Abstract
BACKGROUND:
Sleep difficulties are common among older adults, and clinical management of sleep difficulties commonly includes sleep medication (pharmacological and nonpharmacological). Our research examines sleep medication use and incident dementia over 8 years using nationally representative data from older adults ages 65 years and older in the United States.
METHODS:
We used data collected from the National Health and Aging Trends Study (NHATS), a nationally-representative longitudinal study of Medicare beneficiaries. Routine sleep medication use (pharmacological and non-pharmacological) was defined as use “most nights” or “every night.” Participants were screened for dementia with validated instruments that assessed memory, orientation, and executive function. We conduct prospective analyses to examine the relationship between routine sleep medication use and incident dementia using Cox proportional hazards modeling and estimated survival curves. Analyses controlled for age, sex, marital status, education, and chronic conditions.
RESULTS:
Among respondents at baseline (n= 6,373), most participants (21%) were age 70 to 74 years of age. Participants were 59% female and the sample comprised non-Hispanic White (71%). At baseline, 15% of our study sample reported using sleep medication routinely, which is representative of 4.6 million older adults in the US. Covariate adjusted proportional hazard models revealed that routinely using sleep medication was associated with incident dementia (HR=1.30, 95%CI: 1.10 to 1.53, p<.01).
CONCLUSIONS:
Our study observed, in a nationally representative study of older adults in the US across 8 years of data that 15% of older adults report routinely using sleep medication, yet routine use of sleeping medication was associated with incident dementia across the follow-up interval. Future research may examine behavioral approaches to improving sleep among older adults.
Keywords: Sleep medication, sleep medicine, gerontology, dementia
INTRODUCTION
There is a greater burden of sleep difficulties (e.g., difficulty falling asleep, waking up too early) among older adults age 65 and older than any other age group in the United States (US).1 Those experiencing sleep difficulties may seek medications to aid with their difficulties, either healthcare provider-administered psychotropic medication,2 or over the counter (OTC) agents such as non-pharmacological medications (e.g., diphenhydramine hydrochloride, diphenhydramine citrate, or doxylamine succinate) as well as herbal and nutritional supplements (e.g., valerian, melatonin).3 However, sleeping medications – particularly psychotropic medications – are strongly discouraged by major medical associations, including the American Geriatrics Society, attributable in part to the growing evidence that sleep medication use is associated with cognitive impairment and decline.4 We examine nationally representative data from adults in the US to determine use of prescription or non-prescription sleep medication (sleep medication use) prevalence across 8 years of data, and the relationship between routine medication use and incident dementia.
Research suggests sleep medication use is common among older adults. Data from a large prescription repository show that approximately 9% of community-dwelling older adults report a prescription for sleep medication, with one third of those who report long-term sleep medication use (i.e., over 120 days).5 Another study found 16% of adults above the age of 60 reported a prescription for sleep medication, 85% of which reported obtaining the prescription from their healthcare providers.6 Evidence from the Medical Expenditures Panel Survey, a nationally representative survey in the US, shows that the number of individuals who reported prescription sleep medication use increased 67% (8.1 million to 13.5 million adults) between 1996 and 2013.7 In a cohort study of community-dwelling adults with diagnosed insomnia, 19% reported prescription sleep medication use and 27% reported OTC medication use.8
Research has examined specific pharmacological medications, such as benzodiazepines, and their relationship to various individual health and safety-related concerns among older adults, including risk of motor vehicle crashes,9 falls,10 and mortality.11 Further, older adults are more susceptible to adverse reactions to pharmacological sleep medications than younger adults,12 and there is a greater risk of misuse of pharmacological sleep medications among older adults than in younger adults.13 Research suggests that non-pharmacological medications for sleep have adverse outcomes. Research conducted among hospitalized older adults found that pharmacological (e.g., benzodiazepines) and non-pharmacological (e.g., diphenhydramine) medications resulted in an 18% and 22% respective increased rate of delirium among the sample.14 Also, research conducted among community-dwelling older adults found that non-pharmacological medication use was associated with lower cognitive function scores as compared to those not taking these components over a 10-year follow up period.15
Moreover, there is growing evidence of a relationship between pharmacological sleep medication use and increased risk for Alzheimer’s disease (AD), the most common dementia. In a sample of 2,336 older adults in Finland, researchers observed a significant association between pharmacological sleep medication use (e.g., benzodiazepines) for over 60 days and worse cognitive function.16 Also, a meta-analysis found that benzodiazepine use presents a 78% higher risk for AD compared to those not reporting use of these pharmacological medications.17 The association between benzodiazepine use and AD in this meta-analysis focused on older adults residing outside the US (France,18–20 Taiwan,21 the United Kingdom,22 and Canada23,24), while only one study examined a US sample.25 Further, the research to date has predominantly focused on benzodiazepine use and has employed study designs assessing sleep medication at baseline; participants typically did not complete regular assessment between baseline and follow-up.16
While the available literature and meta-analytic findings show a relationship between sleep medication use and AD, two gaps in the literature persist. First, there is limited evidence from studies using representative data from US-based older adults on the relationship between sleep medication use and dementia. Second, there are only a limited number of studies that prospectively evaluate the relationship between sleep medication use and dementia. To address these gaps, we analyze longitudinal data collected using a nationally representative sample of Medicare beneficiaries in the US to examine the prevalence of sleep medication among older adults. We also prospectively examine the relationship between routine sleep medication use and incident dementia across 8 years of follow-up, positing that there will be a strong association between routine sleep medication use and incident risk for dementia.
