Abstract
Background:
The effects of the docosahexaenoic acid (DHA) on cardiovascular disease are controversial and a mechanistic understanding of how this ω-3 polyunsaturated fatty (ω-3 PUFA) regulates platelet reactivity and the subsequent risk of a thrombotic event is warranted. In platelets, DHA is oxidized by 12-lipoxygenase (12-LOX) producing the oxidized lipids (oxylipins) 11-HDHA and 14-HDHA. We hypothesized that 12-LOX DHA-oxylipins may be involved in the beneficial effects observed in dietary supplemental treatment with ω-3 PUFAs or DHA itself.
Objectives:
To determine the effects of DHA, 11-HDHA and 14-HDHA on platelet function and thrombus formation, and to elucidate the mechanism by which these ω-3 PUFAs regulate platelet activation.
Methods and results:
DHA, 11-HDHA and 14-HDHA attenuated collagen-induced human platelet aggregation, but only the oxylipins inhibited αIIbβ3 activation and decreased α-granule secretion. Furthermore, treatment of whole blood with DHA and its oxylipins impaired platelet adhesion and accumulation to a collagen-coated surface. Interestingly, thrombus formation was only diminished in mice treated with 11-HDHA or 14-HDHA, and mouse platelet activation was inhibited following acute treatment with these oxylipins or chronic treatment with DHA, suggesting that under physiologic conditions, the effects of DHA are mediated through its oxylipins. Finally, the protective mechanism of DHA oxylipins was shown to be mediated via activation of protein kinase A.
Conclusions:
This study provides the first mechanistic evidence of how DHA and its 12-LOX oxylipins inhibit platelet activity and thrombus formation. These findings support the beneficial effects of DHA as therapeutic intervention in atherothrombotic diseases.
Keywords: DHA, 12-lipoxygenase, platelet, thrombosis, ω-3 PUFAs
Introduction
Long-chain polyunsaturated omega-3 fatty acids (ω-3 PUFAs) have been widely recommended based on evidence that supplementation with ω-3 PUFAs enhances cardio protection in patients at cardiovascular risk [1-4]. Since 2002, the American Heart Association (AHA) has recommended an increase in dietary ω-3 intake plus dietary supplements for triglyceride-lowering treatment [5], and more recently, the AHA has extended the recommendation of ω-3 PUFA supplementation to patients with prevalent coronary heart disease [6]. ω-3 PUFA supplements include fish oil, the primary source of nonprescription ω-3 supplements, and pharmaceutical preparations such as ω-3 ethyl esters [6, 7]. All these supplements provide high levels of eicosapentaenoic (EPA) and docosahexaenoic (DHA) fatty acids [8], long-chain forms of ω-3 PUFAs. Putative mechanisms proposed for the cardio protection include regulation of lipid levels, mainly a triacylglycerol-lowering effect [8, 9], reduction of blood pressure [10, 11], reduction of procoagulant activity in the blood [12, 13], prevention of endothelial dysfunction [14, 15], inhibition of platelet aggregation [16] and adhesion [17, 18], reduction of thromboxane A2 (TxA2) formation [19] and attenuation of thrombus formation [19-21].
Several studies have attributed the beneficial effects to EPA [22-27] and recently, the REDUCE-IT trial demonstrated that supplementation with synthetic EPA (icosapent ethyl) decreases the number of cardiovascular events and deaths in individuals at increased risk [28-30]. Regarding DHA, to date, no clinical trials investigating the effects of this fatty acid on individuals at risk of a cardiovascular event have been reported. Furthermore, although clinical studies have suggested that DHA may attenuate systemic inflammation by reducing inflammatory mediators [31], reducing blood pressure in hypertensive individuals [10], and decreasing triglyceride levels in the blood [9, 31], the overall findings are controversial with some reports suggesting beneficial properties of DHA while others report either no effect or detrimental pathological effects with administration of DHA [32-34]. A mechanistic understanding of how DHA regulates platelet function is therefore warranted.
In platelets, oxygenases can produce bioactive metabolites through the metabolism of PUFAs [35]. DHA is a known substrate for the two major oxygenases in platelets, cyclooxygenase-1 (COX-1) [36] and 12-lipoxygenase (12-LOX) [37]. Although DHA has been reported to be a poor substrate for COX-1 [36, 38], 12-LOX readily oxygenates DHA, producing the bioactive oxylipins, 11-HpDHA (11S-hydroperoxydocosahexaenoic acid) and 14-HpDHA (14S-hydroperoxydocosahexaenoic acid), which are immediately reduced to 11-HDHA (11S-hydroxydocosahexaenoic acid) and 14-HDHA (14S-hydroxydocosahexaenoic acid) by glutathione peroxidases [35, 39-41]. In this study, we used purified DHA, 11-HDHA and 14-HDHA to investigate their effects on platelet function and thrombus formation and to elucidate the mechanism by which these ω-3 fatty acids regulate platelet reactivity. We demonstrate that DHA and its 12-LOX-derived oxylipins exert their antiplatelet effects through the attenuation of platelet aggregation, adhesion and accumulation. Interestingly, using an in vivo thrombosis model, we show that only treatment with DHA oxylipins, 11-HDHA and 14-HDHA, significantly attenuated thrombus formation. In support, we demonstrate that acute treatment with DHA oxylipins or chronic treatment with DHA inhibit platelet activation ex vivo, suggesting that under physiologic conditions, the antithrombotic effects of DHA are mediated through its bioactive metabolites. Finally, for the first time, we demonstrate that the activation of protein kinase A (PKA) is one of the mechanisms underlying the antiplatelet and antithrombotic effects of the DHA oxylipins, 11-HDHA and 14-HDHA.
Methods
Preparation of washed human platelets
All studies involving human subjects have been reviewed and approved by the University of Michigan Institutional Review Board. A written informed consent was obtained from self-reported healthy donors prior to the blood draws. Whole blood was collected via venipuncture into vacutainers containing sodium citrate (3.2%; Greiner Bio-One, Monroe, NC). Platelets were isolated via serial centrifugation. Whole blood was centrifuged at 200g for 10 minutes to isolate platelet-rich plasma (PRP). PRP was treated with acid citrate dextrose (2.5% sodium citrate tribasic, 1.5% citric acid, and 2.0% D-glucose) and apyrase (0.02 U/ml) and then centrifuged for 10 minutes at 2000g to pellet the platelets [42]. Platelets were resuspended in Tyrode’s buffer (10 mM N-2-hydroxyethylpiperazine-N9-2-ethanesulfonic acid, 12 mM sodium bicarbonate, 127 mM sodium chloride, 5mM potassium chloride, 0.5 mM monosodium phosphate, 1 mM magnesium chloride, and 5mM glucose) at 3x108 platelets/ml as determined by a complete blood cell counter (Hemavet 950FS; Drew Scientific, Miami Lakes, FL).
Experimental animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Michigan. The C57BL/6 wild-type (WT) control mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and housed in the research facility at the University of Michigan. Male and female mice in this study ranged in age between 8-12 weeks old. From mice treated with acute administration of DHA and oxylipins, whole blood was collected from the inferior vena cava while mice were anesthetized. Citrated whole blood was centrifuged at 200 g for 5 minutes to isolate PRP. PRP was adjusted to 3 x 108 platelets/ml with the use of autologous platelet poor plasma (PPP) to be used in flow cytometer study. From mice used for the aggregation study with DHA treatment in vitro (see Supplemental Data) or oral gavage mice, whole blood was collected and centrifuged, as described above, to isolate PRP. PRP was treated with acid citrate dextrose (2.5% sodium citrate tribasic, 1.5% citric acid, and 2.0% D-glucose) and apyrase (0.1 U/ml), and then centrifuged for 8 minutes at 2000g to pellet the platelets. Washed platelets were resuspended in Tyrode’s buffer at 3 x 108 platelets/ml to be used in flow cytometer and aggregation studies. Platelets were recalcified to a final concentration of 1mM with CaCl2 3 minutes before stimulation in aggregation studies.
Synthesis and Purification of Oxylipins
14-HpDHA and 11-HpDHA were synthesized in 200 mL of 25 mM HEPES buffer (pH 8.0), using h12-LOX. The absorbance increase at 234 nm was monitored until it reached completion and quenched with 0.4% (v/v) glacial acetic acid. For the reduction of 14-HpDHA and 11-HpDHA to 14-HDHA and 11-HDHA, trimethylphosphite was added in molar excess before extracting. The solution was extracted 3 times with 100 mL dichloromethane and evaporated to dryness. The docosanoid products were purified with a normal phase Phenomonex silica column (5 μm, 250 mm x 10 mm) and an isocratic mixture of 99% hexane, 1% isopropanol and 0.1% trifluoroacetic acid. The purity was checked by LC-MS/MS to be greater than 95%.
Dietary supplementation in mice
At 8 weeks, C57BL/6 wild-type mice (male and female) were given an oral gavage daily with vehicle control (polyethylene glycol 300) or DHA (50 mg/kg) (Nu-Chek Prep, Inc) for 4 weeks prior to blood collection and platelet preparation.
