Abstract
Background: Studies comparing the cardiac consequences of hydrophilic and lipophilic statins in experimental and clinical practice settings have produced inconsistent results. In particular, evidence focusing on diabetic patients after acute myocardial infarction (AMI) is lacking.
Methods and Results: From the Osaka Acute Coronary Insufficiency Study (OACIS) registry database, 1,752 diabetic patients with AMI who were discharged with a prescription for statins were studied. Long-term outcomes were compared between hydrophilic and lipophilic statins, including all-cause death, recurrent myocardial infarction (re-MI) and admission for heart failure (HF) and a composite of these (major adverse cardiac events; MACE). During a median follow-up period of 1,059 days, all-cause death, non-fatal re-MI, admission for HF, and MACE occurred in 95, 89, 112 and 249 patients, respectively. Although there was no significant difference between statins in the risk of all-cause death, re-MI and MACE, the risk of HF admission was significantly lower in patients with hydrophilic than lipophilic statins before (adjusted hazard ratio [aHR], 0.560; 95% CI: 0.345–0.911, P=0.019) and after (aHR, 0.584; 95% CI: 0.389–0.876, P=0.009) propensity score matching. Hydrophilic statin use was consistently associated with lower risk for HF admission than lipophilic statins across the subgroup categories.
Conclusions: In the present diabetic patients with AMI, hydrophilic statins were associated with a lower risk of admission for HF than lipophilic statins.
Key Words: Hydrophilic and lipophilic, Myocardial infarction, Prognosis, Secondary prevention, Statin
Thanks to their effect in decreasing low-density lipoprotein cholesterol (LDL-C) level, statins, 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase inhibitors, are widely used in clinical practice settings following acute myocardial infarction (AMI) as a class I indication for secondary prevention.1 Statins in clinical use are divided by pharmacokinetic properties into hydrophilic and lipophilic types. To date, however, studies on the cardiac consequences of these statins in experimental and clinical practice settings have been conflicting. Several reports have reported better outcomes for hydrophilic statins in non-ischemic heart failure (HF)2 and ST-elevation myocardial infarction (STEMI),3 whereas others have suggested favorable outcomes for lipophilic statins in patients with HF.4,5 In contrast, other comparisons of the 2 types of statins have shown no difference: a meta-analysis of coronary artery disease (CAD) showed no difference in major adverse cardiac events (MACE), MI, cardiovascular death, or all-cause mortality,6 while a large clinical study in patients with STEMI found no difference in a composite of death due to any cause, non-fatal MI, non-fatal stroke, unstable angina and congestive HF requiring hospital admission, or in any type of coronary revascularization.7 Further, no study has yet examined whether either statin type is associated with lower risk of long-term outcomes in diabetic patients after AMI, a group considered at higher risk than those without diabetes mellitus (DM). Statin treatment has been reported to be beneficial in diabetic patients with AMI.8,9 Further, a recent report found that hydrophilic statins increased adiponectin and decreased hemoglobin A1c, suggesting that these agents may be associated with lower risk in subsequent outcomes in diabetic patients after AMI through a protective effect of adiponectin and improved glucose metabolism.2 Here, we investigated whether hydrophilic statin use is associated with lower risk for long-term outcomes in diabetic patients with AMI.
Methods
The Osaka Acute Coronary Insufficiency Study (OACIS) is a prospective, multicenter, observational study in which 25 collaborating hospitals in the Osaka region of Japan record demographic, procedural, and outcome data and collect blood samples from patients with AMI. The OACIS is registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) in Japan (ID: UMIN000004575), and the study protocol was approved by the institutional review board of each participating hospital. A detailed description of the OACIS has been published elsewhere.10–14
A total of 11,072 patients with AMI were registered in the OACIS registry between April 1998 and September 2012. Of these, 913 patients who died as inpatients, 6,346 without DM, and 1,999 who did not receive statins at discharge were excluded. A further 62 patients whose data were not available for a diagnosis of DM and medication at discharge were also excluded. The remaining 1,752 diabetic patients following AMI were analyzed as the study subjects. Investigative cardiologists and research coordinators collected patient demographic and clinical data during hospitalization from medical records and by direct interview of patients, their family members and treating physicians, and then added their findings onto the case report form. After written informed consent for enrollment in OACIS was obtained, all in-hospital data were transmitted to the data collection center (Department of Internal Medicine and Therapeutics, Osaka University Graduate School of Medicine) for processing and analysis. Collaborating hospitals were encouraged to enroll consecutive patients with AMI irrespective of treatment strategy or outcome. After discharge, follow-up clinical data were obtained at 3 and 12 months after the onset of AMI and annually thereafter. Information on long-term outcomes including all-cause death, recurrent MI (re-MI) and admission for HF was collected by visiting the research outpatient clinic or by verbal or written contact with the patient or family members. DM was defined as a history of DM, fasting blood sugar ≥126 mg/dL at discharge, hemoglobin A1c measured during hospitalization ≥6.5% or use of anti-diabetic drugs. Long-term outcomes after discharge included re-MI, admission for HF, all-cause death, and a composite of these (MACE).
