Abstract
The human nervous system is one of the most complicated systems in nature. Complex nonlinear behaviours have been shown from the single neuron level to the system level. For decades, linear connectivity analysis methods, such as correlation, coherence and Granger causality, have been extensively used to assess the neural connectivities and input-output interconnections in neural systems. Recent studies indicate that these linear methods can only capture a small amount of neural activities and functional relationships, and therefore cannot describe neural behaviours in a precise or complete way. In this review, we highlight recent advances in nonlinear system identification of neural systems, corresponding time and frequency domain analysis, and novel neural connectivity measures based on nonlinear system identification techniques. We argue that nonlinear modelling and analysis are necessary to study neuronal processing and signal transfer in neural systems quantitatively. These approaches can hopefully provide new insights to advance our understanding of neurophysiological mechanisms underlying neural functions. These nonlinear approaches also have the potential to produce sensitive biomarkers to facilitate the development of precision diagnostic tools for evaluating neurological disorders and the effects of targeted intervention.
Introduction
The human nervous system is a complicated network comprised of more than 10 billion neurons, with trillions of synapses connecting them. Neuronal information processing is complex at different levels, from the microscopic pre- and post-synaptic cellular interactions to the macroscopic interactions between large populations of neurons in, for instance, sensory processing and motor response (Stanley, 2005). The behaviour of a single neuron is highly nonlinear, showing a step-like ‘none-or-all’ firing response (Koch and Segev, 2000), while the behaviour of neurons in a population could be relatively similar. Therefore, the nonlinear response of each individual neuron may be smoothed out by the distribution of membrane thresholds across the population, known as the pool effect (Rosenbaum et al., 2010). This effect typically occurs in a mono-synaptic neural system such as the cortico-spinal tract where the supraspinal motor command is linearly transferred to the motor output due to the pool effect of motor units (Negro and Farina, 2011). However, multi-synaptic neural systems, such as the somatosensory system, have been reported highly nonlinear, showing harmonic responses to periodic stimuli (Langdon et al., 2011; Yang et al., 2016a; Ren et al., 2019; Yang et al., 2020b). Cross-frequency coupling in the corticothalamic interactions has also been reported when characterising essential tremor (He et al., 2016). Nonlinear behaviours in neural systems are thought to be associated with various neural functions, including neuronal encoding, neural processing of synaptic inputs, communication between different neuronal populations and functional integration (Friston, 2001; Yang et al., 2018; Fell and Axmacher, 2011; French and Korenberg, 1989).
Various functional and effective connectivity measures have been developed (Pereda et al., 2005) to characterise such linear and nonlinear functional integration in neural networks, from large-scale neurophysiological signals. These signals, such as electroencephalogram (EEG) and magnetoencephalogram (MEG) from the brain, electromyogram (EMG) from muscles, measure neural activities from macro-scale neuronal populations. While functional connectivity measures, e.g. correlation, coherence, mutual information, only quantify the undirected statistical dependencies among signals from different areas, effective connectivity attempts to quantify the directed causal influences of one neural system over another, either at a synaptic or population level (Friston, 2011). Mostly, effective connectivity measures are based on models of neural interactions or coupling (although there exist model-free measures like transfer entropy (Dimitrov et al., 2011)) and is often time-dependent (dynamic). Therefore, effective connectivity has a strong link with dynamic modelling, also known as system identification in control systems theory (Ljung, 1999; Pintelon and Schoukens, 2012; Schoukens and Ljung, 2019), and corresponding model-based causality analysis.
Nonlinear system identification techniques have been formally applied to study neuronal information processing and neural systems since the 1970s. Some pioneering work includes: the nonlinear dynamic modelling of the retinal neuron chains in receptive-field responses (Marmarelis and Naka, 1973a,b), the identification of nonlinear synaptic interactions (Brillinger et al., 1976), the identification of neural systems using stimulus-response and white-noise approach (Marmarelis and Marmarelis, 1978; Marmarelis and Naka, 1972; Marmarelis, 2012), the development of nonlinear systems analytic approach based on functional power (or Volterra/Wiener) series to study central nervous system function and hippocampal formation (Sclabassi et al., 1988b,a), and nonlinear identification of stretch reflex dynamics (Kearney and Hunter, 1988). Until now, many linear and nonlinear system identification methods have then been proposed and developed in the neuroscience context. Nevertheless, recent studies indicate that linear methods can only capture a small amount of neural activities and functional relationships, and therefore cannot describe neural behaviours in a precise or complete way (Vlaar et al., 2016, 2017). Nonlinear approaches provide us with useful tools to explore the nonlinear nature of neural systems (Friston, 2001). In this review article, we highlight the need and recent advances in nonlinear system identification of neural systems, as well as novel neural connectivity analysis methods based on nonlinear system identification techniques. A diagram that summarises the linear and nonlinear functional and effective connectivity measures and their links with system identification is provided in Figure 1.
