Abstract
Purpose:
Differences in tumor biology, genomic architecture, and health care delivery patterns contribute to the breast cancer mortality gap between White and Black patients in the US. Although this gap has been well documented in previous literature, it remains uncertain how large the actual effect size of race is for different survival outcomes and the four breast cancer subtypes.
Methods:
We established a breast cancer patient cohort at the University of Chicago Comprehensive Cancer Center. We chose five major survival outcomes to study: overall survival, recurrence-free survival, breast-cancer-specific survival, time-to-recurrence and post-recurrence survival. Cox proportional hazards models were used to estimate the hazard ratios between Black and White patients, adjusting for selected patient, tumor and treatment characteristics, and also stratified by the four breast cancer subtypes.
Results:
The study included 2795 stage I-III breast cancer patients (54% White and 38% Black). After adjusting for selected patient, tumor and treatment characteristics, Black patients still did worse than White patients in all five survival outcomes. The racial difference was highest within the HR−/HER2+ subgroup, in both overall survival (hazard ratio = 4.00, 95% CI: 1.47–10.86) and recurrence-free survival (hazard ratio = 3.00, 95% CI: 1.36–6.60), adjusting for age at diagnosis, cancer stage and comorbidities. There was also a significant racial disparity within the HR+/HER2− group in both overall survival and recurrence-free survival.
Conclusions:
Our study confirmed that racial disparity existed between White and Black breast cancer patients in terms of both survival and recurrence, and found that this disparity was largest among HR−/HER2+ and HR+/HER2− patients.
Keywords: Breast cancer, racial disparity, subtype, survival, recurrence
INTRODUCTION
Breast cancer continues to be the most common malignancy and remains the second leading cause of cancer death among women in the US [1]. Despite the fact that breast cancer mortality rate has been decreasing, national statistics show that this improvement in survival mostly favored White patients compared with the other racial groups, with Black patients still experiencing high mortality rates [1]. To understand the causes of this racial disparity, previous studies have shown that Black women are more likely to be diagnosed with breast cancer with poorer prognostic features, such as advanced stage, larger tumor size, and more comorbidities [2]. Access to care and treatment, and socioeconomic factors are also relevant [3], and some even argue that if equal treatment were received, equal outcomes would follow [4].
Despite the many studies conducted to understand the causes of this racial disparity, there are still unanswered questions on this topic. One important knowledge gap is how racial disparities differ across the four breast cancer subtypes: HR+/HER2− [estrogen receptor (ER) or progesterone receptor (PR) positive (together referred as hormone receptor (HR) positive) and human epidermal growth factor receptor 2 (HER2) negative]; HR+/HER2+ (ER or PR positive and HER2 positive); HR−/HER2+ (ER and PR negative and HER2 positive); and TNBC (triple-negative breast cancer, ER and PR negative and HER2 negative). Previous studies have already shown that subtypes can contribute to racial disparity. For example, it is well known that TNBC is the most aggressive breast cancer subtype and Black women are more likely to have TNBC compared to White women [5–7]. However, whether racial disparity exists within each subtype remains unclear as previous studies have reported inconsistent findings [6, 8–10].
Another question that has been under-addressed is post-recurrence survival. Several early studies found that age at recurrence, disease-free-interval (the time from initial diagnoses to recurrence), recurrence site (local vs. distant), as well as tumor size, axillary lymph node status and ER/PR status at diagnosis can predict patient survival after disease recurrence [11–13]. However, very few of them, if any, have investigated whether or not racial disparities persist in post-recurrence survival and what factors could explain the disparity.
This study aims to answer these questions by using a multi-ethnic cohort of breast cancer patients established at the University of Chicago. Through analyzing the clinical and epidemiological data collected via electronic medical records and interviews, we examined differences in multiple survival outcomes between non-Hispanic Black (Black) and non-Hispanic White (White) patients within the four breast cancer subtypes. We also examined racial differences in post-recurrence survival.
METHODS
Study population
The Chicago Multiethnic Epidemiologic Breast Cancer Cohort (ChiMEC) study was initiated as a hospital-based case-control study designed to investigate high-penetrance breast cancer susceptibility genes and common genetic variants for breast cancer, and was continued as a follow-up of the breast cancer cohort. The study protocol was approved by the institutional review board of the University of Chicago. In brief, patients with breast cancer diagnosed or treated at the University of Chicago Hospitals were enrolled at the high-risk clinic since 1992 and the breast center since 2008, with most of them coming from the Chicago metropolitan area (82.4% of the patients living in Illinois and 12.7% in Northwest Indiana in the study). Clinical, pathological, and treatment data were collected via electronic medical records. Epidemiological risk factor data were collected via questionnaire. A biobank was established, including blood and tumor samples, fresh frozen tissue and formalin-fixed, paraffin-embedded tumor blocks. The enrollment and follow-up of the patients are on-going. The flowchart of the study’s inclusion criteria is shown in Supplemental Figure 1. Of the 4372 breast cancer patients who were diagnosed between 1993 to 2019 and enrolled in the cohort, 3483 had invasive non-metastatic (stage I-III) breast cancer and were considered for this study. Patients diagnosed before 2000 and those with missing subtype information were excluded from the analysis. Native American patients and male patients were also excluded from the analysis because of the small sample size. After these exclusions, 2795 patients were in the analytical cohort.
