Abstract
Bacterial cell division is orchestrated by the divisome, a protein complex centered on the tubulin homolog FtsZ. FtsZ polymerizes into a dynamic ring that defines the division site, recruits downstream proteins, and directs peptidoglycan synthesis to drive constriction. Recent studies have documented treadmilling of FtsZ polymer clusters both in cells and in vitro. Emerging evidence suggests that FtsZ dynamics are regulated largely by intrinsic properties of FtsZ itself and by the membrane anchoring protein FtsA. While FtsZ dynamics are broadly required for Z-ring assembly, their role(s) during constriction may vary among bacterial species. These recent advances set the stage for future studies to investigate how FtsZ dynamics are physically and/or functionally coupled to peptidoglycan metabolic enzymes to direct efficient division.
Introduction
Cell division in bacteria is a carefully controlled process that requires the coordination of multiple complex systems. In coordination with chromosome replication and segregation, cells must localize peptidoglycan (PG) cell wall enzymes to the site of division and coordinate their activities to synthesize PG, which is thought to be the driving force underlying constriction. The process of cytokinesis is carried out by the divisome, a protein complex responsible for directing division through spatiotemporal control of PG enzymes [1].
At the heart of the divisome lies FtsZ, an essential, polymerization-competent tubulin homolog that localizes to the division site to form a structure known as the “Z-ring”. The site of Z-ring assembly defines the site of division, and FtsZ acts to both recruit and regulate roughly a dozen essential cell division proteins that mediate constriction. Through signals that are not yet clear, the assembled divisome is activated to synthesize peptidoglycan, and the Z-ring constricts in diameter throughout the division process [1].
In recent years, significant advances have been made in understanding the nature, regulation, and function of the dynamic properties of FtsZ and the Z-ring at the polymer and cellular levels. This review aims to highlight recent work that has advanced our understanding of how the dynamics of this critical protein are regulated, and how FtsZ dynamics affect and contribute to the process of division.
FtsZ is dynamic on a cellular scale and within the assembled Z-ring
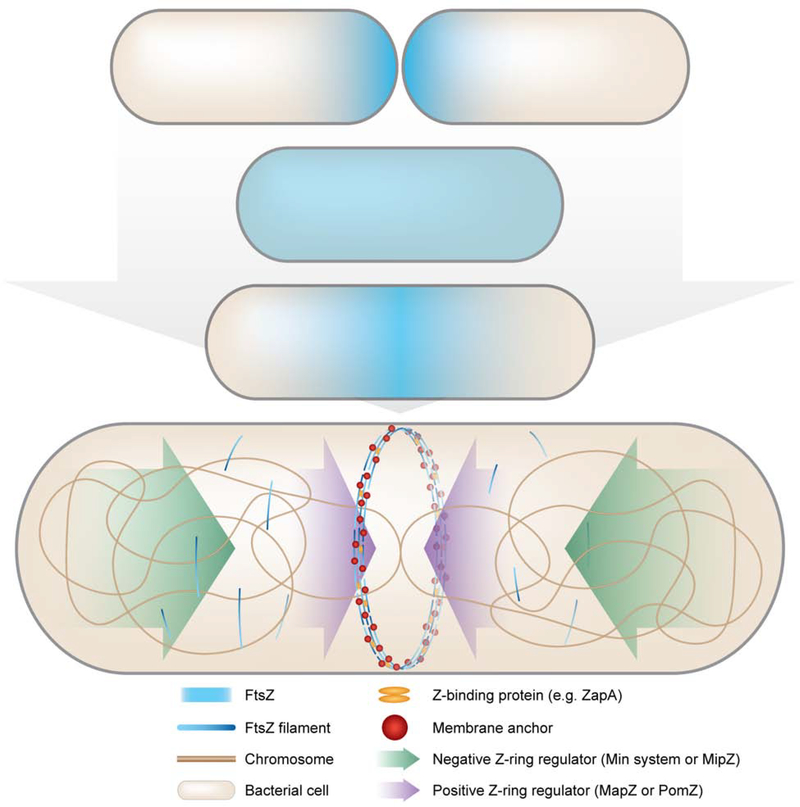
The Z-ring marks the site of cell division, and therefore Z-ring assembly and constriction must be coordinated with cell cycle events like chromosome replication and segregation. Z-ring assembly occurs during S phase and requires a shift in FtsZ polymer concentration toward mid-cell and away from the poles. In most bacteria, mid-cell assembly of FtsZ is driven by extrinsic positioning systems that exploit and modify intrinsic FtsZ polymer dynamics (Figure 1). The Min system in Escherichia coli and Bacillus subtilis and MipZ in Caulobacter crescentus function by localizing factors that prevent FtsZ polymerization near cell poles, effectively constraining Z-ring formation to mid-cell. Conversely, some bacteria instead rely on positive regulators of Z-ring assembly, such as PomZ in Myxococcus xanthus and MapZ in Streptococcus pneumoniae [2]. Walker et al. recently examined fluorescently labeled FtsZ through the cell cycle and observed that FtsZ forms short, transient, membrane-anchored assemblies moving mostly circumferentially along the length of the cell preceding Z-ring assembly [3]. The presence of transient precursors implies that FtsZ polymers stochastically sample the membrane via interactions with membrane anchors in the early stages of Z-ring assembly. The combined actions of Z-ring positioning systems and recruitment of additional factors promote maturation of these transient structures into a stably localized Z-ring at mid-cell (Figure 1).
Figure 1: A host of localization systems contribute to Z-ring assembly throughout the cell cycle.
Early in the cell cycle, FtsZ (blue haze) is rapidly redistributed from the poles throughout the cell. Prior to the subsequent division event as the chromosome (bronze line) is being replicated, the division site is selected by spatially regulated polymerization of FtsZ into a mid-cell ring. Depending on the organism, negative (green arrows) Z-ring regulators (Min system or MipZ) promote disassembly of FtsZ polymers near the poles, or positive (purple arrows) Z-ring regulators (MapZ or PomZ) promote local mid-cell assembly of FtsZ polymers (blue gradient lines). Z-ring assembly and condensation are further facilitated by association with membrane anchors (red spheres) and Z-binding proteins (orange ellipses), e.g. ZapA, at mid-cell.
