Abstract
In this study, quantative nuclear magnetic resonance (qNMR) method was used to determine the content of rosuvastatin in tablet. Linearity, range, limit of detection (LOD), limit of quantification (LOQ), accuracy, and precision were determined in validation study of rosuvastatin. Furthermore, validation study of rosuvastatin was performed with high performance liquid chromatography (HPLC). Uncertainties of qNMR and HPLC methods were determined using per EURACHEM/CITAC Guide CG 4 (3th edition), quantifying uncertainty in analytical measurement. qNMR and HPLC methods were linear in the ranges of 0.10 - 5.00 mg/mL and 0.001 - 0.0995 mg/mL, respectively and these lineraties indicate very good linearity performance with regression coefficients (R2 value) above > 0.99. Moreover, LOD and LOQ values using qNMR method were observed as 0.25 mg/mL and 0.80 mg/mL, respectively. These values using HPLC method were found as 0.00051 µg/mL and 0.001695 µg/mL, respectively. The strengths and weaknesses of qNMR method and HPLC method were determined with spectral emphasis on the role of identical reference standards in qualitive and quantitative analyses. It was found that qNMR method is simple, efficient, reliable, and accurate method. Moreover, qNMR method is an easy, practical, and useful method for the validation and optimization of rosuvastatin in the tablet.
Keywords: qNMR, proton nuclear magnetic resonance, high performance liquid chromatography, rosuvastatin, validation
1. Introduction
High performance liquid chromatography (HPLC) and mass spectroscopy have been used as a quantitative analysis method for many years. However, nuclear magnetic resonance (NMR) spectroscopy was developed in recent years to determine contents in the metabolite, drugs, etc., and this method has been described as quantitative nuclear magnetic resonance (qNMR) spectroscopy or qNMR method [1-6]. Although the sensitivity of qNMR method is lower than chromatographic methods, qNMR method has some advantages over chromatographic methods [7]. In order to create calibration graph, qNMR method does not need a pure reference material [8]; qNMR method performs structural characterization instead of destroying the sample, and the analysis of mixtures can be performed without preisolation process using qNMR method. The basis of qNMR is the relationship between integral value of signal and the proton numbers. Moreover, signal to noise ratio of signal can be used to quantification of material. In order to perform more reliable and more accurate quantification of sample, internal standard material can be used. However, the most important point here is that signals of internal standard material and signals of sample are not definitely overlapping with each other [9-13].
Chemical name and empirical formula of rosuvastatin are bis{(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)ami-no]pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhept-6-enoic acid} calcium salt, (C22H27FN3O6S)2Ca, respectively. Moreover, molecular weight of rosuvastatin calcium is 1001.14 g/mol and chemical structure is given in Figure 1 [14,15]. Rosuvastatin was used to prevent u1d88. Rosuvastatin has been shown to possess a number of advantageous pharmacological properties, including enhanced HMG-CoA reductase binding characteristics and selective uptake into/activity in hepatic cells. In the literature, amount of rosuvastatin in tablets was determined by high-performance liquid chromatography (HPLC) [16-19]. However, any research about determination of amount of rosuvastatin in tablet form using qNMR has not been reported in the literature.
Figure 1.
Chemical structure of Rosuvastatin calcium.
In this study, amount of rosuvastatin in tablet form was determined using qNMR method as an alternative to HPLC. In addition, amount of rosuvastatin in tablet form was examined with HPLC. Excellent quantitative results were shown in the determination of rosuvastatin in tablet and pure rosuvastatin using qNMR. As a result, it is shown that qNMR method is an accurate, precise, and selective method for the determination of the amount of rosuvastatin in tablet.
2. Experimental
2.1. Chemicals and reagents
Rosuvastatin calcium (98%) and ellagic acid (95%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Deuterodimethyl sulfoxide (DMSO-d6, D 99.3% and 0.03% TMS) was purchased from Cambridge Isotope Laboratories Inc. (Andover, MA, USA). HPLC grade acetonitrile was purchased from VWR International (Bois, France). Ortho-phosphoric acid (85%) was purchased from Merck (Darmstadt, Germany). Ultrapure water was generated from Milli-Q system (Merck, Millipore, Billerica, MA, USA). Colnar tablet containing 5 mg rosuvastatin was bought from Sanovel (İstanbul, Turkey). Standard 5-mm NMR tubes were purchased from Norell Inc. (Landisville, NJ, USA).
