Abstract
Objective
A survival benefit was demonstrated for ramucirumab (RAM) in patients with unresectable hepatocellular carcinoma (uHCC) and α-fetoprotein (AFP) concentrations ≥400 ng/mL who had previously received sorafenib (SOR). However, it is unclear whether RAM has a similar efficacy in patients with uHCC that progresses after lenvatinib (LEN) treatment. This study aimed to evaluate the early anti-tumor response to RAM as a second-line treatment for advanced uHCC after LEN treatment.
Methods
We retrospectively assessed the efficacy and safety of RAM at 6 weeks after initiation. The therapeutic effects were evaluated according to the Response Evaluation Criteria in Solid Tumors version 1.1.
Patients
We evaluated 7 patients with uHCC who received RAM as a second- or third-line treatment after LEN failure.
Results
The disease control rate (DCR) was 28.6% (2 of 7 patients). After the initiation of RAM, a rapid disease progression resulted in 1 patient death after 19 days. The median progression-free survival (PFS) was 41 days. There were no grade 3 or 4 treatment-related adverse events. At 6 weeks, there was no deterioration in the modified albumin-bilirubin (mALBI) grade. In patients with an imaging response of stable disease (SD), the rate of AFP production decreased from the baseline.
Conclusion
RAM may have a therapeutic potential for the suppression of uHCC progression in patients previously treated with LEN, as well as for maintaining the liver function during treatment. Evaluating the AFP trends may therefore be useful for predicting RAM effectiveness.
Keywords: hepatocellular carcinoma, high malignant potential, lenvatinib, liver function, ramucirumab
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cause of cancer death in Japan (1). For many years, the prognosis of advanced HCC was poor, with few treatment options. Sorafenib (SOR) has long been used as a first-line systemic chemotherapy (2,3), but in March 2018, lenvatinib (LEN) was newly approved in Japan. As a first-line therapy, LEN achieved better objective response rates and a better progression-free survival (PFS) than SOR in the phase 3 REFLECT trial, and it is therefore now widely used (4).
Regorafenib (REG) was the first agent approved as a second-line systemic therapy after SOR (5). Further, Ramucirumab (RAM) was newly approved in June 2019 as a second-line systemic therapy after SOR in Japan. RAM showed a survival benefit in patients with HCC who had α-fetoprotein (AFP) concentrations ≥400 ng/dL and who were either intolerant to or had shown a radiologic progression on SOR (HR, 0.71; p=0.0199) (6). New drugs (RAM and LEN) have diversified the systemic therapeutic strategy of HCC. However, an effective second-line treatment for patients with LEN failure has not yet been established.
It is unclear whether RAM has a similar efficacy in patients with HCC who progress after LEN, because the REACH-2 trial was conducted only in patients with SOR failure. A previous study showed that the percentage of patients who were candidates for second-line treatment after SOR failure was 35% for REG and 23.3% for RAM (7). Although the target population of patients who are candidates for second-line treatment with RAM may be small, as there is no alternative second-line treatment, it is important to determine the efficacy of RAM after LEN failure. If RAM could be an effective second-line treatment after LEN, we could then predict the treatment response before decompensation of the liver function, and we could treat patients with additional lines of therapy as needed. Therefore, assessing the response to RAM in patients with disease progression after LEN is crucial to enable us to take full advantage of the benefits of sequential systemic therapy.
This study aimed to evaluate the early anti-tumor response and clinical benefit of RAM as a second-line treatment for uHCC after progression on LEN.
Materials and Methods
Study population
From June 2019 to December 2019, we selected 7 patients with HCC initiated ramucirumab (CyramzaⓇ, Eli Lilly, Indianapolis, USA) as a second- or third-line treatment after lenvatinib (LenvimaⓇ, Eisai, Tokyo, Japan). The patients met the following inclusion criteria: 1) Barcelona Clinic Liver Cancer (BCLC), stages A to C, refractory or not amenable to locoregional therapy, 2) Child-Pugh class A liver disease, 3) an observation period of 14 days, 4) serum AFP concentrations ≥400 ng/mL (as measured by a local laboratory), 5) no other advanced cancer complications, 6) LEN as a previous systemic treatment, 7) 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) performed before the initiation of systemic treatment.
