Abstract
The recent impressive clinical responses to antibody-based immunotherapy have prompted the identification of clinically relevant tumor antigens that can serve as targets in solid tumors. Among them, B7-H3, a member of the B7 ligand family, represents an attractive target for antibody-based immunotherapy: it is overexpressed on differentiated malignant cells and cancer initiating cells, with limited heterogeneity, and high frequency (60% of 25,000 tumor samples) in many different cancer types, but has a limited expression at low level in normal tissues. In non-malignant tissues, B7-H3 has a predominantly inhibitory role in adaptive immunity, suppressing T-cell activation and proliferation. In malignant tissues, B7-H3 inhibits tumor antigen-specific immune responses, leading to a pro-tumorigenic effect. B7-H3 also has non-immunological pro-tumorigenic functions, such as promoting migration and invasion, angiogenesis, chemoresistance and endothelial-to-mesenchymal transition as well as affecting tumor cell metabolism. As a result, B7-H3 expression in tumors is associated with poor prognosis. Although experimental B7-H3 silencing reduces cancer cell malignant potential, there has been limited emphasis on the development of B7-H3 blocking antibodies, most likely because the B7-H3 receptor remains unknown. Instead, many antibody-based strategies utilizing distinct effector mechanisms to target B7-H3-expressing cancer cells have been developed. These strategies have demonstrated potent anti-tumor activity and acceptable safety profiles in preclinical models. Ongoing clinical trials are assessing their safety and efficacy in patients. Identification of the B7-H3 receptor will improve our understanding of its role in tumor immunity, and will suggest rational strategies to develop blocking antibodies which may enhance the therapeutic efficacy of tumor immunity.
Keywords: B7-H3, CAR T-cells, immunotherapy, solid tumors, tumor antigen
INTRODUCTION
Monoclonal antibodies (mAbs), alone or as part of novel engineered structures, have had impressive clinical successes in cancer treatment (1). Furthermore, mAbs have facilitated the identification and molecular characterization of tumor antigens (TA) that can serve as antibody-based immunotherapy targets. Many TAs with a selective, although not specific expression on malignant cells have been identified. Among them, B7-H3, also known as CD276 or B7RP-2, a member of the B7 ligand family, appears to be the “right” TA for antibody-based immunotherapy (2), given that it is i) highly expressed with limited heterogeneity on differentiated tumor cells, ii) expressed on cancer initiating cells (CIC); the latter play a major role in metastasis and recurrence, and must be eradicated for a therapy to be effective (3) (Fig. 1), and iii) expressed on tumor-associated vasculature (TAV) and stroma; therefore, B7-H3 immunotargeting is expected to disrupt the tumor microenvironment (TME) and to inhibit neoangiogenesis. In contrast, B7-H3 has a restricted distribution in normal tissues. These characteristics have prompted significant interest in evaluating B7-H3 as a target of mAb-based immunotherapy.
Figure 1. B7-H3 expression on tumorigenic cancer initiating cells isolated from human cancer cell lines.
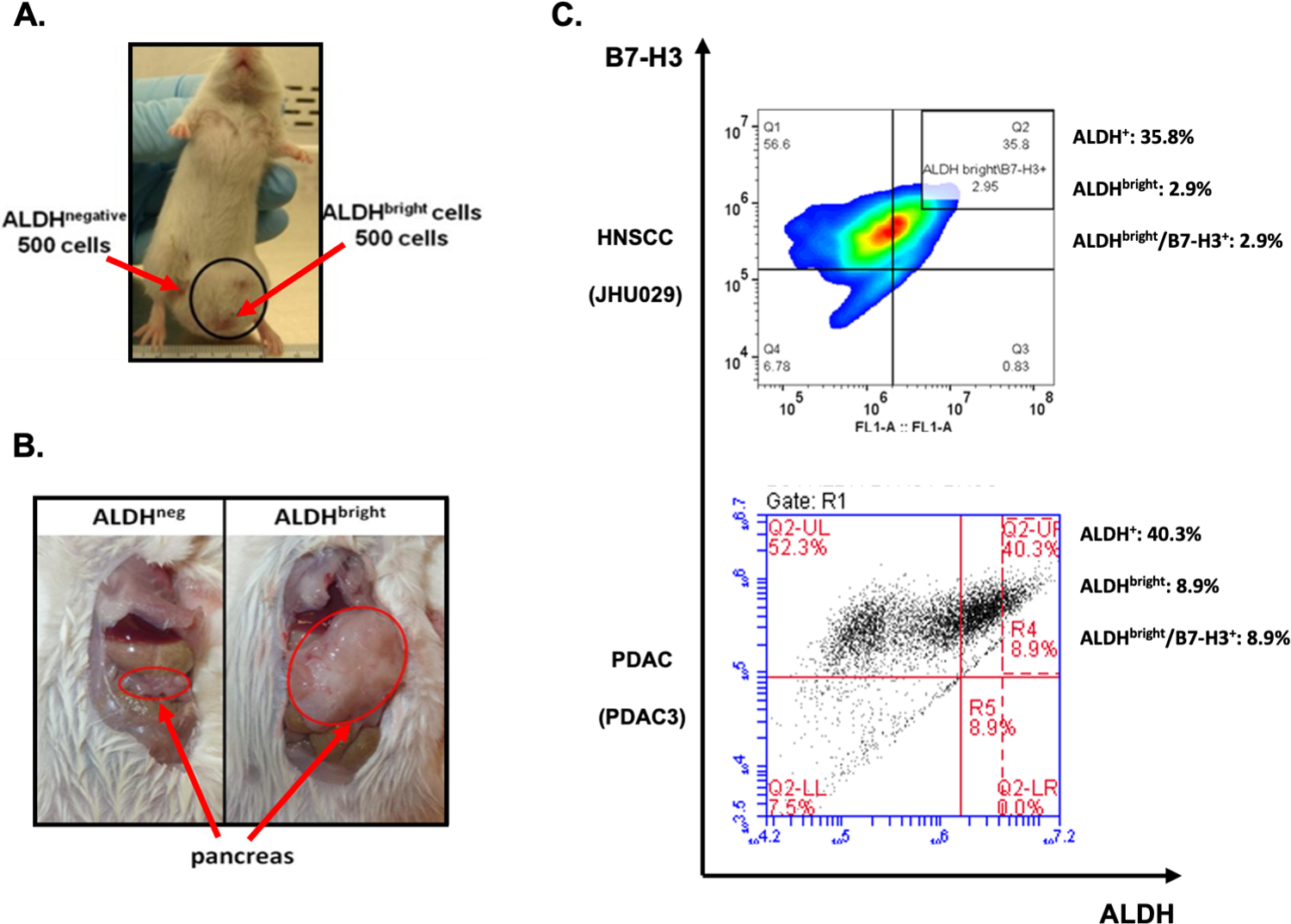
High ALDH activity (ALDHbright cells) is used as a marker to identify cancer initiating cells (CICs). Immunodeficient NOD/SCID mice were challenged with 500 ALDHbright and ALDHneg cells sorted from a (A) HNSCC or (B) PDAC cell line. In both models, tumors developed only in anatomic sites injected with ALDHbright cells, but not ALDHneg tumor cells. CICs from HNSCC (JHU029) and PDAC (PDAC3) human cell lines stained with the B7-H3-specific mAb 376.96 demonstrate high B7-H3 expression (C). ALDH: aldehyde dehydrogenase; HNSCC: head and neck squamous cell cancer; PDAC: pancreatic ductal adenocarcinoma. ALDHbright cells are identified as those ALDH+ cells with twice the mean fluorescence intensity of the ALDH+ population.
STRUCTURE AND REGULATION
B7-H3 is a 316 amino-acid long type I transmembrane protein encoded by a gene in chromosome 9 in mice and 15q24 in humans. It exists in two isoforms determined by its extracellular domain. In mice, the extracellular domain comprises a single pair of immunoglobulin variable (IgV)-like and immunoglobulin constant (IgC)-like domains, whereas in humans it comprises one or two identical pairs due to exon duplication (4–6). Its intracellular domain is short without any known signaling motif (Fig. 2).
Figure 2. B7-H3 structure.