FtsZ is also highly dynamic within the assembled Z-ring, with molecules in the ring turning over with a half-time of ~10–30 s as measured by fluorescence recovery after photobleaching (FRAP) [4,5]. Notably, the recovery time is significantly extended upon expression of a GTPase-deficient FtsZ variant, indicating a dependence of polymer dynamics on FtsZ’s GTPase activity. This observation is supported by previous studies that established FtsZ’s ability to form dynamic polymers upon binding and hydrolyzing GTP in vitro [6].
Recently, studies in several bacterial species have characterized a specific dynamic mode of FtsZ known as treadmilling, previously observed in vitro [7], wherein subunits are added to one end of a filament cluster and dissociated from the other resulting in the apparent directional motion of FtsZ clusters while single molecules of FtsZ are stationary. Using total internal reflection fluorescence microscopy (TIRFM) or imaging Z-rings in vertically-immobilized bacteria, FtsZ treadmilling has been observed in vivo with rates of ~30–40 nm/s - corresponding to roughly 7–9 FtsZ monomers/s - depending on the organism [8–12]. Cell geometry does not appear to affect FtsZ dynamics, as recovery times following photobleaching and treadmilling rates of FtsZ in E. coli cells induced to acquire different diameters or shapes are similar to those in wild-type cells [13]. However, perturbations that decrease or increase GTPase activity lead to a decrease or increase in treadmilling rates, respectively [8,9,12]. Interestingly, while treatment of cells with the GTPase inhibitor PC190723 eliminates treadmilling motion in both Staphylococcus aureus and B. subtilis [8,12], FtsZ structures remain dynamic by FRAP [14], suggesting the existence of yet uncharacterized dynamic modes beyond treadmilling.
Intrinsic regulation of FtsZ polymer dynamics
How are FtsZ dynamics regulated to potentiate the observed in vivo behaviors? In vitro, FtsZ assembles into straight or gently curved single protofilaments under physiological salt and buffer conditions [6]. Early studies employing transmission electron microscopy, GTPase assays, and light scattering to examine FtsZ assembly in solution identified GTP binding and hydrolysis as key factors driving polymer dynamics. Increasing Mg2+, Ca2+, or DEAE-dextran (a polycationic crowding agent) concentrations results in more stable polymers that form thick bundles or sheets, apparently by favoring abundant lateral filament interactions and reducing polymer disassembly [6].
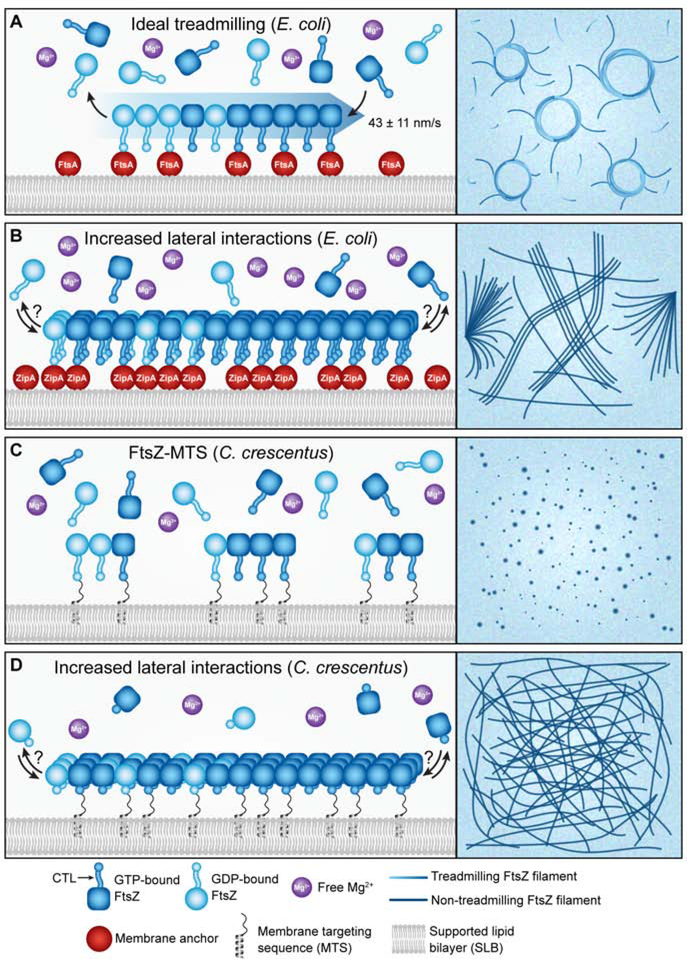
The implementation of supported lipid bilayers (SLBs) coupled with TIRFM has allowed the in vitro study of FtsZ dynamics and superstructure in the physiologically relevant context of membrane association. Loose and Mitchison first observed treadmilling of purified E. coli FtsZ on SLBs with rates similar to those subsequently observed in vivo (~40 nm/s). They found that treadmilling requires sufficient local FtsZ concentration, GTP hydrolysis, and association with the SLB through a membrane anchoring protein, in their case E. coli FtsA [7] (Figure 2A). Wagstaff et al. recently provided a structural explanation for both cooperative assembly and treadmilling of single FtsZ protofilaments, proposing that both are enabled by a switch between open and closed FtsZ conformations that depends on its polymerization state [15]. These concepts have also been explored by Corbin and Erickson in the design of a Monte Carlo model for FtsZ dynamics that accurately recapitulates properties of FtsZ nucleation and treadmilling in vitro and in vivo and corroborates the hypothesis that these processes are driven by a conformational switch [16].
Figure 2: FtsZ treadmilling on SLBs depends on membrane association, GTPase activity, and proper lateral interactions.
