Abstract
Tuft cells are rare chemosensory epithelial cells that monitor their environment and relay messages to the surrounding tissue via secretion of neuro- and immunomodulatory molecules. In the small intestine tuft cells detect helminth infection, protist colonization, and bacterial dysbiosis, and initiate a type 2 immune response characterized by tissue remodeling. In the airways, tuft cells sense bacteria, allergens, and noxious stimuli and drive evasive behavior, neuroinflammation, and anti-bacterial responses. Here we summarize the most recent tuft cell research and discuss how these findings have provided insight into tuft cell diversity. Built around a core program of chemosensing, tuft cell receptors and effector functions are tuned to the unique environmental exposure and physiology of their surrounding tissue.
Introduction
Tuft cells were first identified in the 1950s by their unique morphology—the eponymous apical tuft of long microvilli—in electron microscopy studies of epithelial cells in rodent airways and gastro-intestinal tract [1,2]. Yet the function of tuft cells remained enigmatic until recent research uncovered their chemosensing capacity and contribution to immune regulation [3–8]. Found in all endoderm-derived columnar epithelia, these morphologically similar cells were given different names in different tissues—microvillus cells in the olfactory epithelium, solitary chemosensory cells in the respiratory epithelium, brush cells in the trachea, tuft cells in the gastrointestinal tract—but share a common transcriptional and developmental identity driven by the transcription factor POU2F3 (POU Class 2 Homeobox 3) [6,9–11]. For the purpose of clarity, we will refer to all tuft-like cells as tuft cells. The shared gene signature consists of a chemosensing pathway and genes for synthesizing soluble neuro-immune mediators [12**,13]. Thus, chemosensory tuft cells are poised in numerous epithelial barriers to sense luminal stimuli and respond with secretion of neuro- and immunomodulatory molecules (Figure 1).
Figure 1: Tuft cell ligands and effector functions are tissue-specific.
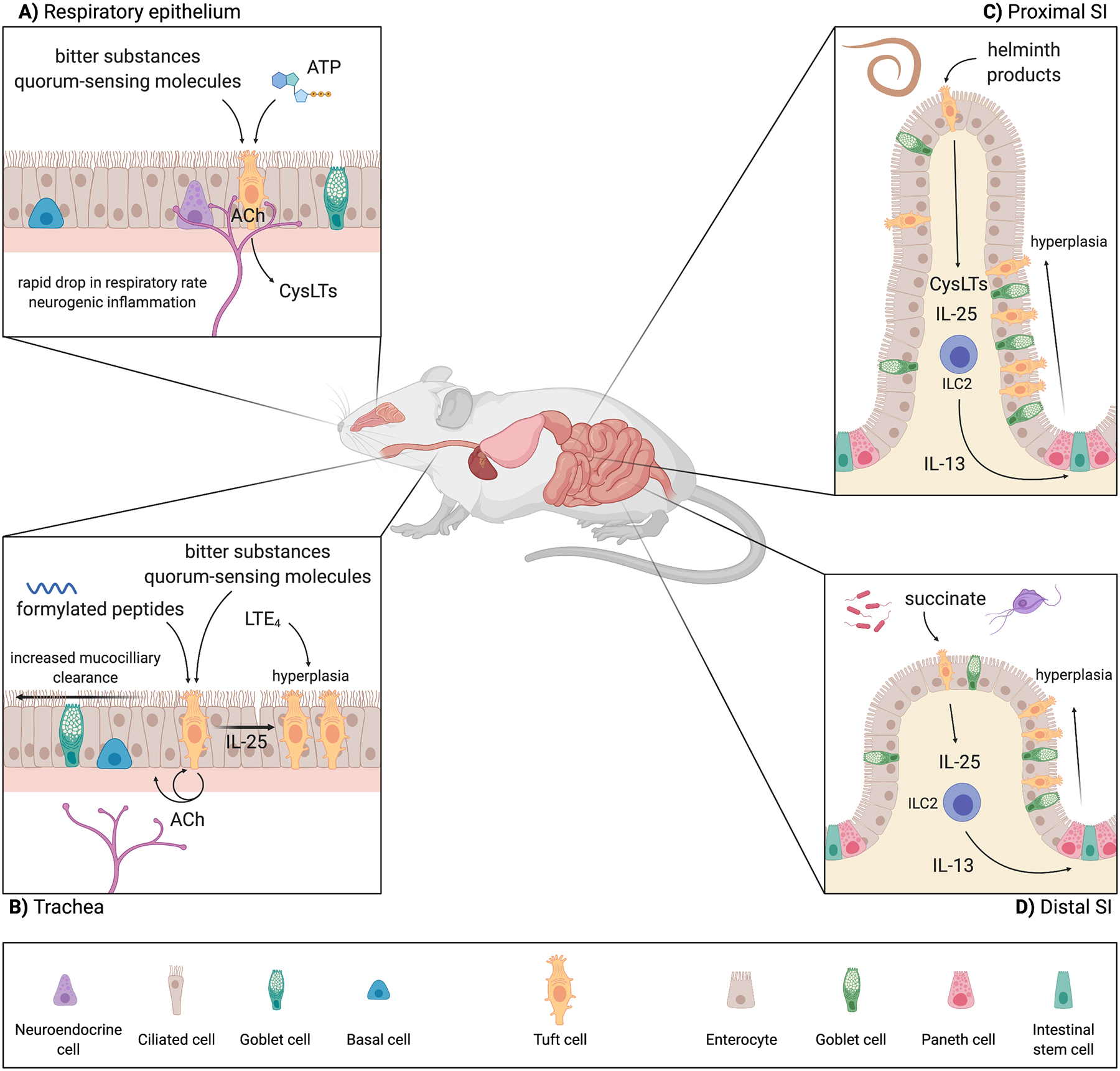
A) In the respiratory epithelium, tuft cells sense irritants, bacterial dysbiosis, and tissue homeostasis through bitter substances, quorum-sensing molecules, and ATP, respectively. These tufts cells are innervated, making ACh an effective effector molecule with which tuft cells can modulate breathing rate as well as neurogenic inflammation. Additionally, respiratory tuft cells can alter tissue immunity through ACh and CysLTs. B) Tracheal tuft cells sense bitter ligands and bacterial secreted formylated peptides. In the trachea, tuft cells are not as innervated but still use ACh to modulate tissue physiology, in this case through autocrine and paracrine signaling to epithelial cells to regulate mucociliary clearance. There is some evidence of tuft cell hyperplasia in the trachea downstream of LTE4 and IL-25, but the mechanism is not fully elucidated. C) The ligands and receptors by which tuft cells in the SI sense luminal helminths are still unknown. Nevertheless, SI tuft cells are critical for the initiation of rapid type 2 immune responses to luminal helminths. These helminths primarily reside in the proximal SI, but the type 2 immune response and resulting tuft and goblet cell hyperplasia is seen throughout the SI. D) In the distal SI, tuft cells sense Tritrichomonas protists and bacterial dysbiosis though the metabolite succinate. Tuft cells in this portion of the SI seem to be particularly poised to sense succinate, as they express higher levels of Sucnr1 compared to tuft cells in the proximal SI. Accordingly, tuft cell hyperplasia following succinate treatment is most prominent in the distal SI, although still occurs throughout the SI. Abbreviations: Acetylcholine (ACh); Choline acetyltransferase (ChAT); Cysteinyl leukotrienes (CysLTs); Leukotriene E4 (LTE4); Group 2 innate lymphoid cell (ILC2); Small intestine (SI); Succinate receptor (SUCNR1). Created with BioRender.com.