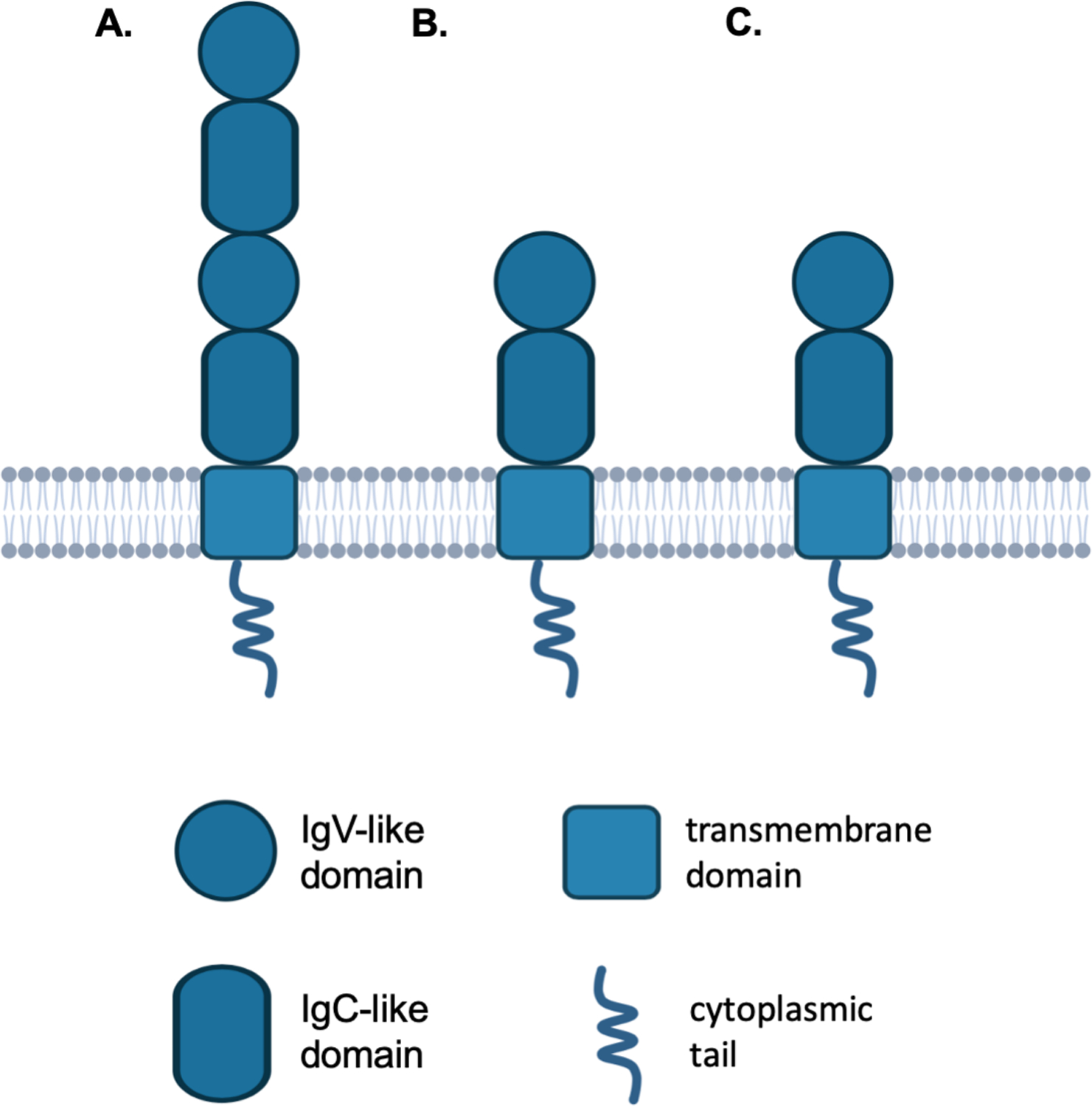
B7-H3 is a type I transmembrane protein composed of an extracellular, a transmembrane and a short intracellular domain. Human B7-H3 exists in two isoforms as determined by its extracellular domain: 4IgB7-H3 which comprises two identical pairs of IgV-like and IgC-like domains (A), and 2IgB7-H3 which comprises a single pair of IgV-like and IgC-like domains (B). 4IgB7-H3 is the predominant isoform in humans. B7-H3 in mouse is expressed only as the 2IgB7-H3 isoform (C). IgV: immunoglobulin variable; IgC: immunoglobulin constant.
B7-H3 shares 20–27% amino-acid identity with other B7 family ligands (7). B7-H3 has maintained its amino-acid sequence through phylogenetic evolution, as it has an 88% amino-acid sequence homology with its murine counterpart (5). Despite this similarity, no human B7-H3-specific antibody cross-reacts with the endogenous murine B7-H3 expressed on murine cells. However, human B7-H3-specific mAbs cross-react with murine B7-H3 expressed by transfected human cells (8), likely because of different B7-H3 glycosylation in the two species and the role of carbohydrates in the expression of epitopes recognized by human B7-H3-specific mAbs (9). Thus, caution should be exercised in interpreting toxicity of strategies which test human B7-H3-specific mAbs in murine models, unless the mAb used has been shown to cross-react with the endogenous B7-H3 expressed by normal murine tissues.
The molecular weight of B7-H3 protein moiety is ~45–66kDa, while that of the glycosylated 4Ig-hB7-H3 isoform is ~100kDa (10). A B7-H3 “soluble” isoform has been detected in plasma, a questionable statement, since there is insufficient evidence about its solubility and B7-H3 is also expressed on exosomes (11). The correlation of B7-H3 level in cancer patients’ serum with clinicopathologic variables (12) suggests its use as a non-invasive biomarker for diagnosis, prognosis, and/or treatment response.
B7-H3 mRNA is expressed in most normal tissues. In contrast, B7-H3 protein has a very limited expression in normal tissues (13,14) because of its post-transcriptional regulation by microRNAs (miRNA). Specifically, miRNA-29 suppresses B7-H3 expression in normal tissues by targeting the B7-H3 3’-untranslated mRNA region. Similarly, miRNA-29, miRNA-124, miRNA-199a and miRNA-1253 regulate B7-H3 expression in neuroblastoma (15), osteosarcoma (16), cervical cancer (17) and medulloblastoma (18), respectively. Furthermore, high miRNA-187, miRNA-1301-3p, miRNA-335-5p and miRNA-28-5p expression downregulates B7-H3 expression in CRC; this change leads to reduced aggressiveness, lower pathologic stage and decreased metastatic potential (19,20).
FUNCTIONS AND INTERACTIONS WITH MOLECULAR PATHWAYS
A. Immunological and non-immunological functions in non-malignant tissues
B7-H3 may inhibit NK-cell activation and have a pro-inflammatory role leading to cytokine release from monocytes and/or macrophages, in a Toll-like receptor (TLR)-2 and TLR-4-dependent manner (21). Nevertheless, B7-H3 involvement in innate immunity remains questionable.
In adaptive immunity, B7-H3 plays a role in T-cell regulation (22). Surprisingly, the molecular structure of its receptor remains unidentified, although soluble B7-H3 has been shown to bind to CD4+ T, CD8+ T, NK, and NKT-cells (23) and the extent of its binding is increased upon T-cell activation (2). In mice, B7-H3 binds to the CD8+ T-cell trem-like transcript 2 (TLT-2) receptor leading to their activation (24), however, such interaction has not been found with human B7-H3 (25). At any rate, the B7-H3 receptor differs from CD28, CTLA-4 (cytotoxic T lymphocyte-associated protein 4), PD-1 (programmed cell death protein 1) and ICOS (inducible T cell costimulatory), receptors known to bind other B7 family ligands (2,5).
B7-H3 was initially considered necessary for T-cell co-stimulation (2). Their activation was associated with worse outcomes in auto-immune and infectious diseases (26). The co-stimulatory B7-H3 role is further supported by detection of B7-H3-positive mononuclear cells in acute renal rejection biopsies (27). Lastly, B7-H3 expression on mononuclear cells promoted cardiac allograft rejection in mice through CD4+ and CD8+ T-cell activation, while B7-H3 deletion in combination with immunosuppression decreased allograft rejection (27).
Nevertheless, B7-H3 likely has a predominantly inhibitory role in adaptive immunity, suppressing T-cell activation and proliferation. Indeed, B7-H3 expression on antigen-presenting cells (APCs) reduces CD4+ and CD8+ T-cell activation, as well as effector cytokine release (28). Furthermore, B7-H3 decreases T-cell activation and function by inhibiting the expression of major transcriptional factors such as Nuclear Factor-Kappa B (NF-κB). The B7-H3 inhibitory function is further supported by the capacity of APCs to stimulate T-cells in B7-H3-deficient mice (7). In addition, B7-H3 silencing accelerates auto-immune encephalomyelitis via T-helper (Th)-type 1 immune response upregulation (7). Similarly, in graft-versus-host-disease, cardiac allograft transplantation and hypersensitivity reaction models, B7-H3 blockade with anti-B7-H3 mAb MIH35 or gene deletion resulted in uncontrolled Th-type 1 responses and worse outcomes (26,29,30).