Model for polymer behavior (left) and representative experimental view (right) of each scenario are represented. A. Treadmilling of E. coli FtsZ (blue gradient arrow and lines) occurs on SLBs when FtsZ can bind (dark blue) and hydrolyze (light blue) GTP and the local concentration of FtsZ at the membrane, facilitated by a membrane anchor (red spheres) or a membrane targeting sequence (not shown), is sufficient to allow for both polymerization and depolymerization. Treadmilling FtsZ is typically observed in either single clusters or chiral swirls, from each of which treadmilling rates can be determined. B. E. coli FtsZ forms asters or bundles/mesh-like structures under conditions of high E. coli ZipA or high free Mg2+ concentrations, respectively. Treadmilling has not been observed under these conditions, although polymers are still dynamic. C. C. crescentus FtsZ fused to a membrane targeting sequence (FtsZ-MTS) forms small dynamic clusters that do not appear to move by treadmilling under conditions tested thus far. D. C. crescentus FtsZ lacking its C-terminal linker (ΔCTL-MTS) forms stable, mesh-like structures that are more stable compared to WT.
Building on the above observations, Ramirez-Diaz et al. observed that FtsZ fused to a membrane targeting sequence (FtsZ-MTS) exhibits treadmilling on SLBs within a narrow range of Mg2+ concentrations, below which FtsZ cannot bind GTP and above which FtsZ instead forms dense mesh-like structures with increased single molecule residence times [17] (Figure 2B). These structures are likely facilitated by increased lateral interactions favored by high concentrations of free Mg2+ and are similar to the structures formed by a hyper-bundling variant of C. crescentus FtsZ-MTS on SLBs [18] (Figure 2D). These observations suggest a model in which treadmilling activity is highly dependent on local concentrations of FtsZ, GTP, and Mg2+ at the membrane, which brings into question what happens in vivo when these requirements are not met. In contrast to E. coli FtsZ, wild-type C. crescentus FtsZ-MTS does not appear to move directionally on SLBs. Instead, it forms discrete clusters that form and disassemble rapidly, suggesting potential species-specific differences in the intrinsic propensity of FtsZ clusters to undergo treadmilling motion [18] (Figure 2C).
In addition to the GTPase domain, FtsZ includes a conserved C-terminal peptide (CTC), a disordered C-terminal linker (CTL) between the GTPase domain and CTC and, sometimes, a short variable sequence at the extreme C-terminus (CTV), each of which may regulate polymer dynamics. In vitro assembly of FtsZ lacking its C-terminal linker (ΔCTL) results in bundled polymers that exhibit greater stability as compared to wild-type FtsZ from C. crescentus [18–20] (Figure 2C–D) and B. subtilis [21]. Production of ΔCTL in vivo results in disruptions in Z-ring morphology and cell division in B. subtilis [22], E. coli [23], and C. crescentus [19,24]. Taken together, these observations indicate a role for the CTL in regulating interfilament interactions, and consequently, FtsZ dynamics. Conversely, replacement or removal of the CTC, CTV, or both results in either a decrease (B. subtilis [25]) or no change (E. coli and C. crescentus) in bundling propensity and a modest decrease in GTPase rate [20,25]. Thus, the FtsZ C-terminus influences polymer dynamics and lateral interactions, serving as an intrinsic regulator of Z-ring dynamics in addition to the GTPase domain.
The role of binding partners in FtsZ dynamics: is bundling relevant in cells?
FtsZ binding partners have long been known to affect FtsZ’s localization and structure in cells and polymer dynamics and/or superstructure in purified in vitro systems. Specifically, a number of proteins can induce bundling – here defined as association of protofilaments into stable multi-filament structures with tight lateral interactions - of FtsZ filaments in vitro and generally reduce the apparent GTPase activity of FtsZ in the process [26]. The relevance of FtsZ bundling in cells is unclear, however, and mounting evidence suggests that at least some of the proteins previously described as “bundling” proteins likely impact Z-ring organization or function through bundling-independent mechanisms – and/or without impacting FtsZ dynamics - in cells.
One of the best-studied of these is the conserved coiled coil protein ZapA. Purified ZapA causes FtsZ polymers to form stable, multi-filament bundles under some conditions in vitro [27,28], and ZapA promotes formation of coherent Z-rings in vivo [27,29]. These observations led to a model wherein ZapA serves as a bundling protein that stabilizes the Z-ring by promoting lateral interactions and slowing polymer turnover. However, Caldas et al. recently demonstrated that ZapA does not affect FtsZ dynamics on SLBs in vitro, though it promotes organization of polymers into coherent superstructures [30]. These observations are consistent with findings in E. coli, C. crescentus, and B. subtilis that indicate that ZapA does not stabilize bundled FtsZ polymers in cells, but contributes to alignment of FtsZ clusters into a coherent, condensed ring [31–33].
The evolving model for the mechanism by which ZapA influences Z-ring assembly in cells calls into question the physiological relevance of bundling and polymer stability that can be induced by other FtsZ-binding proteins in vitro. Indeed, in C. crescentus the FtsZ binding protein FzlA can induce formation of hyperstable, helical bundles of FtsZ in vitro, but FzlA mutants that lack this activity are still able to support cell division, suggesting it may not be relevant in cells [34]. Similarly, EzrA and SepF, which can inhibit or promote FtsZ bundling in vitro, respectively, are dispensable in B. subtilis and do not affect FtsZ filament dynamics in cells [33].