DHA and oxylipins acute administration in mice
At 10-12 weeks, C57BL/6 wild-type mice (male and female) received an intravenous injection with DHA, 11-HDHA or 14-HDHA (15 mg/kg respectively) or the equivalent volume of dimethyl sulfoxide (DMSO) (Fisher Scientific, Fair Lawn, NJ) (Control) dissolved in a formulation of 5% DMSO in sterile 0.9% sodium chloride (Baxter, Deerfield, IL) 10 minutes prior to the induction of thrombosis (male) or blood draw (male and female).
Platelet aggregation
Human washed platelets were incubated with DHA, 11-HDHA, 14-HDHA (0.5-10 μM respectively) or the equivalent volume of DMSO (Control) for 10 minutes. Following incubation, human platelets were stimulated with collagen (0.625-2 μg/ml) or thrombin (0.1-1 nM). For animal studies, washed platelets from oral gavage mice were stimulated with collagen (1 μg/ml) while washed platelets from mice used for aggregation study with DHA treatment in vitro (see Supplemental Data) were incubated with DHA or DMSO (Control) for 10 minutes prior stimulation with collagen (1 μg/ml). Aggregation was measured in a lumi-aggregometer (Model 700D; Chrono-log). Light transmission was monitored in real time for 10 minutes at 37°C under stirring conditions (1100 rpm).
Flow cytometry
Washed human platelets were treated with DHA, 11-HDHA, 14-HDHA (5-10 μM respectively) or the equivalent volume of DMSO (Control) and were incubated with a FITC-conjugated antibody specific for the active conformation of αIIbβ3, PAC-1 (BioLegend, San Diego, CA), and with a PE-conjugated CD62P antibody specific for P-selectin (BD Pharmingen, Franklin Lakes, NJ) expressed on the platelet surface. PRP from mice treated with acute administration of DHA and oxylipins or washed platelets from oral gavage mice were incubated with a FITC-labeled rat anti-mouse P-selectin (CD62P) monoclonal antibody (Emfret Analytics, Eibelstadt, Germany), and with a PE-labeled rat anti-mouse integrin αIIbβ3 (GPIIbIIIa, CD41/61) monoclonal antibody (Emfret Analytics, Eibelstadt, Germany). Platelets were stimulated by the addition of various concentrations of convulxin (12.5-100 ng/ml for human platelets and 25 ng/ml for mouse platelets) (purchased from Kenneth Clemetson, Theodor Kocher Institute, University of Berne, Bern, Switzerland). Samples were incubated at room temperature in the dark for 10 minutes, fixed with 2% paraformaldehyde and fluorescence intensity was measured via flow cytometry (Accuri C6, BD Biosciences).
Vasodilator-stimulated phospho-protein (VASP) phosphorylation
Washed human platelets were treated with DHA and its oxylipins (10 μM), iloprost (1 nM) (Cayman Chemicals, Ann Arbor, MI), 12S-hydoxyeicosatetraenoic (12(S)-HETrE) (25 μM) , prostacyclin (PGI2) (1 nM) (Sigma-Aldrich, St. Louis, MO) or DMSO (Control) for 1 and 10 minutes at 37°C. Following incubation, reactions were stopped by the addition of 5X Laemmli sample buffer (Tris 1.5 M, pH 6.8; 10% sodium dodecyl sulfate, 50% glycerol, 25% β-mercaptoethanol, 0.6% bromophenol blue). The platelet lysate was boiled for 10 minutes and samples were run on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Western blots were performed with phosphorylated serine 157 and total VASP antibodies (Enzolife Sciences, Farmingdale, NY).
Ex vivo microfluidic perfusion flow chamber
Microfluidic perfusion chamber slides (μ-slide VI 0.1, ibidi, Martinsried, Germany) were coated with 100 μg/ml collagen type I (Chrono-log, Havertown, PA) overnight at 4°C. Freshly drawn citrated whole blood was incubated with 5-10 μM of DHA, 11-HDHA, or 14-HDHA, or DMSO (Control) and fluorescently labeled by incubating with 2μM of 3,3’-dihexyloxacarbocyanine iodide (DiOC6) (Thermo Fischer Scientific, Waltham, MA) for 10 minutes at 37°C. Stained whole blood was recalcified with 5 mM CaCl2 and immediately perfused at arterial shear (1800 seconds−1) through a coated microfluidic slide heated to 37°C using a syringe pump (Harvard Apparatus, Holliston, MA). Platelet adhesion and accumulation were recorded in real time for 4 minutes under an inverted fluorescent microscope (Zeiss Axio Observer Z1 Marianas; 40X objective). Platelet accumulation was quantified by mean fluorescence intensity using Slidebook 6.0 (Intelligent Imaging Innovations).
Laser-induced cremaster arteriole thrombosis model
At 10-12 weeks, C57BL/6 wild-type mice (male) were anesthetized by intraperitoneal injection of ketamine/xylazine (100 mg/kg) and a tracheal tube was inserted under a dissecting microscope to facilitate breathing as described previously [43, 44]. Fluorescent labeling of circulating platelets and detection of fibrin in vivo were achieved by intravenous injection of anti-platelet (DyLight 488 GP1bβ antibody 0.1 μg/g; EMFRET Analytics) and anti-fibrin (Alexa Fluor 647 0.3 μg/g; a gift from Rodney Camire at Children’s Hospital of Philadelphia) via a jugular vein catheter. The cremaster muscle was surgically prepared and cremaster arteriole (30-50 μm diameter) blood flow was visualized under a 63X water immersion objective with a Zeiss Axio Examiner Z1 multi-channel fluorescent microscope with constant perfusion of preheated bicarbonate-buffered saline [45, 46]. Mice were intravenously treated with DHA, 11-HDHA, 14-HDHA (15 mg/kg, respectively) or with the equivalent volume of DMSO (Control) dissolved in a formulation of 5% DMSO in sterile 0.9% sodium chloride 10 minutes prior to the induction of thrombosis. Vascular injury was induced by a laser ablation system (Ablate! photoablation system; Intelligent Imaging Innovations, Denver, CO, USA) and images of thrombus formation were acquired in real-time using a high-speed sCMOS camera. Multiple independent thrombi were induced in the cremaster arterioles in each mouse and platelet accumulation and fibrin formation were analyzed for the change in fluorescent intensity over the course of thrombus formation using the Slidebook 6.0 (Intelligent Imaging Innovations) program.
Statistics.
Paired and unpaired two-tailed student t tests, one- and two-way analysis of variance (ANOVA) and two factor mixed-effects model were performed with Prism 8 (GraphPad Software) to analyze the data. Multiple statistical analyses were used in this study, and the statistical test used in each assay is reported in the figure legends. Data represent mean values ± SEM or values ± SD as described in the figure legends.
Results
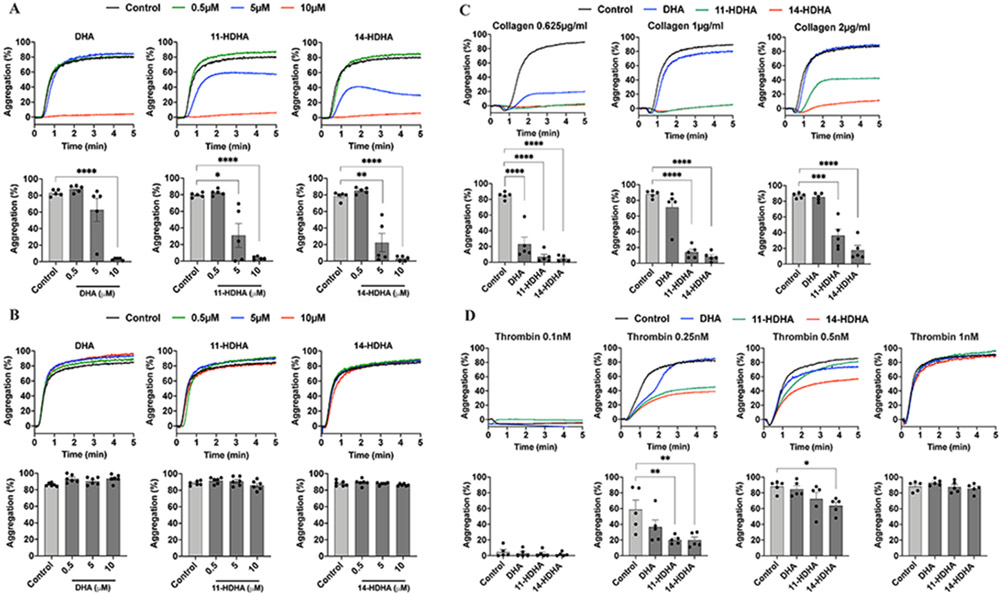
DHA and its 12-LOX-derived oxylipins regulate collagen-induced platelet aggregation.