B7-H3 has also a non-immunological function in normal tissues: it is highly expressed on osteoblasts during embryogenesis, and is crucial for osteoblastic differentiation and bone mineralization (31).
B. Role in tumor microenvironment
B.1. Inhibitor of tumorigenesis
B7-H3 was first demonstrated to have anti-tumor activity in syngeneic murine lymphoma and mastocytoma where it induced CD8+ T-cell and NK-cell activation-mediated tumor regression (32,33). Furthermore, B7-H3 transfection to CRC cells grafted to mice reduced their metastatic potential and prolonged the survival of tumor-bearing mice (34), indicating that B7-H3 upregulation on tumor cells can lead to immune cell recruitment and activation. Indeed, in mice, B7-H3 has been suggested to bind to the TLT-2 receptor on CD8+ T-cells leading to their activation, and IFN-γ and IL-2 secretion (24).
Such evidence has been rarely found in humans, since an association between B7-H3 expression and favorable clinical course of the disease has been described in only 3 of the 61 analyzed studies (gastric (35) and pancreatic cancer (36), acute myeloid leukemia (37)). Whether this difference between mouse and man reflects the lack in humans of B7-H3 binding to T cell TLT-2 receptors (25) and/or the differential expression of the 4 and 2Ig B7-H3 isoforms in the two species, remains to be determined.
B.2. Promoter of tumorigenesis
B7-H3 appears to promote tumorigenesis mainly via immunological mechanisms. Preclinical and clinical evidence indicates that B7-H3 inhibits TA-specific immune responses, leading to a pro-tumorigenic effect. Indeed, B7-H3 expression in tumors correlates with aggressive biology, low tumor-infiltrating T-lymphocyte density and poor prognosis (38,39). Nevertheless, B7-H3-targeting strategies to date do not focus on blocking this inhibition, but rather on eliminating B7-H3-expressing cells. This tumorigenic effect may be mediated by immunoglobulin-like transcript (ILT)-4 overexpression on cancer cells. The latter increases B7-H3 expression via PI3K/AKT/mTOR pathway activation (40). B7-H3 is also correlated with activated regulatory T-cell (Treg) infiltration in non-small cell lung cancer (NSCLC) (41) and suppression of NK-cell-mediated glioma cell lysis (42).
Several mechanisms may explain the dual anti-/pro-tumorigenic B7-H3 role. Firstly, B7-H3, like other checkpoint inhibitor ligands, might interact with both stimulatory and inhibitory receptors (43). Secondly, the differential B7-H3 function may reflect its different binding affinity for the interacting receptors. Finally, tumor cells may express aberrant B7-H3 isoforms or splice variants with different immune functions.
B7-H3 promotes cancer progression and invasion also through non-immunological mechanisms. Indeed, siRNA-induced B7-H3 downregulation on melanoma cells, as well as on breast and prostate cancer cells decreases their in vitro migration and invasiveness (10,44). In contrast, high B7-H3 expression on CRC cells is associated with increased matrix metalloproteinase (MMP)-9 expression, thereby promoting tumor cell migration and invasion. Inhibition of the JAK2/STAT3 pathway, which regulates MMP-9 expression, decreases CRC cell in vitro aggressiveness (45). Furthermore, B7-H3 affects the metastatic potential of human melanoma cells in mice most likely by modulating the metastasis-associated proteins MMP-2 and STAT3 (46). Lastly, B7-H3 promotes angiogenesis in CRC, by upregulating vascular endothelial growth factor A expression through NF-κB pathway activation (47).
B7-H3 enhances chemoresistance, as shown by the decreased apoptosis of breast cancer and CRC cells treated with paclitaxel and oxaliplatin/5-FU, respectively, via JAK/STAT3/survivin pathway activation (48,49), or specifically in CRC by reducing the G2/M phase arrest (50). Similarly, B7-H3 silencing increases pancreatic ductal adenocarcinoma (PDAC) cell gemcitabine sensitivity (51). Therefore, B7-H3 is a potential chemoresistance target.
B7-H3 promotes epithelial-to-mesenchymal transition (EMT) in glioma and hepatoma cells through JAK2/STAT3/Slug pathway activation (52,53). Lastly, B7-H3 affects the metabolism of triple negative breast cancer cells: decreased B7-H3 expression reduces tumor cell glycolytic capacity and increases their AKT/mTOR inhibitor sensitivity (54). Consistent with these activities, B7-H3 protein expression correlated to significant positive enrichment in EMT, UV response, protein secretion, WNT/β-catenin signaling, and Notch signaling proteins in 378 cancer cell lines included in the Cancer Cell Line Encyclopedia (55) (Supplementary Tables 1–3).
EXPRESSION IN NORMAL AND MALIGNANT TISSUES, AND ASSOCIATION WITH MALIGNANT DISEASE PROGNOSIS
Immunohistochemical staining with mAb 8H9 of a large number of multiple normal tissues has detected only heterogeneous nonspecific cytoplasmic staining in stomach, liver, pancreas and adrenal cortex (56). Similarly, we have also reported only weak cytoplasmic immunohistochemical staining of salivary gland acinar cells, gastric epithelial cells, and adrenal gland cells (8).
In contrast, B7-H3 is highly expressed with limited heterogeneity in all cancer types tested. Representative results obtained by immunohistochemical and flow cytometric analysis of malignant and normal cells stained with the B7-H3-specific mAb 376.96 are shown in Fig. 3. In a comprehensive literature review comprising 94 studies on 21 cancer types including 26,703 patients, we found that the cumulative frequency of B7-H3 positivity among all tumors is 60% (Fig. 4). B7-H3 displays high expression on stromal fibroblasts and TAV even in tumors with low B7-H3 expression. Indeed, B7-H3 TAV expression frequency ranged between 86%–98% in hepatoma, CRC, renal cell carcinoma, and melanoma, with frequency in ovarian cancer outlying at 44% (unpublished).
Figure 3. Higher B7-H3 expression on solid cancers than on normal tissues, as determined by immunohistochemical and flow cytometric staining with the B7-H3-specific mAb 376.96.
