Abstract
The RAS-RAF-MEK-ERK pathway is the most well-studied of the mitogen-activated protein kinase (MAPK) cascades and is critical for cell proliferation, differentiation, and survival. Abnormalities in regulation resulting from mutations in components of this pathway, particularly in upstream proteins RAS and RAF, are responsible for a significant fraction of human cancers and nearly all cutaneous melanomas. Activation of receptor tyrosine kinases by growth factors and various extracellular signals to the sequential activation of RAS, RAF, MEK, and finally ERK, which activates numerous transcription factors and facilitates oncogenesis in the case of aberrant pathway activation. While extensive studies have worked to elucidate the activation mechanisms and structural components of upstream MAPK components, comparatively less attention has been directed towards the kinases, MEK and ERK, due to the infrequency of oncogenic activating mutations in these kinases. However, acquired drug resistance has become a major issue in the treatment of RAS- and RAF- mutated cancers. Targeting the terminal kinases in the MAPK cascade has shown promise for overcoming many of these resistance mechanisms and improving treatment options for patients with MAPK-aberrant cancers. Here, we will describe the role of MEK and ERK in MAPK signaling and summarize the current understanding of their interaction and activation mechanisms. We will also discuss existing targeted approaches for MEK and ERK, and the benefits of alternative strategies. Areas requiring further exploration will be highlighted to guide future research endeavors and aid in the development of alternative therapeutic strategies to combat surmounting drug resistance in treating MAPK-mediated cancers.
Graphical Abstract
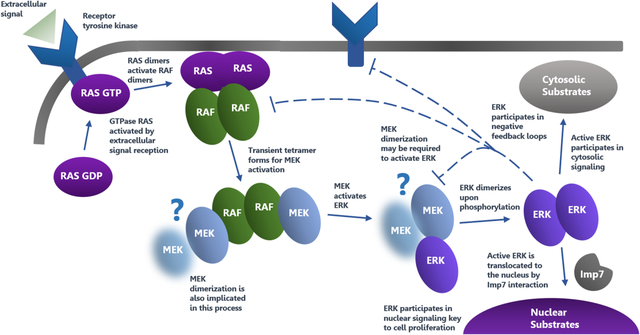
Visual Overview
Shown is an overview of the MAPK pathway highlighting the various mechanisms of activation and phosphorylation, as well as the importance of oligomerization and feedback regulation. Question marks indicate areas of uncertainty in the present model and arrows show sequential activation and signal transmission steps.
I. Introduction
The mitogen-activated protein kinase (MAPK) signal transduction cascades are highly conserved central regulators of cell proliferation, cell cycle progression, and survival, which are activated in response to numerous extracellular signals including growth factors, cytokines, and stress inducers (1). There are presently four MAPK cascades identified in eukaryotic cells: the extracellular signal-regulated kinase (ERK) cascade, the p38MAPK cascade, the c-Jun N-terminal kinase cascade, and the ERK5 cascade. While the p38MAPK and c-Jun N-terminal cascades are principally involved in transducing stress-related stimuli, the ERK cascade is predominately involved in the transmission of mitogenic signals. The most well-studied of the MAPK pathways, the RAS-RAF-MEK-ERK signal transduction cascade, is aberrantly activated in over one-third of all human cancers and 90% of cutaneous melanomas (2, 3). Upon signaling by receptor tyrosine kinases, G protein-coupled receptors, and other membrane receptors, the GTPase, RAS, is activated, and it subsequently recruits the RAF kinases to the plasma membrane for activation (4). Active RAF kinases phosphorylate the downstream mitogen-activated protein kinase kinase (MEK) (5). A transient tetramer, consisting of two RAF-MEK dimers, is formed to facilitate MEK activation by RAF (6). Active MEK then dually phosphorylates ERK, which can translocate to the nucleus to activate a wide variety of transcription factors and other nuclear substrates, alongside its cytosolic targets (7, 8). Due to their prominent role in cancer development and progression, the ERK pathway components, particularly the oncogenic RAS and RAF, have been extensively studied and promising targets for drug design (1, 9, 10).
There are two identified isoforms of both MEK and ERK. Due to the high sequence conservation and functional similarity between the two isoforms, they shall henceforth be referred to as MEK1/2 and ERK1/2, or simply MEK and ERK. MEK1/2 is a dual-specificity protein kinase, classified as part of the serine/threonine protein kinase family (11), that is capable of catalyzing the phosphorylation of select threonine and tyrosine residues of ERK1/2. Active MEK1/2 phosphorylates ERK on both a tyrosine and threonine residue on the TEY motif of ERK1/2 (7, 8, 12). ERK1/2 is a proline-directed protein-serine/threonine kinase (7, 8). Analysis of ERK catalytic activity and substrate interactions revealed that ERK exhibits specificity in substrate phosphorylation for serine/threonine contained within a conserved Pro-XXX-Ser/Thr-Pro sequence (13). While MEK1/2 is characterized by highly narrow substrate specificity, with ERK1/2 as its only known downstream target (1), ERK1/2 has extremely broad substrate specificity and is capable of activating both nuclear and cytosolic targets, many of which are transcription factors essential for cell proliferation and survival, or are involved in negative feedback loops (14). Given the exclusivity of the MEK/ERK interaction and the existence of ERK1/2 as the sole substrate of MEK1/2, ERK kinases play a critical role in mediating the downstream effects of MEK activation in the context of both normal and oncogenic pathway signaling.
This review will characterize the roles of MEK and ERK as components of the MAPK cascade. It will discuss the structural properties and subcellular localization of MEK and ERK, highlight key features of the MEK/ERK interaction and activation mechanism, and review current therapeutic approaches and existing small-molecule inhibitors directed towards MEK and ERK. In light of extensive drug resistance to approved RAF and MEK inhibitors and the persistent inefficacy of current targeting strategies, we will suggest alternative approaches that take advantage of the MEK/ERK network that could inform future research and drug development.
II. Structural and Functional Components of MEK and ERK
Kinases of the MAPK pathway share the highly conserved general features of protein kinases (relevant reviews (8, 15)) that were first identified in cyclic AMP-dependent protein kinase A (PKA) (16). They consist of a small N-terminal lobe and a larger C-terminal lobe separated by a catalytic cleft where MgATP binds for active site phosphoryl transfer (Fig. 1). The N-lobes of MEK and ERK contain five β-sheets as well as a highly flexible conserved glycine-rich loop that aids in positioning bound ATP for cleavage and phosphoryl transfer (Fig. 1). The N-lobe also contains a conformationally-sensitive αC-helix that is critical to the activation state of the kinase (Fig. 1). Following the glycine-rich loop is a conserved valine (V81/85 in MEK1/2 and V56/39 in ERK1/2) that engages in a hydrophobic interaction with the adenine of ATP (Fig. 2). The C-terminal lobe contains four β-sheets and six conserved α-helices. Located within the β3-strand of the N-lobe is an Ala-XXX-Lys motif (residues 95–97/99–101 in MEK1/2 and 69–71/52–54 in ERK1/2). This β3-strand lysine forms a critical salt-bridge with a conserved glutamate from the αC-helix (residue 114/118 in MEK1/2 and 88/71 in ERK1/2) that is necessary but not sufficient for kinase activation (Fig. 1). Relative to the inactive conformation, the αC-helix of an active kinase is positioned farther inward, in order to form the salt-bridge necessary for stabilizing the active conformation; this is labeled the “αC-helix in” conformation. The activation segments of MEK and ERK begin with a conserved DFG motif and end with a canonical APE motif (in MEK1 this motif is SPE).
Fig. 1: Key Structural Features of ERK.
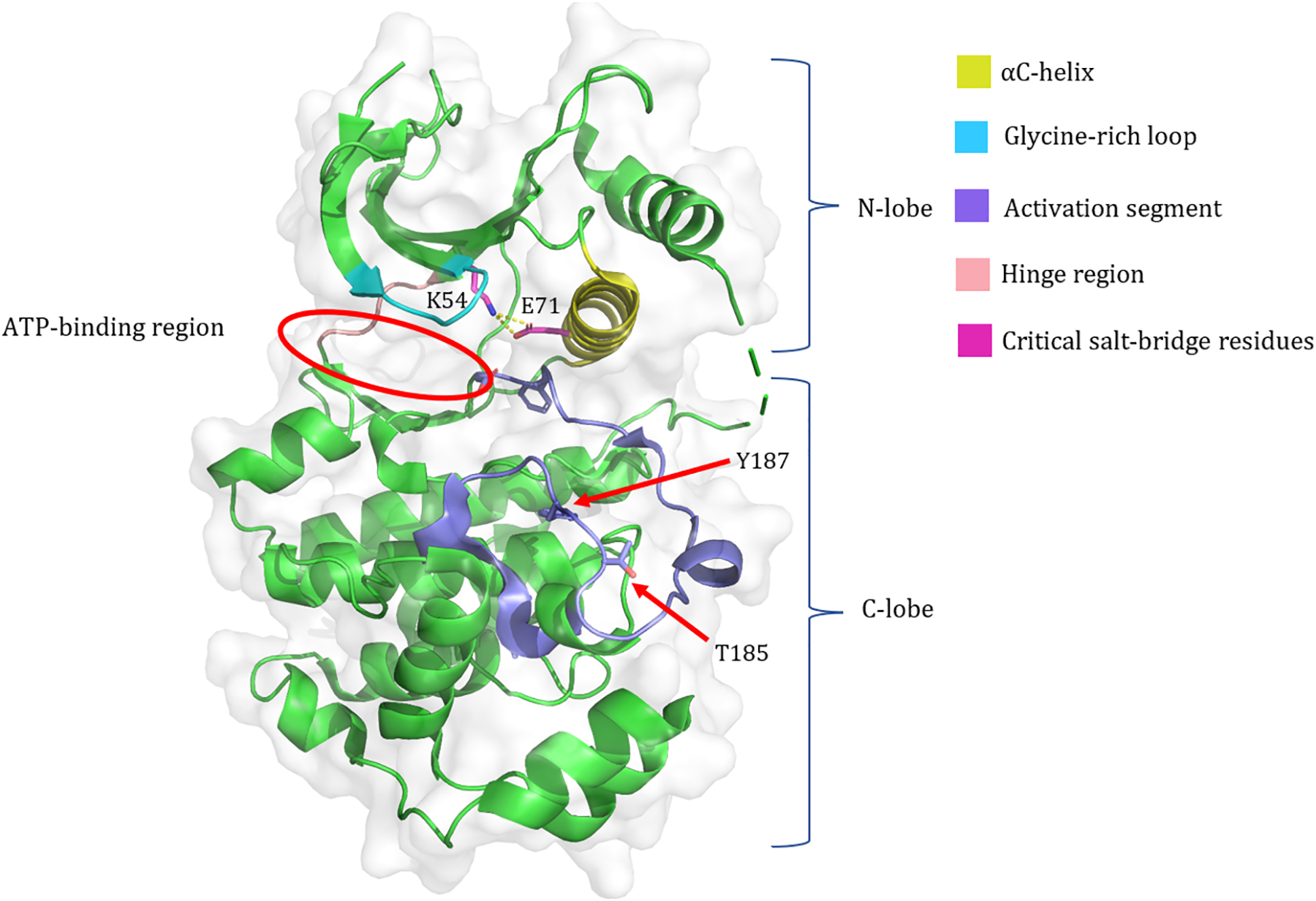
The crystal structure of human ERK2 (PDB ID: 5NHP). Important structural components discussed in the text are highlighted, four critical residues are labeled, and the N- and C- lobes and the ATP-binding region are indicated.
Fig. 2: ATP Binding Interactions of ERK.
