Abstract
Purpose
Immunotherapy is currently ineffective for nearly all pancreatic ductal adenocarcinomas(PDAC), largely due to its tumor microenvironment(TME) that lacks antigen experienced T effector cells(Teffs). Vaccine-based immunotherapies are known to activate antigen-specific Teffs in the peripheral blood. To evaluate the effect of vaccine therapy on the PDAC TME, we designed a neoadjuvant and adjuvant clinical trial of an irradiated, granulocyte-macrophage colony-stimulating factor(GM-CSF)-secreting, allogeneic PDAC vaccine(GVAX).
Patients and Method
Eighty-seven eligible patients with resectable PDAC were randomly assigned(1:1:1) to receive GVAX alone or in combination with two forms of low-dose cyclophosphamide(Cy). Resected tumors following neoadjuvant immunotherapy were assessed for the formation of tertiary lymphoid aggregates(TLA) in response to treatment. The clinical endpoints are disease-free survival(DFS) and overall survival(OS).
Results
The neoadjuvant treatment with GVAX either alone or with two forms of low dose Cy is safe and feasible without adversely increasing the surgical complication rate. Patients in Arm A who received neoadjuvant and adjuvant GVAX alone had a trend toward longer median OS(35.0 months) than that(24.8 months) in the historical controls who received adjuvant GVAX alone. However, Arm C, who received low dose oral Cy in addition to GVAX, had a significantly shorter DFS than Arm A. When comparing patients with OS>24 months to those with OS<15 months, longer OS was found to be associated with higher density of intratumoral TLA.
Conclusion
It is safe and feasible to use a neoadjuvant immunotherapy approach for PDACs to evaluate early biologic responses. In-depth analysis of TLAs is warranted in future neoadjuvant immunotherapy clinical trials.
Keywords: vaccines, pancreatic cancer, intratumoral lymphoid aggregates
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) has become the 3rd leading cause of cancer deaths in the US (1). While immunotherapy has become a breakthrough therapy in treating many different types of malignant diseases and demonstrated effectiveness in at least a subset of patients, it is currently ineffective for nearly all pancreatic cancer patients (2,3). Immune tolerance mechanisms are established early in the development of pancreatic cancer (4). By the time the tumor is invasive, the tumor microenvironment (TME) is infiltrated with multiple immunosuppressive cells, including tumor-associated macrophages (TAMs), myeloid derived suppressive cells (MDSCs), and CD4+ FoxP3+ T regulatory cells (Tregs), but lacks quality antigen experienced T effector cells (Teffs) (5–7). Thus, the first requirement for achieving an effective antitumor immune response is the successful induction and activation of cancer targeted Teffs capable of infiltrating into the TME.
Vaccine-based immunotherapies are known to activate antigen-specific Teffs in the peripheral blood; however, little is known about the effect of vaccines on the TME. We developed a human granulocyte macrophage-colony stimulating factor (GM-CSF)-secreting whole-cell pancreatic cancer vaccine (GVAX) to promote T cell responses against a range of tumor associated antigens (8–11). Prior work has shown that combining GVAX with low-dose cyclophosphamide (Cy) to deplete Tregs results in higher avidity mesothelin-specific T cell responses and longer survival in patients with metastatic PDAC compared to GVAX alone (9). However, these prior studies were not designed to directly evaluate the effects of GVAX treatment on the PDAC TME. To evaluate changes in the PDAC TME, we designed a clinical trial comparing GVAX given in the neoadjuvant and adjuvant setting as a single agent, or in combination with two dosing schedules of low dose Cy (13). We previously reported the primary immunologic endpoint analysis for the first 39 patients who were evaluable (13). Immunohistochemical analysis (IHC) of tumor tissue resected from GVAX-treated patients identified the formation of immunotherapy-induced intratumoral tertiary lymphoid aggregates (organized lymphoid structures) that were not observed in tumors resected from unvaccinated patients.
We have completed the enrollment of 87 eligible patients to this clinical trial and have followed the subjects for an extended period for clinical outcomes. This study represents the first neoadjuvant immunotherapy clinical trial for resected PDAC. Here, we report the clinical outcomes of this clinical trial and the correlation between the density of lymphoid aggregates and clinical outcomes.
