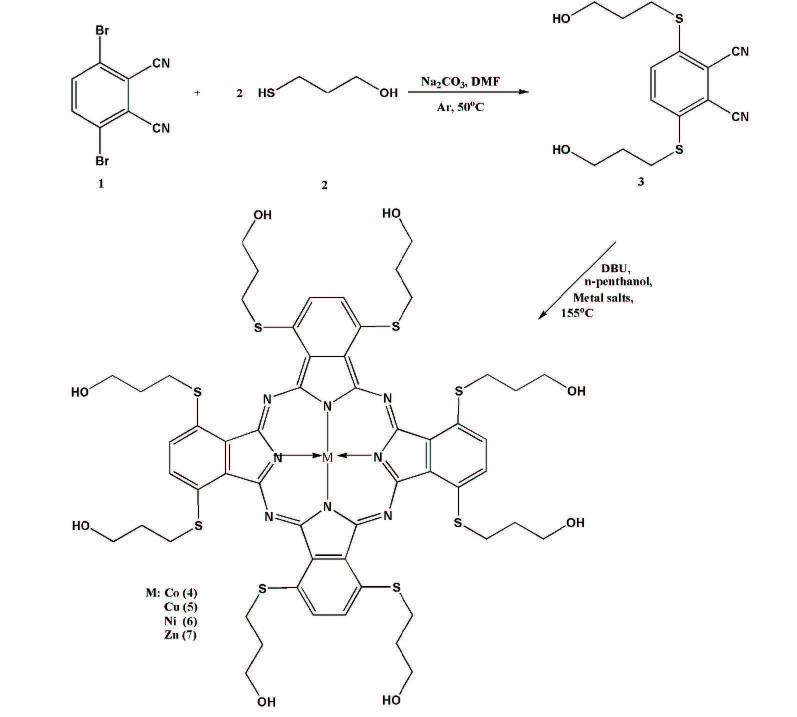
Abstract
The current study describes the synthesis, electrochemical, computational, and photochemical properties of octa (3-hydroxypropylthio) substituted cobalt (II) ( 4 ), copper (II) ( 5 ), nickel (II) ( 6 ) and zinc(II) ( 7 ) phthalocyanine derivatives. These novel compounds were characterized by elemental analysis,1H,13C NMR, FT-IR, UV-Vis, and MS. The redox behaviors of these metallo-phthalocyanines were investigated by the cyclic voltammetric method. The optimized molecular structure and gauge-including atomic orbital (GIAO)1H and13C NMR chemical shift values of these phthalocyanines in the ground state had been calculated by using B3LYP/6–31G(d,p) basis set. The outcomes of the optimized molecular structure were given and compared with the experimental NMR values. The photochemical properties including photodegradation and singlet oxygen generation of zinc(II) phthalocyanine were studied in DMSO solution for the determination of its photosensitizer behaviors.
Keywords: Metallo-phthalocyanines, cyclic voltammetry, computational chemistry, photodynamic therapy, photochemical properties
1. Introduction
Phthalocyanines (Pcs) and their metal complexes have been studied for a long time, and they are still the matters of intense investigation. They show various exceptional properties and they havepotential applications in different scientific and innovative areas like nonlinear optics [1], electrochromic imaging systems [2], chemical detectors [3–5], solar cells [6], photovoltaic optics, molecular electronics [7], liquid crystals [8], semiconductors [9], laser dyes [10], optical storage devices [11], catalyst [12] and photodynamic therapy (PDT) [13]. The developing utilization of phthalocyanines as cutting edge materials in the recent decade and they have empowered the blend of new materials which vary as far as the central metal ion and peripheral substituents [14].
Electrochemical properties of phthalocyanines in the electrolytic solution, are dependent ontheir energy values of the HOMOs and LUMOs of the frontier orbitals [15]. Electrochemical properties of the proposed compounds may have the possible potential usage in electrocatalysis, electrosensing, and electrochromic devices. Electron donating alkylthio substituted phthalocyanines are also inherently electron-rich p-type semiconductors [16,17].
The numerous applications of zinc(II) phthalocyanines in the field of medicine, molecular electronics, magnetic devices, chemical sensors depend on their photophysical and photochemical properties. The photochemical properties of these compounds, especially, singlet oxygen quantum yield and photostability were also investigated for photodynamic therapy applications [18]. Therefore, they are widely used in cancer treatment as novel generation photosensitizers. Photosensitizers are desirable to have a long wavelength. In this manner, they have an effective curing performance over deep skin cancer types. However, due to having low energy, photosensitizers decrease the possible harmful effect of light irradiation. [19,20]. Phthalocyanines were known as second-generation photosensitizers in PDT of cancer. They have long-wavelength absorption and highly effective singlet oxygen generation abilities. For this reason, they are suitable for use in cancer treatment [21].
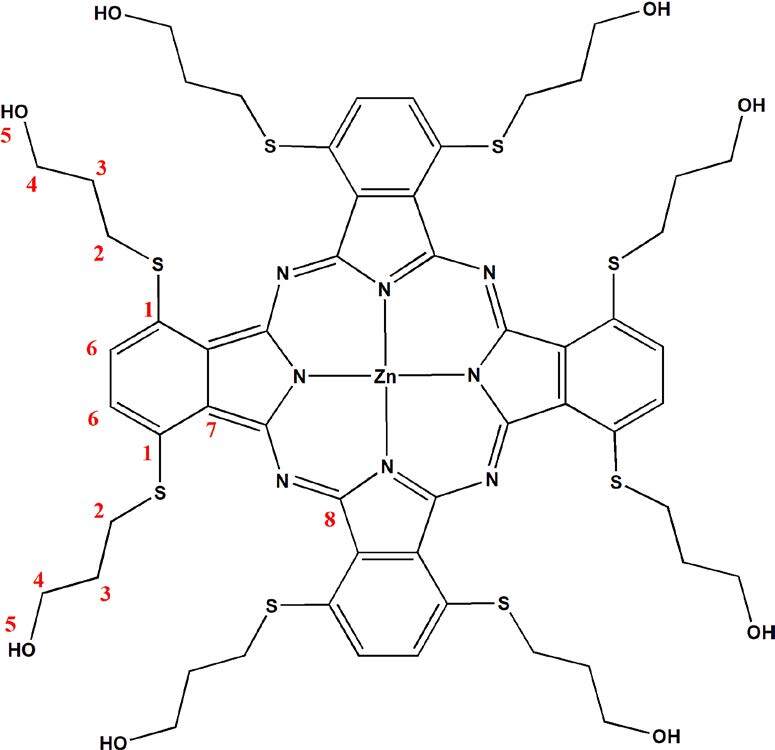
In this study, the novel metallo-phthalocyanines wherein the 3-hydroxypropylthio groups connected to nonperipheral positions of the Pc macrocycle were synthesized. Electron donating sulfur groups are known to shift the Q-band to the long wavelength in nonperipheral positions that is desirable for potential PDT applications. The newly synthesized compounds have been characterized by1H,13C NMR, UV-Vis, FT-IR, micrOTOF mass, electrochemical and computational studies as well as elemental analysis. The photochemical properties such as singlet oxygen generation and photodegradation of zinc(II) phthalocyanine was also investigated to determine possible usage of this compound as a photosensitizer for cancer treatment by photodynamic therapy technique. The theoretical1H and13C NMR data of the optimized geometry were also compared with the experimental chemical shift values.