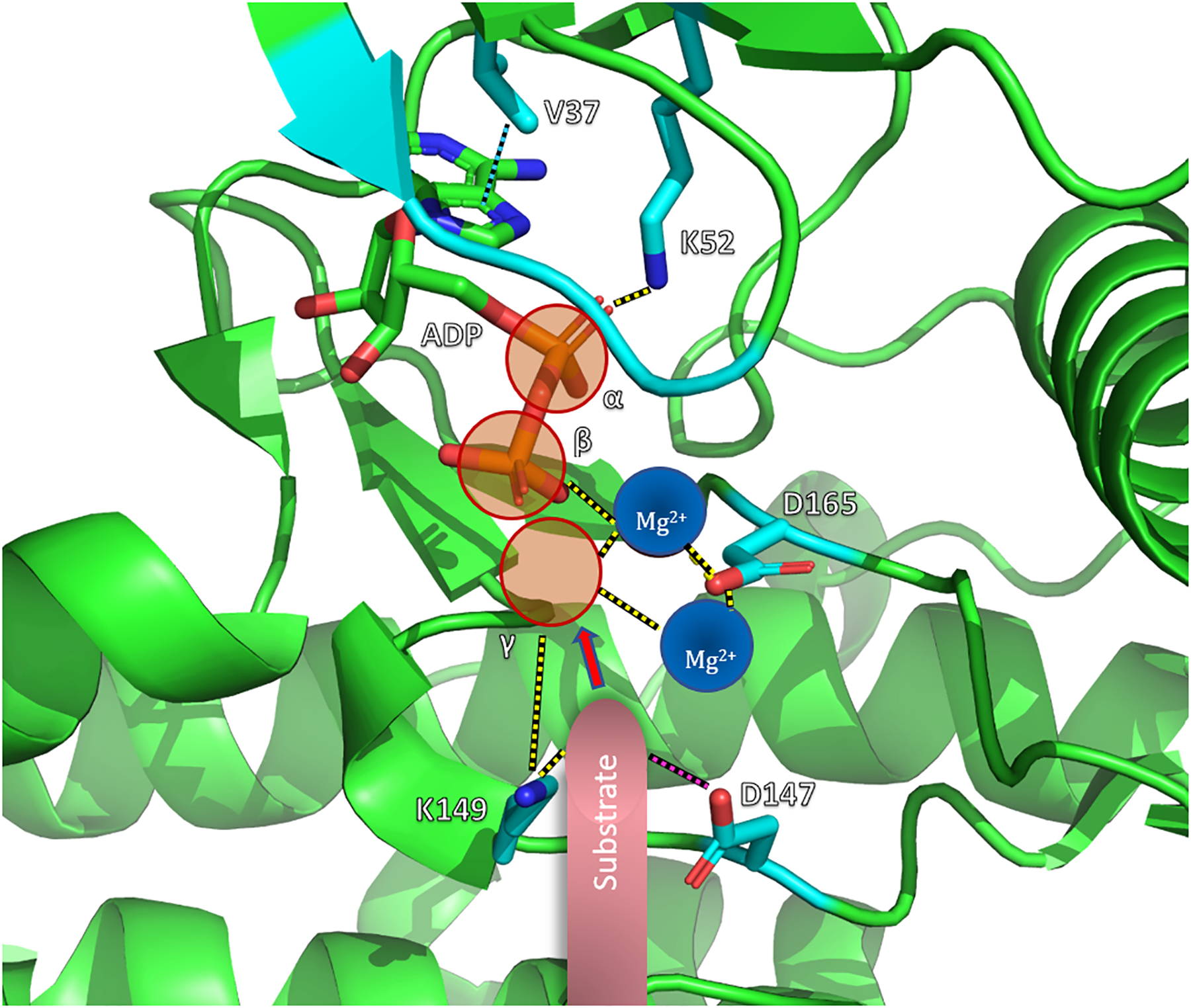
The figure is adapted from a crystal structure of ADP-bound Rat ERK2 (PDB ID: 4GVA). The key residues believed to facilitate ATP binding and phosphoryl transfer are indicated. Electrostatic interactions are represented by yellow dashed lines. The pink dashed line between D147 and substrate indicates its proposed role in the deprotonation of substrate for nucleophilic attack, indicated by the pink arrow. D165 is the residue from the DFG motif discussed in the text. The hydrophobic interaction between the conserved valine and adenine is shown as a cyan dashed line. The theoretical locations of two bound Mg2+ cofactors are shown and the γ-phosphate that would be present in ATP is represented by a transparent red sphere. The labeled residues correspond to Rat ERK2 (add 2 to obtain the corresponding human ERK2 residues).
The N-lobe and C-lobe of MEK and ERK are separated by a flexible hinge region (Fig. 1) that allows for rotation of the lobes relative to each other. The catalytically active conformation requires the inward rotation of the N- and C- lobes towards each other to bring distant active site residues closer and form a closed active site. Minimal outward rotation of the N- and C- lobes is required to allow ATP binding and ADP release during the catalytic cycle. The αC-helix within the N-lobe plays a role in stabilizing the active conformation by forming the Lys-Glu salt-bridge as mentioned above. In the C-lobe, the D of the DFG motif is also critical to assuming the active conformation, as the aspartate sidechain must rotate inward to coordinate Mg2+ in the active site (Fig. 2). In the inactive conformation, the aspartate faces outwards, labeled “DFG-out”. An aspartate residue within the catalytic loop (residue 190/194 in MEK1/2 and 166/149 in ERK1/2) is believed to deprotonate the protein substrate and facilitate its nucleophilic attack on the γ-phosphate of ATP (Fig. 2).
Phosphorylation of activation segment residues is required to induce the conformational changes necessary to assemble a functional active site. Contained within the activation segment of MEK are two serine residues that are phosphorylated by RAF kinases for activation; these residues are S218 and S222 of MEK1 and S222 and S226 of MEK2. While both residues are required for activation, the dephosphorylation of either leaves MEK fully activated (17, 18). The activation of ERK requires dual phosphorylation on both a tyrosine and threonine residue contained on a TEY motif within the activation segment (Fig. 1) of ERK (residues T202/185 and Y204/187 on ERK1/2). Phosphorylation of both residues is required for the full activation of ERK (7).
Analysis of the inactive and active conformations of dozens of protein kinases gave rise to the classification of certain key residues into larger structural motifs known as regulatory and catalytic spines (Fig. 3), or R-spines and C-spines respectively (19, 20). These sets of residues are another highly conserved feature of protein kinases and have been identified in both MEK and ERK (8, 15). The configuration of the residues classified as part of these spines determines the overall spine alignment, which has been shown to be highly correlated with the activation state of the kinase. In both MEK and ERK, a residue from the β4-strand, C-helix, activation loop, catalytic loop, and F-helix make up the five residues that comprise the R-spine (Fig. 3). Four of these residues are hydrophobic and the fifth is a conserved aspartate residue. Eight hydrophobic residues make up the C-spine, and these residues are located in β-strands 2, 3, and 7, as well as in the D-helix and F-helix (Fig. 3). Residues from both spines are key to catalysis, as the R-spine stabilizes the binding site for interactions with protein substrates and the C-spine positions ATP within the active site for phosphoryl transfer (2). A bent R-spine is associated with the inactive conformation of the kinase.
Fig. 3: R- and C-spines of ERK2.
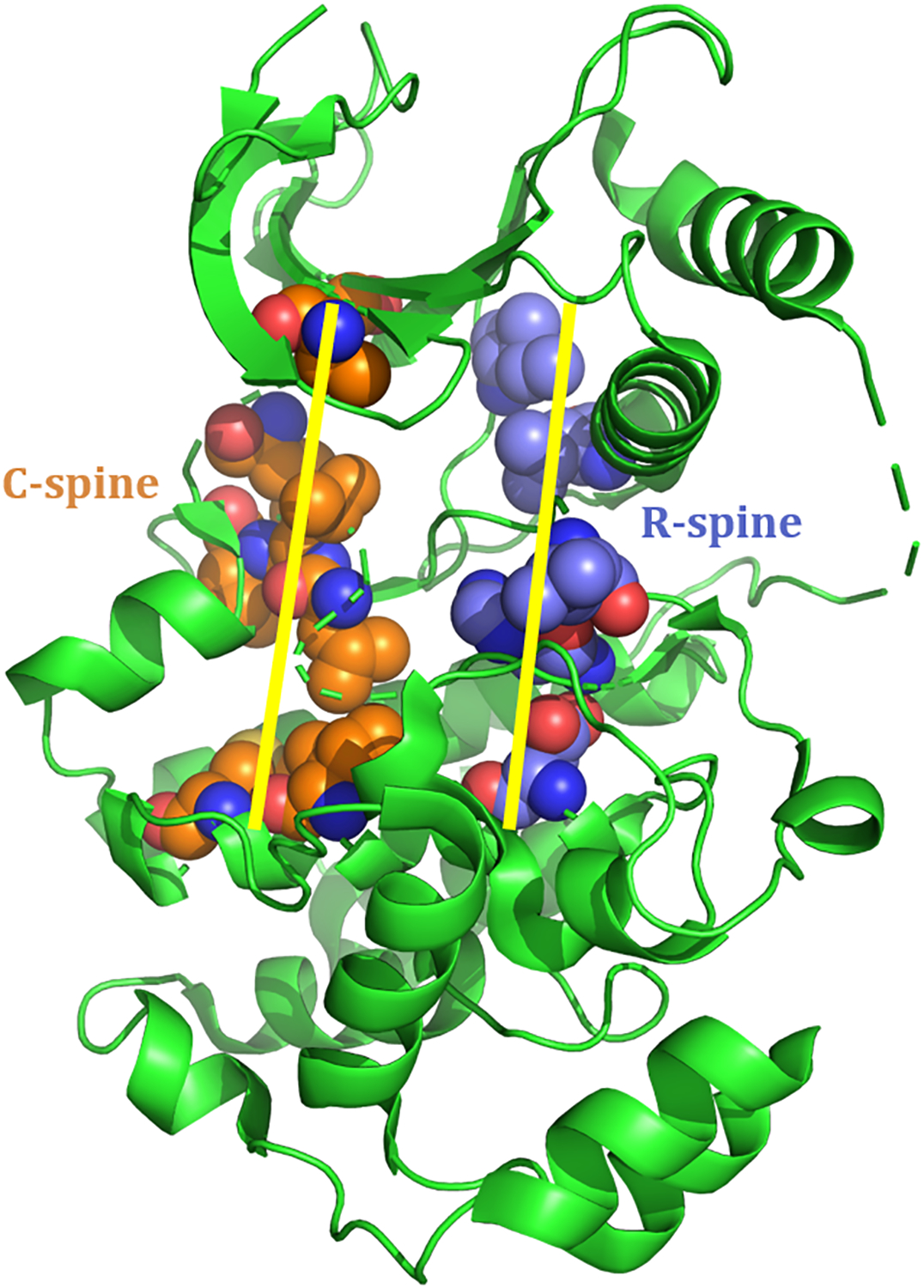
The residues represented here as spheres comprise the R- and C-spines of ERK2. R-spine residues are colored blue and C-spine residues are colored orange. This is adapted from a crystal structure of human ERK2 (PDB ID: 5NHP).
The N-terminal sequence of MEK contains an αA-helix involved in autoinhibitory interactions, a nuclear export sequence (NES), and an ERK binding domain. The A-helix of MEK, first identified in a structural and mechanistic analysis of MEK1, has an autoinhibitory role, as it makes extensive contacts with the kinase domain and effectively stabilizes the inactive “αC-helix out” conformation (21). The contacts are mainly hydrophobic and are largely confined to the N-lobe. Only two residues contact the C-terminal lobe, therefore it is likely that the A-helix residues do not necessarily affect the relative movements of the N- and C- lobes in their stabilization of the “αC-helix out” conformation. Notably, a class of identified oncogenic mutants of MEK consists of deletions, substitutions, and helix-breaking mutations of A-helix residues, as well as mutations in the residues that interact with A-helix residues. These mutations relieve the autoinhibition that is critical in maintaining the low basal activity of MEK and are therefore associated with hyperactive de-regulated signaling (22, 23). The N-terminal nuclear export sequence of MEK is key in localizing MEK to the cytoplasm, which is critical to the role of MEK as a cytoplasmic anchor of ERK (discussed below). An ERK-binding domain is located in the N-terminus of MEK, and studies have demonstrated that the N-terminal region is necessary for MEK to interact with ERK (24). Located in the C-terminal domain of MEK1 and MEK2 is a proline-rich insert that is not present in other members of the MEK family. Deletion of this region has no effect on MEK activation or binding to RAF, but significantly decreases the ability of MEK to bind and phosphorylate ERK (25). Additionally, unique to MEK1 is a threonine residue (Thr292) which can be phosphorylated by ERK1/2 to induce feedback inhibition of the pathway (26).
A majority of the substrates, phosphatases, scaffolds, and regulators that interact with ERK, including MEK1, possess either of two conserved sequence motifs labeled the D-docking domain or F-docking domain (8). Regions of ERK that interact with these sites are identified as D-site recruitment sites (DRS) and F-site recruitment sites (FRS) respectively (8). The DRS of ERK is located on the backside of the region containing the catalytic cleft and consists of largely negatively charged residues that interact with the positively charged residues of the D-docking domain of interacting proteins (Fig. 4). The FRS of ERK (Fig. 4) consists of mainly aromatic and hydrophobic residues that interact with FXFP or FXF sequences in the F-docking domains.
Fig. 4: DRS and FRS of ERK2.