Covariates
Demographic data were collected through survey at baseline and confirmed by medical records, including race, ethnicity, gender and age at diagnosis. Clinical, pathological, and treatment data were collected using medical records, including date of diagnosis, year of diagnosis, AJCC stage, histological grade, statuses of ER, PR, and HER2, chemotherapy, and radiotherapy. When conducting stratified analysis by subtype, PR status was additionally adjusted for patients in the two HR+ subtypes. This is because previous literature has shown that ER+/PR− tumors could be associated with poorer survival than ER+/PR+ tumors, although they are both classified as HR+ subtypes [14, 15]. The statuses of ER/PR were based on pathological report. As the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) issued guidelines in 2010, lowering the cutoff point for ER/PR positivity from 10% positive nuclei to 1% positive nuclei [16], most borderline ER+ or PR+ (1%−9% positive) in this study were classified as positive. Comorbidity status was represented as Deyo/Charlson comorbidity index [17], which was a cumulative score of 15 health conditions at date of breast cancer diagnosis. Patient insurance status was defined using their primary payer information at diagnosis based on the medical records.
Survival outcomes
Patients were followed via several routes including hospital-based cancer registry, records of clinical visits, and periodic search of National Death Index. Cause of death (COD) was defined based on the following criteria: A patient was considered as having died of breast cancer if the patient: 1) had evidence of breast cancer at last follow up and had no other co-morbidities, 2) had evidence of breast cancer at last follow up and had local/regional or distant recurrence or no remission, or 3) had distant metastasis and had no other co-morbidities. A patient was identified as having died from other diseases if the patient 1) had no evidence of breast cancer at last follow up and had other co-morbidities, 2) had no evidence of breast cancer at last follow up and had no recurrence or only in situ recurrence. For other situations, COD was considered as unknown.
We examined five major survival outcomes: 1) overall survival, defined as the time from the date of diagnosis to the date of death from any causes or date of last follow-up; 2) recurrence-free survival, defined as the time from the date of diagnosis to the first appearance of one of the following: invasive recurrence of breast cancer (local, regional or distant), death from any causes or date of last follow-up; 3) breast-cancer-specific survival, defined as the time from the date of diagnosis to the date of death due to breast cancer or date of last follow-up; 4) time-to-recurrence, defined as the time from the date of diagnosis to the date of recurrence or date of last follow-up; 5) post-recurrence survival, defined as the time from the date of recurrence to the date of death from any causes or date of last follow-up. We also examined death-of-other-causes survival, defined as the time from the date of diagnosis to the date of death due to causes other than breast cancer or date of last follow-up.
Statistical analysis
Baseline tumor, patient and treatment characteristics were compared between racial/ethnic groups using chi-square tests for categorical variables and analysis of variance for continuous variables. Cox proportional hazards models were used to estimate the hazard ratios and the corresponding 95% confidence intervals (CI) between Black and White patients in overall survival, recurrence-free survival, breast-cancer-specific survival, death-of-other-causes, time-to-recurrence analysis as well as post-recurrence survival. Selected patient, tumor and treatment characteristics were sequentially adjusted for to understand their individual contribution to racial disparities. We also conducted subgroup analyses to compare the Black and White patients using Kaplan-Meier graphs and Cox models, stratifying by the four main breast cancer subtypes. Statistical analyses were conducted using the STATA 16 software package (StataCorp, College Station, TX).
RESULTS
Of the 2795 invasive non-metastatic breast cancer patients analyzed, 1521 (54.4%) were non-Hispanic White and 1067 (38.2%) were non-Hispanic Black. Given that there were only 121 Asian patients and 86 Hispanic patients, the following analysis will only focus on the Black and White patients. The mean age at diagnosis was older for Black (59.6 years) than White patients (55.5 years) (Table 1). As expected, the percentage of TNBC patients was much higher among Blacks (25.8%) compared to Whites (14.1%), while the proportion of HR+/HER2− patients was higher in White (69.8%) compared to Black patients (57.5%). For other tumor characteristics, compared to Whites, Black patients were more likely to be diagnosed with a higher grade and at a more advanced stage; while they were not significantly different in terms of receiving chemotherapy and radiotherapy. In addition, Black patients were more likely to have higher comorbidity burden, and more likely to rely on Medicaid and Medicare rather than private insurance.
Table 1.