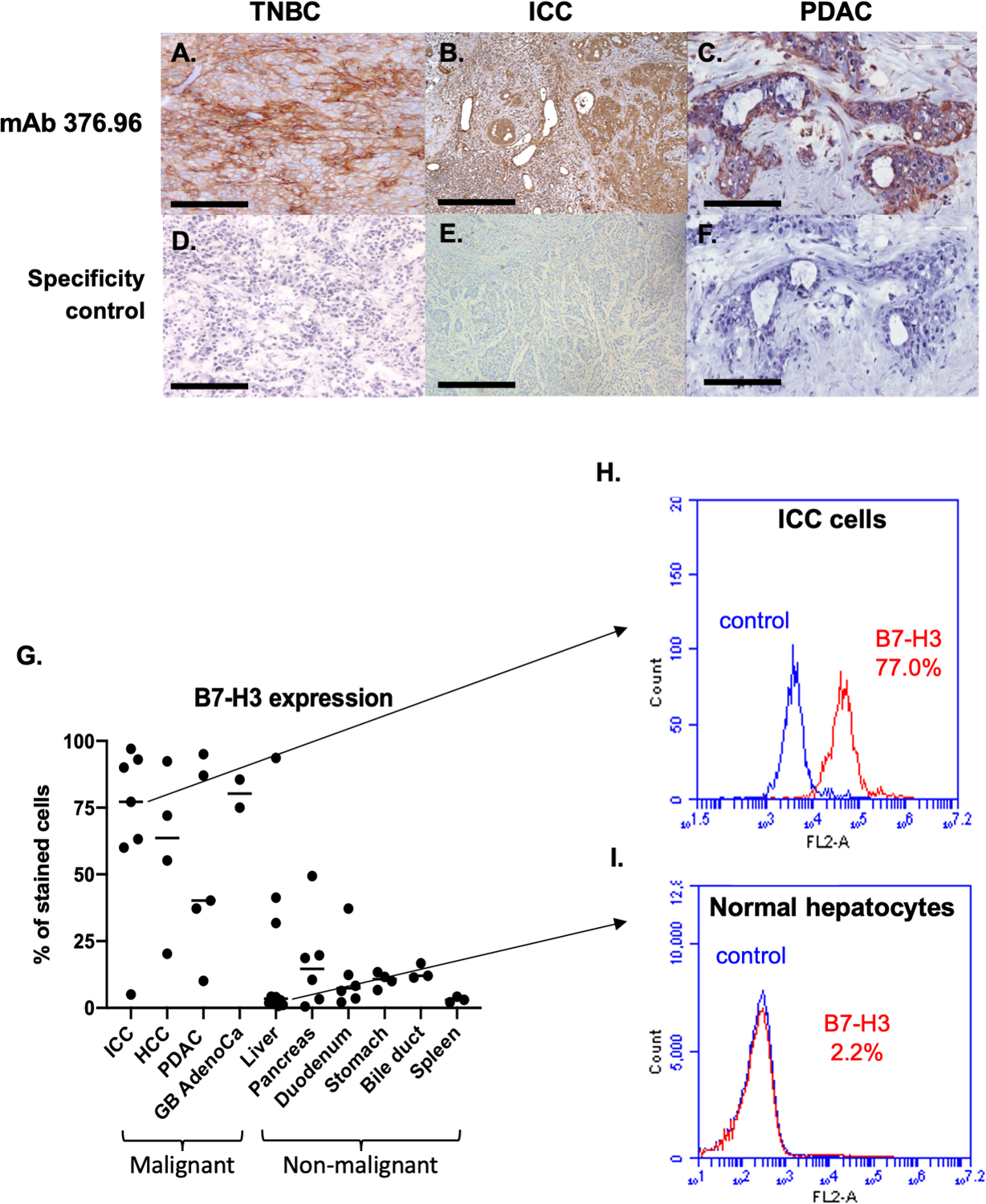
Representative micrographs of immunohistochemical staining with the B7-H3-specific mAb 376.96 of frozen tissue sections of TNBC (A), ICC (B), and PDAC (C) tumors are shown. Slides stained with the secondary antibody were used as a negative control (D, E, and F, respectively). (Magnification 200X). Surgically resected tumor specimens have a higher frequency of B7-H3-positive cells compared to normal tissues as determined by flow cytometric analysis (G). Surgically resected specimens were incubated with collagenase IV and single cell suspension was obtained. Cells were then stained with the B7-H3-specific mAb 376.96 (1ug/mL) and then analyzed by flow cytometry. Each dot on the plot represents a specimen from a different patient and the horizontal bars indicate the median value of each group. The frequency of B7-H3-positive cells was significantly higher in malignant tissues (Mann-Whitney U test, p<0.001). Of note, the liver specimen with the highest percentage of B7-H3-positive cells had underlying chronic hepatitis C, and the pancreas specimen with 50% B7-H3-positive cells had underlying chronic pancreatitis. Surgically removed ICC cells have a high frequency of B7-H3-positive cells (H), while normal liver cells adjacent to cancer tissue have a low frequency of B7-H3-positive cells (I), as determined by flow cytometric analyses. Cells were stained with the B7-H3-specific mAb 376.96 and rabbit anti-mouse IgG-PE antibodies. The isotype-matched mAb F3-C25 was used as a control for mAb 376.96. Stained cells were subjected to flow cytometry analysis. The percentage of cells stained with the B7-H3-specific mAb 376.96 are shown in each histogram. TNBC: triple negative breast cancer; ICC: intrahepatic cholangiocarcinoma; PDAC: pancreatic ductal adenocarcinoma; HCC: hepatocellular carcinoma; GB AdenoCa: gallbladder adenocarcinoma.
Figure 4. High frequency of B7-H3 expression in all the cancer types tested.
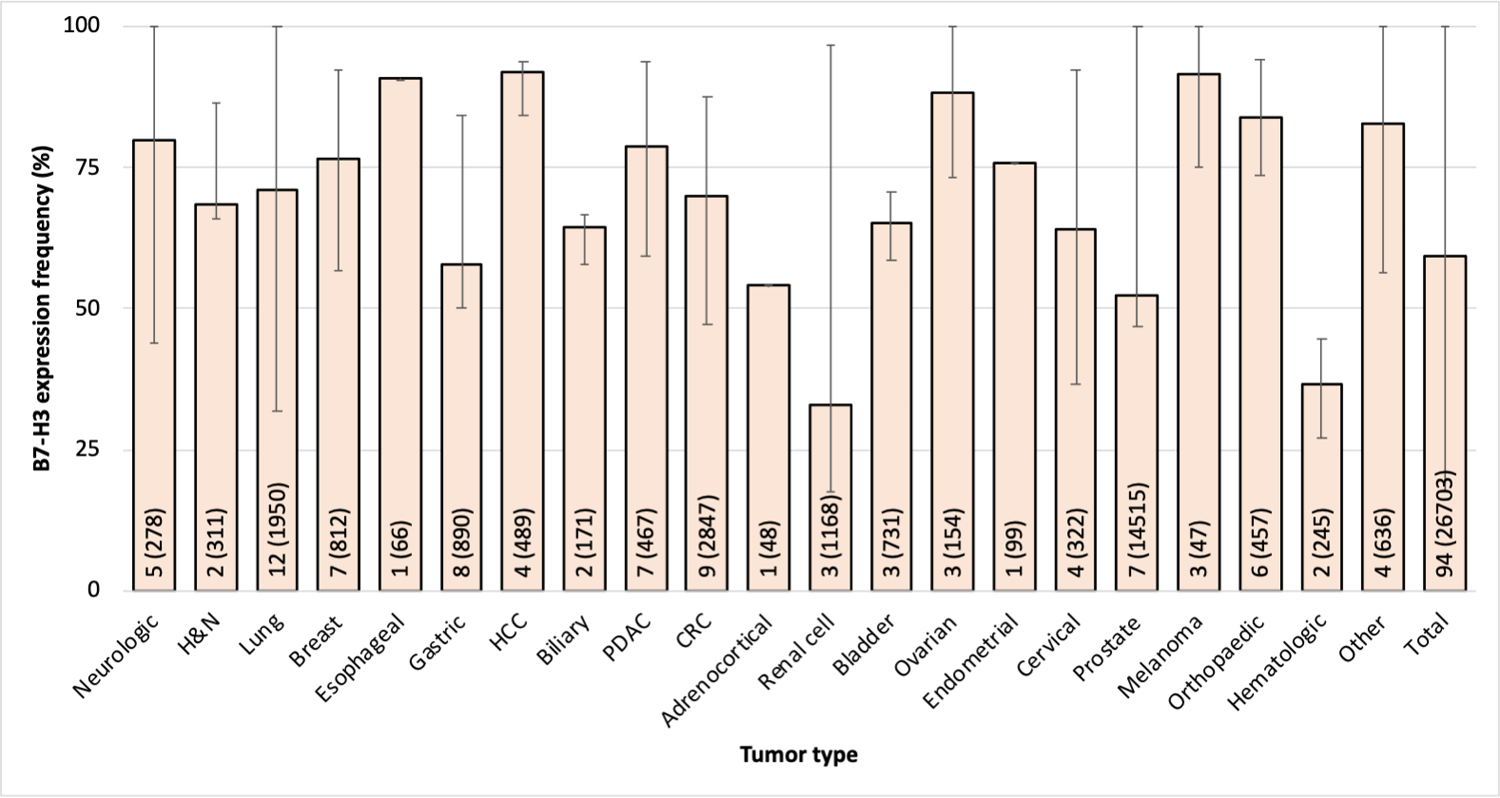
B7-H3 expression was assessed in surgically resected primary tumors by immunohistochemical staining with B7-H3-specific antibodies. Bar graphs represent the average of the frequency of B7-H3 expression in each cancer type yielded from a pooled data analysis. The number of included studies (number of tested samples) for each cancer type is presented within each bar. Error bars represent the range of B7-H3 expression frequency reported. B7-H3 expression was defined as positive by the criteria used by each individual study. H&N: head and neck; HCC: hepatocellular carcinoma; CRC: colorectal cancer. The tumors labeled as “other” include small round blue cell tumors of childhood, pediatric brain and solid tumors, Ewing’s family of tumors and renal angiomyolipoma.
Additionally, of the 61 studies that associated B7-H3 expression in tumors with prognosis, 67% demonstrated an association of positive/high expression of B7-H3 with poor prognosis, 28% found no correlation, and only 3 (5%) (35–37) demonstrated an association of positive/high B7-H3 expression with better prognosis (unpublished).
ATTRACTIVE IMMUNOTHERAPY TARGET
The recent advances in molecular biology and antibody engineering have allowed targeting B7-H3 utilizing multiple effector mechanisms. Most of these strategies have been tested in vitro and in mice generating encouraging safety and/or anti-tumor activity data, paving the way for B7-H3-targeting clinical trials (Table 1).
Table 1.