METHOD
We analyzed data from the National Health and Aging Trends Study (NHATS), an annual in-home, computer-assisted, longitudinal, nationally representative survey of community-dwelling Medicare beneficiaries 65 years and older drawn from the Medicare enrollment database using a complex sampling design. We analyzed all available years, covering 8 years of data from 2011 to 2018. The study began in 2011, with a core interview administered annually.
Participants
Adults aged 65 and older were sampled from the Medicare enrollment file and first interviewed in 2011. NHATS also used proxy respondents for those individuals who were unwilling or unable to complete an interview, which has been shown to reduce attrition bias in longitudinal studies with older adults.26 The baseline sample comprised 7,609 older adults in the US. We drop those who screened positive for dementia at baseline (n=1,236) in order to examine the relationship between sleep medication use and incident dementia prospectively. We also make use of the survey weights for a full sample of 30,903,122 for our assessment of the prevalence of sleep medication use among respondents in this nationally representative sample.27 Additional information regarding the study design and content are available in greater detail elsewhere.28 All respondents provided consent, and the study protocol was approved by the Johns Hopkins University Institutional Review Board (IRB).
Measures
Sleep medication.
We examined sleep medication use among older adults based on participants’ response to the question, “In the last month how often did you take medication to help you sleep?” Participants recorded their response on a scale from “every night: 7 nights a week” (1) to “most nights: 5–6 nights a week” (2), “some nights: 2–4 nights a week” (3), “rarely: once a week or less” (4), and “never” (5). We reverse-coded responses so that higher values indicated greater use. We created a new variable for medication use so that 0 represented “never” or “rarely” or “some nights” and 1 represented routine use of sleep medication (“most nights” or “every night”).
Incident dementia.
Dementia screening was conducted with either the patient or a proxy report. Participants rated their memory then performed a memory-related activity (immediate and delayed 10-word recall). Next, participants responded to items related to orientation performed a clock drawing test to assess executive function. For proxy interviews, an 8-item informant screener for dementia called the AD8 was administered.29 Consistent with previous literature, based on their responses, participants screened either negative or positive for probable dementia.30–32 Sensitivity of the NHATS probable dementia screening measure has been determined in previous research to be 66%, and specificity is 87%, with respect to a clinical dementia diagnosis.31,33
Statistical Analyses
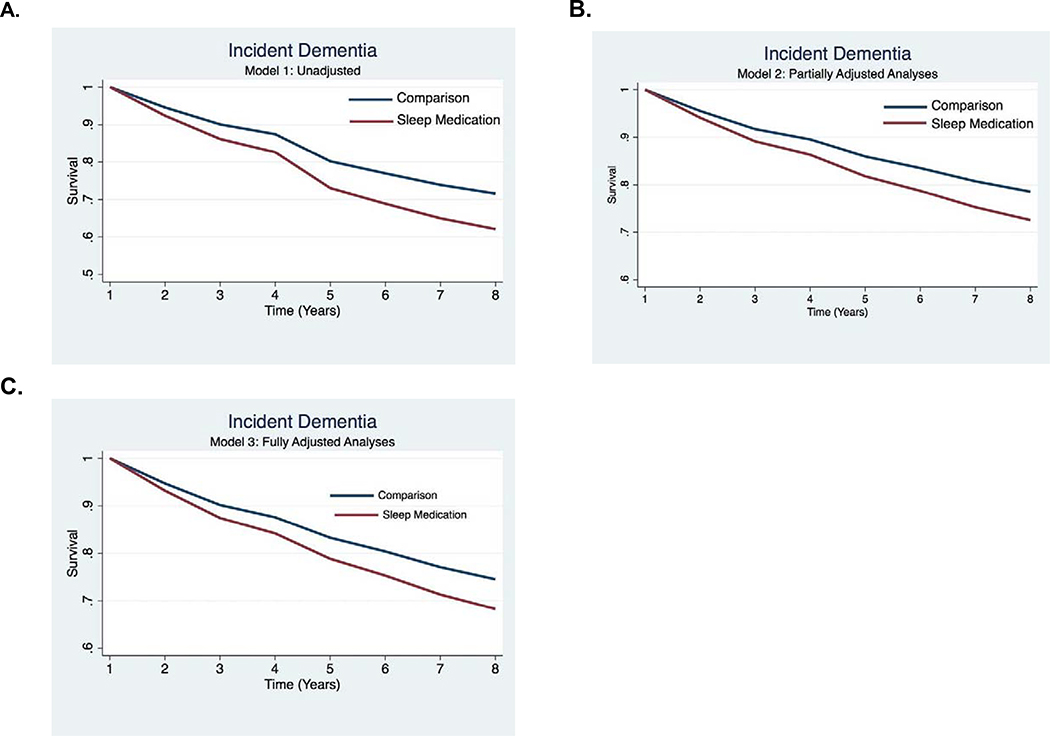