METHODS
Study Design
This was a single-institution study of patients with PDAC who underwent pancreaticoduodenectomy at the Johns Hopkins Hospital (Baltimore, Maryland). Eligible patients with resectable PDAC received GVAX administered intradermally either alone or in combination with immunomodulatory doses of cyclophosphamide (Cy) as neoadjuvant and adjuvant treatment in addition to adjuvant chemotherapy and/or radiation therapy at specified intervals. Pancreatic GVAX consists of 2 allogeneic pancreatic tumor cell lines that have been modified with a plasmid vector encoding the cDNA for hGM-CSF. The whole-cell vaccines deliver a range of antigens without the need for specific knowledge of the relevant target antigens. The GM-CSF simultaneously recruits and provides maturation signals to antigen-presenting cells (APC) attracted to the local vaccine site. The recruited APCs then orchestrate the immune response by processing tumor antigens expressed by GVAX and presenting them to the patient’s Teff cells. Studies evaluating GVAX in patients with both resected and metastatic PDAC have shown that GVAX induces systemic T cell responses specific to mesothelin, an antigen that is expressed commonly by PDAs and also by GVAX, in a subset of patients that are associated with longer survival (10,12). The study was approved by the Johns Hopkins Institutional Review Board (IRB) and Institutional Biosafety Committee (IBC), as well as the FDA Center for Biologics Evaluation and Research and the National Institutes of Health Recombinant DNA Advisory Committee (J0810, NCT00727441). Informed written consent was obtained from all patients. The trial was conducted according to the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference of Harmonization.
Between July 2008 and February 2015, 87 eligible patients (Supplementary Table S1) enrolled in this clinical trial. Both female and male patients were equally enrolled. Patients were randomized 1:1:1 into three treatment arms. In Arm A, patients received GVAX alone; in Arm B, patients received GVAX plus a single intravenous dose of cyclophosphamide at 200 mg/m2 one day before each vaccination; in Arm C, patients received GVAX plus oral cyclophosphamide at 100 mg once daily for one week on and one week off starting on the day of vaccination. Up to six GVAX treatments were administered, and all the patients remained in their initial treatment arms throughout the study. Each vaccine was injected equally into six intradermal areas in both lower limbs and the non-dominant upper limb, as described previously (10).
Patient Population
The main eligibility requirements before surgery included: suspected or confirmed diagnosis of pancreatic ductal adenocarcinoma; deemed to be surgically resectable; no known second malignancies within five years of diagnosis of pancreatic cancer (other than carcinoma-in-situ of the cervix, superficial skin cancer, or superficial bladder cancer); Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; no clinical evidence of metastases; no serious autoimmune or allergic disease requiring treatment with systemic corticosteroids; adequate hematologic, hepatic, and renal function; and human immunodeficiency virus-negative status. The main eligibility requirements post-surgery included: histologic diagnosis of pancreatic ductal adenocarcinoma after R0 or R1 resection; enrollment within ten weeks of surgery; ECOG performance status of 0 or 1; no clinical evidence of metastases; no serious autoimmune or allergic disease requiring treatment with systemic corticosteroids; no systemic corticosteroids within one month before receiving the immunotherapy; adequate hematologic, hepatic, and renal function; and human immunodeficiency virus-negative status. All 87 subjects were evaluated for safety endpoints. As pre-planned in the clinical protocol, patients evaluable for efficacy endpoints were determined immediately following the surgery and were only those who had both a pathological diagnosis of pancreatic ductal adenocarcinoma excluding any mixed histology or non-pancreatic ductal adenocarcinoma, and a clinical diagnosis of the AJCC stage I or II pancreatic ductal adenocarcinoma excluding those with R2 resection or with distant metastases.
Procedures and Treatment
After obtaining informed consent, baseline studies were performed for tumor assessment (computed tomography [CT] scan and carbohydrate antigen [CA] 19–9 serum marker level measurements) and toxicity evaluation (complete blood counts with differential and platelets, complete chemistry profile, absolute eosinophil count, and serum amylase), according to the schema in Supplementary Fig. S1. Patients received the first vaccine two weeks before the surgical resection, second vaccine six to ten weeks after pancreaticoduodenectomy, and third to sixth vaccines four weeks apart, beginning four weeks after the completion of adjuvant chemotherapy and/or radiation. Patients were assessed by CT scan (every two to three months) and measurement of CA 19–9 levels. Patients began adjuvant chemotherapy four weeks following the second vaccine. Adjuvant chemotherapy was administered as per standard of care but modified as needed at the discretion of their primary oncologists. Treatment with standard of care adjuvant radiation was determined by each primary oncology team.
Assessment of Toxicities
Toxicities were graded using the National Cancer Institute’s cancer clinical trials common toxicity criteria CTC v.3. Toxicities were identified by medical history, physical examination, and review of the laboratory studies (performed complete blood count with differential, chemistry profile, and serum amylase) biweekly during vaccine cycles one through three, then monthly during vaccine cycles four through six. Postoperative complications within 30 days of surgery were graded by the Clavien-Dindo Criteria (15).
Comparison Cohorts
We compared our clinical outcomes to a similar patient cohort from a previously published clinical trial of GVAX (J9988, NCT00084383). In this clinical trial, patients who underwent the Whipple surgery received five GVAX treatments in a sequential combination with adjuvant chemoradiation therapy (CRT) at the Johns Hopkins Hospital between 1999 and 2004 (10). The schedule of the five GVAX treatments in J9988 was similar to that of the five postoperative GVAX treatments in the current study.