2. Experimental
All information about the used materials, equipment, synthesis, electrochemical measurements singlet oxygen and photodegradation quantum yields as photophysical properties and theoretical calculations were showed in the “Supplementary materials”.
3. Results and discussion
3.1. Synthesis and characterization
3,6-bis(3-hydroxypropylthio)phthalonitrile (3) was synthesized via a condensation reaction of 3,6-dibromophthalonitrile [22] with 3-mercapto propanol under very convenient conditions with a better yield than the first synthesis result (38%) (Scheme 1) [23]. This compound was prepared previously by the SNAr reaction of 3,6-(4’-methylphenyl-sulfanyloxy) phthalonitrile with 3-mercapto-propanole. In the1H NMR spectrum of this compound, resonances at δ = 3.49, 1.73, 3.17, and 4.67 ppm should be related to OCH2, CH2, SCH2, and –OH protons, respectively. The aromatic protons appeared as a dublet at δ = 7.81–7.78 ppm as expected (Figure S1).13C NMR spectrum of 3 showed the presence of characteristic carbon resonances of C≡N groups at δ = 116.95 ppm, that can be attributed to the formation of 3,6-disubstituted phthalonitrile. The other chemical shifts at δ = 132.9, 141.2, 145.3, 59.6, 31.9, and 29.7 ppm could be related CN-ArC, ArC, S-ArC, OCH2, SCH2, and CH2moieties, respectively (Figure S2). These NMR signals (Table 1) are in accordance with the published results [23]. In the FT-IR spectrum of this molecule showed the characteristic vibrations for the C≡N groups at 2220 cm-1(Figure S3).
Scheme 1.
The synthesis route of the phthalonitrile and metallophthalocyanines.
Table 1.
1H and13C chemical shifts of compound 3 (experimental and theoretical values).
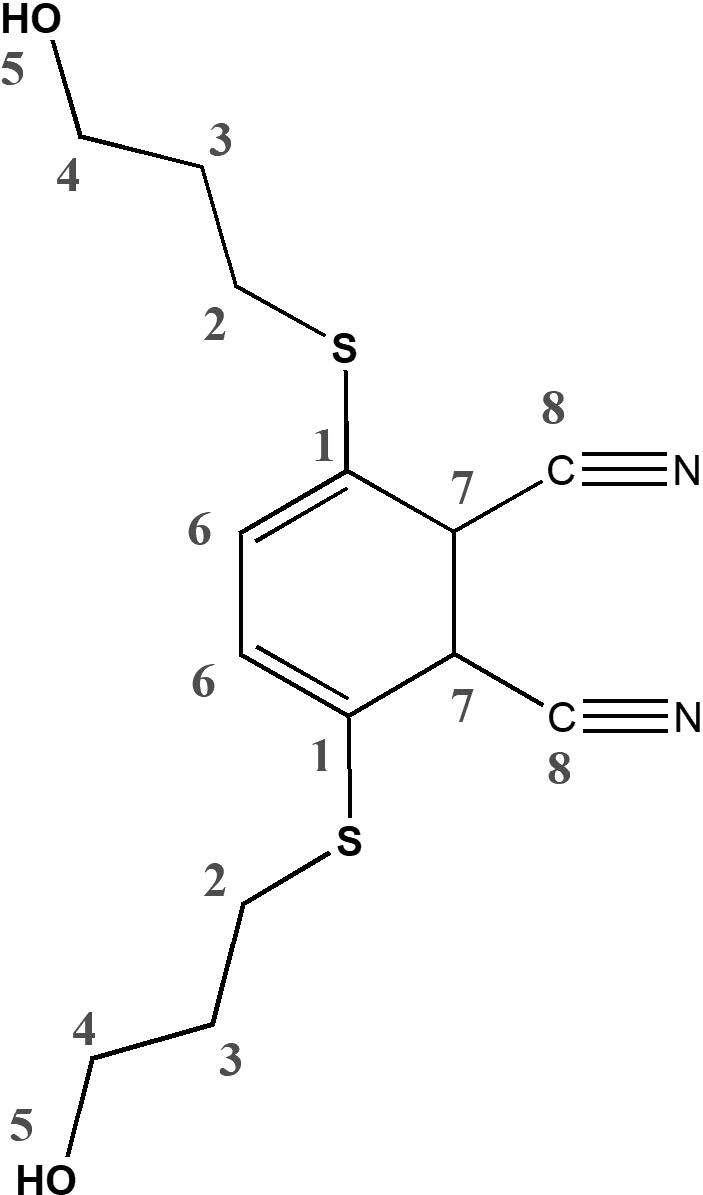 |
Atoms | Exp. | Gas phase | DMSO |
| C1 | 145.27 | 141.99 | 143.74 | |
| C2 | 31.87 | 32.06 | 32.31 | |
| C3 | 29.74 | 32.13 | 32.39 | |
| C4 | 59.55 | 62.29 | 61.59 | |
| C6 | 132.99 | 119.57 | 123.10 | |
| C7 | 141.15 | 113.72 | 111.04 | |
| C8 | 116.05 | 105.13 | 108.02 | |
| H2 | 3.17 | 2.08 | 3.08 | |
| H3 | 1.73 | 2.74 | 1.98 | |
| H4 | 3.49 | 4.01 | 4.02 | |
| H5 | 4.67 | 0.19 | 0.80 | |
| H6 | 7.81 | 7.11 | 7.63 |
Metallo-phthalocyanines ( MPc ) (4 –7) were synthesized by the reaction of 3 with anhydrous metal salts (CoCl2, NiCl2, CuCl2, and Zn(OAc)2) in n-pentanol in the presence of catalytic amounts of DBU under an argon atmosphere (Scheme 1). The shared features of all new products were performed by spectroscopic methods and elemental analysis such as UV-Vis, FT-IR,1H NMR (for compounds 3, 6, and 7),13C NMR (for compounds 3 and 7) and MS (micrOTOF).
In the1H NMR spectra of nickel(II) (6) and zinc(II) (7) phthalocyanines in DMSO-d6, the characteristic resonances of aromatic protons were observed at δ = 7.74–7.58 or 7.98 ppm, respectively. The other signals of compound 6 and 7 due to hydroxypropyl groups as multiplets at δ = 1.95 (6) or singlet at 2.11 ppm (7) for -CH2- protons, broad chemical shift at δ = 3.66 (6) and dublet at 3.77 ppm (7) for OCH2 protons, broad singlet at δ = 3.33 (6) ppm as superimposed H2O proton, and singlet δ = 3.45 (7) ppm for SCH2 protons and broad peaks at δ = 4.70 (6), 4.75 (7) ppm concerning OH (Figures S4 and S5).13C NMR spectra concerning C≡N signals at δ = 116.9 ppm belonging to precursor compound (3) disappeared in the case of NiPc and ZnPc formations. In addition to that, the appearance of novel signals at δ = 145.5 ppm and δ = 152.7 related to the inner core of phthalocyanines also indicated the formulation of metallo-phthalocyanine structures (Figures S6 and S7). The other13C NMR data of these molecules were almost identical to those of the precursor molecule (3) as anticipated. In the MS spectra of NiPc and ZnPc measured by the micrOTOF technique proved proposed structure due to the molecular ion peaks which observed at m/z = 1291.9 [M]+ and 1298.9 [M]+, respectively (Figures S8 and S9). In the FT-IR spectra of the compounds (4–7) (Figures S10–S13), the stretching vibrations concerning C≡N groups at 2221 cm-1belong to phthalonitrile (3) disappear after the cyclotetramerization reaction. The deformation of these vibrations confirmed the formation of phthalocyanines. The rest of the FT-IR spectra showed very close similarity to the starting compound. The mass spectra of compounds 4 and 5 recorded by micrOTOF technique also confirmed the molecular ion peaks at m/z = 1291.0 [M+H]+ and 1297.2 [M]+, respectively (Figures S14 and S15).
