Abstract
Background:
Prior literature in cystic fibrosis (CF) has shown a 10-year survival gap between Canada and the US. We hypothesized that differential access to, and survival following lung transplantation may contribute to the observed gap.
Objective:
To compare CF transplant outcomes between Canada and the US, and estimate the potential contribution of transplant to the survival gap.
Methods:
Data from the Canadian CF Registry and US CF Foundation Patient Registry supplemented with data from United Network for Organ Sharing were used. The probability of surviving post-transplant between 2005–2016 was calculated using the Kaplan-Meier method. Survival by insurance status at the time of transplant and transplant center volume in the US were compared to Canada using Cox Proportional Hazard models. Simulations were used to estimate the contribution of transplant to the survival gap.
Results:
Between 2005 and 2016, there were 2,653 patients in the US and 470 in Canada who underwent lung transplantation for CF. The 1-, 3- and 5-year survival rates were 88.3%, 71.8% and 60.3% in the US compared to 90.5%, 79.9% and 69.7% in Canada. Patients in the US were also more likely to die on the waitlist (p<0.01) compared to patients in Canada. If the proportion of patients transplanted and post-transplant survival in the US were to increase to that observed in Canada, we estimate that the survival gap would decrease from 10.8 years to 7.5 years.
Conclusions:
Differences in waitlist mortality and post-transplant survival can explain up to a third of the survival gap observed between the US and Canada.
INTRODUCTION
Although survival in cystic fibrosis (CF) has improved dramatically over the last several decades, the rate of improvement varies by country.(1–3) In fact, a growing survival gap for individuals living with CF was identified between Canada and the United States (US). Using data between 2009 to 2013, people with CF in Canada had a 10.8 year survival advantage compared to people living in the US, although the cause is yet to be determined.(4) We know that life-saving therapy such as lung transplantation has the potential to impact survival rapidly and we hypothesized that differential access to, and survival following lung transplantation may contribute to the survival gap observed in our previous paper.(4) Independently published reports suggest survival differences may exist after lung transplant when comparing Canada to the US. Between 2005 to 2015, the one- and five-year post-transplant survival for individuals with CF in the US were reported to be 89% and 60%, respectively.(5) Although over a different time period, the Canadian one- and five-year post-transplant survival between 1988 and 2012 were reported to be 88% and 67%, respectively.(6) Direct comparison of independently published survival statistics can be difficult to interpret as differences in population characteristics and study definitions may introduce bias.(7) Prior work by our group showed that CF lung transplant recipients in the US had worse nutritional parameters pre-transplant than similar CF patients in Canada however post-transplant outcomes were not conducted in this earlier study.(8)
In order to understand the potential impact of transplant on the survival gap observed between Canada and the US, the current study aim was to compare differences in health outcomes for individuals with CF who were listed or received a lung transplant. Specifically, objectives were to: (a) systematically compare CF transplant survival metrics (including death rate on the waiting list, time to transplant, and post-transplant survival) between Canada and the US; (b) examine the impact of insurance status and surgical volume of the transplant center on post-transplant survival; and (c) quantify the potential contribution of transplant on the survival gap between countries.
METHOD
This population-based cohort study used prospectively collected Canadian CF Registry (CCFR) and US CF Foundation (CFF) Patient Registry (US CFFPR) data supplemented with data from the Thoracic subset of the United Network for Organ Sharing database (UNOS) from 1984 to 2016, inclusive. Details concerning the record linkage between the CFFPR and UNOS can be found in the online supplement. As the lung allocation score (LAS) for triaging transplant recipients was implemented in the US in 2005, we focused on the post-LAS period of 2005 to 2016.
Each CF center that submits data to the registry obtains patient consent for data to be collected. This study was approved at St. Michael’s Hospital, Toronto, Ontario (Research Ethics Board # 14–148), the University of Washington (Institutional Review Board #2270), and Seattle Children’s Hospital (Institutional Review Board # 15294). A detailed description of both registries as well as information on how individuals are accrued and monitored within each registry is outlined in a previous publication.(4)
A unified Canada-US-UNOS data set was created after harmonizing data definitions and data collection methods within each registry.(4) US patients were grouped into the following hierarchical insurance categories: 1) Medicare/Medicaid if Medicare and/or Medicaid were indicated at any point within three years prior to the transplant date, 2) Other insurance if other insurance options (e.g. private) were indicated, and no Medicare/Medicaid was selected in the three-year window, 3) Missing insurance if no insurance or missing insurance for every year of the three-year window. There are more than 60 lung transplant centers in the US and 4 centers in Canada. US transplant centers were categorized as high volume transplant centers for those centers in UNOS that did more than 26 lung transplants per year, and low volume otherwise.(9) Transplant center volume was determined for all underlying diagnoses as well as CF-specific diagnosis. Canadian transplant centers were all high volume (>26 transplants per year).
Statistical Analyses
Continuous variables were summarized by reporting the median and interquartile range (IQR) while categorical variables were summarized by reporting the frequency and proportion. Standardized differences of greater than 10 were deemed important.(10) Unless otherwise stated, all p-values are unadjusted, two-sided, and assessed for significance at p<0.05.
Time to death after lung transplant was calculated from the date of first lung transplant to the date of death. Patients were censored at December 31st of their last year of follow-up. We further explored differences in post-transplant survival by stratifying according to insurance status and transplant center volume. Post-transplant survival estimates censored at repeat transplant (instead of including survival time after additional lung transplants) as well as survival after excluding patients with Burkholderia cepacia complex are shown in the online supplement.
In order to calculate the proportion of deaths on the waiting list, we included all patients who had a transplant listing date and calculated the proportion who died. Time on the transplant waiting list was calculated for two groups and compared between countries. Group 1: all patients who ultimately received a transplant (i.e. time from listing date until transplant date) and Group 2: time from listing date until death for those who died waiting for a lung transplant. In addition, the proportion of deaths on the waiting list for Group 2 was calculated for each country.