Statistical Considerations
Descriptive statistics (means, ranges, counts, and percentages) were used to describe the study population. Counts and proportions were used to summarize the number of individuals and the number of cycles with adverse events. Disease-free survival (DFS) was defined as the time from the date of randomization until clinical evidence of disease (e.g., CT scan). Individuals were censored with respect to DFS at the date of the last follow-up with documented disease status if they withdrew from the study, they had no evidence of disease at the time of analysis, or they were lost to follow-up. To obtain a conservative estimate of DFS, an individual was also censored at the date of the last follow-up with a documented disease status if death occurs and the disease status was unknown. Overall survival (OS) was defined as the time from the date of randomization until death, regardless of cause. Individuals were censored with respect to OS when they were last confirmed to be alive if they withdrew from the study, were lost to follow-up, or were alive at the time of analysis. Comparisons between the OS and DFS of the active treatment arms and the control group were made using Kaplan-Meier curves and log-rank tests. The Cox model was used for univariate and multivariate survival analysis. Wilcoxon test was used for comparison of immunologic endpoints between different groups. P values from multiple testing were adjusted using the Benjamini-Hochberg method at level 0.05. Statistical analyses were performed using R version 2.5.1.
The clinical outcomes in this study, including OS and DFS, were secondary endpoints of the trial. The trial was powered to detect the level of peripheral mesothelin-specific T cell response (primary endpoint, unpublished). The sample size was calculated separately for each arm. A sample size of thirteen patients in each arm was estimated to provide 90% power to detect a mesothelin response rate of 50% from a null rate of 10% with a two-sided type I error rate of 5%. Considering a dropout rate of 40% following surgery and another 25% following chemoradiation for each arm, 29 patients per arm (87 total) were needed to obtain 13 patients that were evaluable for the immunologic endpoint.
RESULTS
Patent enrollment and treatment
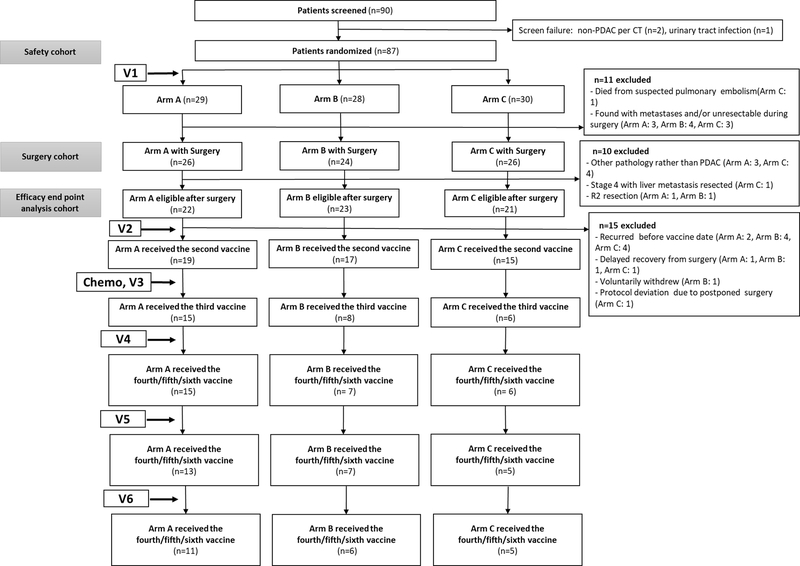
Among 90 patients who were screened, 87 patients were deemed eligible, were randomized to one of three arms (Arm A: 29, Arm B: 28 and Arm C: 30), and received the first vaccine two weeks +/− four days before surgery (Fig. 1). Three patients failed screening criteria for eligibility, two were diagnosed as non-PDAC per preoperative CT, and one had urinary tract infection. Seventy-six patients (Arm A: 26, Arm B: 24 and Arm C: 26) successfully underwent pancreaticoduodenectomy. For the 11 who did not have a surgical resection, one patient died from suspected pulmonary embolism prior to the planned operation date, ten patients were found to be unresectable during surgery or to have metastases which were not radiographically identified before the surgery, and the procedure was aborted. Among the 76 patients who had pancreaticoduodenectomy, ten were deemed ineligible to continue on the study and were taken off study postoperatively: two patients were found to have ampullary cancer, one to have neuroendocrine tumor, two to have undifferentiated carcinoma, one to have adenosquamous carcinoma, one to have autoimmune pancreatitis, one to have stage 4 with both primary PDAC and the liver metastasis resected, and two to have grossly residual tumors (R2 resection). These patients’ preoperative CT scans did not distinguish these other pancreatic conditions from PDAC. As a result, 66 patients (Arm A: 22, Arm B: 23 and Arm C: 21) were considered to be evaluable as pre-planned in the clinical protocol for the efficacy endpoints (Table 1) and were subsequently evaluated for their eligibility for the second vaccination. Ten patients were found to have recurrence when they were screened for the second vaccination. Three patients had a delayed recovery (more than ten weeks) from the surgery and thus were not eligible for study continuation. One patient voluntarily withdrew from the study. One patient had postponed surgery that was a protocol deviation due to cardiac issues, and postoperatively died likely of cardiac disease. The 51 patients remaining on the study received the second immunotherapy followed by the standard adjuvant chemotherapy and/or radiotherapy (Supplementary Table S2). Twenty-nine patients remained disease-free following adjuvant chemotherapy and/or radiotherapy received the third immunotherapy. A total of 28, 25, and 22 patients received PDAC GVAX cycle four, five, and six treatments respectively.