Demographic and Clinical Characteristics of 2795 Breast Cancer Patients in the ChiMEC Cohort by Race and Ethnicity
| Factor | White (n= 1521) | Black (n=1067) | Asian (n=121) | Hispanic (n=86) | p-value |
|---|---|---|---|---|---|
| Age at diagnosis, mean (sd) | 55.5 (12.8) | 59.6 (14.2) | 50.7 (12.7) | 53.4 (14.8) | <0.001 |
| Year at diagnosis, mean (sd) | 2011.4 (4.7) | 2010.6 (4.8) | 2011.9 (4.2) | 2012.1 (4.5) | <0.001 |
| Breast Cancer Subtype | <0.001 | ||||
| HR+/Her2− | 1062 (69.8%) | 613 (57.5%) | 81 (66.9%) | 59 (68.6%) | |
| HR+/Her2+ | 157 (10.3%) | 102 (9.5%) | 21 (17.4%) | 8 (9.3%) | |
| HR−/Her2+ | 88 (5.8%) | 77 (7.2%) | 8 (6.6%) | 3 (3.5%) | |
| TNBC | 214 (14.1%) | 275 (25.8%) | 11 (9.1%) | 16 (18.6%) | |
| PR Status among HR+ patients | 0.140 | ||||
| PR− | 198 (16.2%) | 150 (21.0%) | 15 (14.7%) | 10 (14.9%) | |
| PR+ | 1020 (83.7%) | 563 (78.7%) | 87 (85.3%) | 57 (85.1%) | |
| missing | 1 (0.1%) | 2 (0.3%) | 0 (0.0%) | 0 (0.0%) | |
| Tumor Grade | <0.001 | ||||
| 1 | 224 (14.7%) | 123 (11.5%) | 16 (13.2%) | 11 (12.8%) | |
| 2 | 655 (43.1%) | 413 (38.7%) | 53 (43.8%) | 35 (40.7%) | |
| 3 | 462 (30.4%) | 434 (40.7%) | 38 (31.4%) | 30 (34.9%) | |
| missing | 180 (11.8%) | 97 (9.1%) | 14 (11.6%) | 10 (11.6%) | |
| Tumor Stage | 0.021 | ||||
| 1 | 718 (47.2%) | 432 (40.5%) | 52 (43.0%) | 33 (38.4%) | |
| 2 | 595 (39.1%) | 453 (42.5%) | 53 (43.8%) | 40 (46.5%) | |
| 3 | 208 (13.7%) | 182 (17.0%) | 16 (13.2%) | 13 (15.1%) | |
| Carlson Comorbidity Index | <0.001 | ||||
| 0 | 1270 (83.5%) | 747 (70.0%) | 108 (89.3%) | 68 (79.0%) | |
| 1 | 104 (6.8%) | 122 (11.4%) | 9 (7.4%) | 6 (7.0%) | |
| >2 | 147 (9.7%) | 198 (18.6%) | 4 (3.3%) | 12 (14.0%) | |
| Type of surgery | <0.001 | ||||
| Lumpectomy | 766 (50.4%) | 573 (53.7%) | 44 (36.4%) | 41 (47.7%) | |
| Mastectomy | 723 (47.5%) | 430 (40.3%) | 74 (61.2%) | 43 (50.0%) | |
| No or unknwon | 32 (2.1%) | 64 (6.0%) | 3 (2.5%) | 2 (2.3%) | |
| Chemotherapy | 0.26 | ||||
| No | 718 (47.2%) | 468 (43.9%) | 52 (43.0%) | 35 (40.7%) | |
| Yes | 803 (52.8%) | 599 (56.1%) | 69 (57.0%) | 51 (59.3%) | |
| Radiotherapy | 0.15 | ||||
| No | 559 (36.8%) | 388 (36.4%) | 55 (45.5%) | 27 (31.4%) | |
| Yes | 950 (62.4%) | 678 (63.5%) | 65 (53.7%) | 59 (68.6%) | |
| missing | 12 (0.8%) | 1 (0.1%) | 1 (0.8%) | 0 (0.0%) | |
| Insurance | <0.001 | ||||
| Uninsured | 6 (0.4%) | 4 (0.4%) | 0 (0.0%) | 1 (1.2%) | |
| Any Medicaid | 42 (2.8%) | 260 (24.4%) | 5 (4.1%) | 10 (11.6%) | |
| Medicare | 377 (24.8%) | 374 (35.1%) | 19 (15.7%) | 20 (23.3%) | |
| Private Insurance | 1056 (69.4%) | 396 (37.1%) | 94 (77.7%) | 53 (61.6%) | |
| Other/Unknown | 40 (2.6%) | 33 (3.0%) | 3 (2.5%) | 2 (2.3%) | |
The median follow-up time of the cohort was 6.9 years (interquartile range: 3.9–10.5) and Black and White patients had similar duration of follow-up. Although there was no minimal amount of follow-up time required for the study, the most recent date of diagnosis for the patients was October 10, 2019 while the date of last contact was March 11, 2020, providing us with the opportunity to follow up the patients for a sufficient amount of time. Among the cases that were alive, 99.8% had a follow-up time of more than 3 months. Overall, 311 patients had recurrent breast cancers and 472 patients died, among which 229 died of breast cancer. The hazard ratios estimated in Cox proportional hazards models sequentially adjusting for important patient and tumor characteristics are provided in Table 2. After adjusting for age at diagnosis, tumor stage, subtype, grade, comorbidities, treatment received (whether received chemotherapy or not, whether received radiotherapy or not), calendar year at diagnosis and insurance status, Black patients still had a significantly worse outcome than White patients in overall survival, recurrence-free survival and breast-cancer-specific survival. The racial disparity was most severe in breast-cancer-specific survival (adjusted hazard ratio = 1.66, 95% CI: 1.23–2.24), adjusting for the covariates mentioned above. Through sequentially adjusting for these characteristics, we found that age at diagnosis, tumor stage, subtype, comorbidities and insurance status were the most important factors that contributed to the racial disparity.
Table 2.