Clinical trials targeting B7-H3 on solid tumors with multiple effector mechanisms.
| Trial number | Title | Status |
|---|---|---|
| NCT01391143 | Safety Study of MGA271 in Refractory Cancer | Completed |
| NCT02381314 | Safety Study of Enoblituzumab (MGA271) in Combination with Ipilimumab in Refractory Cancer | Completed |
| NCT02982941 | Enoblituzumab (MGA271) in Children with B7-H3-expressing Solid Tumors | Completed |
| NCT02923180 | Neoadjuvant Enoblituzumab (MGA271) in Men with Localized Intermediate and High-Risk Prostate Cancer | Active not recruiting |
| NCT04129320 | Enoblituzumab Plus MGA012 in Squamous Cell Carcinoma of the Head and Neck | Not yet recruiting |
| NCT02475213 | Safety Study of Enoblituzumab (MGA271) in Combination with Pembrolizumab or MGA012 in Refractory Cancer | Active not recruiting |
| NCT03729596 | MGC018 With or Without MGA012 in Advanced Solid Tumors | Recruiting |
| NCT02628535 | Safety Study of MGD009 in B7-H3-expressing Tumors | Terminated |
| NCT03406949 | MGD009/MGA012 Combination in Relapsed/Refractory Cancer | Active not recruiting |
| NCT01502917 | Convection-Enhanced Delivery of 124I-8H9 for Patients with Non-Progressive Diffuse Pontine Gliomas Previously Treated with External Beam Radiation Therapy | Recruiting |
| NCT01099644 | Intraperitoneal Radioimmunotherapy with 131I-8H9 for Patients with Desmoplastic Small Round Cell Tumors and Other Solid Tumors Involving the Peritoneum | Active not Recruiting |
| NCT00089245 | Radiolabeled Monoclonal Antibody Therapy in Treating Patients with Refractory, Recurrent, or Advanced CNS or Leptomeningeal Cancer | Active not recruiting |
| NCT04022213 | A Study of the Drug I131-Omburtamab in People with Desmoplastic Small Round Cell Tumors and Other Solid Tumors in the Peritoneum | Recruiting |
| NCT04167618 | 177Lu-DTPA-Omburtamab Radioimmunotherapy for Recurrent or Refractory Medulloblastoma | Not yet recruiting |
| NCT04145622 | Study of DS-7300a in Participants with Advanced Solid Malignant Tumors | Recruiting |
| NCT04185038 | Study of B7-H3-Specific CAR T Cell Locoregional Immunotherapy for Diffuse Intrinsic Pontine Glioma/Diffuse Midline Glioma and Recurrent or Refractory Pediatric Central Nervous System Tumors | Recruiting |
| NCT04077866 | B7-H3 CAR-T for Recurrent or Refractory Glioblastoma | Recruiting |
| NCT04432649 | Targeting CD276 (B7-H3) Positive Solid Tumors by 4SCAR-276 | Recruiting |
| NCT04385173 | Pilot Study of B7-H3 CAR-T in Treating Patients with Recurrent and Refractory Glioblastoma | Recruiting |
| NCT04483778 | B7H3 CAR T Cell Immunotherapy for Recurrent/Refractory Solid Tumors in Children and Young Adults | Recruiting |
| NCT04315246 | 177Lu-DTPA-Omburtamab Radioimmunotherapy for Leptomeningeal Metastasis from Solid Tumors | Not yet recruiting |
A. Blocking mAbs
The significant changes induced in cancer cells by silencing B7-H3 (38,39) and the impressive clinical effect of mAbs blocking checkpoint molecules has provided a strong rationale to test B7-H3-specific inhibitory mAbs in solid tumors. B7-H3 blocking with mAbs has been shown to increase CD8+ T and NK-cell tumor infiltration and reduce tumor growth, and/or prolong survival in mouse models of hematopoietic cancers, melanoma (57), CRC (58), and more recently ovarian cancer (23). However, translation to a clinical setting has been hampered by lack of human B7-H3-specific blocking mAbs.
B. Radioimmunotherapy
B7-H3-specific mAbs have been used as carriers to selectively target radioisotopes to tumors. In an early single-arm imaging study in patients with B7-H3-positive tumors, 131I-murine mAb 8H9 (omburtamab, Y-mAbs) displayed moderate hepatic uptake (NCT00582608). Although no hepatotoxicity was reported, to avoid potential toxicity and to increase the therapeutic index, in subsequent studies systemic administration was replaced with compartmental administration. Specifically, in Phase I trials, intrathecal omburtamab in metastatic central nervous system neuroblastoma (NCT00089245) and intraperitoneal 131I-mAb 8H9 in desmoplastic small round cell tumors (NCT01099644) were well-tolerated (59). Convection-enhanced brainstem delivery of 124I-mAb 8H9 to diffuse pontine glioma resulted in negligible systemic exposure and no toxicity (NCT01502917). To facilitate rapid radioactivity clearance, mAb 8H9 was conjugated with the chelator diethylenetriamine pentaacetate and radiolabeled with lutetium-177 (60).
Furthermore, the B7-H3-specific mAb 376.96 has been conjugated with 212Pb, a source of α-particles. Ovarian tumor-bearing mice treated with this conjugate, alone or with carboplatin, survived 2–3 times longer than controls (61). This conjugate also inhibited human PDAC cell growth in vitro and patient-derived xenograft growth in mice (62).
C. Antibody drug conjugates (ADCs)
The ADC approach has been tested utilizing MGC018 (humanized B7-H3 mAb with a cleavable linker-duocarmycin payload, MacroGenics) which delivers duocarmycin to tumors. A Phase I/II trial is assessing its safety alone or in combination with an anti-PD-1 mAb in B7-H3-expressing solid tumors (NCT03729596). DS-7300a (Daiichi Sankyo), a B7-H3-specific mAb conjugated to four topoisomerase I inhibitor particles is being tested in a Phase I/II trial (NCT04145622).
D. mAbs mediating cellular cytotoxicity
The fully humanized mAb enoblituzumab (MGA271, MacroGenics) bearing an Fc domain engineered to enhance its anti-tumor function by increasing binding to the activating receptor CD16A and reducing that to the inhibitory receptor CD32B (63), was the first mAb tested against B7-H3-expressing tumors. Enoblituzumab was effective in multiple cancer types through antibody-dependent cellular cytotoxicity (ADCC) (63). Interim results from a Phase I trial (NCT01391143) including patients with B7-H3-expressing tumor or TAV cells, demonstrated that enoblituzumab was well-tolerated with no dose-limiting toxicity (64). A Phase II trial is assessing neoadjuvant enoblituzumab in prostate cancer (NCT02923180). Furthermore, a Phase I trial (NCT02475213) demonstrated that enoblituzumab with pembrolizumab in head and neck squamous cell cancer (HNSCC), NSCLC, urothelial cancer and melanoma was safe; the frequency of immune-related adverse events was comparable to pembrolizumab alone (65). Enoblituzumab with ipilimumab was also tested in a Phase I trial in NSCLC and melanoma (NCT02381314, results pending). More recently, a novel Fc enhanced bispecific anti-B7-H3 mAb/PD-1 fusion protein showed promising results in mice (66).
E. CD3-engaging bispecific antibodies (BsAbs)
BsAbs have been tested utilizing a B7-H3 mAb scFv linked to an anti-CD3 mAb scFv to recruit and activate T-cells against tumor cells (67). Only obrindatamab (MGD009, MacroGenics), a humanized CD3xB7-H3 dual affinity protein has been tested in humans in advanced B7-H3-expressing tumors (NCT02628535). In 2018 the FDA imposed a partial hold on this study due to hepatic adverse events likely due to cytokine release syndrome secondary to increased T-cell activation. These events were uncomplicated and short-lived, and the hold was lifted in 2019. However, the trial was terminated in November 2019 by MacroGenics due to administrative reasons (E. Bonvini, personal communication). Another B7-H3xCD3 BsAb created by coupling an anti-human B7-H3 mAb with an anti-CD3 mAb showed potent cytotoxicity toward hematological malignant cells in vitro (68).
F. Tri-specific Killer Engagers (TriKEs)
TriKEs form an antigen-specific immunological synapse between NK and tumor cells, thereby triggering NK-cell-mediated tumor cell lysis (69). TriKEs are composed of either 3 scFvs of antibodies with different specificity or 2 scFvs (CD16-specific and TA-specific) and a cytokine, most frequently IL-15. Vallera et al. have generated a B7-H3/IL-15 TriKE using the scFv of the B7-H3-specific mAb 376.96. This strategy demonstrated significant tumor burden decrease in vitro and in mice against HNSCC, PDAC and ovarian cancer (70,71). A second-generation TriKE has been bioengineered by the same group, with human IL-15 as a modified crosslinker between an anti-B7-H3 scFv and a humanized camelid anti-CD16 single domain antibody. The latter allows improved function of the IL15 moiety, improving NK-cell activation and proliferation, and augmenting killing of ovarian cancer cells in vitro and in mice (72).
