipients with resolved HBV, the risk of HBV reactivation is higher with allogeneic HSCT (allo-HSCT) than with autologous HSCT (auto-HSCT) (7). HBV reactivation usually develops a few months after chemotherapy or immunosuppressive therapy. Long-term HBV-DNA monitoring after transplantation is recommended (4), because the time interval between HSCT and HBsAg-positive conversion can be long. However, currently, there is no definitive indication for how long the viral load should be monitored in such cases. We herein present the case of a patient who experienced HBV reactivation 55 months after chemotherapy including rituximab and autologous peripheral blood stem cell transplantation (auto-PBSCT), and de novo hepatitis B improved with tenofovir alafenamide fumarate (TAF) therapy.
Case Report
A 54-year old woman presented to our hospital with a continuous low-grade fever. Her workup revealed stage IVB diffuse large B-cell lymphoma (DLBCL). Her laboratory findings were negative for HBs-Ag and HBV-DNA, but was positive for HBs-Ab and HBc-Ab. She had no family history of HBV infection. She underwent 4 courses of rituximab, cyclophosphamide (CPM), doxorubicin, vincristine (VCR), and prednisolone (R-CHOP) therapy, 2 courses of rituximab, methotrexate, procarbazine, and VCR (R-MPV) therapy, 1 course of rituximab, CPM, doxorubicin, etoposide, vincristine, and prednisolone (R-CHOEP) therapy, and 2 courses of rituximab, CPM, high-dose cytarabine, dexametazone, and etoposide (R-CHASE) therapy. Furthermore, she underwent one course of rituximab, melphalan, CPM, etoposide, and dexametazone (R-LEED) therapy, and auto-PBSCT. She achieved complete remission. During the period of chemotherapy and auto-PBSCT, her serum level of HBV-DNA remained undetectable.
After chemotherapy and auto-PBSCT, she was monitored regularly every 1-3 months and was not receiving immunosuppressive therapy. Her serum HBV-DNA levels became positive 55 months after the last treatment, increasing to 4.1 log IU/mL. HBs-Ag, hepatitis B envelope antigen (HBeAg), and hepatitis B core-related antigen (HBcrAg) also became positive without elevation of serum transaminase. The HBV genotype was group C (Table 1). She had no history of blood transfusion and was sexually inactive. We diagnosed her to have HBV reactivation. Although we recommended that she start nucleotide analogue therapy (NA), she refused and requested a reexamination one month later.
Table 1.
Laboratory Findings at 55 Months Following Chemotherapy and Auto-PBSCT.
| Variable | Variable | |||||
|---|---|---|---|---|---|---|
| White blood cells (/µL) | 4,140 | Total bilirubin (mg/dL) | 0.5 | |||
| Red blood cells (104/µL) | 355 | AST (IU/L) | 21 | |||
| Hematocrit (%) | 33.6 | ALT (IU/L) | 19 | |||
| Hemoglobin (g/dL) | 11.2 | LDH (IU/L) | 154 | |||
| Platelets (104/µL) | 28.0 | ALP (IU/L) | 240 | |||
| PT-INR | 1.01 | GGT (IU/L) | 41 | |||
| PT (%) | 97 | HBsAg (IU/mL) | 4.63 | |||
| Total protein (g/dL) | 6.9 | HBV-DNA (log IU/mL) | 4.1 | |||
| Alubmin (g/dL) | 3.8 | HBeAg | 11.4 | |||
| C-reactive protein (mg/dL) | 1.26 | HBeAb (%) | 0.1 | |||
| BUN (mg/dL) | 14 | HBcrAg (log U/mL) | 5.5 | |||
| Creatinine (mg/dL) | 0.45 | HBV genotype | C |
auto-PBSCT: autologous peripheral blood stem cell transplantation, PT: prothrombin time, PT-INR: prothrombin time-international normalized ratio, BUN: blood urea nitrogen, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, GGT: gamma-glutamyl transpeptidase, HBsAg: hepatitis B surface antigen, HBeAg: hepatitis B envelope antigen, HBeAb: anti-HBe antibody, HBcrAg: hepatitis B core-related antigen
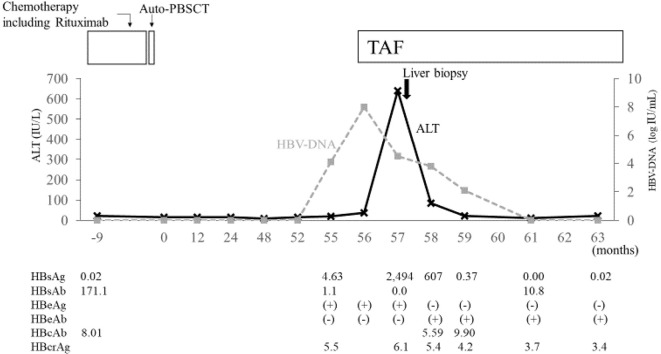
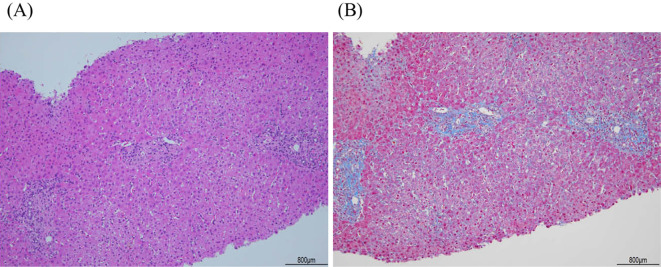