We further explored differences in post-transplant survival by adjusting for patient and clinical characteristics using a multivariable Cox proportional hazards (PH) model. The model adjusted for time-independent patient characteristics (country, sex, race, age at diagnosis, pancreatic status (insufficient vs. sufficient), presence of CFRD pre-transplant) as well as baseline clinical factors that were measured pre-transplant (body mass index (BMI), forced expiratory volume in one second (FEV1), pulmonary exacerbations, and microbiology). Pre-transplant clinical factors were the last recorded measurement in the 3-years prior to lung transplantation. In addition, we included lung transplant centre volume (both total and CF-specific) as a covariate both as a categorical variable as well as a continuous variable in a separate adjusted model. We excluded individuals under the age of 6 years from the multivariable model because lung function measurements were not available. Missing lung function and BMI measurements were imputed using multiple imputation which included the following variables: country, gender, age at diagnosis, the presence of Burkholderia cepacia complex, the presence of Pseudomonas aeruginosa, pancreatic status, CFRD, age at transplant, use of BiPAP, use of home oxygen, FEV1 % predicted, BMI (value and percentile), number of pulmonary exacerbations and race. A variable was not used in the imputation strategy if that was the variable being imputed.(11)
In order to quantify the contribution that lung transplant may have on the US-Canada survival gap, we used simulation analysis to estimate the median age of survival in the US if the same proportion of patients in the US were transplanted and if the post-transplant survival was the same as in Canada and compared this to the estimated median age of survival when using the original data. In order to match the observed transplant recipient rate to that of Canada, we simulated all non-transplanted patients who died with an FEV1 < 40% between 2005–2016 in the US such that they received a transplant at their original date of death. An FEV1 < 40% was chosen as this is considered severe lung disease and it would be plausible for these patients to be considered for lung transplantation.(12, 13) The post-transplant survival time for these patients was simulated to to reflect current Canadian post-transplant survival. We repeated these simulations 100 times and calculated the 95th percentile interval. Additional details concerning the simulation are provided in the online supplement.
RESULTS
Post-transplant survival: 2005–2016
Between 2005 and 2016, there were 2,653 (6.9%) patients in the US and 470 (9.1%) in Canada who underwent lung transplantation for CF (Figure 1). A total of 18 (<1%) lung-liver transplants (13 from US, 5 from Canada) and 10 (<1%) heart-lung transplants (10 from US) were included. There were 1,017 (38.3%) and 137 (29.1%) post-transplant deaths recorded in the US and Canada, respectively. A total of 357/596 (59.8%) and 3364/5576 (60.3%) deaths occurred during 2005 and 2016 without evidence of being listed for or receiving a transplant in Canada and the US respectively. Demographic and clinical characteristics of the lung transplant recipients are presented in Table 1. In the US, there were a higher proportion of pediatric patients transplanted compared to Canada, more CFRD, home O2, BiPAP use and more pulmonary exacerbations. More patients had B. cepacia complex infection in Canada compared to the US.
Figure 1:
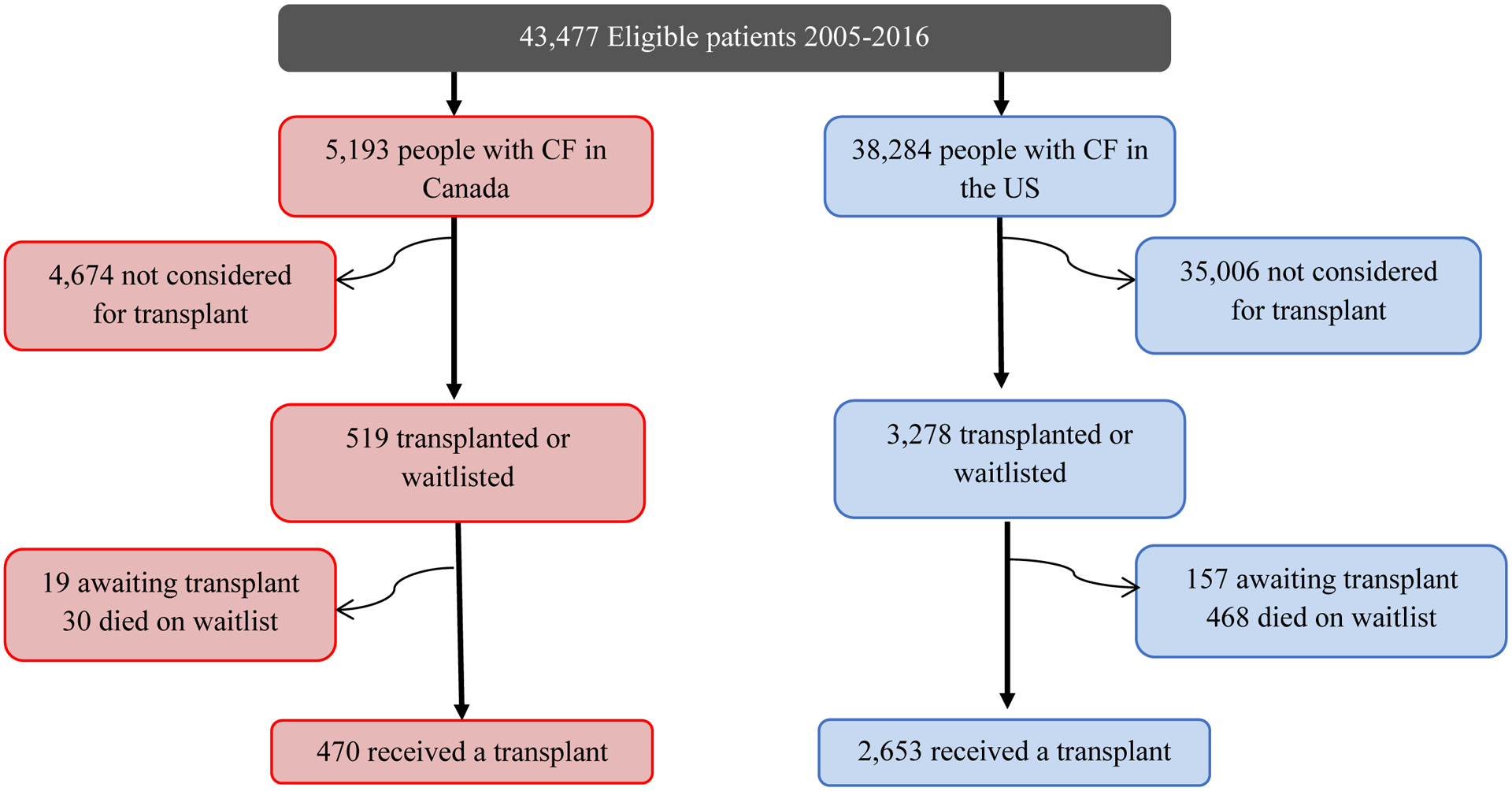
Flow diagram of study population
Table 1:
Clinical/demographic characteristics at baseline and summary of outcomes, Transplanted Patients, 2005 to 2016
| Female | 226 (48.1) | 1,301 (49.0) |
| Adult | 445 (94.7) | 2,370 (89.3) |
| Missing | 5 (1.1) | 289 (10.9) |
| Insufficient | 445 (94.7) | 2,609 (98.3) |
| Missing | 1 (0.2) | 0 (0.0) |
| Yes (ever pre-transplant) | 90 (19.1) | 79 (3.0) |
| Yes | 191 (40.6) | 1,437 (54.2) |
| Missing | 30 (6.4) | 274 (10.3) |
| Missing | 35 (7.4) | 361 (13.6) |
| Yes | 249 (53.0) | 1,612 (60.8) |
| Yes | 18 (3.8) | 282 (10.6) |
| 3+ | 237 (50.4) | 1,589 (59.9) |
| 23 (4.9) | 224 (9.5) | |
Number are N (%) unless otherwise specified.
Abbreviations: B. cepacia complex, Burkholderia cepacia complex; BMI, body mass index; CF, cystic fibrosis; CFRD, cystic fibrosis-related diabetes; DF508, delta F508 genotype; FEV1 % predicted, forced expiratory volume in 1 second percent predicted; LTFU, lost-to-follow-up; PEx, pulmonary exacerbation; Std Diff, standardized difference
Toatl number of patients followed in the time period from Canada was 5,193 and 38,256 from the US. Clinical values are summarized based on the last recorded measurement in the 3-years prior to lung transplantation.
The standardized difference is the mean difference as a percentage of the average standard deviations. A standardized difference greater than 10 is generally used to determine those variables that remain sufficiently different between the two countries.
The patient’s first transplant of any type is considered only. Pediatric patients are those < 18 years of age, adult patients are 18 years of age and older.
Percent predicted FEV1 was calculated using the GLI reference values using the subject’s FEV1 value from the most recent year of follow-up.
BMI categories are defined using the WHO classification. The patient’s BMI in the most recent year of follow-up was used. BMI was classified as 1) underweight if their BMI percentile was less than 12% for pediatrics (defined as age<19) or BMI under 18.5 kg/m2 for adults, 2) adequate weight if their BMI percentile was 12–84% for pediatrics or BMI 18.5–24.9 kg/m2 for adults, or 3) overweight if their BMI percentile was over 84% for pediatrics or BMI over 24.9 kg/m2 for adults.
BiPAP use in the year prior to transplant.
Number of pulmonary exacerbations in the year prior to transplant.
Lost to follow-up is defined as patients who are alive but whose last available year of data occurs more than 2 years before the cohort end year. That is, a patient would be considered lost to follow-up if their last available reporting year is 2014 or earlier.
Median post-transplant survival was 7.8 years (95% CI 7.2–8.4) for the US. The median post-transplant survival time for Canada is greater than 12 years but the exact time could not be estimated as the curve did not cross the 50% mark.(Figure 2). The 1-, 3- and 5-year survival rates were 88.3%, 71.8% and 60.3% in the US compared to 90.5%, 79.9% and 69.7% in Canada.
Figure 2:
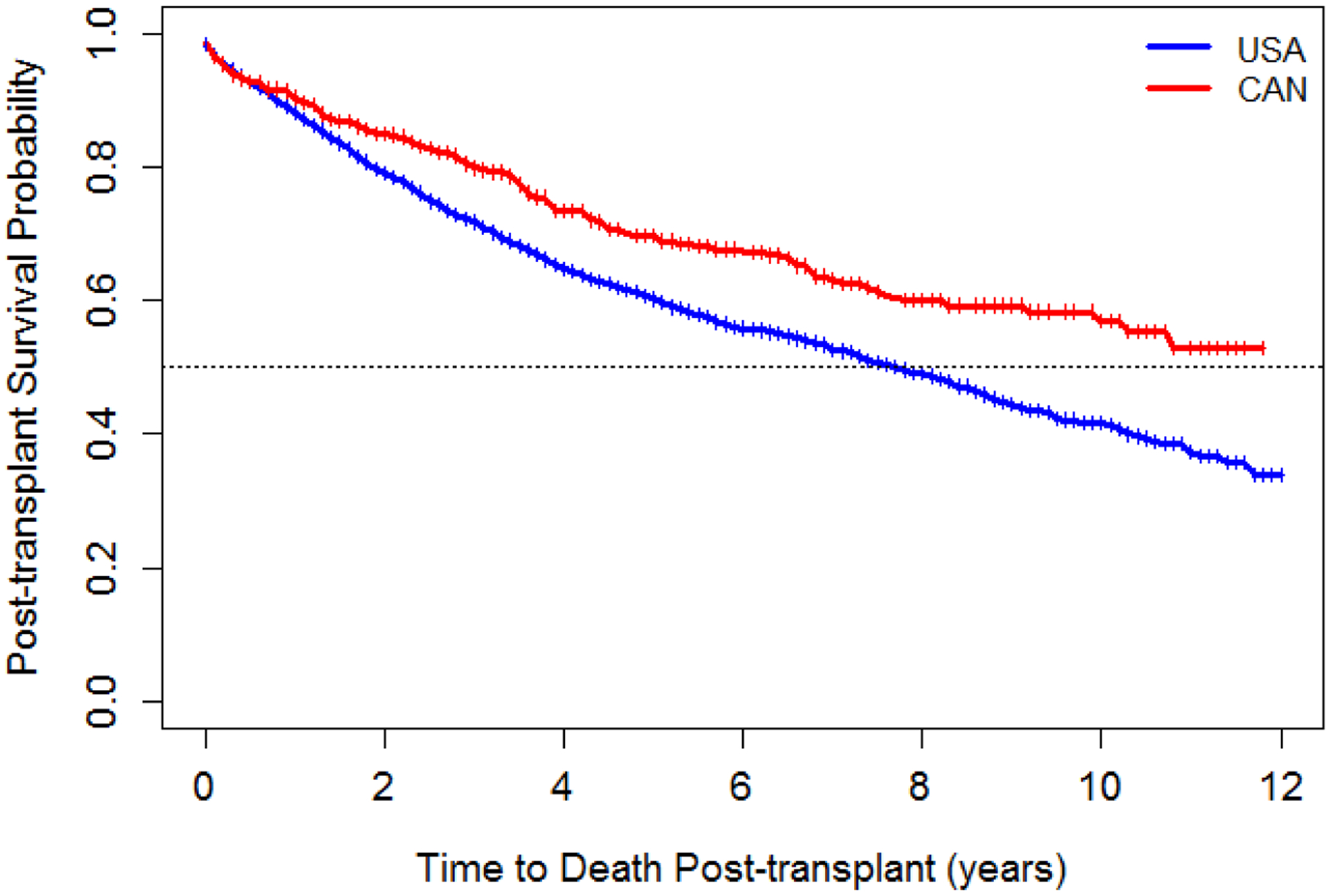
Post-transplant survival after first lung transplant by country, 2005–2016*
*The vertical bars on these figures represent the timepoints at which each patient was censored without experiencing the event
Between 2005 and 2016, out of 2,994 US and 463 Canadian patients with listing dates, there were 474 (15.8%) and 30 (6.5%) deaths on the wait list in the US and Canada, respectively. The median wait list time for those that received a transplant (Group 1) was 3.6 months (IQR 1.2–10.8) versus 7.2 months (IQR 2.4–15.6) in the US and Canada respectively. Median time on the waitlist for those who died prior to transplant (Group 2) was 4.8 months (IQR: 1.2–12) in the US and 2.4 months (IQR: 1.2–7.2) in Canada.
Survival by insurance status is shown in Figure 3. US patients were classified by insurance status with 1,577 (59.4%) on Medicare/Medicaid, 920 (34.7%) on other insurance (891/920 or 96.8% indicated private insurance) and 156 (5.9%) patients with missing insurance status. Post-transplant survival by insurance status revealed worse survival for those on Medicare/Medicaid compared to other insurance. The median survival for those with other insurance was 10.2 years (95% CI 9.1–11.1) whereas for those on Medicare/Medicaid it was 6.6 years (95% CI 5.7–7.4). Of note, survival curves for Canada and US patients with other insurance were not statistically different from one another (HR=1.11, 95% CI: 0.91–1.36) whereas US patients on Medicare/Medicaid were 1.56 (95% CI: 1.30–1.88) times more likely than people with CF in Canada to die after transplant.
Figure 3:
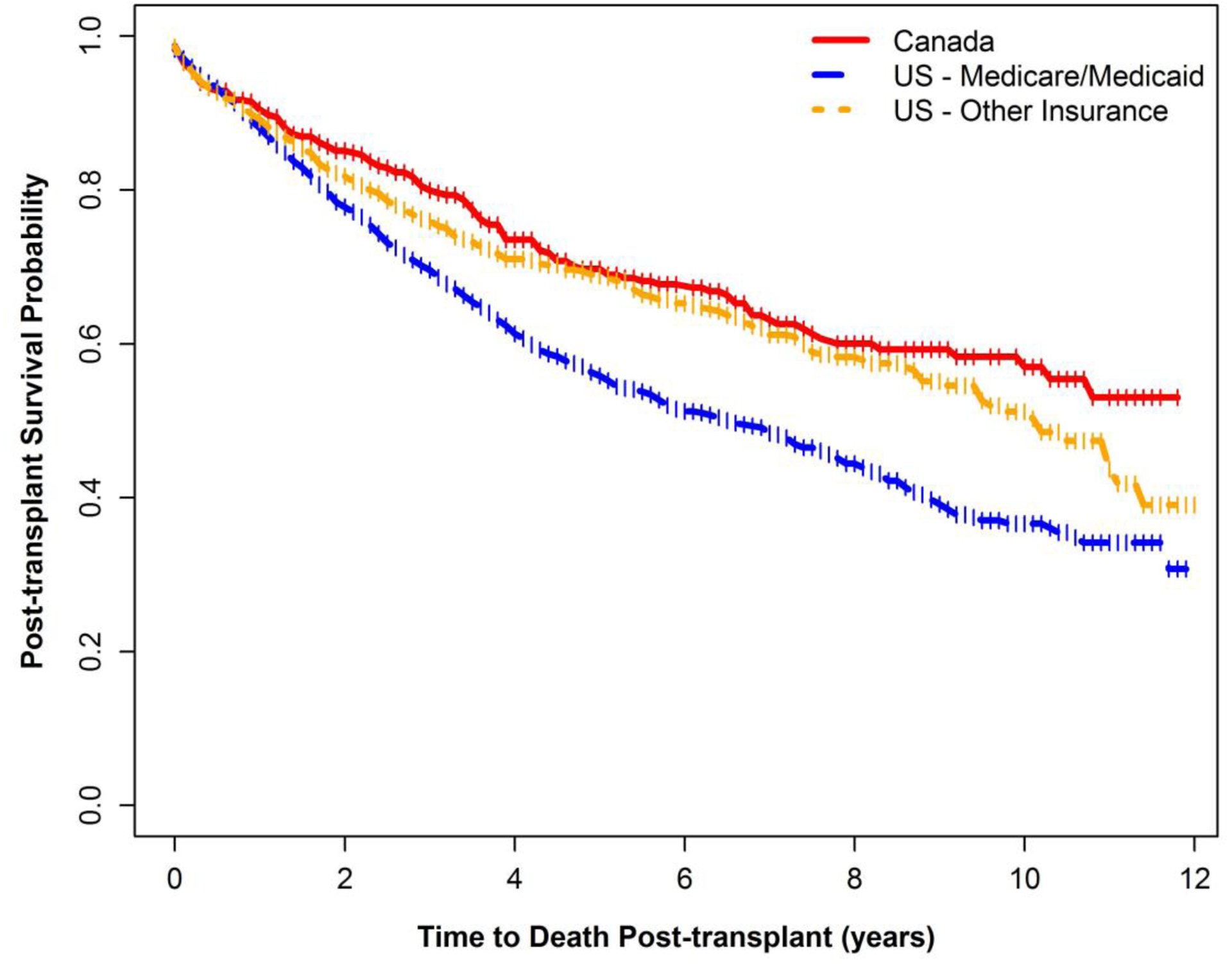
Post-transplant survival after first lung transplant by country and insurance status, 2005–2016*
*The vertical bars on these figures represent the timepoints at which each patient was censored without experiencing the event
There were 1,658 (62.4%) patients transplanted at a high transplant-volume centers in the US, 955 (36.0%) transplanted at low-volume centers and 40 (1.5%) patients who did not have transplant center indicated and could not be assigned a transplant center volume. The median post-transplant survival for US high-volume centers was 8.7 years (95% CI 7.9-N/A) compared to 6.2 years (95% CI 5.1–7.0) at US low-volume centers. Patients transplanted at a low-volume US center were 1.32 times (95% CI 1.16–1.49) more likely to die compared to patients transplanted at a high-volume US center. Patients at high-volume centers in the US were significantly more likely to die following transplant compared to Canadian patients (HR=1.32, 95% CI: 1.10–1.59), whereas US patients transplanted at low-volume centers were 1.74 times more likely to die following transplant compared to Canadian patients (95% CI: 1.44–2.11) (Table S3 in supplement).
The 1-, 3-, and 5-year survival estimates from the sensitivity analyses excluding those with B. cepacia complex infection prior to transplant and censoring at the time of second transplant can be found in the Supplement (Table S5).
Multivariable model
After adjusting for confounding variables and factors known to be associated with post-transplant survival, US patients were 1.5 times more likely to die post-transplant compared to people with CF in Canada (adjusted HR 1.53, 95% CI 1.26–1.84). (Table 2) Other pre-transplant factors associated with an increased risk of death after transplant included CFRD (HR 1.14 (95% CI 1.01–1.29), B. cepacia complex (HR 1.40, 95% CI 1.03–1.91), being underweight (HR 1.34, 95% CI 1.18–1.52), and age at diagnosis < 2 years (HR 1.19, 95% CI 1.04–1.35). The results were unchanged after adjusting for transplant center volume (both total volume and CF-specific volume) as well as when used as a continuous or categorical variable within the model (see Supplement Tables S1–S4).
Table 2:
Cox modeling for time to death after first lung transplant, 2005–2016
| Variable | Unadjusted HR | 95% CI | Adjusted HR | 95% CI |
|---|---|---|---|---|
| Country (US vs. CAN) | 1.45 | 1.22–1.74 | 1.53 | 1.26–1.84 |
| Gender (Female vs. Male) | 1.07 | 0.96–1.20 | 1.004 | 0.89–1.13 |
| Age at dx (<2 years vs. ≥ 2years) | 1.25 | 1.09–1.42 | 1.19 | 1.04–1.35 |
| Pancreatic Status (I vs. S) | 1.22 | 0.81–1.85 | N/Aa | |
| CFRD (pre-transplant) | 1.18 | 1.05–1.33 | 1.14 | 1.01–1.29 |
| PSA alone vs Neither | 0.92 | 0.78–1.08 | 0.89 | 0.76–1.05 |
| Race (Caucasian vs Other) | 0.81 | 0.58–1.12 | 0.83 | 0.6–1.16 |
| Underweight vs Adequate Weight | 1.35 | 1.19–1.52 | 1.34 | 1.18–1.52 |
| FEV1 % Predicted (pre-transplant) | 1.005 | 1.00–1.01 | 1.005 | 1–1.01 |
| 3+ | 1.17 | 1.00–1.37 | 1.15 | 0.98–1.35 |
Abbreviations: B. cepacia complex, Burkholderia cepacia complex; BMI, body mass index; CAN, Canada; CF, cystic fibrosis; CFRD, cystic fibrosis-related diabetes; CI, confidence interval; DF508, delta F508 genotype; Dx, diagnosis; FEV1 % predicted, forced expiratory volume in 1 second percent predicted; I, insufficient; N/A, not available; PSA, Pseudomonas aeruginosa; PEx, pulmonary exacerbation; S, sufficient; US, United States.
A significant correlation was seen between CFRD pre-transplant and pancreatic status, therefore, both variables could not be added to the multivariable model at the same time. Only CFRD was included in final model.
Microbiology is categorized into three mutually exclusive groups: if patient has ever grown B. cepacia complex then classified as B. cepacia complex, if they have grown PSA but never B. cepacia complex, they are classified as PsA alone and if they have never grown B. cepacia complex or PSA they are classified as neither.
BMI categories: Patients are classified as overweight if they are older than 19 years of age and their BMI is more than 24.9 kg/m2 or if they are under 19 years of age and their bmi percentile is more 85% or higher and they are classified as underweight if they are older than 19 years of age and their BMI is less than 18.5 kg/m2 or they are younger than 19 years of age and their bmi percentile is less than 12%.
Reference group for pulmonary exacerbations is none.
Simulation study: contribution of transplant to survival gap
The overall median age of survival for people with CF (including post-transplant survival time) in the 5-year window 2012–2016 was 41.8 years for the US and 52.6 years for Canada with a survival gap of 10.8 years between countries (see Supplement). If the proportion of transplanted patients in the US and the post-transplant survival was increased to match that of Canada during the 2005–2016 period (i.e. 9.1% transplanted and 12 years post-transplant survival) the resulting median age of survival in the US increased to 45.1 years (95% percentile interval (PI) 44.5–45.9) thus reducing the survival gap to 7.5 years. Therefore, assuming a “best case scenario”, by increasing the transplant rate and post-transplant survival in the US to match that seen in Canada there is potential to decrease the survival gap by 30.6%, from 10.8 years to 7.5 years.
DISCUSSION
Our study demonstrates that differences in wait-list mortality and post-transplant survival between the US and Canada contribute to the observed survival gap. A smaller proportion of the CF population in the US as compared to Canada is listed for transplant, and individuals in the US are twice as likely to die waiting for a transplant. In addition, people with CF in Canada on average live 4 years longer post-transplant than recipients in the US. Simulation analysis suggest that these factors may explain nearly one-third of the overall survival gap seen between the two countries.
The significantly higher number of deaths on the waiting list in the US raises the possibility that individuals may be referred or listed too late for this life-saving therapy. For instance, we previously showed that a higher proportion of US patients were malnourished pre-transplant compared to CF patients in Canada.(8) Merlo et al. showed that CF patients who were transplanted since the LAS was implemented had a higher risk of post-transplant death compared to those pre-LAS suggesting that patients were sicker going into transplant.(14) Thus, although the LAS seems to have reduced deaths on the wait-list in the US, it also may have led to patients being sicker at time of transplant. Recent guidelines have recommended earlier referral to transplantation for CF patients as well as including CF-specific variables to the LAS in order to improve discrimination among waitlisted CF patients.(12, 15) US CFFPR data from 2001–2008 showed decreased likelihood of referral for transplant among individuals with Medicaid insurance or those with less than a high school education.(16) Given the known association of Medicaid insurance status with socioeconomic status (SES), it is reasonable to infer that socioeconomic factors play a role in access to transplant in the US. Implementing strategies to increase access and reduce barriers to lung transplantation in those with end-stage lung disease may reduce deaths on the waiting list and narrow the survival gap between the countries.
The reasons for differential survival post-lung transplantation between Canada and the US are likely multi-factorial and beyond the scope of this paper. Possible causes include differential use of strategies (i) to bridge patients to transplant(17, 18), (ii) to expand the donor pool such as the use of ex-vivo lung perfusion(19, 20), donation after cardiac death, or more recently accepting hepatitis C virus positive donors, (iii) to determine timing of transplant (e.g. the severity of illness prior to transplant), or (iv) to manage patients post-transplant (i.e. use of plasmapheresis or IVIg in patients with antibodies). Improved survival in Canada compared to the US has been documented in other solid organ transplants. Kim et al. showed that mortality after renal transplant was significantly higher in Canada compared to the US.(21) Similar to our study, the mortality risk in the first year after transplant was similar in both countries but significantly higher mortality was seen in the US beyond the first year. Another potential contributing factor may be the involvement of CF centers in the care of post-transplant CF patients. In Canada, although the transplant team is heavily involved in clinical care following lung transplantation, primary CF centers often remain involved in the management of non-pulmonary complications including CFRD and gastrointestinal complications. There is variability in the US with respect to CF center involvement in post-transplant care for individuals with CF and CF Foundation guidelines for post-transplant management are currently in progress in the US. This study confirms that high transplant-volume centers in the US have better post-transplant survival compared with low-volume centers, which is consistent with previous research (9, 22); however, high-volume centers still have shorter survival than Canadian centers. Therefore, in addition to surgical experience, other aspects of transplant care between the countries must be investigated in order to understand the differences in outcomes related to transplant volume. Further targeted research is required to fully understand differences in the peri- and post-transplant period that may contribute to the survival differences. Future targeted research is necessary to fully understand the factors associated with the post-transplant survival differences reported in this study.
Beyond lung transplant center volume, additional center-specific factors most likely impact post-transplant survival. Although such granularity exists within UNOS data a comparable national transplant database does not exist within Canada. The Canadian CF Registry does not capture detailed information regarding the surgical procedure nor the transplant specific information on donors and recipients making it impossible to evaluate the impact of such factors between the two countries. It is also important to note that a 3-year difference in life expectancy has been documented between Canada and the US in the general population. One might expect that this discrepancy may also translate to the CF population and contribute to the CF survival gap. However, one must keep in mind that the 10-year survival gap seen in CF is in median survival age while the survival gap in the general population refers to life expectancy. These are two distinct metrics that cannot be directly compared as they represent different aspects of survival.
International comparisons between Canada and the US in cancer patients showed important discrepancies with better survival in Canada even when socioeconomic status was taken into consideration.(23) Some researchers have suggested that the differences in the health care system between the countries significantly contribute to the observed disparities although this is yet to be proven. Within our study, individuals receiving Medicare/Medicaid within 3 years prior to their lung transplant had significantly worse post-transplant survival than those patients who had other insurance, which was primarily comprised of private insurance coverage. More importantly, those with private insurance had similar post-transplant survival to people with CF in Canada, a pattern that was seen in our earlier study that examined overall survival differences.(4) Medicare/Medicaid has been used in prior studies as a surrogate for SES and social determinants of health.(24, 25) The Canadian registry does not have a direct measure of SES per se. Since people with CF in both Canada and the US can be expected to incur significant expenses, including relocation costs in order to receive a transplant, it is possible that the impact of social inequality is greater for people living in the US compared with Canada. While insurance coverage for pre-transplant clinic visits is not likely limited for Medicaid/Medicare patients in the US(16, 24), it is possible that other psychosocial determinants of health may play a role in access to pre- and post-transplant care. Differences between Canada and the US in post-transplant outpatient clinic attendance have not been well-studied. Access to medications after transplant may be limited by an inability to afford co-pays in the US, which may disproportionately affect individuals with lower SES whereas post-transplant medications are generally covered by insurance or government plans in Canada. Furthermore, there are additional complex factors beyond insurance markers of socioeconomic status that contribute to worsened health outcomes. These include food insecurity, housing instability, mental health and addiction issues, adherence to therapy, as well as unstable social/family environments which could significantly impair one’s ability to maintain their health. These factors exist in both countries and targeted research to evaluate the differences and impact of these factors is warranted. Finally, it is worth noting that a significant proportion of deaths in both countries occurred without evidence of referral to or receipt of lung transplantation. Although these patients may have had factors that precluded transplantation, some individuals may not have been considered at all. This represents an opportunity to improve access to this potentially life-saving therapy within both countries and is a focus of future research. Post-transplant survival appeared to be similar between the two countries in the first year after which the survival curves diverged. It is possible that differences in the approach to surveillance or monitoring post-transplant between the countries impact the development of chronic lung allograft dysfunction (CLAD) which is the main long-term cause of death post-transplant. Furthermore, differences between the countries with respect to colon cancer screening or complication rates of CFRD (i.e. renal failure, poor glycemic control) may contribute to worse outcomes. Detailed post-transplant data were not available with the CF registries to adequately evaluate these issues thus further study in this area is needed to address these important questions.
The strengths of our study include population-based longitudinal data captured within both registries with low rates of loss to follow up. The linkage to UNOS allowed for more accurate capture of key data such as transplant and vital status. Further, the differential survival was seen across several sub-group analyses suggesting the results are robust. Limitations of our study must also be acknowledged. Probabilistic linkage of the US CFFPR to UNOS may have resulted in mismatched patients. However, over 85% of the linked patients matched on last name, first name, gender and date of birth suggesting that matches were accurate for the majority of linked patients. Adjusting for post-transplant clinical factors was not possible because neither registry had complete post-transplant clinical data. By design, waitlist mortality may be slightly overestimated as transplanted patients who were missing a listing date were excluded from the calculation. Excluding those patients in the calculation creates a potential bias towards the null therefore the reported difference in the deaths on the waiting list is conservative. Further, we estimate a small number of individuals are missing listing dates thus we are confident this has a negligible impact on our results. Finally, outcomes of heart-lung/lung-liver transplant recipients may differ from lung only transplant patients; however, this represented a very small proportion (<1%) of our cohort therefore the impact is expected to be minimal.
In conclusion, to our knowledge this is the first study to systematically compare post-transplant survival and health outcomes for individuals with CF in Canada and the US. Differential rates of death without transplant and post-transplant survival rates explain ~30% of the survival gap between the two countries. Further, private health insurance and high-volume transplant centers in the US are associated with better survival. Targeted research is needed to investigate differences in peri- and post-transplant management of transplanted patients to further understand the remaining reasons for differential transplant-related survival and ultimately to implement strategies to further bridge the overall survival gap between the countries.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge the financial support of the US CFF which made this study possible (Grant #STEPHE14A0). Also, we are grateful to Cystic Fibrosis Canada and the US CFF for providing registry data for this project. In addition, we would like to acknowledge and thank all of the CF patients and families in the US and Canada who consent to be part of their respective national CF patient registries as well as the CF clinic staff who spend many hours inputting the data.
Funding
CHG receives funding from the Cystic Fibrosis Foundation, the NIH (R01HL103965, R01HL113382, R01AI101307, U M1HL119073, P30DK089507) and the FDA (R01FD003704). KJR receives funding from the Cystic Fibrosis Foundation (RAMOS17A0) and the NIH (K23HL138154).
Footnotes
Conflict of Interest Statement
Conflict of interest statement: Several authors (JSO, A. Fink, BCM, A. Faro, KP, AE) work at the Cystic Fibrosis Foundation (CFF) in the United States. Multiple authors (KJR, BSQ, CHG, ALS) receive grant funding from the US CFF. ALS receives funding from CF Canada outside of this work; as well as personal fees from Vertex Pharmaceuticals Inc. BSQ reports personal fees from Vertex Pharmaceuticals and from Proteostasis Therapeutics, grants from CF Canada, grants from CF Foundation, grants from Gilead Sciences, grants from BC Lung Association, all of which are outside of the submitted work. None of these financial relationships influenced the interpretation or reporting of the current study.
REFERENCES
- 1.Simmonds NJ. Ageing in cystic fibrosis and long-term survival. Paediatric respiratory reviews 2013; 14 Suppl 1: 6–9. [DOI] [PubMed] [Google Scholar]
- 2.Stephenson AL, Tom M, Berthiaume Y, Singer LG, Aaron SD, Whitmore GA, Stanojevic S. A contemporary survival analysis of individuals with cystic fibrosis: a cohort study. The European respiratory journal 2015; 45: 670–679. [DOI] [PubMed] [Google Scholar]
- 3.Stephenson AL, Stanojevic S, Sykes J, Burgel PR. The changing epidemiology and demography of cystic fibrosis. Presse medicale (Paris, France : 1983) 2017; 46: e87–e95. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson AL, Sykes J, Stanojevic S, Quon BS, Marshall BC, Petren K, Ostrenga J, Fink AK, Elbert A, Goss CH. Survival Comparison of Patients With Cystic Fibrosis in Canada and the United States: A Population-Based Cohort Study. Annals of internal medicine 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramos KJK SG; Bradford MC;, Somayaji R; Morrell ED; Pilewski JM; Lease ED; Mulligan MS; Aitken MA; Gries CJ; Goss CH;. Underweight patients with cystic fibrosis have acceptable survival after lung transplantation: a UNOS registry study. Chest 2019; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephenson AL, Sykes J, Berthiaume Y, Singer LG, Aaron SD, Whitmore GA, Stanojevic S. Clinical and demographic factors associated with post-lung transplantation survival in individuals with cystic fibrosis. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2015; 34: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 7.Sykes J, Stanojevic S, Goss CH, Quon BS, Marshall BC, Petren K, Ostrenga J, Fink A, Elbert A, Stephenson AL. A standardized approach to estimating survival statistics for population-based cystic fibrosis registry cohorts. Journal of clinical epidemiology 2016; 70: 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quon BS, Sykes J, Stanojevic S, Marshall BC, Petren K, Ostrenga J, Fink A, Elbert A, Faro A, Goss CH, Stephenson AL. Clinical characteristics of cystic fibrosis patients prior to lung transplantation: An international comparison between Canada and the United States. Clinical transplantation 2018; 32: e13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilic A, Gleason TG, Kagawa H, Kilic A, Sultan I. Institutional volume affects long-term survival following lung transplantation in the USA. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery 2019. [DOI] [PubMed] [Google Scholar]
- 10.Austin PC, Mamdani MM. A comparison of propensity score methods: a case-study estimating the effectiveness of post-AMI statin use. Statistics in medicine 2006; 25: 2084–2106. [DOI] [PubMed] [Google Scholar]
- 11.van Buuren S, Groothuis-Oudshoorn K. MICE: Multivariate imputation by chained equations in R. J Statistical Software 2011; 45: 1–67. [Google Scholar]
- 12.Ramos KJ, Smith PJ, McKone EF, Pilewski JM, Lucy A, Hempstead SE, Tallarico E, Faro A, Rosenbluth DB, Gray AL, Dunitz JM. Lung transplant referral for individuals with cystic fibrosis: Cystic Fibrosis Foundation consensus guidelines. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society 2019; 18: 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapnadak SG, Dimango E, Hadjiliadis D, Hempstead SE, Tallarico E, Pilewski JM, Faro A, Albright J, Benden C, Blair S, Dellon EP, Gochenour D, Michelson P, Moshiree B, Neuringer I, Riedy C, Schindler T, Singer LG, Young D, Vignola L, Zukosky J, Simon RH. Cystic Fibrosis Foundation consensus guidelines for the care of individuals with advanced cystic fibrosis lung disease. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society 2020. [DOI] [PubMed] [Google Scholar]
- 14.Merlo CA, Weiss ES, Orens JB, Borja MC, Diener-West M, Conte JV, Shah AS. Impact of U.S. Lung Allocation Score on survival after lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 2009; 28: 769–775. [DOI] [PubMed] [Google Scholar]
- 15.Lehr CJ, Skeans M, Dasenbrook EC, Fink A, Fernandez G, Faro A, Valapour M. Effect of Including Important Clinical Variables on Accuracy of the Lung Allocation Score for Cystic Fibrosis and Chronic Obstructive Pulmonary Disease. American journal of respiratory and critical care medicine 2019. [DOI] [PubMed] [Google Scholar]
- 16.Ramos KJ, Quon BS, Psoter KJ, Lease ED, Mayer-Hamblett N, Aitken ML, Goss CH. Predictors of non-referral of patients with cystic fibrosis for lung transplant evaluation in the United States. Journal of cystic fibrosis : official journal of the European Cystic Fibrosis Society 2016; 15: 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyoda Y, Bhama JK, Shigemura N, Zaldonis D, Pilewski J, Crespo M, Bermudez C. Efficacy of extracorporeal membrane oxygenation as a bridge to lung transplantation. The Journal of thoracic and cardiovascular surgery 2013; 145: 1065–1071. [DOI] [PubMed] [Google Scholar]
- 18.Hoopes CW, Kukreja J, Golden J, Davenport DL, Diaz-Guzman E, Zwischenberger JB. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. The Journal of thoracic and cardiovascular surgery 2013; 145: 862–867; discussion 867–868. [DOI] [PubMed] [Google Scholar]
- 19.Cypel M, Yeung JC, Liu M, Anraku M, Chen F, Karolak W, Sato M, Laratta J, Azad S, Madonik M, Chow CW, Chaparro C, Hutcheon M, Singer LG, Slutsky AS, Yasufuku K, de Perrot M, Pierre AF, Waddell TK, Keshavjee S. Normothermic ex vivo lung perfusion in clinical lung transplantation. The New England journal of medicine 2011; 364: 1431–1440. [DOI] [PubMed] [Google Scholar]
- 20.Cypel M, Keshavjee S. Extending the donor pool: rehabilitation of poor organs. Thoracic surgery clinics 2015; 25: 27–33. [DOI] [PubMed] [Google Scholar]
- 21.Kim SJ, Schaubel DE, Fenton SS, Leichtman AB, Port FK. Mortality after kidney transplantation: a comparison between the United States and Canada. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 2006; 6: 109–114. [DOI] [PubMed] [Google Scholar]
- 22.Thabut G, Christie JD, Kremers WK, Fournier M, Halpern SD. Survival differences following lung transplantation among US transplant centers. Jama 2010; 304: 53–60. [DOI] [PubMed] [Google Scholar]
- 23.Gorey KM. Breast cancer survival in Canada and the USA: meta-analytic evidence of a Canadian advantage in low-income areas. International journal of epidemiology 2009; 38: 1543–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. American journal of respiratory and critical care medicine 2001; 163: 1331–1337. [DOI] [PubMed] [Google Scholar]
- 25.Quon BS, Psoter K, Mayer-Hamblett N, Aitken ML, Li CI, Goss CH. Disparities in access to lung transplantation for patients with cystic fibrosis by socioeconomic status. American journal of respiratory and critical care medicine 2012; 186: 1008–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