Figure 1:
Overview of the linear and nonlinear functional and effective connectivity (causality) measures and their links with system identification methods. The linear functional connectivity, linear system identification and linear causality measures are first reviewed in the ‘Linear connectivity and system identification’ section. The nonlinear and nonlinear time-varying system identification approaches (in both time and frequency domains) are then investigated in the ‘Nonlinear system identification of neural systems’ section. The recently proposed nonlinear function connectivity measures and nonlinear causality measures (based on nonlinear system identification) are introduced in the ‘Nonlinear neural connectivity analysis’ section. The abbreviations in the diagram are defined as: autoregressive (AR), autoregressive with exogenous input (ARX), frequency response function (FRF), nonlinear autoregressive (moving average) model with exogenous inputs (NAR(MA)X), artificial neural networks (ANN), deep neural networks (DNN), generalised frequency response function (GFRF), output frequency response function (OFRF), time-varying (TV), Granger causality analysis (GCA), partial directed coherence (PDC), directed transfer function (DTF), spectral Granger causality and directed coherence (DCOH), Granger causality (GC), dynamic causal modelling (DCM), nonlinear partial directed coherence (NPDC).
Nonlinearity in the neuronal level and neural systems
At a single neuron level, the action potential spike is the principal basis of information encoding, which allows signal transmission across different neuronal populations (French and Korenberg, 1989). The spike timing is thought to be associated with the coding scheme in neural systems (Fetz, 1997). The nonlinear nature of the neuronal process of synaptic input influences the temporal firing behaviour of individual neurons. Different types of neurons have their own repertoire of ion channels that are responsible for their characteristic nonlinear firing patterns and associated neural functions. For example, persistent inward currents mediated by their voltage-gated sodium and calcium channels are an important source of the nonlinear behaviour of spinal motoneurons. They are instrumental in generating the sustained force outputs required for postural control (Hounsgaard et al., 1988). Activation of the L-type calcium channels in nigral dopaminergic neurons results in intrinsic bursting behaviour (Sinha et al., 2020b), exhibiting low-dimensional determinism and likely encodes meaningful information in the awake state of the brain Jeong et al. (2012). The nonlinearity of the neuronal transfer function mediated by its component ion channels can generate various types of nonlinear output patterns such as harmonic, subharmonic and/or intermodulation of input patterns.
Despite plenty of knowledge of the nonlinear behaviour of a single neuron, the input-output relation at the neural system level is yet to be understood entirely. The system-level neural response is a composite output of collective neuronal activities from a large number of neurons. In a neuronal population, the pool effect can reduce the nonlinearity generated from each individual neuron, by smoothing the neuronal dynamics from a scale of milliseconds (spikes) to 10 milliseconds (local field potentials) or to 100 milliseconds (large-scale neurophysiological activities or signals such as EEG) (Ros et al., 2014). Such effects have been previously demonstrated through both a computational model and an in vivo study in the human motor system, where the motor command can be transmitted linearly via the mono-synaptic corticospinal tract when more than five motoneurons are activated (Negro and Farina, 2011). However, a small amount of nonlinearity may still be present (Yang et al., 2016b). A recent study simulated nonlinear neuronal dynamics on a large-scale neural network that captured the inter-regional connections of neocortex in the macaque. The authors applied information-theoretic measures to identify functional networks and characterised structure-function relations at multiple temporal scales (Honey et al., 2007). The nonlinearity in each neuronal population can cumulatively increase if the system involves multiple synaptic connections (Sinha et al., 2020a). A recent study in hemiparetic stroke shows that the nonlinearity in the motor system increases due to an increased usage of multi-synaptic indirect motor pathways, e.g. cortico-reticulospinal tract (Li et al., 2019), following damage to the mono-synaptic corticospinal tract (Yang et al., 2020a).