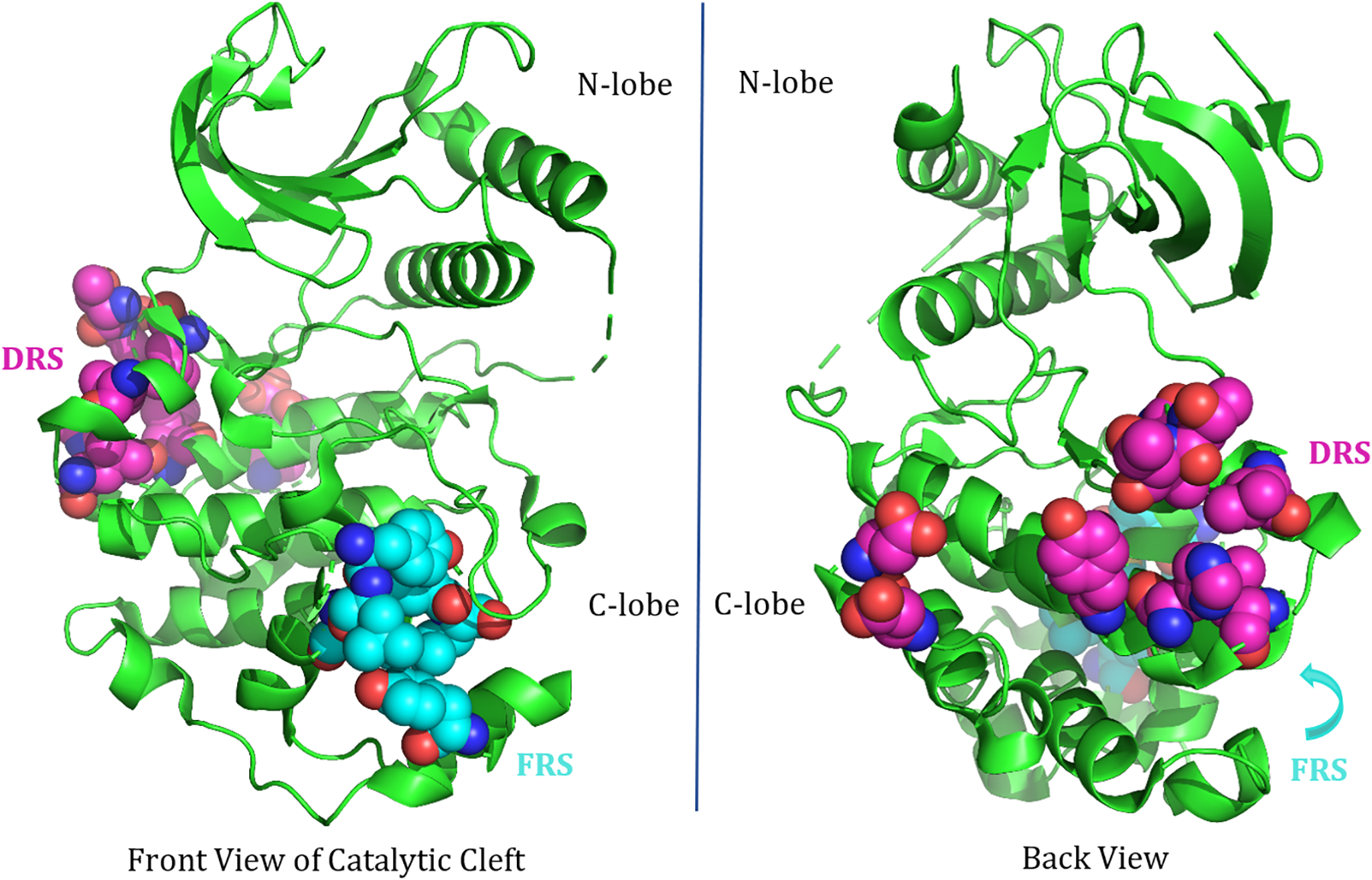
The residues represented as spheres here comprise the D-site recruitment site (pink) and F-site recruitment site (cyan) of ERK2. The DRS is located on the backside of the catalytic cleft and has a predominately negatively charged exterior, allowing it to interact with predominately positively charged D-docking site residues of interacting proteins. The residues present in the DRS of human ERK2 are L115, L121, H125, Y128, L157, T159, T160, D318, and D321. The FRS residues are mainly hydrophobic: M199, L200, Y233, L234, L237, Y263. This is adapted from a crystal structure of human ERK2 (PDB ID: 5NHP).
The MEK/ERK interaction is highly specific and conformationally sensitive, and it involves the interaction of several key regions of both MEK and ERK. A previous study identified multiple spatially distant regions of ERK that are believed to be involved in conferring high affinity and specificity to interactions with MEK. Located within the C-terminal region is an insert domain that is unique to MAP kinases and has been shown to be critical to phosphorylation by MEK in vitro (27). In addition, while the phosphorylation lip, containing the TEY motif, is critical to the phosphorylation interaction, it surprisingly contributes little to the specificity of the MEK/ERK interaction (28). The N-terminal sequence of ERK has also been shown to be important for association with MEK, ERK kinase activity, and subcellular localization (29). Deletion and mutagenesis studies on this region, specifically of residues 19–25 in ERK2, rendered the kinase inactive and led to aberrant nuclear localization due to the inability to associate with its cytoplasmic anchor, MEK (discussed below) (29). The mutant ERK2 was unable to bind MEK in vivo, as revealed through co-immunoprecipitation experiments. Intriguingly, this ERK2 mutant still showed significant phosphorylation by MEK. An interesting question is whether the MEK-ERK association for active phosphorylation and for inactive cytoplasmic anchoring might be different in terms of affinity and might involve the interaction of different domains or residues of MEK and ERK. As a structure for either the active MEK-ERK complex or the inactive cytoplasmic anchoring complex has yet to be resolved, it is difficult to determine precisely the nature of their interaction and the particular contribution of this N-terminal region, along with other implicated regions, to their interaction.
III. Cytoplasmic Anchoring and Nuclear Transport of ERK
In addition to catalyzing ERK phosphorylation, MEK functions as a cytoplasmic anchor for ERK. MEK contains a nuclear export sequence (NES) that localizes it to the cytoplasm. Several studies have shown that inactive ERK exists in the cytosol bound to MEK, and, in the absence of MEK, ERK is localized to the nucleus (24, 30). Once phosphorylated, ERK dissociates from MEK and either translocates to the nucleus or remains localized in the cytoplasm bound to scaffold proteins. The initial translocation of ERK requires ERK activation by phosphorylation, as nuclear translocation was not observed in the presence of MEK inhibitors but phosphorylated ERK was able to localize to the nucleus in the absence of MEK (31, 32). Live-cell imaging studies revealed that, upon activation, ERK localizes to the nucleus, however there is no significant movement of MEK into the nucleus, supporting a MEK-independent translocation (30).
The mechanism by which ERK translocates to the nucleus is still unclear. Significant discrepancies existed among previous reports, however recent studies have clarified and refined the current model. A widespread set of theories had been proposed in the past, including nucleoporin-mediated transport, dimerization-dependent and independent transport, and both active and passive transport (33–35). Consideration of conventional transport mechanisms was unyielding, as ERK did not appear to contain a nuclear localization sequences (NLS) and showed no evidence of binding to an NLS-containing shuttling protein. However, a nuclear translocation sequence (NTS) was recently identified in ERK (36). While an NLS is often identified as a cluster of basic residues, typically lysine or arginine, that facilitate binding to transport protein β-karyopherin importin-β and adaptor protein importin-α, which function to shuttle the protein across the nuclear membrane (37), the NTS of ERK instead contains a three-residue, phosphorylatable motif, Ser-Pro-Ser (SPS). The most recent model for the nuclear translocation of ERK involves phosphorylation on the SPS motif of the NTS of ERK, which facilitates binding to importin-7 (36, 38). Importin-7 is then proposed to escort ERK through the nuclear pore via interactions with nucleoporins and, upon entry, the GTPase, Ran, helps ERK dissociate from importin-7. As mentioned above, TEY phosphorylation of ERK is necessary for nuclear transport. Consistent with the current accepted mechanism, TEY phosphorylation is believed to be required only for dissociation from cytoplasmic anchoring complexes and not for the subsequent shuttling of ERK to the nucleus, however further investigation is needed to elucidate the precise role of TEY phosphorylation in nuclear transport. It may be valuable to assess whether importin-7 recognition and binding is affected by the phosphorylation state of Tyr and Thr of TEY and whether cytosolic unbound, unphosphorylated-TEY, phospho-SPS ERK can be transported effectively, since dissociation from the anchoring complex is not a limiting factor.
IV. Spatiotemporal Control in ERK Signaling
The duration of ERK localization in the nucleus is one identified means of signal output differentiation in ERK signaling. While phosphorylation of ERK is necessary and sufficient for the initial rapid translocation to the nucleus (31, 32), sustained nuclear signaling and accumulation of ERK in the nucleus requires transcriptional induction by ERK and the neosynthesis of short-lived proteins, possibly nuclear anchoring proteins (31). Taken together, these studies suggest that ERK oscillates between two high affinity binding states, interacting with anchor proteins in both the cytoplasm (MEK and various scaffold proteins) and in the nucleus, and that the nuclear anchor proteins have transient expression and duration, thus prompting the return of ERK to the cytoplasm after a regulated time period (39). However, additional research is needed to verify this model and identify the alleged nuclear anchor proteins. The molecular interactions facilitating the export of ERK back to the cytoplasm following activation remain unclear. Previous study has suggested that ERK exits the nucleus via an NES-dependent mechanism that is highly sensitive to the activation state of ERK, and that NES-containing MEK might serve as the escort (40, 41). However, subsequent studies have been unable to definitively validate this proposal since the amount of MEK localized to the nucleus is relatively small compared to ERK, which does not provide substantial support for this theory (42). Further investigation into the nuclear export of ERK is needed to elucidate the molecular mechanisms that facilitate the dynamic localization of ERK.
Cytoplasmic trapping is an additional suggested mechanism of spatial regulation of ERK signaling (39). Preferential cytoplasmic localization and reduced nuclear signaling promote alternate signaling outputs. Cytoplasmic trapping of ERK by PEA15, a phosphoprotein enriched in diabetes, was shown to block cell proliferation in astrocytes, and this inhibition was released in the absence of PEA15 (43). In addition, β-arrestin has been shown to bind and facilitate cytoplasmic retention of active ERK, increasing cytoplasmic substrate activation while decreasing ERK-dependent transcription (44). The ERK module is subject to extensive spatial and temporal regulation, enabling the differentiation of signaling outputs.
V. Overview of the Nuclear and Cytosolic Targets of ERK Signaling
Extensive efforts have been made to identify and characterize the extremely large and diverse substrate pool of ERK1/2. Over 600 direct substrates of ERK, including transcription factors, cytoskeletal elements, apoptotic regulators, kinases and phosphatases, mRNA splicing and transcription machinery, and additional signaling proteins, have been identified and compiled in recent decades in the literature, as well as catalogued in online databases (45–48). The nuclear targets of ERK consist largely of transcription factors involved in cell proliferation and are key in oncogenic transformation (47). Upon mitogenic stimulation and MAPK activation, ERK translocates to the nucleus and can induce the expression of numerous immediate early genes (IEGs), including transcription factors, C-Fos, Myc, and Fra, via activation of transcription factor substrates such as Elk1 (49–51). Additionally, sustained ERK activation can facilitate the subsequent phosphorylation of these unstable IEG transcription factors by ERK, which leads to their stabilization and accumulation and promotes cell proliferation (46, 52, 53). Sustained ERK activation can also facilitate the inhibition of the antiproliferative Tob and Foxo3a proteins, allowing for cell cycle progression (54, 55). While ERK signaling is capable of both promoting and inhibiting apoptosis in different cellular and signaling contexts, hyperactive ERK signaling is generally associated with anti-apoptotic outcomes (56). For example, ERK phosphorylation has been shown to inhibit the pro-apoptotic Bim protein or stabilize the anti-apoptotic protein Mcl-1 (57, 58). As evidenced by the small representative set of targets discussed here, the nuclear substrates of ERK are predominately responsible for the ability of ERK signaling to promote cell proliferation and survival.
Regarding cytosolic ERK signaling, active ERK retained in the cytoplasm is capable of interacting with cytoskeletal elements, including the focal adhesion protein, paxillin, which has also been shown to play a scaffolding role in mediating RAF/MEK/ERK complexation and activation localized to focal adhesions (45, 46, 59–61). Paxillin-bound ERK is able to, in turn, phosphorylate paxillin, which increases the association of paxillin with the focal adhesion kinase (FAK) and enhances cell spreading and adhesion upon hepatocyte growth factor stimulation (59–61). Active ERK can also phosphorylate both ribosomal S6 kinases (RSKs), which propagate ERK signaling and contribute to cell migration and proliferation, and MSKs, which are capable of regulating IEGs (62–64). The prominent roles of effectors downstream of ERK in modulating critical processes such as cell proliferation, migration, and survival reveal the tremendous oncogenic potential of prolonged and de-regulated ERK signaling.