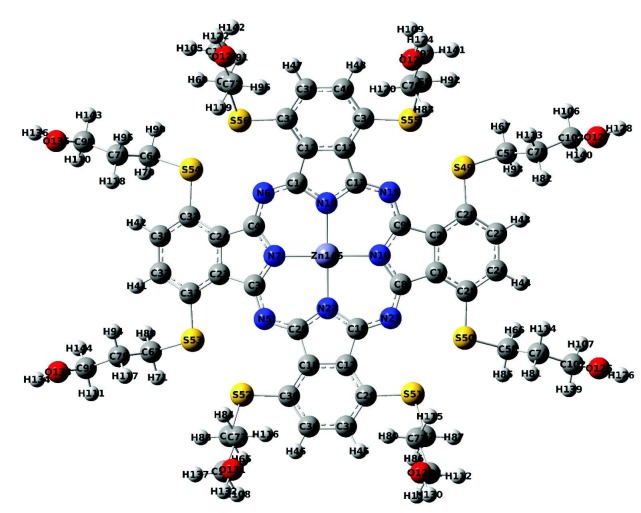
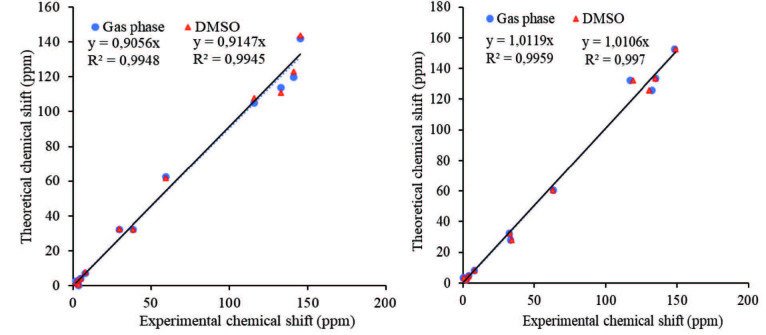
Theoretical1H and13C NMR chemical shifts of the compound 3 and ZnPc were calculated from B3LYP/6–31G (d,p) (see in the optimized molecular structure of the ZnPc in the ground state, Figure 1). The calculated NMR resonances concerning phthalonitrile compound 3 and ZnPc were given in Tables 1 and 2, respectively. The optimized geometric parameters of the ZnPc compound (bond lengths, bond angles, and dihedral angles) by B3LYP methods with 6–31G(d,p) as the basis set were presented in Tables S1 and S2. The correlations (Figure 2) between the experimental and calculation of the chemical shift values of the compounds are described by the equations of dcal (ppm) = 0,953 dexp + 0,6972 (R2 = 0,983) for compound 3 (Table 1) and dcal(ppm) = 0,9738 dexp + 0,6852 (R2 = 0,9915) for ZnPc (Table 2), respectively.
Figure.
Optimized geometry of the ZnPc in the ground state.
Figure 2.
The correlation graphs between the experimental and theoretical 1H and 13C chemical shift values of the molecules in DMSO.
Table 2.
1H and13C chemical shift of ZnPc (experimental and theoretical values).
 |
Atoms | Exp. | Gas phase | DMSO |
| C1 | 133.66 | 135.07 | 134.94 | |
| C2 | 32.26 | 32.36 | 32.78 | |
| C3 | 28.23 | 33.48 | 34.10 | |
| C4 | 60.41 | 63.14 | 62.96 | |
| C6 | 125.57 | 117.07 | 119.32 | |
| C7 | 132.69 | 132.46 | 130.59 | |
| C8 | 152.68 | 148.46 | 149.21 | |
| H2 | 3.45 | 3.07 | 3.19 | |
| H3 | 2.11 | 2.46 | 2.19 | |
| H4 | 3.77 | 4.07 | 4.08 | |
| H5 | 4.75 | 0.19 | 0.75 | |
| H6 | 7.98 | 7.79 | 7.99 |
3.2. Ground state electronic absorption spectra
Phthalocyanine compounds show two strong absorption bands in their electronic absorption spectroscopy that correlate to π → π* transitions. One of them is the so-called Q-band and seen at around 600–800 nm, and the other is called as B band and arise approximately 300–450 nm [24]. The ground-state electronic absorption spectra of Ni(II), Co(II), Cu(II) and Zn(II) phthalocyanines were measured in DMSO (Figure 3). The most of phthalocyanines show characteristic absorption band in the visible region approximately at around 600–750 nm named the Q-band and in the UV region at around 300–400 nm named B or Soret band [25]. The Q-band absorption in DMSO of the four metallated phthalocyanine can be aligned in the sequence Ni(II) > Cu(II) > Zn(II) > Co(II). The Q-bands were seen at 811, 797, 792, and 767 nm for compounds 6, 5, 7, and 4, respectively. These single absorptions in lower energy regions should be related π→π* transitions of the phthalocyanine cores. The Q-bands of compounds are significantly red-shifted among the metallo-phthalocyanines. It is well known that the electron-releasing groups such as alkylthio are bound to eight α-benzo positions of the phthalocyanine skeleton, the Q-band absorptions shift to longer wavelength. The transition metal ions have been settled in the phthalocyanine core may be expected they greatly affect absorption properties. Ni(II), Cu(II) and Co(II) have a similar electronegativity [26], so that the effect of electronegativity on the red-shifted is inferred to be similar [27]. The absorption maxima of 4 and 7 shifts to the shorter wavelength in the order of Co(II) and Zn(II) as central metals in the phthalocyanine core [28]. Ni(II) phthalocyanines have maximum red as an unusual shift among the metallatedphthalocyanines can be attributed the perfect planarity of the d8 electronic configuration of this metal [14].
Figure 3.