Assessing the input-output relation in neural systems, e.g. sensory, motor and cognitive processes, is essential to a better understanding of the nervous system. For instance, it could help to gain a better insight into the normal and pathological neural functions. It is well known that a linear system generates only iso-frequency interactions between an input and the output, e.g. the coupling of neuronal oscillations at a specific frequency band (Fries, 2015). For decades, correlation and coherence measures have been used to identify the linear interaction in neural systems. More recently, various studies indicate the input-output neural interactions can cross different frequency components or bands, which is named cross-frequency coupling (Jensen and Colgin, 2007; Canolty and Knight, 2010; Hyafil et al., 2015; Aru et al., 2015) and is a distinctive feature of a nonlinear system. In the following sections, we review both the linear and nonlinear approaches for identifying neural systems and associating neural connectivity, especially from a system identification perspective.
Linear connectivity and system identification
The nervous system is a highly cooperative network composed of different groups of neurons. Neural connectivity, i.e., the synchronization of neural activity across these groups, is crucial to the coordination among distant, but functionally related, neuronal groups (Varela et al., 2001). Linear neural connectivity can be assessed by determining the signal correlation or causality between the recorded neural signals. This section reviews commonly used linear connectivity, system identification methods and their interconnections in studying neural systems.
Correlation and coherence
The most widely used measure of interdependence between two time series in the time domain is the cross-correlation function (Pereda et al., 2005), which measures the linear correlation between two signals or stochastic processes X and Y with discrete observations x(t) and y(t), at t = 1, 2, …, N, as a function of their delay time:
| (1) |
where N is the number of samples and τ the time lag between two signals. This function ranges from −1 (complete linear inverse correlation) to 1 (complete linear direct correlation). The value of τ that maximizes this function can be used to estimate the linearly related delay between signals. The well-known Pearson correlation coefficient is equal to Cxy(τ) when τ = 0. The linear dependence between two signals in the frequency domain is usually measured by the spectral coherence. The coherence between two signals at frequency f is defined as:
| (2) |
where Sxy(f) is the cross-spectral density between x and y, and Sxx(f) and Syy(f) the auto-spectral density of x and y respectively. The cross-spectral and auto-spectral densities are the Fourier transforms of the cross-correlation and auto-correlation functions of the two signals. Values of coherence are always between 0 and 1. The correlation and coherence measures have been widely applied to EEG, MEG or EMG signals to characterise the neuronal interactions, from the firing of cortical neuron spike trains to complicated neural systems (for reviews see (Pereda et al., 2005; Bastos and Schoffelen, 2016)).
System identification and causality
Unlike functional connectivity, effective connectivity emphasises on the directional causal influences between neural areas or signals. Here, we first introduce the classical Granger causality and its link with the time-domain linear system identification, i.e. regression models of bivariate time series. The frequency-domain causality measures can then be linked with the frequency response function of linear systems.
Considering two signals or variables X and Y, the interactions of the signals can be described by bivariate linear autoregressive with exogenous input (ARX) models jointly,
| (3) |
where p and q are the model order of y and x regressors; ex(t) and ey(t) are the uncorrelated model prediction errors over time. A linear causal influence from X to Y defined by Granger can be expressed as a log ratio of the prediction error variances of the corresponding restricted (AR) and unrestricted (ARX) models:
| (4) |
where x− and y− denotes contributions from lagged input and output terms, respectively; y−22 denotes the variance of ey when there are only regression terms of Y. The linear ARX models (3) can be re-written in matrix form and mapped to the frequency domain by Fourier transformation:
| (5) |
where the components of the coefficient matrix A(f) are with fs the sampling frequency and δlm the Kronecker delta function. We can re-write the above equation by inverting the coefficient matrix G(f) = A−1(f) and moving the so-called transfer function matrix G(f) to the right-hand-side the equation. Different frequency-domain Granger causality measures, such as partial directed coherence (PDC), directed transfer function (DTF), spectral Granger causality and directed coherence (DCOH) (Baccalá and Sameshima, 2001; Chicharro, 2011; Gourévitch et al., 2006), can then be expressed as a function of the elements of either the coefficient matrix A(f) or the transfer function matrix G(f) (Baccala and Sameshima, 2001; Chicharro, 2011). By dividing both sides of (5) with the corresponding diagonal elements in the coefficient matrix A, the off-diagonal elements in the transformed coefficient matrix are actually related to the negative frequency response functions (FRFs) of linear ARX systems, if one signal is treated as the input while the other is treated as the output. For instance,
| (6) |
the FRF, HX→Y (f), describes the input-output relationship, i.e., with input X and output Y, of the (noise-free) ‘system’ in the frequency domain. It is also known as the ‘transfer function’ in linear system theory. Frequency-domain Granger causality measure, e.g. PDC, can be expressed as a function of the FRFs of the corresponding linear ARX and AR models:
| (7) |
Establishing such a link between the causality measures and linear system identification, in both time and frequency domains, is crucial to the further development of nonlinear model-based causality measures via nonlinear system identification, which will be investigated in the ‘Nonlinear system identification of neural systems’ and the ‘Nonlinear neural connectivity analysis’ sections.