Hazard Ratios between Black and White Patients for Different Survival Outcomes Sequentially Adjusted for Demographic and Clinical Characteristics among 2795 patients
| Variables adjusted in model | Hazard Ratio for Black vs. White (95% CI) |
|||
|---|---|---|---|---|
| Overall Survival | Recurrence-Free Survival | Breast-Cancer-Specific Survival | Time-to-Recurrence Analysis | |
| Unadjusted | 2.14 (1.77–2.58) | 1.85 (1.57–2.19) | 2.31 (1.76–3.03) | 1.51 (1.20–1.91) |
| Adjusted for age at diagnosis | 1.81 (1.50–2.19) | 1.69 (1.43–2.00) | 2.27 (1.73–2.99) | 1.60 (1.27–2.02) |
| + stage | 1.73 (1.43–2.09) | 1.61 (1.36–1.90) | 2.08 (1.58–2.74) | 1.47 (1.16–1.85) |
| + subtype | 1.63 (1.34–1.97) | 1.51 (1.27–1.79) | 1.83 (1.39–2.42) | 1.32 (1.04–1.67) |
| + grade | 1.61 (1.33–1.96) | 1.49 (1.26–1.77) | 1.81 (1.37–2.40) | 1.30 (1.03–1.65) |
| + Carlson comorbidity index | 1.51 (1.24–1.84) | 1.40 (1.18–1.66) | 1.78 (1.35–2.36) | 1.28 (1.01–1.63) |
| + chemotherapy | 1.50 (1.24–1.83) | 1.39 (1.17–1.66) | 1.77 (1.34–2.35) | 1.28 (1.01–1.63) |
| + radiotherapy | 1.53 (1.26–1.86) | 1.41 (1.19–1.68) | 1.80 (1.36–2.39) | 1.29 (1.02–1.64) |
| + year at diagnosis | 1.53 (1.25–1.86) | 1.41 (1.18–1.67) | 1.84 (1.39–2.43) | 1.28 (1.01–1.63) |
| + insurance status | 1.35 (1.10–1.66) | 1.30 (1.08–1.56) | 1.66 (1.23–2.24) | 1.23 (0.96–1.59) |
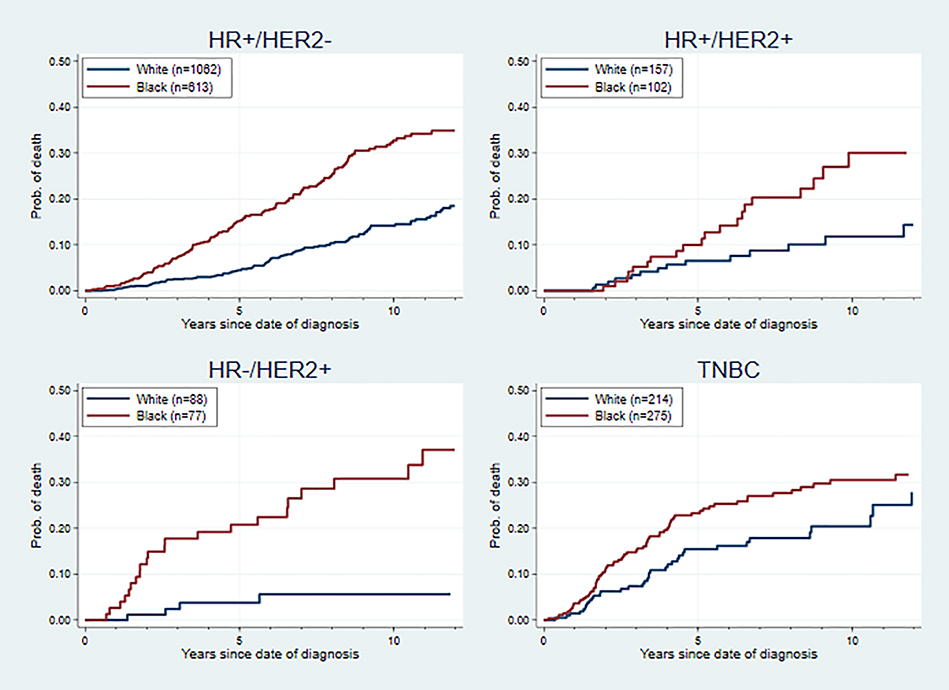
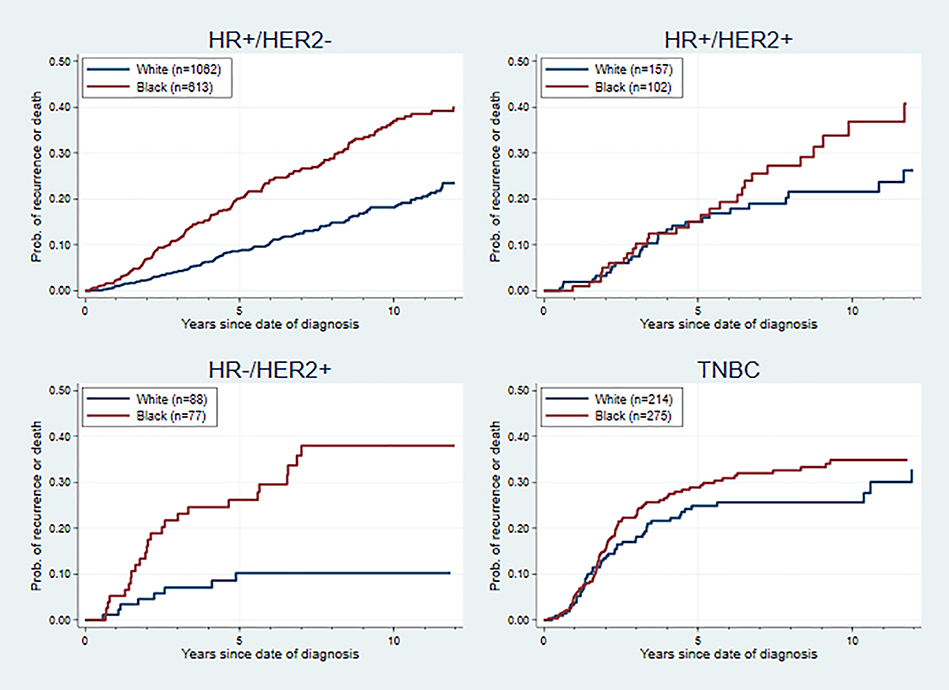
Racial disparities in outcomes were found in all molecular subtypes, but the effect sizes varied. As is shown in Figures 1 and 2, the difference in hazards between Blacks and Whites was most extreme within the HR−/HER2+ subgroup, in terms of overall survival (hazard ratio = 4.00, 95% CI: 1.47–10.86) and recurrence-free survival (hazard ratio = 3.00, 95% CI: 1.36–6.60), adjusting for age at diagnosis, cancer stage and comorbidities. After adjusting for age, stage, comorbidities and PR status (only for the HR+ subtypes), there was a significant racial disparity within the HR+/HER2− subgroup in overall survival and recurrence-free survival, while no significant racial disparities in hazards were found for the HR+/HER2+ and TNBC subgroups. The same pattern in racial disparity was also found in breast-cancer-specific survival, death-of-other-causes survival, and time-to-recurrence analysis (Supplemental Figures 2–4).