2.2. Instruments
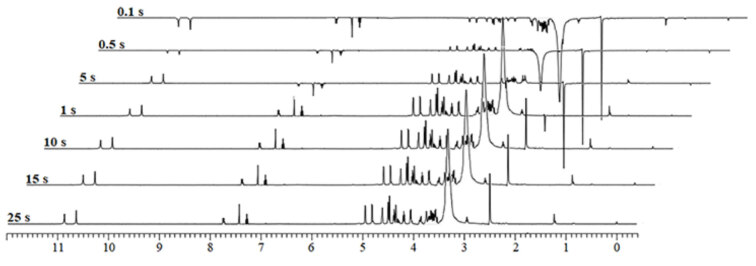
NMR spectra were collected with JEOL ECZ 500R (JEOL Ltd., Tokyo, Japan) at room temperature and proton resonance frequency was adjusted to 500.13 MHz. Tetra methylsilane (TMS) and DMSO- d6were used as internal standard and solvent, respectively. In the qNMR method, pulse angle was used as 90°. Data points, number of scan and spectral width were adjusted as 48 K, 32, and 12.5 kHz, respectively. Relaxation time was adjusted to 25 s. All chemical shifts were reported in parts per million (ppm) relative to dimethyl sulfoxide (DMSO- d6) at 2.51 ppm and TMS at 0.00 ppm. Phase and baseline distortions of spectra were adjusted automatically by JEOL Delta NMR Software (version 5.1.3). Moreover, each NMR experiment was repeated three times including sample preparation.
Thermo Scientific Dionex Ultimate 3000 HPLC (Germering, Germany) system equipped with gradient pump, a variable wavelength programmable PDA detector was used. In the optimized procedure, the separation was performed at flow rate 0.8 ml/min through a Zorbax ODS C18 analytical column (5 µm, 150 mm × 4.6 mm) (GL Science Inc., Japan) and the column compartment temperature was adjusted to 30 ℃. The mobile phases were used as isocratic system. Separation was achieved using a mobile phase acetonitrile: water (40: 60, v/ v) and pH of mobile phase was adjusted at 3.5 with phosphoric acid [20]. Rosuvastatin detection was carried out at 242 nm wavelength. The injection volume was 5 µL and analysis time was 12 min (including reequilibration). All mobile phases were degassed and filtered through 0.2 µm membrane filter (GUS, Standfor, ME, USA) before use.
2.3. Preparation of samples
In order to prepare standard solution for qNMR, 0.12, 2.14, 2.93, 4.24, and 4.91 mg (± 0.01 mg) rosuvastatin standard sample and 1.02 mg (±0.01 mg) ellagic acid (internal standard) were weighed using Ohaus AX224 (Ohous Cor., USA), and then these samples were put into the 5 mm NMR tube. 1 mL DMSO- d6solvent was added on each sample using pipet tip (Eppendorf, Hamburg, Germany). The solution was mixed until a clear solution was obtained. Each solution was prepared 3 times to obtain more accurate results.
In order to prepare tablet solution for qNMR, 20 mg ± 0.01 mg commercial rosuvastatin tablet in powder form was weighed and was put into 5 mm NMR tube. Ellagic acid was added on rosuvastatin tablet powder, and obtained mixture powder was dissolved in 1 mL DMSO- d6.
2.4. HPLC analysis of rosuvastatin
1 mg ( ± 0.001 mg) rosuvastatin was accurately weighed and dissolved in 1 mL ( ± 0.001 mL) deionized water. Obtained solution was filtered with 0.22 µm membrane filter (GVS, Standfor, ME, USA). This solution was used as the stock solution of standard. Sample solution was prepared using the same method. Obtained solution was diluted with the distilled water for different concentration. The sample and standard solutions were injected to HPLC.
3. Results and discussion
The most important factor in the qNMR method is spin-lattice relaxation time (T1). In this study, T1relaxation time measurement experiments of rosuvastatin and ellagic acid were performed with inversion recovery method [21]. Obtained 1H NMR signals in relaxation time measurement experiments were given in Figure S1. T1relaxation times of signals observed at 6.51 ppm, 4.19 ppm, and 3.54 ppm of rosuvastatin were measured as 1.877 s, 1.991 s, and 1.467 s, respectively. T1relaxation time of CH, OH (2), and OH (3) groups of ellagic acid were measured as 2.221 s, 1.596 s, and 1.569 s, respectively, and therefore, the relaxation times were set as 25 s in all1H NMR experiments depending on the resulting relaxation times (Figure 2).