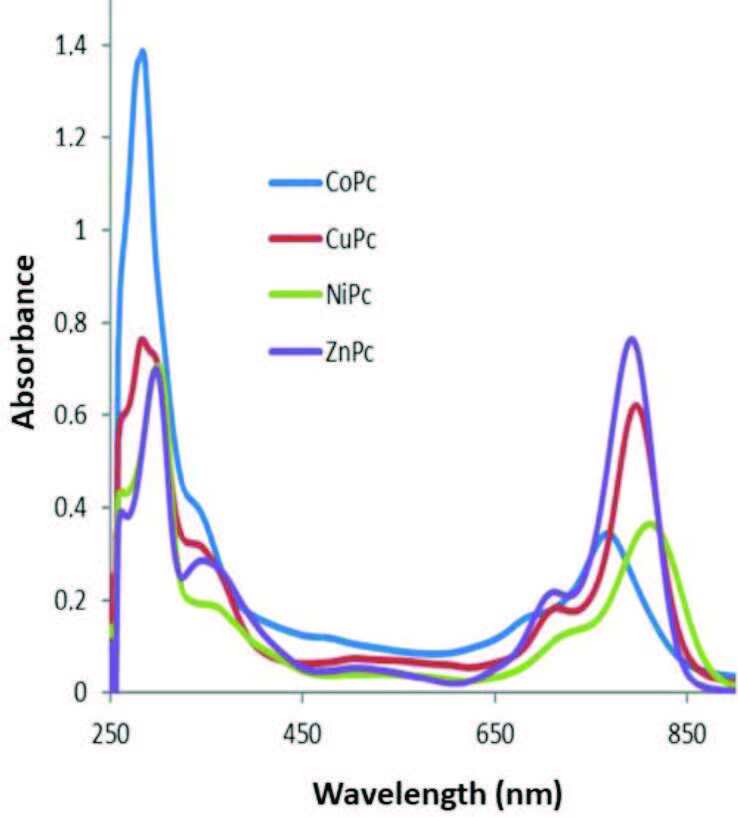
The UV-Vis spectra of MPc (1 × 10-5 M in DMSO).
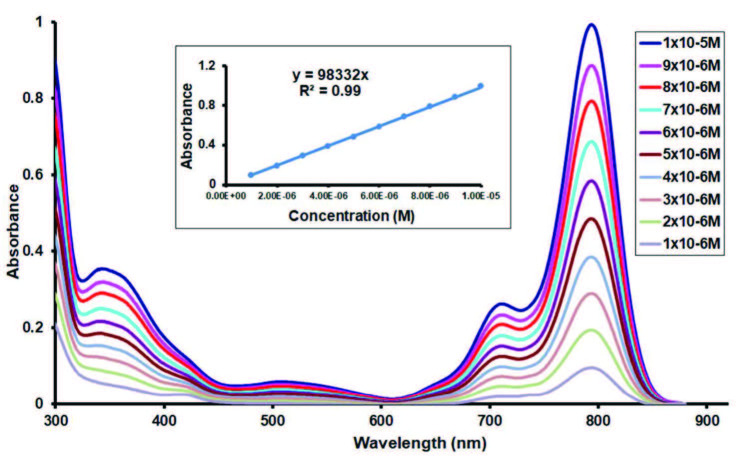
Aggregation is usually portrayed as a coplanar association of rings proceeding from monomer to dimer and higher order complexes. There are lots of parameters for aggregation in phthalocyanines; concentration, the nature of the solvent, nature of the substituents, complexed metal ions, and temperature [29]. The Q-band absorption maximum was independent of concentration and followed the Beer–Lambert law with a constant extinction coefficient in the studied concentration range [Figure 4 as an example for ZnPc (7)] for all studied metallo-phthalocyanines and these phthalocyanines did not exhibit any aggregation in the studied concentration range.
Figure 4.
Electronic absorption spectral changes for complex 7 in DMSO at different concentrations (Inset: Plot of absorbance versus concentration).
3.3 Electrochemical studies
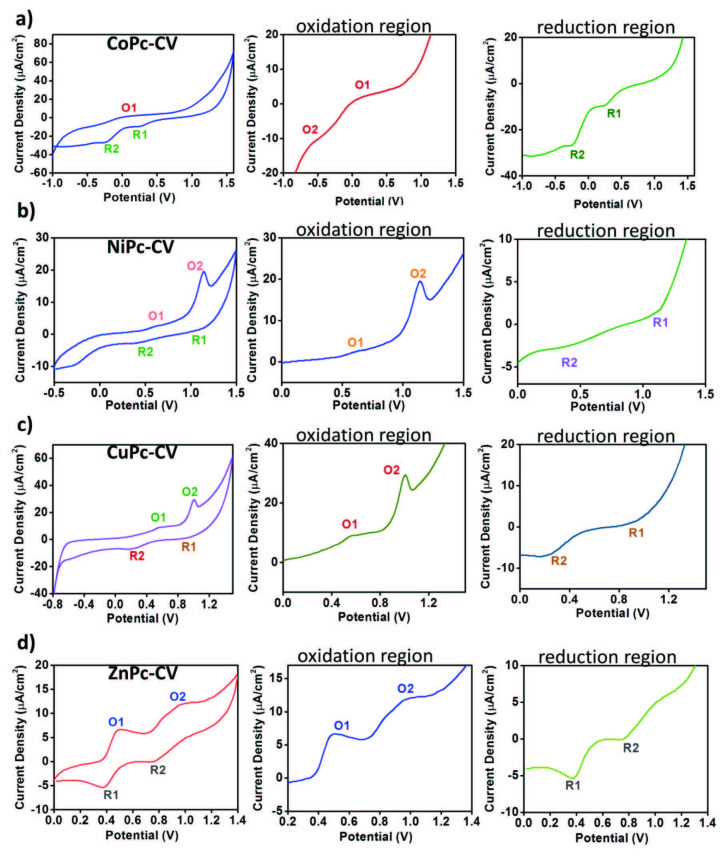
Voltammetric analyses of metallo-phthalocyanines have been performed with cyclic voltammetry (CV) as mentioned above. Figure 5 demonstrates the CV responses of the synthesized metallo-phthalocyanines recorded in the cathodic and anodic potential side in DCM:DMF (0.8:0.2)/TBP6 electrolyte system on an ITO working electrode.
CoPc gives nonquasi-reversible metal-based reduction at 0.25 V (R1). Also, it is thought that nonquasi-reversible Pc based oxidation and reduction reactions were observed at 0.1 V(O1) and –0.25 V(R2). Most of the studies in the literature on reduction properties of the MPc complexes including that such complexes have two reduction processes as one metal- and one ring-based [30,31].
Cyclic voltammetry graph of NiPc showed two oxidation peaks at 1.14 and 0.62 V and consecutive reduction peaks at 1.00 V and 0.38 V. When the electrochemical behavior of CuPc is examined, oxidation and reduction peak values have found to be lower than those of NiPc . In terms of CuPc , these values are 1.0 V and 0.56 V for oxidation and 0.18 V and 0.88 V for reduction. ZnPc had the lowest oxidation and reduction peak potential values among those of the other studied phthalocyanine derivatives. Compared to NiPc which has the oxidation peak values at 1.14 V and 0.62 V, ZnPc had lower redox peak values at 0.98 V and 0.49V. This result may be due to the smaller atomic radius of Zn metal comparing to the other metals’ atomic radii.
Figure 5.
CV responses of synthesized metallo-phthalocyanines a) CoPc, b) NiPc, c) CuPc, d) ZnPc recorded in the cathodic and anodic potential side in DCM:DMF (0.8:0.2)/ TBP6 electrolyte system on an ITO working electrode.