Background
The structural features and ontogeny of tuft cells have been reviewed extensively elsewhere [14,15*]. Here we provide a brief summary of the chemosensory and immune function of tuft cells. The taste chemosensing pathway expressed in tuft cells was identified in the 1990s, well before tuft cell function was understood [13,16]. Canonically, this pathway is activated by G protein-coupled taste receptors (TAS1R, TAS3R, and the TAS2R family) signaling through GNAT3 (G Protein Subunit Alpha Transducin 3), PLCB2 (Phospholipase C Beta 2) and ITPR3 (Inositol 1,4,5-Trisphosphate Receptor Type 3). The resulting Ca2+ flux opens the membrane cation channel TRPM5 (Transient Receptor Potential Cation Channel Subfamily M Member 5), which depolarizes the cell and drives release of ATP to activate adjacent neurons [17]. The core transcriptional signature shared by tuft cells from at least 5 tissues includes Gnat3, Plcb2 and Trpm5 [12**]. Defined tuft cell ligands include bacterial quorum sensing molecules (QSM), ATP, succinate, and others. As discussed below, engagement of upstream receptors and some of the cytosolic components of this pathway in tuft cells is context dependent, but to date it appears that all tuft cell-mediated phenotypes require TRPM5. Nearly all tuft cells also express Chat (choline acetyltransferase), the enzyme that synthesizes the neurotransmitter acetylcholine (ACh), which contributes to some of the known tuft cell functions in the airways and urogenital tract [3,5,18].
An immune function for tuft cells was first described in the airways, where their chemosensing capacity has been linked to secretion of antimicrobial peptides and mast cell degranulation via neuronal stimulation [5,19**]. Airway tuft cells also mediate evasion of noxious substances via transient breathing cessation [3,4]. An immune role for intestinal tuft cells was first reported when they were identified as the sole source of the cytokine interleukin 25 (IL-25) [6–8]. In the presence of helminths or Tritrichomonas protists, small intestine (SI) tuft cell-derived IL-25 directly activates group 2 innate lymphoid cells (ILC2s) in the lamina propria. Upon activation, ILC2s produce the canonical type 2 cytokines IL-5, −9, and −13 to coordinate classic manifestations of type 2 inflammation. Among its many targets, IL-13 signals to intestinal stem cells, biasing their lineage commitment toward tuft and goblet cells, resulting in hyperplasia of both cell types and activation of a feed-forward tuft-ILC2 circuit. Since the intestinal epithelium is almost completely renewed every 5 days, activation of the tuft-ILC2 circuit results in rapid remodeling of the intestinal epithelium, which contributes to helminth clearance. Recent studies that have further refined our understanding of the intestinal tuft-ILC2 circuit and of airway tuft cells are briefly summarized below.
Intestines update
One question to arise from initial studies was how activation of the tuft-ILC2 circuit is regulated. Tuft cells constitutively express Il25 mRNA, yet the feed-forward tuft-ILC2 circuit is not activated in the absence of tuft cell ligands [8,13]. Two mutually nonexclusive models have therefore emerged to explain how the tuft-ILC2 circuit is turned on downstream of chemosensing by tuft cells. Luo et al. found that IL-25 release is regulated by chemosensing: when measured from scraped intestinal villi samples, IL-25 release was reduced when villi were pretreated with a PLCB2 inhibitor or when taken from a Trpm5-deficient animal [20*]. Additionally, IL-25 is reduced if villi are pretreated with the vesicular transport inhibitor, Brefeldin A, suggesting IL-25 may be released through vesicles following tuft cell stimulation.
Regardless of the mechanism for IL-25 release, McGinty et al. hypothesized that additional signals are needed for optimal activation of ILC2s during helminth infection [21*]. Indeed, lung ILC2s must integrate multiple signaling cascades that separately mobilize the transcription factors NF-κB and NFAT to achieve optimal activation [22,23]. Cysteinyl leukotrienes (CysLTs), lipid signaling molecules rapidly synthesized by arachidonate 5-lipoxygenase (ALOX5) and leukotriene C4 synthase (LTC4S), can mobilize NFAT in lung ILC2s. Although synthesis of CysLTs is canonically thought to be restricted to hematopoietic cells, tuft cells express all the necessary enzymes as part of their core signature [12**]. In the SI, if tuft cells lack the ability to synthesize CysLTs, tuft cell hyperplasia, goblet cell responses, and worm clearance are delayed during helminth infection [21*]. Therefore, tuft cells are equipped to optimally activate ILC2s using two effector molecules, IL-25 and CysLTs. This has added a new layer to the communication between tuft cells and the underlying immune system during helminth infection.
The tuft-ILC2 circuit is also activated by intestinal colonization with Tritrichomonas protists. Tritrichomonas sp. are found as part of the commensal flora of many vivariums and their impact on intestinal immune responses has only recently come to light [7,24,25]. Tritrichomonas sp. were shown to activate the tuft-ILC2 circuit via TRPM5, but the upstream receptor and ligand remained unknown [7]. Unlike taste cells, SI tuft cells express few, if any, canonical taste receptors at homeostasis, suggesting that they must use different receptors to activate the chemosensing pathway. One GPCR enriched in SI tuft cells is the receptor for extracellular succinate (SUCNR1). Surprisingly, succinate provided in the drinking water of mice is sufficient to drive tuft cell hyperplasia and other hallmarks of intestinal type 2 immune responses [12**,26**,27**].