One month later, elevated HBV-DNA (8.0 log IU/mL) and transaminase levels (AST 35 IU/L; ALT 37 IU/L) were found (Fig. 1). She was started on TAF. Her peak transaminase levels were observed 1 month after treatment initiation (AST 445 IU/L; ALT 640 IU/L). A liver biopsy revealed slight cross-linked fibrosis between portal veins and a lymphocyte infiltrate in the portal vein area, with a moderate degree of inflammation (Fig. 2). During the course of TAF therapy, the patient's transaminase and HBV-DNA levels gradually decreased. After five months, HBV-DNA, and HBs-Ag were no longer detectable, while the HBs-Ab became positive. However, HBcrAg remained detectable and this, TAF therapy continued.
Figure 1.
Clinical course of the patient’s condition. Solid thick black arrow indicates the time of liver biopsy. Solid think black arrows indicate the time of chemotherapy including rituximab and auto-PBSCT. auto-PBSCT: autologous peripheral blood stem cell transplantation
Figure 2.
Histological findings of the liver biopsy specimen. (A) Moderate lymphocytic infiltrate is observed in the portal areas of hepatic lobules with slightly piecemeal necrosis (Hematoxylin and Eosin staining; magnification, ×100) (B) Slight portal fibrosis with portal-to-portal bridging. (Masson’s trichrome staining; magnification, ×100).
Discussion
In this case, the patient developed hepatitis due to HBV reactivation 55 months after the end of chemotherapy and auto-PBSCT. HBV reactivation is a common and potentially fatal complication in patients with past HBV infection undergoing immunosuppressive therapy (1-3). Patients receiving chemotherapy, including rituximab and auto-PBSCT, are at a high risk of HBV reactivation (4-6). To prevent HBV reactivation in patients receiving HSCT, the Japan Society of Hepatology (JSH) guidelines recommend that monthly HBV-DNA monitoring be performed during treatment and for at least 12 months after its completion. It also recommends long-term HBV-DNA monitoring after HSCT. HBV reactivation is not uncommon in HSCT recipients. In particular, patients undergoing allo-HSCT are at a very high risk of HBV reactivation, and the time interval between transplantation and HBs-Ag conversion can be long (8,9). The risk of HBV reactivation is lower with auto-HSCT than with allo-HSCT (7); however, the rate of reactivation is still 5.4-16.7% in patients with resolved HBV infection (7,10,11). Rare cases have been reported where HBV reactivation occurred more than 1 year after auto-HSCT (12,13) (Table 2). Auto-HSCT is accepted as a comparatively effective treatment for blood diseases, and the current protocol for patients receiving auto-HSCT therapy is to source stem cells from peripheral blood (14). Our patient developed de novo hepatitis due to HBV reactivation 55 months after the end of chemotherapy and auto-PBSCT. This prompts us to question the current recommended timeframe of viral load monitoring in HSCT recipient. The prophylactic use of NA in HBs-Ag negative, HBc-Ab positive HSCT recipients is recommended by the European Association for the Study of the Liver (EASL), and the American Association for the Study of Liver Diseases (AASLD) guidelines (5,6), although the optimal duration of prophylaxis is not known. The incidence of HBV reactivation after HSCT in patients with resolved HBV infection is about 40% (9), suggesting that more than half of HSCT patients do not require NA prophylactic administration. Thus, NA prophylaxis for all patients may not be an economically sound option. Recently, it has been reported that an ultra-high sensitivity HBsAg assay can become an alternative method to diagnose HBV reactivation, because such an assay is quick, easy, and effective (15). As reported in this study, the ultra-high sensitivity HBsAg assay has been useful for long-term follow-ups.
Table 2.