Figure 2.
T1 relaxation time measurements of rosuvastatin and ellagic acid.
3.1. Validation
3.1.1. Specificity and selectivity
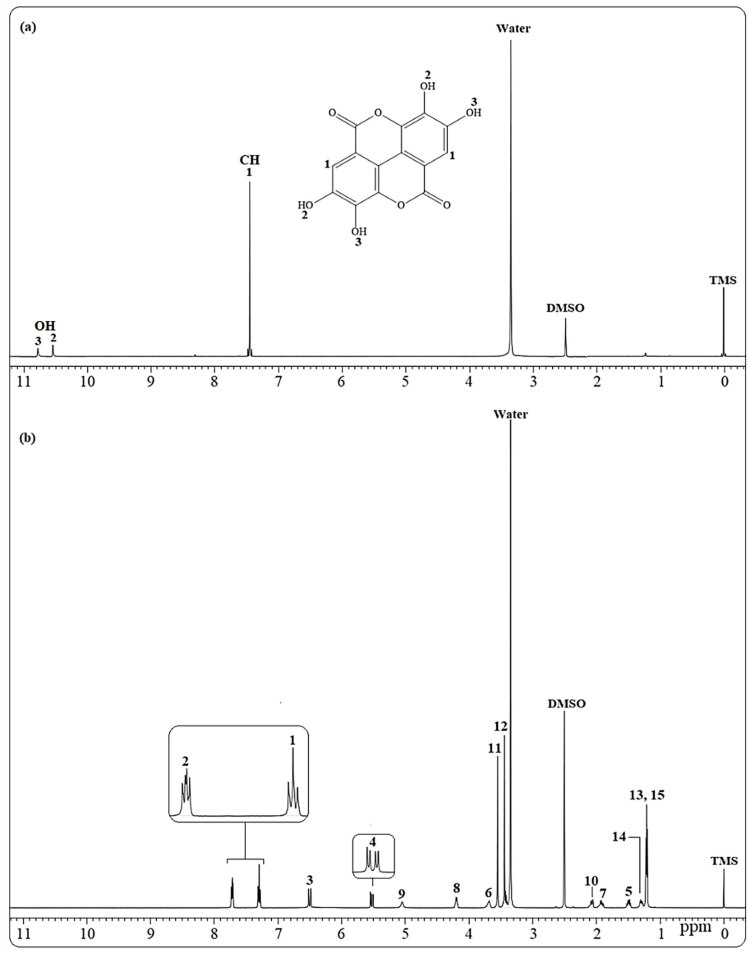
In order to determine interferences in sample solutions, specificity and selectivity were performed with NMR. Internal standard (ellagic acid), standard sample (rosuvastatin), and tablet of rosuvastatin samples were dissolved in DMSO-d6and1H NMR spectra were collected using NMR for specificity study. Obtained spectra are given in Figures 3 and 4. As can be seen from the obtained spectra, NMR signals of internal standard sample, rosuvastatin, and solvent did not overlap with each other. On the other hand, used signals did not affect each other when the qNMR method is used. Obtained spectra shows a convincing specificity and selectivity of the qNMR method for rosuvastatin validation and optimization studies.
Figure 3.
1H NMR spectra of (a) ellagic acid and (b) standard rosuvastatin in DMSO-d6.
Figure 4.
1H NMR spectra of (a) rosuvastatin tablet form and (b) rosuvastatin tablet and ellagic acid in DMSO-d6
3.1.2. Linearity and range
In order to perform linearity study of rosuvastatin, firstly five different concentration solutions containing rosuvastatin (0.12, 2.14, 2.93, 4.24 and 4.91 mg rosuvastatin were dissolved in 1 mL DMSO-d6) were prepared and then 1H NMR spectra of samples with different concentrations were obtained. Calibration graphs in qNMR methods were obtained using a ratio of the analyte to internal standard (IS) for peaks appeared at 6.51 ppm, 4.19 ppm, and 3.54 ppm, and calibration curves were obtained using these ratios with the sample concentration for each signal. The calibration curves are given in Figure S1. The correlation coefficients of quantitative protons observed at 6.51 ppm, 4.19 ppm, and 3.54 ppm were calculated as 0.9947, 0.9944, and 9963, respectively. According to the results obtained, a perfect linearity of the qNMR method was observed. In this study, the calibration graph drawn for 3.54 ppm, which has the best r2 value, was used.