As a component of the citric acid cycle, mammalian succinate is usually stored intracellularly. However, bacteria, protists, and helminths can release succinate as a byproduct of anaerobic fermentation [28]. Correspondingly, activation of the tuft-ILC2 circuit by Tritrichomonas sp. is completely Sucnr1-dependent [12**]. Additionally, microbial dysbiosis caused by antibiotic or laxative treatment can induce tuft cell hyperplasia in a Sucnr1-dependent manner [27**]. This response may depend on the composition of the microbiome and relative contribution of succinate-producing vs. - consuming bacteria. Intriguingly, although succinate can be detected in supernatants of in vitro cultured N. brasiliensis and Tritrichomonas sp., the in vivo sensing of helminths is entirely Sucnr1-independent [12**,27**]. This suggests another, possibly redundant, sensor and ligand pair leads to TRPM5 activation in tuft cells during helminth infection.
Beyond immune responses to helminths and protists, roles for tuft cells in other settings of intestinal inflammation are yet to be well studied. In a model of food allergy where sensitization occurs through tape stripping of the skin, the combination of systemic IL-33 from keratinocytes and IL-25 from intestinal tuft cells activates ILC2s in the intestine [29*]. ILC2-derived IL-13 drives tuft hyperplasia and expansion of mast cells, which induce anaphylaxis upon oral challenge. While it remains unclear how IL-25 release from intestinal tuft cells is triggered during the skin sensitization phase of this allergy model, it raises the possibility that chronic activation of the tuft-ILC2 circuit by stimuli such as Tritrichomonas sp. colonization could increase susceptibility to food allergy.
Airway update
In the current paradigm, airway tuft cells act as chemosensory sentinels that sense noxious environmental ligands via the TAS2R family of taste receptors and secrete ACh. In turn, ACh signals on afferent neurons to control breathing and inflammation [3–5]. Specifically, tuft cell activation in the nasal epithelium causes mast cell degranulation, but there is scant evidence linking airway tuft cells to the initiation of type 2 inflammation more broadly [5]. Defined tuft cell ligands either indirectly indicate bacterial dysbiosis (e.g. QSMs) or are themselves noxious substances (e.g. denatonium and other TAS2R ligands).
Several recent studies have expanded on this basic paradigm with the discovery of novel tuft cell ligands and effector functions. Perniss et al. reported that, consistent with the theme of sensing dangerous bacterial growth, tracheal tuft cells detect formylated peptides produced by pathogenic bacteria of the lung [30**]. Stimulation of explanted tracheas with formylated peptides drives mucociliary clearance, an innate process that sweeps bacteria up and out of the trachea. This response requires release of ACh from tuft cells to stimulate neighboring ciliated cells. The formylated peptide receptor remains unknown, but the authors excluded non-redundant functions for canonical formyl peptide receptors and taste receptors, including all TAS2Rs. Nonetheless, the canonical taste transduction pathway, including PLCB2, ITPR3, and TRPM5 is required. The findings were complemented by another tracheal study showing that bitter ligands and QSMs also enhanced mucociliary clearance via ACh signaling, though questions remain since Perniss et al. found that mucociliary clearance induced by those same ligands was mostly tuft cell-independent [30**,31*]. In addition, the study showed that autocrine ACh signaling regulates tuft cell intracellular calcium levels much like it does in taste cells [32]. Tuft cell sensing of bacteria has also been extended to the gingiva [33]. These tuft cells, which express GNAT3 but not ChAT, control oral bacterial community composition and density in an ACh-independent manner, perhaps through induction of anti-microbial peptides.
Bankova et al. expanded on the concept of tuft cells and type 2 immunity by reporting that tracheal tuft cells modestly increased in number following aeroallergen sensitization. The mechanism for this expansion remains unresolved, but seems to be largely IL-13-independent [34]. More recent work by the same group further implicates airway tuft cells in type 2 immunity with the finding that nasal tuft cells are activated by host-derived ATP through P2Y2 (P2Y Purinoceptor 2) during aeroallergen sensitization [35*]. As in the intestine, chemosensing induced CysLT synthesis in tuft cells. Tuft cell-deficient mice failed to produce CysLTs and had modestly reduced eosinophilia following Alternaria sensitization, though tuft cell-specific targeting of LTC4S was not performed. Future studies are needed to determine if tuft cells produce CysLTs upon stimulation with previously defined bitter and quorum-sensing ligands, or if effector functions are somehow segregated based on stimulus type.
Lastly, a study by Rane et al. has expanded the repertoire of airway tuft cells with the seminal finding that influenza A (IAV) infection induces tuft cell differentiation in the deep lung, where they are not found during homeostasis [36**]. These pulmonary tuft cells arise from p63+ stem cells that are mobilized to repair the severe damage caused by IAV infection. While they appear to be bonafide tuft cells capable of responding to succinate and denatonium, numerous questions remain: what signals drive their differentiation (i.e. do they require IL-13?), what effector molecules do they produce, and what functions do they perform, if any?
Human tuft cells
As in rodents, tuft cells were first identified in humans by electron microscopy. Reports were sparse, but cells with tuft-like morphology were spotted in the airways and intestines by the 1970s [37,38]. Human tuft cells were then ignored for decades, until advances in rodent models once again spurred progress. Tuft cells expressing taste receptors, GNAT3, and TRPM5 were identified in human sinonasal epithelium and air-liquid interface culture systems allowed for functional studies [39]. In a pair of seminal papers, Cohen and colleagues demonstrated that tuft cells flux calcium in response to the bitter ligand denatonium, that this Ca2+ flux was propagated to neighboring cells via gap junctions, and that antimicrobial peptides were secreted from the epithelium as a result [19**,40]. Interestingly, sweet taste transduction inhibited these responses, suggesting complex integration of environmental signals by tuft cells.
More recently, this same group examined IL-25 expression and tuft cell replication in settings of allergic inflammation, such as chronic rhinosinusitis with nasal polyps and eosinophilic fungal disease [41–43]. They found that tuft cell frequency and IL-25 expression are increased in such inflamed epithelia, and that IL-13 is sufficient to induce IL-25 and drive tuft cell replication in vitro. The authors suggest that just as in the mouse intestine, there could be a feed forward epithelial-immune circuit in the airways.