Limitation of linear approaches on identifying neural system
Linear connectivity and system identification allow the assessment of communication between neuronal populations at the same oscillatory frequency band or similar neuronal firing patterns. The applications of linear approaches have been thoroughly reviewed previously (Sakkalis, 2011; Blinowska, 2011). However, it is not clear how much information is missing when using the linear approach since the behaviour of various neural systems can be highly nonlinear (Friston, 2001; Yang et al., 2018). When one uses a linear measure to investigate a neural system, the nonlinear neural interaction is ignored, especially between the neuronal populations which have very different mean firing rates such as the central nervous system and the periphery. A recent study reported that in the human somatosensory system over 80% of the cortical response to wrist joint sensory input comes from nonlinear interactions, where a linear model explains only 10% of the cortical response (Vlaar et al., 2016). Therefore, nonlinear connectivity and modelling approaches are needed to investigate neural systems in a complete way.
Nonlinear system identification of neural systems
It is often impossible to derive a mechanistic model of a neural system, due to the complexity of the underlying biological process and many unobservable state variables. In this section, we focus on the generic nonlinear model representations of a single-input and single-output (SISO) neural dynamic system, its identification process in the time domain, and corresponding frequency-domain analysis. We first investigate the identification of nonlinear time-invariant systems, and then time-varying nonlinear systems.
Time-domain nonlinear system identification
Volterra series
The Volterra series model is a direct generalisation of the linear convolution integral and provides an intuitive representation for a nonlinear input-output system. The output y(t) of a SISO nonlinear system can be expressed as a Volterra functional of the input signal u(t):
| (8) |
where y(n)(t) is the nth-order output and M is the maximum order of the system’s nonlinearity; hn(τ1, · · ·, τn) is the nth-order impulse response function or Volterra kernel, which describes nonlinear interactions among n copies of input and generalises the linear convolution integral to deal with nonlinear systems. Neurobiologically, Volterra series can be directly interpreted as the effective connectivity - ‘the influence that one neural system exerts over another, either at a synaptic or population level’ (Friston, 1994). The first-order kernel describes the linear ‘driving’ efficacy or linear synchronous interactions, and the second- or higher-order kernels represent the ‘modulatory’ influence or asynchronous interactions (Friston, 2001). The Fourier transform of the first-order kernel is the FRF (or transfer function) that describes the interactions in the same frequencies, while the frequency-domain counterparts of the higher-order kernels are the GFRFs (to be discussed in the ‘Frequency-domain nonlinear system analysis: nonlinear frequency response functions’ section) which quantify the cross-frequency interactions.
Practically, to deal with a large number of Volterra series coefficients, a regularization strategy is often employed in the estimation procedure (Birpoutsoukis et al., 2017). Volterra model has been widely used in physiological systems, including neural systems, modelling. Some recent examples include the study of nonlinear interactions in the hippocampal-cortical neurons (Song et al., 2007), in the spectrotemporal receptive fields of the primary auditory cortex (Pienkowski and Eggermont, 2010), in the sensory mechanoreceptor system (Vlaar et al., 2016), in the human somatosensory system (i.e. the cortical response to the wrist joint sensory input) indicating the dominance of nonlinear response (Vlaar et al., 2017), in multiple-input and multiple-output (MIMO) spiking neural circuits (Lazar and Slutskiy, 2015) and hippocampal memory prostheses (Song et al., 2016). The Volterra model also has a strong theoretical link with the NARMAX model (Billings, 2013) and the dynamic causal modelling (Friston et al., 2003) to be discussed next.