We computed descriptive statistics for demographic factors. To examine the proportion of older adults in the US routinely using sleep medications, we compute descriptive statistics for sleep medication use in each year of the study. We do so with and without population weights. We also compute descriptive statistics for routine sleep medication use by dementia status. We examined routine sleep medication use and incident dementia using Cox proportional hazards modeling to estimate the hazard ratios (HRs) with 95% confidence intervals (Cis) and estimated survival curves. We define the first dementia screening result as the event, after which we censor participants. We enter sleep medication use, which changes over time, in our analyses, as the predictor. First, we examined the relationship between routine sleep medication use and dementia without covariates (Model 1). Second, we examined the relationship between routine sleep medication use and dementia while controlling for demographic covariates, including age (0= 65 up to 75 years of age; 1= 75 years of age and above), sex (0=male, 1=female), race (0=white, 1=non-white), and relationship status (0=widowed, single, or divorced, 1=living with partner or married). Third, we examined the relationship between sleep medication use and incident dementia while controlling for Model 2 demographic covariates and health conditions (0=no chronic conditions; 1=heart attack, 2=depression, 3=hypertension, 4=stroke, 5=diabetes). Depressive symptoms were measured using the Patient Health Questionnaire-2.34 Finally, in the estimated survival curves display time to a positive dementia screening result as a function of exposure to sleep medication use in unadjusted (Model 1), partially adjusted (Model 2), and fully adjusted models (Model 3, Figures 2A–C). Statistical analyses were performed in Stata (Version 16, College Station, TX).
Figure 2. Estimated survival curves displaying the relationship between sleep medication use and incident dementia over 8 years in unadjusted analyses (2A), partially adjusted analyses (2B), and fully adjusted analyses (2C).
Model 1: The model does not include any covariates.
Model 2: The model includes adjustment for demographic factors (age, sex, marital status, and education).
Model 3: The model includes adjustment for Model 2 factors and health conditions.
RESULTS
Table 1 displays demographic characteristics of the sample. The majority of the sample was 70–74 years of age (21%). The sample was predominantly non-Hispanic White (71%), followed by Black/African American (21%), Hispanic/Latino (5%) and more than one race or other (3%). The sample was slightly more female (59%) than male (41%). Among the sample half were married (51%) followed by widowed (21%) and divorced (11%).
Table 1.
Baseline demographic and health characteristics of the study sample.
| N | % | ||
|---|---|---|---|
| Age | 65–69 years | 1,345 | 19% |
| 70–74 years | 1,503 | 21% | |
| 75–79 years | 1,370 | 20% | |
| 80–84 years | 1,334 | 19% | |
| 85–89 years | 845 | 12% | |
| >90 years | 612 | 9% | |
| Race | White | 4,986 | 71% |
| Black | 1,446 | 21% | |
| Hispanic | 169 | 5% | |
| Other | 344 | 3% | |
| More than one race | 64 | 1% | |
| Sex | Male | 2840 | 41% |
| Female | 4,169 | 59% | |
| Marital Status | Married | 3,217 | 51% |