DHA and its oxylipins, 11-HDHA and 14-HDHA, were directly assessed for their ability to regulate agonist-induced human platelet aggregation (Fig 1A, B). To determine if DHA and its oxylipins directly regulate platelet activity, washed human platelets were stimulated with either collagen (0.625 μg/ml) or thrombin (1 nM) in the presence of increasing concentrations of DHA and its oxylipins (0-10 μM). DHA and its oxylipins were observed to attenuate human platelet aggregation in response to EC80 concentration of collagen in a dose-dependent manner (Fig 1A). In contrast, DHA, 11-HDHA and 14-HDHA were observed to be less sensitive to thrombin stimulation of platelets at even the highest concentration of DHA or oxylipins tested (Fig 1B). In order to determine whether the observed inhibitory effect of DHA oxylipins on platelet aggregation is specifically a collagen-mediated response, washed human platelets were stimulated with increasing concentrations of collagen (0.625-2 μg/ml) or decreasing concentrations of thrombin (0.1-1 nM) in the presence of 10 μM DHA and its 12-LOX-derived oxylipins. Interestingly, 11-HDHA and 14-HDHA significantly attenuated human platelet aggregation in response to higher concentrations of collagen (Fig 1C) and low concentrations of thrombin 0.25nM (11-HDHA and 14-HDHA) and 0.5nM (14-HDHA) (Fig 1D).
Figure 1. DHA and its oxylipins regulate platelet aggregation:
The effects of DHA and its 12-LOX oxylipins, 11-HDHA and 14-HDHA, on platelet aggregation were assessed by incubating human platelets with DMSO (Control) or increasing concentrations (0.5, 5 and 10 μM) of DHA, 11-HDHA or 14-HDHA for 10 minutes prior to stimulation with A) collagen 0.625 μg/ml (n=5) and B) thrombin 1 nM (n=6). The effects of DHA and its 12-LOX oxylipins on platelet aggregation stimulated with increasing concentrations of collagen or decreasing concentrations of thrombin were assessed by incubating human platelets with DMSO (Control) or 10 μM of DHA, 11-HDHA or 14-HDHA for 10 minutes prior to stimulation with C) collagen 0.625-2 μg/ml (n=5) and D) thrombin 0.1-1 nM (n=5). Data represents mean ± SEM. A two-tailed, paired t test was performed. Asterisks denote statistical differences between Control and treated groups: *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
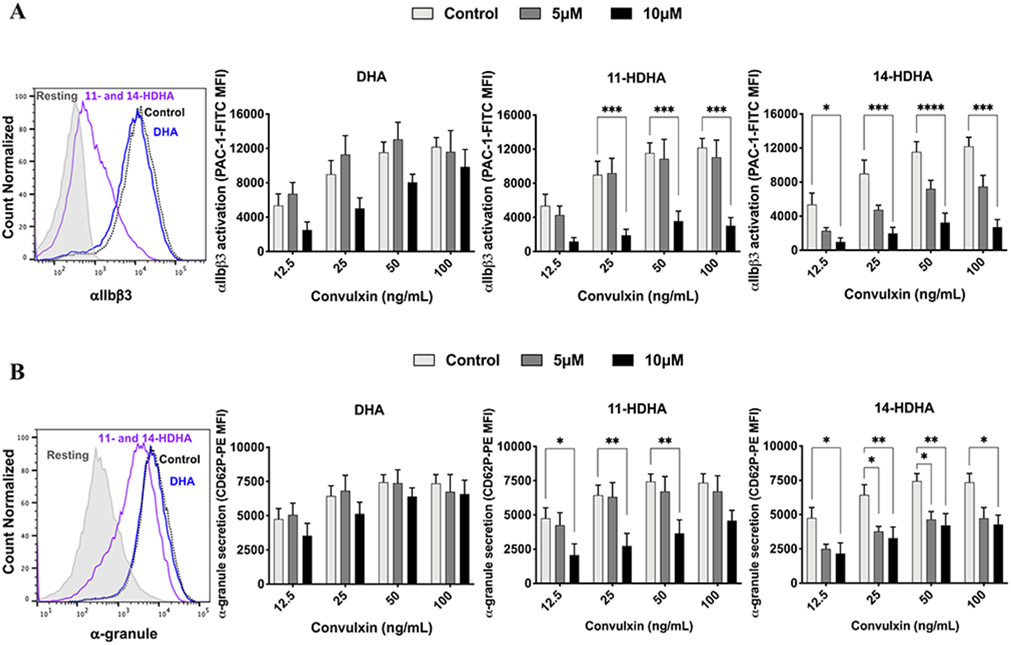
11-HDHA and 14-HDHA attenuate platelet aggregation through inhibition of integrin αIIbβ3 and α-granule secretion.
Since DHA, 11-HDHA and 14-HDHA were found to significantly attenuate platelet aggregation following stimulation with collagen (Fig 1A), the ability of these oxylipins to affect integrin activation was assessed. Activation of integrin αIIbβ3, an essential integrin that becomes active in platelet activation, was measured in response to activation of the collagen receptor GPVI in the presence or absence of DHA or 11-HDHA or 14-HDHA. Human platelets were treated with DHA or 11-HDHA or 14-HDHA at levels previously found to be achievable following ω-3 fatty acid supplementation (5 and 10 μM) [47, 48] for 10 minutes prior to stimulation of GPVI by increasing concentrations of convulxin, a direct activator of GPVI. Active αIIbβ3 was measured by flow cytometry following GPVI stimulation and, in contrast to aggregation experiments, DHA treatment had no observable inhibitory effect on αIIbβ3 activation. However, treatment with either 12-LOX oxylipin of DHA, 11-HDHA or 14-HDHA, significantly attenuated αIIbβ3 activation at both low and high concentrations of convulxin (Fig 2A). To determine whether α-granule secretion was also inhibited by treatment with DHA or its 12-LOX oxylipins, the surface expression of P-selectin, a marker for α-granule secretion, was assessed following stimulation with increasing concentrations of convulxin (Fig 2B). Similar to observations in Figure 2A, DHA treatment had no observable inhibitory effect at low or high concentrations of convulxin, while both 11-HDHA and 14-HDHA (5 and 10 μM) decreased α-granule secretion following stimulation with convulxin.
Figure 2. DHA oxylipin regulation of integrin activation and granule secretion:
11-HDHA and 14-HDHA inhibit αIIbβ3 activation and decrease α-granule secretion in human platelets upon stimulation with convulxin. Role of DHA and its oxylipins in A) αIIbβ3 activation (n=5) and B) α-granule secretion (n=5) were measured after treatment with DMSO (Control) or increasing concentrations (5 and 10 μM) of DHA, 11-HDHA or 14-HDHA for 10 minutes prior to stimulation with convulxin (12.5 – 100 ng/ml) for 10 minutes. Data represent mean ± SEM. Two-way statistical ANOVA with Tukey’s multiple comparisons test. Asterisks denote statistical differences between Control and treated groups: *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. MFI, mean fluorescence intensity.
DHA 12-LOX-derived oxylipin signals through PKA.
12(S)-HETrE, a 12-LOX-derived oxylipin of the ω-6 fatty acid dihomo-gamma-linolenic acid (DGLA), has been previously shown to inhibit platelet activation and thrombosis through activation of PKA [43, 49]. To determine if the antiplatelet effect of DHA oxylipins signal at least in part through a similar mechanism, VASP (S157) phosphorylation, the major substrate of PKA, was measured in human platelets treated with DMSO (Control), DHA, 11-HDHA, or 14-HDHA (10 μM, respectively), iloprost (1 nM), 12(S)-HETrE (25 μM) or PGI2 (1 nM) prior to lysis. In order to assess VASP phosphorylation, platelets were treated for 1 minute (Fig 3A) or 10 minutes (Fig 3B). As expected, treatment with iloprost, a synthetic prostacyclin analog used as a positive control, robustly induced phosphorylation of VASP. Furthermore, 12(S)-HETrE and PGI2, an endogenous prostaglandin of the prostacyclin receptor, increased VASP phosphorylation. Interestingly, while treatment with DHA had no effect, VASP phosphorylation was significantly enhanced following treatment with either 11-HDHA or 14-HDHA for both 1 and 10 minutes.
Figure 3. DHA oxylipin activation of cAMP and PKA:
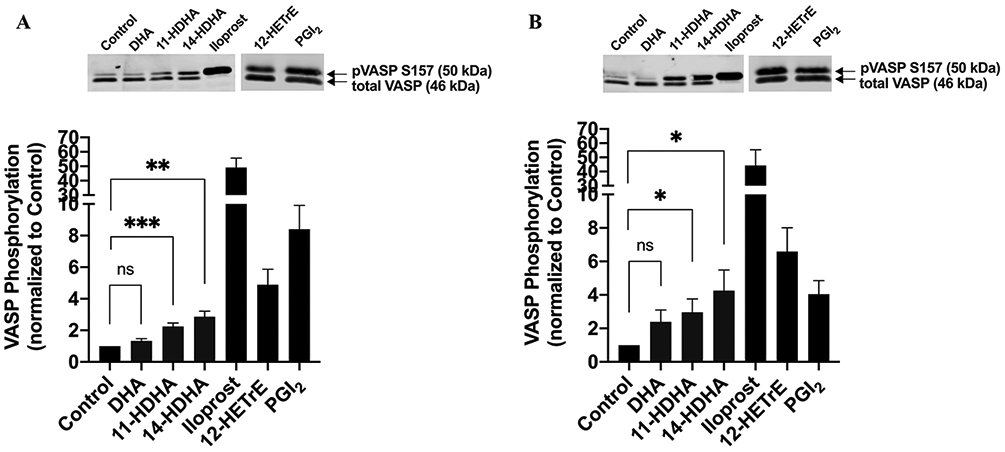
11-HDHA and 14-HDHA activate PKA-dependent signaling pathway in platelets. Human platelets were treated with DMSO (Control), 10 μM of DHA, 11-HDHA or 14-HDHA, 1 nM of Iloprost, 25 μM of 12(S)-HETrE or 1 nM of PGI2 for A) 1 minute (n=8 in all groups, n=6 in 12(S)-HETrE and PGI2) or B) 10 minutes (n=8 in DMSO, DHA, 11-HDHA and 14-HDHA, n=5 in Iloprost, n=6 in 12(S)-HETrE and PGI2), and VASP phosphorylation was assessed by western blotting. The level of phosphorylated VASP was normalized to the level of total VASP in each sample, and data were reported as fold change in VASP phosphorylation relative to the Control. A two-tailed, paired t test was performed and data represent mean ± SEM. Asterisks denote statistical differences between Control and treated groups (DHA, 11-HDHA and 14-HDHA): *P<0.05, **P<0.01, ***P<0.001. ns indicates not significant.