Membrane anchors: mixed effects on FtsZ dynamics
Proteins that facilitate association of FtsZ with the membrane are important for Z-ring assembly and function, and they impact FtsZ dynamics and superstructure. The two best characterized of these are the broadly conserved actin homolog FtsA and the γ-proteobacterial protein ZipA. In E. coli, both FtsA and ZipA bind to the CTC of FtsZ and, although both are individually essential for cell division, either of the two is sufficient for Z-ring assembly [35]. ZipA comprises a short N-terminal transmembrane sequence, a proline- and glutamine-rich central domain, and a large cytosolic C-terminal region that binds the FtsZ CTC. Early experiments demonstrated that ZipA can induce bundling of FtsZ filaments in vitro [36]. In vitro experiments using SLBs have since observed that ZipA is sufficient to recruit FtsZ to a membrane where it ultimately forms straight bundles and asters [7,37] (Figure 2B). While these bundles are dynamic, the lack of individual clusters under these conditions makes it unclear whether treadmilling occurs in FtsZ-ZipA structures. Lowering the ZipA concentration yielded rings that appeared to be dynamic in one study [38] and more static in another [39], but treadmilling was not documented in either case. As ZipA can bind to both monomers and polymers of FtsZ with comparable affinity [7,40], ZipA likely functions to increase the local concentration of FtsZ at the membrane to facilitate polymerization.
FtsA is a widely conserved actin homolog that interacts with downstream divisome proteins, is implicated in regulating constriction activation, and was shown to treadmill with FtsZ in B. subtilis [1,8]. Purified E. coli FtsZ forms co-assemblies with FtsA and exhibits treadmilling activity on SLBs (Figure 2A), with higher FtsA concentrations favoring short, highly dynamic FtsZ polymers [7]. Interestingly, FtsA stimulates dynamics of membrane-associated FtsZ assemblies without affecting the apparent GTPase rate [13]. FtsA overproduction results in a division defect in all species tested and dispersed Z-rings in E. coli [41] and C. crescentus [24], suggesting that overabundant FtsA destabilizes the Z-ring, an effect similar to the destabilization of SLB-associated filaments observed in vitro. Collectively, the above observations lead to the hypothesis that FtsA not only serves as a membrane anchor, but also destabilizes FtsZ polymers and facilitates treadmilling.
While FtsA has been reported in several studies to form polymers in vitro [42,43], the role of FtsA self-assembly remains poorly understood. FtsA binds and hydrolyzes ATP, which is required for its activation of FtsZ dynamics on SLBs [7]. An FtsA variant deficient in ATP hydrolysis fails to self-assemble in vitro and results in shorter cell length when produced with a GFP tag in E. coli [44], suggesting that monomers of FtsA promote cell division. Indeed, FtsA variants predicted to be deficient in self-interaction are able to suppress deletion of multiple divisome components in E. coli, including ZipA [45,46], and can partially suppress the toxic effects of deleting the CTL in C. crescentus [24]. These mutants have also been observed to facilitate bundling of FtsZ in vitro and suppress a bundling-defective FtsZ variant in vivo [47]. Overall, FtsA monomers appear to allow FtsZ lateral interactions in vitro, while FtsA polymers may destabilize FtsZ filaments and stimulate FtsZ dynamics on membranes.
While genetic evidence suggests distinct roles for monomeric versus polymeric FtsA, the presence of FtsA polymers in cells has been enigmatic. Structures proposed to be FtsA filaments parallel to FtsZ filaments were recently observed using cryo-electron tomography (cryoET) in both vegetatively growing and sporulating B. subtilis [48], suggesting that polymers may indeed be present in vivo in this organism. However, FtsA polymers have not been detected in wild-type cells of C. crescentus or E. coli by cryoET, although FtsZ polymers are readily observed [43,49,50]. The in vivo polymerization state of FtsA, and how it relates to the regulation of FtsZ dynamics and function, therefore remains an important outstanding question.
So what? The function of FtsZ dynamics in division
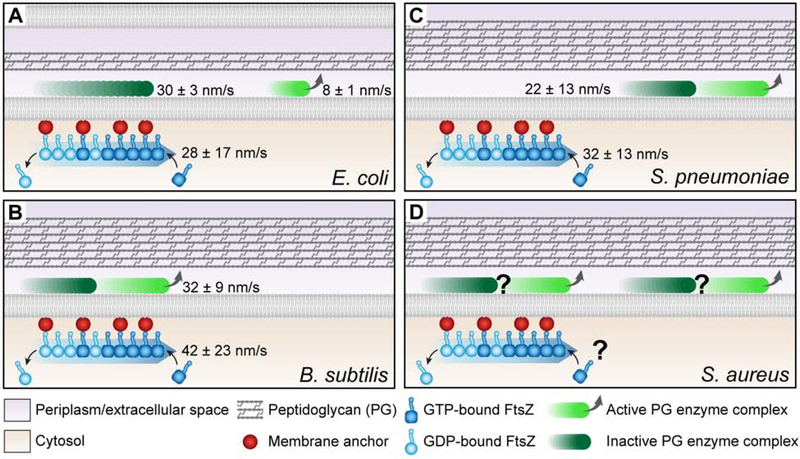
The study of FtsZ dynamics has yielded a plethora of insights into its function, as well as the function of the divisome as a whole. Dynamic FtsZ filaments are clearly required for division site selection, as spatial regulators of Z-ring assembly exploit the dynamic nature of FtsZ polymers. However, what is the relationship between FtsZ dynamics in the Z-ring and the PG enzymes that drive constriction (Figure 3 and Table 1)? The rate of FtsZ treadmilling is correlated with movement of PG remodeling complexes in E. coli and B. subtilis, but not in S. pneumoniae [8–10] (Figure 3A–C). The rate of treadmilling impacts the rate of PG metabolism and constriction in B. subtilis, suggesting FtsZ-mediated regulation of PG synthase activity [7]. Conversely, in E. coli, FtsZ dynamics primarily serve to distribute PG enzymes evenly about the circumference of the cell to maintain septum geometry [8]. Yang and colleagues recently discovered that in E. coli the PG synthase FtsW exists in two motile populations: an actively synthesizing, slow-moving population independent of FtsZ and an inactive, fast-moving population that is likely associated with FtsZ [51] (Figure 3A). This distribution model is supported by evidence of a diffusion and capture mechanism that uses FtsZ treadmilling to direct movement of the E. coli divisome regulators FtsN and FtsQ on SLBs [52] and PG synthesis enzymes in vivo [51,53].