| Living w/ partner | 138 | 2% | |
| Separated | 103 | 2% | |
| Divorced | 710 | 11% | |
| Widowed | 1,980 | 21% | |
| Never married | 220 | 4% | |
| Education | High school | 31 | 1% |
| Some college | 318 | 50% | |
| College | 2,435 | 39% | |
| Grad. degree | 669 | 10% | |
| Conditions | Heart attack | 1,164 | 15% |
| Hypertension | 5,108 | 67% | |
| Stroke | 892 | 12% | |
| Diabetes | 1,925 | 25% | |
| Depression | 3,495 | 28% | |
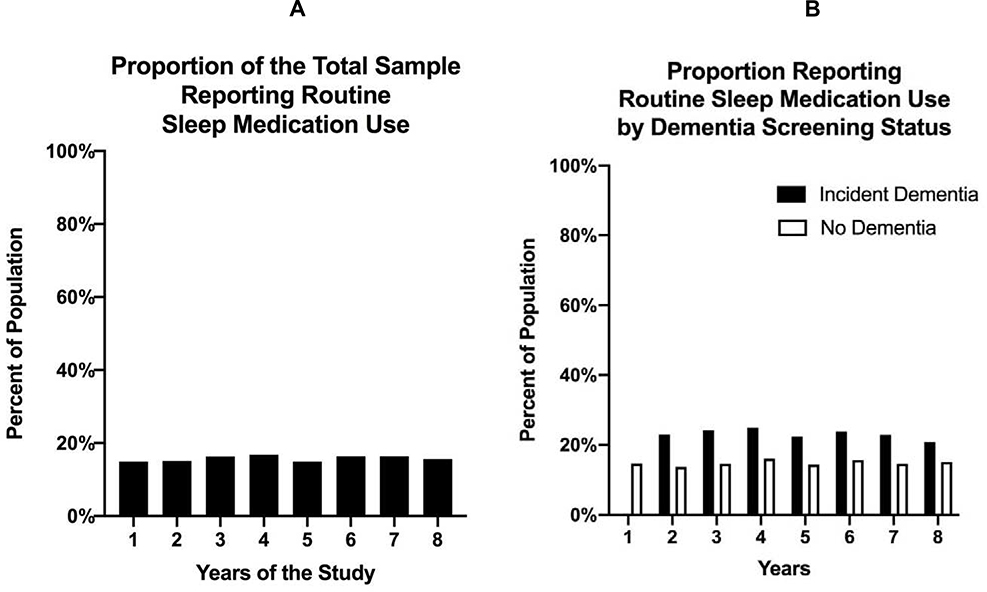
Prevalence of sleep medication across the 8 years of the study is shown graphically in Figures 1A and 1B. In Year 1, 71% of the sample reported sleep medication use either “never,” “rarely,” or “some nights.” Without the population weights, this represented 5,462 individuals. With the population weights, this represented 26.3 million older adults. In Year 1, 15% of the sample reported sleep medication use “most nights” or “every night.” Without population weights, this represented 900 older individuals. With the population weights, this represented 4.6 million older adults. As shown in Figure 1A, routine sleep medication use (i.e., either “most nights” or “every night”) is relatively stable at 15% of the total population across all study years. As shown in Figure 1B, routine sleep medication use is consistently higher among those with dementia than those without dementia across the study years.
Figure 1. Descriptive statistics summarizing the proportion of those reporting routine sleep medication use among the total sample (Figure 1A) and by dementia screening result (Figure 1B).
Table 2 displays results from the Cox proportional hazard model. Compared to never using sleep medication, routine sleep medication use was associated with greater risk for incident dementia in follow-up years in all models (Model 1, unadjusted: HR=1.42, 95%CI: 1.27–1.60, p<.001; Model 2: partially adjusted HR= 1.33, 95%CI: 1.12–1.60, p<.001; and Model 3: HR=1.30,95%CI: 1.10–1.52, p=.002). The relationship between routine sleep medication use and incident dementia is also displayed using estimated survival curves for each model (unadjusted: Figures 2A; partially adjusted: Figure 2B; fully adjusted: Figure 2C).
Table 2.
Cox proportional hazard model results examining the relationship between routine sleep medication use and incident dementia across 8 years of data.
| Model 1 Unadjusted | Model 2 Partially adjusted | Model 3 Fully adjusted | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | Lower | Upper | P-value | OR | Lower | Upper | P-value | OR | Lower | Upper | P-value | |
| Incident Dementia | ||||||||||||
| (0 = negative screening for dementia; 1 = positive screening for dementia) | ||||||||||||
| Sleep Medication | ||||||||||||
| Never/rarely | Reference | Reference | Reference | |||||||||
| Most nights/every night | 1.42 | 1.27 | 1.60 | <.001 | 1.33 | 1.12 | 1.60 | <.001 | 1.30 | 1.10 | 1.52 | .002 |
Model 1: No covariates.
Model 2: Demographic covariates and chronic conditions.
Model 3: Demographic, chronic health conditions and self-reported sleep difficulties (falling asleep and waking up at night).