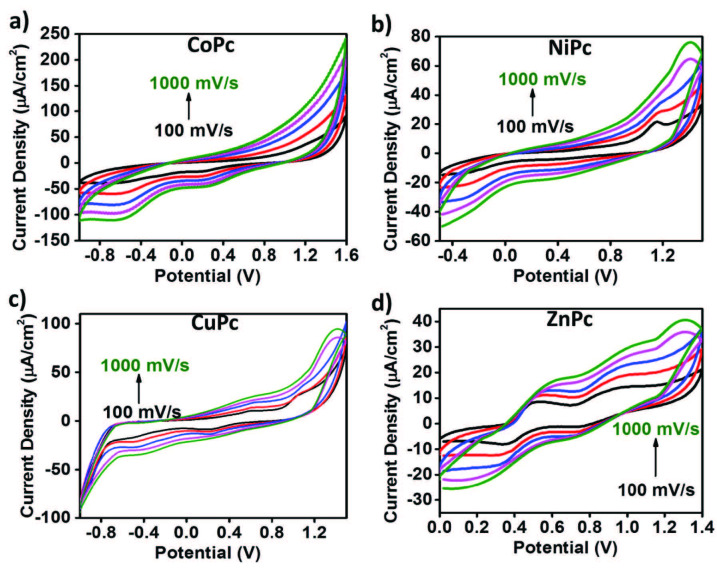
The speed of the applied potential can be changed by changing the scan rate in cyclic voltammetry experiments. Higher peak current values were obtained at high scan rates due to a reduction in the size of the diffusion layer [32]. Figure 6 shows a series of cyclic voltammograms recorded at different scan rates for an electrolyte solution containing metallo-phthalocyanine. The linearity of the peak current values with the square root of the scan rates proved that the electrochemical reaction on the electrode surfaces was diffusion controlled as expected [32].
Figure 6.
CV responses of synthesized metallo-phthalocyanines at different scan rates a) CoPc, b) NiPc, c) CuPc, d) ZnPc recorded in DCM:DMF (0.8:0.2)/TBP6 electrolyte system.
3.4. HOMO-LUMO studies
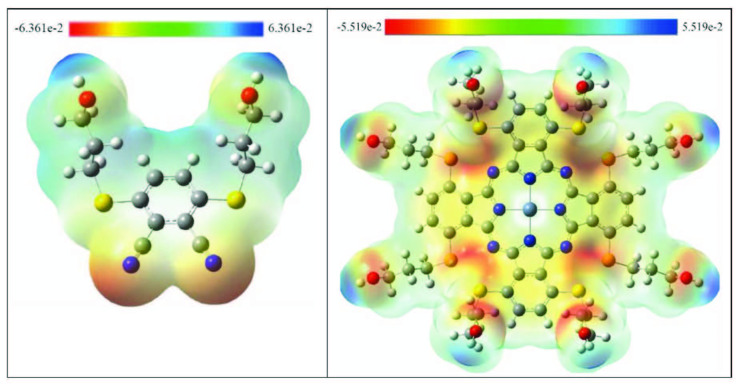
The electrostatic potential map of a molecule supply knowledge about the electron acceptor and electron donor regions. This knowledge may help us to see the relationships between the atoms regarding intramolecular and intermolecular hydrogen bonds. The distinctive values of the electrostatic potential at the area of the map are referred to by varied colours: blue refers to the most positive electrostatic potential, red refers to the most electronegative electrostatic potential site and green refers to the zero potential sites.
The electronic properties of a molecule can be calculated depending on HOMO and LUMO energies. In the calculations, the electron affinity ( A =–LUMO) and the ionization potential ( I =–HOMO) are the basic parameters. The other parameters such as absolute electronegativity (c=( I+A )/2), softness ( S =( I-A )/2), and absolute hardness (h=( I-A )/2) can be calculated accordingly.
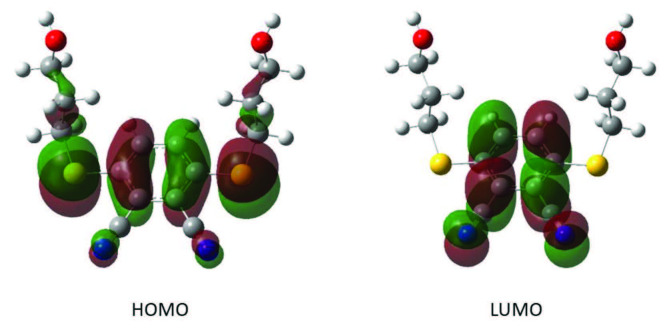
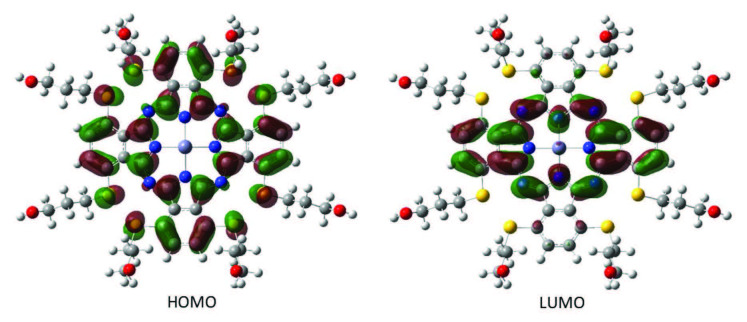
The dispersions of the HOMO and LUMO orbitals calculated for the B3LYP/6–31G(d, p) level for the compounds 3 and ZnPc were shown in Figures 7 and 8, respectively. In our calculations, ZnPc had a total of 1588 orbitals out of which 339 were filled and the rest were 1249 empty orbitals. The orbital numbered as 339 accounted for HOMO and 340 accounted for LUMO orbitals. The corresponding energy values were calculated as –4.29 eV for the HOMO and –2.42 eV for the LUMO energies with B3LYP/6–31G(d, p) level. The parameters for the 3,6-bis-(3-hydroxypropylthio)phthalonitrile were calculated at the same levels and the results were presented in Table 3.
Figure 7.
The HOMO and LUMO energies of the compound 3 with B3LYP/6–31G(d,p) basis set in gas phase.
Figure 8.
The HOMO-LUMO energies of the ZnPc.
Table 3.
Electronic properties for the phthalonitrile (3) and ZnPc compounds.