Statistical Analysis
Categorical variables were compared using the chi-squared test with continuity correction or Fisher’s exact test. Continuous variables are presented as median (IQR) or mean±SD and were compared using the unpaired t-test or 2-tailed Wilcoxon rank sum test between patients with a hydrophilic or lipophilic statin at discharge. To minimize differences in baseline characteristics between the 2 groups, patients were matched in a 1-to-1 manner on the basis of propensity scores, which were calculated for each patient using a logistic regression model15 that included a total of 14 variables (age, male sex, body mass index [BMI], hypertension, smoking, prior MI, STEMI, peak creatine kinase ≥3,000 IU/L, Killip ≥II on admission, reperfusion therapy, ejection fraction [EF] <40% before discharge, angiotensin-converting enzyme inhibitor or angiotensin receptor blockers, β-blockers, and antiplatelets at discharge). According to the propensity score, patients were selected using a 5-to-1 digit-matching technique using the nearest-neighbor method.16,17 Long-term outcomes of re-MI, admission for HF, all-cause death and MACE were assessed using Kaplan-Meier analysis and compared using the log-rank test. Cox regression analysis was performed to assess whether treatment with either statin was associated with a lower risk of these events. Propensity score was incorporated as a variable into the models before matching. Subgroup analysis was performed in patients after propensity score matching to clarify an interaction between statin treatment and subgroup category for long-term outcome, if statistical significance for the outcomes was observed in the overall patients. All analyses were performed using SPSS version 23 (SPSS, Chicago, IL, USA). Statistical significance was defined as P<0.05 and P for interaction <0.1.
Results
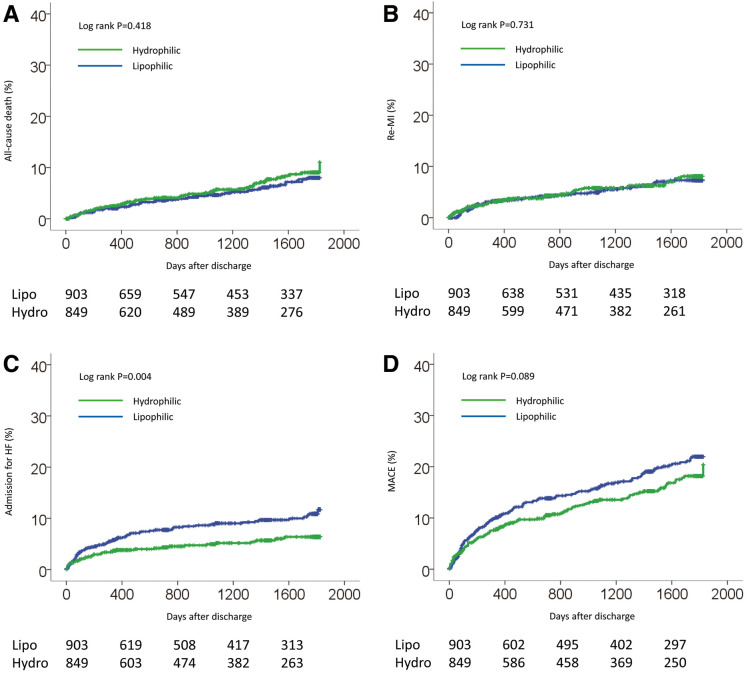
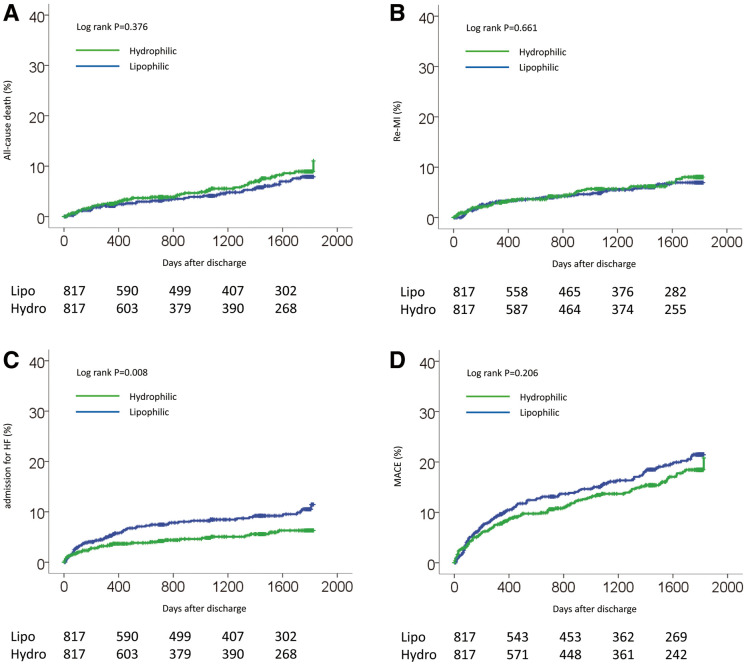
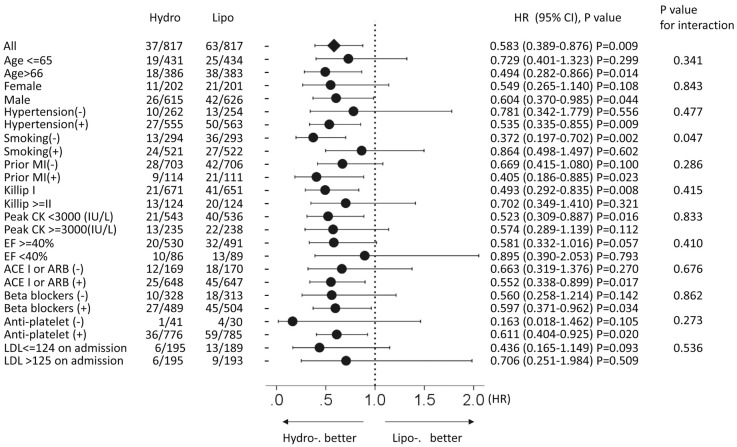
From a large database of the OACIS registry, we identified 1,752 patients who fulfilled the inclusion criteria of the present study. Of these, pravastatin and rosuvastatin, which are categorized as hydrophilic statins, were prescribed to 596 and 253 patients, respectively, while simvastatin, atorvastatin, pitavastatin and fluvastatin, which are categorized as lipophilic statins, were prescribed to 123, 567, 139 and 74 patients, respectively. Baseline characteristics are listed in Table 1. Before propensity score matching, compared with the lipophilic statin group, hydrophilic statin group patients were more likely to be older and more likely to have lower cholesterol on admission. There was no significant difference between groups in sex, BMI, coronary risk factors (smoking and hypertension), Killip class ≥II, or reperfusion therapy between the 2 types of statin. There was also no significant difference in EF measured on echocardiography before discharge