DISCUSSION
Using a nationally representative sample of Medicare beneficiaries in the US, we examined the routine sleep medication use across 8 years cross-sectionally. Results of these analyses showed that approximately 15% of older adults overall report routinely sleep medication use, or approximately 4.6 million older adults. In prospective analyses across the follow-up interval we found that, after removing those with dementia at baseline, those reporting routine sleep medication use were at 30% greater risk of incident dementia over subsequent years, compared to those without routine use of sleeping medication, after controlling for confounders. The results showed that routine sleep medication use was associated with incident dementia in fully adjusted models controlling for relevant demographic factors, health conditions, and sleep difficulties. Our results contribute to previous work which has shown that sleep medication use (both pharmacological and non-pharmacological), although commonly administered, is associated with a host of subsequent adverse outcomes among older adults.7,9,11,15,20
There are several plausible mechanisms that could account for the increased risk of incident dementia among those who reported routinely using sleep medications. First, routine sleeping medication use may be a proxy for insomnia or disturbed sleep, which may increase risk or accelerate the development of dementia, for research has shown that during sleep there is greater clearance of neurotoxic waste (β-amyloid) than during wakefulness.35 Second, routine sleep medication use may increase risk for dementia directly via sleep medication-induced alterations to sleep architecture, as shown in previous research,36 and therefore accelerate cognitive decline. Third, sleep disturbance is an early symptom of dementia and sleeping medication use may then be a proxy for an individual who is at risk for developing dementia. Fourth, while our analysis removed those with dementia at baseline, dementia brain pathology could have commenced well before this study. Finally, an alternative hypothesis is that an unmeasured variable is associated with both sleep medication use and dementia, which is affecting the relationship observed between sleep medication and incident dementia.
Our results show that routine sleep medication use is commonly reported among populations of older adults. This is concerning, particularly because evidence-based behavioral therapies that may be effective for promoting and enhancing sleep among older adults that may be used in lieu of sleep medications, which include cognitive behavioral therapy for insomnia (CBTI).37 Further, CBTI has been validated in low-cost web-based formats,38,39 and studies have been undertaken to tailor CBTI materials to older individuals.40 CBTI and related programs are promising, and future research may consider tailoring these programs to older adults and their unique needs. Researchers who examine CBTI among older adults, however, should be advised that their successful implementation can yield minimal if any increase in objectively monitored sleep duration, particularly because restriction of time in bed in a key tenant of CBTI curricula.41 Instead, researchers may emphasize other outcomes such as sleep efficiency or patient-reported sleep quality.
In conclusion, high quality geriatric care must be a top priority with an aging population, as noted by the Institute of Medicine.42 Our findings provide further support and evidence that sleep medications are all too commonly administered, yet associated with greater risk for incident dementia, and that the US healthcare system is in need of creative solutions for addressing poor sleep among older individuals.
Future Research
These findings suggest a number of opportunities for future research. First, this epidemiologic study documented an association between routine sleep medication use and incident dementia in a nationally representative sample of community-dwelling older adults in the US. In so doing, our study offered strong preliminary evidence for an association between sleep medication and incident dementia among older adults. However, future research may explore the causal link between sleep medication use (either prescription or OTC) and incident dementia in randomized controlled trial. Second, our results contribute to the growing literature to suggest that sleep medication use is associated with greater risk for incident dementia. Future research may examine the severity of the underlying motivation for sleep medication use (i.e., the sleep difficulty and its severity). Third, there is a large body of evidence to suggest that behavioral treatments for sleep difficulties are effective, such as CBTI.39 Therefore, behavioral treatments for sleep difficulties may be designed that explicitly consider the unique challenges among older adult populations. Another opportunity for future research is to explore the indications for sleep medications among older adults. This may be interesting, for research in a general adult population has shown that sleep difficulties actually represent a small proportion (12%) of the indications for sleep medication prescription.7
Limitations
While strengths of our study include prospective, nationally representative data on sleep medication and dementia among older adults in the US, there are limitations. First, sleep medication use in our analysis is obtained via self-reported. Access to actual sleep medication prescription data would be a substantial improvement and eliminate potential self-report bias. However, widely used validated scales, such as the Pittsburgh Sleep Quality Index,43 measure self-reported sleep medication in a similar manner as we did in the current study.44 Second, the measurement of sleep medication is limited in that it does not report either the type or the dose of sleep medication, or the indications for sleep medication prescription. It is possible, that some participants had varied conceptions of what constitutes a “medication to help you sleep,” as they were asked in the present study. Nevertheless, the measure assessing sleep medication did have strengths, such as a scale assessing frequency of use in a typical week.
It is important to emphasize the epidemiological nature of our results, which would benefit from further exploration in randomized controlled trials. Another limitation is that sleep difficulty is a common manifestation of early dementia, for which someone may seek pharmacological intervention. Therefore, it is possible that there is confounding in our interest in the use of sleep medication and its association to the development of dementia. Further, dementia pathology may commence years before a diagnosis of dementia is administered. Also, sleep medication use leads to worse performance on cognitive testing, such as the questionnaires used to screen for dementia in this study, and therefore could have resulted in a false diagnosis of dementia. Although the incident dementia variable in this study was created in accordance with the NHATS study protocol, it was based on self-reported survey measures as opposed to an objective measure, such as a health record. While the dementia screening measures used in this study have been shown to be validated proxies for assessing the a clinical diagnosis of dementia,30 it is nevertheless a limitation that our primary outcome is not a clinical diagnosis. Further, incident dementia that was identified among participants in our study could have been due to AD, but there are other potential causes that may be studied in future research. Finally, although we control for depression in our study, there may be bi-directional interactions between sleep difficulties, depression, and dementia that may be explored in future studies.