Figure 1. Overall Survival Curve for Black and White Patients by Breast Cancer Subtype.
Cox proportional hazards models were used adjusting for age at diagnosis, cancer stage, PR status and comorbidities in HR+/HER2− and HR+/HER2+ subtypes; adjusting for age at diagnosis, cancer stage and comorbidities in HR−/HER2+ and TNBC subtype. The adjusted hazard ratio (95% CI) was 1.56 (1.22–2.00) for HR+/HER2− subtype, 1.42 (0.74–2.70) for HR+/HER2+ subtype, 4.00 (1.47–10.86) for HR−/HER2+ subtype, and 1.26 (0.84–1.88) for TNBC subtype.
Figure 2. Recurrence-Free Survival Curve for Black and White Patients by Breast Cancer Subtype.
Cox proportional hazards models were used adjusting for age at diagnosis, cancer stage, PR status and comorbidities in HR+/HER2− and HR+/HER2+ subtypes; adjusting for age at diagnosis, cancer stage and comorbidities in HR−/HER2+ and TNBC subtypes. The adjusted hazard ratio (95% CI) was 1.53 (1.22–1.91) for HR+/HER2− subtype, 1.04 (0.61–1.77) for HR+/HER2+ subtype, 3.00 (1.36–6.60) for HR−/HER2+ subtype, and 1.06 (0.75–1.50) for TNBC subtype.
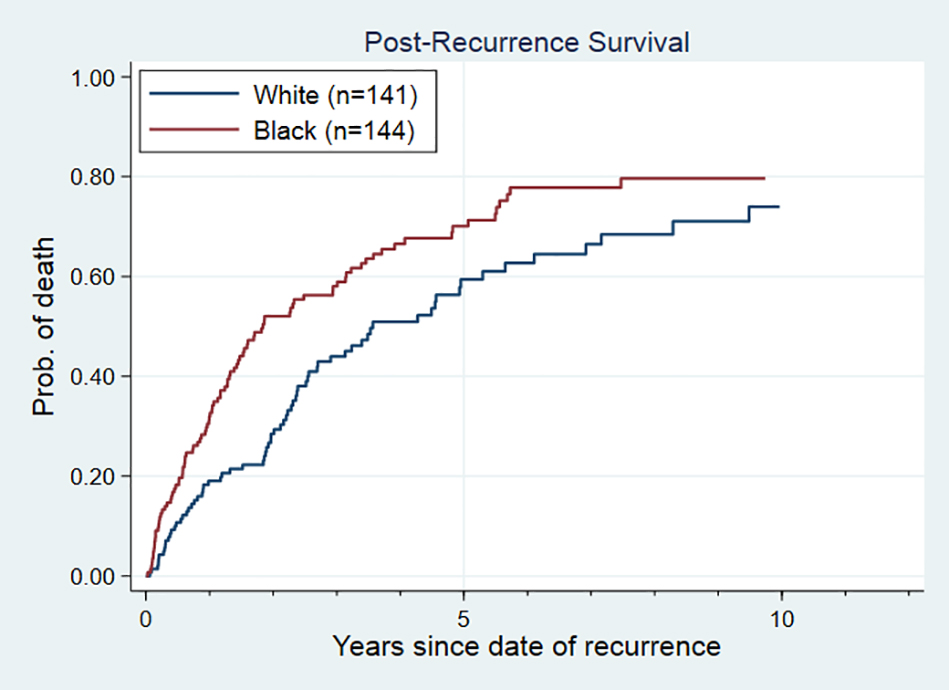
In the post-recurrence survival analysis for recurrent patients only, we found the existence of a strong racial disparity in post-recurrence survival (Figure 3). The median survival time for Black patients after recurrence was 1.8 years (95% CI: 1.4–3.0), while for White patients it was 3.6 years (95% CI: 2.6–5.3), with an unadjusted hazard ratio of 1.55 (95% CI: 1.15–2.11). Through sequentially adjusting for important patient and tumor characteristics (Table 3), we found that age at recurrence, recurrence site, disease-free-interval and insurance status contributed the most in explaining the survival difference between Black and White recurrent patients. After adjusting for these factors, a weak racial disparity persisted (hazard ratio= 1.31, 95% CI: 0.93–1.84), although not statistically significant.
Figure 3. Post-Recurrence Survival Curve for Black and White among 311 Patients with Recurrent Breast Cancer.
In Cox proportional hazards models adjusting for age at recurrence, recurrence site, disease-free-interval, cancer stage, cancer subtype, year at recurrence and insurance status, the adjusted hazard ratio (95% CI) was 1.31 (0.93–1.84).