Apart from the ability of oncogenic ERK signaling to promote proliferation and survival via the diverse functions of its downstream effectors, of note are the additional ERK-dependent mechanisms by which MAPK-mutant tumors escape or resist suppression by the immune system. Recent work revealed that non-small cell lung cancer (NSCLC) cells harboring mutations in KRAS (RAS isoform), which facilitate overactivated MAPK signaling, induce apoptosis of T cells via the upregulation of programmed death-ligand 1 (PD-L1) by a mechanism dependent on ERK activity (65). Treatment with an ERK inhibitor reversed these effects and was correlated with a decreased survival of KRAS-mutant tumor cells (65). In addition, KRAS mutant cells have been shown to enhance the secretion of cytokines, IL10 and TGFβ1, which negatively regulate T cell immunity by inducing suppressive regulatory T cells (Tregs) (66). This facilitates immune evasion and tumorigenesis. The expression of IL10 and TGFβ1 occurs as a result of ERK activation of substrate AP-1 (66). Therefore, these KRAS mutant tumors are able to escape immune recognition via a mechanism dependent on the downstream MEK/ERK/AP-1 signaling axis. MEK/ERK/STAT3 signaling has also been shown to facilitate the upregulation of Tregs in several cancers (67–69). The complex, largely ERK-mediated, mechanisms by which oncogenic MAPK-related cancers evade destruction by the immune system have important consequences for developing and optimizing immunotherapy approaches, small-molecule kinase inhibition strategies, as well as combination-based therapies.
VI. Current Understanding of MEK/ERK Activation Mechanisms
In the absence of pathway stimulation, MEK and one of the RAF isoforms, BRAF, form face-to-face heterodimers (6). None of the other RAF isoforms, ARAF and CRAF, have been shown to exhibit this interaction, which raises the question of how they are able to eventually bind and activate MEK. Either RAF dimers or RAF-MEK heterodimers are recruited to the plasma membrane upon upstream RAS activation, and dimeric RAS activates RAF dimers (9). A transient MEK-RAF-RAF-MEK tetramer then forms to facilitate RAF activation via cis-autophosphorylation of the RAF activation loop (70, 71). Recent work has suggested that MEK may also need to be a dimer to be phosphorylated by RAF and to phosphorylate ERK (22). Additional studies are needed to verify this in vivo and affirm the inability of MEK monomers to be phosphorylated by RAF, as well as elucidate the mechanism by which MEK activates ERK. It is possible that MEK monomers dock on the RAF-MEK complex and begin to assemble MEK homodimers with RAF-complexed MEK. However, if there is indeed a transition from RAF-MEK tetramers to MEK homodimers, the point of dissociation, and the state at which phosphorylation occurs, remains unclear. Additionally, further studies are needed to clarify whether both MEK protomers in the homodimer must be active to phosphorylate ERK or whether monophosphorylated dimers are capable of activating ERK. Following RAF dissociation, activated MEK then phosphorylates ERK. In the context of this model, several questions arise given the established role of MEK as a cytoplasmic anchor for ERK and the existence of MEK-ERK heterodimers in quiescent cells. Can MEK be activated as a dimer with ERK or must MEK-ERK cytoplasmic anchoring complexes first dissociate before RAF recruitment and MEK activation? What cellular conditions influence the dynamic equilibrium between MEK-ERK heterodimers and BRAF-MEK heterodimers in quiescent cells? Additional research is needed to fully elucidate the RAS-RAF-MEK-ERK activation mechanism and resolve these remaining questions.
The MEK/ERK interaction is highly specific and complex formation requires full length ERK in its native conformation; MEK1/2 is unable to phosphorylate an assortment of related protein-serine/threonine or protein-tyrosine kinase substrates and cannot interact in vitro with ERK1/2 peptides containing the TEY phosphorylation segment (7). The phosphorylation of ERK is ordered, with Tyr phosphorylated before Thr (72). Additionally, while phosphorylation of both Thr and Tyr is required for full ERK activation (7), monophosphorylated ERK species have been shown to exhibit intermediate activity in vitro (73). While pTyr ERK2 has been shown to associate with the Golgi complex and influence its structure during the G2/M phase of the cell cycle in HeLa cells, the biological role of monophosphorylated ERK species remains unclear (74). In addition, previous work has shown that dimerization is critical to upstream RAF function and MEK activation by RAF (70, 75), yet significantly less studies have focused on the MEK/ERK interaction. Recent work has suggested the importance of MEK1/2 dimerization in ERK1/2 activation (22), yet the mechanism by which MEK1/2 phosphorylates and activates ERK1/2, achieving its high conformational selectivity for native ERK1/2, as well as the unique resulting dual phosphorylation of Tyr followed by Thr, remains an intriguing question for further study.
Previous work has shown that, in vitro, MEK achieves dual phosphorylation via a nonprocessive mechanism by which the pERK ▪ MEK complex dissociates after the first phosphorylation event and then pERK associates with another active MEK to catalyze the second phosphorylation on Thr (12). However, under certain cellular conditions, mainly molecular crowding, it has been shown that this mechanism switches to “quasi-processive” phosphorylation by which the diffusional restriction due to crowding significantly increases the probability that the same MEK that catalyzed the first phosphorylation will then re-associate to catalyze the second (76). It had previously been proposed that scaffold proteins might facilitate the stabilization of the MEK/ERK complex such that both phosphorylation events could occur in one molecular collision via a fully processive mechanism, but previous study has shown that this is unlikely since the knockdown of various scaffolds, such as KSR1, MP-1, and β-arrestin 1/2, had no effect on processivity (76).
VII. MEK and ERK hetero- and homodimers
While MEK1 and MEK2 have largely indistinguishable structures and roles, a point of differentiation lies in their negative feedback loop participation. It was demonstrated that MEK1 and MEK2 form heterodimers that are responsive to negative feedback inhibition by ERK via phosphorylation on Thr292 of MEK1 (26). This prompts dephosphorylation of the activation loop serine residues, and the phosphorylation states of both MEK1 and MEK2 can be regulated via the MEK1-MEK2 heterodimer. Intriguingly, an analogous regulatory residue is not present in MEK2, and therefore in the absence of MEK1 or in the case of inhibition of MEK1-MEK2 heterodimers, this feedback inhibition is abolished, increasing the amount of phosphorylated MEK and the duration of ERK signaling. To date, only MEK1 is known to have this role in feedback inhibition with ERK. A question that arises, given the existence of MEK1-MEK2 heterodimers, is if, as recent work suggests, MEK must be a dimer to phosphorylate ERK, does it do so as a MEK1-MEK2 heterodimer or as a homodimer of each isoform? Additionally, since MEK1 and MEK2 have been shown to have slightly different signaling niches (73, 77), how might they exhibit differential signaling whilst still remaining responsive to negative feedback loops via their heterodimeric interaction? It would be valuable to assess the ability of heterodimeric MEK to activate ERK relative to monomeric and homodimeric MEK to help elucidate the biological roles of each and determine which interactions are most likely responsible for ERK phosphorylation in vivo.
ERK2 subunits have been shown to associate in vitro via co-immunoprecipitation and sedimentation experiments. A crystal structure of phosphorylated ERK2 (PDB: 2ERK) reveals that it can form a symmetrical homodimer with both active sites accessible (78). Nonhelical leucine zipper-like interactions are present in the dimer interface (Fig. 5), stabilized by residues on the phosphorylation segment and C-terminus that undergo a conformational change upon phosphorylation (32, 78). The dimer-interface consists of 11 residues from each monomer; residues L333, L334, and L336 on each protomer engage in leucine zipper-like interactions, and two key salt bridges between residues H176 and E343 link the monomers as shown in Fig. 5 (79). Due to the extensive spatial hydrophobic contacts and key electrostatic interactions facilitating ERK dimerization, a single mutation is not enough to disrupt the ERK homodimer (79).
Fig. 5: Active ERK2 homodimer interface.
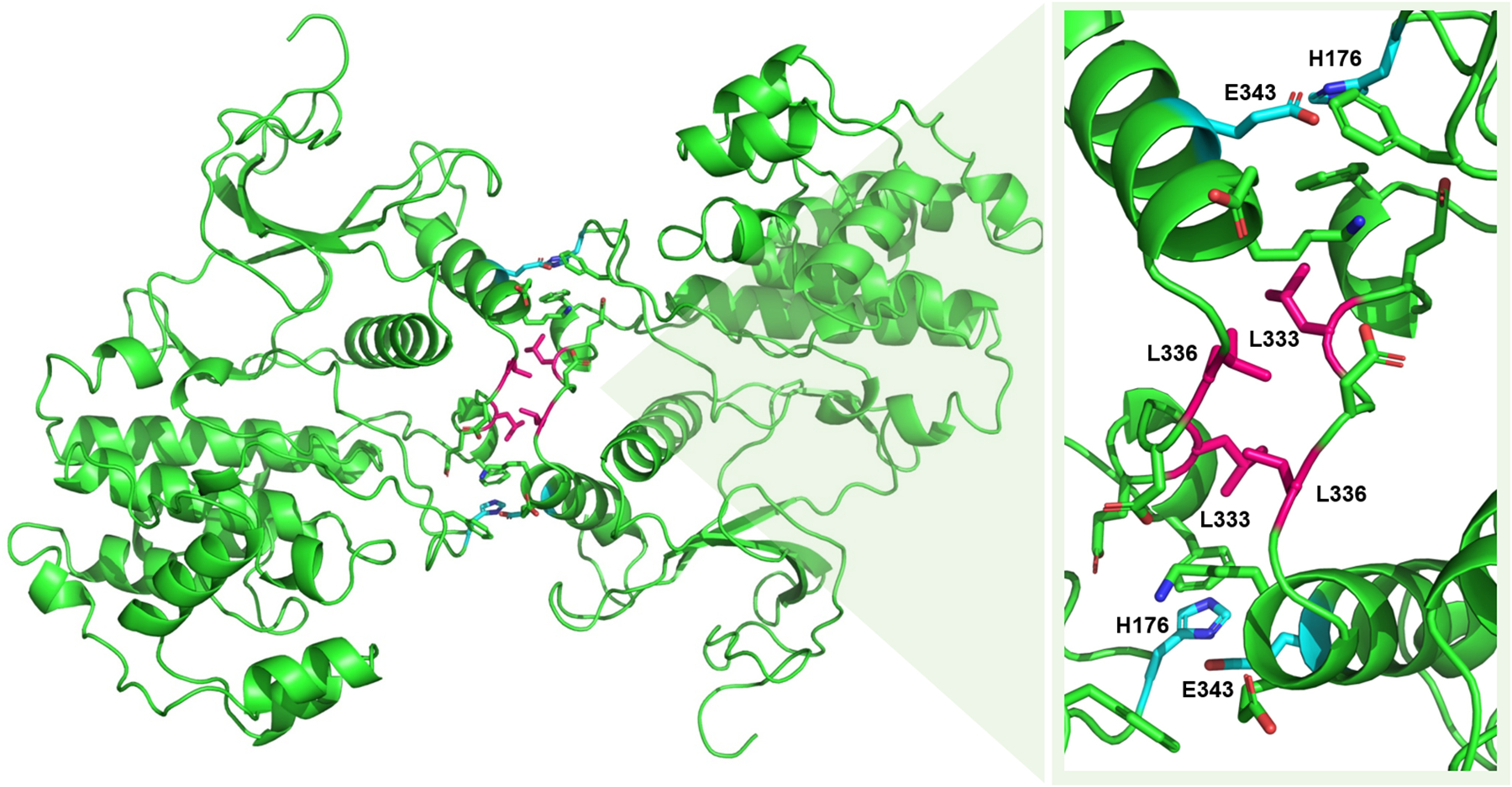
A crystal of the phosphorylated ERK2 homodimer is shown to the left (PDB ID: 2ERK). A close-up of the dimer interface is shown at the right with key residues labelled. Select residues engaging in leucine zipper-like interactions are shown in pink. The two sets of residues forming a critical salt-bridge are shown in cyan. Figure adapted from Wilsbacher et al. 2006 (79).
It has been demonstrated that β3-αC loop deletions in RAF and MEK increase homodimerization and oncogenic activation independent of upstream kinases (22, 70). MEK1 mutants with β3-αC loop deletions (Fig. 6) exhibit enhanced homodimerization and are activated via intradimer transphosphorylation (22). The enhanced formation of face-to-face homodimers of mutant MEK1, where the active site of one protomer aligns to catalyze activation loop phosphorylation of the other, facilitates this interaction. It would be intriguing to determine whether ERK β3-αC loop deletion mutants exhibit enhanced homodimerization and MEK-independent activity. MEK-independent activity would require ERK deletion mutants to self-activate upon dimerization via intra-dimer transphosphorylation. This is unlikely, however, due to the fact that wild-type ERK1/2 has been shown to form homodimers in which both active sites are accessible (Fig. 5) (32, 78), unlike the face-to-face alignment in MEK (80). The dimerization alignment of ERK1/2 heterodimers has not been determined due to the high instability of ERK1/2 heterodimers (32). Therefore, it is possible that, if ERK1/2 β3-αC loop deletion mutants exhibit enhanced dimerization as seen with MEK and RAF β3-αC loop deletion mutants, they could be capable of forming a heterodimer exhibiting the necessary dimer alignment for transphosphorylation.