Figure 3: The relationships between FtsZ treadmilling and PG enzyme complex movement and activity vary across organisms.
A. E. coli bears both fast, inactive (dark green) and slow, active (light green) moving PG enzyme populations, and FtsZ treadmilling rates correlate with the former. B. B. subtilis inactive and active PG enzyme complexes comprise a single population of moving molecules that correlates with and depends on FtsZ treadmilling. C. S. pneumoniae inactive and active PG enzyme complexes comprise a single population of moving molecules that does not correlate with or depend on FtsZ treadmilling. D. S. aureus has a moving population of PG enzyme complexes and treadmilling FtsZ, but the rates of each have yet to be determined.
Table 1:
Impacts of perturbing FtsZ GTPase activity, either due to mutation or treatment with PC190723 following Z-ring assembly
| Species | PG Enzyme Recruitment | Constriction | PG enzyme movement rate | Septum morphology |
|---|---|---|---|---|
| E. coli | Still occurs | Still occurs | Fast, inactive population correlates with FtsZ treadmilling rate; slow, active population does not | Aberrant morphology |
| B. subtilis | Still occurs | Still occurs if already initiated; rate correlates with FtsZ treadmilling rate | Correlates with FtsZ treadmilling rate | No reported change |
| S. pneumoniae | Still occurs | Fails for GTPase-deficient FtsZ; ??? for PC addition after start of constriction | Does not correlate with FtsZ treadmilling rate | No reported change |
| S. aureus | Still occurs | Still occurs if already initiated | ??? | No reported change |
Interestingly, FtsZ treadmilling is only required until constriction has initiated in B. subtilis and S. aureus, as treatment with PC190723 after that point does not prevent continued constriction [12,54]. A requirement for dynamic FtsZ only early in the constriction process is similar to the observation in fission yeast that actin is dispensable for completion of cytokinesis if the septum has progressed sufficiently, with septum assembly being sufficient at later stages [55]. Collectively, these findings suggest that FtsZ dynamics are broadly required for division site selection and Z-ring assembly, and that they contribute to efficiency and directionality of constriction through distribution and, in some organisms, regulation of PG synthesis complexes.
Conclusions and Outlook
Recent investigations into the mechanisms and functions of FtsZ dynamics have honed our understanding of the role of this crucial cell division mediator. Notably, treadmilling motion has been characterized in a number of bacterial species. FtsZ dynamics appear to be predominantly regulated by its intrinsic polymerization properties including GTPase activity and lateral interactions, but the membrane anchor FtsA also likely contributes to treadmilling and possibly other dynamic behaviors in vivo. In contrast, other binding partners may not function to regulate dynamics, but to promote assembly of a localized, condensed ring that can efficiently direct division. While FtsZ dynamics are broadly important for division site selection in diverse bacterial species, their function within an assembled and constricting Z-ring may be more species-specific, regulating protein distribution in some organisms and activity in others.
Of course, there are important questions that have yet to be addressed. Which, if any, dynamic behaviors beyond treadmilling are important for FtsZ’s function? Studies conducted until now have focused on treadmilling out of necessity, as it is relatively easy to observe on a macro scale both in vitro and in vivo. There may be other dynamic modes that complement treadmilling, as evidenced by the observation that Z-rings remain dynamic even after inhibition of treadmilling. Characterizing additional dynamic behaviors and connecting each – including treadmilling – to specific aspects of FtsZ function in cells will require the development of methods to disrupt them individually without affecting GTPase rate, as this drives global FtsZ dynamics. Also perplexing is whether treadmilling occurs through association and disassociation of FtsZ monomers, oligomers, polymers, or all of the above with clusters of FtsZ. Although addition and loss of monomers is intuitive, in vivo treadmilling rates are faster than would be predicted from in vitro GTPase rates [56] if monomers are solely involved. Finally, significant differences have been reported for the impact of FtsZ dynamics on cell wall synthesis in different species. Are these the result of divergent evolution, or is there a conserved mechanism of division that can be modulated depending on growth conditions and other factors? We have a long way to go in terms of understanding how Z-ring structure and dynamics are regulated, and by extension, the effects that regulation has on cell division.
Highlights.
Z-ring spatial regulators exploit intrinsic properties of FtsZ polymer dynamics
Treadmilling requires GTP hydrolysis, low levels of Mg2+, and membrane association
FtsA concentration affects FtsZ polymer dynamics and Z-ring architecture
FtsZ dynamics are crucial for cell wall synthase recruitment and distribution
In some species, FtsZ treadmilling regulates cell wall synthesis rate
Acknowledgments
We thank the members of the Goley lab and Joshua McCausland, Dr. Ryan McQuillen, and Dr. Xinxing Yang from the Xiao Lab for their feedback on this article.
Funding
This work was supported by the National Institutes of Health [grant numbers R01GM108640, R35GM136221, T32GM007445].
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mahone CR, Goley ED: Bacterial cell division at a glance. J Cell Sci 2020, 133:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schumacher MA: Bacterial Nucleoid Occlusion: Multiple Mechanisms for Preventing Chromosome Bisection During Cell Division. Subcell Biochem 2017, 84:267–298. [DOI] [PubMed] [Google Scholar]
- [3].Walker BE, Männik J, Männik J: Transient Membrane-Linked FtsZ Assemblies Precede Z-Ring Formation in Escherichia coli. Curr Biol 2020, 30:499–508.e6.* The authors combine a “mother machine” with time-lapse imaging to create a high-throughput method for observing E. coli FtsZ filaments throughout the cell over the entire cell cycle. They find that FtsZ forms short polymers that are transiently associated with the membrane along the length of the cell prior to Z-ring formation.