Table 3.
Hazard Ratios between Black and White in Post-Recurrence Survival among 311 Patients with Recurrent Breast Cancer Sequentially Adjusted for Demographic and Clinical Characteristics
| Variables adjusted in model | Hazard Ratio for Black vs. White (95% CI) |
|---|---|
| Unadjusted | 1.55 (1.15–2.11) |
| Adjusted for age at recurrence | 1.46 (1.07–1.98) |
| + recurrence site | 1.58 (1.16–2.15) |
| + disease-free-interval | 1.47 (1.08–2.01) |
| + stage | 1.50 (1.09–2.05) |
| + subtype | 1.51 (1.10–2.06) |
| + year at recurrence | 1.51 (1.10–2.06) |
| + insurance status | 1.31 (0.93–1.84) |
Discussion
Based on a cohort of breast cancer patients recruited at the University of Chicago with a median follow-up time of 6.9 years, we found substantial racial differences between White and Black patients in overall survival, recurrence-free survival, breast-cancer-specific survival and time-to-recurrence. We also found racial differences in post-recurrence survival. Adjusting for selected patient, tumor and treatment characteristics reduced, but did not eliminate these racial differences. Adjusting for the calendar year at diagnosis (or calendar year at recurrence in post-recurrence survival analysis) did not make much difference in the effect size, implying that the treatment improvement over time did not substantially contribute to reducing racial disparities.
It is noteworthy that, of the survival outcomes examined, the disparity between Black and White patients was strongest in terms of breast-cancer-specific mortality, while fairly weak in time-to-recurrence survival. The risk of recurrence is related to the first line treatment received, while breast-cancer-specific mortality could also be affected by the later lines of treatments. Therefore, a possible explanation of why the racial disparity was greater in breast-cancer-specific mortality is that racial disparity has been reinforced through multiple sequences of treatments. Indeed, we confirmed that racial difference also persisted in post-recurrence survival. This could be related to difference in treatment quality, adherence to treatment as well as access to primary care after tumor eradication. This could also explain why racial difference was greatly reduced after controlling for insurance status in post-recurrence survival, since insurance status could be viewed as an indicator of the patient’s socioeconomic status and the quality of treatment they have received.
Another major finding of the study is that the strength of racial differences varied among the four breast cancer subtypes. In terms of both overall survival and recurrence-free survival, a significant disparity was found within the HR+/HER2− group while not in the HR+/HER2+ group. This is consistent with what was shown in previous literature [6, 8–10]. The study also found no significant racial differences within the TNBC subgroup after adjusting for age, stage and comorbidities. This is consistent with most previous studies [8, 9]. One large study using the California Cancer Registry found that significant racial differences in outcomes existed among stage III TNBC patients, although it did not observe any racial differences among TNBC patients of other stages [6].
The most interesting finding of the study is that a large gap in survival and recurrence risk was found within HR−/HER2+ patients. Previous studies addressing this question have conflicting results, with some being consistent with our findings that HR−/HER2+ patients experience the largest racial disparity [8], some finding a weak association [10], while some found no significant racial disparity [6, 9]. A possible explanation to reconcile this is that the results will be unstable in studies [10] if HR+ and HR− patients were compared without further stratifying by HER2 status, as we have shown that racial disparity could be very different for HR−/HER2+ and TNBC patients. Short follow-up time [6] or small sample size [9] might also affect a study’s power to observe survival differences.
What are the underlying reasons for racial differences in outcomes among HR−/HER2+ and HR+/HER2− breast cancer patients to be most severe? Both subtypes can be treated effectively through targeted therapy [18], suggesting that racial difference may exist in response to targeted therapy. This could be related to difference in toxicity level -- as previous research has suggested, Black patients have a higher rate of cardiotoxicity when undergoing HER2-targeted therapy like trastuzumab compared to White patients [19–21]. In addition, the response rate to hormonal therapy is also determined by the actual percentage of ER positivity [22, 23], and studies showed that Black and White patients have differentiated distributions of ER positivity with immunohistochemistry defined ER+ tumors. One study showed that, among ER-positive patients, “borderline ER+” (patients with ER-positivity between 1–10%) were more prevalent in Blacks than in Whites (7.2% in White vs. 13.2% in Black) [23], although no significant racial difference in proportion of ER positivity was found in some other studies [24, 25]. Nevertheless, even if the proportion of “borderline patients” were similar, since “borderline ER+” were pathologically heterogeneous within themselves, Black patients with “borderline ER+” tumors were found to be associated with poorer endocrine therapy response compared to their White counterparts [25]. Another explanation lies in the difference in adherence to treatment, since suboptimal adherence is a major threat to the efficacy of the treatment. Previous literature has pointed out that in general, Black patients were more likely to be undertreated than White patients due to various socio-economic reasons like lack of access, financial barriers and lower trust of the health care system [4, 6, 10, 26]. This is especially a problem in hormonal treatments like tamoxifen, where the length of a complete course of treatment is 5 years, although whether there is racial disparity in adherence to hormonal treatment remained unclear [27–29]. Therefore, our next step of research is to further explore the adherence to treatment for Black and White patients in the ChiMEC cohort using detailed clinical records.
A major strength of this study is that it established a cohort of breast cancer patients that are diverse in terms of their racial/ethnic origin. This is of great significance as the cohort has a fairly large proportion of Black patients, which enable us to investigate the racial disparities experienced by breast cancer patients and within individual subtypes. In addition, detailed clinical records have been collected for these patients who have been followed by a fairly long period of time, providing the study the power to investigate the effects of tumor and treatment characteristics with both accurate data and also to observe long-term outcomes.