NARMAX model
Although Volterra series can provide an intuitive representation for nonlinear systems, there are several critical limitations including i) it cannot represent severely nonlinear systems; ii) the order of the Volterra series expansion can be very high in order to achieve a good approximation accuracy; however iii) the estimation of high order Volterra kernel requires a large number of data and can be computationally very expensive. Nonlinear Autoregressive Moving Average Model with Exogenous Inputs (NARMAX) model (Leontaritis and Billings, 1985; Billings, 2013) has therefore been developed as an alternative to the Volterra series. NARMAX model normally contains a much smaller number of terms due to the inclusion of output delay terms, and its identification process is computationally more efficient. Similar to the Volterra series, a polynomial Nonlinear Autoregressive Model with Exogenous Inputs, NARX (the simplest NARMAX) model, can be expressed as a summation of a series of output terms with different orders of nonlinearity:
| (9) |
where p + q = n, ki = 1, …, K, and . The number of model terms depends on the order of input and output (q and p) and the maximum lags (K). The NARX model structure and parameters are typically identified based on the forward regression with the orthogonal least squares (FROLS) method (Chen et al., 1989). In cases where the system under study is stochastic with unknown coloured noise, noise moving average (MA) models should be employed to form a full NARMAX model. The identified model can be statistically validated using nonlinear correlation tests (Billings and Voon, 1983; He et al., 2014a).
A wide range of nonlinear systems can be represented by NARMAX method, including systems with exotic nonlinear behaviours such as subharmonics, bifurcations, and chaos, as observed in the human nervous system (Breakspear, 2017). Until now, NARMAX methodology has been employed to develop dynamic models for nonlinear sensory processing circuit from spiking neuron data (Florescu and Coca, 2018) as an improvement to the previous Volterra model-based studies (Lazar and Slutskiy, 2015), to investigate the somatosensory afferent pathways from muscles to the brain (Tian et al., 2018; Gu et al., 2020); as well as to study the corticothalamic nonlinear interactions during tremor active and resting states (He et al., 2016). Apart from efficient time-domain predictive modelling, NARMAX also provides an essential base for the nonlinear frequency-domain analysis, nonlinear time-varying modelling, and nonlinear causality analysis to be discussed in the following sections.
Dynamic causal modelling
Most of the effective connectivity models, e.g. linear and nonlinear autoregressive models, are directly identified from functional neurophysiological signals. However, sometimes it would be more accurate and meaningful to identify the causal interactions of the underlying neuronal activities at the level of neuronal dynamics (Stephan et al., 2007). The aim of dynamic causal modelling (DCM) (Friston et al., 2003, 2019) is to infer such connectivity among brain regions (or sources) under different experimental factors or inputs. A DCM comprises typically two parts: a neuronal part that describes dynamics among neuronal sources and a measurement part that describes how the source dynamics generate measurable observations, e.g. EEG or MEG (Kiebel et al., 2008; David et al., 2006). Therefore, DCM can be naturally expressed as a nonlinear state-space model with hidden states denoting unobserved neuronal dynamics and the observation equation (e.g. the lead-field) assumed linearly in the states. The effective connectivity among those sources can be identified via Bayesian model selection and Bayesian inference of the neuronal model parameters. One strength of DCM is its biophysical and neuronal interpretation of how the neurophysiological signals are generated from the underlying neuronal system, through the generative or forward (state-space) modelling. Due to the complexity and computational cost of Bayesian model selection, DCM is more suitable to investigate the connectivity among pre-defined regions of interest, rather than exploratory analysis of relatively large brain or neural networks (Stephan et al., 2010). Compared to the hypothesis-driven DCM, the NARMAX or Volterra models are more flexible in terms of model structure identification and their direct frequency-domain mapping (to be discussed) is a powerful tool to study the nonlinear cross-frequency interactions between neurological regions.