- [4].Stricker J, Maddox P, Salmon ED, Erickson HP: Rapid assembly dynamics of the Escherichia coli FtsZ-ring demonstrated by fluorescence recovery after photobleaching. Proc Natl Acad Sci 2002, 99:3171–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Anderson DE, Gueiros-Filho FJ, Erickson HP: Assembly Dynamics of FtsZ Rings in Bacillus subtilis and Escherichia coli and Effects of FtsZ-Regulating Proteins. J Bacteriol 2004, 186:5775–5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Romberg L, Levin PA: Assembly dynamics of the bacterial cell division protein FtsZ: poised at the edge of stability. Annu Rev Microbiol 2003, 57:125–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Loose M, Mitchison TJ: The bacterial cell division proteins FtsA and FtsZ selforganize into dynamic cytoskeletal patterns. Nat Cell Biol 2014, 16:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bisson-Filho AW, Hsu Y-P, Squyres GR, Kuru E, Wu F, Jukes C, Sun Y, Dekker C, Holden S, VanNieuwenhze MS, et al. : Treadmilling by FtsZ filaments drives peptidoglycan synthesis and bacterial cell division. Science 2017, 355:739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang X, Lyu Z, Miguel A, McQuillen R, Huang KC, Xiao J: GTPase activity-coupled treadmilling of the bacterial tubulin FtsZ organizes septal cell wall synthesis. Science (80- ) 2017, 355:744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Perez AJ, Cesbron Y, Shaw SL, Bazan Villicana J, Tsui H-CT, Boersma MJ, Ye ZA, Tovpeko Y, Dekker C, Holden S, et al. : Movement dynamics of divisome proteins and PBP2x:FtsW in cells of Streptococcus pneumoniae. Proc Natl Acad Sci U S A 2019, 116:3211–3220.** This study characterizes Streptococcus pneumoniae FtsZ treadmilling in both mature and nascent Z-rings and establishes that filament velocity is dependent on GTP hydrolysis, as in other organisms studied. The authors also observe movement of the peptidoglycan synthesis complex bPBP2x:FtsW and determine that it does not correlate with FtsZ treadmilling.
- [11].Li Y, Shao S, Xu X, Su X, Sun Y, Wei S: MapZ Forms a Stable Ring Structure That Acts As a Nanotrack for FtsZ Treadmilling in Streptococcus mutans. ACS Nano 2018, 12:6137–6146.* This study is the first to observe FtsZ treadmilling in S. mutans. The authors also demonstrate that while the Z-ring positioning protein MapZ is important for FtsZ localization, it does not affect treadmilling dynamics.
- [12].Monteiro JM, Pereira AR, Reichmann NT, Saraiva BM, Fernandes PB, Veiga H, Tavares AC, Santos M, Ferreira MT, Macário V, et al. : Peptidoglycan synthesis drives an FtsZ-treadmilling-independent step of cytokinesis. Nature 2018, 554:528–532.** The authors evaluate the localization of S. aureus components involved in peptidoglycan precursor synthesis and incorporation, revealing that MurJ recruitment to the septum, which is crucial for septal peptidoglycan synthesis, depends on DivIB, DivIC, and FtsL. They also observe FtsZ treadmilling and demonstrate that FtsZ dynamics are only required for constriction in the early steps of cytokinesis.
- [13].Söderström B, Badrutdinov A, Chan H, Skoglund U: Cell shape-independent FtsZ dynamics in synthetically remodeled bacterial cells. Nat Commun 2018, 9:4323.* The authors treat E. coli cells with A22 and measure FtsZ treadmilling rates and recovery after photobleaching to determine that FtsZ dynamics are independent of cell diameter and other changes in envelope geometry.
- [14].Adams DW, Wu LJ, Czaplewski LG, Errington J: Multiple effects of benzamide antibiotics on FtsZ function. Mol Microbiol 2011, 80:68–84. [DOI] [PubMed] [Google Scholar]
- [15].Wagstaff JM, Tsim M, Oliva MA, García-Sanchez A, Kureisaite-Ciziene D, Andreu JM, Löwe J: A Polymerization-Associated Structural Switch in FtsZ That Enables Treadmilling of Model Filaments. MBio 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Corbin LC, Erickson HP: A Unified Model for Treadmilling and Nucleation of Single-Stranded FtsZ Protofilaments. Biophys J 2020, doi: 10.1016/j.bpj.2020.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ramirez-Diaz DA, García-Soriano DA, Raso A, Mücksch J, Feingold M, Rivas G, Schwille P: Treadmilling analysis reveals new insights into dynamic FtsZ ring architecture. PLoS Biol 2018, 16:e2004845.** This study demonstrates that E. coli FtsZ can treadmill when associated with a supported lipid bilayer via a membrane targeting sequence. In addition, they demonstrate that treadmilling only occurs within a narrow range of free Mg2+ concentrations, balancing the ability to bind and hydrolyze GTP with increased propensity for filament bundling, which precludes treadmilling.
- [18].Sundararajan K, Vecchiarelli A, Mizuuchi K, Goley ED: Species- and C-terminal linker-dependent variations in the dynamic behavior of FtsZ on membranes in vitro. Mol Microbiol 2018, 110:47–63.** This study is the first to examine dynamics of FtsZ from an organism other than E. coli (specifically C. crescentus) on supported lipid bilayers and demonstrates that, unlike E. coli FtsZ, it assembles into small, dynamic clusters. The authors also show that C. crescentus FtsZ lacking its C-terminal linker forms mesh-like structures and exhibits slower turnover compared to wildtype.