The study had several limitations. First, since we only controlled for insurance status to represent the patient’s socioeconomic status, it remains unknown what other unmeasured socioeconomic factors are relevant in explaining the racial disparities. In addition, we were unable to adjust for any difference in lifestyle factors that could have affected survival outcomes. Being unable to account for individual socio-economic and lifestyle factors is a common drawback when the data source is mainly clinical records. Therefore, we also attempt to collect data on some relevant socio-economic and lifestyle factors through interview and surveys. Since this process is still ongoing and not all of the patients in the study have completed their questionnaires, these epidemiological data will be analyzed in future studies. Another limitation is that the determination of cause of death was not based on death certificates, but assigned by the researcher based on the patient’s cancer status at last follow-up and whether they had other comorbidities. However, even though misclassification could exist because of the rather conservative way of assignment we are taking, there is no reason to suspect it to be differentiated between racial groups. Thus, information bias should not be a major threat to our study. Lastly, the sample size and number of events within the HR+/HER2+ subgroup and the HR−/HER2+ subgroup are rather small. This limits the study’s power to detect a statistically significant difference in hazards between Black and White HR+/HER2+ patients, despite that a difference seems to exist according to the Kaplan-Meier curves. Meanwhile, the strong racial disparity found among HR−/HER2+ patients need to be confirmed by further studies of larger sample size so that more confounders can be adjusted for.
In conclusion, the study showed explicitly that Black breast cancer patients experienced worse outcomes in terms of both survival and recurrence compared to White patients, especially for HR−/HER2+ and HR+/HER2− patients. Adjusting for selected patient, tumor and treatment characteristics reduced, but did not eliminate, this racial disparity. This implies that future studies should be conducted to assess the role of other biological and socioeconomic factors that could account for the persistence of this disparity (e.g. access to care, quality of care) and help in developing intervention methods to close the disparity gap.
Supplementary Material
Acknowledgement
This project was supported by National Cancer Institute (P20 CA233307), the Breast Cancer Research Foundation, and Susan G. Komen for the Cure (SAC110026). The authors are grateful to the participants of the ChiMEC study.
Footnotes
Compliance with Ethical Standards
Olufunmilayo I. Olopade is a cofounder at CancerIQ and serves on the Scientific Advisory Board for Tempus. The other authors made no disclosures.
The study protocol was approved by Intuitional Review Board of the University of Chicago. All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Noone AMHN, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) (2018) SEER Cancer Statistics Review, 1975–2015. In:National Cancer Institute, Bethesda, MD [Google Scholar]
- 2.Silber JH, Rosenbaum PR, Clark AS, Giantonio BJ, Ross RN, Teng Y, Wang M, Niknam BA, Ludwig JM, Wang W, Even-Shoshan O, Fox KR (2013) Characteristics associated with differences in survival among black and white women with breast cancer. JAMA 310:389–397. doi: 10.1001/jama.2013.8272 [DOI] [PubMed] [Google Scholar]
- 3.Prieto D, Soto-Ferrari M, Tija R, Pena L, Burke L, Miller L, Berndt K, Hill B, Haghsenas J, Maltz E, White E, Atwood M, Norman E (2019) Literature review of data-based models for identification of factors associated with racial disparities in breast cancer mortality. Health Syst (Basingstoke) 8:75–98. doi: 10.1080/20476965.2018.1440925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wheeler SB, Reeder-Hayes KE, Carey LA (2013) Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist 18:986–993. doi: 10.1634/theoncologist.2013-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL (2019) Breast cancer statistics, 2019. CA Cancer J Clin 69:438–451. doi: 10.3322/caac.21583 [DOI] [PubMed] [Google Scholar]
- 6.Tao L, Gomez SL, Keegan TH, Kurian AW, Clarke CA (2015) Breast Cancer Mortality in African-American and Non-Hispanic White Women by Molecular Subtype and Stage at Diagnosis: A Population-Based Study. Cancer Epidemiol Biomarkers Prev 24:1039–1045. doi: 10.1158/1055-9965.EPI-15-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly B, Olopade OI (2015) A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin 65:221–238. doi: 10.3322/caac.21271 [DOI] [PubMed] [Google Scholar]
- 8.O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, Dressler LG, Geradts J, Millikan RC (2010) Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res 16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma H, Lu Y, Malone KE, Marchbanks PA, Deapen DM, Spirtas R, Burkman RT, Strom BL, McDonald JA, Folger SG, Simon MS, Sullivan-Halley J, Press MF, Bernstein L (2013) Mortality risk of black women and white women with invasive breast cancer by hormone receptors, HER2, and p53 status. BMC cancer 13:225. doi: 10.1186/1471-2407-13-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jemal A, Robbins AS, Lin CC, Flanders WD, DeSantis CE, Ward EM, Freedman RA (2018) Factors That Contributed to Black-White Disparities in Survival Among Nonelderly Women With Breast Cancer Between 2004 and 2013. J Clin Oncol 36:14–24. doi: 10.1200/JCO.2017.73.7932 [DOI] [PubMed] [Google Scholar]