G. CAR T-cells and CAR NK-cells
CAR T-cells permit rapid generation of polyclonal T-cells with TA-specificity and potent cytotoxicity, and can recognize tumor cells independently of HLA class I antigen expression (73). B7-H3 CAR T-cells engineered with various B7-H3-specific scFvs have demonstrated potent in vitro anti-tumor activity against multiple cancer types (8,74–77). However, their efficacy remains limited in mice. This limitation which is common with CAR T-cells targeting different TA in mice and humans likely reflects the negative impact of TME escape mechanisms on tumor cell - CAR T-cell interactions (78). To counteract this limitation, combinatorial strategies with agents which can enhance the anti-tumor activity of CAR T-cells and/or tumor cell susceptibility to the effector mechanism are being tested. Preclinical studies suggest an acceptable safety profile. B7-H3 CAR T-cells are being evaluated in trials targeting glioblastoma (NCT04077866) and pediatric glioma (NCT04185038). More recently, NK-cells have been used to generate CAR NK-cells which have controlled growth of human NSCLC cells grafted in mice and prolonged their survival (79).
CONCLUSION
B7-H3 has a complex, still not entirely clear, role in the TME with several immunological and non-immunological functions. Endeavors to identify its receptor are of outmost importance; this information will elucidate its role in the TME, but, most importantly, will facilitate the development of B7-H3 blocking reagents. To date, this area remains unexploited, since most B7-H3-targeting strategies focus on eliminating B7-H3-expressing tumor cells. Blocking the B7-H3-mediated inhibition on T-cells is expected to greatly improve TA-specific immune responses and in turn, significantly ameliorate the disease course. As observed with other checkpoint inhibition strategies, B7-H3-blocknig mAbs may prove to be a significant addition to the anti-tumor strategy armamentarium.
B7-H3 is highly expressed, with limited heterogeneity, on cancer cells in primary tumors. Additional data on B7-H3 expression in metastases – crucial for designing B7-H3-targeting therapies – are needed. Other areas needing additional investigation include i) the assessment of the value of B7-H3 serum level as a diagnostic, prognostic and monitoring marker, and ii) the evaluation of B7-H3-targeting ability to eliminate CICs, given their postulated role in metastasis and recurrence.
The attractiveness of B7-H3 as a target for cancer antibody-based therapy has stimulated the development of B7-H3-targeting immunotherapeutic strategies. Preclinical results are encouraging. Additionally, data from clinical trials demonstrate a favorable safety profile with limited toxicity. Multiple ongoing clinical trials are evaluating the therapeutic efficacy of B7-H3-targeting strategies, alone or in combination with other checkpoint inhibitors. This information will assess its value as a target of antibody-based immunotherapy.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr. Ezio Bonvini for reviewing the manuscript, for his constructive suggestions and for the update he provided regarding the status of the clinical trials conducted by MacroGenics.
This work was supported by the National Institutes of Health (grant RO1DE028172 [S. Ferrone]), by the National Cancer Institute (grants RO3CA223886 [S. Ferrone] and RO3CA231766 [S. Ferrone, C. Ferrone]), and by the Department of Defense (grants W81XWH-16-1-0500 [S. Ferrone] and W81XWH-20-1-0315 [S. Ferrone]).
Abbreviation list
- ADC
antibody drug conjugate
- ADCC
antibody-dependent cellular cytotoxicity
- APC
antigen presenting cells
- BsAb
bispecific antibody
- CAR
chimeric antigen receptor
- CIC
cancer initiating cell
- CRC
colorectal cancer
- CTLA-4
cytotoxic T lymphocyte-associated protein 4
- EMT
epithelial-to-mesenchymal transition
- HNSCC
head and neck squamous cell cancer
- ICOS
inducible T-cell costimulatory
- IgC
immunoglobulin constant domain
- ILT
immunoglobulin-like transcript
- IgV
immunoglobulin variable domain
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- miRNA
microRNA
- MMP
matrix metalloproteinase
- NF-κB
nuclear factor kappa b
- NSCLC
non-small cell lung cancer
- PDAC
pancreatic ductal adenocarcinoma
- PD-1
programmed cell death protein 1
- TA
tumor antigen
- TAV
tumor-associated vasculature
- Th
T helper
- TLR
toll-like receptor
- TLT
trem-like transcript
- TME
tumor microenvironment
- Treg
regulatory T cell
- TriKE
tri-specific killer engager
Footnotes
Conflicts of interest
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Kruger S, Ilmer M, Kobold S, Cadilha BL, Endres S, Ormanns S, et al. Advances in cancer immunotherapy 2019 - latest trends. J Exp Clin Cancer Res 2019;38(1):268 10.1186/s13046-019-1266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol 2001;2(3):269–74 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 3.Visvader JE. Cells of origin in cancer. Nature 2011;469(7330):314–22 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 4.Steinberger P, Majdic O, Derdak SV, Pfistershammer K, Kirchberger S, Klauser C, et al. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J Immunol 2004;172(4):2352–9 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- 5.Sun M, Richards S, Prasad DV, Mai XM, Rudensky A, Dong C. Characterization of mouse and human B7-H3 genes. J Immunol 2002;168(12):6294–7 10.4049/jimmunol.168.12.6294. [DOI] [PubMed] [Google Scholar]
- 6.Picarda E, Ohaegbulam KC, Zang X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin Cancer Res 2016;22(14):3425–31 10.1158/1078-0432.CCR-15-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol 2003;4(9):899–906 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 8.Du H, Hirabayashi K, Ahn S, Kren NP, Montgomery SA, Wang X, et al. Antitumor Responses in the Absence of Toxicity in Solid Tumors by Targeting B7-H3 via Chimeric Antigen Receptor T Cells. Cancer Cell 2019;35(2):221–37 e8 10.1016/j.ccell.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai K, Wilson BS, Bigotti A, Natali PG, Ferrone S. A 94,000-dalton glycoprotein expressed by human melanoma and carcinoma cells. J Natl Cancer Inst 1982;68(5):761–9. [PubMed] [Google Scholar]
- 10.Chen YW, Tekle C, Fodstad O. The immunoregulatory protein human B7H3 is a tumor-associated antigen that regulates tumor cell migration and invasion. Curr Cancer Drug Targets 2008;8(5):404–13 10.2174/156800908785133141. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Liu P, Wang Y, Nie W, Li Z, Xu W, et al. Characterization of a soluble B7-H3 (sB7-H3) spliced from the intron and analysis of sB7-H3 in the sera of patients with hepatocellular carcinoma. PLoS One 2013;8(10):e76965 10.1371/journal.pone.0076965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie C, Liu D, Chen Q, Yang C, Wang B, Wu H. Soluble B7-H3 promotes the invasion and metastasis of pancreatic carcinoma cells through the TLR4/NF-kappaB pathway. Sci Rep 2016;6:27528 10.1038/srep27528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vigdorovich V, Ramagopal UA, Lazar-Molnar E, Sylvestre E, Lee JS, Hofmeyer KA, et al. Structure and T cell inhibition properties of B7 family member, B7-H3. Structure 2013;21(5):707–17 10.1016/j.str.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seaman S, Zhu Z, Saha S, Zhang XM, Yang MY, Hilton MB, et al. Eradication of Tumors through Simultaneous Ablation of CD276/B7-H3-Positive Tumor Cells and Tumor Vasculature. Cancer Cell 2017;31(4):501–15 10.1016/j.ccell.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu H, Cheung IY, Guo HF, Cheung NK. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer Res 2009;69(15):6275–81 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Kang FB, Sun N, Wang J, Chen W, Li D, et al. The tumor suppressor miR-124 inhibits cell proliferation and invasion by targeting B7-H3 in osteosarcoma. Tumour Biol 2016;37(11):14939–47 10.1007/s13277-016-5386-2. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Feng KX, Li H, Wang L, Xia H. MicroRNA-199a Inhibits Cell Proliferation, Migration, and Invasion and Activates AKT/mTOR Signaling Pathway by Targeting B7-H3 in Cervical Cancer. Technol Cancer Res Treat 2020;19:1533033820942245 10.1177/1533033820942245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanchan RK, Perumal N, Atri P, Chirravuri Venkata R, Thapa I, Klinkebiel DL, et al. MiR-1253 exerts tumor-suppressive effects in medulloblastoma via inhibition of CDK6 and CD276 (B7-H3). Brain Pathol 2020;30(4):732–45 10.1111/bpa.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Zhao Y, Xu M, Zhou F, Yan J. Serum miR-1301-3p, miR-335-5p, miR-28-5p, and their target B7-H3 may serve as novel biomarkers for colorectal cancer. J BUON 2019;24(3):1120–7. [PubMed] [Google Scholar]
- 20.Wang ZS, Zhong M, Bian YH, Mu YF, Qin SL, Yu MH, et al. MicroRNA-187 inhibits tumor growth and invasion by directly targeting CD276 in colorectal cancer. Oncotarget 2016;7(28):44266–76 10.18632/oncotarget.10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang G, Wang J, Kelly J, Gu G, Hou J, Zhou Y, et al. B7-H3 augments the inflammatory response and is associated with human sepsis. J Immunol 2010;185(6):3677–84 10.4049/jimmunol.0904020. [DOI] [PubMed] [Google Scholar]