Other black-box neural nonlinear system identification methods
Apart from the aforementioned three important generic nonlinear model representations, other black-box modelling approaches have also been applied in the ‘neural system identification’ context. For example, artificial neural networks (ANNs), e.g. recurrent, multilayer perceptron, fuzzy, probabilistic neural networks, have often been used as alternatives to classical system identification models. ANNs have been applied to predict neural responses in visual cortex (Lehky et al., 1992; Lau et al., 2002), and to improve the prediction of synaptic motor neuron responses (Meruelo et al., 2016). More recently, deep neural networks (DNNs), such as convolutional neural network (CNN) or recurrent neural network (RNN), are employed to model sensory neural responses, to understand neural computations and to learn feature spaces for neural system identification (McIntosh et al., 2016; Batty et al., 2016; Klindt et al., 2017; Kell and McDermott, 2019; Keshishian et al., 2020). Nevertheless, in the current neuroscience literature, ANNs and DNNs are applied more towards automatic feature extraction and classification problems rather than traditional ‘system identification’. For instance, automatic detection and diagnosis of neurological disorders via a combination of ANN with other nonlinear feature extraction techniques such as approximate entropy and wavelet (Srinivasan et al., 2007; Ocak, 2009; Guo et al., 2010; Subasi, 2006), or direct implementation of DNNs (Zhou et al., 2018; Emami et al., 2019). Nonparametric Bayesian approaches like Gaussian process (GP) is closely related to ANN. GP has recently been used for system identification purpose (Kocijan et al., 2005; Gregorčič and Lightbody, 2008, 2009) and applied to analyse neurophysiological signals (Wu et al., 2015), such as the use of GP modelling for EEG-based seizure detection and prediction (Faul et al., 2007) and heteroscedastic modelling of noisy high-dimensional MEG data (Fyshe et al., 2012). Compared with ANN, GP can be applied to model datasets with small sample size and it has a relatively small number of hyperparameters. Additionally, due to its Bayesian nature, GP can incorporate prior knowledge and specifications into the modelling and can directly capture the model uncertainty. Another well-known system identification paradigm is the nonlinear state-space model (Verdult, 2002; Schön et al., 2011). Its strength in dynamic (latent) state estimation and sequential inference process makes it a suitable candidate in the identification of certain neural systems. The state-space models have been applied to infer neural spiking activity induced by an implicit stimulus observed through point processes (Smith and Brown, 2003), to perform optimal decoding given multi-neuronal spike train data and tracking nonstationary neuron tuning properties (for a review, see (Paninski et al., 2010)), and to perform source localization from neurophysiological signals like MEG and EEG (Fukushima et al., 2012; Barton et al., 2008). All of those black-box modelling approaches are usually flexible and accurate in quantifying complex and long-range nonlinear interactions. In comparison, the advantages of NARMAX and Volterra models are their modelling simplicity, interpretability of nonlinear interactions in the time-domain (e.g. the order of nonlinearity, phase delay), and frequency-domain mapping and analysis (e.g. energy transfer, intermodulations).
Frequency-domain nonlinear system analysis: nonlinear frequency response functions
Many nonlinear effects, such as harmonics, intermodulations and energy transfer, can only be accurately and intuitively characterised in the frequency domain. Thus, it is important to ‘map’ the identified time-domain nonlinear models to the frequency domain for further analysis. A multidimensional Fourier transform of the nth-order Volterra kernel in (8) yields the so-called nth-order generalised frequency response function (GFRF), Hn(f1, · · ·, fn), which is a natural extension of the linear frequency response function to the nonlinear case (Billings, 2013). The output spectrum Y (f) of a nonlinear system can then be expressed as a function of the input spectrum X(f) and GFRF, known as the output frequency response function (OFRF) (Lang and Billings, 1996; Billings, 2013):
| (10) |
Compared with the Volterra series, the GFRFs can be more efficiently computed from the identified time-domain NARMAX model (9) and corresponding model parameters (Billings and Tsang, 1989). As shown in Figure 2, the peaks in 1st-order GFRF indicate the well-known ‘resonance frequencies’ of the linear part of the system; and the peaks (or ridges) in the 2nd-order GFRF would indicate nonlinear harmonics (f1 + f2 when f1 = f2) or inter-modulation effects (f1 ± f2 when f1 ≠ f2) in the output spectrum, and so on. Since , the nth OFRF Yn(f) represents the nth-order (linear or nonlinear) contribution from the input to the output spectrum. Practically, by comparing the OFRF with the spectrum of the output signal, obtained from a classical nonparametric estimation such as fast Fourier transform, one can also ‘validate’ the accuracy of the time-domain modelling in addition to the aforementioned NARMAX model validation (He et al., 2016).
