Abstract
Background: The clinical frailty scale (CFS) predicts late mortality in patients undergoing transcatheter aortic valve replacement. We evaluated the CFS and other parameters associated with 1-year mortality after balloon aortic valvuloplasty (BAV).
Methods and Results: Between January 2013 and May 2018, 148 patients with severe aortic stenosis (AS) who underwent BAV at the present hospital were enrolled. We recorded pre-procedural CFS grade, baseline characteristics, echocardiographic, and hemodynamic parameters. To investigate the potential risk to patients before BAV, we evaluated the Society of Thoracic Surgeons (STS) score. After patients who underwent surgical aortic valve replacement, transcatheter aortic valve replacement or repeat BAV were excluded, we investigated 1-year survival. Of 127 patients, 41 (32.3%) died ≤1 year after BAV, 8 of whom (19.5% of all-cause deaths) had cardiac deaths. Higher grade of CFS and STS score significantly correlated with 1-year mortality. Severe frailty and the high operative risk group (CFS ≥7 and STS score ≥8.7%) had an extremely poor prognosis (1-year mortality, 81.2%).
Conclusions: In this BAV cohort, severe frailty was a predictor of 1-year mortality in elderly patients with severe AS.
Key Words: Aortic stenosis, Balloon aortic valvuloplasty, Clinical frailty scale
The incidence of aortic stenosis (AS) is increasing with the aging of the population, and is associated with poor survival without aortic valve replacement once symptoms develop.1 Furthermore, elderly patients with AS experience progressive symptoms with reduced functional status and quality of life caused by frailty.
Balloon aortic valvuloplasty (BAV) is less invasive than surgery, and is thought to be a bridge to surgical or transcatheter aortic valve replacement in hemodynamically unstable patients or in patients who are unsuitable because of severe comorbidities.2 In early reports, the immediate efficacy of BAV for high-risk patients with severe AS was demonstrated from the standpoint of hemodynamic improvement and palliation of symptoms.3,4 Recent reports suggested that BAV can lead to improvement of cardiac function that may have impact on further definitive treatment and prognosis.5,6
Frailty, defined as a syndrome of impaired physiologic reserve and decreased resistance to stressors, is associated with morbidity and mortality,7 and is emerging as an important arbiter of clinical decision-making in elderly AS patients. As in transcatheter aortic valve replacement (TAVR) candidates, frailty was not associated with increased periprocedural complications; but it was associated with an increased risk of death ≤1 year after the procedure.8 The clinical frailty scale (CFS), a semiquantitative tool that provides a generally accepted clinical definition of frailty,9 accurately reflects clinical frailty and is a useful predictor of mortality in elderly Japanese patients after TAVR.10
In this report, we investigated the usefulness of the CFS for predicting the outcome of BAV.
Methods
Patients and Study Design
This was a single-center retrospective study that enrolled consecutive patients with symptomatic or asymptomatic severe AS undergoing BAV at Awaji Medical Center from January 2013 to May 2018. Patients underwent BAV as a bridge to surgical aortic valve replacement (SAVR) or TAVR, for risk reduction for non-cardiac surgery, for destination therapy when considered unsuitable for SAVR or TAVR due to severe comorbidity, or for diagnostic therapy to determine the implications of AS symptoms. Patient characteristics including frailty factors, echocardiography data, procedural information, complications, and in-hospital, 30-day and 1-year mortality rates were retrospectively recorded. To evaluate frailty, we used the CFS and Canadian Study of Health and Aging grading criteria.9 The CFS ranges from 1, very fit; to 9, terminally ill. We categorized patients into 5 groups as follows: non-frail, CFS 1–3; vulnerable, CFS 4; mildly frail, CFS 5; moderately frail, CFS 6; and severely frail, CFS ≥7, as reported previously.10
To estimate the risk stratification for AS, we calculated the Society of Thoracic Surgeons (STS) score11,12 for each patient.
The primary endpoint was 1-year mortality, divided into cardiac death and non-cardiac death. The secondary endpoint was cardiovascular mortality.
The procedures followed were in accordance with the Declaration of Helsinki and the ethics standards of the responsible committee on human experimentation.
BAV Procedure
All BAV procedures were performed with local anesthesia at the puncture site. There were 2 approaches for the balloon catheter, antegrade or retrograde. An antegrade trans-septal approach using the Inoue balloon (TORAY, Japan) was performed as previously reported.13 Briefly, balloon devices were delivered through the 14-Fr catheters via the femoral vein, and temporary pacing was delivered via the contralateral femoral vein with a 6-Fr catheter. A snare catheter was secured to an extra-stiff 0.032-inch guidewire from the right femoral vein through the right atrium, left atrium, left ventricle, and then across the aortic valve, providing enough support to deliver and control the balloon devices. Although the Inoue balloon was the first choice for the antegrade approach, in cases where it was difficult to pass the Inoue balloon, the VACS II (Osypka AG, Germany) or TYSHAK (NumED CANADA, Canada) were selected. For the conventional retrograde arterial approach, the VACS II, TYSHAK, MAXI LD (Cardinal health Japan, Japan), or MUSTANG (Boston Scientific, Ireland) were chosen according to operator discretion. Balloons were advanced from the femoral artery. At the present institution, the antegrade approach is the standard option, and the retrograde approach is considered in cases of acute heart failure or unstable hemodynamics, or of unsuitability for the femoral venous approach. The AcuNav (Siemens Medical Solutions, USA) was used for the jugular venous 8-Fr approach for guidance for the atrial septum puncture, to observe the aortic valve at inflation time, and to monitor for complications such as cardiac tamponade and aortic regurgitation. We used contrast-enhanced multidetector computed tomography to measure the size and area of the aortic annulus.
Statistical Analysis
Statistical analysis was conducted using MedCalc ver. 18.2.1 (MedCalc Software, Mariakerke, Belgium). Continuous variables are expressed as mean±SD or median (IQR). Differences in the continuous parameters between the 2 groups were calculated using unpaired t-test. Categorical variables are expressed as frequency counts, and intergroup comparisons were made using Fisher’s exact test. Multivariate logistic regression analysis was used to determine the significant factors predicting 1-year mortality. Factors with P<0.05 on univariate analyses were included in this multiple logistic regression model. To explore the optimal cut-off of STS score associated with 1-year mortality after BAV, we used receiver operating characteristic (ROC) curve analysis. Cumulative incidences of clinical event rates were estimated using Kaplan-Meier analysis and the differences were assessed with the log-rank test. P<0.05 was considered statistically significant.
Results
Patient Enrollment
Of a total of 148 patients, 7 underwent repeat BAV, 11 underwent SAVR, and 3 underwent TAVR ≤1 year after the initial BAV. Finally, 127 patients were included in this study (Figure 1).
Figure 1.
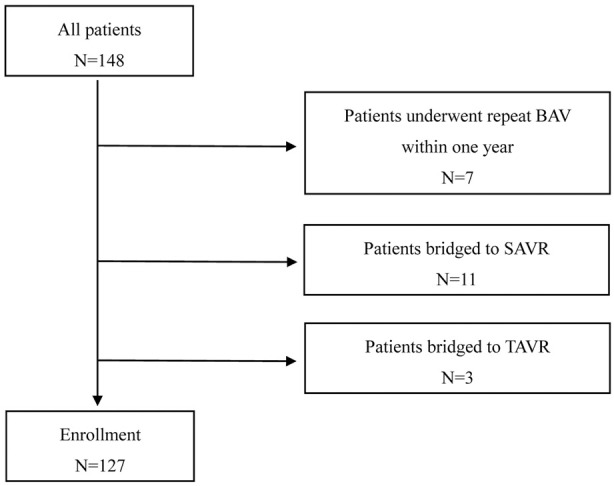
Subject selection. BAV, balloon aortic valvuloplasty; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
Baseline Characteristics
Baseline characteristics are summarized in Table 1. Mean age was 86.8 years, and 34% were male. Of the 127 patients, 9.4% were categorized as CFS 1–3 (n=12), 29.9% were CFS 4 (n=38), 23.6% were CFS 5 (n=30), 18.9% were CFS 6 (n=24), and 18.1% were CFS 7–9 (n=23). Mean STS score was 11.4. There were significant differences between the survivor (n=86) and non-survivor (n=41) groups in terms of sex, New York Heart Association (NYHA) class, STS score and CFS. With respect to pre-procedural laboratory data, serum albumin was higher in the survivor group than in the non-survivor group (3.4 g/dL vs. 3.1 g/dL, P<0.05). Serum creatinine and brain natriuretic peptide were significantly lower in the survivor group than in the non-survivor group. With respect to comorbidities, there were no significant differences between the groups except for smoking (P=0.008) and hemodialysis (P=0.018).
Table 1.
Baseline Characteristics in Elderly AS Patients
| Overall (n=127) |
Survivor (n=86) |
Non-survivor (n=41) |
P-value | |
|---|---|---|---|---|
| Age (years) | 86.8±5.6 | 86.7±4.9 | 87.0±6.9 | 0.77 |
| Male | 43 (33.9) | 23 (26.7) | 20 (48.8) | 0.017 |
| BMI (kg/m2) | 20.7±3.3 | 21.0±3.5 | 20.1±3.0 | 0.21 |
| BSA (m2) | 1.41±0.17 | 1.42±0.18 | 1.40±0.17 | 0.75 |
| NYHA class III/IV | 69 (54.3) | 38 (44.2) | 31 (75.6) | 0.001 |
| STS score (%) | 11.4±9.1 | 9.0±6.8 | 16.6±11.0 | <0.0001 |
| CFS | 0.0021 | |||
| 1–3 | 12 (9.4) | 11 (12.8) | 1 (2.4) | |
| 4 | 38 (29.9) | 27 (31.4) | 11 (26.8) | |
| 5 | 30 (23.6) | 24 (27.9) | 6 (14.6) | |
| 6 | 24 (18.9) | 16 (18.6) | 8 (19.5) | |
| 7–9 | 23 (18.1) | 8 (9.3) | 15 (36.6) | |
| Preprocedural laboratory data | ||||
| Hemoglobin (g/dL) | 10.9±1.9 | 11.0±2.0 | 10.7±1.7 | 0.43 |
| Albumin (g/dL) | 3.3±0.5 | 3.4±0.5 | 3.1±0.4 | 0.0025 |
| Creatinine (mg/dL) | 2.1±5.6 | 1.4±1.4 | 3.7±9.5 | 0.025 |
| eGFR (mL/min/1.73 m2) | 44.3±24.8 | 46.0±21.5 | 40.8±30.6 | 0.007 |
| BNP (pg/mL) | 423 (177–915) | 308 (137–690) | 798 (339–1,700) | 0.0002 |
| Comorbidity | ||||
| Hypertension | 92 (72.4) | 64 (74.4) | 28 (68.3) | 0.53 |
| Diabetes mellitus | 35 (27.6) | 28 (32.6) | 7 (17.1) | 0.089 |
| Dyslipidemia | 42 (33.1) | 31 (36.0) | 11 (26.8) | 0.32 |
| Smoking | 20 (15.7) | 8 (9.3) | 12 (29.3) | 0.008 |
| CAD | 20 (15.7) | 13 (15.1) | 7 (17.1) | 0.80 |
| PAD | 11 (8.7) | 5 (5.8) | 6 (14.6) | 0.17 |
| CVD | 17 (13.4) | 12 (14.0) | 5 (12.2) | 1.00 |
| Hemodialysis | 12 (9.4) | 4 (4.7) | 8 (19.5) | 0.018 |
| AF | 41 (32.3) | 25 (29.1) | 16 (39.0) | 0.31 |
Data given as mean±SD, n (%) or median (IQR). AF, atrial fibrillation; AS, aortic stenosis; BMI, body mass index; BNP, brain natriuretic peptide; BSA, body square area; CAD, coronary artery disease; CFS, clinical frailty scale; CVD, cerebrovascular disease; eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association; PAD, peripheral artery disease; STS, Society of Thoracic Surgeons.
Echocardiography Data
Echocardiographic data are listed in Table 2. The average left ventricular ejection fraction (LVEF) and aortic valve area (AVA) were 54.5±12.7%, and 0.68±0.15 cm2, respectively. Although not statistically significant, there was a trend toward a higher LVEF in the survivor group than in the non-survivor group (survivor, 56.0±11.1%; non-survivor, 51.3±15.4%; P=0.061). In contrast, there were no significant differences in AS parameters such as AVA, peak velocity, or mean pressure gradient between the groups.
Table 2.
Echocardiography and Periprocedural BAV Data in Elderly AS Patients
| Overall (n=127) |
Survivor (n=86) |
Non-survivor (n=41) |
P-value | |
|---|---|---|---|---|
| Echocardiography data | ||||
| LAD (mm) | 42.7±6.1 | 42.3±6.1 | 43.8±6.3 | 0.38 |
| EDD (mm) | 43.7±6.7 | 43.9±6.9 | 43.1±6.2 | 0.55 |
| ESD (mm) | 29.8±7.3 | 29.8±7.4 | 29.6±7.3 | 0.87 |
| LVEF (%) | 54.5±12.7 | 56.0±11.1 | 51.3±15.4 | 0.061 |
| E velocity (m/s) | 88.6±35.6 | 86.7±35.0 | 92.6±37.0 | 0.40 |
| E/e’ | 19.4±10.2 | 18.8±8.0 | 21.0±13.9 | 0.29 |
| TRPG (mmHg) | 38.3±15.1 | 37.8±15.4 | 39.4±14.5 | 0.60 |
| AR ≥grade 2 | 12 (9.8) | 9 (10.8) | 3 (7.7) | 0.75 |
| MR ≥grade 2 | 58 (46.8) | 36 (42.9) | 22 (55.0) | 0.25 |
| AVA (cm2) | 0.68±0.15 | 0.68±0.15 | 0.68±0.18 | 1.00 |
| Indexed AVA (cm2/m2) | 0.46±0.14 | 0.45±0.14 | 0.47±0.14 | 0.55 |
| AV mean gradient (mmHg) | 36.7±17.0 | 37.4±16.4 | 35.4±18.4 | 0.56 |
| AV peak gradient (mmHg) | 64.8±28.4 | 65.3±27.4 | 63.9±30.8 | 0.79 |
| AV peak velocity (m/s) | 3.9±0.9 | 3.9±0.9 | 3.9±1.0 | 0.63 |
| LVOT diameter (mm) | 20.2±1.9 | 20.0±1.9 | 20.4±1.9 | 0.33 |
| LVOT VTI (cm2) | 19.0±7.3 | 19.4±7.6 | 18.2±6.8 | 0.37 |
| Stroke volume (mL) | 58.0±18.4 | 57.8±17.5 | 58.4±20.6 | 0.88 |
| Periprocedural data | ||||
| Antegrade approach | 86 (67.7) | 62 (72.1) | 24 (58.5) | 0.16 |
| Procedure time (min) | 83 (73–105) | 90 (74–109) | 77 (70–96) | 0.095 |
| Antegrade approach (min) | 91 (77–109) | 95 (78–109) | 82 (74–104) | 0.19 |
| Retrograde approach (min) | 70 (59–96) | 63 (57–103) | 71 (62–88) | 0.91 |
| Max balloon size (mm) | 20.2±2.4 | 20.1±4.7 | 20.3±5.2 | 0.62 |
| Number of balloon inflation | 7.0 (4.0–13.0) | 7.0 (4.8–13.0) | 6.0 (4.0–12.3) | 0.50 |
| Major axis (mm) | 25.6±2.5 | 25.6±2.5 | 25.6±2.5 | 0.91 |
| Minor axis (mm) | 20.0±2.3 | 19.7±2.1 | 20.7±2.5 | 0.052 |
| Area (mm2) | 416±76 | 412±80 | 427±63 | 0.47 |
| Annulus diameter (mm) | 22.5±2.0 | 22.5±2.1 | 22.5±1.9 | 0.93 |
| Pre-hemodynamic data | ||||
| Cardiac index (L/min/m2) | 2.5±0.9 | 2.6±0.9 | 2.3±0.6 | 0.16 |
| Peak to peak gradient (mmHg) | 48.3±30.2 | 48.5±29.5 | 47.6±32.4 | 0.90 |
| AVA (cm2) (Gorlin formula) | 0.58±0.19 | 0.59±0.20 | 0.53±0.17 | 0.21 |
| Post-hemodynamic data | ||||
| Cardiac index | 2.8±0.8 | 2.8±0.8 | 2.6±0.8 | 0.14 |
| Peak to peak gradient (mmHg) | 24.0±19.0 | 24.1±19.0 | 23.9±19.2 | 0.97 |
| AVA (cm2) (Gorlin formula) | 0.94±0.5 | 0.97±0.59 | 0.84±0.37 | 0.33 |
| Delta AVA (cm2) | 0.33±0.53 | 0.35±0.58 | 0.27±0.35 | 0.50 |
| Delta indexed AVA (cm2/m2) | 0.21±0.38 | 0.23±0.42 | 0.15±0.24 | 0.28 |
Data given as mean±SD, n (%) or median (IQR). AR, aortic regurgitation; AS, aortic stenosis; AV, aortic valve; AVA, aortic valve area; BAV, balloon aortic valvuloplasty; E, peak early diastolic velocity; e’, peak velocity of the early diastolic wave; EDD, end-diastolic dimension; ESD, end-systolic dimension; LAD, left atrial dimension; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; MR, mitral regurgitation; TRPG, tricuspid regurgitation pressure gradient; VTI, velocity-time integral.
Periprocedural Data
Periprocedural patient characteristics are listed in Table 2. The antegrade approach was used in 86 patients (67.7%), and the retrograde approach was used in 41 patients (32.3%). There were no prognostic differences between those 2 approaches. Mean annulus diameter measured on multi-slice computed tomography (MSCT) was 22.5 mm. Maximum balloon size was 20.2 mm, smaller than the mean annulus diameter. Cardiac index and AVA were significantly greater after the procedure (cardiac index: before, 2.5±0.9 L/min/m2; after, 2.8±0.8 L/min/m2; P<0.0001, AVA: before, 0.58±0.19 cm2; after, 0.94±0.5 cm2; P<0.0001). No periprocedural parameters, including AS severity and cardiac function, differed between the groups.
Outcomes and Complications
Outcomes and complications are listed in Table 3. Forty-one patients died in ≤1 year and the all-cause 1-year mortality rate was 32.3%. Of these patients, 8 (19.5% of all deaths) died of cardiac events. The in-hospital mortality rate was 11.0%, and the 30-day mortality rate was 11.0%. Cardiovascular mortality was 2.4%, and 3.1%, respectively. Cardiac tamponade was observed in 4 patients, and all of those patients had the pericardial drainage tube removed in ≤24 h. Symptomatic cerebral infarction in ≤48 h after the procedure was observed in 2 patients (1.6%).
Table 3.
Outcomes and Complications After BAV
| Overall (n=127) |
|
|---|---|
| 1-year mortality | |
| All-cause mortality | 41 (32.3) |
| Cardiovascular death | 8 (6.3) |
| Pneumonia | 12 (9.4) |
| Sepsis (cholecystitis, arthritis) | 3 (2.4) |
| Cancer | 3 (2.4) |
| GI bleeding | 2 (1.6) |
| Unknown (decrepitude) | 7 (5.5) |
| In-hospital mortality | |
| All-cause mortality | 14 (11.0) |
| Cardiovascular death | 3 (2.4) |
| Pneumonia | 3 (2.4) |
| Sepsis (cholecystitis, arthritis) | 1 (0.8) |
| Cancer | 0 (0.0) |
| GI bleeding | 0 (0.0) |
| Unknown (decrepitude) | 0 (0.0) |
| 30-day mortality | |
| All-cause mortality | 14 (11.0) |
| Cardiovascular death | 4 (3.1) |
| Pneumonia | 2 (1.6) |
| Sepsis (cholecystitis, arthritis) | 1 (0.8) |
| Cancer | 0 (0.0) |
| GI bleeding | 0 (0.0) |
| Unknown (decrepitude) | 0 (0.0) |
| Complications | |
| Bleeding | 2 (1.6) |
| Symptomatic stroke | 2 (1.6) |
| Cardiac tamponade | 4 (3.1) |
| AR | 1 (0.8) |
Data given as n (%). GI, gastrointestinal. Other abbreviations as in Table 2.
Logistic Regression Analysis for 1-Year Mortality
Logistic regression analysis for predicting 1-year mortality is given in Table 4. We analyzed the parameters that clarified significant differences between survivor and non-survivor groups (Tables 1–3). On multivariable analysis, high CFS grade, high STS score, low albumin, and smoking habit were independent predictors of 1-year mortality (Table 4).
Table 4.
Logistic Regression Analysis for 1-Year Mortality After BAV in Elderly AS Patients
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age | 1.01 | 0.94–1.08 | 0.77 | |||
| Male | 2.61 | 1.20–5.67 | 0.02 | |||
| NYHA III/IV | 3.92 | 1.71–8.98 | 0.001 | |||
| STS score | 1.11 | 1.05–1.18 | 0.0002 | 1.07 | 1.00–1.14 | 0.04 |
| CFS | 1.73 | 1.27–2.35 | 0.0005 | 1.75 | 1.17–2.62 | 0.007 |
| Smoking | 4.03 | 1.50–10.9 | 0.006 | 7.73 | 1.95–30.7 | 0.004 |
| Albumin | 0.29 | 0.12–0.67 | 0.004 | 0.30 | 0.11–0.80 | 0.02 |
| eGFR | 0.99 | 0.98–1.01 | 0.27 | |||
| BNP | 1.00 | 1.00–1.00 | 0.009 | |||
| LVEF | 0.97 | 0.94–1.00 | 0.07 | |||
Abbreviations as in Tables 1,2.
Cumulative 1-Year Mortality According to CFS Grade
Kaplan-Meier analysis of cumulative mortality of the 5 groups on the basis of CFS is presented in Figure 2. Cumulative 1-year mortality rates in each group were 8.3%, CFS 1–3; 28.9%, CFS 4; 20.0%, CFS 5; 33.3%, CFS 6; and 65.2%, CFS 7–9. Increase in CFS grade was significantly associated with increasing risk of 1-year mortality (log-rank P=0.0004).
Figure 2.
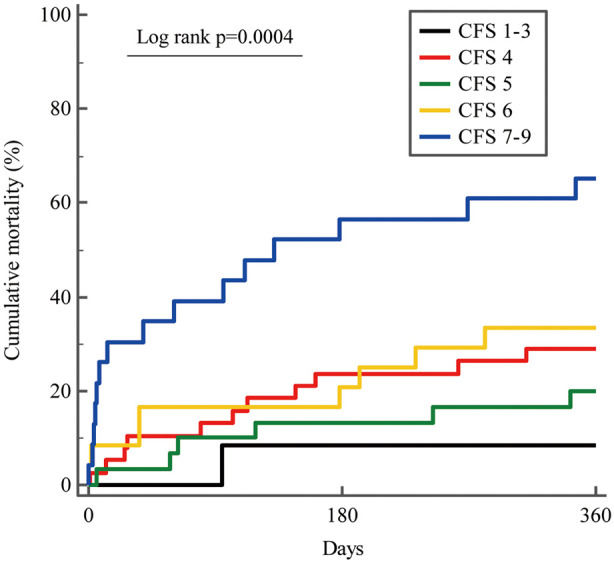
Kaplan-Meier analysis of 1-year all-cause mortality after balloon aortic valvuloplasty according to clinical frailty scale grade in elderly Japanese patients with severe aortic stenosis.
STS Score Predicting 1-Year Mortality
From the ROC curve analysis, the optimal STS score cut-off for 1-year mortality was 8.7%, with an area under the curve of 0.780, a specificity of 65.1%, and a sensitivity of 82.9% (Figure 3).
Figure 3.
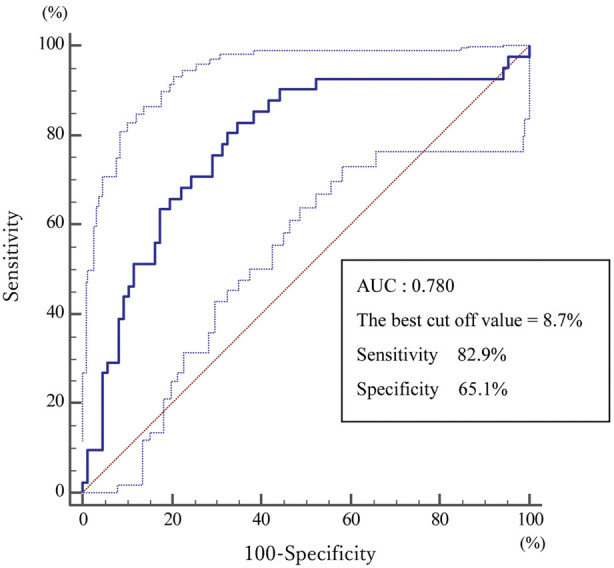
Receiver operating characteristic curve analysis of Society of Thoracic Surgeons score predicting 1-year mortality after balloon aortic valvuloplasty in elderly Japanese patients with severe aortic stenosis. AUC, area under the curve.
CFS and STS Scores Predicting 1-Year Mortality
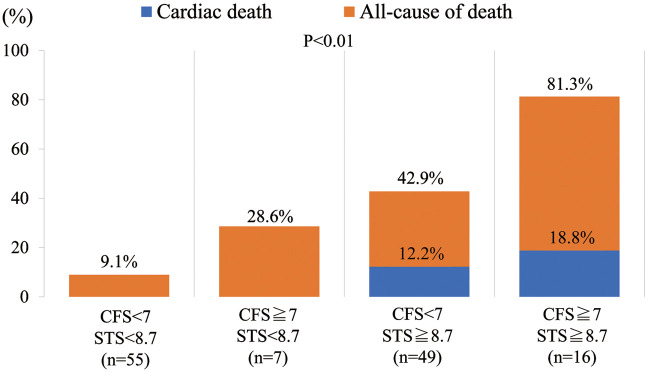
Figure 4 shows 4 groups classified according to the CFS cut-offs ≥7 or <7, and STS score ≥8.7% or <8.7%. Of the 127 patients, 55 had CFS <7 and STS score <8.7, 7 patients had CFS ≥7 and STS score <8.7, 49 had CFS <7 and STS score ≥8.7, and 16 had CFS ≥7 and STS score ≥8.7. For these 4 groups, 1-year mortality was 9.1%, 28.6%, 42.9%, and 81.3%, respectively (P<0.0001), and cardiovascular mortality was 0%, 0%, 12.2%, and 18.8%, respectively (P=0.035).
Figure 4.
One-year mortality after balloon aortic valvuloplasty in elderly Japanese patients with severe aortic stenosis according to clinical frailty scale (CFS) and Society of Thoracic Surgeons (STS) score cut-offs.
Discussion
This study explored the CFS and other parameters associated with 1-year mortality after BAV. The main findings of this study are as follows: (1) 1-year mortality after BAV was 32.3%, and 19.5% of those patients died of cardiac event; (2) high CFS grade, high STS score, low albumin, and smoking habit were independent predictive factors for 1-year mortality; (3) severe frailty and higher risk status (CFS ≥7, STS score ≥8.7%) were fatal (1-year mortality, 81.3% ), while moderate or less frailty and lower risk status (CFS <7, STS score <8.7%) were associated with a good prognosis (1-year mortality, 9.1%).
Mid-Term Effect of BAV
Although early reports of BAV demonstrated acute improvement of hemodynamics and a high success rate,3,4 studies in the past few decades showed poor outcome in the mid–long term after BAV (1-year mortality, 56–64%).6,14,15 In contrast, other recent reports noted a better prognosis (1-year mortality, 83–84%).16,17 Although it was unclear whether there were differences in outcome after BAV, these were primarily caused by patient-related matters. In this study, 1-year mortality after BAV was 32.3%, and, of those deaths, cardiac death accounted for only 19.5%. The data may indicate that BAV controlled the cardiac issues due to severe AS and that the mortality was primarily associated with the severity of comorbidities. In general, hemodialysis patients have worse outcomes than non-dialysis patients. We evaluated 1-year mortality excluding hemodialysis patients (Supplementary Table 1). On multivariable analysis, high CFS grade, male gender, and NYHA III/IV were independent predictors of 1-year mortality.
Although the long-term effect of BAV was unclear, BAV may possibly control the disease 1 year or more after the procedure.
Transthoracic echocardiography is the best imaging modality for less invasive and accurate assessment of AS after BAV. The serial echocardiography parameters 1, 3, 6, and 12 months after BAV are listed in Supplementary Table 2. Echocardiographic parameters of AS severity, such as AVA, peak velocity, and pressure gradient, gradually approached pre-procedural levels, but stroke volume and velocity time integral did not. The discrepancy between AS and cardiac output parameters may indicate fewer cardiovascular events despite “severe” AS parameters.
Risk of Severe Frailty in AS
The potential prognostic value of the simple CFS grading tool for risk stratification before TAVR in elderly patients has been confirmed in a Japanese multicenter registry study.10 CFS grade was associated with increasing 30-day and cumulative 1-year mortality. CFS grade is a semiquantitative tool that provides a generally accepted clinical definition of frailty that can be applied easily even by non-geriatricians; it is accessible for evaluation of elder patients by any clinician.9 In this study, we assessed CFS grading for the prediction of 1-year mortality after BAV, and the cumulative mortality was very similar to that of the population in which TAVR had been performed. Given that severe AS patients are usually of an advanced age, frailty may be very important in the short to mid-term outcome regardless of the type of procedure. CFS grading is useful for its simplicity and competent assessment in clinical settings.
In this study, 50 patients (39%) were classified as non-frail or vulnerable (CFS, 1–4). Of those patients, 11 were treated for risk reduction before non-cardiac surgery, 8 for mental disorders caused by dementia, and 11 for old age (≥90 years old). Although physiological frailty is very important for selecting a strategy for AS patients, these indications or patient status may be essential from a clinical viewpoint.
Risk Stratification According to STS Score
Risk scoring methods, including STS score and EuroSCORE II,18 have been commonly used for the prediction of postoperative mortality and morbidity after cardiac surgery. For patients undergoing TAVR, these risk scoring methods are also useful and accurate for risk stratification.19 In this study, STS score ≥8.7% was the best cut-off value for predicting 1-year mortality. The STS scoring method includes age, sex, LVEF, NYHA class, use of inotropic agents, and others. Such parameters indicate patients who are “too sick”; AS patients who are too sick would have a poor prognosis regardless of whether they underwent SAVR, TAVR, or BAV. A recent report on BAV showed that time matters were key to survival for severe AS patients with cardiogenic shock.20 The “too late” AS patients also had extremely poor prognosis.20 In the present study, 41 patients (32.3%) died ≤1 year after BAV, and 14 (11.0%) died ≤30 days after BAV. Most deaths in the short term may have been in “too sick, too late” patients.
BAV Procedure
In the TAVR era, contrast-enhanced MSCT is the well-established method for assessing the size of the aortic complex, including aortic annular diameter and calcium deposition, for indicating adequate target balloon size, and for avoiding complication risks such as annulus rupture or massive aortic regurgitation. Furthermore, trans-esophageal or intra-cardiac echocardiography gives clear real-time imaging during the procedure.17,21 In the present BAV procedure, we used AcuNav via the jugular or femoral veins, and observed aortic valves, trans-septal puncture, aortic regurgitation, pericardial effusion, and the balloon catheter positioning without anesthesia. As with imaging-guided percutaneous coronary intervention, imaging-guided BAV will become the mainstream procedure for adequate and safe performance.
Recently, the antegrade approach for BAV via the trans-septal route was reported to have favorable clinical performance, and the advantage of the procedure was possibly due to the utilization of the Inoue balloon with several inflations of gradually increasing diameter.16 In the present study, 67.7% of all BAV were performed using the antegrade approach, and the number of mean inflation times was 7. Although it was unclear whether there was a difference in outcome after BAV, the current method, including accurate CT measurement, intra-cardiac echocardiography guiding, Inoue balloon usage, and multiple balloon inflations, may account for the improved 1-year mortality.
Complications
Complications associated with BAV are sometimes serious, and serious adverse events are not rare (15.6%).22 Subsequently, the ACC/AHA guidelines discourage BAV before non-cardiac surgery.23 Although the most frequent drawback of BAV was vascular complications caused by large-sized sheaths, recently, several types of hemostatic devices have been used effectively.24 At the present institution we usually use the HemCon® bandage (HemCon Medical Technologies, Portland, OR, USA) for the puncture site of the femoral artery. A total of 67.7% of the present patients underwent the antegrade approach via the femoral vein, and this may be feasible for decreasing vascular complications. Aortic regurgitation and cerebral infarction were the most disturbing complications related to this procedure. Both complications were also rare (1–2%),5,22 similar to the present results, but closely linked to the BAV procedure. In 148 patients, severe aortic regurgitation was present in 2. Fortunately, the hemodynamics in those 2 patients did not collapse or require emergency surgery. One patient underwent SAVR in an elective setting, and the other patient was followed up without SAVR or TAVR. Both cases involved bicuspid aortic valves. Given that the aortic valve orifice is elliptic in bicuspid aortic valves, a balloon size fitted to the surgical valve ring may be deemed inappropriate.
In this study, cardiac tamponade occurred in 4 patients (3.1%). All underwent pericardial drainage, and their hemodynamics stabilized immediately. Approximately 20–50 mL of bloody effusion was removed, and the drainage tube was removed the next day in all cases. The hemodynamics slowly stabilized after the procedure. In consideration of the time course and good response to the drainage, the bleeding was thought to be right-sided bleeding and was mainly caused by intracardiac echocardiography or temporary pacing lead.
Further analysis is needed to clarify the mechanism and trends related to these complications.
Study Limitations
Several limitations should be noted. First, this study had a relatively limited sample size, raising the possibility of selection bias. Second, it was a retrospective, single-center study. Third, the survival rate could have been influenced not only by valvular disease but also by severe comorbidities. Fourth, the number of patients who were bridged to TAVR was very small, primarily because of the lack of facilities for TAVR in Awaji Island. More than 40 BAV were performed in 1 year at the present institution, therefore there may have been some bias regarding the indication for BAV in patients with severe AS.
Conclusions
The CFS is a simple assessment tool, useful for predicting 1-year mortality in AS patients after BAV. Severely frail advanced-age patients would have poor prognosis despite the improvement of AS.
IRB Information
The present study was approved by the ethics committee of Hyogo Prefectural Awaji Medical Center Ethics Committee (reference no. 30-59).
Supplementary Files
Supplementary Table 1. Logistic regression analysis for 1-year mortality excluding hemodialysis patients Supplementary Table 2. Serial echocardiographic parameters 1-, 3-, 6-, 12-month after BAV (n=63)
References
- 1. Davies SW, Gershlick AH, Balcon R.. Progression of valvar aortic stenosis: A long-term retrospective study. Eur Heart J 1991; 12: 10–14. [DOI] [PubMed] [Google Scholar]
- 2. Falk V, Baumgartner H, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al.. Corrigendum to “2017 ESC/EACTS Guidelines for the management of valvular heart disease” [Eur J Cardiothorac Surg 2017; 52: 616–664]. Eur J Cardiothorac Surg 2017; 52: 832. [DOI] [PubMed] [Google Scholar]
- 3. Cribier A, Savin T, Saoudi N, Rocha P, Berland J, Letac B.. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: An alternative to valve replacement? Lancet 1986; 1: 63–67. [DOI] [PubMed] [Google Scholar]
- 4. Cribier A, Savin T, Berland J, Rocha P, Mechmeche R, Saoudi N, et al.. Percutaneous transluminal balloon valvuloplasty of adult aortic stenosis: Report of 92 cases. J Am Coll Cardiol 1987; 9: 381–386. [DOI] [PubMed] [Google Scholar]
- 5. Daniec M, Nawrotek B, Sorysz D, Rakowski T, Dziewierz A, Rzeszutko L, et al.. Acute and long-term outcomes of percutaneous balloon aortic valvuloplasty for the treatment of severe aortic stenosis. Catheter Cardiovasc Interv 2017; 90: 303–310. [DOI] [PubMed] [Google Scholar]
- 6. Szerlip M, Arsalan M, Mack MC, Filardo G, Worley C, Kim RJ, et al.. Usefulness of balloon aortic valvuloplasty in the management of patients with aortic stenosis. Am J Cardiol 2017; 120: 1366–1372. [DOI] [PubMed] [Google Scholar]
- 7. Pedone C, Costanzo L, Cesari M, Bandinelli S, Ferrucci L, Antonelli Incalzi R.. Are performance measures necessary to predict loss of independence in elderly people? J Gerontol A Biol Sci Med Sci 2016; 71: 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, Schnell S, et al.. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: A single-center experience. JACC Cardiovasc Interv 2012; 5: 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al.. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimura T, Yamamoto M, Kano S, Kagase A, Kodama A, Koyama Y, et al.. Impact of the clinical frailty scale on outcomes after transcatheter aortic valve replacement. Circulation 2017; 135: 2013–2024. [DOI] [PubMed] [Google Scholar]
- 11. O’Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al.. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 2: Isolated valve surgery. Ann Thorac Surg 2009; 88: S23–S42. [DOI] [PubMed] [Google Scholar]
- 12. Shahian DM, O’Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al.. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: Part 3: Valve plus coronary artery bypass grafting surgery. Ann Thorac Surg 2009; 88: S43–S62. [DOI] [PubMed] [Google Scholar]
- 13. Sakata Y, Syed Z, Salinger MH, Feldman T.. Percutaneous balloon aortic valvuloplasty: Antegrade transseptal vs. conventional retrograde transarterial approach. Catheter Cardiovasc Interv 2005; 64: 314–321. [DOI] [PubMed] [Google Scholar]
- 14. O’Neill WW.. Predictors of long-term survival after percutaneous aortic valvuloplasty: Report of the Mansfield Scientific Balloon Aortic Valvuloplasty Registry. J Am Coll Cardiol 1991; 17: 193–198. [DOI] [PubMed] [Google Scholar]
- 15. Lieberman EB, Bashore TM, Hermiller JB, Wilson JS, Pieper KS, Keeler GP, et al.. Balloon aortic valvuloplasty in adults: Failure of procedure to improve long-term survival. J Am Coll Cardiol 1995; 26: 1522–1528. [DOI] [PubMed] [Google Scholar]
- 16. Sakata Y, Matsubara K, Tamiya S, Hayama Y, Usui K.. The efficacy and safety of antegrade Inoue-balloon aortic valvuloplasty to treat calcific critical aortic stenosis. J Invasive Cardiol 2015; 27: 373–380. [PubMed] [Google Scholar]
- 17. Mizutani K, Hara M, Ishikawa H, Nishimura S, Ito A, Iwata S, et al.. Safety and efficacy of simultaneous biplane mode of 3-dimensional transesophageal echocardiography-guided antegrade multiple-inflation balloon aortic valvuloplasty in patients with severe aortic stenosis. Circ J 2017; 81: 748–754. [DOI] [PubMed] [Google Scholar]
- 18. Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, et al.. EuroSCORE II. Eur J Cardiothorac Surg 2012; 41: 734–744; discussion 744–745. [DOI] [PubMed] [Google Scholar]
- 19. Biancari F, Juvonen T, Onorati F, Faggian G, Heikkinen J, Airaksinen J, et al.. Meta-analysis on the performance of the EuroSCORE II and the Society of Thoracic Surgeons scores in patients undergoing aortic valve replacement. J Cardiothorac Vasc Anesth 2014; 28: 1533–1539. [DOI] [PubMed] [Google Scholar]
- 20. Debry N, Kone P, Vincent F, Lemesle G, Delhaye C, Schurtz G, et al.. Urgent balloon aortic valvuloplasty in patients with cardiogenic shock related to severe aortic stenosis: Time matters. EuroIntervention 2018; 14: e519–e525. [DOI] [PubMed] [Google Scholar]
- 21. Shimada Y, Ito K, Yano K, Tanaka C, Nakashoji T, Tonomura D, et al.. Real-time transesophageal echocardiography facilitates antegrade balloon aortic valvuloplasty. Cardiovasc Diagn Ther 2016; 6: 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ben-Dor I, Pichard AD, Satler LF, Goldstein SA, Syed AI, Gaglia MA Jr, et al.. Complications and outcome of balloon aortic valvuloplasty in high-risk or inoperable patients. JACC Cardiovasc Interv 2010; 3: 1150–1156. [DOI] [PubMed] [Google Scholar]
- 23. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, et al.. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the management of patients with valvular heart disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017; 135: e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 24. Dall’Ara G, Santarelli A, Marzocchi A, Bacchi Reggiani ML, Sabattini MR, Moretti C, et al.. Vascular complications after balloon aortic valvuloplasty in recent years: Incidence and comparison of two hemostatic devices. Catheter Cardiovasc Interv 2018; 91: E49–E55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Logistic regression analysis for 1-year mortality excluding hemodialysis patients Supplementary Table 2. Serial echocardiographic parameters 1-, 3-, 6-, 12-month after BAV (n=63)