Conclusion
Using a nationally representative sample of older adults representing approximately 25 million older adults, we analyzed prospectively the relationship between routine sleep medication use and incident dementia across 8 years. Results from our study show that 15% of our sample, representing 4.6 million older individuals, report routinely using sleep medications. Our results also showed that routine use of sleep medication was associated with incident dementia over the span of 8 years. Although sleep medications are commonly used to treat sleep disturbance among older adults in the US, their use is discouraged by several national organization and behavioral strategies have been shown to be efficacious. Future research may consider behavioral approaches to improving sleep among older adults.
ACKNOWLEDGEMENTS
Funding
This work was supported by awards K01HL150339, U54MD000538, K07AG052685, R01AG056531, R01AG056031.
Sponsor’s Role
N/a
ABBREVIATIONS
- AD
Alzheimer’s Disease
- IRB
Institutional Review Board
- NHATS
National Health and Aging Trends Study
- OTC
Over the counter
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest: Dr. Rapoport has received consulting fees from Jazz Pharmaceuticals, Eight Mattress, and Fischer and Paykel Healthcare. Dr. Robbins has received consulting fees from Denihan Hospitality, Rituals Cosmetics, Sleep Cycle, and by Nacht. Dr. Czeisler reports grants from Mary Ann & Stanley Snider via Combined Jewish Philanthropies, grants from National Football League Charities, grants from Optum, grants from Philips Respironics Inc, grants from ResMed Foundation, grants from San Francisco Bar Pilots, other from Schneider Inc, grants from Sysco Corp, grants from Teva Pharmaceuticals Industries Ltd, personal fees from Bose Corporation, non-financial support from Boston Celtics, personal fees from Boston Red Sox, personal fees from Columbia River Bar Pilots, personal fees from Institute of Digital Media and Child Development, personal fees from Klarman Family Foundation, personal fees from Samsung Electronics, personal fees and other from Vanda Pharmaceuticals Inc, personal fees from Zurich Insurance Company Ltd, personal fees from McGraw Hill, other from Cephalon Inc, personal fees from Washington State Board of Pilotage Commissioners, personal fees from Ganésco Inc, grants from Jazz Pharmaceuticals Plc Inc, grants from Regeneron Pharmaceuticals, grants from Sanofi SA, personal fees from New England Journal of Medicine, personal fees from Teva Pharma Australia, personal fees from AARP, personal fees from American Academy of Dental Sleep Medicine, personal fees from Eisenhower Medical Center, personal fees from M. Davis and Company, personal fees from Physician's Seal, personal fees from UC San Diego, personal fees from University of Washington, personal fees from Guy A. Ricciardulli, Attorney at Law, personal fees from Kessinger Law Group PLLC, personal fees from Law Offices Of Robert Hamparyan, personal fees from Law Offices of Rossman, Baumberger, Reboso & Spier, P.A., personal fees from Mcelfish Law Firm, personal fees from Millberg Gordon Stewart PLLC, personal fees from University of Washington, personal fees from Hupy and Abram, SC, personal fees from King & Spalding LLP, personal fees from Law Offices of Power, Rogers & Smith LLP, personal fees from Lewis Brisbois Bisgaard Smith LLP, personal fees from Nurenberg Paris Heller & McCarthy, personal fees from United States Aircraft Insurance Group, personal fees from University of Michigan, personal fees from Zehl and Associates PC, personal fees from Cole, Cole & Easley PC, non-financial support from CurtCo Media Labs LLC, personal fees from Haglund Kelley LLP, personal fees from Marshall Dennehey Warner Coleman Goggin, personal fees from Maryland Sleep Society, personal fees from Morgan & Meyers PLC, personal fees from National Sleep Foundation, personal fees from Nurenberg Paris Heller & McCarthy, personal fees from Ostroff Injury Law PC, personal fees from Pratt Clay LLC, personal fees from Lyon Gorsky Gilbert Livingston LLP, personal fees from Drake Law Firm, personal fees from Segal Law Firm, personal fees from Clement Law Firm, personal fees from Sleep Research Society, personal fees from Tencent, personal fees from Casper Sleep Inc, grants from Dayzz Ltd, grants from Teva Pharma Australia PTY Ltd, outside the submitted work;Inaddition,Dr.Czeisler has a patent Actiwatch-2 and Actiwatch-Spectrum devices with royalties paid to Philips Respironics Inc.