| Phthalonitrile (3) | ZnPc | |||||
|---|---|---|---|---|---|---|
| Electronic parameters | 6–31g(d) | 6–31g(d,p) | 6–31g+(d,p) | 6–31g(d) | 6–31g(d,p) | 6–31g+(d,p) |
| eV | 4.3009 | 4.2996 | 4.1644 | 1.6878 | 1.6857 | 1.6504 |
| l(Å) | 288.28 | 288.36 | 297.72 | 734.6 | 735.51 | 751.25 |
| Oscillator strengths | 0.1665 | 0.1458 | 0.2951 | 0.4347 | 0.4356 | 0.4374 |
| HOMO (au) | –0.22186 | –0.22195 | –0.23012 | –0.15748 | –0.15774 | –0.16678 |
| LUMO (au) | –0.07989 | –0.08017 | –0.09137 | –0.08906 | –0.08939 | –0.09940 |
| ΔE=LUMO-HOMO | 3.86 | 3.86 | 3.78 | 1.86 | 1.86 | 1.83 |
| TD/LUMO-HOMO | 4.30 | 4.30 | 4.16 | 1.69 | 1.69 | 1.65 |
| I (eV) | 6.04 | 6.04 | 6.26 | 4.29 | 4.29 | 4.54 |
| A (eV) | 2.17 | 2.18 | 2.49 | 2.42 | 2.43 | 2.70 |
| χ (eV) | 4.11 | 4.11 | 4.37 | 3.35 | 3.36 | 3.62 |
| Hardness(η) | 1.93 | 1.93 | 1.89 | 0.93 | 0.93 | 0.92 |
| Softness(s) | 0.52 | 0.52 | 0.53 | 1.07 | 1.08 | 1.09 |
| µ = -(I + A)/2 = - χ | –1.93 | –1.93 | –1.89 | –0.93 | –0.93 | –0.92 |
| ω = µ2 / (2η) | 0.966 | 0.965 | 0.944 | 0.465 | 0.465 | 0.458 |
| Dipole moment (debye) | 12.084518 | 12.036222 | 12.379576 | 6.017804 | 5.869903 | 7.233204 |
| Polarizability (α) (a.u) | 211.008667 | 212.453118 | 237.359667 | 724.789333 | 729.525000 | 792.483000 |
| Hyperpolarizability (β) (a.u) | 214.054379 | 215.011406 | 574.413256 | 387.135619 | 394.940022 | 1518.758510 |
As shown in Figure 9, the red region was localized on the nitrogen atoms and vicinity of the sulphur atoms in both the phthalonitrile and the ZnPc , whereas the blue region was delocalized on the OH groups. Hence, it was found that the ZnPc was useful to both bond metallically, and it has intermolecularly interacted. This result also supports the evidence of the charge analyses part.
Figure 9.
The MAP surface obtained at B3LYP/6–31G(d,p) level for phthalonitrile and ZnPc compounds.
NBO analysis is a tool for the determination of intramolecular interactions. The NBO analysis is used to specify the interactions between filled and empty orbitals of a molecule with the help of DFT method [33–35]. The NBO analysis, especially charge transfer, indicates the role of intermolecular orbital interaction in the compound. In tandem with this, the stabilization energy E(2) linked with electron delocalization between donor and acceptor is predicted for each donor NBO (i) and acceptor NBO (j) as follows:
where qi is the orbital occupancy of the ith donor, Ej and Ei are the diagonal elements (orbital energies) and F(i,j) is the off-diagonal NBO Fock matrix element. The hyper conjugative σ→σ* interactions play an extremely significant role in the molecule represent the weak departures from a strictly localized natural Lewis structure that constitutes the primary “noncovalent” effects [36]. The results of the NBO analysis of the ZnPc collected with B3LYP/6–31G(d,p) basis set presented in Table S3.
The interactions between C25-C26 (π*) and π*(C1-C2), C27-C28 (π*) and π*(C1-C2), the stabilization of 275.24 kcal/mol, which denotes larger delocalization. According to Table S3, C25-C26 is rich in electrons since close to the electron release group. That is why it is a donor. In contrast, C1-C2 is acceptor because the electron is poor. The interaction between the C8-N10 (σ *) π*(C19-N21), C3-N5 (π*) π*(C23-C31), C4-N7 (π*) π*(N6-C14), C13-N16 (π*) π*(C9-N15), C19-N21 (π*) π*(C17-C29), C20-N22(π*) π*(C3-N5) also represent the larger delocalization. The E(2) value is essential chemically and may be exploited as a measure of the intramolecular delocalization.
The calculated visible absorption maxima at TD–B3LYP/6–31G(d,p) of λ which are a function of the electron availability were displayed in Table 3. The most likely transition for the molecule is the HOMO-LUMO transition at 339→340 because the maximum f = 0.4356 (oscillator strength) value is in the excited state-1 at 735.51 nm. HOMO-LUMO+1 transition was calculated at 339→341 molecular orbital, excited state-2: 735.21 nm. Typically, the energy bandgap in inorganic materials is ~ 1.5 eV, in organic materials is in the range of 1.5–3.5 eV. In accordance with this, the compound is capable of being a potential molecule for inorganic semiconductor materials [37]. Additionally, according to ligand, this value reveals that the compound becomes more conductive electrically.
3.5. Singlet oxygen generation properties
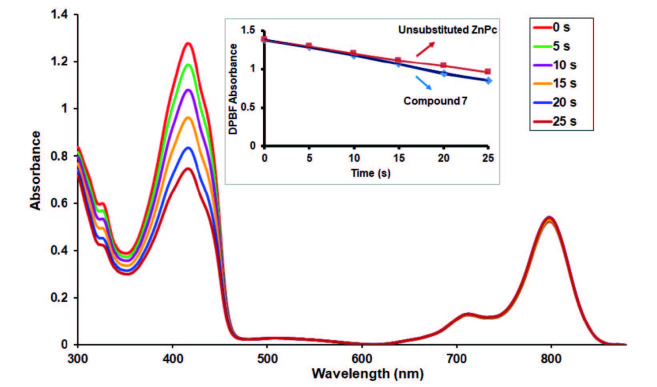
PDT is a treatment for cancer where light, molecular oxygen, and photosensitizer are used in combination to produce cytotoxic forms of oxygen such as singlet oxygen. The generation of singlet oxygen is the key to show PDT potential of the compounds [38]. The singlet oxygen production of studied zinc(II) phthalocyanine (7) was determined with the chemical method in DMSO. 1,3-diphenylisobenzofuran (DPBF) was used as a singlet oxygen scavenger which causes the formation of endoperoxide species. A time-dependent decrease of DPBF absorbance at 417 nm was observed for phthalocyanine photosensitizer 7. There was no change in the Q-band intensity during the ΦΔdeterminations and it supports that studied phthalocyanine did not show any degradation by used light irradiation (Figure 10). The singlet oxygen production of studied zinc(II) phthalocyanine (7) was found higher compared to unsubstituted zinc(II) phthalocyanine in DMSO (Figure 10, inset). The singlet oxygen generation properties of other metallo-phthalocyanines (4, 5, and 6) studied in this work did not investigate due to paramagnetic behavior of the used Co, Ni, and Cu metal ions the cavities of these phthalocyanines because paramagnetic metal ions reduce the photoactivity of the molecules.
Figure 10.
Electronic absorption spectral changes during singlet oxygen determination. This determination was for compound 7 in DMSO at a concentration of 1.0 × 10-5 M using DPBF at a concentration of 1.0 × 10-4 M (Inset: Plot of DPBF absorbance versus time).
The synthesis of the phthalocyanine compounds bearing different alkylthio groups on the phthalocyanine ring were given in the literature but the photochemical properties of these derivatives were studied limitedly. On the other hand, the phthalocyanine derivatives substituted at the nonperipheral octa positions of the phthalocyanine macrocycle are very rare in the literature. The studied zinc(II) phthalocyanine (7) showed higher singlet oxygen production in comparison with other octa nonperipheral substituted photosensitizers containing different alkylthio groups such as 2-propoxy, benzyloxy or 3,5 bis(benzyloxy)benzyloxy groups [39]. Similarly, the studied zinc(II) phthalocyanine (7) showed higher singlet oxygen production in comparison with alkylthio substituted pyridoporphyrazines [40]. Additionally, the phthalocyanine 7 exhibited similar singlet oxygen generation when compared to the nonperipherally octa-sulfanyl substituted zinc phthalocyanine [41].