However, since R2 values in other calibration charts are very close to each other, they can be used as a calibration chart. On the other hand, it was observed that there was no difference between the groups because of P > 0.05 in the ANOVA test.
3.1.3. Accuracy
The accuracy study of qNMR method was determined using recovery test. Different amounts of pure rosuvastatin were added into tablet rosuvastatin sample in which quantity of rosuvastatin was known.
The accuracy was determined using the following formula [19]. Moreover, ms(weight of rosuvastatin in tablet), mx(obtained weight of rosuvastatin), m0(weight of added rosuvastatin), and RSD (relative standard deviations) according to 3 different signals were given in Table 1. The average recovery values and RSDs show that the qNMR can provide an ideal accuracy for rosuvastatin determination.
Table 1.
Recovery test results of rosuvastatin.
| No | Rosuvastatin | Recovery (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| mo (mg) | ms (mg) | mx (mg) | 6.51 ppm | 4.19 ppm | 3.54 ppm | |||
| 6.51 ppm | 4.19 ppm | 3.54 ppm | ||||||
| 1 | 0.627 | 0.805 | 1.424 | 1.425 | 1.421 | 99.63 | 99.38 | 99.50 |
| 2 | 0.629 | 1.012 | 1.635 | 1.640 | 1.639 | 99.41 | 99.90 | 9980 |
| 3 | 0.641 | 1.231 | 1.868 | 1.862 | 1.864 | 99.67 | 99.51 | 99.35 |
| 4 | 0.601 | 1.801 | 2.415 | 2.418 | 2.414 | 100.72 | 100.89 | 100.67 |
| 5 | 0.621 | 2.310 | 2.936 | 2.938 | 2.933 | 100.22 | 100.13 | 100.87 |
| Average | - | - | - | - | - | 99.93 | 99.96 | 100.04 |
| RSD % | - | - | - | - | - | 1.06 | 1.02 | 1.11 |
3.1.4. Precision
Precision study was determined using RSD of repeatability and inter-day and intra-day precision. The repeatability was tested using three different amounts of rosuvastatin and each experiment was repeated 3 times including sample preparation. Inter-day precision and intra-day precision were shown in Table 2 and Table 3, respectively.
Table 2.
Inter-day precision of rosuvastatin.
| Rosuvastatinamounts(mg) | 0th Hour | 6th Hour | 12th Hour | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure amounts (mg) | RSD % | Measure amounts (mg) | RSD % | Measure amounts (mg) | RSD % | |||||||
| 6.51ppm | 4.19ppm | 3.54ppm | 6.51ppm | 4.19ppm | 3.54ppm | 6.51ppm | 4.19ppm | 3.54ppm | ||||
| 0.805 | 0.804 | 0.803 | 0.802 | 0.12 | 0.803 | 0.801 | 0.799 | 0.10 | 0.799 | 0.798 | 0.797 | 0.13 |
| 1.231 | 1.229 | 1.226 | 1.227 | 0.11 | 1.228 | 1.230 | 1.227 | 0.13 | 1.227 | 1.231 | 1.230 | 0.16 |
| 2.310 | 2.299 | 2.304 | 2.301 | 0.14 | 2.302 | 2.305 | 2.301 | 0.16 | 2.304 | 2.302 | 2.305 | 0.18 |
Table 3.
Intra-day precision of rosuvastatin.
| Rosuvastatin amounts(mg) | Day 1 | Day 2 | Day 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure amounts (mg) | RSD % | Measure amounts (mg) | RSD % | Measure amounts (mg) | RSD % | |||||||
| 6.51 ppm | 4.19 ppm | 3.54 ppm | 6.51 ppm | 4.19 ppm | 3.54 ppm | 6.51 ppm | 4.19 ppm | 3.54 ppm | ||||
| 0.805 | 0.799 | 0.797 | 0.800 | 0.15 | 0.796 | 0.799 | 0.801 | 0.10 | 0.796 | 0.794 | 0.797 | 0.12 |
| 1.231 | 1.226 | 1.227 | 1.225 | 0.13 | 1.225 | 1.226 | 1.224 | 0.15 | 1.214 | 1.213 | 1.215 | 0.14 |
| 2.310 | 2.297 | 2.304 | 2.305 | 0.17 | 2.305 | 2.303 | 2.305 | 0.17 | 2.284 | 2.282 | 2.285 | 0.19 |
3.1.5. Limit of detection (LOD) and limit of quantification (LOQ)
LOD and LOQ values were determined using standard deviation of the response σ and slope S of calibration curve using following equations.