Fig. 6: β3-αC Region of MEK1.
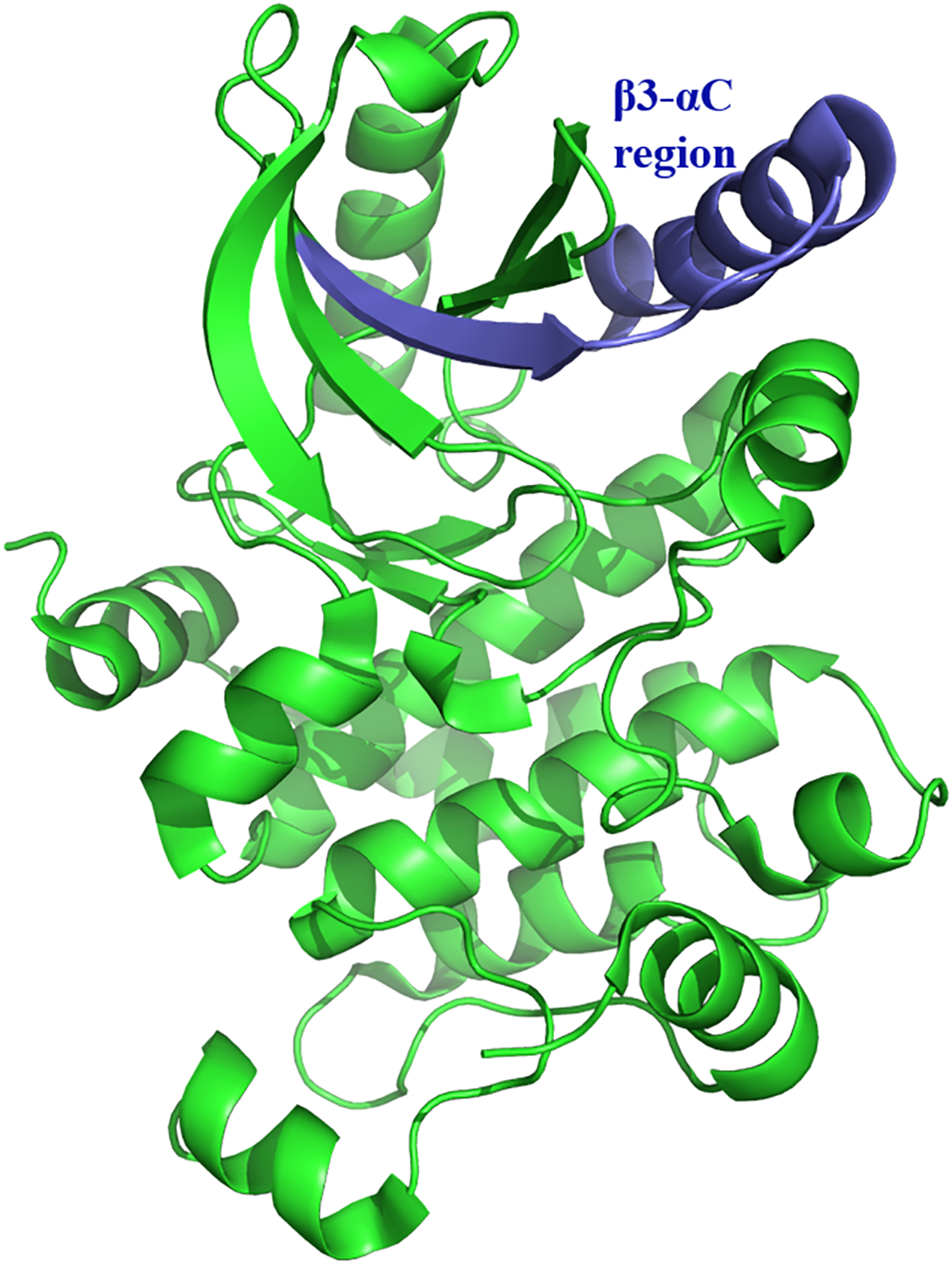
The β3-αC Region of MEK1 is shown in blue (PDB ID: 3EQI).
ERK1/2 has been shown to form homodimers with either phosphorylated or unphosphorylated ERK1/2, however the dimerization equilibrium is shifted in favor of homodimers in which both protomers are active (32). Active ERK1/2 has been shown to homodimerize upon phosphorylation both in vitro and in vivo. Among bi-phosphorylated and mono-phosphorylated ERK1 dimers and phosphorylated ERK1 monomers, the bi-phosphorylated species exhibit significantly higher activity levels both in vivo and in vitro, and thus are responsible for full ERK activation (81). It has been verified that MEK must be a dimer in order to be phosphorylated by RAF and to phosphorylate ERK, however preliminary studies have shown that ERK does not need to be a dimer in order to be phosphorylated by MEK, despite subsequent dimerization upon activation (22). Additional structural studies of the MEK/ERK interaction are necessary since there is presently no published structure of the MEK/ERK complex that would aid in determining the biologically relevant protomer alignments, key interface residues, and oligomeric status.
VIII. Feedback Regulation of the MEK/ERK Pathway
Pathway inhibition resulting from MEK1 T292 phosphorylation by ERK, as mentioned above, is just one element of the extensive, largely ERK-mediated, regulatory network modulating MAPK pathway activity. The most well-studied and central components of this network are highlighted below and summarized in Fig. 7 to provide context for subsequent discussion of ERK as a therapeutic target. The intricacy and abundance of both intrinsic and compensatory feedback interactions that provide positive and negative regulation of the MEK/ERK module often present the greatest challenges to therapeutic pathway inhibition. Broadly, the feedback regulation of the MEK/ERK module can be subdivided into two types: the first consisting of a rapid system of intercomponent phosphorylations and the second involving the de novo synthesis of regulatory proteins (82). Of all the MAPK components, ERK is the foremost regulator and is involved in inhibitory feedback loops with nearly every pathway component. In addition to its inhibitory interactions with MEK1, ERK has been shown to phosphorylate both CRAF and BRAF, impairing their ability to be recruited with other active RAFs to the membrane for RAS activation (83, 84). Numerous exchange factors and adaptor proteins that are involved in RAS binding to receptor tyrosine kinases are also inhibited by ERK-dependent phosphorylation (82). Phosphorylation of the guanine nucleotide exchange factor, SOS, decreases RAS activation and can be facilitated by ERK directly or via the activation of ribosomal S6 kinase 2 (RSK2), a cytosolic substrate of ERK (85, 86). The negative feedback loop involving SOS effectively facilitates the conversion of active RAS to the inactive RAS-GDP form within minutes, even in the presence of continuous receptor tyrosine kinase stimulation (87). In addition, ERK has been shown to directly phosphorylate receptor tyrosine kinases, as well as the most well-studied scaffold protein, KSR (88, 89). KSR is capable of binding RAF, MEK1/2, and ERK1/2. While its complex mechanism of action has not yet been fully elucidated, KSR is believed to enhance MAPK signaling, as well as exert control over the specificity, subcellular localization, and duration of the interactions of MAPK components. KSR forms inactive complexes with MEK under quiescent conditions and has been shown to enhance MEK translocation to the plasma membrane upon RAS and RAF activation, as well as allosterically activate and stabilize RAF-MEK binding for MEK activation (89, 90). Phosphorylation of KSR impairs its ability to bind RAF and MEK and localize at the plasma membrane, and results in a decrease in active ERK species (89). KSR is also subject to additional regulation via interactions with factors outside the MAPK pathway (91). Aside from the largely inhibitory mechanisms of MAPK feedback regulation, the RAF kinase inhibitor protein (RKIP) is involved in a positive feedback loop. RKIP has been shown to interfere with RAF/MEK binding and behaves analogous to a competitive inhibitor of MEK phosphorylation by RAF (92, 93). EGF-induced ERK activation was shown to correspond with a decreased binding of RKIP to RAF, and therefore a decreased inhibition of ERK signaling (93). In addition to Protein Kinase C phosphorylation of RKIP, ERK has been implicated in the phosphorylation of RKIP, which impairs its ability to bind RAF and inhibit MEK activation (93, 94). However, studies are needed to provide direct evidence of this interaction in vivo. Apart from the rapid feedback provided from these phosphorylation and binding interactions, the transcription of regulatory elements also contributes to the intricate MAPK feedback network, and the long-term effects it mediates are involved in steady-state activity and temporal control of signaling.
Fig. 7: Feedback Network involving the MEK/ERK Module.
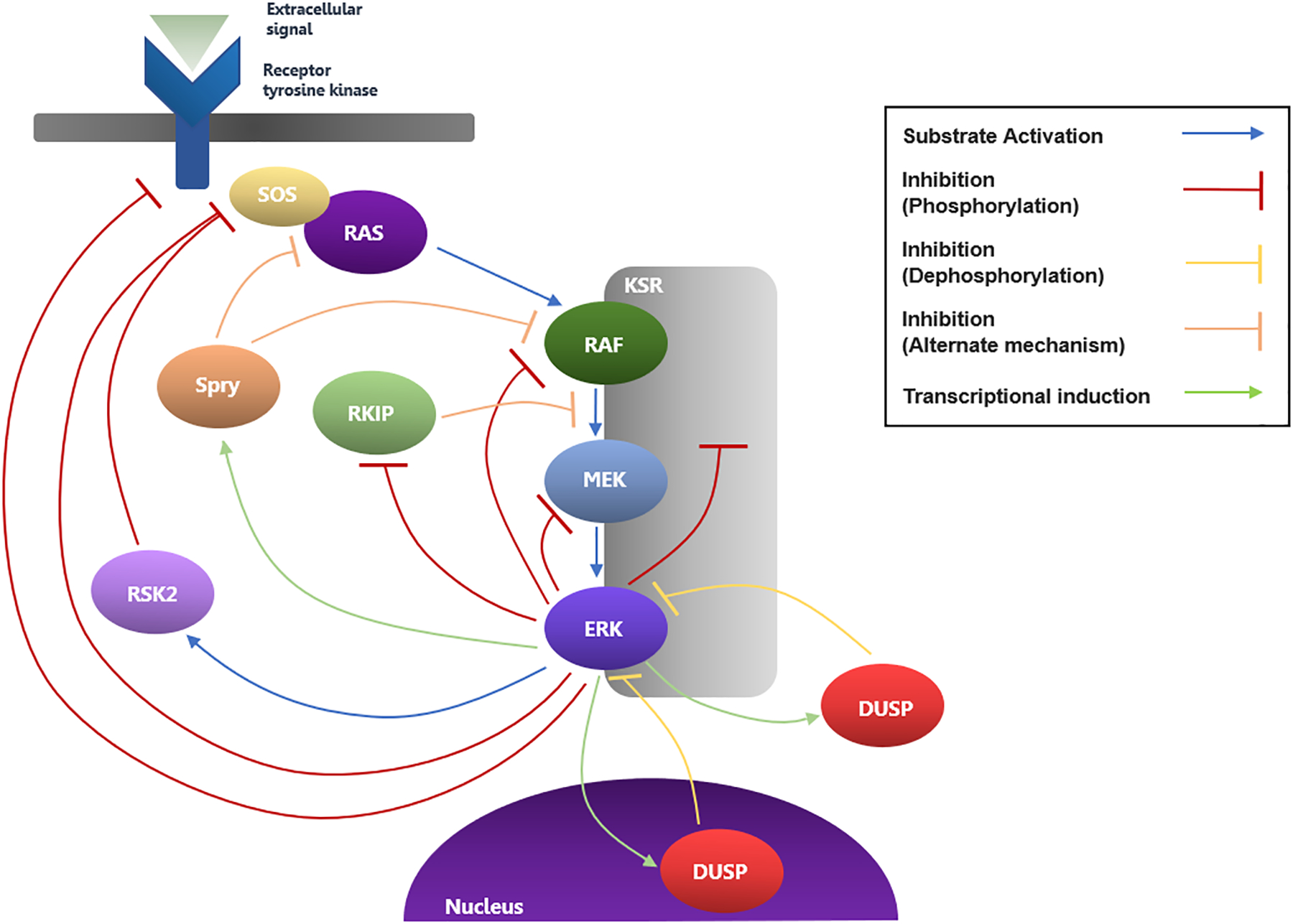
The main MAPK regulatory loops involving the components discussed in the text are depicted here. The dimerization states of the MAPK components and the additional regulatory phosphorylation interactions beyond transcriptional induction proposed between ERK and DUSPs and Sprys are excluded here for simplicity.