References
- 1.Bixler EO, Vgontzas AN, Lin H-M, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90(8):4510–4515. doi: 10.1210/jc.2005-0035 [DOI] [PubMed] [Google Scholar]
- 2.Aparasu RR, Mort JR, Brandt H. Psychotropic prescription use by community-dwelling elderly in the United States. J Am Geriatr Soc. 2003;51(5):671–677. [DOI] [PubMed] [Google Scholar]
- 3.Culpepper L, Wingertzahn MA. Over-the-Counter Agents for the Treatment of Occasional Disturbed Sleep or Transient Insomnia: A Systematic Review of Efficacy and Safety. Prim Care Companion CNS Disord. 2015;17(6). doi: 10.4088/PCC.15r01798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2015;63(11):2227–2246. doi: 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 5.Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136–142. doi: 10.1001/jamapsychiatry.2014.1763 [DOI] [PubMed] [Google Scholar]
- 6.Neutel CI, Patten SB. Sleep medication use in Canadian seniors. Can J Clin Pharmacol. 2009;16(3):e443–452. [PubMed] [Google Scholar]
- 7.Bachhuber MA, Hennessy S, Cunningham CO, Starrels JL. Increasing Benzodiazepine Prescriptions and Overdose Mortality in the United States, 1996– 2013. Am J Public Health. 2016;106(4):686–688. doi: 10.2105/AJPH.2016.303061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillai V, Cheng P, Kalmbach DA, Roehrs T, Roth T, Drake CL. Prevalence and Predictors of Prescription Sleep Aid Use among Individuals with DSM-5 Insomnia: The Role of Hyperarousal. Sleep. 2016;39(4):825–832. doi: 10.5665/sleep.5636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemmelgarn B, Suissa S, Huang A, Boivin JF, Pinard G. Benzodiazepine use and the risk of motor vehicle crash in the elderly. JAMA. 1997;278(1):27–31. [PubMed] [Google Scholar]
- 10.de Vries OJ, Peeters G, Elders P, et al. The elimination half-life of benzodiazepines and fall risk: two prospective observational studies. Age Ageing. 2013;42(6):764–770. doi: 10.1093/ageing/aft089 [DOI] [PubMed] [Google Scholar]
- 11.Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: a matched cohort study. BMJ Open. 2012;2(1):e000850. doi: 10.1136/bmjopen-2012-000850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naranjo CA, Herrmann N, Mittmann N, Bremner KE. Recent advances in geriatric psychopharmacology. Drugs Aging. 1995;7(3):184–202. doi: 10.2165/00002512-199507030-00004 [DOI] [PubMed] [Google Scholar]
- 13.Gurvich T, Cunningham JA. Appropriate use of psychotropic drugs in nursing homes. Am Fam Physician. 2000;61(5):1437–1446. [PubMed] [Google Scholar]
- 14.Rothberg MB, Herzig SJ, Pekow PS, Avrunin J, Lagu T, Lindenauer PK. Association between sedating medications and delirium in older inpatients. J Am Geriatr Soc. 2013;61(6):923–930. doi: 10.1111/jgs.12253 [DOI] [PubMed] [Google Scholar]
- 15.Basu R, Dodge H, Stoehr GP, Ganguli M. Sedative-hypnotic use of diphenhydramine in a rural, older adult, community-based cohort: effects on cognition. Am J Geriatr Psychiatry. 2003;11(2):205–213. [PubMed] [Google Scholar]
- 16.Virta JJ, Heikkilä K, Perola M, et al. Midlife sleep characteristics associated with late life cognitive function. Sleep. 2013;36(10):1533–1541, 1541A. doi: 10.5665/sleep.3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Islam MM, Iqbal U, Walther B, et al. Benzodiazepine Use and Risk of Dementia in the Elderly Population: A Systematic Review and Meta-Analysis. NED. 2016;47(3–4):181–191. doi: 10.1159/000454881 [DOI] [PubMed] [Google Scholar]
- 18.Paterniti S, Dufouil C, Alpérovitch A. Long-term benzodiazepine use and cognitive decline in the elderly: the Epidemiology of Vascular Aging Study. J Clin Psychopharmacol. 2002;22(3):285–293. [DOI] [PubMed] [Google Scholar]
- 19.Shash D, Kurth T, Bertrand M, et al. Benzodiazepine, psychotropic medication, and dementia: A population-based cohort study. Alzheimers Dement. 2016;12(5):604–613. doi: 10.1016/j.jalz.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 20.Lagnaoui R, Bégaud B, Moore N, et al. Benzodiazepine use and risk of dementia: A nested case–control study. Journal of Clinical Epidemiology. 2002;55(3):314–318. doi: 10.1016/S0895-4356(01)00453-X [DOI] [PubMed] [Google Scholar]
- 21.Wu C-S, Wang S-C, Chang I-S, Lin K-M. The association between dementia and long-term use of benzodiazepine in the elderly: nested case-control study using claims data. Am J Geriatr Psychiatry. 2009;17(7):614–620. doi: 10.1097/JGP.0b013e3181a65210 [DOI] [PubMed] [Google Scholar]
- 22.Imfeld P, Bodmer M, Jick SS, Meier CR. Benzodiazepine Use and Risk of Developing Alzheimer’s Disease or Vascular Dementia: A Case-Control Analysis. Drug Saf. 2015;38(10):909–919. doi: 10.1007/s40264-015-0319-3 [DOI] [PubMed] [Google Scholar]