- [19].Sundararajan K, Miguel A, Desmarais SM, Meier EL, Casey Huang K, Goley ED: The bacterial tubulin FtsZ requires its intrinsically disordered linker to direct robust cell wall construction. Nat Commun 2015, 6:7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sundararajan K, Goley ED: The intrinsically disordered C-terminal linker of FtsZ regulates protofilament dynamics and superstructure in vitro. J Biol Chem 2017, 292:20509–20527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cohan MC, Eddelbuettel AMP, Levin PA, Pappu R V.: Dissecting the Functional Contributions of the Intrinsically Disordered C-terminal Tail of Bacillus subtilis FtsZ. J Mol Biol 2020, 432:3205–3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Buske PJ, Levin PA: A flexible C-terminal linker is required for proper FtsZ assembly in vitro and cytokinetic ring formation in vivo. Mol Microbiol 2013, 89:249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gardner KAJA, Moore DA, Erickson HP: The C-terminal linker of Escherichia coli FtsZ functions as an intrinsically disordered peptide. Mol Microbiol 2013, 89:264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Barrows JM, Sundararajan K, Bhargava A, Goley ED: FtsA Regulates Z-Ring Morphology and Cell Wall Metabolism in an FtsZ C-Terminal Linker-Dependent Manner in Caulobacter crescentus. J Bacteriol 2020, 202:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Buske PJ, Levin PA: Extreme C terminus of bacterial cytoskeletal protein FtsZ plays fundamental role in assembly independent of modulatory proteins. J Biol Chem 2012, 287:10945–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Huang K-H, Durand-Heredia J, Janakiraman A: FtsZ ring stability: of bundles, tubules, crosslinks, and curves. J Bacteriol 2013, 195:1859–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gueiros-Filho FJ, Losick R: A widely conserved bacterial cell division protein that promotes assembly of the tubulin-like protein FtsZ. Genes Dev 2002, 16:2544–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Low HH, Moncrieffe MC, Löwe J: The Crystal Structure of ZapA and its Modulation of FtsZ Polymerisation. J Mol Biol 2004, 341:839–852. [DOI] [PubMed] [Google Scholar]
- [29].Dajkovic A, Pichoff S, Lutkenhaus J, Wirtz D: Cross-linking FtsZ polymers into coherent Z rings. Mol Microbiol 2010, 78:651–68. [DOI] [PubMed] [Google Scholar]
- [30].Caldas P, López-Pelegrín M, Pearce DJG, Budanur NB, Brugués J, Loose M: Cooperative ordering of treadmilling filaments in cytoskeletal networks of FtsZ and its crosslinker ZapA. Nat Commun 2019, 10.** This study examines the effects of ZapA on FtsZ dynamics on supported lipid bilayers and determines that while ZapA stabilizes the architecture of FtsZ/FtsA filaments, it does not affect filament orientation or treadmilling velocity.
- [31].Buss J, Coltharp C, Huang T, Pohlmeyer C, Wang S-C, Hatem C, Xiao J: In vivo organization of the FtsZ-ring by ZapA and ZapB revealed by quantitative super-resolution microscopy. Mol Microbiol 2013, 89:1099–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Woldemeskel SA, McQuillen R, Hessel AM, Xiao J, Goley ED: A conserved coiled-coil protein pair focuses the cytokinetic Z-ring in Caulobacter crescentus. Mol Microbiol 2017, 105:721–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Squyres GR, Holmes MJ, Barger SR, Pennycook BR, Ryan J, Yan VT, Garner EC: Dynamics of bacterial cell division: Z ring condensation is essential for cytokinesis. bioRxiv 2020, doi: 10.1101/2020.06.30.180737.** This study combines observation of FtsZ filament tracking and subunit lifetimes to investigate the effects of the FtsZ binding proteins (ZBPs) EzrA, SepF, and ZapA in B. subtilis on Z-ring assembly and FtsZ dynamics. The authors conclude that while the ZBPs are necessary for complete Z-ring condensation, they do not affect FtsZ treadmilling dynamics or polymer length.
- [34].Lariviere PJ, Szwedziak P, Mahone CR, Löwe J, Goley ED: FzlA, an essential regulator of FtsZ filament curvature, controls constriction rate during Caulobacter division. Mol Microbiol 2018, 107:180–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hale CA, de Boer PA: Recruitment of ZipA to the septal ring of Escherichia coli is dependent on FtsZ and independent of FtsA. J Bacteriol 1999, 181:167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hale CA, Rhee AC, de Boer PAJ: ZipA-Induced Bundling of FtsZ Polymers Mediated by an Interaction between C-Terminal Domains. J Bacteriol 2000, 182:5153–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mateos-Gil P, Márquez I, López-Navajas P, Jiménez M, Vicente M, Mingorance J, Rivas G, Vélez M: FtsZ polymers bound to lipid bilayers through ZipA form dynamic two dimensional networks. Biochim Biophys Acta - Biomembr 2012, 1818:806–813. [DOI] [PubMed] [Google Scholar]
- [38].Krupka M, Sobrinos-Sanguino M, Jiménez M, Rivas G, Margolin W: Escherichia coli ZipA Organizes FtsZ Polymers into Dynamic Ring-Like Protofilament Structures. MBio 2018, 9:1–15.* This study examines the effects of Zaps, FtsA, and ZipA on FtsZ dynamics in E. coli and finds that excess ZipA cannot complement loss of Zaps and that physiological levels of ZipA on a supported lipid bilayer supports the formation of dynamic FtsZ swirls, suggesting that ZipA affects FtsZ filament architecture without altering dynamics.
- [39].García-Soriano DA, Heermann T, Raso A, Rivas G, Schwille P: The speed of FtsZ treadmilling is tightly regulated by membrane binding. Sci Rep 2020, 10:10447.* This study reiterates the requirements of membrane binding and GTP hydrolysis for E. coli FtsZ to form dynamic structures on supported lipid bilayers and observes that different structures arise when different membrane targeting sequences are used. They also observe that physiological levels of ZipA promote formation of FtsZ rings, but note that these are not dynamic, in contrast to findings by Krupka et al.