or in medication at discharge, except for antiplatelets, which were significantly lower in the hydrophilic statin group than in lipophilic statin group. After propensity score matching, the differences between the groups were attenuated and all baseline characteristics became well-balanced, with P>0.05, except for total cholesterol level. Factors on multivariate logistic regression analysis associated with prescription of a hydrophilic statin at discharge were older age, smoking habit, and non-prescription of antiplatelet therapy at discharge (Table 2). During a median follow-up period of 1,059 days (IQR, 330–1,784 days), no significant difference was observed in the incidence of all-cause death, re-MI or MACE between the groups before propensity score matching (Table 3; Figure 1A,B,D). In contrast, admission for HF was significantly lower in the hydrophilic statin group than in the lipophilic statin group (Table 3; Figure 1C). On multivariate Cox regression analysis there was no significant difference in the risk of MACE, all-cause death or re-MI between the statins, whereas hydrophilic statins were significantly associated with a lower risk of admission for HF than lipophilic statins (Table 4). This was consistent with the results after propensity score matching (Figure 2; Tables 3,5). There was no significant interaction between type of statin and admission for HF in the subgroups other than presence or absence of smoking and treatment for admission for HF (Figure 3). The lower risk with hydrophilic statins compared with lipophilic statins for admission for HF appeared to be consistent across the subgroups.
Table 1.
Baseline Characteristics vs. Statin and PSM Status
| Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|
| Hydrophilic (n=849) |
Lipophilic (n=903) |
All (n=1,752) |
P-value | Hydrophilic (n=817) |
Lipophilic (n=817) |
All (n=1,634) |
P-value | |
| Age (years) | 64.8±10.9 | 63.6±11.2 | 64.2±11.1 | 0.029 | 64.7±10.9 | 64.2±10.8 | 64.4±10.9 | 0.347 |
| Male | 75.1 | 75.6 | 75.4 | 0.812 | 75.3 | 75.4 | 75.3 | 0.954 |
| BMI (kg/m2) | 24.8±3.8 | 25±4.2 | 24.9±4.0 | 0.323 | 24.8±3.8 | 24.9±4.2 | 24.9±4 | 0.564 |
| Hypertension | 68.4 | 69.5 | 69.0 | 0.603 | 67.9 | 68.9 | 68.4 | 0.670 |
| Smoking | 64.5 | 61.8 | 63.1 | 0.238 | 63.9 | 64.0 | 64.0 | 0.959 |
| Prior MI | 13.9 | 14.0 | 13.9 | 0.974 | 14.0 | 13.6 | 13.8 | 0.829 |
| STEMI | 84.7 | 84.7 | 84.7 | 0.982 | 85.0 | 83.8 | 84.4 | 0.488 |
| Creatine (mg/dL) | 1.1±1.1 | 1.1±1.2 | 1.1±1.1 | 0.862 | 1.1±1.1 | 1.0±1.1 | 1.1±1.1 | 0.691 |
| eGFR <60 mL/min/1.73 m2 | 40.9 | 38.7 | 39.8 | 0.386 | 40.8 | 38.1 | 39.4 | 0.304 |
| eGFR (mL/min/1.73 m2) | 66.2±25.1 | 68.8±31.6 | 67.6±28.7 | 0.083 | 66.3±25.2 | 69.1±31.6 | 67.7±28.7 | 0.071 |
| Blood sugar (mg/dL) | 222±97 | 225±90 | 224±93 | 0.583 | 223±97 | 224±91 | 223±94 | 0.789 |
| Total cholesterol (mg/dL) | 204±48 | 210±52 | 207±50 | 0.005 | 204±48 | 210±52 | 207±50 | 0.021 |