- 22.Hofmeyer KA, Ray A, Zang X. The contrasting role of B7-H3. Proc Natl Acad Sci U S A 2008;105(30):10277–8 10.1073/pnas.0805458105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai D, Li J, Liu D, Hong S, Qiao Q, Sun Q, et al. Tumor-expressed B7-H3 mediates the inhibition of antitumor T-cell functions in ovarian cancer insensitive to PD-1 blockade therapy. Cell Mol Immunol 2020;17(3):227–36 10.1038/s41423-019-0305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, Azuma M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci U S A 2008;105(30):10495–500 10.1073/pnas.0802423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leitner J, Klauser C, Pickl WF, Stockl J, Majdic O, Bardet AF, et al. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur J Immunol 2009;39(7):1754–64 10.1002/eji.200839028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo L, Zhu G, Xu H, Yao S, Zhou G, Zhu Y, et al. B7-H3 Promotes Pathogenesis of Autoimmune Disease and Inflammation by Regulating the Activity of Different T Cell Subsets. PLoS One 2015;10(6):e0130126 10.1371/journal.pone.0130126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Fraser CC, Kikly K, Wells AD, Han R, Coyle AJ, et al. B7-H3 promotes acute and chronic allograft rejection. Eur J Immunol 2005;35(2):428–38 10.1002/eji.200425518. [DOI] [PubMed] [Google Scholar]
- 28.Prasad DV, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, et al. Murine B7-H3 is a negative regulator of T cells. J Immunol 2004;173(4):2500–6 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 29.Ueno T, Yeung MY, McGrath M, Yang S, Zaman N, Snawder B, et al. Intact B7-H3 signaling promotes allograft prolongation through preferential suppression of Th1 effector responses. Eur J Immunol 2012;42(9):2343–53 10.1002/eji.201242501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veenstra RG, Flynn R, Kreymborg K, McDonald-Hyman C, Saha A, Taylor PA, et al. B7-H3 expression in donor T cells and host cells negatively regulates acute graft-versus-host disease lethality. Blood 2015;125(21):3335–46 10.1182/blood-2014-09-603357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suh WK, Wang SX, Jheon AH, Moreno L, Yoshinaga SK, Ganss B, et al. The immune regulatory protein B7-H3 promotes osteoblast differentiation and bone mineralization. Proc Natl Acad Sci U S A 2004;101(35):12969–73 10.1073/pnas.0405259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun X, Vale M, Leung E, Kanwar JR, Gupta R, Krissansen GW. Mouse B7-H3 induces antitumor immunity. Gene Ther 2003;10(20):1728–34 10.1038/sj.gt.3302070. [DOI] [PubMed] [Google Scholar]
- 33.Luo L, Chapoval AI, Flies DB, Zhu G, Hirano F, Wang S, et al. B7-H3 enhances tumor immunity in vivo by costimulating rapid clonal expansion of antigen-specific CD8+ cytolytic T cells. J Immunol 2004;173(9):5445–50 10.4049/jimmunol.173.9.5445. [DOI] [PubMed] [Google Scholar]
- 34.Lupu CM, Eisenbach C, Lupu AD, Kuefner MA, Hoyler B, Stremmel W, et al. Adenoviral B7-H3 therapy induces tumor specific immune responses and reduces secondary metastasis in a murine model of colon cancer. Oncol Rep 2007;18(3):745–8. [PubMed] [Google Scholar]
- 35.Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, et al. Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol 2006;12(3):457–9 10.3748/wjg.v12.i3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loos M, Hedderich DM, Ottenhausen M, Giese NA, Laschinger M, Esposito I, et al. Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer 2009;9:463 10.1186/1471-2407-9-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guery T, Roumier C, Berthon C, Renneville A, Preudhomme C, Quesnel B. B7-H3 protein expression in acute myeloid leukemia. Cancer Med 2015;4(12):1879–83 10.1002/cam4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L, Chen J, Xu B, Wang Q, Zhou W, Zhang G, et al. B7-H3 expression associates with tumor invasion and patient’s poor survival in human esophageal cancer. Am J Transl Res 2015;7(12):2646–60. [PMC free article] [PubMed] [Google Scholar]
- 39.Brustmann H, Igaz M, Eder C, Brunner A. Epithelial and tumor-associated endothelial expression of B7-H3 in cervical carcinoma: relation with CD8+ intraepithelial lymphocytes, FIGO stage, and phosphohistone H3 (PHH3) reactivity. Int J Gynecol Pathol 2015;34(2):187–95 10.1097/PGP.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 40.Zhang P, Yu S, Li H, Liu C, Li J, Lin W, et al. ILT4 drives B7-H3 expression via PI3K/AKT/mTOR signalling and ILT4/B7-H3 co-expression correlates with poor prognosis in non-small cell lung cancer. FEBS Lett 2015;589(17):2248–56 10.1016/j.febslet.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 41.Jin Y, Zhang P, Li J, Zhao J, Liu C, Yang F, et al. B7-H3 in combination with regulatory T cell is associated with tumor progression in primary human non-small cell lung cancer. Int J Clin Exp Pathol 2015;8(11):13987–95. [PMC free article] [PubMed] [Google Scholar]
- 42.Lemke D, Pfenning PN, Sahm F, Klein AC, Kempf T, Warnken U, et al. Costimulatory protein 4IgB7H3 drives the malignant phenotype of glioblastoma by mediating immune escape and invasiveness. Clin Cancer Res 2012;18(1):105–17 10.1158/1078-0432.CCR-11-0880. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Kang FB, Shan BE. B7-H3-mediated tumor immunology: Friend or foe? Int J Cancer 2014;134(12):2764–71 10.1002/ijc.28474. [DOI] [PubMed] [Google Scholar]
- 44.Yuan H, Wei X, Zhang G, Li C, Zhang X, Hou J. B7-H3 over expression in prostate cancer promotes tumor cell progression. J Urol 2011;186(3):1093–9 10.1016/j.juro.2011.04.103. [DOI] [PubMed] [Google Scholar]
- 45.Liu F, Zhang T, Zou S, Jiang B, Hua D. B7H3 promotes cell migration and invasion through the Jak2/Stat3/MMP9 signaling pathway in colorectal cancer. Mol Med Rep 2015;12(4):5455–60 10.3892/mmr.2015.4050. [DOI] [PubMed] [Google Scholar]
- 46.Tekle C, Nygren MK, Chen YW, Dybsjord I, Nesland JM, Maelandsmo GM, et al. B7-H3 contributes to the metastatic capacity of melanoma cells by modulation of known metastasis-associated genes. Int J Cancer 2012;130(10):2282–90 10.1002/ijc.26238. [DOI] [PubMed] [Google Scholar]
- 47.Wang R, Ma Y, Zhan S, Zhang G, Cao L, Zhang X, et al. B7-H3 promotes colorectal cancer angiogenesis through activating the NF-κB pathway to induce VEGFA expression. Cell Death Dis 2020;11(1):55 10.1038/s41419-020-2252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu H, Tekle C, Chen YW, Kristian A, Zhao Y, Zhou M, et al. B7-H3 silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3 phosphorylation. Mol Cancer Ther 2011;10(6):960–71 10.1158/1535-7163.MCT-11-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang T, Jiang B, Zou ST, Liu F, Hua D. Overexpression of B7-H3 augments anti-apoptosis of colorectal cancer cells by Jak2-STAT3. World J Gastroenterol 2015;21(6):1804–13 10.3748/wjg.v21.i6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma Y, Wang R, Lu H, Li X, Zhang G, Fu F, et al. B7-H3 promotes the cell cycle-mediated chemoresistance of colorectal cancer cells by regulating CDC25A. J Cancer 2020;11(8):2158–70 10.7150/jca.37255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao X, Zhang GB, Gan WJ, Xiong F, Li Z, Zhao H, et al. Silencing of B7-H3 increases gemcitabine sensitivity by promoting apoptosis in pancreatic carcinoma. Oncol Lett 2013;5(3):805–12 10.3892/ol.2013.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang FB, Wang L, Jia HC, Li D, Li HJ, Zhang YG, et al. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int 2015;15:45 10.1186/s12935-015-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhong C, Tao B, Chen Y, Guo Z, Yang X, Peng L, et al. B7-H3 Regulates Glioma Growth and Cell Invasion Through a JAK2/STAT3/Slug-Dependent Signaling Pathway. Onco Targets Ther 2020;13:2215–24 10.2147/ott.S237841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nunes-Xavier CE, Karlsen KF, Tekle C, Pedersen C, Oyjord T, Hongisto V, et al. Decreased expression of B7-H3 reduces the glycolytic capacity and sensitizes breast cancer cells to AKT/mTOR inhibitors. Oncotarget 2016;7(6):6891–901 10.18632/oncotarget.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nusinow DP, Szpyt J, Ghandi M, Rose CM, McDonald ER 3rd, Kalocsay M, et al. Quantitative Proteomics of the Cancer Cell Line Encyclopedia. Cell 2020;180(2):387–402 10.1016/j.cell.2019.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Modak S, Kramer K, Gultekin SH, Guo HF, Cheung NK. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res 2001;61(10):4048–54. [PubMed] [Google Scholar]
- 57.Lee YH, Martin-Orozco N, Zheng P, Li J, Zhang P, Tan H, et al. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res 2017;27(8):1034–45 10.1038/cr.2017.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu H, Shi T, Wang M, Li X, Gu Y, Zhang X, et al. B7-H3 inhibits the IFN-γ-dependent cytotoxicity of Vγ9Vδ2 T cells against colon cancer cells. Oncoimmunology 2020;9(1):1748991 10.1080/2162402x.2020.1748991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Modak S, Carrasquillo J, LaQuaglia M, Pat Z, Heaton T, Cheung N-K, et al. Abstract CT006: Intraperitoneal radioimmunotherapy for desmoplastic small round cell tumor: Results of a phase I study (NCT01099644). Cancer Research 2018;78(13 Supplement): 10.1158/1538-7445.Am2018-ct006. [DOI] [Google Scholar]
- 60.CommissionSecuritiesandExchange. 2018. Y-mAbs Therapeutics, Inc <https://www.sec.gov/Archives/edgar/data/1722964/000104746918005786/a2236551zs-1.htm>.