The predominant genomic mechanisms of MAPK feedback regulation that have been elucidated to date include the transcriptional induction of dual-specificity phosphatases (DUSPs), which dephosphorylate and inactivate ERK1/2, and the tumor suppressor Sprouty (Spry) proteins, which decrease MAPK signaling via complex mechanisms, likely involving the direct inhibition of RAF (95, 96). DUSPs are capable of binding to both phosphorylated and unphosphorylated ERK1/2, and cytoplasmic DUSP variants 6,7, and 9 are ERK1/2 selective, while nuclear variants 1,2,4, and 5 can bind both ERK1/2 and the related stress-induced MAPK relatives, JNK and p38 (95). DUSP variants 2,4, and 5 have been shown to bind and sequester inactive ERK1/2 in the nucleus, while DUSP6 has been shown to do so in the cytoplasm (97–99). Numerous members of the DUSP family are transcriptionally induced as a direct result of ERK activation, therefore forming a negative regulatory loop. For example, DUSP6 is induced in an ERK-dependent manner, following fibroblast growth factor (FGF) treatment, by the binding of ERK-activated Ets transcription factors (100). Adding another layer of complexity, it has been demonstrated that the activities or stabilities of numerous DUSP variants can be modulated by subsequent ERK-dependent phosphorylation. C-terminal ERK-dependent phosphorylation on DUSP1 and DUSP4 increases stability, enhancing phosphatase activity (101, 102). Structural studies of DUSP6 revealed key electrostatic contacts with the DRS of ERK that conferred specificity towards ERK1/2 (103). Regarding the second major contributor to the delayed regulation network, Sprouty proteins, previous study revealed that among four mammalian isoforms, Spry2 exhibited the most potent inhibition of FGF-induced MAPK signaling at the level of RAF activation (104). Another study suggested that Spry1 and Spry2 inhibit ERK signaling by preventing the activation of RAS by receptor tyrosine kinases (105). Subsequent study revealed that Spry4 directly binds to the kinase domain of RAF to inhibit forms of RAS-independent RAF activation and ERK signaling, but that it is unable to inhibit epidermal growth factor (EGF)-induced RAS-dependent ERK signaling (106). Given the present findings, it appears that the different isoforms may exhibit preferential regulation for specific cellular and activation contexts. While the precise mechanisms of inhibition of the various Spry isoforms, including whether they act upstream or downstream of RAS, are still a point of discrepancy, several studies have confirmed the role of Spry in a rapid negative feedback loop regulating ERK signaling (96, 107). An examination of human tumor cells revealed an increased presence of Spry genes, and FGF-induced Spry expression was shown to be positively correlated with, and dependent on, ERK activation (96). Not only is Spry activity transcriptionally regulated, but further study revealed that ERK activation is associated with phosphorylation of select Spry residues, and dephosphorylation of these residues abolishes the inhibitor activity of Spry (107, 108). Furthermore, regardless of phosphorylation status, Spry2 was shown to be unable to bind oncogenic BRAFV600E mutants (108), suggesting a possible means by which this mutant circumvents negative feedback regulation. For additional work detailing the regulation mechanisms of the MAPK pathway, see the following reviews (10, 82, 109–111). The involvement of ERK in nearly every component of the intricate regulatory loops of the MAPK pathway speaks to its importance in maintaining physiological MAPK signaling and in the complex resistance mechanisms that emerge in the case of hyperactive MAPK signaling upon inhibition of upstream components.
IX. MEK and ERK inhibitors
While upstream RAS and the RAF kinases are frequently mutated in MAPK-driven cancers, mutations in MEK and ERK are significantly less common, constituting less than 1% of all observed MAPK oncogenic genomic alterations. However, despite the infrequency of MEK/ERK activating mutations, inhibitors targeting these downstream effectors are of growing interest due to the significant problem of acquired resistance to RAF inhibitor therapies and the dependency of multiple tumor types on the MAPK pathway. There are currently four FDA-approved inhibitors of MEK, cobimetinib, trametinib, binimetinib, and selumetinib, which are all classified as ATP non-competitive, type III allosteric protein kinase inhibitors (112–114). They bind to a site that is adjacent to, but not overlapping, the ATP-binding site of MEK, and are thought to form a hydrogen bond with the β3 lysine and engage in hydrophobic contacts with the conformation-sensitive αC-helix, G-rich loop, and activation segment (2, 115). These interactions are based off a crystal structure of MEK bound to cobimetinib (PDB ID: 4AN2) and MEK bound to selumetinib (PDB ID: 4U7Z) since a structure of MEK bound to trametinib or binimetinib has yet to be published. The mostly recently approved inhibitor, selumetinib, received approval for the treatment of neurofibromatosis type 1 in children exhibiting symptomatic plexiform neurofibromas. Cobimetinib in combination with the RAF inhibitor, vemurafenib, and trametinib in combination with the RAF inhibitor, dabrafenib, are presently two of the approved treatment options for patients with BRAFV600E mutant melanomas. Notably and despite the fact that the RAS genes are the most frequently mutated oncogenes in human cancer and have been extensively studied for several decades, there are no clinically effective drugs for RAS-mutated cancers to date. The existing approved RAF and MEK treatment options mentioned above exhibit efficacy only in patients with tumors harboring BRAF V600E or V600K mutations, and the search for effective anti-RAS drugs has been met with little success. However, recent exploration into the efficacy of ERK inhibition in these RAS mutated cancers holds promise for the development of effective combination therapeutic strategies (116).
While there are presently no FDA-approved ERK inhibitors, there are several candidate compounds in development or currently in clinical trials. The ERK inhibitor GDC-0994 has shown potent efficacy in combination with the MEK inhibitor, cobimetinib, in RAS and RAF mutant cell lines, and the dual-mechanism inhibitor SCH-772984 has proven capable of both inhibiting ERK phosphorylation by MEK and preventing ERK kinase activity (117, 118). The ATP-competitive ERK inhibitor, BVD-523 (ulixertinib), is currently the furthest through clinical trials. Ulixertinib inhibits the kinase activity of ERK but does not prevent phosphorylation of ERK by MEK. Intriguingly, while ulixertinib has been shown to paradoxically raise levels of ERK phosphorylation due to inhibition of negative feedback loops, it still significantly inhibits cell proliferation in cancer cell lines (119). Ulixertinib inhibits ERK activation of downstream effectors with high specificity, and exhibits potent antitumor activity in multiple cancer cell lines (119). This study showed that ulixertinib also exhibits a modest level of synergy in combination with either BRAF inhibitor dabrafenib or vemurafenib against BRAFV600E cancer cell lines (119). The tolerance to this combination in clinical application remains to be determined, but it may be comparable to BRAF/MEK inhibitor combination therapy, although broader toxicity is a potential obstacle. In BRAFV600E cancer cell lines, sensitivity to combination treatment with ulixertinib lasted significantly longer than with trametinib or dabrafenib, or even with the dabrafenib/trametinib combination, all of which quickly lose their efficacy with the emergence of upstream resistant mutations (119). Given that the emergence of KRAS or MEK mutations are a major source of acquired resistance to MAPK pathway inhibitors, studies have also assessed the sensitivity of resistant KRAS or MEK mutant cancer cell lines to ulixertinib and determined that, despite proving resistant to MEK inhibitors, several of the tested BRAFV600E cell lines, harboring clinically relevant KRAS or MEK resistant mutations, were responsive to ulixertinib treatment (119). In conclusion, ERK inhibitors could be more effective in cases where upstream mutations induce pathway reactivation and resistance. While their tolerance and efficacy in the clinic remains to be determined, inhibitors targeting the terminal kinase of the MAPK pathway hold promise for improving targeting specificity and circumventing existing resistance mechanisms.
X. Targeting Approaches
Additional means of interference with MEK/ERK signal transduction, apart from allosteric inhibition of a site adjacent to the ATP-binding site, are currently being explored in hopes of enhancing specificity, reducing cytotoxicity, and improving clinical efficacy. While previous MEK inhibitors have largely been developed for allosteric inhibition, additional targeting strategies require consideration in light of persistent drug resistance limiting the duration of effective inhibition. If future studies are able to validate the importance of MEK dimerization in ERK activation, small-molecule compounds that interfere with the dimer interface of MEK might present a promising alternative strategy for therapeutic intervention in MAPK-mutant cancers.
A recent focus is designing therapeutic strategies to preferentially target nuclear ERK signaling over cytosolic signaling. Ideally, this would prevent further oncogenic activation while avoiding the harmful side effects induced upon inhibition of vital cytosolic pathways. Recent work proposing inhibition of ERK dimerization presented the first means of selective nuclear inhibition (120). A previous study had shown that dimerization is a prerequisite for pathological autophosphorylation on residue T188 of mouse ERK2, which is a critical event in executing nuclear translocation and signaling (121). In a subsequent study, an ERK2-dimerization inhibitory peptide (EDI) was created that selectively bound to inactive ERK2 and prevented T188 phosphorylation without interfering with ERK activation by MEK on the TEY motif (120). Given that EDI-mediated interference with dimerization did not interfere with TEY phosphorylation, dimerization of ERK must not be required for activation by MEK. Recent work also supports this conclusion (22). In cardiomyocytes treated with EDI, the nuclear translocation and signaling of ERK were inhibited, but, strikingly, cytoplasmic signaling remained unaffected (120). The use of EDI to repress oncogenic activation of the RAF/MEK/ERK cascade in a heart failure model demonstrated that interference with ERK dimerization inhibits the pro-hypertrophic effects of upregulated ERK nuclear signaling while preserving cytosolic pathways and thus avoiding the severe cardiotoxic side effects typically induced with full suppression of the cascade (120). The pathological overabundance of T188-phosphorylated ERK species has also been identified in human colon and lung cancers. EDI effectively repressed cancer cell proliferation in these tumors, to an extent comparable to global kinase inhibition (120). Together, these results suggest the validity of interfering with ERK dimerization and T188 phosphorylation as a targeting strategy to enable preferential inhibition of oncogenic nuclear signaling without inducing cardiotoxic side effects. It is important to note that the preservation of cytosolic signaling in this approach should theoretically enable ERK to retain its participation in negative feedback loops, which may prevent the development of acquired drug resistance.
Given the essential role of ERK nuclear translocation and signaling in inducing cell proliferation, interfering with this translocation has been investigated as another therapeutic strategy to inhibit growth and induce apoptosis in cancer cells. Analysis of the underlying resistance mechanisms reducing RAF and MEK inhibitor efficacy in patients reveals that inhibition of ERK negative feedback loops is likely a significant factor in the development of acquired resistance (122). For example, the PI3K-AKT pathway becomes hyperactivated in the absence of the negative feedback loops and cross-talk with the MAPK pathway (123). Negative feedback interactions and cross-talk involving ERK occur mainly in the cytoplasm. As discussed above, an approach allowing for preferential inhibition of nuclear signaling and preservation of cytosolic interactions of ERK could be an extremely valuable treatment strategy. A previous study employed a myristoylated NTS-derived phosphomimetic peptide designed to compete with phospho-NTS ERK binding to importin-7 (124). The peptide exhibited high specificity for the ERK-importin-7 interaction, abolished the ability of ERK to bind with importin-7, and effectively prevented nuclear signaling interactions (124). Cytosolic interactions, including phosphorylation on the TEY motif of ERK or activation of substrate RSK, remained unaffected. In addition, the peptide selectively reduced cell viability and proliferation in cancer cells while exhibiting no effect on immortalized cells. Its strength was shown to be comparable to existing ERK inhibitors currently under clinical testing. Interestingly, the peptide exhibited the strongest effect in BRAF melanoma cells and was capable of inducing apoptosis, although the mechanism by which this occurs remains unclear. It may be analogous to the mechanism by which BRAF inhibitors induce apoptosis in BRAF mutant melanomas (125–127). This study provided the first proof of concept for the targeted inhibition of ERK nuclear translocation as a means to inhibit cancer cell proliferation while preserving the negative feedback loops, TEY activation, and cytosolic signaling of ERK.
XI. Concluding Remarks
Despite extensive efforts in developing clinically promising inhibitors, determining structures, and unveiling activation mechanisms for upstream MAPK components, relatively little focus has been directed towards understanding and elucidating the functional interactions of the downstream kinases, MEK and ERK. While oncogenic pathway activation due to mutations in these kinases is extremely uncommon, their importance as a means of inhibiting hyperactivated MAPK signaling cannot be understated, particularly in light of the persistent issue of limited responsiveness to existing inhibitors and acquired drug resistance in the clinic. Relatively recent efforts to explore alternative therapeutic strategies produced the RAF-MEK inhibitor combination therapy, which was capable of prolonging responses and delaying the emergence of resistance in patients (2, 128). However, innovative and unconventional approaches will be required to restore sensitivity and successfully fight aberrant MAPK activation in RAF- and MEK-inhibitor resistant cancers. Preliminary studies of potential small-molecule ERK inhibitors have shown promise, as these inhibitors may be able to circumvent many mechanisms of resistance by targeting the terminal kinase in the MAPK cascade. Additionally, a preferential inhibition of the nuclear pathways of ERK, which are largely responsible for activation and oncogenesis, may reduce toxicity and the harmful side effects associated with MAPK inhibition. The development and improvement of inhibitors directed towards MEK or ERK will require future research that can provide insight into the activation mechanism and structurally critical components of the MEK/ERK interaction.