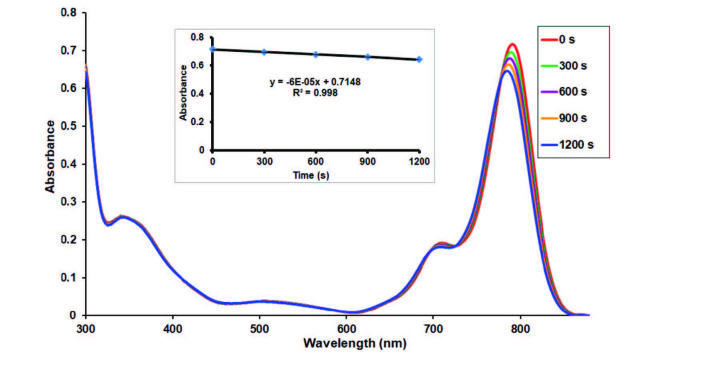
3.6. Photodegradation studies
Photodegradation quantum yield can be used to study the stability of photosensitizer during the photocatalytic reaction in PDT [42]. The current study shows that photodegradation properties of studied zinc(II) phthalocyanine (7) were determined in DMSO by monitoring the collapse of their absorption bands underused light irradiation with increasing time (Figure 11). The Φd value of zinc(II) phthalocyanine was found the order of 3.79 × 10–5 (between 10–3 and 10–6 for ideal photosensitizer) in DMSO [21]. The Φd value of the investigated zinc(II) phthalocyanine (7) was found slightly higher than unsubstituted zinc(II) phthalocyanine (Φd = 2.61 × 10–5) [18]. On the other hand, the phthalocyanine 7 exhibited lower photodegredation quantum yield value when compared to the non-peripherally octa-sulfanyl substituted zinc phthalocyanine [41] which means that the studied zinc(II) phthalocyanine (7) exhibited higher stability to light irradiation.
Figure 11.
The electronic absorption spectral changes of zinc(II) phthalocyanine (7) in DMSO under light irradiation revealing the vanishing of the Q-band at 5 min intervals (Inset: Plot of absorbance vs. time).
4. Conclusion
In this study, the phthalonitrile derivative substituted with 3-hydroxypropylthio groups at 3 and 6 positions as ligand and its non-peripheral octa substituted metallo-phthalociyanines [M =Zn(II), Ni(II), Cu(II) and Co(II)] were synthesized and characterized. Electrochemical properties of the proposed compounds also investigated because of these kinds of compounds show the possible potential usage in electro-catalysis, electrosensing, and electrochromic devices. When the ΔE values in Table 3 are compared, the ZnPc compound is electrically more conductive than phthalonitrile. In this electrical conductivity, zinc(II) plays an important role. Besides, the molecular geometry and GIAO1H and13C NMR chemical shift values of the molecule in the ground state had been estimated by applying B3LYP with 6–31G(d,p) basis set. Also, the photochemical properties such as singlet oxygen generation and photodegradation under light irradiations were studied for the determination of possible photosensitizer ability of zinc(II) phthalocyanine derivative 7. These properties of the other studied metallo-phthalocyanines did not investigate because of the paramagnetic behavior of metal ions (Co, Ni, and Cu) in the cavities of these phthalocyanines. The absorbance of the new zinc(II) phthalocyanine (7) was studied in DMSO solutions at different concentrations for determination of the most suitable concentration for further photochemical properties. The singlet oxygen production of this phthalocyanine (7) was determined in DMSO using a chemical method. The singlet oxygen production of studied zinc(II) phthalocyanine (7) was found higher compared to unsubstituted zinc(II) phthalocyanine in DMSO. In this study, the photodegradation behavior of the zinc(II) phthalocyanine (7) was determined in DMSO. The Φd value of zinc(II) phthalocyanine was found in the order of 3.79 × 10–5 in DMSO. This value is slightly higher than unsubstituted zinc(II) phthalocyanine [37,43].
Supplementary Materials
Acknowledgments
The authors are grateful to Pamukkale University–PAUBAP (Project No. 2020HZDP002) as they supported this project financially.
References
- Torre GD Vazquez P Agullo-Lopez F Torres T. Phtahlocyanines and related compounds: Organic targets for nonlinear optical applications. Journal of Materials Chemistry . 1998;8:1671–1683. [Google Scholar]
- Phthalocyanines Properties and Applications 3 1993.
- Phthalocyanines Properties and Applications 1 1989.
- Parra V Bouvet M Brunet J Rodríguez-Méndez ML Saja JA On the effect of ammonia and wet atmospheres on the conducting properties of different lutetium bisphthalocyanine thin films. Thin Solid Films . 2008;5:9012–9019. [Google Scholar]
- Bouvet M. Phthalocyanine-based field-effect transistors as gas sensors. Analytical and Bioanalytical Chemistry . 2006;384:366–373. doi: 10.1007/s00216-005-3257-6. [DOI] [PubMed] [Google Scholar]
- Yang F Forrest SR Photocurrent generation in nanostructured organic solar cells. ACS Nano . 2008;2:1022–1032. doi: 10.1021/nn700447t. [DOI] [PubMed] [Google Scholar]
- Forrest SR Ultrathin organic films grown by organic molecular beam deposition and related techniques. Chemical Review . 1997;97:1793–1896. doi: 10.1021/cr941014o. [DOI] [PubMed] [Google Scholar]
- Phthalocyanines Properties and Applications 2 1993.
- Bilgin A Ertem B Gök Y. Synthesis and characterization of new metal-free and metallophthalocyanines containing spherical or cylindrical macrotricyclic moieties. Polyhedron . 2005;24:1117–1124. [Google Scholar]
- Phthalocyanines Properties and Applications 4 1996.
- Emmelius M Pawlowski G Vollmann HW Materials for optical data storage. Angewandte Chemie International Edition . 1989;28:1445–1471. [Google Scholar]
- Yılmaz F Özer M Kani İ Bekaroğlu Ö Catalytic activity of a thermoregulated, phase-seperable Pd(II)-perfluoroalkylphthalocyanine complex in an organic/fluorous biphasic system: hydrogenation of olefins. Catalysis Letters . 2009;130:642–647. [Google Scholar]
- Rosenthal I Phthalocyanines as photodynamic sensitizers. Photochemistry and Photobiology . 1991;53:859–870. doi: 10.1111/j.1751-1097.1991.tb09900.x. [DOI] [PubMed] [Google Scholar]
- Özçeşmeci İ Okur Aİ Gül A New phthalocyanines bearing tetra(hydroxylethylthio) functionalities. Dyes and Pigments . 2007;75:761–765. [Google Scholar]
- Necedova MM Martinicka A Magdolen P Novakova V Zahradnik P Phthalocyanine-triphenylamine dyads: synthesis, electrochemical, spectral and DFT study. Dyes and Pigments . 2017;141:448–456. [Google Scholar]
- Wannebroucq A Meunier-Prest R Chambron JC Braxhais CH Suisse JM Synthesis and characterization of fluorophthalocyanines bearing four 2-(2-thienyl)ethoxy moieties: from the optimization of the fluorine substitution to chemosensing. RCS Advances . 2017;7:41272–41281. [Google Scholar]
- Tuncer S Koca A Gül A Avcıata U -Dithiaheterocycle-fused porphyrazines: Synthesis, characterization, voltammetric and spectroelectrochemical properties. Dyes and Pigments . 2009;1:144–151. [Google Scholar]
- Gurol I Durmuş M Ahsen V Nyokong T Synthesis, photophysical and photochemical properties of substituted zinc phthalocyanines. Dalton Transactions . 2007;34:3782–3791. doi: 10.1039/b704345g. [DOI] [PubMed] [Google Scholar]
- Schumann C Nalewey MA Pong AJ Chan KJ A multifunctional theronostic platform based on phthalocyanine-loaded dendrimer for imge-guided drug delivery and phthadynamictheraphy. Molecular Pharmaceuties . 2013;10:3946–3958. doi: 10.1021/mp400397t. [DOI] [PubMed] [Google Scholar]
- Shao J Dai Y Zhao W Xie J Xue J Intracellular distribution and mechanism of photosensitizer Zn(II)-phthalocyanine solubilized in cremophor EL against human hepatocellular carcinoma HepG2 cells. Cancer Letters . 2013;330:49–56. doi: 10.1016/j.canlet.2012.11.017. [DOI] [PubMed] [Google Scholar]
- and photophysical charactrization. Photosensitizers in Medicine, Environment and Security . 2012. pp. 135–266.