| HDL-C (mg/dL) | 45.6±12.5 | 46.5±12.3 | 46.1±12.4 | 0.155 | 45.6±12.5 | 46.5±12.4 | 46±12.5 | 0.187 |
| LDL-C (mg/dL) | 128±45 | 128±42 | 128±43 | 0.856 | 128±45 | 128±42 | 128±43 | 0.869 |
| HbA1c (NGSP) (%) | 7.7±1.7 | 7.9±1.7 | 7.8±1.7 | 0.137 | 7.7±1.7 | 7.9±1.7 | 7.8±1.7 | 0.171 |
| Triglyceride | 140±105 | 143±108 | 141±107 | 0.554 | 140±106 | 144±111 | 142±108 | 0.471 |
| Peak CK (IU/L) | 1,807 (929–3,384) |
1,893 (919–3,663) |
1,832 (923–3,525) |
0.459 | 1,824 (942–3,461) |
1,839 (913–3,627) |
1,828 (927–3,536) |
0.798 |
| Peak CK ≥3,000 IU/L | 29.9 | 31.0 | 30.5 | 0.632 | 30.2 | 30.7 | 30.5 | 0.816 |
| Killip ≥II (%) | 15.8 | 17.2 | 16.5 | 0.442 | 15.6 | 16.0 | 15.8 | 0.827 |
| Reperfusion (%) | 94.3 | 94.1 | 94.2 | 0.861 | 94.5 | 94.7 | 94.6 | 0.817 |
| Emergency PCI (%) | 91.5 | 90.7 | 91.1 | 0.546 | 91.6 | 91.3 | 91.4 | 0.860 |
| Stent implantation | 72.9 | 75.4 | 74.2 | 0.231 | 73.3 | 76.1 | 74.7 | 0.190 |
| EF before discharge <40% | 13.8 | 16.3 | 15.0 | 0.205 | 14.0 | 15.3 | 14.6 | 0.499 |
| EF before discharge (%) | 52.7±11.4 | 52.3±12 | 52.5±11.7 | 0.562 | 52.6±11.4 | 52.7±11.9 | 52.6±11.6 | 0.883 |
| ACEI | 47.7 | 45.3 | 46.5 | 0.312 | 48.1 | 46.0 | 47.1 | 0.399 |
| ARB | 34.0 | 35.7 | 34.9 | 0.477 | 33.9 | 36.8 | 35.4 | 0.214 |
| ACE or ARB | 79.2 | 77.5 | 78.3 | 0.407 | 79.3 | 79.2 | 79.3 | 0.951 |
| β-blockers | 59.4 | 62.3 | 60.9 | 0.201 | 59.9 | 61.7 | 60.8 | 0.447 |
| Diuretics | 28.4 | 31.7 | 30.1 | 0.126 | 28.5 | 31.2 | 29.8 | 0.243 |
| Antiplatelets | 94.1 | 96.6 | 95.4 | 0.015 | 95.0 | 96.3 | 95.6 | 0.185 |
| Insulin | 19.7 | 22.3 | 21.1 | 0.232 | 19.9 | 22.2 | 21.0 | 0.298 |
| Propensity score | 0.485±0.082 | 0.500±0.082 | 0.493±0.082 | <0.001 | 0.487±0.078 | 0.492±0.078 | 0.489±0.078 | 0.258 |
Data given as mean±SD, % or median (IQR). ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; BMI, body mass index; CK, creatine kinase; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; NGSP, National Glycohemoglobin Standardization Program; PCI, percutaneous coronary intervention; PSM, propensity score matching; STEMI, ST- elevation myocardial infarction.
Table 2.
Factors Associated With Hydrophilic Statin Prescription at Discharge
| OR | 95% CI | P-value | |
|---|---|---|---|
| Age | 1.021 | 1.008–1.035 | 0.001 |
| Male | 1.017 | 0.736–1.405 | 0.919 |
| BMI | 1.004 | 0.970–1.040 | 0.803 |
| Hypertension | 0.850 | 0.649–1.113 | 0.237 |
| Smoking | 1.422 | 1.070–1.890 | 0.015 |
| Prior MI | 0.989 | 0.691–1.417 | 0.954 |
| STEMI | 0.730 | 0.511–1.043 | 0.084 |
| Peak CK ≥3,000 IU/L | 1.033 | 0.789–1.353 | 0.813 |
| Killip ≥2 | 0.834 | 0.596–1.166 | 0.288 |
| Reperfusion therapy | 1.217 | 0.624–2.375 | 0.564 |
| EF before discharge <40% | 0.778 | 0.546–1.109 | 0.165 |
| ACEI or ARB | 1.362 | 0.988–1.879 | 0.060 |
| β-blockers | 0.970 | 0.750–1.254 | 0.815 |
| Antiplatelet agents | 0.399 | 0.224–0.709 | 0.002 |
OR, odds ratio. Other abbreviations as in Table 1.
Table 3.
Incidence of Long-Term Outcomes After Discharge vs. Statin and PSM Status
| Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|
| Hydrophilic (n=849) |
Lipophilic (n=903) |
All (n=1,752) |
P-value† | Hydrophilic (n=817) |
Lipophilic (n=817) |
All (n=1,634) |
P-value† | |
| All-cause death | 49 (5.8) | 46 (5.1) | 95 (5.4) | 0.418 | 47 (5.8) | 40 (4.9) | 87 (5.3) | 0.376 |
| Re-MI | 44 (5.2) | 45 (5.0) | 89 (5.1) | 0.731 | 42 (5.1) | 39 (4.8) | 81 (5.0) | 0.661 |
| Admission for HF | 39 (4.6) | 73 (8.1) | 112 (6.4) | 0.004 | 37 (4.5) | 63 (7.7) | 100 (6.1) | 0.008 |
| MACE‡ | 107 (12.6) | 142 (15.7) | 249 (14.2) | 0.089 | 105 (12.9) | 124 (15.2) | 229 (14.0) | 0.206 |
Data given as n (%). †Log-rank. ‡Composite of all-cause death, re-MI and admission for HF. HF, heart failure; MACE, major adverse cardiac events; PSM, propensity score matching; re-MI, recurrent myocardial infarction.
Figure 1.
Cumulative incidence of (A) all-cause death, (B) recurrent myocardial infarction (re-MI), (C) admission for heart failure (HF), and (D) a composite of these (major adverse cardiac events; MACE) in diabetic patients with acute myocardial infarction stratified according to statin use at discharge, before propensity score matching.
Table 4.
Adjusted HR of Hydrophilic Statin for Outcomes Before PSM
| HR | 95% CI | P-value | |
|---|---|---|---|
| Model 1 | |||
| All-cause death | 1.131 | 0.632–2.023 | 0.679 |
| Re-MI | 1.191 | 0.717–1.976 | 0.499 |
| Admission for HF | 0.560 | 0.345–0.911 | 0.019 |
| MACE‡ | 0.804 | 0.584–1.107 | 0.181 |
| Model 2 | |||
| All-cause death | 1.147 | 0.761–1.728 | 0.512 |
| Re-MI | 1.060 | 0.694–1.621 | 0.786 |
| Admission for HF | 0.583 | 0.391–0.869 | 0.008 |
| MACE‡ | 0.817 | 0.633–1.053 | 0.118 |
†Compared with lipophilic statins (reference group, HR=1). ‡Composite of all-cause death, re-MI and admission for HF. Model 1, adjusted for age, male sex, BMI, hypertension, smoking, prior MI, STEMI, peak CK ≥3,000 IU/L, Killip class ≥2, reperfusion therapy, EF before discharge <40%, ACEI or ARB, β-blockers and antiplatelet agents. Model 2, adjusted for propensity score. Abbreviations as in Tables 1,3.
Figure 2.
Cumulative incidence of (A) all-cause death, (B) recurrent myocardial infarction (re-MI), (C) admission for heart failure (HF), and (D) a composite of these (major adverse cardiac events; MACE), in diabetic patients with acute myocardial infarction stratified by statin use at discharge, after propensity score matching.
Table 5.
Unadjusted HR of Hydrophilic Statin for Outcomes After PSM
| HR | 95% CI | P-value | |
|---|---|---|---|
| All-cause death | 1.209 | 0.793–1.844 | 0.377 |
| Re-MI | 1.102 | 0.713–1.705 | 0.661 |
| Admission for HF | 0.584 | 0.389–0.876 | 0.009 |
| MACE | 0.846 | 0.652–1.097 | 0.206 |
†Compared with lipophilic statins (reference group, HR=1). ‡Composite of all-cause death, re-MI and admission for HF. Abbreviations as in Table 3.
Figure 3.
Subgroup analysis after propensity score matching of admission for heart failure (HF) stratified according to the prescription of hydrophilic and lipophilic statins at discharge. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CK, creatine kinase; EF, ejection fraction; LDL, low-density lipoprotein; MI, myocardial infarction.
Discussion
We examined whether hydrophilic statin use was associated with better long-term outcomes than lipophilic statin use in 1,752 diabetic patients with AMI who were discharged alive using the OACIS registry database. We found that (1) the risk of HF admission was significantly lower in patients taking hydrophilic statins than in those with lipophilic statins both before and after propensity score matching, although there was no significant difference in the risk of all-cause death, non-fatal re-MI or MACE between the 2 types of statin; and (2) there was no heterogeneity between statins and various subgroups for HF admission, with all subgroups showing a lower risk with hydrophilic statins than with lipophilic statins after propensity score matching.
Inconsistent results for clinical outcomes between hydrophilic and lipophilic statins have been reported. A meta-analysis by Bonsu et al involving 10,966 HF patients found that lipophilic statins were associated with lower risk of all-cause mortality (OR, 0.50; 95% CI: 0.11–0.89, P=0.01), cardiovascular mortality (OR, 0.61; 95% CI: 0.25–0.97, P=0.009) and hospitalization for worsening HF (OR, 0.52; 95% CI: 0.21–0.83, P=0.0005) than the hydrophilic statin rosuvastatin.5 In contrast, Chitose et al reported that compared with lipophilic atorvastatin, the hydrophilic statin rosuvastatin was beneficial for myocardial salvage, with greater improvement in left ventricular EF and lower brain natriuretic peptide (BNP) at 6 months after STEMI.3 Moreover, Sakamoto et al reported that hydrophilic pravastatin was superior to lipophilic statins at preventing new Q-wave appearance and in reducing cardiovascular events in normocholesterolemic Japanese patients after AMI.18 Meanwhile, Izawa et al observed no significant difference in a composite of death due to any cause, non-fatal MI, non-fatal stroke, unstable angina and congestive HF requiring hospital admission, or in any type of coronary revascularization at 2 years after AMI in patients treated with hydrophilic pravastatin and lipophilic atorvastatin.7 Unlike these studies, which included patients regardless of DM status, the present study focused on diabetic patients following AMI. The present findings are partially consistent with previous studies that found no significant difference between the 2 types of statins in the risk of all-cause death6,7 or re-MI.6,7 In contrast, we observed that hydrophilic statin use was associated with lower risk of admission for HF following AMI than lipophilic statins. With regard to the difference between subsequent MACE rate of 31% in the ALPS-AMI7 and of 14% in the current study, the main reason for this could be the difference in the definition of outcomes between the 2 studies. Revascularization was included in the MACE in the ALPS-AMI study, but not in the current study. Although all-cause death was similar between the 2 studies, re-MI and admission for HF were higher in the current study than in the ALPS-AMI study, possibly due to the present study having a longer follow-up period and the more severe conditions related to DM.
Although we cannot determine the mechanism of this association of hydrophilic statins with lower risk of admission for HF than lipophilic statins, several possibilities may be considered. Demyanets et al reported that lipophilic but not hydrophilic statins induce a pro-apoptotic state in human adult cardiac myocytes in vitro.19 They showed that whereas lipophilic statins downregulated mRNA of Mcl-1, an inhibitor of apoptosis, by 49%, and reduced Mcl-1 protein expression, hydrophilic statins had no such effect. Also, it has been speculated that lipophilic statins might potentially be harmful, likely through the inhibition of CoQ10 biosynthesis, leading to disturbances in cardiac energy metabolism.20 This phenomenon may negate the beneficial lipid-lowering effect of statins on cardiovascular protection. Sugiyama et al reported that pravastatin improved glucose metabolism associated with an increase in adiponectin,21 which is known to have anti-atherogenic properties in patients with CAD,22 and to protect against the development of systolic dysfunction after AMI through its ability to suppress cardiac hypertrophy and interstitial fibrosis, and also to protect against myocyte and capillary loss, as seen in an experimental model in mice.23 Kai et al showed that switching from the lipophilic simvastatin to the hydrophilic pravastatin led to an increase in plasma adiponectin without a change in serum LDL-C.24 Sustained hyperglycemia has been reported to increase the glycation of interstitial proteins such as collagen, which in turn results in altered myocardial stiffness and impaired contractility through impaired calcium homeostasis, upregulation of the renin-angiotensin system, increased oxidative stress, altered substrate metabolism and mitochondrial dysfunction.24,25 These direct and indirect mechanisms on the myocardium may explain the present observation of lower risk of readmission for HF in association with the use of hydrophilic statins. Randomized controlled trials (RCT) are needed to confirm these observations.
With regard to factors associated with the prescription of hydrophilic statin at discharge, older age, smoking habit, and non-prescription of antiplatelet therapy at discharge were extracted on multivariate logistic regression analysis (Table 2). Although we cannot determine why these factors were extracted, one possibility is simply that it is a chance observation, on the basis that few physicians might consider the differences in drug actions between hydrophilic and lipophilic statins based on these factors.
Study Limitations
Several limitations of this study warrant mention. First, it was conducted under an observational rather than randomized design, leading to the possibility of bias in the receipt of statin type. To minimize this potential bias, we first analyzed the whole population, then performed propensity score matching analysis. We obtained similar results with robustness between the whole and propensity score-matched groups, suggesting that any such bias might be minimal. Nonetheless, the clinical question of whether hydrophilic statin use reduces risk for long-term outcomes in DM patients with AMI needs to be evaluated in RCT, because there still exists potential bias even after propensity score matching in the current observational study. Second, we did not have information on drug dosage at discharge, discontinuation or adverse event rates of the drugs, or laboratory data during the follow-up period, including those for LDL-C, BNP, or C-reactive protein, which would help clarify the mechanisms underlying the observations.
Conclusions
Compared with lipophilic statins, hydrophilic statin use was associated with lower risk of admission for HF in diabetic patients with AMI. Confirmation of these results in an RCT is warranted.
Acknowledgments
We thank Mariko Kishida, Rie Nagai, Nanase Muraoka, Hiroko Takemori, Akiko Yamagishi, Kumiko Miyoshi, Chizuru Hamaguchi, Hiroko Machida, Mariko Yoneda, Nagisa Yoshioka, Mayuko Tomatsu, Kyoko Tatsumi, Tomoko Mizuoka, Shigemi Kohara, Junko Tsugawa, Junko Isotani, Sachiko Ashibe, and all the other OACIS research coordinators and nurses for their excellent assistance with data collection.