LOD = 3.3σ / S LOQ = 10σ / S
LOD and LOQ values were calculated as 0.25 mg/mL and 0.80 mg/mL, respectively.
3.1.6. Robustness
Robustness refers to the effect of experimental results caused by the variations acquisition parameters. In this study, robustness was assessed on the difference analysis from the single variable test of the following acquisition parameters: the number of scans, the relaxation delay, the pulse width, the data points, and spectral width. Robustness results are given in Table 4, and robustness results show that analysis parameters do not significantly change the results compared with the optimized state.
Table 4.
Robustness results of rosuvastatin.
| Parameters | Optimized | Changed values | Difference (%) | ||
|---|---|---|---|---|---|
| 6.51 ppm | 4.19 ppm | 3.54 ppm | |||
| Number of scan | 16 | 8 | 0.21 | 0.22 | 0.25 |
| 32 | 0.24 | 0.25 | 0.29 | ||
| Number of prescan | 4 | 0 | 0.29 | 0.28 | 0.25 |
| 8 | 0.33 | 0.35 | 0.27 | ||
| Data points | 16K | 8K | 0.42 | 0.37 | 0.34 |
| 32K | 0.41 | 0.39 | 0.39 | ||
| Spectral width | 20 ppm | 15 ppm | 0.50 | 0.57 | 0.52 |
| 25 ppm | 0.52 | 0.55 | 0.54 | ||
| Relaxation time | 25 s | 5 s | 0.31 | 0.36 | 0.35 |
| 25 s | 0.33 | 0.39 | 0.31 | ||
3.1.7. The determination of rosuvastatin in tablet
Amount of rosuvastatin in the tablet was determined by the qNMR method and by HPLC. Obtained results were given in Table 5. A difference was observed between the results obtained from two different signals used in the qNMR method. A difference was observed between the results obtained from two different signals used in the qNMR method. Depending on this situation, their interaction with other protons in the environment or their interaction with the solvent is also different. Therefore, the T1 relaxation times are also different from each other. As a result, the difference in the relaxation times can cause different amounts of rosuvastatin in tablet form. In addition, the results obtained by the NMR method and the results obtained by HPLC are the perfect match, and the results obtained by both methods are similar.
Table 5.
The determination of rosuvastatin using qNMR method and HPLC.
| Sample | qNMR (n = 3) | HPLC (n = 3) | |||
|---|---|---|---|---|---|
| Signals (ppm) | % | RSD% | % | RSD% | |
| Rosuvastatin 5mg tablet | 6.51 ppm | 100.01 | 0.54 | 100.7 | 0.5 |
| 4.19 ppm | 99.56 | 0.48 | |||
| 3.54 ppm | 99.92 | 0.45 | |||
Amount of rosuvastatin in the tablet was calculated using the following formula [9,20]:
In this formula, Ixand Istdshow the integrated value of rosuvastatin and ellagic acid. Nxand Nstdshow the number of protons of used quantitative signals of rosuvastatin and ellagic acid. Mxand Mstdindicate the molecular weight of rosuvastatin and ellagic acid. mtablet and mstd denote the mass of rosuvastatin and ellagic acid. T and L are average tablet weight and amount of rosuvastatin in the tablet, respectively. Pstdis the purity of ellagic acid.