In principle, the inclusion criteria were based on those of the REACH-2 trial. In the REACH-2 trial, SOR was the only prior systemic treatment for HCC that was allowed, and it must have been discontinued at least 14 days before randomization because of either intolerance or disease progression (6); however, in our study, 6/7 patients continued the previous systemic treatment until RAM was initiated. In addition, in our clinical setting, one patient was in poor clinical condition, with an Eastern Cooperative Oncology Group (ECOG) performance status of 2.
The patients received intravenous RAM (8 mg/kg) every 14 days until either disease progression or unacceptable toxicity occurred.
Adverse events were graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE; version 4.0).
Treatment was generally discontinued for grade 4 clinical adverse events.
Diagnosis of HCC
The diagnosis of HCC was based predominantly on a dynamic computed tomography (CT) image analysis. A hyperattenuating liver nodule in the arterial phase of the dynamic study and washout in the portal or delayed phase resulted in a diagnosis of HCC (8).
FDG-PET/CT imaging analysis of HCC
Before initiating systemic treatment, FDG-PET/CT was performed with a dedicated whole-body PET scanner (Biograph mCT Flow 40, Siemens Healthcare, Bayern, Germany). Using a software package for a semi-quantitative analysis (SYNAPSE VINCENT ver.4, FUJIFILM Medical, Tokyo, Japan), we focused the volume of interest (VOI) along the outline of the tumor, and the maximum standardized uptake value (SUVmax) and the mean standardized uptake value (SUVmean) in each intrahepatic target tumor were calculated. To analyze the normal liver activity, three non-overlapping spherical VOIs (two in the right lobe and one in the left lobe), measuring 1 cm3, were focused on using the axial PET images, avoiding the areas of the HCC, as seen on dynamic CT. The tumor-to-liver uptake ratio (TLR) was calculated using the following equation:
TLR=SUVmax of the tumor/SUVmean of the normal liver
Based on previous reports (9,10), we selected a TLR≥2 to indicate a high malignant potential and defined it as “PET-positive”.
Treatment response evaluation and follow-up protocol
The CT or magnetic resonance imaging (MRI) and local laboratory assessments were performed at baseline, before each treatment cycle and after 6 weeks to determine the objective response. The tumor response was assessed in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) (version 1.1) (11) and modified RECIST (mRECIST) (12). The serum AFP levels were measured on 10 to 20 days before the initiation of RAM, on the starting day, and then 2 weeks and 6 weeks after the initiation of RAM.
The rate of change in AFP concentrations was calculated using the following equation:
Rate of change in AFP concentration=change from baseline in AFP concentration during the evaluation period (μg/L)/length of the evaluation period (days)
The modified albumin-bilirubin (ALBI) grade (mALBI) (13) score and grade were used to assess the hepatic reserve function.
Statistical analysis
A statistical analysis was performed using the IBM SPSS software program (ver. 26.0 SPSS, IBM, Chicago, USA). Data were expressed as the median and range. Differences in TLR between each group were analyzed by the Mann-Whitney U test. P values <0.05 were considered to indicate statistical significance.
Ethical considerations
This retrospective non-interventional study was approved by the Institutional Review Board, Toranomon Hospital (protocol number: 1438-H/B). The study was performed in accordance with the Declaration of Helsinki.
Results
Clinical profiles and laboratory data
Table shows the patient baseline characteristics. In one case (case 3), RAM was initiated as a third-line treatment (SOR/LEN/RAM); the other cases initiated RAM as a second-line treatment (LEN/RAM).
Table.
Clinical Profiles and Features of Patients with HCC Treated with Ramucirumab.
| Case | Sex | Age (y) |
Etiology | Treatment line | PS | EHM | MVI | FDG-uptake | AFP at baseline (μg/L) | Modified ALBI grade at baseline | Waiting time (day) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 67 | HCV | 2nd | 1 | - | - | Positive | 2,4142.7 | 2b | 218 |
| 2 | Male | 50 | HBV | 2nd | 1 | - | Vv2 | Positive | 948.1 | 1 | 15 |
| 3 | Male | 67 | HCV | 3rd | 0 | Bone Lymph nodule Lung | - | Positive | 891.5 | 2b | 267 |
| 4 | Male | 84 | HCV | 2nd | 2 | Lung | Vp3 | Positive | 543.4 | 2b | 127 |
| 5 | Male | 67 | HBV | 2nd | 0 | - | - | Positive | 3,5851.3 | 2b | 66 |
| 6 | Male | 71 | HBV | 2nd | 1 | Bone | - | Positive | 538.7 | 2b | 235 |
| 7 | Female | 71 | HCV | 2nd | 1 | - | - | Negative | 590.5 | 1 | 184 |
HCC: Hepatocellular carcinoma, PS: performance status, EHM: extrahepatic metastasis, MVI: macrovascular invasion, FDG: 18F-fluorodeoxyglucose, AFP: α-fetoprotein, HCV: hepatitis C viral infection, HBV: hepatitis B viral infection
The median waiting period (time form LEN failure to RAM starting) was 184 days (range=15-267 days). Before the initiation of RAM, 6 patients received bridging therapy after LEN failure; 4 patients underwent transcatheter arterial chemoembolization (TACE), 1 patient underwent surgical resection, and 1 patient received radiation therapy.