- 61.Kasten BB, Arend RC, Katre AA, Kim H, Fan J, Ferrone S, et al. B7-H3-targeted (212)Pb radioimmunotherapy of ovarian cancer in preclinical models. Nucl Med Biol 2017;47:23–30 10.1016/j.nucmedbio.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kasten BB, Gangrade A, Kim H, Fan J, Ferrone S, Ferrone CR, et al. (212)Pb-labeled B7-H3-targeting antibody for pancreatic cancer therapy in mouse models. Nucl Med Biol 2018;58:67–73 10.1016/j.nucmedbio.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loo D, Alderson RF, Chen FZ, Huang L, Zhang W, Gorlatov S, et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res 2012;18(14):3834–45 10.1158/1078-0432.CCR-12-0715. [DOI] [PubMed] [Google Scholar]
- 64.Powderly J, Cote G, Flaherty K, Szmulewitz RZ, Ribas A, Weber J, et al. Interim results of an ongoing Phase I, dose escalation study of MGA271 (Fc-optimized humanized anti-B7-H3 monoclonal antibody) in patients with refractory B7-H3-expressing neoplasms or neoplasms whose vasculature expresses B7-H3. J Immunother Cancer 2015;3(Suppl 2): 10.1186/2051-1426-3-S2-O8. [DOI] [Google Scholar]
- 65.Charu Aggarwal AJ, Ferris Robert, Antonia Scott, Rahma Osama, Tolcher Anthony, Cohen Roger B., Lou Yanyan, Hauke Ralph, Vogelzang Nicholas, Zandberg Dan, Kalebasty Arash, Atkinson Victoria, Adjei Alex, Seetharam Mahesh, Birnbaum Ariel, Weickhardt Andrew, Ganju Vinod, Bondarenko Riva, Peng Linda, Wu Tony, Currence Scott, Baughman Jan, Bonvini Ezio, Goldberg Stacie, Wigginton Jon, Lakhani Nehal. A Phase 1, Open-Label, Dose Escalation Study of Enoblituzumab in Combination with Pembrolizumab in Patients with Select Solid Tumors. SITC. Washington, D.C.2018. [Google Scholar]
- 66.Xu Y, Xiao Y, Luo C, Liu Q, Wei A, Yang Y, et al. Blocking PD-1/PD-L1 by an ADCC enhanced anti-B7-H3/PD-1 fusion protein engages immune activation and cytotoxicity. Int Immunopharmacol 2020;84:106584 10.1016/j.intimp.2020.106584. [DOI] [PubMed] [Google Scholar]
- 67.Weidle UH, Kontermann RE, Brinkmann U. Tumor-antigen-binding bispecific antibodies for cancer treatment. Semin Oncol 2014;41(5):653–60 10.1053/j.seminoncol.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 68.Sun X, Yu Y, Ma L, Xue X, Gao Z, Ma J, et al. T cell cytotoxicity toward hematologic malignancy via B7-H3 targeting. Invest New Drugs 2020;38(3):722–32 10.1007/s10637-019-00819-y. [DOI] [PubMed] [Google Scholar]
- 69.Davis ZB, Vallera DA, Miller JS, Felices M. Natural killer cells unleashed: Checkpoint receptor blockade and BiKE/TriKE utilization in NK-mediated anti-tumor immunotherapy. Semin Immunol 2017;31:64–75 10.1016/j.smim.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inc GB. 2018 03/06. GT BIOPHARMA ANNOUNCES POSTIVE RESULTS FOR TWO NEXT GENERATION TRIKES IN SOLID TUMORS. <https://www.gtbiopharma.com/news-media/press-releases/detail/157/gt-biopharma-announces-postive-results-for-two-next>. Accessed 2020 03/06.
- 71.Kontos F, Kurokawa T, Vallera DA, Ferrone S, Ferrone CR. IL-15/B7-H3 TriKEs-Based Immunotherapy for Pancreatic Ductal Adenocarcinoma. Journal of the American College of Surgeons 2019;229(4):S176 10.1016/j.jamcollsurg.2019.08.388. [DOI] [Google Scholar]
- 72.Vallera DA, Ferrone S, Kodal B, Hinderlie P, Bendzick L, Ettestad B, et al. NK-Cell-Mediated Targeting of Various Solid Tumors Using a B7-H3 Tri-Specific Killer Engager In Vitro and In Vivo. Cancers (Basel) 2020;12(9) 10.3390/cancers12092659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai L, Michelakos T, Yamada T, Fan S, Wang X, Schwab JH, et al. Defective HLA class I antigen processing machinery in cancer. Cancer Immunol Immunother 2018;67(6):999–1009 10.1007/s00262-018-2131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nehama D, Di Ianni N, Musio S, Du H, Patane M, Pollo B, et al. B7-H3-redirected chimeric antigen receptor T cells target glioblastoma and neurospheres. EBioMedicine 2019;47:33–43 10.1016/j.ebiom.2019.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tang X, Zhao S, Zhang Y, Wang Y, Zhang Z, Yang M, et al. B7-H3 as a Novel CAR-T Therapeutic Target for Glioblastoma. Mol Ther Oncolytics 2019;14:279–87 10.1016/j.omto.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Z, Jiang C, Liu Z, Yang M, Tang X, Wang Y, et al. B7-H3-Targeted CAR-T Cells Exhibit Potent Antitumor Effects on Hematologic and Solid Tumors. Mol Ther Oncolytics 2020;17:180–9 10.1016/j.omto.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng M, Yu L, Hu J, Zhang Z, Wang H, Lu D, et al. Efficacy of B7-H3-Redirected BiTE and CAR-T Immunotherapies Against Extranodal Nasal Natural Killer/T Cell Lymphoma. Transl Oncol 2020;13(5):100770 10.1016/j.tranon.2020.100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martinez M, Moon EK. CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment. Front Immunol 2019;10:128 10.3389/fimmu.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang S, Cao B, Zhou G, Zhu L, Wang L, Zhang L, et al. Targeting B7-H3 Immune Checkpoint With Chimeric Antigen Receptor-Engineered Natural Killer Cells Exhibits Potent Cytotoxicity Against Non-Small Cell Lung Cancer. Front Pharmacol 2020;11:1089 10.3389/fphar.2020.01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