Figure 1.
The CONSORT diagram of the numbers of patients receiving standard of care treatments and vaccine (V) treatments
Table 1.
Demographic and clinicopathologic characteristics of patients evaluable for the primary efficacy analysis (i.e. eligible after surgery).
| Variables | All Patients | Arm A | Arm B | Arm C |
|---|---|---|---|---|
| N=66 | N=22 | N=23 | N=21 | |
| Age, years | ||||
| Median (IQR) | 62 [56, 74] | 59.5 [54, 70] | 61 [57, 74] | 65 [61, 75] |
| Sex, n (%) | ||||
| Male | 40 (61) | 16 (73) | 14 (61) | 10 (48) |
| Female | 22 (39) | 6 (27) | 9 (39) | 11 (52) |
| Race, n (%) | ||||
| White | 60 (91) | 19 (86) | 22 (96) | 19 (90) |
| Non-White | 6 (9) | 3 (14) | 1 (4) | 2 (10) |
| Tumor size, cm | ||||
| Median (IQR) | 3.52 (1.16) | 3.21 (1.06) | 3.44 (1.03) | 3.94 (1.31) |
| Tumor grade, n (%) | ||||
| G1: Well | 5 (8) | 2 ( 9) | 3 (13) | 0 (0) |
| G2: Moderate | 35 (53.0) | 15 (68) | 8 (35) | 12 (57) |
| G3: Poor | 25 (38) | 5 (23) | 11 (48) | 9 (43) |
| G4: Undifferentiated | 1 ( 1) | 0 (0) | 1 (4) | 0 ( 0.0) |
| LN, n (%) | ||||
| Positive | 53 (80) | 16 (72) | 18 (78) | 19 (91) |
| Negative | 13 (20) | 6 (28) | 5 (22) | 2 (9) |
| PLN, n (%) | ||||
| Median (IQR) | 3 [1, 5] | 2 [0, 5] | 3 [2, 5] | 3 [1, 6] |
| TLN, n (%) | ||||
| Median (IQR) | 20 [15, 27] | 18.50 [14, 22] | 19 [16, 24] | 26 [18, 28] |
| Stage, n (%)* | ||||
| I | 5 (8) | 3 (14) | 1 (4) | 1 (5) |
| II | 61 (92) | 19 (86) | 22 (96) | 20 (95) |
| Margin, n (%) | ||||
| R0 | 46 (70) | 14 (64) | 18 (78) | 14 (67) |
| R1 | 20 (30) | 8 (36) | 5 (22) | 7 (33) |
IQR: interquartile range (1st –3rd quartile); LN: lymph node; PLN: positive lymph node; TLN: total lymph node
AJCC 7th edition
All patients developed local reactions at the vaccine intradermal injection sites, including erythema, induration, tenderness, and itchiness, as anticipated based on a previous trial with the same vaccine (Supplementary Table S3)(10). None developed open wounds at the injection sites. Most of these reactions were self-limited. Treatment-related high grade (grades >= 3a) adverse events occurred in six patients (seven events) (Supplementary Table S4). The patient with autoimmune pancreatitis did not experience worsening autoimmune pancreatitis after he received the immunotherapy. Postoperative complications are summarized in Supplementary Table S5. There were no unusual patterns of postoperative complications. There were no delays in surgery due to immunotherapy related adverse events. These results suggest that neoadjuvant vaccine therapy with or without Cy is safe and feasible for neoadjuvant administration.
Efficacy Analysis
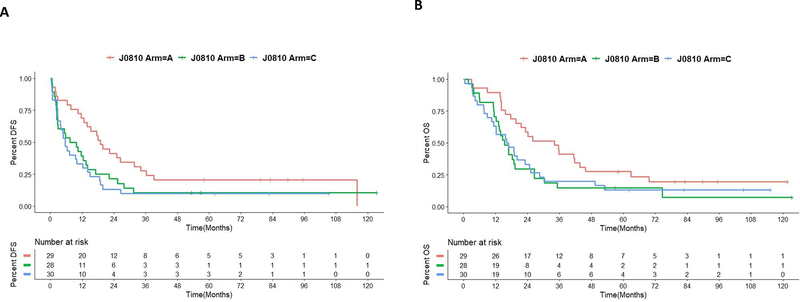
All 87 patients’ DFS and OS are reported in Fig. 2 and Table 2, although it should be noted that 21 of them were not considered evaluable due to non-PDAC histology or non-resectable stage of their disease. The remaining 66 patients were considered evaluable for efficacy analysis according to the clinical protocol (Fig. 1, Table 1). When stratified by treatment, the patients in Arm A had a median DFS of 18.4 months (Fig. 3, Supplementary Table S6, and Table S7). In contrast, patients in Arm B had a median DFS of 11.7 months, although the difference between Arms A and B were not significant (p = 0.29). Patients in Arm C only had a median DFS of 7.2 months, which was significantly shorter than that in Arm A (p=0.02) (Fig. 3, Supplementary Table S6 and Table S7); and this inferior outcome in Arm C appeared to be independent of other prognostic factors (Supplementary Table S8). Therefore, it seems that low metronomic oral doses of Cy in combination with GVAX results in an inferior outcome when compared to GVAX alone.
Figure 2. Clinical outcomes for all 87 randomized patients.
A. Kaplan-Meier survival curves of disease-free survival (DFS) of all 87 patients. B. Kaplan-Meier survival curves of overall survival (OS) of all 87 patients
Table 2.
Median disease-free survival (DFS) and overall survival (OS) of all 87 patients in the J0810 study
| DFS |
OS |
||||||
|---|---|---|---|---|---|---|---|
| Study Arm | n | Events | Median (months) | 95% Confidence Interval | Events | Median (months) | 95% Confidence Interval |
| A | 29 | 24 | 18.92 | (13.87,34.1) | 23 | 34.2 | (21.6,45.7) |
| B | 28 | 25 | 8.54 | (2.66,17.1) | 24 | 15.4 | (13.2,26.5) |
| C | 30 | 27 | 5.56 | (2.67,14.0) | 26 | 16.5 | (11.3,25.2) |
Figure 3. Clinical outcomes of 66 evaluable patients.
A. Kaplan-Meier survival curves of disease-free survival (DFS) of 66 patients evaluable for efficacy endpoint. B. Kaplan-Meier survival curves of overall survival (OS) of 66 patients evaluable for efficacy endpoint.
The median OS in Arm A was 35.0 months, which was longer than that in Arm B but not statistically significant (p=0.3) (Fig. 3, Supplementary Table S6 and Table S7). Therefore, the single intravenous dose of Cy may adversely affect both the DFS and OS of patients receiving GVAX. The median OS in Arm A was also longer than the median OS of 18.9 months in Arm C (p=0.42), further suggesting that low metronomic oral doses of Cy may adversely affect the outcome of the GVAX treatment. The results were similar after controlling for other prognostic factors (Supplementary Table S9). Subgroup analysis of patients who remained disease-free at the postoperative imaging reevaluation and had received at least two vaccines yielded similar findings (Supplementary Fig. S2, Table S10, and Table S11). Nevertheless, the sample size may be too small to draw definitive conclusions.
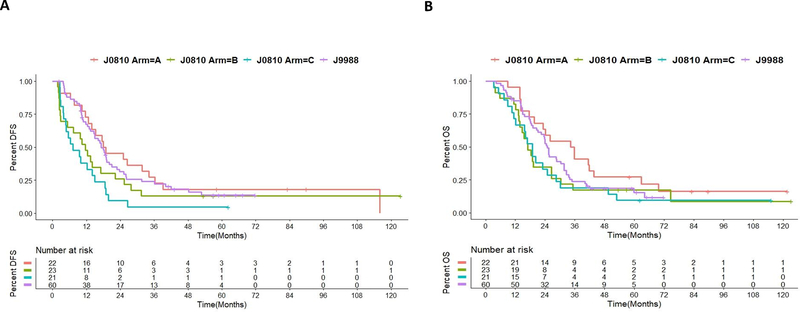
Patients in this study were also compared with those in the previous phase 2 adjuvant study J9988 with 60 patients (10). Arm C had significantly worse DFS than J9988, but Arms A and B had similar DFS. There was no significant difference in OS among Arms A, B, C, and J9988. OS and DFS appeared to be potentially longer in Arm A of this study (Supplementary Table S6) than either arm in the ESPAC4 study (16). It should be noted that the majority of 22 patients in Arm A received single-agent gemcitabine (Supplementary Table S2), which was standard of care adjuvant therapy when this study was conducted.
Density of tertiary lymphoid aggregate (TLA) correlates with survival following neoadjuvant vaccine therapy
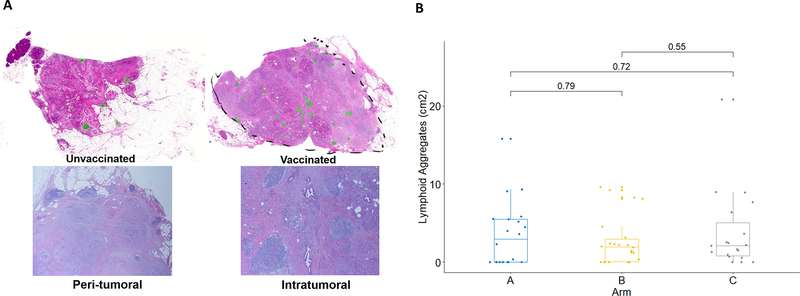
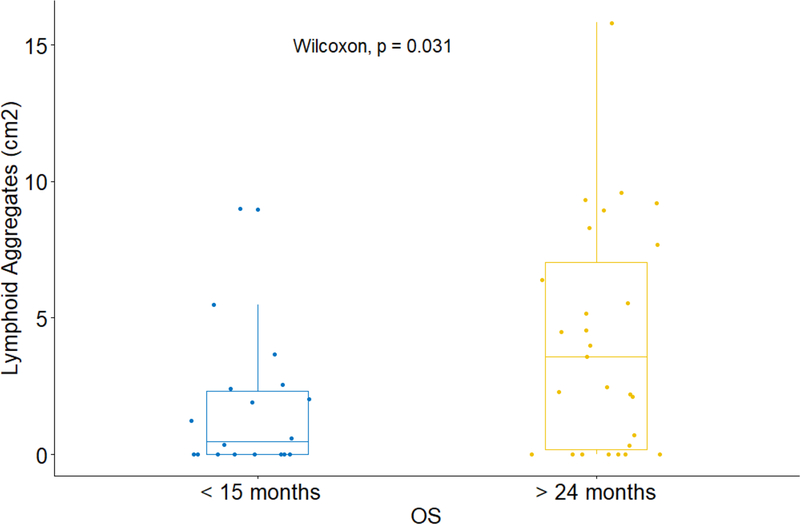
Previously, we showed that GVAX treatment induced intratumoral TLAs in a subset of patients (13) (Fig. 4). Here, we assessed the relationship between the formation of intratumoral TLAs and the survival of patients following GVAX treatment. We analyzed TLAs in the PDAC tumors from all of the evaluable patients for the efficacy endpoint and examined the association between the density of TLAs and OS. One patient who likely died of a cardiac condition, as described above, was excluded because the patient did not have disease recurrence before death. There was no statistically significant difference in the TLA density between different arms (Fig. 4), suggesting that Cy did not affect TLA formation. Among all evaluable patients, there was a non-significant trend towards longer DFS and OS in patients with higher intratumoral TLA density than those with lower intratumoral TLA density, using median TLA density as the cutoff (Supplementary Table S12). It would be difficult to determine whether the patients whose OS between 15 and 24 months are considered to be survivors with better or worst outcomes. After we removed the patients whose OS was between 15 and 24 months, we found that the LA density in patients with OS > 24 months is significantly higher than that in patients with OS < 15 months. Therefore, we chose these cutoffs to present our data. When we compared patients whose OS were longer than 24 months with those whose OS was shorter than 15 months (all censored patients had a follow-up over 24 months), we observed a significant difference (Wilcoxon test, p=0.03) in the densities of their intratumoral TLAs between these two groups. Longer OS was associated with a higher density of TLA (Fig. 5). It should be noted that in either group of patients with shorter or longer OS, some patients developed essentially no TLAs (density was 0) following vaccine therapy, and some patients had no TLAs results available. Our results suggest that vaccine-induced TLAs may be a prognostic factor for patients who received GVAX. It remains to be investigated whether other characteristics of TLAs are evidences of clinical benefit from treatment with GVAX.
Figure 4. Tertiary lymphoid aggregates in PDACs.
A. Hematoxylin and eosin stain of representative untreated PDAC (unvaccinated) and PDAC following vaccine therapy (vaccinated) were shown. Peritumoral and intratumoral lymphoid aggregates were circled in green in lower magnitude images. Selected regions with lymphoid aggregates were also shown in higher magnitude. B. Comparison of the density of intratumoral lymphoid aggregates in PDACs between each two of three arms.
Figure 5.
Comparison of the density of intratumoral lymphoid aggregates between PDACs with overall survival (OS) less than 15 months and those with OS greater than 24 months.
DISCUSSION
This study demonstrates the feasibility of performing a neoadjuvant PDAC clinical trial to evaluate the early biologic response to experimental therapies, including immunotherapy, when only one treatment is given before surgical resection. The availability of surgically resected tumors after treatment has provided the opportunity to conduct comprehensive analyses of tumors and assess changes in the TME in response to treatment. The results show that neoadjuvant treatment with GVAX, either alone or with two forms of low dose Cy, is safe and feasible without adversely increasing the surgical complication rate. Our study also suggests that neoadjuvant GVAX treatment without Cy does not adversely affect the survival of the patients. Nevertheless, neoadjuvant and/or adjuvant usage of low dose Cy appears to negatively affect patient survival compared to GVAX alone, although the sample size is too small to draw a definitive conclusion about Cy. We do not know the mechanism underlying the negative effect of Cy on this vaccine. Preclinical studies provided evidence that low dose Cy depleted Tregs when given with GVAX (17–21). Although Cy was shown to reduce Tregs in patient specimens, this did not translate into improved clinical outcomes. It is possible that the neoadjuvant Cy treatment decreased the effector T cells available in the peripheral blood, which might be important for the surgical outcome as previous studies showed that low preoperative lymphocyte count was associated with poorer survival following the surgical resection of PDACs (22,23). Nevertheless, the sample size was too small to draw definitive conclusions. Our prior work did show that combining GVAX with low dose Cy was associated with increased survival in patients with metastatic PDAC compared to GVAX alone (9). Therefore, additional assessment of the role of low dose Cy by analyzing the specimens collected from this clinical trial is warranted before incorporating low dose Cy in conjunction with GVAX in future studies.
The main pitfall of this study is that 21 (24%) patients were not considered to be evaluable following surgery. It was anticipated that some of the patients would be found to have unresectable diseases intraoperatively. The percentage of patients (N = 10, 11%) who were found to have unresectable diseases in this study is similar to published studies (8%)(24). Nevertheless, many of the unevaluable patients (N = 7, 33%) had a pathological diagnosis other than PDAC and were subsequently removed from the study. This may be inevitable for similar PDAC window-of-opportunity studies, particularly when the preoperative tissue diagnosis is not a routine practice for resectable pancreatic masses. As most of these pathological diagnoses are known to be associated with better outcomes than PDACs, we excluded them from the efficacy analysis. There is a potential bias by using the eligible criteria after surgery to exclude the patients who do not have the pathologic diagnosis of PDAC. However, the analysis of the entire cohort has similar results.
Our study did observe comparable survival in patients treated with GVAX alone, when compared to adjuvant chemotherapy studies. The majority of patients in this trial received single-agent gemcitabine as adjuvant chemotherapy. There was no difference in the percentage of patients who received adjuvant chemotherapy/radiation and the type of adjuvant therapy that they received among different treatment arms. Therefore, it is possible that the increased survival rate in Arm A is due to the effect of GVAX, particularly neoadjuvant GVAX and that the survival benefit from GVAX was offset by the usage of Cy in Arms B and C.
This study was not powered to compare the efficacy between the treatment arms. The clinical outcome comparisons between different cohorts within this study, and with the J9988 or the ESPAC-4 study may be difficult to interpret with the small sample size and the patient heterogeneity. Therefore, as per the above discussion, the conclusions made in this study need to be further validated with a larger study.
The primary objective of this clinical trial was to assess changes in the PDAC TME with GVAX neoadjuvant therapy. Our previously published analysis of the first 39 evaluable patients on this trial reported the significant finding of the formation of TLAs just two weeks after one treatment of GVAX (13). Subsequent studies have shown that TLAs were associated with better prognosis in melanoma patients receiving immune checkpoint inhibitors (25). The current study showed that a higher density of TLAs might be associated with better OS, further supporting that a subgroup of patients may benefit from vaccine therapy if vaccine therapy is able to induce a higher density of TLAs. It is possible that the higher density of TLAs is just a surrogate of better prognosis of resected PDACs independent of vaccine therapy. Whether vaccine-induced TLAs play a role in mediating antitumor activity remains to be established. A more thorough analysis of TLAs should provide additional information on the role of TLAs in the TME in response to immunotherapy.
Based on the results of the current study, we opened a neoadjuvant clinical trial platform study to evaluate other immunomodulating agents in combination with Cy/GVAX. In one three-arm study (NCT02451982), we are testing GVAX and low dose Cy with or without PD-1 blockade antibody and CD137 agonist antibody for the neoadjuvant and adjuvant treatment of resectable PDAC patients. Another phase 2 study (NCT02648282) is testing GVAX and low dose Cy with PD-1 blockage antibody and stereotactic body radiation therapy as neoadjuvant therapy for locally advanced PDAC patients. Through these new, ongoing neoadjuvant studies, we will continue to validate the findings in the current study while testing the new strategies of immunotherapy combinations.
Supplementary Material
Translational Relevance.
This study represents the first neoadjuvant window-of-opportunity platform clinical trial for studying the biological effects of experimental therapies on tumor cells and the tumor microenvironment of solid tumors. We previously reported the primary immunologic endpoint analysis for the first 39 patients in this clinical trial of a pancreatic cancer vaccine-based immunotherapy for resectable pancreatic ductal adenocarcinoma. Since the initial publication of the primary immunologic endpoint, many neoadjuvant window-of-opportunity clinical trials have been developed after this clinical trial model. Here, we report the safety, feasibility and efficacy results of this clinical trial including the long-term follow-up data and the potential association of vaccine-induced intratumoral tertiary lymphoid aggregates with patients’ overall survival. Thus, the results of this clinical trial support the application of a neoadjuvant clinical trial platform to assess the early biological response to experimental therapeutics.
Acknowledgments
Grant information: This work was supported mainly by the Viragh Foundation and the Skip Viragh Pancreatic Cancer Center at Johns Hopkins (DL, EMJ); NIH grant R01 CA169702 (LZ); NIH grant K23 CA148964 (LZ); NIH grant R01 CA197296 (LZ, EMJ); NIH SPORE grant P50 CA062924 (EMJ, DL, and LZ); NIH Cancer Center Support Grant P30 CA006973.
Conflicts of interest: LZ receives grant support from Bristol-Meyer Squibb, Merck, Astrazeneca, iTeos, Amgen, NovaRock, Inxmed, and Halozyme. LZ is a paid consultant/Advisory Board Member at Biosion, Alphamab, NovaRock, Akrevia, Datarevive, QED, Xilio, Natera, Novagenesis, and Mingruizhiyao. LZ holds shares at Alphamab and Mingruizhiyao. EMJ is a paid consultant for Genocea, CSTONE, Achilles, DragonFly, and Adaptive Biotech. Through a licensing agreement with Aduro Biotech, Dr. Jaffee and the Johns Hopkins University have the potential to receive royalties on the sale of GVAX.
REFERENCES
- 1.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global cancer Observatory: cancer today. Lyon, France: international agency for research on cancer. Cancer Today 2018. [Google Scholar]
- 2.O’Reilly EM, Oh D-Y, Dhani N, Renouf DJ, Lee MA, Sun W, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA oncology 2019;5(10):1431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osipov A, Zaidi N, Laheru DA. Dual Checkpoint Inhibition in Pancreatic Cancer: Revealing the Limitations of Synergy and the Potential of Novel Combinations. JAMA oncology 2019;5(10):1438–9. [DOI] [PubMed] [Google Scholar]
- 4.Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer—science driving clinical progress. Nature Reviews Cancer 2005;5(6):459–67. [DOI] [PubMed] [Google Scholar]
- 5.Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology 2013;144(6):1230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331(6024):1612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res 2007;67(19):9518–27. [DOI] [PubMed] [Google Scholar]
- 8.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. J Clin Oncol 2001;19(1):145–56. [DOI] [PubMed] [Google Scholar]
- 9.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res 2008;14(5):1455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma: a phase II trial of safety, efficacy, and immune activation. Annals of surgery 2011;253(2):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 2013;36(7):382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen YC, Huang LQ, et al. Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. J Exp Med 2004;200(3):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer immunology research 2014;2(7):616–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goc J, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Characteristics of tertiary lymphoid structures in primary cancers. Oncoimmunology 2013;2(12):e26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clavien PA, Barkun J, De Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Annals of surgery 2009;250(2):187–96. [DOI] [PubMed] [Google Scholar]
- 16.Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. The Lancet 2017;389(10073):1011–24. [DOI] [PubMed] [Google Scholar]
- 17.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels J-PH, et al. Recruitment of latent pools of high-avidity CD8+ T cells to the antitumor immune response. The Journal of experimental medicine 2005;201(10):1591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, et al. CD4+ CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. European journal of immunology 2004;34(2):336–44. [DOI] [PubMed] [Google Scholar]
- 19.Hermans IF, Chong TW, Palmowski MJ, Harris AL, Cerundolo V. Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer research 2003;63(23):8408–13. [PubMed] [Google Scholar]
- 20.Leao IC, Ganesan P, Armstrong TD, Jaffee EM. Effective Depletion of Regulatory T Cells Allows the Recruitment of Mesothelin‐Specific CD8+ T Cells to the Antitumor Immune Response Against a Mesothelin‐Expressing Mouse Pancreatic Adenocarcinoma. Clinical and translational science 2008;1(3):228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss VL, Lee TH, Jaffee EM, Armstrong TD. Targeting the right regulatory T-cell population for tumor immunotherapy. Oncoimmunology 2012;1(7):1191–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark E, Connor S, Taylor M, Madhavan K, Garden O, Parks R. Preoperative lymphocyte count as a prognostic factor in resected pancreatic ductal adenocarcinoma. Hpb 2007;9(6):456–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rho SY, Hwang HK, Chong JU, Yoon DS, Lee WJ, Kang CM. Association of preoperative total lymphocyte count with prognosis in resected left‐sided pancreatic cancer. ANZ journal of surgery 2019;89(5):503–8. [DOI] [PubMed] [Google Scholar]
- 24.Gemenetzis G, Groot VP, Blair AB, Ding D, Thakker SS, Fishman EK, et al. Incidence and risk factors for abdominal occult metastatic disease in patients with pancreatic adenocarcinoma. Journal of surgical oncology 2018;118(8):1277–84. [DOI] [PubMed] [Google Scholar]
- 25.Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nature medicine 2018;24(11):1649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.