- 23.Billioti de Gage S, Moride Y, Ducruet T, et al. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ. 2014;349:g5205. doi: 10.1136/bmj.g5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Billioti de Gage S, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231. doi: 10.1136/bmj.e6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray SL, Dublin S, Yu O, et al. Benzodiazepine use and risk of incident dementia or cognitive decline: prospective population based study. BMJ. 2016;352:i90. doi: 10.1136/bmj.i90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weir D, Faul J, Langa K. Proxy interviews and bias in the distribution of cognitive abilities due to non-response in longitudinal studies: a comparison of HRS and ELSA. Longitudinal and life course studies. 2011;2(2):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montaquila J, Freedman VA, Spillman B, Kasper JD. National Health and Aging Trends Study development of round 1 survey weights. NHATS technical paper. 2012;2. [Google Scholar]
- 28.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort Profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014;43(2):576–585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galvin J, Roe C, Xiong C, Morris J. Validity and reliability of the AD8 informant interview in dementia | Neurology. 2006;67(11):1942–1948. doi: 10.1212/01.wnl.0000247042.15547.eb [DOI] [PubMed] [Google Scholar]
- 30.Kasper J, Freedman V, Spillman B. Classification of Persons by Dementia Status in the National Health and Aging Trends Study. In: Technical Paper #5. Johns Hopkins University School of Public Health. NHATS.org [Google Scholar]
- 31.Hunt LJ, Covinsky KE, Yaffe K, et al. Pain in Community-Dwelling Older Adults with Dementia: Results from the National Health and Aging Trends Study. J Am Geriatr Soc 2015;63(8):1503–1511. doi: 10.1111/jgs.13536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachman DL, Wolf PA, Linn R, et al. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992;42(1):115–119. doi: 10.1212/wnl.42.1.115 [DOI] [PubMed] [Google Scholar]
- 33.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of Dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phipps AI, Bhatti P, Neuhouser ML, et al. Pre-diagnostic sleep duration and sleep quality in relation to subsequent cancer survival. J Clin Sleep Med. 2016;12(4):495–503. doi: 10.5664/jcsm.5674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. science. 2013;342(6156):373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maestri M, Carnicelli L, Tognoni G, et al. Non-rapid eye movement sleep instability in mild cognitive impairment: a pilot study. Sleep Med 2015;16(9):1139–1145. doi: 10.1016/j.sleep.2015.04.027 [DOI] [PubMed] [Google Scholar]
- 37.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive Behavioral Therapy for Treatment of Chronic Primary Insomnia: A Randomized Controlled Trial. JAMA. 2001;285(14):1856–1864. doi: 10.1001/jama.285.14.1856 [DOI] [PubMed] [Google Scholar]
- 38.Espie CA, Kyle SD, Miller CB, Ong J, Hames P, Fleming L. Attribution, cognition and psychopathology in persistent insomnia disorder: outcome and mediation analysis from a randomized placebo-controlled trial of online cognitive behavioural therapy. Sleep Med. 2014;15(8):913–917. doi: 10.1016/j.sleep.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 39.Ritterband LM, Thorndike FP, Ingersoll KS, et al. Effect of a Web-Based Cognitive Behavior Therapy for Insomnia Intervention With 1-Year Follow-up: A Randomized Clinical Trial. JAMA Psychiatry. 2017;74(1):68–75. doi: 10.1001/jamapsychiatry.2016.3249 [DOI] [PubMed] [Google Scholar]
- 40.Chen Y-X, Hung Y-P, Chen H-C. Mobile application–assisted cognitive behavioral therapy for insomnia in an older adult. Telemedicine and e-Health. 2016;22(4):332–334. doi: 10.1089/tmj.2015.0064 [DOI] [PubMed] [Google Scholar]
- 41.Bathgate CJ, Edinger JD, Wyatt JK, Krystal AD. Objective but Not Subjective Short Sleep Duration Associated with Increased Risk for Hypertension in Individuals with Insomnia. SLEEP. 2016;39(05):1037–1045. doi: 10.5665/sleep.5748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Field MJ, Cassel CK. Approaching death: improving care at the end of life. Health Prog. 2011;92(1):25. [PubMed] [Google Scholar]
- 43.Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 44.Min Y, Kirkwood CK, Mays DP, Slattum PW. The Effect of Sleep Medication Use and Poor Sleep Quality on Risk of Falls in Community-Dwelling Older Adults in the US: A Prospective Cohort Study. Drugs Aging. 2016;33(2):151–158. doi: 10.1007/s40266-015-0339-9 [DOI] [PubMed] [Google Scholar]