Sources of Funding
This work was supported by Grants-in-Aid for University and Society Collaboration (19590816 and 19390215) from the Japanese Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan.
IRB Information
Osaka University Research Ethics Committee (reference No: 425).
Disclosures
Yasuhiko S. has received honoraria from Kowa. S.H. has received honoraria from Kowa and Daiichi-Sankyo. H.M. has received honoraria from Daiichi-Sankyo. T. Kitamura has received honoraria from AstraZeneca and Bristol-Myers Squibb. A.S. has received honoraria from Daiichi-Sankyo. M.H. has received honoraria from Boehringer-Ingelheim, Bayer, and Tanabe-Mitsubishi Pharma. I.K. has received honoraria from Pfizer, Daiichi-Sankyo, and MSD, and research grants from Kowa, Astellas, Daiichi-Sankyo and Mitsubishi Tanabe Pharma. Yasushi S. has received honoraria from Pfizer, Daiichi-Sankyo, Bristol-Myers Squibb, Kowa, Mitsubishi Tanabe Pharma, Astellas, MSD, Shionogi, AstraZeneca, and Novartis. Yasuhiko S., I.K. are members of Circulation Reports’ Editorial Team. The other authors declare no conflicts of interest.
Appendix. OACIS Investigators
Chair
Yasushi Sakata, Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine, 2-2 Yamada-oka, Suita 565-0871, Japan
Secretariat
Shungo Hikoso (Chief), Daisaku Nakatani, Hiroya Mizuno, Shinichiro Suna, Tomoharu Dohi, Takayuki Kojima, Akihiro Sunaga, Bolrathanak Oeun, Hirota Kida, Sugako Mitsuoka, Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine, Suita, Japan
Participating Hospitals of the OACIS (in alphabetical order)
Higashiosaka City Medical Center, Osaka, Japan; Ishinkai Yao General Hospital, Osaka, Japan; Japan Community Healthcare Organization Osaka Hospital, Osaka, Japan; Japan Community Healthcare Organization Osaka Minato Central Hospital, Osaka, Japan; Kaizuka City Hospital, Osaka, Japan; Kansai Rosai Hospital, Hyogo, Japan; Kashiwara Municipal Hospital, Osaka, Japan; Kawachi General Hospital, Osaka, Japan; Kitaosaka Hospital, Osaka, Japan; Kobe Ekisaikai Hospital, Hyogo, Japan; Meiwa Hospital, Hyogo, Japan; National Hospital Organization Osaka Minami Medical Center, Osaka, Japan; National Hospital Organization Osaka National Hospital, Osaka, Japan; Osaka Daiichi Hospital, Osaka, Japan; Osaka General Hospital of West Japan Railway Company, Osaka, Japan; Osaka General Medical Center, Osaka, Japan; Osaka Kaisei Hospital, Osaka, Japan; Osaka Police Hospital, Osaka, Japan; Osaka Rosai Hospital, Osaka, Japan; Osaka University Hospital, Osaka, Japan; Saiseikai Senri Hospital, Osaka, Japan; Sakurabashi Watanabe Hospital, Osaka, Japan; Settsu Iseikai Hospital, Osaka, Japan; Teramoto Kinen Hospital, Osaka, Japan; Yao Municipal Hospital, Osaka, Japan.
References
- 1. O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al.. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127: e362–e425. [DOI] [PubMed] [Google Scholar]
- 2. Tsutamoto T, Yamaji M, Kawahara C, Nishiyama K, Fujii M, Yamamoto T, et al.. Effect of simvastatin vs. rosuvastatin on adiponectin and haemoglobin A1c levels in patients with non-ischaemic chronic heart failure. Eur J Heart Fail 2009; 11: 1195–1201. [DOI] [PubMed] [Google Scholar]
- 3. Chitose T, Sugiyama S, Sakamoto K, Shimomura H, Yamashita T, Hokamaki J, et al.. Effect of a hydrophilic and a hydrophobic statin on cardiac salvage after ST-elevated acute myocardial infarction: A pilot study. Atherosclerosis 2014; 237: 251–258. [DOI] [PubMed] [Google Scholar]
- 4. Bonsu KO, Reidpath DD, Kadirvelu A.. Effects of statin treatment on inflammation and cardiac function in heart failure: An adjusted indirect comparison meta-analysis of randomized trials. Cardiovasc Ther 2015; 33: 338–346. [DOI] [PubMed] [Google Scholar]
- 5. Bonsu KO, Reidpath DD, Kadirvelu A.. Lipophilic statin versus rosuvastatin (hydrophilic) treatment for heart failure: A meta-analysis and adjusted indirect comparison of randomised trials. Cardiovasc Drugs Ther 2016; 30: 177–188. [DOI] [PubMed] [Google Scholar]
- 6. Bytyci I, Bajraktari G, Bhatt DL, Morgan CJ, Ahmed A, Aronow WS, et al.. Hydrophilic vs lipophilic statins in coronary artery disease: A meta-analysis of randomized controlled trials. J Clin Lipidol 2017; 11: 624–637. [DOI] [PubMed] [Google Scholar]
- 7. Izawa A, Kashima Y, Miura T, Ebisawa S, Kitabayashi H, Yamamoto H, et al.. Assessment of lipophilic vs. hydrophilic statin therapy in acute myocardial infarction: ALPS-AMI study. Circ J 2015; 79: 161–168. [DOI] [PubMed] [Google Scholar]
- 8. Keech A, Colquhoun D, Best J, Kirby A, Simes RJ, Hunt D, et al.. Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose: Results from the LIPID trial. Diabetes Care 2003; 26: 2713–2721. [DOI] [PubMed] [Google Scholar]
- 9. Goldberg RB, Mellies MJ, Sacks FM, Moye LA, Howard BV, Howard WJ, et al.. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: Subgroup analyses in the cholesterol and recurrent events (CARE) trial. The Care Investigators. Circulation 1998; 98: 2513–2519. [DOI] [PubMed] [Google Scholar]
- 10. Matsumoto S, Sakata Y, Suna S, Nakatani D, Usami M, Hara M, et al.. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ Res 2013; 113: 322–326. [DOI] [PubMed] [Google Scholar]
- 11. Nakatani D, Sakata Y, Suna S, Usami M, Matsumoto S, Shimizu M, et al.. Incidence, predictors, and subsequent mortality risk of recurrent myocardial infarction in patients following discharge for acute myocardial infarction. Circ J 2013; 77: 439–446. [DOI] [PubMed] [Google Scholar]
- 12. Nakatani D, Sakata Y, Mizuno H, Shimizu M, Suna S, Usami M, et al.. Impact of diabetes mellitus on rehospitalization for heart failure among survivors of acute myocardial infarction in the percutaneous coronary intervention era. Circ J 2009; 73: 662–666. [DOI] [PubMed] [Google Scholar]
- 13. Kurotobi T, Sato H, Kinjo K, Nakatani D, Mizuno H, Shimizu M, et al.. Reduced collateral circulation to the infarct-related artery in elderly patients with acute myocardial infarction. J Am Coll Cardiol 2004; 44: 28–34. [DOI] [PubMed] [Google Scholar]
- 14. Sakata Y, Nakatani D, Shimizu M, Suna S, Usami M, Matsumoto S, et al.. Oral treatment with nicorandil at discharge is associated with reduced mortality after acute myocardial infarction. J Cardiol 2012; 59: 14–21. [DOI] [PubMed] [Google Scholar]
- 15. D’Agostino RB Jr.. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17: 2265–2281. [DOI] [PubMed] [Google Scholar]
- 16. Villamizar NR, Darrabie MD, Burfeind WR, Petersen RP, Onaitis MW, Toloza E, et al.. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009; 138: 419–425. [DOI] [PubMed] [Google Scholar]
- 17. Seicean S, Seicean A, Alan N, Plana JC, Budd GT, Marwick TH.. Cardioprotective effect of beta-adrenoceptor blockade in patients with breast cancer undergoing chemotherapy: Follow-up study of heart failure. Circ Heart Fail 2013; 6: 420–426. [DOI] [PubMed] [Google Scholar]
- 18. Sakamoto T, Kojima S, Ogawa H, Shimomura H, Kimura K, Ogata Y, et al.. Usefulness of hydrophilic vs lipophilic statins after acute myocardial infarction: Subanalysis of MUSASHI-AMI. Circ J 2007; 71: 1348–1353. [DOI] [PubMed] [Google Scholar]
- 19. Demyanets S, Kaun C, Pfaffenberger S, Hohensinner PJ, Rega G, Pammer J, et al.. Hydroxymethylglutaryl-coenzyme A reductase inhibitors induce apoptosis in human cardiac myocytes in vitro. Biochem Pharmacol 2006; 71: 1324–1330. [DOI] [PubMed] [Google Scholar]
- 20. Ichihara K, Satoh K.. Disparity between angiographic regression and clinical event rates with hydrophobic statins. Lancet 2002; 359: 2195–2198. [DOI] [PubMed] [Google Scholar]
- 21. Sugiyama S, Fukushima H, Kugiyama K, Maruyoshi H, Kojima S, Funahashi T, et al.. Pravastatin improved glucose metabolism associated with increasing plasma adiponectin in patients with impaired glucose tolerance and coronary artery disease. Atherosclerosis 2007; 194: e43–e51. [DOI] [PubMed] [Google Scholar]
- 22. Matsuzawa Y, Funahashi T, Kihara S, Shimomura I.. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol 2004; 24: 29–33. [DOI] [PubMed] [Google Scholar]
- 23. Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci WS, et al.. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol 2007; 42: 1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kai T, Arima S, Taniyama Y, Nakabou M, Kanamasa K.. Comparison of the effect of lipophilic and hydrophilic statins on serum adiponectin levels in patients with mild hypertension and dyslipidemia: Kinki Adiponectin Interventional (KAI) Study. Clin Exp Hypertens 2008; 30: 530–540. [DOI] [PubMed] [Google Scholar]
- 25. Boudina S, Abel ED.. Diabetic cardiomyopathy revisited. Circulation 2007; 115: 3213–3223. [DOI] [PubMed] [Google Scholar]