3.2. HPLC analysis of the rosuvastatin in the tablet
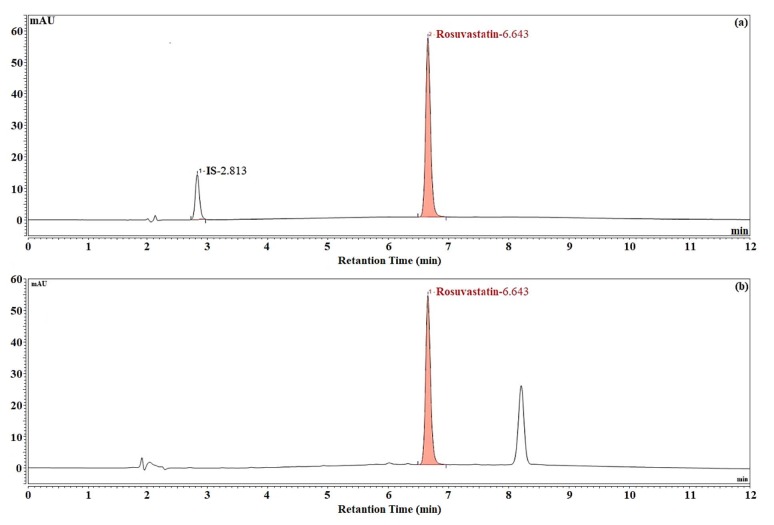
The method was validated in accordance with International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) [22]. One tablet containing rosuvastatin was weighted, and then tablet was powdered in tablet mortar. 1 mg well-mixed powder was weighted from tablet containing 5 mg rosuvastatin and transferred into a 2 mL vial. A total of 2 mL distilled water and caffeic acid as internal standard material were added into vial, and the mixture was sonicated for about 12 min. HPLC chromatograms of rosuvastatin tablet were given in Figure. 5.
Figure 5.
HPLC chromatogram of rosuvastatin (a) and rosuvastatin tablet (b).
Amount of rosuvastatin per unit dose was calculated using fallow formula [17];
where mtablet: powder is the weighted mass of tablet powder sample, T is weight of tablet, P is purity of rosuvastatin.
Linearity was investigated in the range of 2.0 × 10-9- 1.99 × 10-7M for rosuvastatin. The coefficient of variation of inter-day experiments for calibration experiments were 0.9992 for rosuvastatin. Calibration graph of rosuvastatin was given in Figure S2.
For the precision of the method, a standard solution containing 1, 25, and 100 ppm rosuvastatin, and IS were analyzed in three consecutive days with five replicates. The RSD% values were below 2% as seen in Table 6, which shows that the method is precise. Accuracy studies were conducted with three different concentrations for rosuvastatin. Each solution was injected six times a day. The accuracy results of the method are given in Table 7.
Table 6.
Precision of HPLC method.
| Linearity Parameters | Intra day (n = 5) | Inter day(n = 11) | ||
|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | ||
| Mean | 20.108 | 20.761 | 20.741 | 21.105 |
| SD | 0.170 | 0.179 | 0.171 | 0.016 |
| RSD% | 0.845 | 0.864 | 0.829 | 1.377 |
Table 7.
Accuracy of the HPLC method.
| Amount of rosuvastatin (ppm) | Found (ppm) | Recovery% | RSD% |
|---|---|---|---|
| 1 | 1.019 | 101.90 | 0.74 |
| 25 | 25.135 | 100.54 | 0.16 |
| 100 | 101.342 | 101.34 | 0.32 |
The sensitivity of the method was calculated by using the slope of calibration curve and the standard deviation of the response. The limit of detection (LOD) values was calculated as 1.02 × 10-9M, and limit of quantification (LOQ) values were calculated as 3.39 × 10-9M for rosuvastatin. The selectivity of the method was proved with checking the signals at different wavelengths during analysis. No interferences with the peaks of interest were observed.
3.3. Uncertainty evaluation of qNMR and HPLC methods
Uncertainty is very important parameter because uncertainty in the measurement affects the quality and reliability of measured result. The purity of IS and rosuvastatin with reference to tyrosine was calculated in qNMR method. Uncertainty of HPLC method was determined using EURACHEM/CITAC Guide CG 4 (3th edition) quantifying uncertainty in analytical measurement [23, 24]. Uncertainty parameters of qNMR and HPLC methods were given in Tables 8 and 9.
Table 8.
Uncertainty evolution of HPLC method.
| x | U(x) | |
|---|---|---|
| Weighing of the starting sample (mg) | 10.04 | 0.0178 |
| Stock solution (mg/ml) | 1000 | 0.7786 |
| Repeatability | 1 | 0.0016 |
| Combined standard Measurement uncertainty (%) | 2.012 | |
| Expanded standard Measurement uncertainty (%) | 4.014 | |
| Relative measurement uncertainty (%) | 0.58 | |
Table 9.