Acknowledgments
This work was supported by NIH grant R01 CA226888 awarded to R.M. and a R01 CA226888-S1 supplement to L.A.A. We thank Dr. Jessie Villanueva for critical reading of the manuscript.
Abbreviations
- MAPK
mitogen-activated protein kinase
- RAF
rapidly accelerating fibrosarcoma, proto-oncogene serine/threonine protein kinase
- MEK
mitogen-activated protein kinase kinase/ERK kinase
- ERK
extracellular signal-regulated protein kinase
- Imp7
importin-7
- DRS
D-site recruitment site
- FRS
F-site recruitment site
- NES
nuclear export sequence
- NTS
nuclear translocation sequence
- NLS
nuclear localization sequence
- DUSP
dual-specificity phosphatase
- Spry
sprouty protein
Footnotes
Competing interests: The authors declare no competing interests.
References
- 1.Roberts PJ, Der CJ, Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26, 3291–3310 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Roskoski R, Allosteric MEK1/2 inhibitors including cobimetanib and trametinib in the treatment of cutaneous melanomas. Pharmacol. Res 117, 20–31 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Wortzel I, Seger R, The ERK cascade: Distinct functions within various subcellular organelles. Genes and Cancer 2, 195–209 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marais R, Light Y, Paterson HF, Marshall CJ, Ras recruits Raf-1 to the plasma membrane far activation by tyrosine phosphorylation. EMBO J. 14, 3136–3145 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macdonald SG, et al. , Reconstitution of the Raf-1-MEK-ERK signal transduction pathway in vitro. Mol. Cell. Biol 13, 6615–6620 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haling JR, et al. , Structure of the BRAF-MEK Complex Reveals a Kinase Activity Independent Role for BRAF in MAPK Signaling. Cancer Cell 26, 402–413 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Seger R, et al. , Purification and characterization of mitogen-activated protein kinase activator(s) from epidermal growth factor-stimulated A431 cells. J. Biol. Chem 267, 14373–14381 (1992). [PubMed] [Google Scholar]
- 8.Roskoski R, ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol. Res 66, 105–143 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Nan X, et al. , Ras-GTP dimers activate the Mitogen-Activated Protein Kinase (MAPK) pathway. Proc. Natl. Acad. Sci. U. S. A 112, 7996–8001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao Z, et al. , BRAF Mutants Evade ERK-Dependent Feedback by Different Mechanisms that Determine Their Sensitivity to Pharmacologic Inhibition. Cancer Cell 28, 370–383 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S, The protein kinase complement of the human genome. Science (80-. ) 298, 1912–1934 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Burack WR, Sturgill TW, The activating dual phosphorylation of MAPK by MEK is nonprocessive. Biochemistry 36, 5929–5933 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez FA, Raden DL, Davis RJ, Identification of substrate recognition determinants for human ERK1 and ERK2 protein kinases. J. Biol. Chem 266, 22159–22163 (1991). [PubMed] [Google Scholar]
- 14.Mebratu Y, Tesfaigzi Y, How ERK1/2 activation controls cell proliferation and cell death is subcellular localization the answer? Cell Cycle 8, 1168–1175 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roskoski R, MEK1/2 dual-specificity protein kinases: Structure and regulation. Biochem. Biophys. Res. Commun 417, 5–10 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Knighton DR, et al. , Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science (80-. ) 253, 407–414 (1991). [DOI] [PubMed] [Google Scholar]
- 17.Zheng CF, Guan KL, Activation of MEK family kinases requires phosphorylation of two conserved Ser/Thr residues. EMBO J. 13, 1123–1131 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alessi DR, et al. , Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. EMBO J. 13, 1610–1619 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornev AP, Haste NM, Taylor SS, Ten Eyck LF, Surface comparison of active and inactive protein kinases identifies a conserved activation mechanism. Proc. Natl. Acad. Sci. U. S. A 103, 17783–17788 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kornev AP, Taylor SS, Ten Eyck LF, A helix scaffold for the assembly of active protein kinases. Proc. Natl. Acad. Sci. U. S. A 105, 14377–14382 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischmann TO, et al. , Crystal structures of MEK1 binary and ternary complexes with nucleotides and inhibitors. Biochemistry 48, 2661–2674 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Yuan J, et al. , Activating mutations in MEK1 enhance homodimerization and promote tumorigenesis. Sci. Signal 11 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Emery CM, et al. , MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc. Natl. Acad. Sci. U. S. A 106, 20411–20416 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukuda M, Gotoh Y, Nishida E, Interaction of MAP kinase with MAP kinase kinase: Its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 16, 1901–1908 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dang A, Frost JA, Cobb MH, The MEK1 Proline-rich Insert Is Required for Efficient Activation of the Mitogen-activated Protein Kinases ERK1 and ERK2 in Mammalian Cells *. 273, 19909–19913 (1998). [DOI] [PubMed] [Google Scholar]
- 26.Catalanotti F, et al. , A Mek1-Mek2 heterodimer determines the strength and duration of the Erk signal. Nat. Struct. Mol. Biol 16, 294–303 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Wilsbacher JL, Goldsmith EJ, Cobb MH, Phosphorylation of MAP Kinases by MAP / ERK Involves Multiple Regions of MAP Kinases *. 274, 16988–16994 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Robinson MJ, et al. , Contributions of the Mitogen-activated Protein (MAP) Kinase Backbone and Phosphorylation Loop to MEK Specificity *. 271, 29734–29739 (1996). [DOI] [PubMed] [Google Scholar]
- 29.Eblen ST, Catling AD, Assanah MC, Weber MJ, Biochemical and Biological Functions of the N-Terminal, Noncatalytic Domain of Extracellular Signal-Regulated Kinase 2. Mol. Cell. Biol 21, 249–259 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burack WR, Shaw AS, Live cell imaging of ERK and MEK: Simple binding equilibrium explains the regulated nucleocytoplasmic distribution of ERK. J. Biol. Chem 280, 3832–3837 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Lenormand P, Brondello J, Brunet A, Pouysségur J, Activation and Neosynthesis of Nuclear Anchoring Proteins. Cell 142, 625–633 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khokhlatchev AV, et al. , Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 93, 605–615 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Matsubayashi Y, Fukuda M, Nishida E, Evidence for Existence of a Nuclear Pore Complex-mediated, Cytosol-independent Pathway of Nuclear Translocation of ERK MAP Kinase in Permeabilized Cells. J. Biol. Chem 276, 41755–41760 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Adachi M, Fukuda M, Nishida E, Two co-existing mechanisms for nuclear import of MAP kinase: Passive diffusion of a monomer and active transport of a dimer. EMBO J. 18, 5347–5358 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosako H, et al. , Phosphoproteomics reveals new ERK MAP kinase targets and links ERK to nucleoporin-mediated nuclear transport. Nat. Struct. Mol. Biol 16, 1026–1035 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Zehorai E, Yao Z, Plotnikov A, Seger R, The subcellular localization of MEK and ERK-A novel nuclear translocation signal (NTS) paves a way to the nucleus. Mol. Cell. Endocrinol 314, 213–220 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Lange A, et al. , Classical nuclear localization signals: Definition, function, and interaction with importin α. J. Biol. Chem 282, 5101–5105 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zehorai E, Seger R, Beta-like importins mediate the nuclear translocation of MAPKs. Cell. Physiol. Biochem 52, 802–821 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Pouysségur J, Volmat V, Lenormand P, Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem. Pharmacol 64, 755–763 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Adachi M, Fukuda M, Nishida E, Nuclear export of MAP kinase (ERK) involves a MAP kinase kinase (MEK)-dependent active transport mechanism. J. Cell Biol 148, 849–856 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costa M, et al. , Dynamic regulation of ERK2 nuclear translocation and mobility in living cells. J. Cell Sci 119, 4952–4963 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Furuno T, Hirashima N, Onizawa S, Sagiya N, Nakanishi M, Nuclear Shuttling of Mitogen-Activated Protein (MAP) Kinase (Extracellular Signal-Regulated Kinase (ERK) 2) Was Dynamically Controlled by MAP/ERK Kinase After Antigen Stimulation in RBL-2H3 Cells. J. Immunol 166, 4416–4421 (2001). [DOI] [PubMed] [Google Scholar]
- 43.Formstecher E, et al. , PEA-15 Mediates Cytoplasmic Sequestration of ERK MAP Kinase. Dev. Cell 1, 239–250 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM, β-arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J. Biol. Chem 277, 9429–9436 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Yoon S, Seger R, The extracellular signal-regulated kinase: Multiple substrates regulate diverse cellular functions. Growth Factors 24, 21–44 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Eblen ST, Extracellular-Regulated Kinases: Signaling From Ras to ERK Substrates to Control Biological Outcomes, 1st Ed. (Elsevier Inc., 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maik-Rachline G, Hacohen-Lev-Ran A, Seger R, Nuclear erk: Mechanism of translocation, substrates, and role in cancer. Int. J. Mol. Sci 20, 1–18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ünal EB, Uhlitz F, Blüthgen N, A compendium of ERK targets. FEBS Lett. 591, 2607–2615 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J, Molecular, interpretation of ERK signal duration by immediate early gene products. Nat. Cell Biol 4, 556–564 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Cruzalegui FH, Cano E, Treisman R, ERK activation induces phosphorylation of Elk-1 at multiple S/T-P motifs to high stoichiometry. Oncogene 18, 7948–7957 (1999). [DOI] [PubMed] [Google Scholar]
- 51.Gille H, et al. , ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 14, 951–962 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weber JD, Raben DM, Phillips PJ, Baldassare JJ, Sustained activation of extracellular-signal-regulated kinase 1 (ERK1) is required for the continued expression of cyclin D1 in G1 phase. Biochem. J 326, 61–68 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balmanno K, Cook SJ, Sustained MAP kinase activation is required for the expression of cyclin D1, p21(Cip1) and a subset of AP-1 proteins in CCL39 cells. Oncogene 18, 3085–3097 (1999). [DOI] [PubMed] [Google Scholar]
- 54.Maekawa M, Nishida E, Tanoue T, Identification of the anti-proliferative protein Tob as a MAPK substrate. J. Biol. Chem 277, 37783–37787 (2002). [DOI] [PubMed] [Google Scholar]
- 55.Yang JY, et al. , ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat. Cell Biol 10, 138–148 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu Z, Xu S, ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 58, 621–631 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Biswas SC, Greene LA, Nerve growth factor (NGF) down-regulates the Bcl-2 homology 3 (BH3) domain-only protein Bim and suppresses its proapoptotic activity by phosphorylation. J. Biol. Chem 277, 49511–49516 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Domina AM, Vrana JA, Gregory MA, Hann SR, Craig RW, MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene 23, 5301–5315 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Liu ZX, Yu CF, Nickel C, Thomas S, Cantley LG, Hepatocyte growth factor induces ERK-dependent paxillin phosphorylation and regulates paxillin-focal adhesion kinase association. J. Biol. Chem 277, 10452–10458 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Ishibe S, Joly D, Zhu X, Cantley LG, Phosphorylation-dependent paxillin-ERK association mediates hepatocyte growth factor-stimulated epithelial morphogenesis. Mol. Cell 12, 1275–1285 (2003). [DOI] [PubMed] [Google Scholar]
- 61.Ishibe S, Joly D, Liu ZX, Cantley LG, Paxillin serves as an ERK-regulated scaffold for coordinating FAK and Rac activation in epithelial morphogenesis. Mol. Cell 16, 257–267 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Sturgill TW, Ray LB, Erikson E, Maller JL, Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature 334, 715–718 (1988). [DOI] [PubMed] [Google Scholar]