Platelet adhesion and accumulation under arterial shear is attenuated with DHA and its 12-LOX-derived oxylipins.
While Figures 1-3 support a role of DHA oxylipins in regulation of purified platelet activity through inhibition of collagen signaling, the role of DHA or its 12-LOX oxylipins in regulating initial platelet activation through collagen in whole blood under flow has yet to be determined. To identify if the observed inhibitory effects remain under physiological conditions, the ability of human platelets to bind to collagen was assessed ex vivo using a collagen-coated microfluidic chamber. Human whole blood was perfused under arterial flow conditions. Sodium-citrated and recalcified whole blood was treated with DMSO (Control) or varying concentrations of DHA, 11-HDHA, or 14-HDHA (5, 10 and 20 μM, respectively) 10 minutes prior to perfusion (Fig 4A-C). Whole blood treated with increasing concentrations of DHA (10 and 20μM) (Fig 4A), 11-HDHA (20μM) (Fig 4B) and 14-HDHA (10 and 20μM) (Fig 4C) was observed to have significantly attenuated platelet adherence and accumulation.
Figure 4. DHA and its oxylipins negatively regulate platelet adhesion:
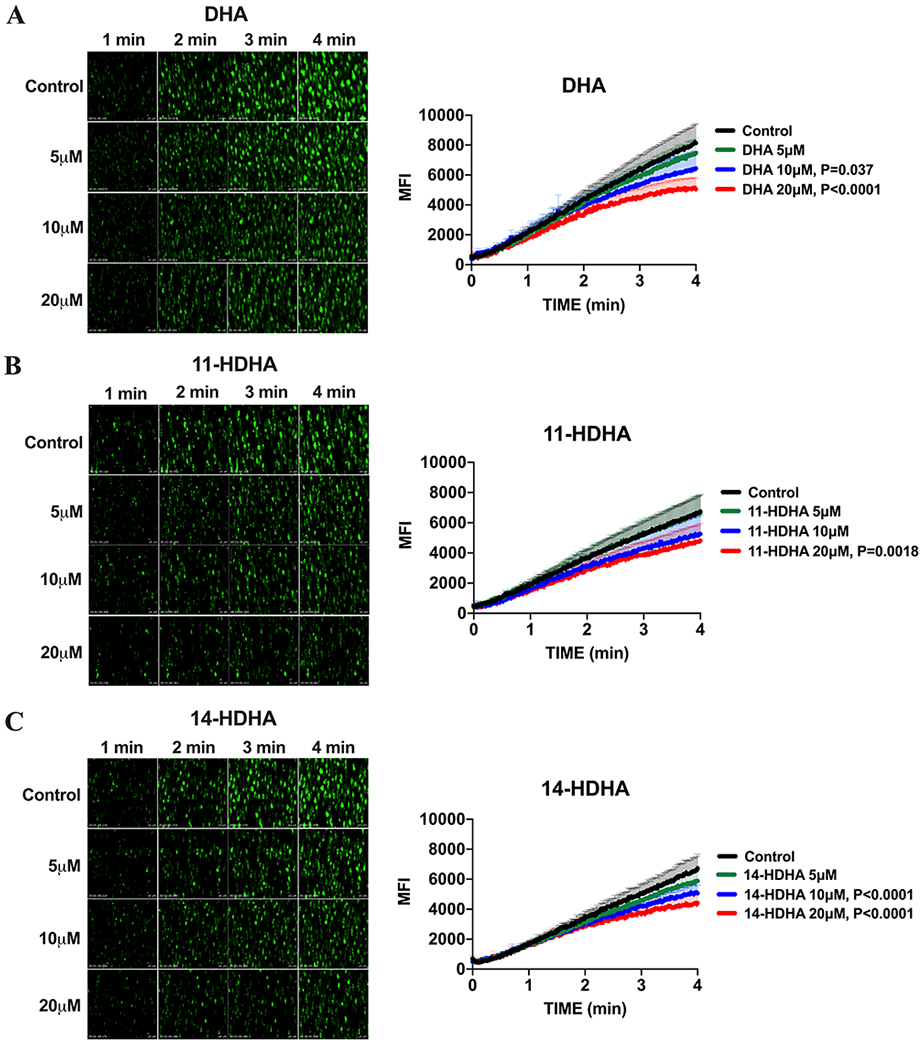
DHA and its 12-LOX oxylipins, 11-HDHA and 14-HDHA, inhibit platelet adhesion and accumulation on a collagen-coated surface under arterial shear flow conditions. Sodium-citrated whole blood was treated with DMSO (Control) or increasing concentrations (5, 10 and 20μM) of A) DHA (n=6), B) 11-HDHA (n=6) or C) 14-HDHA (n=6) for 10 minutes at 37°C prior to perfusion over a collagen-coated surface at arterial shear rate (1800/s) for 4 minutes. Data represent mean ± SEM. P value denote the statistical difference between Control and treated groups. Two-way statistical ANOVA with Dunnett’s multiple comparisons post-test was performed. MFI indicates mean fluorescence intensity.
Acute administration of 11-HDHA and 14-HDHA attenuated thrombus formation in vivo through inhibition of integrin αIIbβ3 activation and α-granule secretion.
To determine if the effects of DHA and its 12-LOX-derived oxylipins on thrombus formation and growth persist under in vivo conditions, platelet accumulation and fibrin formation in growing thrombi were measured in the mouse following laser-induced cremaster arteriole thrombosis. Dynamics of platelet accumulation and fibrin formation within the thrombus at the site of injury significantly differed in animals treated with 11-HDHA and 14-HDHA. While acute administration of DHA in mice had no effect on thrombus formation, thrombus growth was significantly attenuated in mice treated with 11-HDHA and 14-HDHA (Fig 5B; supplemental Videos 3 and 4), as decreased platelet accumulation led to smaller thrombi in response to vascular injury when compared to control. In addition, onset of clot formation was delayed in mice treated with 14-HDHA. Dynamics of fibrin formation showed no difference following treatment with either DHA or its 12-LOX oxylipins (Fig 5C). The anti-thrombotic effect observed in the in vivo thrombosis model was further confirmed by assessment of integrin αIIbβ3 activation and P-selectin surface expression on platelets isolated from mice intravenously treated with DHA, 11-HDHA or 14-HDHA (15mg/kg respectively). We have demonstrated that both 11-HDHA and 14-HDHA significantly attenuated αIIbβ3 activation (Fig 5D) and α-granule secretion (Fig 5E) following stimulation with convulxin. Additionally, DHA was observed to have a small but significant attenuation of αIIbβ3 activation and α-granule secretion.
Figure 5. DHA and its oxylipins attenuate in vivo platelet activation and thrombus formation:
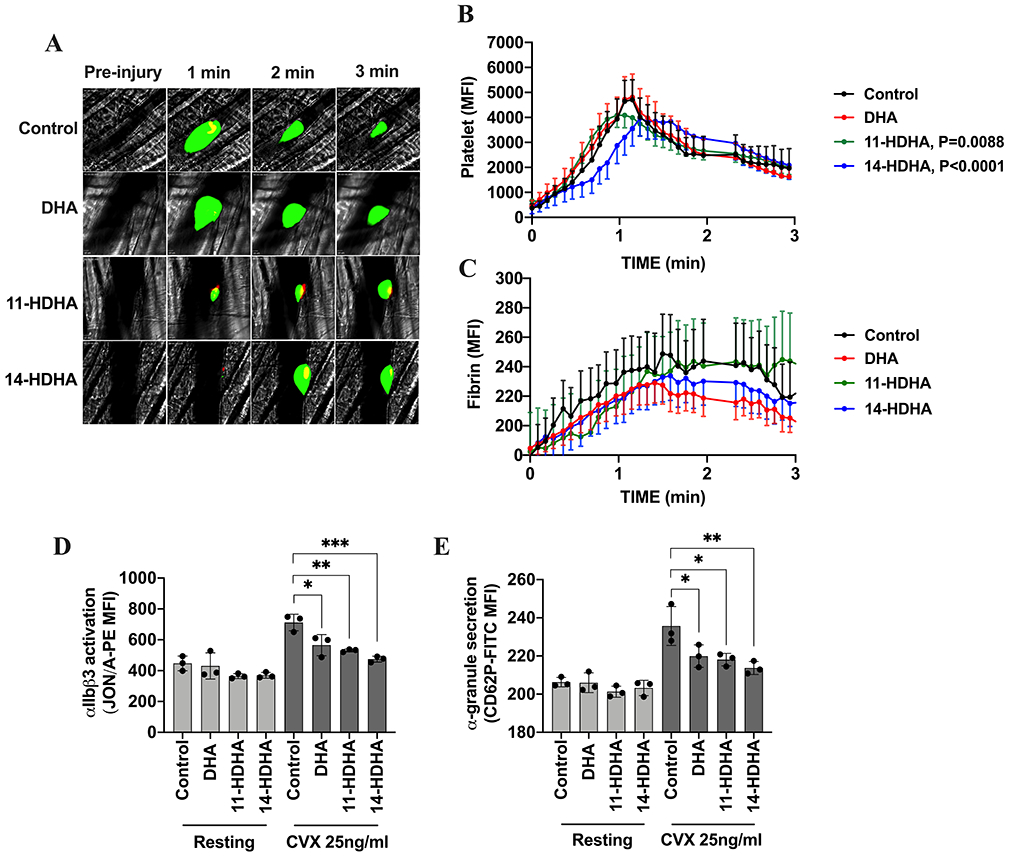
Acute treatment with 11-HDHA and 14-HDHA attenuates thrombus formation in thrombosis model. Representative images (A) of platelet accumulation (green) and fibrin (red) in growing thrombi in cremaster arterioles in mice Control treated with vehicle (DMSO) (n=38) and mice treated with 15mg/kg of DHA (n=27), 11-HDHA (n=36) and 14-HDHA (n=33), respectively (3 mice per group, 8-12 thrombi per mouse). Time after vascular injury are indicated above. Dynamics of platelet accumulation (B) and fibrin formation (C) in thrombi were analyzed by change in fluorescent intensity. Acute treatment with 15mg/kg of DHA, 11-HDHA or 14-HDHA inhibited integrin αIIbβ3 activation and decreased α-granule secretion ex vivo. PRP from treated mice was stimulated with convulxin (25 ng/ml) and D) activation of integrin αIIbβ3 (n=3) and E) α-granule secretion (n=3) was assessed by flow cytometer. Data represent mean ± SEM for in vivo and mean ± SD for ex vivo. P value or asterisks denote the statistical difference between Control and treated groups: *P<0.05, **P<0.01, ***P<0.001. Two factor mixed-effects model analysis was performed for in vivo and one-way ANOVA with Tukey’s multiple comparisons post-test was performed for ex vivo. MFI indicates mean fluorescence intensity, CVX indicates convulxin.
DHA dietary supplementation attenuates collagen-induced platelet aggregation through inhibition of integrin αIIbβ3 activation and α-granule secretion.
To determine whether DHA dietary supplementation regulates platelet function, washed platelets from mice supplemented orally daily with 50 mg/kg of DHA were stimulated with collagen (1 μg/ml) and platelet aggregation was assessed. Aggregation was significantly attenuated in platelets from mice that received DHA (Fig 6A). Accordingly, platelets from treated mice showed significant attenuation of integrin αIIbβ3 activation (Fig 6B) and decrease in α-granule secretion (Fig 6C) following stimulation with convulxin.
Figure 6. Supplementation with DHA attenuates platelet function:
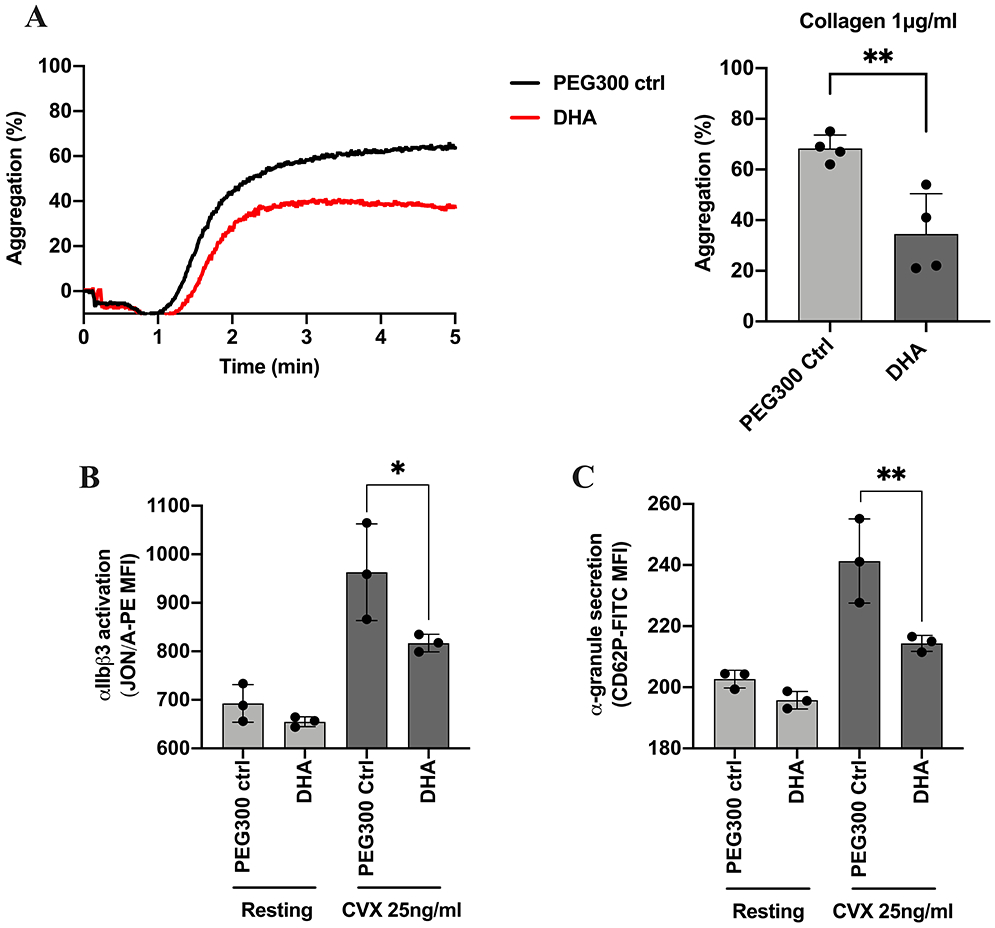
The effect of chronic dietary supplementation with DHA on platelet aggregation was assessed by stimulating washed platelets from mice that were given an oral gavage for 4 weeks with A) collagen 1 μg/ml (n=3). The effect of DHA supplementation on B) integrin αIIbβ3 activation (n=3) and C) α-granule secretion (n=3) was assessed by flow cytometer using washed platelets from treated mice stimulated with convulxin (25 ng/ml). Data represent mean ± SD. Asterisks denote the statistical difference: *P<0.05, **P<0.01. A two-tailed, unpaired t test was performed for aggregation and one-way ANOVA with Tukey’s multiple comparisons post-test was performed for flow cytometer. MFI indicates mean fluorescence intensity, CVX indicates convulxin.
Discussion
DHA is a naturally occurring ω-3 PUFA and its effects on cardiovascular disease have been extensively studied starting with the observation that a cardioprotective benefit may exist for people taking fish oil [3, 6]. Although studies have suggested beneficial effects of DHA in individuals at risk for an ischemic event [9, 31], the mechanism by which this fatty acid regulates platelet function and subsequent risk for a thrombotic event remains unclear. Based on the fact that DHA is considered a poor substrate for COX-1 [36, 38], we reasoned that DHA 12-LOX-derived oxylipins may be involved in the beneficial effects observed in the treatment with fish oil or DHA itself. In this study, we demonstrate that DHA and its bioactive oxylipins, 11-HDHA and 14-HDHA, play a role in the regulation of platelet function and thrombosis. 11-HDHA and 14-HDHA are shown here to attenuate agonist-induced human platelet activation, adhesion and thrombus formation through a pathway that involves activation of PKA-dependent signaling (Fig. 3), a critical mechanism of action for the inhibition of platelet activation [50, 51].
Using two endogenous agonists, collagen and thrombin, that are known to directly activate platelet granule secretion and TxA2 production, leading to platelet aggregation [52], human platelets treated with DHA and its 12-LOX-derived oxylipins were shown to be less sensitive to thrombin-induced aggregation (Fig 1B, D), whereas these ω-3 fatty acids were shown to primarily regulate collagen-mediated platelet activation (Fig 1A, C). This finding is consistent with a previous study that observed no effects on blood coagulation following DHA supplementation [53]. Accordingly, in the in vivo thrombosis model, a difference in fibrin clot formation was not observed following treatment with DHA or its 12-LOX-derived oxylipins, 11-HDHA and 14-HDHA (Fig 5C). In contrast to the subtle effect of oxylipin on thrombin-mediated activation, DHA and both 12-LOX oxylipins were observed to significantly inhibit collagen-induced platelet activation. Several studies have reported conflicting results regarding the anti-aggregatory effects of DHA on platelets. While some studies have indicated that DHA has no effect on platelet activation [33, 53], others [54, 55] have demonstrated that DHA regulates collagen-mediated platelet aggregation which is similar to the findings presented here. Interestingly, using mouse platelets, we demonstrated that treatment with DHA (10 μM) in vitro attenuated platelet aggregation (Fig S1). Ex vivo, platelet integrin αIIbβ3 activation was attenuated (Fig 5D) and α-granule secretion was decreased (Fig 5E) following acute administration of DHA (15 mg/kg). However, in human platelets, although lower concentrations (5μM) of both 11-HDHA and 14-HDHA significantly attenuated platelet aggregation, higher concentrations of DHA (10μM) were required to achieve a similar effect (Fig 1A), suggesting that, in humans, the anti-aggregatory effect of the 12-LOX DHA-oxylipins is more potent compared to its fatty acid precursor. Additionally, we demonstrated that the oxylipins from DHA, but not DHA itself, attenuated biochemical endpoints of human platelet activation including integrin αIIbβ3 activation and α-granule secretion (Fig 2). Consistent with the data presented here, a previous study has indicated that 14-HDHA had a more potent anti-aggregatory effect on platelet activation in response to U-46619 (TxA2 receptor agonist) compared to other hydroxylated fatty acids [56]. However, when the antiplatelet effect of 14-HDHA (the hydroxy form) is compared to 14-HpDHA (the hydroperoxy form), our study suggests that 14-HDHA is less potent than 14-HpDHA. Of particular interest, while 14-HpDHA fully inhibits collagen-mediated platelet aggregation at lower concentrations (1-5 μM) [40], we observed that only treatment with an increased concentration of 14-HDHA (10 μH) fully inhibited platelet activation (Fig 1A). The higher potency of the hydroperoxide was also observed in a previous study from our group using 12(S)-HPETrE and 12(S)-HETrE, oxylipin with anti-aggregatory effect signaling through activation of PKA [42].
Platelet adhesion is the first step in platelet activation and platelet clot formation following initial contact with the injured vessel wall [57, 58]. As previous studies have demonstrated inhibition of platelet adhesiveness following supplementation with fish oil [17, 18, 59], collagen-coated perfusion chambers were used here to determine the effects of DHA and its bioactive oxylipins in whole blood as they flow over collagen at arterial shear rates [44, 60]. In line with the observed in vitro inhibition of platelet activation, DHA, 11-HDHA and 14-HDHA were shown to impair platelet-surface and platelet-platelet interactions under arterial flow conditions (Fig 4). Although the in vitro and ex vivo data in this study support a direct regulation of platelet reactivity mediated by DHA itself as well as its 12-LOX oxylipins, the in vivo thrombosis data demonstrated that only the acute administration of 11-HDHA or 14-HDHA attenuated overall clot formation, with 14-HDHA also delaying the onset of the clot (Fig 5). Additionally, ex vivo platelet activation was significantly attenuated in mice acutely treated with DHA oxylipins (Fig 5D, E) and in mice that received chronic supplementation with DHA (Fig 6). This suggests that under physiologic conditions, it is primarily the 11-HDHA and 14-HDHA oxylipins that play a role in the regulation of thrombus formation. Furthermore, while the current study clearly shows direct effects of 12-LOX-derived oxylipins in regulation of platelet function, we must acknowledge the possibility that the in vivo regulation of clot formation and resolution is due in part to complex metabolism of DHA to pro-resolving mediators as has recently been demonstrated by others [61, 62]. Additionally, synthesis of pro-resolvins from DHA has been implicated in regulation of platelet activation [38, 63] and thrombus resolution in deep vein thrombosis in vivo [64]. Hence, it is possible based on the work with pro-resolving mediators that some of the observations presented here may represent not only 11-HDHA and 14-HDHA regulation of the platelet, but additionally complex metabolism of these oxylipins through a transcellular mechanism which may help to synergistically regulate the antithrombotic effects observed following treatment with DHA oxylipins.
Recently our group [65] has demonstrated that the basal level of DHA in mouse platelets is ~30 μM and in plasma is ~400 μM, whereas several studies with humans have reported that the basal level of DHA in plasma ranges from 70 to 230 μM [66-68]. Additionally, previous studies from our group have observed that 12-LOX-derived oxylipins can be formed from the fatty acid precursor in μM concentrations [43, 69]. Based on these findings, it is reasonable to suggest that the concentration of the fatty acid used in this study is in line with the physiological levels found in humans.
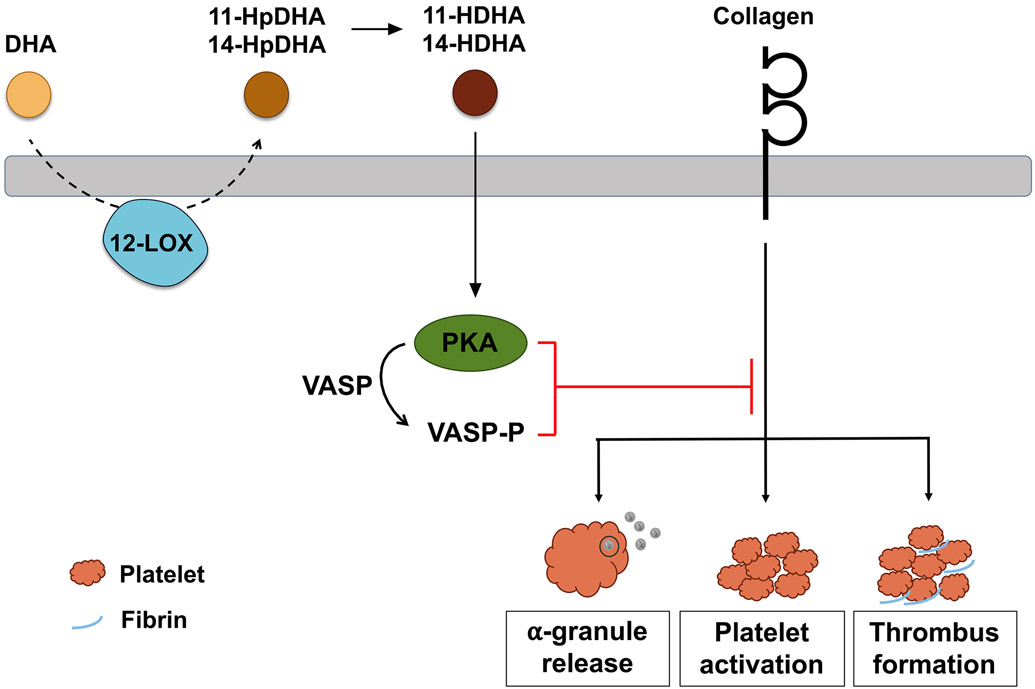
This study delineates for the first time the underlying mechanism by which DHA and its oxylipins regulate uncontrolled platelet activation and 11-HDHA and 14-HDHA attenuate occlusive thrombus formation (Fig 7), which commonly leads to myocardial infarction or stroke. Based on the fact that ω-3 fatty acids are widely recommended in clinics to lower triglyceride levels in the blood [8], these findings represent a granular understanding of how the DHA contained in ω-3 PUFA supplements elicits its beneficial effects as a therapeutic intervention in atherothrombotic diseases through inhibition of platelet activity and clot formation independent from its widely studied triglycerol-lowering effects in the blood.
Figure 7. Model of DHA and oxylipin regulation of platelet function and clot formation:
Schematic overview of the mechanism underlying the inhibitory effect of DHA bioactive oxylipins, 11-HDHA and 14-HDHA, on platelet activation and thrombus formation. In platelets, 12-LOX metabolizes free DHA into 11-HpDHA and 14-HpDHA, which are immediately reduced to the bioactive oxylipins, 11-HDHA and 14-HDHA. Both oxylipins activate protein kinase A (PKA), which phosphorylates a number of proteins, including vasodilator-stimulated phosphoprotein (VASP), leading to inhibition of α-granule release, platelet activation and thrombus formation in response to collagen.
Supplementary Material
Essentials.
Platelet 12-lipoxygenase (12-LOX) oxidizes docosahexaenoic acid (DHA) to form oxylipins.
We investigated how DHA and its oxylipins regulate platelet function and thrombus formation.
DHA 12-LOX oxylipins attenuated platelet activation and clot formation.
DHA 12-LOX oxylipins inhibited platelet reactivity in a GPVI-dependent manner via activation of protein kinase A.
Acknowledgement
We thank Amanda Prieur for recruiting subjects and performing blood draws.
Source of funding
This work was supported in part by research grants from the National Institutes of Health R01 GM105671, R35 GM131835 (M.H. and T.R.H.); R01 HL144660 (M.H.).
Footnotes
Disclosures
There are no conflicts of interest for any author related to the work reported in this manuscript.
CITED LITERATURE
- [1].INVESTIGATORS G-P. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- [2].Guasch-Ferre M, Babio N, Martinez-Gonzalez MA, Corella D, Ros E, Martin-Pelaez S, Estruch R, Aros F, Gomez-Gracia E, Fiol M, Santos-Lozano JM, Serra-Majem L, Bullo M, Toledo E, Barragan R, Fito M, Gea A, Salas-Salvado J. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am J Clin Nutr. 2015;102:1563–73. [DOI] [PubMed] [Google Scholar]
- [3].Petsini F, Fragopoulou E, Antonopoulou S. Fish consumption and cardiovascular disease related biomarkers: A review of clinical trials. Crit Rev Food Sci Nutr. 2019;59:2061–71. [DOI] [PubMed] [Google Scholar]
- [4].Manson JE, Bassuk SS, Cook NR, Lee IM, Mora S, Albert CM, Buring JE, Group VR. Vitamin D, Marine n-3 Fatty Acids, and Primary Prevention of Cardiovascular Disease Current Evidence. Circ Res. 2020;126:112–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kris-Etherton PM, Harris WS, Appel LJ, American Heart Association. Nutrition C. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. [DOI] [PubMed] [Google Scholar]
- [6].Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, Kris-Etherton PM, Jacobson TA, Engler MB, Alger HM, Appel LJ, Mozaffarian D, American Heart Association Nutrition Committee of the Council on L, Cardiometabolic H, Council on E, Prevention, Council on Cardiovascular Disease in the Y, Council on C, Stroke N, Council on Clinical C. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation. 2017;135:e867–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Calder P New evidence that omega-3 fatty acids have a role in primary prevention of coronary heart disease. Journal of Public Health and Emergency. 2017;1:35-. [Google Scholar]
- [8].Skulas-Ray AC, Wilson PWF, Harris WS, Brinton EA, Kris-Etherton PM, Richter CK, Jacobson TA, Engler MB, Miller M, Robinson JG, Blum CB, Rodriguez-Leyva D, de Ferranti SD, Welty FK, American Heart Association Council on Arteriosclerosis T, Vascular B, Council on L, Cardiometabolic H, Council on Cardiovascular Disease in the Y, Council on C, et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation. 2019;140:e673–e91. [DOI] [PubMed] [Google Scholar]
- [9].Mori TA, Burke V, Puddey IB, Watts GF, O’Neal DN, Best JD, Beilin LJ. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71:1085–94. [DOI] [PubMed] [Google Scholar]
- [10].Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993;88:523–33. [DOI] [PubMed] [Google Scholar]
- [11].Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–9. [DOI] [PubMed] [Google Scholar]
- [12].Andriamampandry M, Freund M, Wiesel ML, Rhinn S, Ravanat C, Cazenave JP, Leray C, Gachet C. Diets enriched in (n-3) fatty acids affect rat coagulation factors dependent on vitamin K. C R Acad Sci III. 1998;321:415–21. [DOI] [PubMed] [Google Scholar]
- [13].Larson MK, Tormoen GW, Weaver LJ, Luepke KJ, Patel IA, Hjelmen CE, Ensz NM, McComas LS, McCarty OJ. Exogenous modification of platelet membranes with the omega-3 fatty acids EPA and DHA reduces platelet procoagulant activity and thrombus formation. Am J Physiol Cell Physiol. 2013;304:C273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Das UN. COX-2 inhibitors and metabolism of essential fatty acids. Med Sci Monit. 2005;11:RA233–7. [PubMed] [Google Scholar]
- [15].Jung UJ, Torrejon C, Tighe AP, Deckelbaum RJ. n-3 Fatty acids and cardiovascular disease: mechanisms underlying beneficial effects. Am J Clin Nutr. 2008;87:2003S–9S. [DOI] [PubMed] [Google Scholar]
- [16].Larson MK, Shearer GC, Ashmore JH, Anderson-Daniels JM, Graslie EL, Tholen JT, Vogelaar JL, Korth AJ, Nareddy V, Sprehe M, Harris WS. Omega-3 fatty acids modulate collagen signaling in human platelets. Prostaglandins Leukot Essent Fatty Acids. 2011;84:93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li XL, Steiner M. Fish oil: a potent inhibitor of platelet adhesiveness. Blood. 1990;76:938–45. [PubMed] [Google Scholar]
- [18].Li XL, Steiner M. Dose response of dietary fish oil supplementations on platelet adhesion. Arterioscler Thromb. 1991;11:39–46. [DOI] [PubMed] [Google Scholar]
- [19].Umemura K, Toshima Y, Asai F, Nakashima M. Effect of dietary docosahexaenoic Acid supplementation on platelet function: studies in the rat femoral artery thrombosis model. Platelets. 1994;5:214–8. [DOI] [PubMed] [Google Scholar]
- [20].Andriamampandry MD, Leray C, Freund M, Cazenave J-P, Gachet C. Antithrombotic Effects of (n-3) Polyunsaturated Fatty Acids in Rat Models of Arterial and Venous Thrombosis. Thrombosis Research. 1999;93:9–16. [DOI] [PubMed] [Google Scholar]
- [21].Adili R, Voigt EM, Bormann JL, Foss KN, Hurley LJ, Meyer ES, Veldman AJ, Mast KA, West JL, Whiteheart SW, Holinstat M, Larson MK. In vivo modeling of docosahexaenoic acid and eicosapentaenoic acid-mediated inhibition of both platelet function and accumulation in arterial thrombi. Platelets. 2019;30:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8. [DOI] [PubMed] [Google Scholar]
- [23].Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, Soni PN. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol. 2011;108:682–90. [DOI] [PubMed] [Google Scholar]
- [24].Muhammad K, Morledge T, Sachar R, Zeldin A, Wolski K, Bhatt D. Treatment with w-3 fatty acids reduces serum C-reactive protein concentration. Clinical Lipidology. 2011;6:723–9. [Google Scholar]
- [25].Ballantyne CM, Bays HE, Kastelein JJ, Stein E, Isaacsohn JL, Braeckman RA, Soni PN. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. 2012;110:984–92. [DOI] [PubMed] [Google Scholar]
- [26].Nishio R, Shinke T, Otake H, Nakagawa M, Nagoshi R, Inoue T, Kozuki A, Hariki H, Osue T, Taniguchi Y, Iwasaki M, Hiranuma N, Konishi A, Kinutani H, Shite J, Hirata K. Stabilizing effect of combined eicosapentaenoic acid and statin therapy on coronary thin-cap fibroatheroma. Atherosclerosis. 2014;234:114–9. [DOI] [PubMed] [Google Scholar]
- [27].Nosaka K, Miyoshi T, Iwamoto M, Kajiya M, Okawa K, Tsukuda S, Yokohama F, Sogo M, Nishibe T, Matsuo N, Hirohata S, Ito H, Doi M. Early initiation of eicosapentaenoic acid and statin treatment is associated with better clinical outcomes than statin alone in patients with acute coronary syndromes: 1-year outcomes of a randomized controlled study. Int J Cardiol. 2017;228:173–9. [DOI] [PubMed] [Google Scholar]
- [28].Bhatt DL, Steg PG, Brinton EA, Jacobson TA, Miller M, Tardif JC, Ketchum SB, Doyle RT Jr., Murphy SA, Soni PN, Braeckman RA, Juliano RA, Ballantyne CM, Investigators R-I. Rationale and design of REDUCE-IT: Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial. Clin Cardiol. 2017;40:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr., Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM, Investigators R-I. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- [30].Bhatt DL, Miller M, Brinton EA, Jacobson TA, Steg PG, Ketchum SB, Doyle RT Jr., Juliano RA, Jiao L, Granowitz C, Tardif JC, Olshansky B, Chung MK, Gibson CM, Giugliano RP, Budoff MJ, Ballantyne CM, Investigators R-I. REDUCE-IT USA: Results From the 3146 Patients Randomized in the United States. Circulation. 2020;141:367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Allaire J, Couture P, Leclerc M, Charest A, Marin J, Lepine MC, Talbot D, Tchernof A, Lamarche B. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: the Comparing EPA to DHA (ComparED) Study. Am J Clin Nutr. 2016;104:280–7. [DOI] [PubMed] [Google Scholar]
- [32].von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The Effect of Dietary ω-3 Fatty Acids on Coronary Atherosclerosis. Annals of Internal Medicine. 1999;130:554–62. [DOI] [PubMed] [Google Scholar]
- [33].Cottin SC, Alsaleh A, Sanders TA, Hall WL. Lack of effect of supplementation with EPA or DHA on platelet-monocyte aggregates and vascular function in healthy men. Nutr Metab Cardiovasc Dis. 2016;26:743–51. [DOI] [PubMed] [Google Scholar]
- [34].Kuda O Bioactive metabolites of docosahexaenoic acid. Biochimie. 2017;136:12–20. [DOI] [PubMed] [Google Scholar]
- [35].Yeung J, Hawley M, Holinstat M. The expansive role of oxylipins on platelet biology. J Mol Med (Berl). 2017;95:575–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dong L, Zou H, Yuan C, Hong YH, Kuklev DV, Smith WL. Different Fatty Acids Compete with Arachidonic Acid for Binding to the Allosteric or Catalytic Subunits of Cyclooxygenases to Regulate Prostanoid Synthesis. J Biol Chem. 2016;291:4069–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lagarde M, Guichardant M, Bernoud-Hubac N, Calzada C, Vericel E. Oxygenation of polyunsaturated fatty acids and oxidative stress within blood platelets. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:651–6. [DOI] [PubMed] [Google Scholar]
- [38].Lagarde M, Liu M, Vericel E, Calzada C, Chen P, Driss F, Guichardant M. Docosahexaenoic acid, protectin synthesis: relevance against atherothrombogenesis. Proc Nutr Soc. 2014;73:186–9. [DOI] [PubMed] [Google Scholar]
- [39].Aveldano MI, Sprecher H. Synthesis of hydroxy fatty acids from 4, 7, 10, 13, 16, 19-[1–14C] docosahexaenoic acid by human platelets. J Biol Chem. 1983;258:9339–43. [PubMed] [Google Scholar]
- [40].Freedman C, Tran A, Tourdot BE, Kalyanaraman C, Perry S, Holinstat M, Jacobson MP, Holman TR. Biosynthesis of the Maresin Intermediate, 13S,14S-Epoxy-DHA, by Human 15-Lipoxygenase and 12-Lipoxygenase and Its Regulation through Negative Allosteric Modulators. Biochemistry. 2020;59:1832–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Perry SC, Kalyanaraman C, Tourdot BE, Conrad WS, Akinkugbe O, Freedman JC, Holinstat M, Jacobson MP, Holman TR. 15-Lipoxygenase-1 biosynthesis of 7S,14S-diHDHA implicates 15-Lipoxygenase-2 in biosynthesis of resolvin D5. J Lipid Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ikei KN, Yeung J, Apopa PL, Ceja J, Vesci J, Holman TR, Holinstat M. Investigations of human platelet-type 12-lipoxygenase: role of lipoxygenase products in platelet activation. J Lipid Res. 2012;53:2546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yeung J, Tourdot BE, Adili R, Green AR, Freedman CJ, Fernandez-Perez P, Yu J, Holman TR, Holinstat M. 12(S)-HETrE, a 12-Lipoxygenase Oxylipin of Dihomo-γ-Linolenic Acid, Inhibits Thrombosis via Gαs Signaling in Platelets. Arterioscler Thromb Vasc Biol. 2016;36:2068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Adili R, Tourdot BE, Mast K, Yeung J, Freedman JC, Green A, Luci DK, Jadhav A, Simeonov A, Maloney DJ, Holman TR, Holinstat M. First Selective 12-LOX Inhibitor, ML355, Impairs Thrombus Formation and Vessel Occlusion In Vivo With Minimal Effects on Hemostasis. Arterioscler Thromb Vasc Biol. 2017;37:1828–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Reheman A, Gross P, Yang H, Chen P, Allen D, Leytin V, Freedman J, Ni H. Vitronectin stabilizes thrombi and vessel occlusion but plays a dual role in platelet aggregation. J Thromb Haemost. 2005;3:875–83. [DOI] [PubMed] [Google Scholar]
- [46].Wang Y, Reheman A, Spring CM, Kalantari J, Marshall AH, Wolberg AS, Gross PL, Weitz JI, Rand ML, Mosher DF, Freedman J, Ni H. Plasma fibronectin supports hemostasis and regulates thrombosis. J Clin Invest. 2014;124:4281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Johansen O, Seljeflot I, Hostmark AT, Arnesen H. The effect of supplementation with omega-3 fatty acids on soluble markers of endothelial function in patients with coronary heart disease. Arterioscler Thromb Vasc Biol. 1999;19:1681–6. [DOI] [PubMed] [Google Scholar]
- [48].Plourde M, Chouinard-Watkins R, Rioux-Perreault C, Fortier M, Dang MT, Allard MJ, Tremblay-Mercier J, Zhang Y, Lawrence P, Vohl MC, Perron P, Lorrain D, Brenna JT, Cunnane SC. Kinetics of 13C-DHA before and during fish-oil supplementation in healthy older individuals. Am J Clin Nutr. 2014;100:105–12. [DOI] [PubMed] [Google Scholar]
- [49].Tourdot BE, Adili R, Isingizwe ZR, Ebrahem M, Freedman JC, Holman TR, Holinstat M. 12-HETrE inhibits platelet reactivity and thrombosis in part through the prostacyclin receptor. Blood Adv. 2017;1:1124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Smolenski A Novel roles of cAMP/cGMP-dependent signaling in platelets. J Thromb Haemost. 2012;10:167–76. [DOI] [PubMed] [Google Scholar]
- [51].Raslan Z, Aburima A, Naseem KM. The Spatiotemporal Regulation of cAMP Signaling in Blood Platelets-Old Friends and New Players. Front Pharmacol. 2015;6:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cattaneo M Light transmission aggregometry and ATP release for the diagnostic assessment of platelet function. Semin Thromb Hemost. 2009;35:158–67. [DOI] [PubMed] [Google Scholar]
- [53].Nelson GJ, Schmidt PC, Bartolini GL, Kelley DS, Kyle D. The effect of dietary docosahexaenoic acid on plasma lipoproteins and tissue fatty acid composition in humans. Lipids. 1997;32:1137–46. [DOI] [PubMed] [Google Scholar]
- [54].Woodman RJ, Mori TA, Burke V, Puddey IB, Barden A, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis. 2003;166:85–93. [DOI] [PubMed] [Google Scholar]
- [55].Phang M, Lincz LF, Garg ML. Eicosapentaenoic and docosahexaenoic acid supplementations reduce platelet aggregation and hemostatic markers differentially in men and women. J Nutr. 2013;143:457–63. [DOI] [PubMed] [Google Scholar]
- [56].Croset M, Sala A, Folco G, Lagarde M. Inhibition by lipoxygenase products of TXA2-like responses of platelets and vascular smooth muscle. 14-Hydroxy from 22:6n-3 is more potent than 12-HETE. Biochem Pharmacol. 1988;37:1275–80. [DOI] [PubMed] [Google Scholar]
- [57].Dopheide SM, Yap CL, Jackson SP. Dynamic aspects of platelet adhesion under flow. Clin Exp Pharmacol Physiol. 2001;28:355–63. [DOI] [PubMed] [Google Scholar]
- [58].Coenen DM, Mastenbroek TG, Cosemans J. Platelet interaction with activated endothelium: mechanistic insights from microfluidics. Blood. 2017;130:2819–28. [DOI] [PubMed] [Google Scholar]
- [59].Andrioli G, Carletto A, Guarini P, Galvani S, Biasi D, Bellavite P, Corrocher R. Differential effects of dietary supplementation with fish oil or soy lecithin on human platelet adhesion. Thromb Haemost. 1999;82:1522–7. [PubMed] [Google Scholar]
- [60].Tourdot BE, Stoveken H, Trumbo D, Yeung J, Kanthi Y, Edelstein LC, Bray PF, Tall GG, Holinstat M. Genetic Variant in Human PAR (Protease-Activated Receptor) 4 Enhances Thrombus Formation Resulting in Resistance to Antiplatelet Therapeutics. Arterioscler Thromb Vasc Biol. 2018;38:1632–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128:2657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med. 2009;206:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Chen P, Fenet B, Michaud S, Tomczyk N, Véricel E, Lagarde M, Guichardant M. Full characterization of PDX, a neuroprotectin/protectin D1 isomer, which inhibits blood platelet aggregation. FEBS Letters. 2009;583:3478–84. [DOI] [PubMed] [Google Scholar]
- [64].Cherpokova D, Jouvene CC, Libreros S, DeRoo EP, Chu L, de la Rosa X, Norris PC, Wagner DD, Serhan CN. Resolvin D4 attenuates the severity of pathological thrombosis in mice. Blood. 2019;134:1458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yeung J, Adili R, Yamaguchi A, Freedman CJ, Chen A, Shami R, Das A, Holman TR, Holinstat M. Omega-6 DPA and its 12-lipoxygenase-oxidized lipids regulate platelet reactivity in a nongenomic PPARα-dependent manner. Blood Adv. 2020;4:4522–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Harper CR, Edwards MJ, DeFilippis AP, Jacobson TA. Flaxseed oil increases the plasma concentrations of cardioprotective (n-3) fatty acids in humans. J Nutr. 2006;136:83–7. [DOI] [PubMed] [Google Scholar]
- [67].Pomponi M, Janiri L, La Torre G, Di Stasio E, Di Nicola M, Mazza M, Martinotti G, Bria P, Lippa S, Natili R, Pomponi MF. Plasma levels of n-3 fatty acids in bipolar patients: deficit restricted to DHA. J Psychiatr Res. 2013;47:337–42. [DOI] [PubMed] [Google Scholar]
- [68].Abdelmagid SA, Clarke SE, Nielsen DE, Badawi A, El-Sohemy A, Mutch DM, Ma DW. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS One. 2015;10:e0116195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Holinstat M, Boutaud O, Apopa PL, Vesci J, Bala M, Oates JA, Hamm HE. Protease-activated receptor signaling in platelets activates cytosolic phospholipase A2α differently for cyclooxygenase-1 and 12-lipoxygenase catalysis. Arterioscler Thromb Vasc Biol. 2011;31:435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.