Assessment of the response to RAM
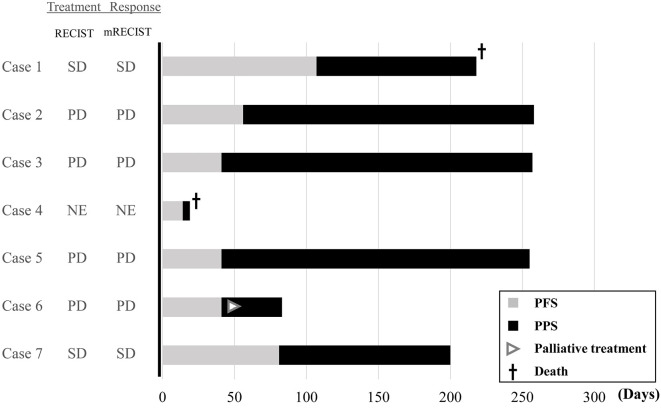
Fig. 1 summarizes the RAM treatment response. At 6 weeks, the treatment response was evaluated for 6 cases; 4 patients had progressive disease (PD), and 2 (cases 1 and 7) experienced stable disease (SD) by RECIST (version 1.1). The antitumor response evaluated by mRECIST was the same as that by RECIST. The median (range) PFS was 41 (14-107) days.
Figure 1.
Overview of patients treated with ramucirumab. Gray bars indicate the progression-free survival (PFS); black bars indicate the post-progression survival (PPS), and the PFS plus PPS equals overall survival (OS) after ramucirumab initiation. The treatment response at 6 weeks after ramucirumab initiation was categorized as NE (not evaluable), SD (stable disease), and PD (progressive disease). RECIST: Response Evaluation Criteria in Solid Tumors, mRECIST: modified RECIST
All 7 patients received a full dose at initiation. However, 1 required a dose reduction due to grade 2 pancytopenia during the observation period.
The data cutoff for this report was end of May 2020. The median follow-up duration was 218 (range, 19-258) days; the median survival time was not attained within the observation period. All patients showed disease progression during the observation period. Two patients died due to disease progression (cases 1 and 4), 4 patients continued to receive subsequent treatments (e.g., re-start LEN therapy, TACE or R0 surgical resection) and 1 started palliative care due to the onset of bone metastases.
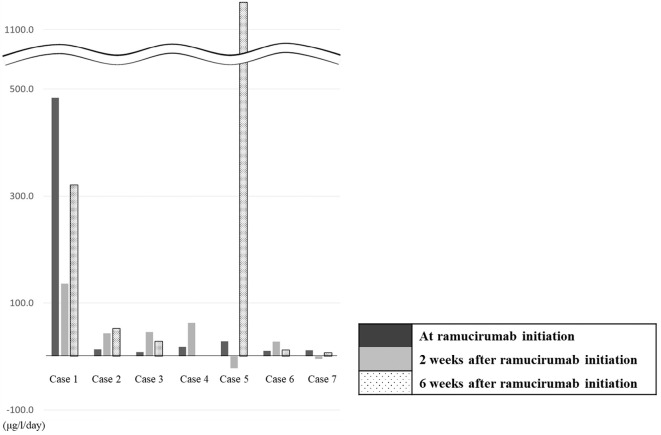
Trend in AFP concentration after RAM initiation
At 6 weeks after RAM initiation, we assessed the rate of change in AFP in 6 patients. Although the AFP value increased in all patients, the rate of change in AFP was lower than that before the initiation of RAM in 2 cases (cases 1 and 7). The trend of rate of change in AFP correlated with the response on imaging (SD by RECIST) (Fig. 2).
Figure 2.