Uncertainty evolution of qNMR method.
| x | U(x) | |
|---|---|---|
| Purity of rosuvastatin (%) | 98.00 | 0.08551 |
| Purity of IS (%) | 95.00 | 0.02717 |
| Purity of reference (%) | 99.00 | 0.00330 |
| Mw of rosuvastatin (g/mol) | 500.57 | 0.02450 |
| Mw of IS (g/mol) | 0.03237 | |
| Mw of reference (g/mol) | 181.19 | 0.00416 |
| Purity of rosuvastatin (%) | 98.00 | |
| Combined standard Measurement uncertainty (%) | 0.087 | |
| Expanded standard Measurement uncertainty (%) | 0.176 | |
| Relative measurement Uncertainty (%) | 0.179 | |
For qNMR, the standard uncertaintyu(Px) can be calculated by the following equation [25]:
u(Pref) was obtained from the certificate of tyrosine, and thenu(Mx) andu(Mstd) were calculated by the following equation:
Uncertainty of purity measurement was evaluated as follows:
The purity of rosuvastatin measured by qNMR can be calculated by the following equation:
Equation of qNMR method for analyte mass is as follows:
I = peak ares of number of proton, N = number of proton, Mstd= molecular weight, mstd= weighed mass, Pstd= purity, Wx= mass of analyte.
4. Conclusions
In order to determine the content of rosuvastatin in the tablet, qNMR method was developed. Moreover, content of rosuvastatin in the tablet was also determined with HPLC. It was concluded that qNMR method could determine identification and quantification of rosuvastatin. The results obtained according to the calibration graphs obtained from different NMR signals are similar to each other. Observation and interpretation of characteristic 1H NMR signals of rosuvastatin ensures authenticity of these samples. Moreover, qNMR method can use to determine impurities and other components without reference materials. On the other hand, retention time was used to identify the rosuvastatin. qNMR method is an accurate, precise, simple, fast, and repeatable method for rosuvastatin determination. As a result, qNMR method and HPLC method confirmed that both methods indicate reasonable agreement in the determination of rosuvastatin in the tablet. Moreover, qNMR method is an easy, practical, and useful method for determination of rosuvastatin in the tablet form. The content determination results of rosuvastatin tablets were identical when comparing the qNMR method to the HPLC method. Moreover, qNMR method could be used as an alternative to or in addition to chromatographic methods for measurement of rosuvastatin in the tablet.
Supplementary Materials
Acknowledgment
This research did not receive any specific grant from funding agencies. We would like to thank Eskisehir Osmangazi University, Central Research Laboratory Research and Application Center (ARUM) since all experiments were performed in ARUM.
References
- Malz F Jancke H Journal of Pharmaceutical and Biomedical Analysis. 2005;38:813–823. doi: 10.1016/j.jpba.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Holzgrabe U Quantitative NMR spectroscopy in pharmaceutical applications. Progress in Nuclear Magnetic Resonance Spectroscopy. 2010;57:229–240. doi: 10.1016/j.pnmrs.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Chen X Guo Y Hu Y Yu B Qi J Quantitative analysis of highly similar salvianolic acids with 1H qNMR for quality control of traditional Chinese medicinal preparation Salvianolate Lyophilized Injection. Journal of Pharmaceutical and Biomedical Analysis. 2016;124:281–287. doi: 10.1016/j.jpba.2016.02.016. [DOI] [PubMed] [Google Scholar]
- Pauli GF qNMR-A versatile concept for the validation of natural product reference compounds. Phytochemical Analysis. 2001;12:28–42. doi: 10.1002/1099-1565(200101/02)12:1<28::AID-PCA549>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Pauli GF Jaki B. U Lankin DC Quantitative 1H NMR: development and potential of a method for natural products analysis. Journal of Natural Products. 2005;68:133–149. doi: 10.1021/np0497301. [DOI] [PubMed] [Google Scholar]