- 63.Deak M, Clifton AD, Lucocq JM, Alessi DR, Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 17, 4426–4441 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hauge C, Frödin M, RSK aand MSK in MAP kinase signalling. J. Cell Sci 119, 3021–3023 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Chen N, et al. , KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol. Immunother 66, 1175–1187 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zdanov S, et al. , Mutant KRAS conversion of conventional T cells into regulatory T cells. Cancer Immunol. Res 4, 354–365 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salaroglio IC, et al. , Zoledronic acid overcomes chemoresistance and immunosuppression of malignant mesothelioma. 6, 1128–1142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kopecka J, et al. , Zoledronic acid-encapsulating self-assembling nanoparticles and doxorubicin : a combinatorial approach to overcome simultaneously chemoresistance and immunoresistance in breast tumors. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salaroglio IC, Mungo E, Gazzano E, Kopecka J, Riganti C, ERK is a Pivotal Player of Chemo-Immune-Resistance in Cancer. 1–31 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuan J, et al. , The dimer-dependent catalytic activity of RAF family kinases is revealed through characterizing their oncogenic mutants. Oncogene 37, 5719–5734 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hu J, et al. , Allosteric activation of functionally asymmetric RAF kinase dimers. Cell 154, 1036–1046 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haystead AJ, Dent P, Wu J, Haystead GMM, Sturgill TW, Ordered phosphorylation. Assessment 306, 17–22 (1992). [DOI] [PubMed] [Google Scholar]
- 73.Zhou B, Zhang ZY, The activity of the extracellular signal-regulated kinase 2 is regulated by differential phosphorylation in the activation loop. J. Biol. Chem 277, 13889–13899 (2002). [DOI] [PubMed] [Google Scholar]
- 74.Cha H, Shapiro P, Tyrosine-phosphorylated extracellular signal-regulated kinase associates with the Golgi complex during G2/M phase of the cell cycle: Evidence for regulation of Golgi structure. J. Cell Biol 153, 1355–1367 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Freeman AK, Ritt DA, Morrison DK, Effects of Raf Dimerization and Its Inhibition on Normal and Disease-Associated Raf Signaling. Mol. Cell 49, 751–758 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aoki K, Yamada M, Kunida K, Yasuda S, Matsuda M, Processive phosphorylation of ERK MAP kinase in mammalian cells. Proc. Natl. Acad. Sci. U. S. A 108, 12675–12680 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou LEI, et al. , MEK1 and MEK2 isoforms regulate distinct functions in pancreatic cancer cells. 251–255 (2010). [DOI] [PubMed] [Google Scholar]
- 78.Canagarajah BJ, Khokhlatchev A, Cobb MH, Goldsmith EJ, Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell 90, 859–869 (1997). [DOI] [PubMed] [Google Scholar]
- 79.Wilsbacher JL, et al. , Characterization of mitogen-activated protein kinase (MAPK) dimers. Biochemistry 45, 13175–13182 (2006). [DOI] [PubMed] [Google Scholar]
- 80.Ohren JF, et al. , Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat. Struct. Mol. Biol 11, 1192–1197 (2004). [DOI] [PubMed] [Google Scholar]
- 81.Philipova R, Whitaker M, Active ERK1 is dimerized in vivo: Bisphosphodimers generate peak kinase activity and monophosphodimers maintain basal ERK1 activity. J. Cell Sci 118, 5767–5776 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lake D, Corrêa SAL, Müller J, Negative feedback regulation of the ERK1/2 MAPK pathway. Cell. Mol. Life Sci 73, 4397–4413 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dougherty MK, et al. , Regulation of Raf-1 by direct feedback phosphorylation. Mol. Cell 17, 215–224 (2005). [DOI] [PubMed] [Google Scholar]
- 84.Ritt DA, Monson DM, Specht SI, Morrison DK, Impact of Feedback Phosphorylation and Raf Heterodimerization on Normal and Mutant B-Raf Signaling. Mol. Cell. Biol 30, 806–819 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Langlois WJ, Sasaoka T, Saltiel AR, Olefsky JM, Negative Feedback Regulation and Desensitization of Insulin- and Epidermal Growth Factor- stimulated p21 ras Activation *. October, 25320–25323 (1995). [DOI] [PubMed] [Google Scholar]
- 86.Douville E, Downward J, EGF induced SOS phosphorylation in PC12 cells involves P90 RSK-2. Oncogene 15, 373–383 (1997). [DOI] [PubMed] [Google Scholar]
- 87.Waters SB, et al. , Desensitization of Ras activation by a feedback disassociation of the SOS-Grb2 complex. J. Biol. Chem 270, 20883–20886 (1995). [DOI] [PubMed] [Google Scholar]
- 88.Kawasaki Y, et al. , Feedback control of ErbB2 via ERK-mediated phosphorylation of a conserved threonine in the juxtamembrane domain. Sci. Rep 6, 1–9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McKay MM, Ritt DA, Morrison DK, Signaling dynamics of the KSR1 scaffold complex. Proc. Natl. Acad. Sci. U. S. A 106, 11022–11027 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lavoie H, et al. , MEK drives BRAF activation through allosteric control of KSR proteins. Nature 554, 549–553 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kolch W, Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol 6, 827–837 (2005). [DOI] [PubMed] [Google Scholar]
- 92.Yeung K, et al. , Mechanism of Suppression of the Raf/MEK/Extracellular Signal-Regulated Kinase Pathway by the Raf Kinase Inhibitor Protein. Mol. Cell. Biol 20, 3079–3085 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shin SY, et al. , Positive- and negative-feedback regulations coordinate the dynamic behavior of the Ras-Raf-MEK-ERK signal transduction pathway. J. Cell Sci 122, 425–435 (2009). [DOI] [PubMed] [Google Scholar]
- 94.Corbit KC, et al. , Activation of Raf-1 Signaling by Protein Kinase C through a Mechanism Involving Raf Kinase Inhibitory Protein *. 278, 13061–13068 (2003). [DOI] [PubMed] [Google Scholar]
- 95.Caunt CJ, Keyse SM, Dual-specificity MAP kinase phosphatases (MKPs): Shaping the outcome of MAP kinase signalling. FEBS J. 280, 489–504 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ozaki K, et al. , Erk pathway positively regulates the expression of sprouty genes. Biochem. Biophys. Res. Commun 285, 1084–1088 (2001). [DOI] [PubMed] [Google Scholar]
- 97.Kucharska A, Rushworth LK, Staples C, Morrice NA, Keyse SM, Regulation of the inducible nuclear dual-specificity phosphatase DUSP5 by ERK MAPK. Cell. Signal 21, 1794–1805 (2009). [DOI] [PubMed] [Google Scholar]
- 98.Caunt CJ, Rivers CA, Conway-Campbell BL, Norman MR, McArdle CA, Epidermal growth factor receptor and protein kinase C signaling to ERK2: Spatiotemporal regulation of ERK2 by dual specificity phosphatases. J. Biol. Chem 283, 6241–6252 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Karlsson M, Mathers J, Dickinson RJ, Mandl M, Keyse SM, Both nuclear-cytoplasmic shuttling of the dual specificity phosphatase MKP-3 and its ability to anchor MAP kinase in the cytoplasm are mediated by a conserved nuclear export signal. J. Biol. Chem 279, 41882–41891 (2004). [DOI] [PubMed] [Google Scholar]
- 100.Ekerot M, et al. , Negative-feedback regulation of FGF signalling by DUSP6/MKP-3 is driven by ERK1/2 and mediated by Ets factor binding to a conserved site within the DUSP6/MKP-3 gene promoter. Biochem. J 412, 287–298 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brondello JM, Pouysségur J, McKenzie FR, Reduced MAP kinase phosphatase-1 degradation after p42/p44(MAPK)- dependent phosphorylation. Science (80-. ) 286, 2514–2517 (1999). [DOI] [PubMed] [Google Scholar]
- 102.Cagnol S, Rivard N, Oncogenic KRAS and BRAF activation of the MEK/ERK signaling pathway promotes expression of dual-specificity phosphatase 4 (DUSP4/MKP2) resulting in nuclear ERK1/2 inhibition. Oncogene 32, 564–576 (2013). [DOI] [PubMed] [Google Scholar]
- 103.Liu S, Sun JP, Zhou B, Zhang ZY, Structural basis of docking interactions between ERK2 and MAP kinase phosphatase 3. Proc. Natl. Acad. Sci. U. S. A 103, 5326–5331 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yusoff P, et al. , Sprouty2 inhibits the Ras/MAP kinase pathway by inhibiting the activation of Raf. J. Biol. Chem 277, 3195–3201 (2002). [DOI] [PubMed] [Google Scholar]
- 105.Gross I, Bassit B, Benezra M, Licht JD, Mammalian Sprouty Proteins Inhibit Cell Growth and Differentiation by Preventing Ras Activation. J. Biol. Chem 276, 46460–46468 (2001). [DOI] [PubMed] [Google Scholar]
- 106.Sasaki A, et al. , Mammalian Sprouty4 suppresses Ras-independent ERK activation by binding to Raf1. Nat. Cell Biol 5, 427–432 (2003). [DOI] [PubMed] [Google Scholar]
- 107.Hanafusa H, Torii S, Yasunaga T, Nishida E, Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat. Cell Biol 4, 850–858 (2002). [DOI] [PubMed] [Google Scholar]
- 108.Brady SC, et al. , Sprouty2 association with B-Raf is regulated by phosphorylation and kinase conformation. Cancer Res. 69, 6773–6781 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Avraham R, Yarden Y, Feedback regulation of EGFR signalling: Decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol 12, 104–117 (2011). [DOI] [PubMed] [Google Scholar]
- 110.Liu F, Yang X, Geng M, Huang M, Targeting ERK, an Achilles’ Heel of the MAPK pathway, in cancer therapy. Acta Pharm. Sin. B 8, 552–562 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ramos JW, The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int. J. Biochem. Cell Biol 40, 2707–2719 (2008). [DOI] [PubMed] [Google Scholar]
- 112.Roskoski R, Classification of small molecule protein kinase inhibitors based upon the structures of their drug-enzyme complexes. Pharmacol. Res 103, 26–48 (2016). [DOI] [PubMed] [Google Scholar]
- 113.Wu PK, Park JI, MEK1/2 Inhibitors: Molecular Activity and Resistance Mechanisms. Semin. Oncol 42, 849–862 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Markham A, Keam SJ, Selumetinib: First Approval. Drugs 80, 931–937 (2020). [DOI] [PubMed] [Google Scholar]
- 115.Robarge KD, et al. , Structure based design of novel 6,5 heterobicyclic mitogen-activated protein kinase kinase (MEK) inhibitors leading to the discovery of imidazo[1,5-a] pyrazine G-479. Bioorganic Med. Chem. Lett 24, 4714–4723 (2014). [DOI] [PubMed] [Google Scholar]
- 116.Ryan MB, Der CJ, Wang-Gillam A, Cox AD, Targeting RAS-mutant Cancers: Is ERK the Key? Trends in Cancer 1, 183–198 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Deng Y, et al. , Discovery of novel, dual mechanism ERK inhibitors by affinity selection screening of an inactive kinase. J. Med. Chem 57, 8817–8826 (2014). [DOI] [PubMed] [Google Scholar]
- 118.Merchant M, et al. , Combined MEK and ERK inhibition overcomes therapy-mediated pathway reactivation in RAS mutant tumors. PLoS One 12, 1–22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Germann UA, et al. , Targeting the MAPK signaling pathway in cancer: Promising preclinical activity with the novel selective ERK1/2 inhibitor BVD-523 (ulixertinib). Mol. Cancer Ther 16, 2351–2363 (2017). [DOI] [PubMed] [Google Scholar]
- 120.Tomasovic A, et al. , Interference with ERK-dimerization at the nucleocytosolic interface targets pathological ERK1/2 signaling without cardiotoxic side-effects. Nat. Commun 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lorenz K, Schmitt JP, Schmitteckert EM, Lohse MJ, A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat. Med 15, 75–83 (2009). [DOI] [PubMed] [Google Scholar]
- 122.Chandarlapaty S, Negative feedback and adaptive resistance to the targeted therapy of cancer. Cancer Discov. 2, 311–319 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Su F, et al. , Resistance to selective BRAF inhibition can be mediated by modest upstream pathway activation. Cancer Res. 72, 969–978 (2012). [DOI] [PubMed] [Google Scholar]
- 124.Plotnikov A, et al. , The nuclear translocation of ERK1/2 as an anticancer target. Nat. Commun 6 (2015). [DOI] [PubMed] [Google Scholar]
- 125.VanBrocklin MW, Verhaegen M, Soengas MS, Holmen SL, Mitogen-activated protein kinase inhibition induces translocation of bmf to promote apoptosis in melanoma. Cancer Res. 69, 1985–1994 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jiang CC, et al. , Apoptosis of human melanoma cells induced by inhibition of B-RAF V600E involves preferential splicing of bimS. Cell Death Dis. 1, 1–14 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Beck D, et al. , Vemurafenib potently induces endoplasmic reticulum stress-mediated apoptosis in BRAFV600E melanoma cells. Sci. Signal 6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Eroglu Z, Ribas A, Combination therapy with BRAF and MEK inhibitors for melanoma: Latest evidence and place in therapy. Ther. Adv. Med. Oncol 8, 48–56 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