Rate of change in AFP concentration (μg/L/day) at 2 and 6 weeks after ramucirumab initiation. The rate of change in the AFP concentration was calculated with the following equation: Rate of change in AFP concentration=change from baseline AFP concentration during the evaluation period (μg/L)/length of the evaluation period (days). One patient was not evaluable at 6 weeks due to death (case 4). AFP: α-fetoprotein
Changes in liver function and body weight at 6 weeks after RAM initiation
At 6 weeks after RAM initiation, we assessed the hematological and biochemical parameters and evaluated the liver function by calculating the Child-Pugh score and mALBI score.
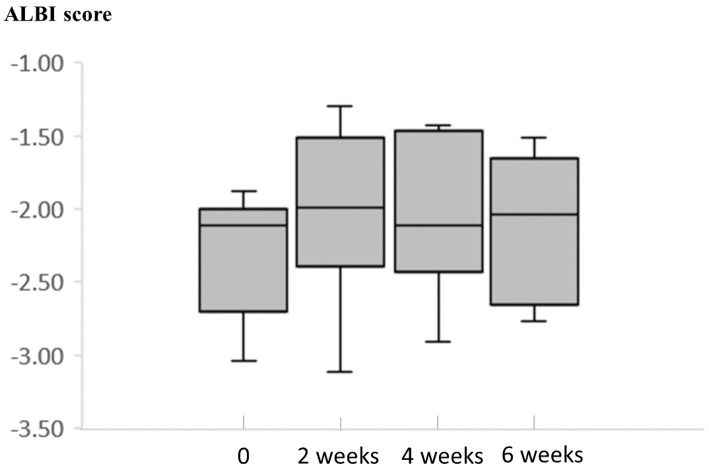
Fig. 3 showed the ALBI score at baseline, after 2 weeks, and after 4 weeks.
Figure 3.
ALBI score at 2, 4, and 6 weeks. We calculated the ALBI score at the initiation of RAM and at 2, 4, and 6 weeks after the initiation of RAM. The median ALBI score at each time point was -2.11, -1.99, -2.11, and -2.04, respectively. ALBI: albumin-bilirubin, RAM: Ramucirumab
At the initiation of RAM, 4 patients had an mALBI grade of 2b; 2 had a grade of 1. There was no deterioration in the mALBI grade after 6 weeks.
After initiating RAM, the adverse effects of LEN (diarrhea, anorexia) decreased.
No grade 3 or 4 treatment-related adverse events were observed.
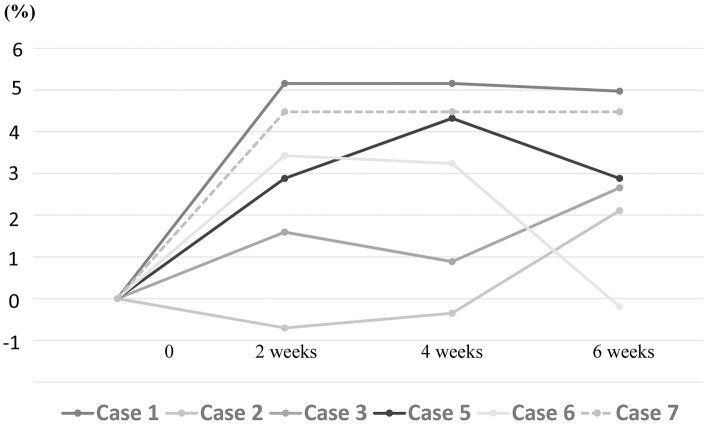
The patients maintained their body weight, and in 5 patients, the body weight improved after 6 weeks (Fig. 4).
Figure 4.
Body weight ratio at 2, 4, and 6 weeks. Body weight improved in 5 patients (cases 1, 2, 3, 5, and 7) after 6 weeks.
Discussion
In the current treatment strategy for advanced HCC, LEN is one of the best first-line molecular targeted agents due to a high treatment response and PFS (4). However, regardless of the efficacy of the molecular targeted agent, the treatment duration is limited. In this situation, there is no established second-line molecular targeted agent for use after progression on LEN.
In this report, we observed an early anti-tumor response to second-line treatment with RAM for advanced HCC that progresses after LEN treatment. The REACH-2 trial did not include patients who received first-line systemic therapy other than SOR. Furthermore, to our knowledge, only one small study has previously reported RAM treatment after LEN failure (14).
Therefore, further verification is required regarding the effects of RAM treatment after LEN failure. Our report of early antitumor efficacy of RAM may help in reevaluating the strategies for patients with HCC after LEN failure.