- Pauli GF Jaki BU. Lankin DC A routine experimental protocol for qHNMR illustrated with Taxol. Journal of Natural Products. 2007;70:589–595. doi: 10.1021/np060535r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler C Napolitano JG McAlpine JB Chen SN Pauli GF Universal quantitative NMR analysis of complex natural samples. Current Opinion in Biotechnology. 2014;25:51–59. doi: 10.1016/j.copbio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goger NG Parlatan HK Basan H Berkkan A Ozden T. Quantitative determination of azathioprine in tablets by 1H NMR spectroscopy. Journal of Pharmaceutical and Biomedical Analysis. 2000;21:685–690. doi: 10.1016/s0731-7085(99)00156-9. [DOI] [PubMed] [Google Scholar]
- Malz F Jancke H Journal of Pharmaceutical and Biomedical Analysis. 2005;38:813–823. doi: 10.1016/j.jpba.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Holzgrabe U Deubner R Schollmayer C Waibel B. Quantitative NMR —applications in drug analysis. Journal of Pharmaceutical and Biomedical Analysis. 2005;38:806–812. doi: 10.1016/j.jpba.2005.01.050. [DOI] [PubMed] [Google Scholar]
- Ohtsuki T Sato K Akiyama N Kawamura H Y. Absolute quantification for benzoic acid in processed foods using quantitative proton nuclear magnetic resonance spectroscopy. Talanta. 2012;99:342–348. doi: 10.1016/j.talanta.2012.05.062. [DOI] [PubMed] [Google Scholar]
- Sun S Jin M Zhou X Ni J Jin X The application of quantitative 1H-NMR for the determination of orlistat in tablets. Molecules. 2017;22:10–10. doi: 10.3390/molecules22091517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberger T. Determination of standard sample purity using the high-precision 1H-NMR process. Analytical and Bioanalytical Chemistry. 2012;403:247–254. doi: 10.1007/s00216-012-5777-1. [DOI] [PubMed] [Google Scholar]
- Adams LS Zhang Y Seeram NP Heber D Chen S. Pomegranate ellagitannin–derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prevention Research. 2010;3:108–113. doi: 10.1158/1940-6207.CAPR-08-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N Wang ZY Mo SL Loo TY Wang DM Ellagic acid, a phenolic compound, exerts anti-angiogenesis effects via VEGFR-2 signaling pathway in breast cancer. Breast Cancer Research and Treatment. 2012;134:943–955. doi: 10.1007/s10549-012-1977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour S Omar S. Validated high-performance liquid chromatographic method for the estimation of rosuvastatin calcium in bulk and pharmaceutical formulations. International Journal of Biomedical Science: IJBS. 2011;7:283–288. [PMC free article] [PubMed] [Google Scholar]
- Kumar TR Shitut NR Kumar PK Vinu MC Kumar VVP Determination of rosuvastatin in rat plasma by HPLC: validation and its application to pharmacokinetic studies. Biomedical Chromatography. 2006;20:881–887. doi: 10.1002/bmc.611. [DOI] [PubMed] [Google Scholar]
- Gomes FP García PL Alves JMP Singh AK Kedor-Hackmann ERM Development and validation of stability-indicating HPLC methods for quantitative determination of pravastatin, fluvastatin, atorvastatin, and rosuvastatin in pharmaceuticals. Analytical Letters. 2009;42:1784–1804. [Google Scholar]
- Liang X Du L Su F Parekh HS Su W. The application of quantitative NMR for the facile, rapid and reliable determination of clindamycin phosphate in a conventional tablet formulation. Magnetic Resonance in Chemistry. 2014;52:178–182. doi: 10.1002/mrc.4048. [DOI] [PubMed] [Google Scholar]
- Kaila HO Ambasana MA Thakkar RS Saravaia HT Shah AK A New improved RP-HPLC method for assay of rosuvastatin calcium in tablets. Indian Journal of Pharmaceutical Sciences. 2010;72:592–598. doi: 10.4103/0250-474X.78526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q Qiu H Guo W Wang D. Zhou X Quantitative 1H-NMR method for the determination of Tadalafil in bulk drugs and its tablets. Molecules. 2015;20:12114–12124. doi: 10.3390/molecules200712114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Q2(R1) 2005.
- 2012.
- Guide to the expression of uncertainty in measurement) available free of charge from the Eurachem Website ( 2014.
- Ün İ Vatansever B Şimşek A Gören AC Comparison of qNMR and HPLC-UV techniques for measurement of Coenzyme Q10 in dietary supplement capsules. Journal of Chemical Metrology. 2016;10:1–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials