Abstract
Nearly a century ago, studies by Hogben and others demonstrated that ovulation in female Xenopus laevis can be induced via injection of mammalian gonadotropins into the dorsal lymph sac, allowing for egg production throughout the year independent of the normal reproductive cycles. Hormonally induced females are capable of producing thousands of eggs in a single spawning, which can then be fertilized to generate embryos or used as a substrate for generation of egg extracts. The protocol for induction of ovulation and subsequent egg collection is straightforward and robust, yet some of its details may vary among labs based on prior training, availability of necessary reagents, or the experimental objectives. As the goal of this protocol is not to describe every single variation possible for acquiring eggs but to provide a simple and clear description that can be easily applied by researchers with no prior working experience with X. laevis, we focus on describing the method we use at the National Xenopus Resource, that is, inducing ovulation in X. laevis via dorsal lymph sac injection of gonadotropic hormones and the stimulation of egg laying through application of gentle pressure to the females.
MATERIALS
Reagents:
Human Chorionic Gonadotropin (hCG) (available from several sources including: National Hormone and Peptide Program, Los Angeles, CA, USA; Sigma-Aldrich, St. Louis, MO, USA; Chorulon brand, Merck Animal Health, Madison, NJ, USA; BioVendor, Asheville, NC, USA)
hCG should be resuspended to a concentration of 1000 U/mL in 1X PBS and stored at −20°C.
Ovine Luteinizing Hormone (oLH) (e.g., National Hormone and Peptide Program, Los Angeles, CA, USA)
oLH should be resuspended to a concentration of 0.4 mg/mL dissolved in 1 X PBS and stored at −20°C.
Pregnant Mare Serum Gonadotropin (PMSG) (e.g., BioVendor, Asheville, NC, USA)
PMSG should be resuspended to a concentration of 100 U/mL in 1X PBS and stored at −20°C. The use of Pregnant Mare Serum Gonadotropin has been shown to improve follicular maturity.
Marc’s Modified Ringer Solution (MMR):
Make 10x stock: 1 M NaCl, 20 mM KCl, 10 mM MgSO4·7H2O, 20 mM CaCl2·2H2O, 50 mM HEPES free acid; adjust the pH to 7.4–7.8 with NaOH. Sterilize via autoclaving. This stock can be diluted as necessary with Type 1 ultrapure water (ASTM International 2018) with no further pH adjustments necessary.
Modified Barth’s Saline (MBS):
Make 10x stock: 800 mM NaCl, 10 mM KCl, 10 mM MgSO4·7H2O, 50 mM HEPES free acid, 25 mM NaHCO3; adjust the pH to 7.8 with NaOH; Sterilize via autoclaving. Make a separate 0.1 M stock of CaCl2, also sterilized via autoclaving. To make culture solutions dilute 10x MBS stock with Type 1 ultrapure water, and add CaCl2 stock to a final concentration of CaCl2 in 1x MBS at 0.7 mM.
Phosphate Buffered Saline (PBS) tablets, 100 g.
Equipment:
1ml syringe with PrecisionGlide Needle, 27 G x 3/8" (e.g., Becton, Dickinson and Company, Franklin Lakes, NJ, USA)
Method:
Priming
-
Perform this 1 to 7 day before planned ovulation.
The priming injection on its own does not induce egg laying. Instead, it promotes consistent production of a high number of mature eggs following the boosting injection.
Remove PMSG stocks from −20°C freezer and allow it to thaw at room temperature or 37°C.
-
Fill the 1ml syringe with 30-50 U of 100 U/ml PMSG.
Smaller inbred J-strain frogs require less hormone and can be primed with 30 U of PMSG while larger wild type frogs can be given 50 U of PMSG. hCG can also be used to prime females at 0.1 of the amount used for boosting or 50U for a wild type female.
-
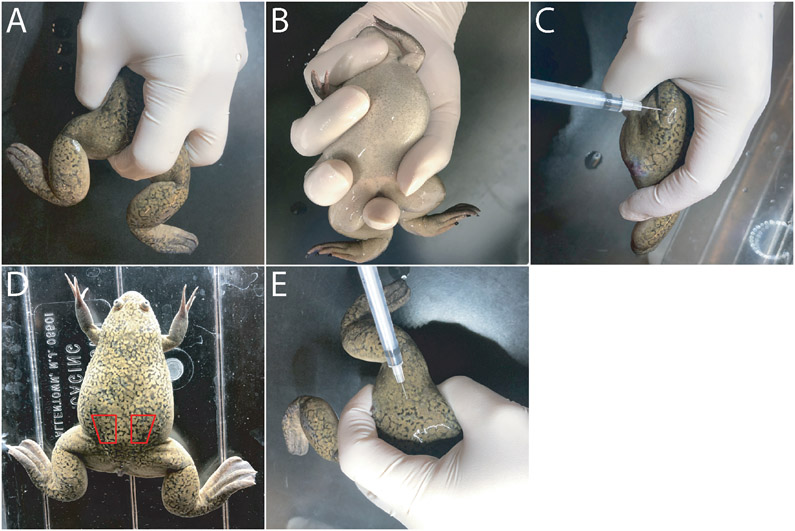
Pick up and restrain the female with one hand in such a way that the dorsal surface of the frog is rested against palm of the hand with the head of the frog pointed towards the wrist (Figure 1A, B). Place the thumb and the middle finger along the posterior sides of the animal abdomen and use the index finger to abduct one of the frog's hind limbs (Figure 1C).
If possible, the palm of the hand should cover the frog’s eyes which will help keep it calm. This hold makes the dorsal lymph sac pocket easily accessible while restraining the animal.
-
With the other hand, insert the needle subcutaneously through the dorsal surface into the dorsal lymph sac in the posterior medial region of the animal proximal to the lateral line “stich marks” and slowly inject the hormone (Figure 1C-E).
Insert the needle at a shallow angle to prevent penetrating muscle.
-
Return the frog to its permanent tank and do not feed until after the eggs have been collected.
Adult frogs can regurgitate food following hormone injection and the presence of solid waste can reduce durability of the laid eggs.
Figure 1. Single hand manual frog restraint for gonadotropin injection and egg collection.
(A) View from the dorsal surface with the index finger in-between both legs, one of the legs being held between the index and the middle fingers, the thumb located around the waist on the other side of the frog. (B) View of the hold from the ventral surface with the index finger between the legs, the middle finger and the thumb located on the waist on the opposite sides of the frog, and the tip of the little finger pressed against the throat to secure the head against the base of the palm of the hand thus aiding to cover the eyes. (C) The frog in fully restrained hold with one of the legs pulled back against the abdomen and a needle ready for injection through the dorsal surface. (D) X. laevis female adult with the general location of the dorsal posterior lymph sacs, the injection target, outlined in red. (E) A more relaxed hold on the female with neither leg fully restrained against the abdomen and with a needle ready for injection through the dorsal surface.
Boosting
-
7.
Perform this the evening before you would like to collect eggs (see 12 for timing details).
The boosting injection promotes the final steps of oocyte maturation and is necessary for induction of ovulation. Females given a boosting injection are likely to spawn, even if they’ve not been given a prior priming injection. The priming injection serves to ensure consistent egg laying response to the boosting injection.
-
8.
Remove oLH or hCG stocks from −20°C freezer and allow them to thaw at room temperature or 37°C.
-
9.
Fill a 1ml syringe with 2 μg of oLH per 1 g of body mass (typically ~200 μg of 0.4 mg/ml oLH) or with 500 U of 1000 U/ml hCG.
Smaller inbred J-strain females require less hormone and can be primed with 120 μg of oLH or 300 U of hCG. Larger wild type females require 200μg of oLH or 500 U of hCG.
-
10.
Inject the frog as described in the Priming section above (see steps 3-5).
-
11.
Prepare a temporary holding tank with system water for the frog to be placed in.
-
12.
Place the frog in the holding tank and incubate at 18°C. Ovulation will begin approximately 8 to 12 hours post injection and should continue for approximately 5 hours.
Onset of ovulation can be delayed by several hours by keeping the female at a lower temperature of 15°C.
Obtaining eggs
-
13.
Remove the frog holding tank from the 18°C incubator and prepare a sterile petri dish for egg collection.
The steps below describe a way of handling a female to promote egg laying and subsequent collection of a batch of eggs into a petri dish. As an alternative, at this point the system water in the frog holding tank can be replaced with egg laying solution, like 1 x MMR or 1 x MBS. Viability of eggs laid into system water is lost rapidly, but can be maintained for at least one hour if laid into egg laying solution. This allows for eggs to be collected throughout the day, without the need to handle the females. The animals tolerate being placed in the egg laying solution well; however, further handling to promote egg laying should be avoided as it does stress the females. This approach is potentially less reliable at producing a large number of viable eggs at exactly the desired time.
-
14.
Similar to the hold used for restraining the animal during hormone injection, gently but firmly pick up the female from the dorsal side with the dominant hand, allowing its head to be concealed by the palm (Figure 1A, B).
The frog should be in the prone position
-
15.
Place the thumb and middle finger along the posterior side of the female. the little finger against the throat of the animal to restrain it further.
-
16.
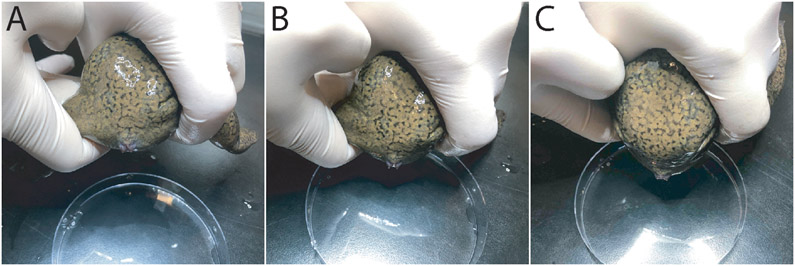
Using the index finger abduct one of the frog’s hind limbs to reflect it rostral-ward, while at the same time abduct the second limb using the other hand (Figure 2A-C).
This will expose the cloaca/vent and allow for the best control when attempting to position the female. The thumb, ring, and index finger should be available to massage the dorsal and ventral lower trunk.
-
17.
Position the female vertically, with the cloaca over the petri dish.
This helps prevent the eggs from running along the body of the female.
-
18.
Use the dominant hand’s ring and middle finger tips to apply pressure to the belly and the other hand’s thumb to apply pressure to the back of the animal.
-
19.
Gently shift the pressure in an anterior to posterior direction to aid the expulsion of eggs.
Simply restraining the female in the described hold, without applying additional pressure is often sufficient for egg expulsion. The hold itself should be tight and stiff enough to prevent the female from breaking loose and the eggs come out as she flexes her leg and abdominal muscles attempting to escape. If no eggs come out when simply holding the animal, gently increase the applied pressure being sure to not hurt the animal and monitor it for stress indicators such as sudden excess of skin surface mucous. Females should only be squeezed once every hour and no more than three times in a single day.
-
20.
Transfer the frog to a clean tank overnight and allow it 24 hours to recover prior to returning it to the system.
When dealing in albino females in particular, even gentle pressure may result in mild bruising around the eyes and the part of the abdomen handled directly. This bruising will disappear within hours and has no long term ill effects on the health of the female. X. laevis are hardy, although care should be taken not to apply such pressure that it leads to injury.
-
21.
Immediately after putting the female back use a transfer pipette to remove any system water that may have dripped into the egg dish during collection.
Egg viability will decline rapidly if they are exposed to a significant amount of system water. If collected eggs are not going to be used immediately, it may be useful to flood the egg collection dish with 1x MMR or 1x MBS which will prevent them from drying out and will help maintain their viability for at least an hour.
Figure 2. Manual two handed restraint of a female ready for egg expulsion.
(A) Initial hold with the female held in the dominant hand. The face of the frog rests against the inside of the base of the palm of the hand, one of the legs is held between the index and the middle finger while the thumb is near the pit of the contralateral arm. The other leg is being restrained between the index and the middle fingers of the other hand. (B) Both hands are used to progressively pull both legs out and forwards toward the head. (C) Fully restrained female with both legs held against the abdomen. The hold is loose enough to allow the female to wiggle and flex its leg and abdominal muscles, yet firm enough to prevent it from breaking loose.
DISCUSSION
The method described here is well established (Wlizla et al. 2018; 2017) and allows for simple and efficient way to collect eggs from female X. laevis at desired times. These eggs can then be used directly in experiments or fertilized via In Vitro Fertilization (IVF) to generate embryos. Developing a correct technique for restraining X. laevis females for hormone injection and egg collection requires some practice but is essential for decreasing distress caused to the animal and for collecting the best quality eggs. Restrained frogs appear to struggle less against the hold when their eyes are covered. For those not experienced in holding frogs a good initial practice may be to place a moist paper towel on a table top, put the female on the paper towel and gently wrap her head to cover her eyes while keeping the posterior dorsal surface still accessible for injection. This way the hard table top surface can be used to aid in immobilizing the animal.
Another aspect of this procedure that requires some practice is determining the amount of pressure that should be applied to the animal during egg expulsion. Too little pressure might not produce any eggs, while too much may cause the eggs to be damaged or cause injury to the animal. If simply holding the female is not productive, some pressure will be necessary. The pressure should be applied in a way where it shifts in a rostral to caudal direction so that it is not simply a squeeze, but instead a massage intended to push the eggs towards and out of the cloaca. As an alternative to handling the female, following the boosting hormone injection she can be placed in a tank containing egg laying solution. The female will lay eggs at a slower rate but they will maintain their viability for at least 1 hour and can be collected directly from the tank; however, it is likely that the total amount of eggs produced will not be as high without the additional manual pressure. Common egg laying solutions include 1 x MMR or 1 x Modified Barth’s Saline (MBS), both of which work equally well for maintaining egg viability. The choice of egg laying solution will depend on prior user experience and experimental design aspects beyond the scope of this chapter.
A final point to address is the use of mammalian gonadotropic hormone injection into the dorsal lymph sac of the X. laevis female as a reliable and effective way for induction of ovulation. hCG has been historically used for this purpose, however, during a shortage of commercially available hCG, we determined that other gonadotropins can stimulate spawning as effectively including PMSG for priming and oLH or human Luteinizing Hormone for boosting (Wlizla et al. 2017). Although, using hormones not sourced in animals may be the more humane option when considering animal welfare, the awareness of these alternatives is useful when dealing with supply disruptions or when trying to find the most economical option.
References
- ASTM International. 2018. ASTM Standard D1193 - 06 (2018). “Standard Specification for Reagent Water.” West Conshohocken, PA. [Google Scholar]
- Wlizla M, Falco R, Peshkin L, Parlow AF, Horb ME. 2017. Luteinizing Hormone is an effective replacement for hCG to induce ovulation in Xenopus. Dev Biol 426: 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlizla M, McNamara S, Horb ME. 2018. Generation and Care of Xenopus laevis and Xenopus tropicalis Embryos. Methods Mol Biol 1865: 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]