Compared with the results of the global phase III clinical trial (REACH-2), the median PFS in our study was short (1.3 vs. 2.8 months in REACH-2), and the disease control rate (DCR) was low (28.6% vs. 59.9% in REACH-2). This could be due to several reasons.
First, there may have been a lead-time bias. Among the 7 patients, 5 (71%) waited over 4 months from disease progression on LEN to initiate RAM; the median waiting time from disease progression was longer than in the REACH-2 trial (6.0 vs. 1.2 months, respectively). Furthermore, 4 patients underwent additional transcatheter arterial chemoembolization (TACE) after disease progression on LEN. Incomplete TACE increases tumor hypoxia leading to the upregulation of hypoxia-inducible factor-1-α (HIF1-α) as well as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF), resulting in increased tumor angiogenesis (15,16). These unfavorable aspects of TACE may have affected the DCR and PFS.
In addition, 85% (6/7) of patients had PET-positive HCC at the time of the initiation of RAM. In previous studies, FDG/PET-CT-positive HCC was reported to be associated with a lower PFS, as it was histologically poorly differentiated (17,18). Therefore, it is likely that our study population included patients with tumors with the potential for rapid progression. In fact, in our study, 4 of 6 PET-positive cases were judged to have early PD (≤41 days), and 1 of 6 cases died due to a rapid disease progression. However, one patient had a relatively long PFS (case 1). Therefore, we performed an additional test to evaluate the difference in the median TLR between the patients with a good (>42 days) and poor (≤41 days) PFS with the Mann-Whitney U test. The median TLR was significantly higher in the poor PFS group than in the good PFS group (2.76 vs. 2.02, respectively; p=0.034).
Recently, the usefulness and positive initial treatment response to LEN for patients with FDG/PET-CT positive HCC has been reported (19). Some unclear points remain regarding the relationship between PET-positive disease and targeted therapy. Thus, it is necessary to study more cases to evaluate the therapeutic effect of RAM in PET-positive cases in the future.
Finally, at the time of RAM initiation, 4 patients (57%) had an mALBI grade of 2b. Patients with mALBI grades of 2b or 3 showed a worse prognosis than those classified as Child-Pugh class A (20).
Kuzuya et al. reported the initial experience of RAM treatment after LEN failure (14). Unlike our study, Kuzuya et al. reported a high DCR (80%) (7). Both the previous study and ours included a small number of patients; thus, individual patient background factors might have influenced the outcomes. Although the previous study did not clarify the waiting time and additional treatments after LEN failure, in our study, almost all patients received other bridging treatments, such as TACE and proton beam therapy, after LEN. In addition, as described above, our study included PET-positive cases, which may suggest histologically poorly differentiated HCC, consistent with a low DCR.
As with clinical trials, RAM treatment did not cause any grade 3 or 4 treatment-related adverse events; at 6 weeks, the mALBI grade did not deteriorate. Additionally, there were no cases of body weight loss. This may be an advantage for a tyrosine kinase inhibitor (TKI)-sequential treatment. In fact, 3 of 4 patients (75%) were able to proceed to the next treatment after the radiological confirmation of disease progression. With regard to LEN therapy, a relationship between a sustained decrease in AFP concentration from 2 to 4 weeks after treatment initiation and achievement of a highly objective response was reported (21). Similarly, in patients with an imaging response of SD, the rate of change in AFP decreased. The evaluation of trends in the AFP concentration may help predict RAM effectiveness.
This study was associated with the limitations of a small sample size and a short follow-up period. In the future, a large-scale analysis with a larger sample size and a longer observation period is necessary.
The pure therapeutic effect of RAM in patients with HCC after LEN failure should be evaluated in the case of sequential TKI treatment. However, in actual clinical practice, patients often receive other bridging treatments to suppress disease progression.
The TACTICS trial (22) showed that SOR in combination with TACE significantly improved the PFS over TACE alone in patients with unresectable HCC. It is thought that our study population therefore reflects the situation in actual clinical practice.
It is important to estimate the effectiveness of each treatment combination according to the current situation. In the future, it will be necessary to study more cases at multiple centers and to evaluate the pure effect of RAM after LEN failure.
In summary, the results of this study suggested that some patients may benefit from RAM after LEN failure and that evaluating the trends in AFP concentrations may be useful for predicting RAM effectiveness. Thus, RAM may have therapeutic utility as a second-line treatment in HCC, not only after SOR, but also after LEN.
Author's disclosure of potential Conflicts of Interest (COI).
Yusuke Kawamura: Honoraria, Eisai. Masahiro Kobayashi: Honoraria, Eisai. Junichi Shindoh: Honoraria, Eisai. Hiromitsu Kumada: Honoraria, Eisai.
Financial Support
This work was supported in part by grants from the Ministry of Health, Labor and Welfare in Japan and Japan Agency for Medical Research and Development.
The sources of funding were the Okinaka Memorial Institute for Medical Research and the Japanese Ministry of Health, Labor and Welfare.
References
- 1.Japan NCC. Center for cancer control and information services [Internet]. [cited 2020 Feb 1]. Available from: https://ganjoho.jp/reg_stat/statistics/stat/summary.html (in Japanese).
- 2. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10: 25-34, 2009. [DOI] [PubMed] [Google Scholar]
- 3. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378-390, 2008. [DOI] [PubMed] [Google Scholar]
- 4. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391: 1163-1173, 2018. [DOI] [PubMed] [Google Scholar]
- 5. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389: 56-66, 2017. [DOI] [PubMed] [Google Scholar]
- 6. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20: 282-296, 2019. [DOI] [PubMed] [Google Scholar]
- 7. Kuzuya T, Ishigami M, Ito T, et al. Clinical characteristics and outcomes of candidates for second-line therapy, including regorafenib and ramucirumab, for advanced hepatocellular carcinoma after sorafenib treatment. Hepatol Res 49: 1054-1065, 2019. [DOI] [PubMed] [Google Scholar]
- 8. Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35: 421-430, 2001. [DOI] [PubMed] [Google Scholar]
- 9. Hatano E, Ikai I, Higashi T, et al. Preoperative positron emission tomography with fluorine-18-fluorodeoxyglucose is predictive of prognosis in patients with hepatocellular carcinoma after resection. World J Surg 30: 1736-1741, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Kitamura K, Hatano E, Higashi T, et al. Preoperative FDG-PET predicts recurrence patterns in hepatocellular carcinoma. Ann Surg Oncol 19: 156-162, 2012. [DOI] [PubMed] [Google Scholar]
- 11. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228-247, 2009. [DOI] [PubMed] [Google Scholar]
- 12. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30: 52-60, 2010. [DOI] [PubMed] [Google Scholar]
- 13. Hiraoka A, Kumada T, Tsuji K, et al. Validation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: a multicenter analysis. Liver Cancer 8: 121-129, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuzuya T, Ishigami M, Ito T, et al. Initial experience of ramucirumab treatment after lenvatinib failure for patients with advanced hepatocellular carcinoma. Anticancer Res 40: 2089-2093, 2020. [DOI] [PubMed] [Google Scholar]
- 15. Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol 49: 523-529, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Li X, Feng GS, Zheng CS, Zhuo CK, Liu X. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol 10: 2878-2882, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seo S, Hatano E, Higashi T, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res 13: 427-433, 2007. [DOI] [PubMed] [Google Scholar]
- 18. Song MJ, Bae SH, Lee SW, et al. 18F-fluorodeoxyglucose PET/CT predicts tumour progression after transarterial chemoembolization in hepatocellular carcinoma. Eur J Nucl Med Mol Imaging 40: 865-873, 2013. [DOI] [PubMed] [Google Scholar]
- 19. Kawamura Y, Kobayashi M, Shindoh J, et al. Pretreatment Heterogeneous Enhancement Pattern of Hepatocellular Carcinoma May Be a Useful New Predictor of Early Response to Lenvatinib and Overall Prognosis. Liver Cancer 1-18, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hiraoka A, Kumada T, Atsukawa M, et al. Important clinical factors in sequential therapy including lenvatinib against unresectable hepatocellular carcinoma. Oncology 97: 277-285, 2019. [DOI] [PubMed] [Google Scholar]
- 21. Kodama K, Kawaoka T, Namba M, et al. Correlation between early tumor marker response and imaging response in patients with advanced hepatocellular carcinoma treated with lenvatinib. Oncology 97: 75-81, 2019. [DOI] [PubMed] [Google Scholar]
- 22. Kudo M, Ueshima K, Ikeda M, et al. Randomized, open label, multicenter, phase II trial comparing transarterial chemoembolization (TACE) plus sorafenib with TACE alone in patients with hepatocellular carcinoma (HCC): TACTICS trial. J Clin Oncol 36 (Suppl): 206-206, 2018. [Google Scholar]