Presenting Clinical Features of 3 Cases of HBV Reactivation in Resolved HBV Patients after Auto-HSCT and Our Patient.
| No | Disease | Sex | Age | Anti-HBs | Period to reactivation | Peak HBV DNA (log IU/mL) | Peak ALT (IU/L) | Treatment of reactivation | Evolution | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HL | M | 68 | Positive | 18 months | >8.2 | ×2UNL | TDF | Resolved | [12] |
| 2 | MM | F | 72 | Negative | 12 months | 7.2 | 21 | LMV | Resolved | [13] |
| 3 | MM | M | 60 | Positive | 20 months | 6.7 | 261 | No treatment | Resolved | [13] |
| 4 | DLBCL | F | 54 | Positive | 55 months | 8.0 | 640 | TAF | Resolved | Our case |
auto-HSCT: autologous hematopoietic stem cell transplantation, anti HBs: anti-hepatitis B surface, HL: hodgkin’s lymphoma, MM: multiple myeloma, DLBCL: diffuse large B-cell lymphoma, ULN: upper limited normal, TDF: tenofovir disoproxil fumarate, LMV: lamivudine, TAF: tenofovir alafenamide fumarate, HBV: hepatitis B virus
As HBV reactivation is a well-known risk for patients receiving immunosuppressive therapy; most patients with past HBV infection are monitored for HBV markers carefully or receive anti-HBV prophylaxis. By adopting this strategy, there have been few reports of pathological HBV reactivation with severe acute fatal hepatitis (16-18). Although the timing of liver biopsy from the onset of de novo hepatitis is almost unchanged, fibrosis was more advanced than that observed in previous cases receiving immunosuppressive therapy for preventing graft-versus-host disease (17,18). The patient in this study did not require receive immunosuppressive therapy, reported to improve clinicopathologic findings for severe hepatitis (19), following the treatment. It has been reported that hepatic fibrosis does not progress after HBsAg seroclearance (20,21), and that fibrosis or chronic changes may sometimes occur before HBV seroclearance. Further study is needed regarding the histopathology, including the progression of liver fibrosis, in patients with HBV reactivation to address these issues.
Certain virologic features have been linked to HBV reactivation. For instance, mutations in the core promoter region (A1762T and G1764A) and in the precore region (G1896A) are reported to be associated with HBV reactivation (22,23). Additionally, a recent study used next-generation sequencing and showed that mutation in the precore region (G1899A) are also associated with HBV reactivation (24). Further studies are required to elucidate the mechanisms that contribute to such virologic features in HBV reactivation.
In the latest JSH, EASL, and AASLD guidelines, entecavir (ETV), tenofovir disoproxil fumarate (TDF), and TAF are recommended as first line anti-HBV treatments for prophylaxis against HBV reactivation (4-6). TAF has demonstrated safety and efficacy in achieving viral suppression in patients with chronic hepatitis B (25-27). In our patient, TAF was used effectively and safely against de novo hepatitis B.
Tamori et al. (28) suggested that NA therapy can be discontinued in patients with HBV reactivation after the HBs-Ab test shows consistent positive results. In our patient, the HBs-Ab test was positive, but HBcr-Ag remained detectable. HBcr-Ag is considered a marker of intrahepatic HBV [covalently closed circular DNA (cccDNA)], and serum HBcrAg positivity is a risk factor for HBV reactivation in patients undergoing immunosuppressive therapy (29). As such, our findings suggest that TAF therapy should be continued until serum HBcrAg becomes undetectable.
In conclusion, we herein described a case of HBV reactivation 55 months after chemotherapy including rituximab and auto-PBSCT. In such high-risk cases, the recommended guidelines should be followed, and careful long-term follow-ups, including the possibility of lifelong observation, are warranted.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We are grateful to all members for the Department of Gastroenterology and Hepatology, Fukuchiyama City Hospital.