- 11.Clark GM, Sledge GW Jr., Osborne CK, McGuire WL (1987) Survival from first recurrence: relative importance of prognostic factors in 1,015 breast cancer patients. J Clin Oncol 5:55–61. doi: 10.1200/JCO.1987.5.1.55 [DOI] [PubMed] [Google Scholar]
- 12.Howell A, Harland RNL, Bramwell VHC, Swindell R, Barnes DM, Redford J, Wilkinson MJS, Crowther D, Sellwood RA (1984) Steroid-Hormone Receptors and Survival after First Relapse in Breast Cancer. The Lancet 323:588–591. doi: 10.1016/s0140-6736(84)90995-4 [DOI] [PubMed] [Google Scholar]
- 13.Insa A, Lluch A, Prosper F, Marugan I, Martinez-Agullo A, Garcia-Conde J (1999) Prognostic factors predicting survival from first recurrence in patients with metastatic breast cancer: analysis of 439 patients. Breast Cancer Res Treat 56:67–78. doi: 10.1023/a:1006285726561 [DOI] [PubMed] [Google Scholar]
- 14.Bae SY, Kim S, Lee JH, Lee HC, Lee SK, Kil WH, Kim SW, Lee JE, Nam SJ (2015) Poor prognosis of single hormone receptor- positive breast cancer: similar outcome as triple-negative breast cancer. BMC cancer 15:138. doi: 10.1186/s12885-015-1121-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM (2003) Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol 21:1973–1979. doi: 10.1200/JCO.2003.09.099 [DOI] [PubMed] [Google Scholar]
- 16.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784–2795. doi: 10.1200/JCO.2009.25.6529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deyo R (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology 45:613–619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 18.Gradishar WJ, Anderson BO, Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A, Giordano SH, Goldstein LJ, Hayes DF, Hudis CA, Isakoff SJ, Ljung BM, Marcom PK, Mayer IA, McCormick B, Miller RS, Pegram M, Pierce LJ, Reed EC, Salerno KE, Schwartzberg LS, Smith ML, Soliman H, Somlo G, Ward JH, Wolff AC, Zellars R, Shead DA, Kumar R, National Comprehensive Cancer Network Breast Cancer P (2014) Breast cancer version 3.2014. J Natl Compr Canc Netw 12:542–590. doi: 10.6004/jnccn.2014.0058 [DOI] [PubMed] [Google Scholar]
- 19.Rugo HS, Brufsky AM, Ulcickas Yood M, Tripathy D, Kaufman PA, Mayer M, Yoo B, Abidoye OO, Yardley DA (2013) Racial disparities in treatment patterns and clinical outcomes in patients with HER2-positive metastatic breast cancer. Breast Cancer Res Treat 141:461–470. doi: 10.1007/s10549-013-2697-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baron KB, Brown JR, Heiss BL, Marshall J, Tait N, Tkaczuk KH, Gottlieb SS (2014) Trastuzumab-induced cardiomyopathy: incidence and associated risk factors in an inner-city population. J Card Fail 20:555–559. doi: 10.1016/j.cardfail.2014.05.012 [DOI] [PubMed] [Google Scholar]
- 21.Litvak A, Batukbhai B, Russell SD, Tsai HL, Rosner GL, Jeter SC, Armstrong D, Emens LA, Fetting J, Wolff AC, Silhy R, Stearns V, Connolly RM (2018) Racial disparities in the rate of cardiotoxicity of HER2-targeted therapies among women with early breast cancer. Cancer 124:1904–1911. doi: 10.1002/cncr.31260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harbeck N, Rody A (2012) Lost in translation? Estrogen receptor status and endocrine responsiveness in breast cancer. J Clin Oncol 30:686–689. doi: 10.1200/JCO.2011.38.9619 [DOI] [PubMed] [Google Scholar]
- 23.Fujii T, Kogawa T, Dong W, Sahin AA, Moulder S, Litton JK, Tripathy D, Iwamoto T, Hunt KK, Pusztai L, Lim B, Shen Y, Ueno NT (2017) Revisiting the definition of estrogen receptor positivity in HER2-negative primary breast cancer. Ann Oncol 28:2420–2428. doi: 10.1093/annonc/mdx397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma H, Lu Y, Marchbanks PA, Folger SG, Strom BL, McDonald JA, Simon MS, Weiss LK, Malone KE, Burkman RT, Sullivan-Halley J, Deapen DM, Press MF, Bernstein L (2013) Quantitative measures of estrogen receptor expression in relation to breast cancer-specific mortality risk among white women and black women. Breast Cancer Res 15:R90. doi: 10.1186/bcr3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benefield HC, Allott EH, Reeder-Hayes KE, Perou CM, Carey LA, Geradts J, Sun X, Calhoun BC, Troester MA (2019) Borderline estrogen receptor-positive breast cancers in black and white women. J Natl Cancer Inst. doi: 10.1093/jnci/djz206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts MC, Wheeler SB, Reeder-Hayes K (2015) Racial/Ethnic and socioeconomic disparities in endocrine therapy adherence in breast cancer: a systematic review. Am J Public Health 105 Suppl 3:e4–e15. doi: 10.2105/AJPH.2014.302490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Partridge AH, Wang PS, Winer EP, Avorn J (2003) Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 21:602–606. doi: 10.1200/JCO.2003.07.071 [DOI] [PubMed] [Google Scholar]
- 28.Shah CH, Balkrishnan R, Diaby V, Xiao H (2019) Examining factors associated with adherence to hormonal therapy in breast cancer patients. Res Social Adm Pharm. doi: 10.1016/j.sapharm.2019.08.005 [DOI] [PubMed] [Google Scholar]
- 29.Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA (2006) Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 99:215–220. doi: 10.1007/s10549-006-9193-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.