- Wang R Zhao Y Zhu C Huang X New Synthesis of 3,6-dibromophthalonitrile and phthalocyanine having eight thienyl substituents at peripheral α-positions. Journal of Heterocyclic Chemistry . 2015;52:1230–1233. [Google Scholar]
- Baygu Y Gök Y. A highly water-soluble zinc(II) phthalocyanines as potential for PDT studies: synthesis and characterization. Inorganic Chemistry Communication . 2018;96:133–138. [Google Scholar]
- 2010. pp. 45–88.
- Kobayashi N Furuyama T Satoh K. Rationally designed phthalocyanines having their main absorption band beyond 1000 nm. Journal of the American Chemical Society . 2011;133:19642–19645. doi: 10.1021/ja208481q. [DOI] [PubMed] [Google Scholar]
- 1978. pp. 16–16.
- Furuyama T Satoh K Kushiya T Kobayashi N. Design properties of phthalocyanine complexes with main-group elements showing main absorption and fluorescence beyond 1000 nm. Journal of the American Chemical Society . 2014;136:765–776. doi: 10.1021/ja411016f. [DOI] [PubMed] [Google Scholar]
- Koçan H Burat AK Synthesis and characterization of [7-(trifluoromethyl)-quinolin-4-yl] oxy-substituted phthalocyanines. Monatshefte für Chemie . 2013;144:171–177. [Google Scholar]
- Engelkamp H Nolte RJM Molecular materials based on crown ether functionalized phthalocyanines. Journal of Porphyrins and Phthalocyanine . 2000;4:454–459. [Google Scholar]
- Nas A Biyiklioglu Z Fandaklı S Sarkı G Yalazan H -yl)phenoxy) substituted cobalt, iron and manganese phthalocyanines: synthesis and electrochemical analysis. Tetra(3-(1 . 2017;466:5–5. [Google Scholar]
- Hanabusa K Shirai H Catalytic functions and application of metallophthalocyanine polymers. ChemInform . 1993;24:197–222. [Google Scholar]
- Elgrishi N Rountree KJ McCarthy BD Rountree ES Eisenhart TT A practical beginner’s guide to cyclic voltammetry. Journal of Chemical Education . 2018;95:197–206. [Google Scholar]
- Francl MM Pietro WJ Hehre WJ Self-consistent molecular orbital methods, XXIII. A polarization-type basis set for second-row elements. The Journal of Chemical Physics . 1982;77:3654–3665. [Google Scholar]
- Hariharan PC Pople JA The influence of polarization functions on molecular orbital hydrogenation energies. Theoretica Chimica Acta . 1973;28:213–222. [Google Scholar]
- Figgen D Rauhut G Dolg M Stoll H Energy-consistent pseudopotentials for group 11 and 12 atoms: adjustment to multi-configuration Dirac–Hartree–Fock data. Chemical Physics . 2005;311:227–244. [Google Scholar]
- Peterson KA Puzzarini C Zn Systematically convergent basis sets for transition metals. II. pseudopotential-based correlation consistent basis sets for the group 11 (Cu. Theoretical Chemistry Accounts . 2005;12:283–296. [Google Scholar]
- Yıldız B Baygu Y Kara I Dal H Gök Y. The synthesis, characterization and computational investigation of new metalloporphyrazine containing 15-membered S4 donor macrocyclic moieties. Tetrahedron . 2016;72:6972–6981. [Google Scholar]
- Shao W Wang H He S Shi L Peng K Photophysical properties and singlet oxygen generation of three sets of halogenated corroles. Journal of Physical Chemistry B . 2012;116:14228–14234. doi: 10.1021/jp306826p. [DOI] [PubMed] [Google Scholar]
- Tillo A Stolarska M Kryjewski M Popenda L Sobotta L Phthalocyanines with bulky substituents at non-peripheral positions-synthesis and physico-chemical properties. Dyes and Pigments . 2016;127:110–115. [Google Scholar]
- Sakamoto K Watabiki S Yoshino S Komoriya T. Synthesis of alkylthio substituted pyridoporphyrazines and their photophysicochemical properties. Journal of Porphyrins and Phthalocyanines . 2017;21:658–664. [Google Scholar]
- Tekdaş DA Kumru U Gürek AG Durmuş M Ahsen V Towards near-infrared photosensitisation: a photosensitising hydrophilic non-peripherally octasulfanyl-substituted Zn phthalocyanine. Tetrahedron Letters . 2012;53:5227–5230. [Google Scholar]
- Erdoğmuş A Durmuş M Uğur AL Avciata O Avciata U Synthesis, photophysics, photochemistry and fluorescence quenching studies on highly soluble substituted oxo-titanium(IV) phthalocyanine complexes. Synthetic Materials . 2010;160:17–17. [Google Scholar]
- Baygu Y Yıldız B Kara I Dal H Gök Y Synthesis, characterization and computational investigation of novel metalloporphyrazines containing 15-memberedO2S2-donor macrocyclic moieties. Journal of Porphyrins and Phtalocyanines . 2018;22:207–220. [Google Scholar]
- 1980.
- S2. Wang R Zhao Y Zhu C Huang X New synthesis of 3,6-dibromophthalonitrile and phthalocyanine having eight thienyl substituents at peripheral α-positions. Journal of Heterocyclic Chemistry . 2015;52:1230–1233. [Google Scholar]
- S3. Baygu Y Gök Y. A highly water-soluble zinc(II) phthalocyanines as potential for PDT studies: synthesis and characterization. Inorganic Chemistry Communication . 2018;96:133–138. [Google Scholar]
- Photosensitizers in Medicine, Environment and Security . 2012. pp. 135–266.
- S5. Spiller W Kliesch H Wöhrle D Hackbarth S Schnurpfeil G Singlet oxygen quantum yields of different photosensitizers in polar solvents and micellar solutions. Journal of Porphyrins and Phthalocyanines . 1998;2:145–158. [Google Scholar]
- S6. Frisch MJ Trucks GW Schlegel HB Scuseria GE Robb MA revision B. Wallingford CT Gaussian . 01;16 [Google Scholar]
- Millam JM. GaussView 6 . pp. 2000–2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials