Abstract
A 74-year-old man presented with abdominal swelling. Computed tomography revealed massive ascites and localized thickening of the small intestinal wall. Enteroscopy showed ulcerative lesions along the circumference of the jejunum. Histological examination showed dense proliferation of large lymphoid atypical cells, and immunohistochemistry showed CD20 and CD10 positivity, CD3 negativity, and Ki67 labeling index >80%. Cytology of the ascitic fluid revealed large lymphoid cells. These findings suggest that small intestine primary diffuse large B-cell lymphoma (DLBCL) caused the ascites. Massive ascites as an initial symptom of primary DLBCL of the jejunum is rare. Herein, we describe this unusual presentation.
Keywords: small intestine, diffuse large B-cell lymphoma, ascites
Introduction
Primary diffuse large B-cell lymphoma (DLBCL) of the small intestine is rare; however, extranodal DLBCL is common in the small intestine. An early and accurate diagnosis is important because of its rapid progression. Symptoms of DLBCL in the small intestine include abdominal pain due to perforated peritonitis, localized abdominal pain without gastrointestinal perforation, melena, ileus, and intussusception (1,2). However, ascites has not been described as a main complaint of small intestinal DLBCL (1).
Exudative ascites are mainly caused by inflammation due to infection or a malignant tumor. There are various primary small intestinal diseases associated with ascites and intestinal wall thickening, including eosinophilic gastroenteritis, intestinal tuberculosis, and some types of malignant tumors (3,4). However, ascites due to DLBCL of the small intestine is yet to be reported. Here, we report a rare case of massive ascites caused by primary DLBCL of the jejunum.
Case Report
A 74-year-old Japanese man presented to our hospital with abdominal swelling. He had undergone surgery and adjuvant chemotherapy for gingival cancer and surgery for lung cancer 5 years and 1 year prior, respectively. The histopathological findings of the resected gingival cancer were moderately differentiated squamous cell carcinoma without bone invasion and with lymphatic invasion and a negative surgical margin. Regarding the resected lung cancer, the findings were adenocarcinoma without vascular invasion and with a negative surgical margin.
Abdominal computed tomography (CT) revealed massive ascites and localized small intestinal wall thickening without peritoneal nodules (Fig. 1). Contrast-enhanced CT could not be performed because of a history of anaphylaxis. Esophagogastroduodenoscopy showed no upper gastrointestinal lesions. His blood test results revealed an increase in serum lactate dehydrogenase (LDH) and soluble interleukin-2 receptor levels, and the other details are presented in Table 1. We conjectured that his pathology was from a small intestinal malignant tumor and neoplastic peritonitis, although colonoscopy was not performed.
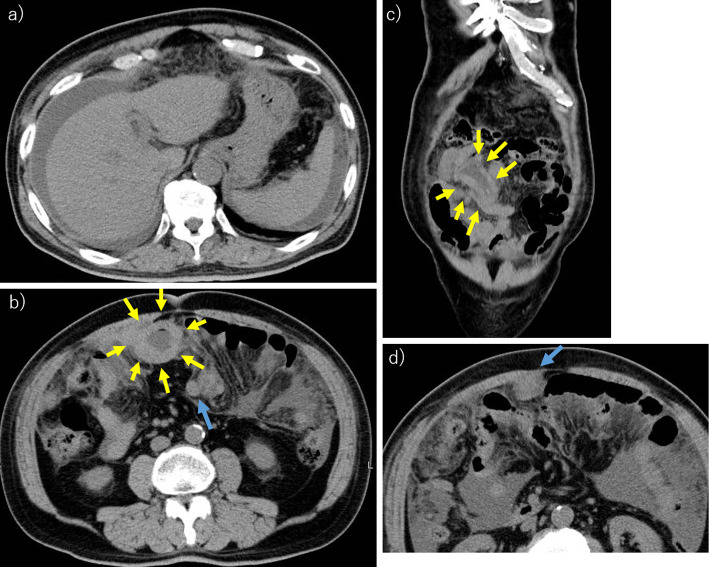
Figure 1.
Abdominal computed tomography. a) The massive ascites. b) The localized small intestinal wall thickening, which is the primary lesion of diffuse large B-cell lymphoma (yellow arrows). There were some intraperitoneal nodules (blue arrow). c) The coronal section at the localized small intestinal wall thickening lesion (yellow arrows). d) There were some intraperitoneal nodules (blue arrow).
Table 1.
Detailed Blood Test Results.
| Items (unit) | Measured value | Reference value range in Osaka Medical College Hospital |
|---|---|---|
| White blood cell counts (/µL) | 6,800 | 3,300-8,600 |
| Red blood cell counts (/µL) | 330 | 386-492×104 |
| Hemoglobin (g/dL) | 9.5* | 11.6-14.8 |
| Platelet count (/µL) | 27.2 | 15.8-34.8×104 |
| Total protein (g/dL) | 6.1* | 6.6-8.1 |
| Albumin (g/dL) | 3.6* | 4.1-5.1 |
| Total bilirubin (mg/dL) | 0.4 | 0.4-1.5 |
| Aspartate aminotransferase (IU/L) | 28 | 13-30 |
| Alanine aminotransferase (IU/L) | 22 | 10-42 |
| Lactate dehydrogenase (IU/L) | 705* | 124-222 |
| Alkaline Phosphatase (IU/L) | 209 | 106-322 |
| Carcinoembryonic antigen (ng/mL) | 0.9 | <0.5 |
| Carbohydrate antigen 19-9 (IU/mL) | 1.6 | <37.0 |
| Squamous cell carcinoma antigen (ng/mL) | 1.1 | <1.5 |
| Soluble interleukin-2 receptor (IU/mL) | 2,210* | 157-474 |
*: outside the reference range.
An ascitic tap revealed bloody ascites. The ascitic fluid count revealed a high number of lymphoma-like cells. Biochemical analysis of the ascitic fluid revealed the marked increase in serum LDH and the soluble interleukin-2 receptor levels; the other details are shown in Table 2. Flow cytometry analysis of the ascitic fluid indicated CD3 negativity and CD19 and CD20 positivity, with a kappa/lambda ratio of 0.00. Cytology of the ascitic fluid showed large lymphoid cells and cytoplasmic vacuolization (Fig. 2). These findings were consistent with ascites caused by DLBCL.
Table 2.
Biochemical Analysis Findings of Ascitic Fluid.
| Items (unit) | Measured value | Reference value range in Osaka Medical College Hospital |
|---|---|---|
| Total protein (g/dL) | 3.4 | Not applicable |
| Lactate dehydrogenase (IU/L) | 4,166 | Not applicable |
| Carcinoembryonic antigen (ng/mL) | <0.5 | <5.0 |
| Carbohydrate antigen 19-9 (IU/mL) | 3.2 | <37.0 |
| Soluble interleukin-2 receptor (IU/mL) | 10,300* | 157-474 |
*: outside the reference range.
Figure 2.
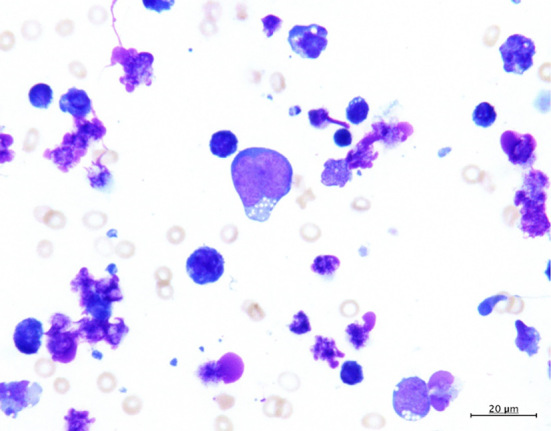
Ascitic fluid cytology reveals large lymphoid cells and cytoplasmic vacuolization.
Transoral double-balloon enteroscopy showed ulcerative lesions around the circumference of the jejunum (Fig. 3a). A small intestine contrast examination using sodium amidotrizoate meglumine solution (gastrografinⓇ), which was performed during endoscopy, revealed that the lumen of this site of the jejunum was irregular (Fig. 3b). An inking was done on the proximal side mucosa of the lesion. Biopsy showed dense proliferation of large lymphoid atypical cells on hematoxylin-eosin staining, and immunostaining findings were as follows: CD20 positive, CD10 positive, CD3 negative, and Ki67 labeling index >80% (Fig. 4).
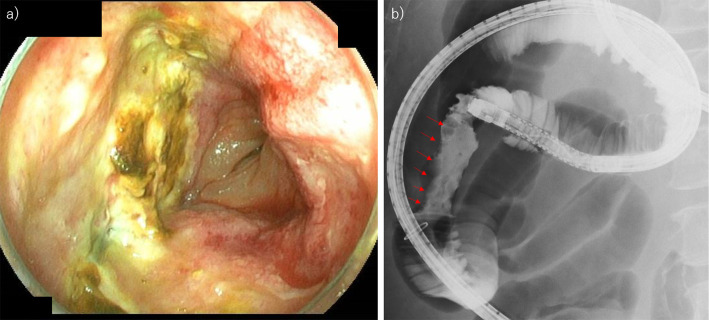
Figure 3.
The appearance of the small intestinal primary diffuse large B-cell lymphoma in the present case. a) Endoscopic findings. The entire circumference of ulcerative lesions with mild narrowing of the lumen of the jejunum. b) Small intestine contrast examination using sodium amidotrizoate meglumine solution (gastrografin®) during the endoscopy also reveals that the lumen of the jejunum is locally irregular (red arrows).
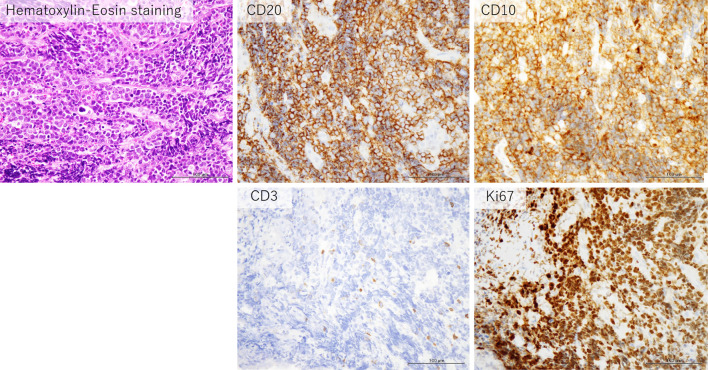
Figure 4.
The histologic examination of the endoscopic biopsy specimen shows dense proliferation of large lymphoid atypical cells with Hematoxylin and Eosin staining, and immunostaining findings are as follows: CD20 positive, CD10 positive, CD3 negative, and a Ki67 labeling index>80%.
Bone marrow examination revealed DLBCL bone marrow infiltration. There were immunoglobulin heavy-chain JH DNA rearrangements and no chromosomal abnormalities. From these findings, we determined that the localized wall thickening of the jejunum was the primary lesion of the DLBCL. The International Prognostic Index (5) (Table 3) was high; age >60 years, stage IV, LDH >reference value range, performance status 2, and more than 1 extra nodal site.
Table 3.
International Prognostic Index.
| Age greater than 60 years |
| Stage III or IV disease |
| Elevated serum LDH* |
| ECOG** performance status of 2, 3, 4 |
| More than 1 extra nodal site |
One point is assigned for each of the above risk factors. The sum the points allotted correlates with the following risk groups: 1) Low risk (0-1 points) - 5-year survival of 73%, 2) Low-intermediate risk (2 points) - 5-year survival of 51%, 3) High-intermediate risk (3 points) - 5-year survival of 43%, 4) High risk (4-5 points) - 5-year survival of 26%.
* Lactate dehydrogenase, ** Eastern Cooperative Oncology Group.
Although he completed half the dose of R-CHEP (cyclophosphamide, adriamycin, etoposide, prednisone, and rituximab) systemic chemotherapy and his abdominal symptoms had improved 3 days after starting chemotherapy, intestinal perforation occurred 9 days after starting chemotherapy, and emergency surgery was performed. The ascitic fluid was found to consist of small amounts of pale yellow, non-bloody, fluid, and the perforation site was consistent with the site of the primary DLBCL lesion. Histologically, small lymphoma masses remained in the resected specimen, but most of the lymphoma cells had cleared with chemotherapy (Fig. 5). The postoperative course was uneventful. Chemotherapy was restarted; however, the lymphoma was refractory to treatment, and the ascites recurred. The patient died 3 months later.
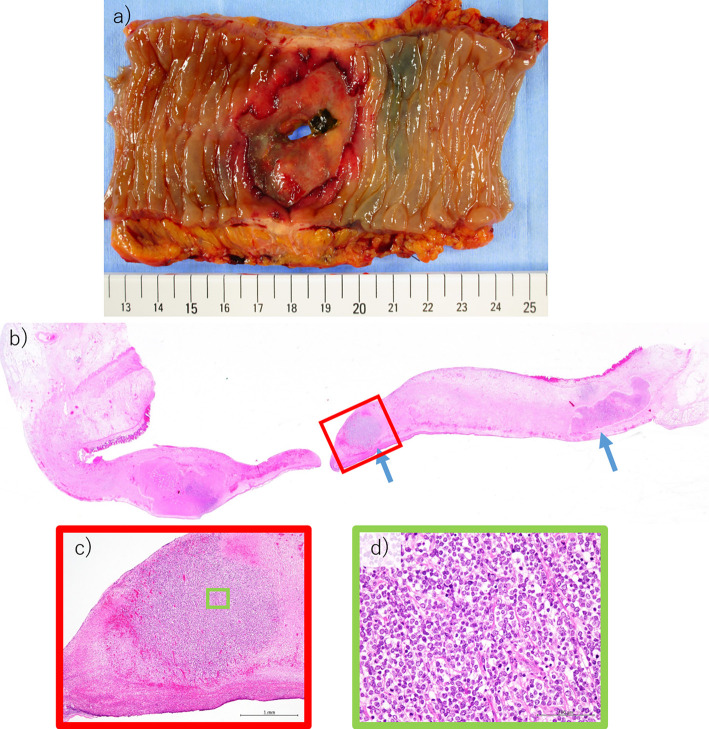
Figure 5.
a) Surgical resection specimen. There was an inking on the proximal side of the perforation site. Therefore, the perforated site was originally the site where the lymphoma was present. b) Histological findings of the resected specimen of the perforation site. Small amount of lymphoma masses remain (blue arrows), but most of the lymphoma cells have cleared following chemotherapy. c) Enlarged view of the red square in b). d) Enlarged view of the green square in c).
Discussion
In this case, massive ascites was the initial symptom of primary DLBCL of the jejunum. To the best of our knowledge, there are no case reports, thus far, on DLBCL in the small intestine with ascites as the main complaint, highlighting the rarity of this case. Although peritoneal lymphomatosis of DLBCL has already been reported (6), to our knowledge, there is no report of peritoneal lymphomatosis in which the primary organ was the small intestine. We considered that the primary organ might be the small intestine in the cases of peritoneal lymphoma of unknown primary origin.
We have experienced nine cases of primary DLBCL of the small intestine over 8 years. The common initial complaints included localized abdominal pain without intestinal perforation (2 cases), ileus (2 cases), intestinal bleeding (2 cases), and intussusception (1 case); only this case presented with ascites. Another patient was asymptomatic, and the diagnosis was incidentally made during a medical checkup.
In this case, it was difficult to suspect malignant lymphoma from our initial medical examination and CT findings. Because the patient had a history of two malignant tumors, we first suspected metastatic recurrence of the gingival or lung cancers, which has been reported previously (7,8). Although there was no dilatation of the intestine proximal to the primary lesion, the possibility of primary small intestinal cancer or a carcinoid could not be ruled out. We considered that a gastrointestinal stromal tumor was unlikely because there were no nodules on the small intestinal wall. Consistent with typical findings of malignant lymphoma, the small intestinal wall was thickened; however, the presence of ascites was not consistent with this diagnosis.
Small intestine malignant lymphoma is rare, accounting for 1-5% of all gastrointestinal malignant tumors. However, it is the most frequently occurring disease (30-40%) among small intestine malignant tumors (9,10). DLBCL occurs most frequently among small intestinal malignant lymphomas. It is classified into the following three types: stenosis type, non-stenosis type, and aneurysmal (dilated) type. The stenosis type is difficult to distinguish, using only CT, from other small intestinal cancers. The non-stenosis and the aneurysmal types are most commonly found with small intestinal primary DLBCL; the present case corresponds to the non-stenosis type.
The Lugano International Conference Classification has generally been used for staging of malignant lymphomas (11). The present case was classified as stage IV since the lymphoma cells had been seeded intraperitoneally. Chemotherapy often destroys lymphoma cells after initial administration, causing intestinal perforation, which worsens the prognosis. However, because there are no reports in which the surgery improves the prognosis of stage IV small intestinal primary DLBCL patients, it is necessary to consider an individualized treatment strategy. In the present case, we chose R-CHEP systemic chemotherapy as the initial treatment because we considered the risks of suture failure and disease progression to be high. We anticipated intestinal perforation and decreased the amount of anticancer drug by 50%, but perforation eventually occurred. No clinical issues arose after the emergency surgery, but the patient died due to the progress of the DLBCL.
This was a rare case of ascites caused by small intestinal primary DLBCL. Hence, it is important to document similar cases and establish appropriate treatment strategies.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Maeshima AM, Taniguchi H, Ito Y, et al. Clinicopathological characteristics of diffuse large B-cell lymphoma involving small and large intestines: an analysis of 126 patients. Int J Hematol 110: 340-346, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Park JW, Song GA, Baek DH, et al. Adult ileocolic intussusception caused by diffuse large B cell lymphoma. Korean J Gastroenterol 75: 46-49, 2020. [DOI] [PubMed] [Google Scholar]
- 3.Ashitani K, Tsuzuki Y, Yamaoka M, et al. Endoscopic features and diagnostic procedures of eosinophilic gastroenteritis. Intern Med 58: 2167-2171, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saliba CC, Tomacruz IDV, Javier MLMM, Co H. Massive infected ascites in an immunocompetent patient with gastrointestinal tuberculosis. BMJ Case Rep 12: e230794, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med 329: 987-994, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Weng SC, Wu CY. Lymphoma presenting as peritoneal lymphomatosis with ascites. J Chin Med Assoc 71: 646-650, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Okamura T, Beppu T, Tokumaru T, et al. Cancer of the mandibular gingiva metastasizing to the small intestine. Auris Nasus Larynx 46: 479-482, 2019. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T, Chino O, Tajima T, et al. Ileal intussusception due to metastasis from squamous cell carcinoma of the lung resected 12 years previously. Tokai J Exp Clin Med 40: 137-140, 2015. [PubMed] [Google Scholar]
- 9.Nakamura S, Matsumoto T, Takeshita M, et al. A clinicopathologic study of primary small intestine lymphoma: prognostic significance of mucosa-associated lymphoid tissue-derived lymphoma. Cancer 88: 286-294, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Mitsui K, Tanaka S, Yamamoto H, et al. Role of double-balloon endoscopy in the diagnosis of small-bowel tumors: the first Japanese multicenter study. Gastrointest Endosc 70: 498-504, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Rohatiner A, d'Amore F, Coiffier B, et al. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol 5: 397-400, 1994. [DOI] [PubMed] [Google Scholar]