References
- 1.Perrillo RP, Gish R, Falck-Ytter YT. American gastroenterological association institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 148: 221-244, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Hui CK, Cheung WW, Zhang HY, et al. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytoxic chemotherapy. Gastroenterology 131: 59-68, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Oketani M, Ido A, Uto H, et al. Prevention of hepatitis B virus reactivation in patients receiving immunosuppressive therapy and chemotherapy. Hepatol Res 42: 627-636, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Drafting Committee for Hepatitis Management Guidelines, the Japan Society of Hepatology. JSH guidelines for the management of hepatitis B virus infection: 2019 update. Hepatol Res 50: 791-816, 2020. [DOI] [PubMed] [Google Scholar]
- 5.Terrault NA, Lok AS, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 67: 1560-1599, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. European Association for the Study of the Liver. EASL 2017 Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 67: 370-398, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Bae SK, Gushima T, Saito N, et al. HBV reactivation after hematopoietic stem cell transplantation and rituximab-containing chemotherapy: a 12-year experience at a single center. Bone Marrow Transplant 54: 629-631, 2019. [DOI] [PubMed] [Google Scholar]
- 8.Matsue K, Aoki T, Odawara J, et al. High risk of hepatitis B-virus reactivation after hematopoietic cell transplantation in hepatitis C core antibody-positive patients. Eur J Haematol 83: 357-364, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Seto WK, Sau-Yan Chan T, Hwang T, et al. Hepatitis B reactivation in occult viral carriers undergoing hematopoietic stem cell plantation: a prospective study. Hepatology 65: 1451-1461, 2017. [DOI] [PubMed] [Google Scholar]
- 10.Tsukune Y, Sasaki M, Odajima T, et al. Incidence and risk factors of hepatitis B virus reactivation in patients with multiple myeloma in an era with novel agents: a nationwide retrospective study in Japan. Blood Cancer J 7: 631, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uhm JE, Kim K, Lim TK, et al. Changes in serologic markers of hepatitis B following autologous hematopoietic stem cell transplantation. Bio Blood Marrow Transplant 13: 463-468, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Papamichalis P, Alexiou A, Boulbou M, Dalekos GN, Rigopoulou EI. Reactivation of resolved hepatitis B virus infection after immunosuppression: is it time to adopt pre-emptive therapy? Clin Res Hepatol Gastroenterol 36: 84-93, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Lee JY, Lim SH, Kim H, et al. Hepatitis B reactivation in multiple myeloma patients with resolved hepatitis B undergoing chemotherapy. Liver Int 35: 2363-2369, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Activities and Outcomes of Hematopoietic Cell Transplantation in Japan (2019) provided by the Japanese Data Center for Hematopoietic Cell Transplantation (JDCHCT) [Internet]. [cited 2020 Jun 17]. Available from: https://www.jdchct.or.jp/en/data/slide/2019/
- 15.Kusumoto S, Tanaka Y, Suzuki R, et al. Ultra-high sensitivity HBsAg assay can diagnose HBV reactivation following rituximab-based therapy in patients with lymphoma. J Hepatol 73: 285-293, 2020. [DOI] [PubMed] [Google Scholar]
- 16.Palmore TN, Shah NL, Loomba R, et al. Reactivation of hepatitis B with reappearance of hepatitis B surface antigen after chemotherapy and immunosuppression. Clin Gastroenterol Hepatol 7: 1130-1137, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiba T, Yokosuka O, Kojima H, et al. Chronic graft-versus-host disease complicated by acute hepatitis B. J Clin Gastroenterol 36: 179-181, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Mawatari S, Uto H, Moriuchi A, et al. Horizontal transmission of de novo hepatitis B between spouses: a case report. Hepatol Res 45: 933-938, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Gregory PB, Knauer CM, Kempson RL, Miller R. Steroid therapy in severe hepatitis. N Engl J Med 294: 681-687, 1976. [DOI] [PubMed] [Google Scholar]
- 20.Ahn SH, Park YN, Park JY, et al. Long-term clinical and histological outcomes in patients with spontaneous hepatitis B surface antigen seroclearance. J Hepatol 42: 188-194, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Arase Y, Ikeda K, Suzuki F, et al. Long-term outcome after hepatitis B surface antigen seroclearance in patients with chronic hepatitis B. Am J Med 119: e9-e16, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Chen PM, Yao NS, Wu CM, et al. Detection of reactivation and genetic mutations of the hepatitis B infections receiving hematopoietic stem cell plantation. Transplantation 74: 182-188, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Kusumoto S, Tanaka Y, Mizokami M, Ueda R. Reactivation of hepatitis B virus reactivation following systemic chemotherapy for malignant lymphoma. Int J Hematol 90: 13-23, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto K, Umemura T, Ito K, et al. Virological factors associated with the occurrence of HBV reactivation in patients with resolved HBV infection analyzed through ultradeep sequencing. J Infect Dis 221: 400-407, 2020. [DOI] [PubMed] [Google Scholar]
- 25.Chang HLY, Fung S, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-positive chronic hepatitis B virus infection: a randomised, double-blined, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 1: 185-195, 2016. [DOI] [PubMed] [Google Scholar]
- 26.Buti M, Gane E, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blined, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol 1: 196-206, 2016. [DOI] [PubMed] [Google Scholar]
- 27.Lok AS, McMahon BJ, Brown RS, et al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology 63: 284-306, 2016. [DOI] [PubMed] [Google Scholar]
- 28.Tamori A, Hino M, Kawamura E, et al. Prospective long-term study of hepatitis B virus reactivation in patients with hematologic malignancy. J Gastroenterol Hepatol 29: 1715-1721, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Seto WK, Wong DK, Chen TS, et al. Association of hepatitis B core-related antigen with Hepatitis B virus reactivation in occult viral carries undergoing high-risk immunosuppressive therapy. Am J Gastroenterol 111: 1788-1795, 2016. [DOI] [PubMed] [Google Scholar]