- [40].Hernández-Rocamora VM, Reija B, García C, Natale P, Alfonso C, Minton AP, Zorrilla S, Rivas G, Vicente M: Dynamic Interaction of the Escherichia coli Cell Division ZipA and FtsZ Proteins Evidenced in Nanodiscs. J Biol Chem 2012, 287:30097–30104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ortiz C, Casanova M, Palacios P, Vicente M: The hypermorph FtsA* protein has an in vivo role in relieving the Escherichia coli proto-ring block caused by excess ZapC+. PLoS One 2017, 12:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Szwedziak P, Wang Q, Freund SMV, Löwe J: FtsA forms actin-like protofilaments. EMBO J 2012, 31:2249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Szwedziak P, Wang Q, Bharat TAM, Tsim M, Löwe J: Architecture of the ring formed by the tubulin homologue FtsZ in bacterial cell division. Elife 2014, 3:e04601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Conti J, Viola MG, Camberg JL: FtsA reshapes membrane architecture and remodels the Z-ring in Escherichia coli. Mol Microbiol 2018, 107:558–576.* This study establishes that association with phospholipids stimulates ATP hydrolysis by FtsA from E. coli and shows that an ATPase-deficient FtsA variant produced in E. coli results in shorter cell length. They also demonstrate that recruitment of polymerized FtsZ to membranes by FtsA is enhanced by addition of ATP, suggesting that FtsA nucleotide hydrolysis, and thus perhaps polymerization, is important for regulating this process.
- [45].Geissler B, Elraheb D, Margolin W: A gain-of-function mutation in ftsA bypasses the requirement for the essential cell division gene zipA in Escherichia coli. Proc Natl Acad Sci U S A 2003, 100:4197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Pichoff S, Shen B, Sullivan B, Lutkenhaus J: FtsA mutants impaired for self-interaction bypass ZipA suggesting a model in which FtsA’s self-interaction competes with its ability to recruit downstream division proteins. Mol Microbiol 2012, 83:151–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Schoenemann KM, Krupka M, Rowlett VW, Distelhorst SL, Hu B, Margolin W: Gain-of-function variants of FtsA form diverse oligomeric structures on lipids and enhance FtsZ protofilament bundling. Mol Microbiol 2018, 109:676–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Khanna K, López-Garrido J, Sugie J, Pogliano K, Villa E: Asymmetric localization of the cell division machinery during Bacillus subtilis sporulation. bioRxiv 2020, doi: 10.1101/2020.07.22.216184.* The authors use cryo-electron tomography to observe FtsZ and FtsA filaments proximal to the membrane in dividing B. subtilis, both during vegetative growth and sporulation. Their observations suggest that the position of FtsA/FtsZ filaments relative to the invaginating septum is differentially regulated depending on B. subtilis’ growth mode.
- [49].Li Z, Trimble MJ, Brun YV, Jensen GJ: The structure of FtsZ filaments in vivo suggests a force-generating role in cell division. EMBO J 2007, 26:4694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yao Q, Jewett AI, Chang Y-W, Oikonomou CM, Beeby M, Iancu CV, Briegel A, Ghosal D, Jensen GJ: Short FtsZ filaments can drive asymmetric cell envelope constriction at the onset of bacterial cytokinesis. EMBO J 2017, 36:1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yang X, McQuillen R, Lyu Z, Phillips-Mason P, De La Cruz A, McCausland JW, Liang H, Demeester KE, Grimes CL, de Boer PAJ, et al. : FtsW exhibits distinct processive movements driven by either septal cell wall synthesis or FtsZ treadmilling in E. coli. bioRxiv 2019, doi: 10.1101/850073.** This study identifies FtsW as the only essential septal monofunctional peptidoglycan glycosyltransferase in E. coli and employs single-molecule tracking to discover that FtsW moves in two populations - active, slow-moving and inactive, fast-moving - and that only movement of the latter correlates with FtsZ treadmilling rates.
- [52].Baranova N, Radler P, Hernández-Rocamora VM, Alfonso C, López-Pelegrín M, Rivas G, Vollmer W, Loose M: Diffusion and capture permits dynamic coupling between treadmilling FtsZ filaments and cell division proteins. Nat Microbiol 2020, 5:407–417.** This study employs lipid-bound cytosolic fragments of the divisome proteins FtsN and FtsQ from E. coli to show that they co-migrate with treadmilling FtsZ-FtsA filaments on a supported lipid bilayer. However, although they move together collectively, the authors observe that individual FtsN/FtsQ peptides exhibit random diffusion and transient confinement, indicating the presence of a diffusion and capture mechanism for movement about the Z-ring.
- [53].McCausland JW, Yang X, Squyres GR, Lyu Z, Bruce KE, Lamanna MM, Soderstrom B, Garner EC, Winkler ME, Xiao J, et al. : Treadmilling FtsZ polymers drive the directional movement of sPG-synthesis enzymes via a Brownian ratchet mechanism. bioRxiv 2019, doi: 10.1101/857813.*This study combines simulations of FtsZ and peptidoglycan synthesis enzyme movement with observations in vertically-immobilized E. coli to propose a Brownian ratchet model for distribution of these enzymes about the division site via FtsZ treadmilling.
- [54].Whitley KD, Jukes C, Tregidgo N, Karinou E, Almada P, Henriques R, Dekker C, Holden S: FtsZ treadmilling is essential for Z-ring condensation and septal constriction initiation in bacterial cell division. bioRxiv 2020, doi: 10.1101/2020.07.01.182006.* This study employs observation of vertically-immobilized B. subtilis to determine that the speed distribution of FtsZ filaments changes as Z-rings mature and begin constriction. They also use an inhibitor of FtsZ activity to show that FtsZ treadmilling is only essential for the distribution of peptidoglycan synthetic enzymes until their arrival at the septum.
- [55].Proctor SA, Minc N, Boudaoud A, Chang F: Contributions of turgor pressure, the contractile ring, and septum assembly to forces in cytokinesis in fission yeast. Curr Biol 2012, 22:1601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Romberg L, Mitchison TJ: Rate-limiting guanosine 5’-triphosphate hydrolysis during nucleotide turnover by FtsZ, a prokaryotic tubulin homologue involved in bacterial cell division. Biochemistry 2004, 43:282–8. [DOI] [PubMed] [Google Scholar]