Whether such a circuit also exists in the human intestine remains completely unknown, but recent discoveries have at least expanded our toolbox for visualization of tuft cells in intestinal biopsies (Table 1). Of note, DCLK1 (Doublecortin-like Kinase 1), one of the most commonly used tuft cell markers in mice, is not a good marker for human tuft cells [44**]. Instead, the markers listed in Table 1 appear to be highly tuft cell-specific. Accordingly, human intestinal tuft cells are likely also a source of eicosanoids and acetylcholine. Tuft cell quantification has not yet been reported in type 2 inflamed human intestine (e.g. during worm infection or allergic disease), but preliminary observations suggest a possible reduction in tuft cells during type 1 inflammation (e.g. duodenitis, Crohn’s, and celiac) [45,46]. Further studies are needed to define the human intestinal tuft cell transcriptome, assess IL-25 protein expression, and determine if IL-13 can also induce human tuft cell hyperplasia. Perhaps organoid culture systems could help to answer these questions. The hope is that tuft cells can be harnessed to treat disease.
Table 1. Markers for human tuft cells.
DCLK1, the marker of choice for mouse tuft cells, is not a reliable marker for human tuft cells. Instead, recent studies have identified numerous proteins that appear to uniquely identify tuft cells among epithelial cells. Concurrent labeling with two or more markers provides the most definitive identification of tuft cells.
| Gene Symbol | Name | Source |
|---|---|---|
| PTGS1 (COX1) | Prostaglandin endoperoxide synthase 1 | [44**,58] |
| HPGDS | Hematopoietic prostaglandin D synthase | [44**,58] |
| ALOX5AP (FLAP) | Arachidonate 5-lipoxygenase activating protein | [44**] |
| ALOX5 (5-LO) | Arachidonate 5-lipoxygenase | [21*] |
| CHAT | Choline acetyltransferas | [44**] |
| CCDC88A (GIRDIN) | Coiled-coil domain containing 88A (**pY1798-specific antibody) | [59] |
| EGFR | Epidermal growth factor receptor (** pY1068-specific antibody) | [21*,60] |
| SOX9 | SRY-box transcription factor 9 | [58] |
| AVIL | advillin | [44**] |
Tuning the tuft cell response
Previous comparisons of tuft cells from across the body have shown they are adapted to their particular tissue location despite sharing a core gene signature. Beyond the specialization revealed by receptor repertoires, new research is extending our understanding of tuft cell diversity by showing that the development and effector functions of tuft cells are not stereotyped, allowing for tuning of the tuft cell response.
Intestines
Although tuft cells are present in both the large and small intestine, activation of the tuft-ILC2 circuit and associated tuft cell hyperplasia has only been reported to occur in the SI, even when type 2 immunity is activated systemically [29*,47,48]. This difference may reflect the distinct developmental programs of tuft cells in each part of the intestine. Tuft cells in the large intestine require Atoh1 (Atonal Homolog BHLH Transcription Factor 1) for development, whereas at least some SI tuft cells can develop independently of Atoh1 [49]. Nevertheless, tuft cells in both locations require Pou2f3, the master tuft cell transcription factor, and express the canonical tuft cell signature genes [12**]. More work is needed to identify a role for tuft cells in the large intestine.
Even within the same tissue, functional differences in tuft cells are emerging (Figure 2A). In the SI, for example, the succinate-sensing pathway predominantly induces tuft cell hyperplasia in the distal SI (ileum), where Tritrichomonas protists and bacteria are most abundant [12**,27**]. Accordingly, ileal tuft cells express the highest levels of Sucnr1 [26**]. By contrast, SUCNR1-independent hyperplasia induced by helminths occurs across the length of the SI, even though the helminths tend to colonize the proximal SI [50]. Differences between succinate sensing and helminth infection also extend to include tuft cell effector functions. Early during helminth infection, tuft cell production of CysLTs is required for optimal ILC2 activation. However, mice lacking CysLT production have no defect in tuft-ILC2 circuit activation when treated with succinate or colonized with Tritrichomonas sp. [21*].
Figure 2: Airway and small intestinal tuft cell signaling.
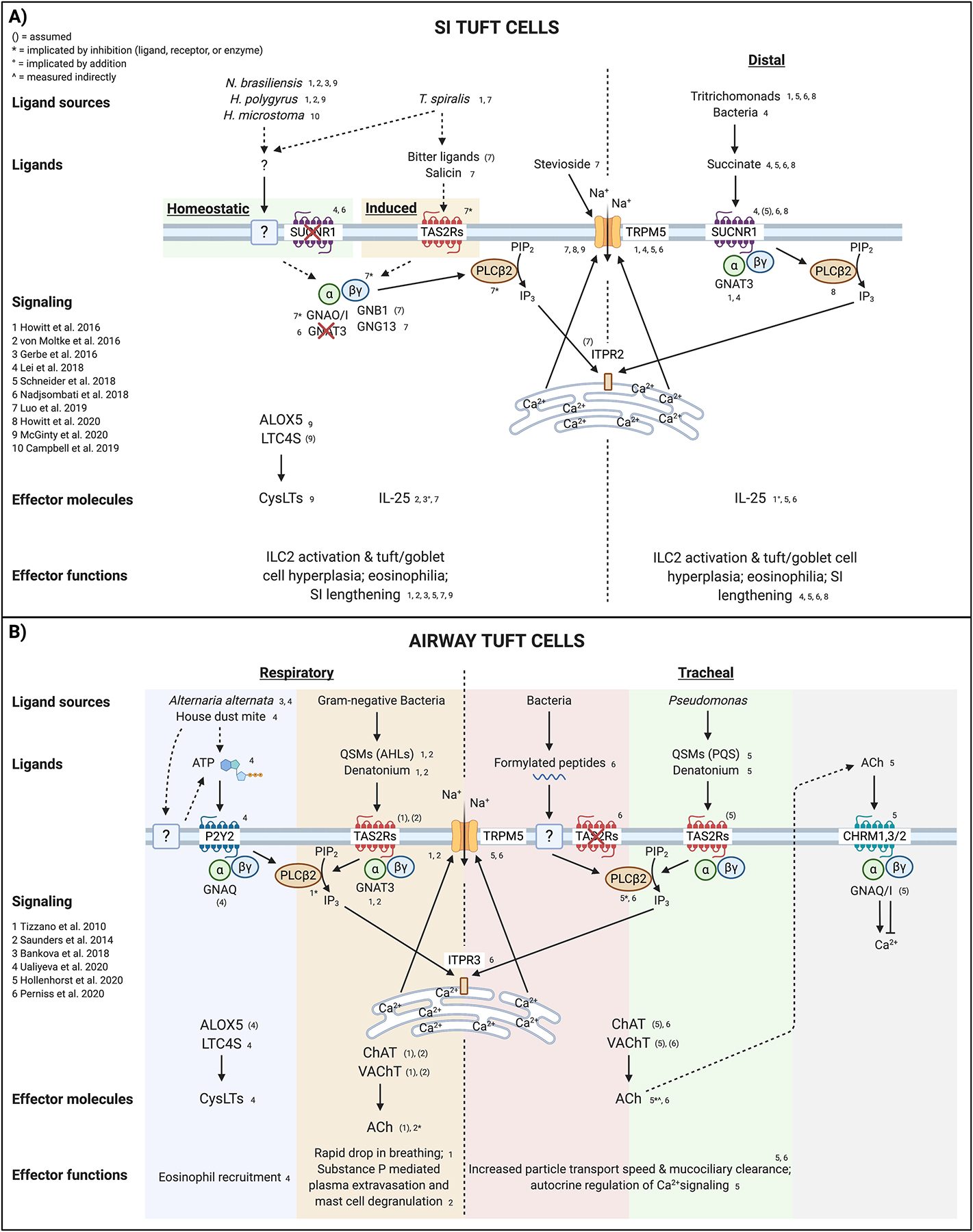
A) Small intestinal tuft cells sense the presence of helminth worms via an unidentified receptor and ligand. Tuft cell-derived IL-25 and CysLTs activate lamina propria ILC2s to drive epithelial remodeling, eosinophilia, and worm clearance. The calcium-gated cation channel TRPM5, downstream of canonical taste transduction in taste cells, is also required for helminth sensing, though Gnat3−/− mice are not affected. Additionally, while some helminths can produce succinate as a metabolic byproduct, Sucnr1−/− mice have no defect in tuft hyperplasia in response to helminth infection. Following initial activation and IL-13 signaling, tuft cells express TAS2Rs, which may sense “bitter” substances found in T. spiralis excretory-secretory products, and result in additional release of IL-25. G-alpha O/I subunits, as well as the G-gamma 13 subunit and PLCB2, have also been implicated in T. spiralis sensing and IL-25 release. Potentiating TRPM5 with stevioside is sufficient to cause release of IL-25. Specifically in the distal SI, tuft cells monitor the metabolite succinate, which can be produced by Tritrichomonas protists as well as dysbiotic bacteria. Unlike helminth sensing, GNAT3 is required for the succinate response, as are PLCB2 and TRPM5, indicating a canonical taste transduction pathway. B) Airway tuft cells have been studied most thoroughly in the respiratory and tracheal epithelia. Respiratory tuft cells (and olfactory tuft cells) use the receptor P2Y2 to sense ATP released from cells via an unknown mechanism following treatment with Alternaria alternata or house dust mite. ATP signaling drives increased intracellular Ca2+ and release of CysLTs that recruit eosinophils and amplify type 2 inflammation. It remains unclear if tracheal tuft cells produce CysLTs. Respiratory tuft cells also express numerous TAS2Rs, which bind denatonium and the “bitter” QSMs produced by certain Gram-negative bacteria. Signaling activates the canonical taste transduction pathway, resulting in release of ACh that signals on peptidergic neurons to rapidly induce a drop in breathing rate and neurogenic inflammation. In the trachea, where tuft cells are less extensively innervated, sensing of bacterial formylated peptides, QSMs, or denatonium activate PLCB2, Ca2+ flux, and TRPM5-dependent ACh release. Via CHRMs, ACh signals in both a paracrine fashion on neighboring ciliated cells to increase ciliary beat frequency and in an autocrine fashion on tuft cells to enhance or suppress the magnitude of Ca2+ flux. While TAS2Rs are assumed to be required for sensing QSMs and denatonium, they are not involved in formylated peptide sensing; the actual receptor remains unidentified. Dashed arrows indicate unconfirmed pathways. Abbreviations: Acetylcholine (ACh); Acyl-homoserine lactones (AHL); Arachidonate 5-lipoxygenase (ALOX5); Bitter taste receptor family (TAS2Rs); Choline acetyltransferase (ChAT); Cysteinyl leukotrienes (CysLTs); G Subunit Alpha Q/I/O (GNAQ/I/O); G Protein Subunit Alpha Transducin 3 (GNAT3); G Subunit Beta 1 (GNB1); G Subunit Gamma 13 (GNG13); Inositol 1,4,5-Trisphosphate Receptor Type 3 (ITPR); Leukotriene C4 synthase (LTC4S); Muscarinic ACh receptors (CHRMs); Phospholipase C Beta 2 (PLCB2); Pseudomonas quinolone signal (PQS); P2Y Purinoceptor 2 (P2Y2); Quorum-sensing molecules (QSMs); Small intestine (SI); Succinate receptor (SUCNR1); Transient Receptor Potential Cation Channel Subfamily M Member 5 (TRPM5); Vesicular acetylcholine transporter (VAChT). Created with BioRender.com.
The activation of different effector functions by succinate and the as yet unidentified helminth ligand(s) may be linked to differential engagement of intracellular signaling pathways. Succinate sensing by tuft cells is Gnat3, Plcb2, and Trpm5-dependent [12**,26**,27**,51]. On the other hand, only Trpm5 is necessary during helminth infection, while Gnat3 is dispensable and the requirement for Plcb2 has not been tested [12**]. Other G protein subunits may also be differentially activated. Tuft cells in the SI express G-protein Subunit Beta 1 (GNB1) and Gamma 13 (GNG13), and mice lacking Gng13 in the intestinal epithelium have reduced tuft cell hyperplasia during Trichinella spiralis infection [20*].
The spatial and functional tuning of tuft cell responses in the SI may reflect different purposes of the type 2 immune response in these contexts. Perhaps, tuft cells sense succinate to monitor the commensal flora and locally regulate interactions with the intestinal epithelium by increasing mucus production through the type 2 immune response. This response is well tolerated, as Tritrichomonas-colonized mice show no ill effects despite chronic tuft and goblet cell hyperplasia in the distal SI. On the other hand, helminths pose a greater risk to the health of the host, causing damage to the intestinal lining, and siphoning host nutrients. The need to expel these parasites is greater and perhaps therefore the tuft-ILC2 circuit is activated along the entire length of the small intestine and potent CysLTs are mobilized.
Airways
Like their SI counterparts, airway tuft cells employ distinct effector functions that are tuned to their location (Figure 2B). Indeed, even in the case of one effector molecule, ACh, there are different outcomes depending on the location. Respiratory tuft cells, which are likely the first to encounter inhaled noxious bitter stimuli or bacteria, use ACh to rapidly decrease the breathing rate and minimize further inhalation [3]. However, once stimuli get to the trachea tuft cells instead use ACh to coordinate the mucociliary mechanism of physical removal [30**,31*]. Future studies should test whether formylated peptides induce mucociliary clearance in the respiratory epithelium or regulate breathing in the trachea.
The outcome of tuft cell activation may also depend on the degree of innervation. Most respiratory tuft cells are closely contacted by peptidergic nerve fibers, while just a quarter of tracheal tuft cells are similarly contacted, and olfactory tuft cells have little to no contact with neurons [3,4,52]. Accordingly, respiratory tuft cells signal directly on the trigeminal nerve to regulate breathing rate and neurogenic inflammation [3,5]. In the trachea, however, ACh instead acts locally in a paracrine fashion on the epithelium by regulating ciliary function [30**,31*]. Further study of olfactory tuft cells is needed to determine what ACh is doing in the complete absence of neuronal contacts, but neighboring epithelial cells flux Ca2+ in response to ACh, suggesting paracrine signaling [53].
Next Directions
Much remains to be learned about the role of tuft cells in immunity and other physiologic functions. We have highlighted some specific questions above, but broader areas of interest start with the on-going search for ligands and effector functions. Most notably, the ligand(s) and receptor(s) that mediate helminth sensing in the SI remain unknown, as does the receptor for formylated peptides in the trachea. Taste receptors have been implicated in helminth sensing, but they are poorly expressed at baseline, suggesting a different receptor mediates the initial detection [20*]. The possibility that tuft cells act as mechanosensors has not been explored, but their similarity to certain morphological features of hair cells in the ear and Merkel cells in the skin is striking [54–56]. On the effector side, it is unclear if tuft cells secrete ATP as their taste receptor cell cousins do. Also, the immune function, if any, of tuft cell-derived acetylcholine in the intestine is completely unknown, as is any function of tuft cells in the gall bladder and colon.
Specifically in the SI, it is unknown how IL-13/STAT6 signaling drives hyper-production of tuft cells from the stem cell compartment and why this only occurs in this one tissue. Indeed, the purpose of tuft cell hyperplasia is itself unknown. It also remains unclear why succinate sensing predominates in the distal SI and is dispensable during helminth infection. Why are tuft cells monitoring microbial metabolism and what are the consequences if this sensing is lost?
Conclusion
We have learned much about tuft cells in recent years and the list of their chemosensory and effector functions is rapidly expanding. While some generalizations can be applied to tuft cells in all tissues, the differences are perhaps more striking. The ligands that activate tuft cells and the effector functions they use to shape the physiology of their surrounding tissue differ widely between organs and even within different regions of the same organ. Clearly, these are versatile cells that have been precisely tuned to their microenvironment.
This review has focused on the immune function of tuft cells, but there is also a growing literature reporting the association of tuft cells with tissue repair and carcinogenesis [36**,57]. Perhaps the coming years will reveal that these seemingly disparate functions are in many ways related. Tissue repair and type 2 immunity are already closely linked and a role in carcinogenesis may similarly be related to a broader role for tuft cells in maintenance and restoration of tissue homeostasis.
Highlights.
Tuft cells reside in the epithelium of airway, intestinal, and urogenital tissues
Tuft cell receptors and effector functions are tuned in a tissue-specific manner
Tuft cells detect noxious substances and ligands from bacteria, helminths, or protists
Tuft cells modulate immune responses and neurologic function
Tools to identify and study human tuft cells are improving
Acknowledgements
MSN is supported by the University of Washington Immunology Training Grant (T32 AI106677). JvM is a Searle Scholar. The laboratory is supported by NIH DP2 OD024087 and R01 AI145848 and the University of Washington.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- [1].Järvi O, Keyriläinen O: On the cellular structures of the epithelial invasions in the gladular stomach of mice caused by intramural application of 20-methylcholantren. Acta Pathol Microbiol Scand 1956, 38:72–73. [PubMed] [Google Scholar]
- [2].Rhodin J, Dalhamn T: Electron microscopy of the tracheal ciliated mucosa in rat. Zeitschrift für Zellforsch und Mikroskopische Anat 1956, 44:345–412. [DOI] [PubMed] [Google Scholar]
- [3].Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill MEA, Silver WL, Kinnamon SC, Finger TE: Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci U S A 2010, 107:3210–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Mühlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, et al. : Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci U S A 2011, 108:9478–9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Saunders CJ, Christensen M, Finger TE, Tizzano M: Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc Natl Acad Sci U S A 2014, 111:6075–6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, et al. : Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 2016, 529:226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Howitt MR, Lavoie S, Michaud M, Blum AM, Tran SV, Weinstock JV, Gallini CA, Redding K, Margolskee RF, Osborne LC, et al. : Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 2016, 351:1329–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].von Moltke J, Ji M, Liang H-E, Locksley RM: Tuft-cell-derived IL-25 regulates an intestinal ILC2–epithelial response circuit. Nature 2016, 529:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yamashita J, Ohmoto M, Yamaguchi T, Matsumoto I, Hirota J: Skn-1a/Pou2f3 functions as a master regulator to generate Trpm5-expressing chemosensory cells in mice. PLoS One 2017, 12:e0189340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ohmoto M, Yamaguchi T, Yamashita J, Bachmanov AA, Hirota J, Matsumoto I: Pou2f3/Skn-1a is necessary for the generation or differentiation of solitary chemosensory cells in the anterior nasal cavity. Biosci Biotechnol Biochem 2013, 77:2154–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yamaguchi T, Yamashita J, Ohmoto M, Aoudé I, Ogura T, Luo W, Bachmanov AA, Lin W, Matsumoto I, Hirota J: Skn-1a/Pou2f3 is required for the generation of Trpm5-expressing microvillous cells in the mouse main olfactory epithelium. BMC Neurosci 2014, 15:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nadjsombati MS, McGinty JW, Lyons-Cohen MR, Jaffe JB, DiPeso L, Schneider C, Miller CN, Pollack JL, Nagana Gowda GA, Fontana MF, et al. : Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity 2018, 49:33–41.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bezençon C, Fürholz A, Raymond F, Mansourian R, Métairon S, Le Coutre J, Damak S: Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol 2008, 509:514–525. [DOI] [PubMed] [Google Scholar]
- [14].von Moltke J: Intestinal Tuft Cells. In Physiology of the Gastrointestinal Tract: Sixth Edition. Elsevier Inc.; 2018:721–733. [Google Scholar]
- [15].O’Leary CE, Schneider C, Locksley RM: Tuft Cells—Systemically Dispersed Sensory Epithelia Integrating Immune and Neural Circuitry. Annu Rev Immunol 2019, 37:47–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Höfer D, Püschel B, Drenckhahn D: Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci U S A 1996, 93:6631–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kinnamon SC, Finger TE: Recent advances in taste transduction and signaling. F1000Research 2019, 8:2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Deckmann K, Filipski K, Krasteva-Christ G, Fronius M, Althaus M, Rafiq A, Papadakis T, Renno L, Jurastow I, Wessels L, et al. : Bitter triggers acetylcholine release from polymodal urethral chemosensory cells and bladder reflexes. Proc Natl Acad Sci U S A 2014, 111:8287–8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee RJ, Kofonow JM, Rosen PL, Siebert AP, Chen B, Doghramji L, Xiong G, Adappa ND, Palmer JN, Kennedy DW, et al. : Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest 2014, 124:1393–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Luo X-CC, Chen Z-HH, Xue J-BB, Zhao D-XX, Lu C, Li Y-HH, Li S-MM, Du Y-WW, Liu Q, Wang P, et al. : Infection by the parasitic helminth Trichinella spiralis activates a Tas2r-mediated signaling pathway in intestinal tuft cells. Proc Natl Acad Sci U S A 2019, 116:5564–5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McGinty JW, Ting HA, Billipp TE, Nadjsombati MS, Khan DM, Barrett NA, Liang HE, Matsumoto I, von Moltke J: Tuft-Cell-Derived Leukotrienes Drive Rapid Anti-helminth Immunity in the Small Intestine but Are Dispensable for Anti-protist Immunity. Immunity 2020, 52:528–541.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lund SJ, Portillo A, Cavagnero K, Baum RE, Naji LH, Badrani JH, Mehta A, Croft M, Broide DH, Doherty TA: Leukotriene C4 Potentiates IL-33–Induced Group 2 Innate Lymphoid Cell Activation and Lung Inflammation. J Immunol 2017, 199:1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].von Moltke J, O’Leary CE, Barrett NA, Kanaoka Y, Austen KF, Locksley RM: Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. J Exp Med 2017, 214:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chudnovskiy A, Mortha A, Kana V, Kennard A, Ramirez JD, Rahman A, Remark R, Mogno I, Ng R, Gnjatic S, et al. : Host-Protozoan Interactions Protect from Mucosal Infections through Activation of the Inflammasome. Cell 2016, 167:444–456.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Escalante NK, Lemire P, Cruz Tleugabulova M, Prescott D, Mortha A, Streutker CJ, Girardin SE, Philpott DJ, Mallevaey T: The common mouse protozoa Tritrichomonas muris alters mucosal T cell homeostasis and colitis susceptibility. J Exp Med 2016, 213:2841–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schneider C, O’Leary CE, von Moltke J, Liang H-E, Ang QY, Turnbaugh PJ, Radhakrishnan S, Pellizzon M, Ma A, Locksley RM: A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell 2018, 174:271–284.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lei W, Ren W, Ohmoto M, Urban JF, Matsumoto I, Margolskee RF, Jiang P: Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc Natl Acad Sci U S A 2018, 115:5552–5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Muller M, Mentel M, van Hellemond JJ, Henze K, Woehle C, Gould SB, Yu R-Y, van der Giezen M, Tielens AGM, Martin WF: Biochemistry and Evolution of Anaerobic Energy Metabolism in Eukaryotes. Microbiol Mol Biol Rev 2012, 76:444–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Leyva-Castillo J-M, Galand C, Kam C, Burton O, Gurish M, Musser MA, Goldsmith JD, Hait E, Nurko S, Brombacher F, et al. : Mechanical Skin Injury Promotes Food Anaphylaxis by Driving Intestinal Mast Cell Expansion. Immunity 2019, 50:1262–1275.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Perniss A, Liu S, Boonen B, Keshavarz M, Ruppert AL, Timm T, Pfeil U, Soultanova A, Kusumakshi S, Delventhal L, et al. : Chemosensory Cell-Derived Acetylcholine Drives Tracheal Mucociliary Clearance in Response to Virulence-Associated Formyl Peptides. Immunity 2020, 52:683–699.e11. [DOI] [PubMed] [Google Scholar]
- [31].Hollenhorst MI, Jurastow I, Nandigama R, Appenzeller S, Li L, Vogel J, Wiederhold S, Althaus M, Empting M, Altmüller J, et al. : Tracheal brush cells release acetylcholine in response to bitter tastants for paracrine and autocrine signaling. FASEB J 2020, 34:316–332. [DOI] [PubMed] [Google Scholar]
- [32].Dando R, Roper SD: Acetylcholine is released from taste cells, enhancing taste signalling. J Physiol 2012, 590:3009–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zheng X, Tizzano M, Redding K, He J, Peng X, Jiang P, Xu X, Zhou X, Margolskee RF: Gingival solitary chemosensory cells are immune sentinels for periodontitis. Nat Commun 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bankova LG, Dwyer DF, Yoshimoto E, Ualiyeva S, McGinty JW, Raff H, von Moltke J, Kanaoka Y, Frank Austen K, Barrett NA: The cysteinyl leukotriene 3 receptor regulates expansion of IL-25–producing airway brush cells leading to type 2 inflammation. Sci Immunol 2018, 3:9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ualiyeva S, Hallen N, Kanaoka Y, Ledderose C, Matsumoto I, Junger WG, Barrett NA, Bankova LG: Airway brush cells generate cysteinyl leukotrienes through the ATP sensor P2Y2. Sci Immunol 2020, 5:eaax7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rane CK, Jackson SR, Pastore CF, Zhao G, Weiner AI, Patel NN, Herbert DR, Cohen NA, Vaughan AE: Development of solitary chemosensory cells in the distal lung after severe influenza injury. Am J Physiol - Lung Cell Mol Physiol 2019, 316:L1141–L1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Watson JH, Brinkman GL: Electron microscopy of the epithelial cells of normal and bronchitic human bronchus. Am Rev Respir Dis 1964, 90:851–866. [DOI] [PubMed] [Google Scholar]
- [38].Moxey PC, Trier JS: Specialized cell types in the human fetal small intestine. Anat Rec 1978, 191:269–285. [DOI] [PubMed] [Google Scholar]
- [39].Barham HP, Cooper SE, Anderson CB, Tizzano M, Kingdom TT, Finger TE, Kinnamon SC, Ramakrishnan VR: Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int Forum Allergy Rhinol 2013, 3:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lee RJ, Hariri BM, McMahon DB, Chen B, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Jiang P, Margolskee RF, et al. : Bacterial D-amino acids suppress sinonasal innate immunity through sweet taste receptors in solitary chemosensory cells. Sci Signal 2017, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Patel NN, Triantafillou V, Maina IW, Workman AD, Tong CCL, Kuan EC, Papagiannopoulos P, Bosso JV, Adappa ND, Palmer JN, et al. : Fungal extracts stimulate solitary chemosensory cell expansion in noninvasive fungal rhinosinusitis. Int Forum Allergy Rhinol 2019, 9:alr.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Patel NN, Kohanski MA, Maina IW, Triantafillou V, Workman AD, Tong CCL, Kuan EC, Bosso JV, Adappa ND, Palmer JN, et al. : Solitary chemosensory cells producing interleukin-25 and group-2 innate lymphoid cells are enriched in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol 2018, 8:900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kohanski MA, Workman AD, Patel NN, Hung LY, Shtraks JP, Chen B, Blasetti M, Doghramji L, Kennedy DW, Adappa ND, et al. : Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2018, 142:460–469.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Schütz B, Ruppert AL, Strobel O, Lazarus M, Urade Y, Büchler MW, Weihe E: Distribution pattern and molecular signature of cholinergic tuft cells in human gastro-intestinal and pancreatic-biliary tract. Sci Rep 2019, 9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Huh WJ, Te Roland J, Asai M, Kaji I: Distribution of duodenal tuft cells is altered in pediatric patients with acute and chronic enteropathy. Biomed Res 2020, 41:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Banerjee A, Herring CA, Chen B, Kim H, Simmons AJ, Southard-Smith AN, Allaman MM, White JR, Macedonia MC, Mckinley ET, et al. : Succinate Produced by Intestinal Microbes Promotes Specification of Tuft Cells to Suppress Ileal Inflammation. Gastroenterology 2020, 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ricardo-Gonzalez RR, Schneider C, Liao C, Lee J, Liang HE, Locksley RM: Tissue-specific pathways extrude activated ILC2s to disseminate type 2 immunity. J Exp Med 2020, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Campbell L, Hepworth MR, Whittingham-Dowd J, Thompson S, Bancroft AJ, Hayes KS, Shaw TN, Dickey BF, Flamar AL, Artis D, et al. : ILC2s mediate systemic innate protection by priming mucus production at distal mucosal sites. J Exp Med 2019, 216:2714–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Herring CA, Banerjee A, McKinley ET, Simmons AJ, Ping J, Roland JT, Franklin JL, Liu Q, Gerdes MJ, Coffey RJ, et al. : Unsupervised Trajectory Analysis of Single-Cell RNA-Seq and Imaging Data Reveals Alternative Tuft Cell Origins in the Gut. Cell Syst 2018, 6:37–51.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF: Cytokine regulation of host defense against parasitic gastrointestinal nematodes: Lessons from studies with rodent models. Annu Rev Immunol 1997, 15:505–533. [DOI] [PubMed] [Google Scholar]
- [51].Howitt MR, Cao YG, Gologorsky MB, Li JA, Haber AL, Biton M, Lang J, Michaud M, Regev A, Garrett WS: The Taste Receptor TAS1R3 Regulates Small Intestinal Tuft Cell Homeostasis. ImmunoHorizons 2020, 4:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Finger TE, Böttger B, Hansen A, Anderson KT, Alimohammadi H, Silver WL: Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci U S A 2003, 100:8981–8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ogura T, Szebenyi SA, Krosnowski K, Sathyanesan A, Jackson J, Lin W: Cholinergic microvillous cells in the mouse main olfactory epithelium and effect of acetylcholine on olfactory sensory neurons and supporting cells. J Neurophysiol 2011, 106:1274–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vollrath MA, Kwan KY, Corey DP: The Micromachinery of Mechanotransduction in Hair Cells. Annu Rev Neurosci 2007, 30:339–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Halata Z, Grim M, Bauman KI: Friedrich Sigmund Merkel and his “Merkel cell”, morphology, development, and physiology: Review and new results. Anat Rec - Part A Discov Mol Cell Evol Biol 2003, 271:225–239. [DOI] [PubMed] [Google Scholar]
- [56].Hoover B, Baena V, Kaelberer MM, Getaneh F, Chinchilla S, Bohórquez DV.: The intestinal tuft cell nanostructure in 3D. Sci Rep 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Middelhoff M, Westphalen CB, Hayakawa Y, Yan KS, Gershon MD, Wang TC, Quante M: Dclk1-expressing tuft cells: Critical modulators of the intestinal niche? Am J Physiol - Gastrointest Liver Physiol 2017, 313:G285–G299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gerbe F, Van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C et al. : Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol 2011, 192:767–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kuga D, Ushida K, Mii S, Enomoto A, Asai N, Nagino M, Takahashi M, Asai M: Tyrosine Phosphorylation of an Actin-Binding Protein Girdin Specifically Marks Tuft Cells in Human and Mouse Gut. J Histochem Cytochem 2017, 65:347–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].McKinley ET, Sui Y, Al-Kofahi Y, Millis BA, Tyska MJ, Roland JT, Santamaria-Pang A, Ohland CL, Jobin C, Franklin JL et al. : Optimized multiplex immunofluorescence single-cell analysis reveals tuft cell heterogeneity. JCI Insight 2017, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]