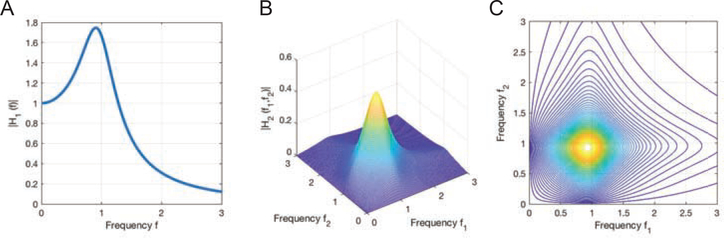
Figure 2:
The GFRFs of an exemplar nonlinear system. (A) The linear 1st-order GFRF, H1(f), shows a ‘resonance’ peak at f = 0.9Hz; (B) and (C) the 3-D and contour plots of the 2nd-order GFRF, H2(f1, f2). It shows a peak at f1 = 0.9Hz, f2 = 0.9Hz, which indicates harmonics at 2f = f1 + f2 = 1.8Hz can be introduced in the output spectrum if input contains a 0.9Hz component.
NARMAX-based frequency-domain analysis method has been applied to quantify the dynamic characteristics of nonlinear sensory processing circuit models from spiking neuron data (Florescu and Coca, 2018), the cross-frequency interactions in the corticothalamic loops with respect to tremor (He et al., 2016), and the characterisation of epileptic brain states (He et al., 2014a). More details will be discussed in the ‘Neurological and clinical applications’ section.
Time-varying nonlinear system identification
Many neurological subsystems are inherently nonstationary, since the brain is a dissipative and adaptive dynamical system (Kaplan et al., 2005; Lehnertz et al., 2017). Modelling and identification approaches of nonstationary processes have been well developed for linear systems, i.e. linear time-varying (LTV) systems. They are primarily based on adaptive recursive methods, such as recursive least squares, least mean squares, and the Kalman filter (Ljung and Gunnarsson, 1990), or based on a finite basis function expansion of the time-varying coefficients (Zou et al., 2003; Tsatsanis and Giannakis, 1993; Niedzwiecki and Klaput, 2002; Zheng et al., 2001). The identification of a nonlinear time-varying system is more sophisticated. The primary difficulty is how to effectively and simultaneously resolve the nonlinear model structure selection and the time-varying coefficient estimation. Approaches based on time-varying Volterra series combining artificial neural networks (Iatrou et al., 1999), principal dynamic modes (Zhong et al., 2007), or sliding-window strategy (Li et al., 2016), have been proposed. However, the model structure selection is still an unsolved issue, and the identification and frequency-domain analysis are computationally costly.
A better strategy is to extend the basis function expansion approach, originally proposed for LTV identification, to nonlinear time-varying cases (Billings and Wei, 2005). The time-varying (TV) parameters in TV-NARX models are first expanded using multi-wavelet basis functions, and TV nonlinear model is transformed into an expanded time-invariant model; the challenging TV model selection and parameter estimation problem can then be solved using the computational efficient FROLS algorithm. To accommodate the stochastic perturbations or additive coloured noise, this procedure can also be extended to more general TV-NARMAX models by introducing a modified extended least squares (ELS) algorithm (He et al., 2015). Several modifications to the TV-NARX model has recently been proposed using different basis functions or model selection procedure (Li et al., 2018; Guo et al., 2019). The corresponding frequency-domain analysis for nonlinear time-varying systems has also been developed based on the identified time-domain TV-NARX or TV-NARMAX model and the TV-GFRF concepts (He et al., 2013, 2015). By fitting TV-NARX models to two fragments of intracranial EEG recordings measured from epileptic patients, the corresponding frequency-domain analysis (i.e. TV-GFRF and TV-OFRF) shows the nonlinear energy transfer effect – the underlying neural system transfers the energy from lower frequencies to higher frequencies as the seizure spreading from the left to the right brain regions over time (He et al., 2013, 2014b).
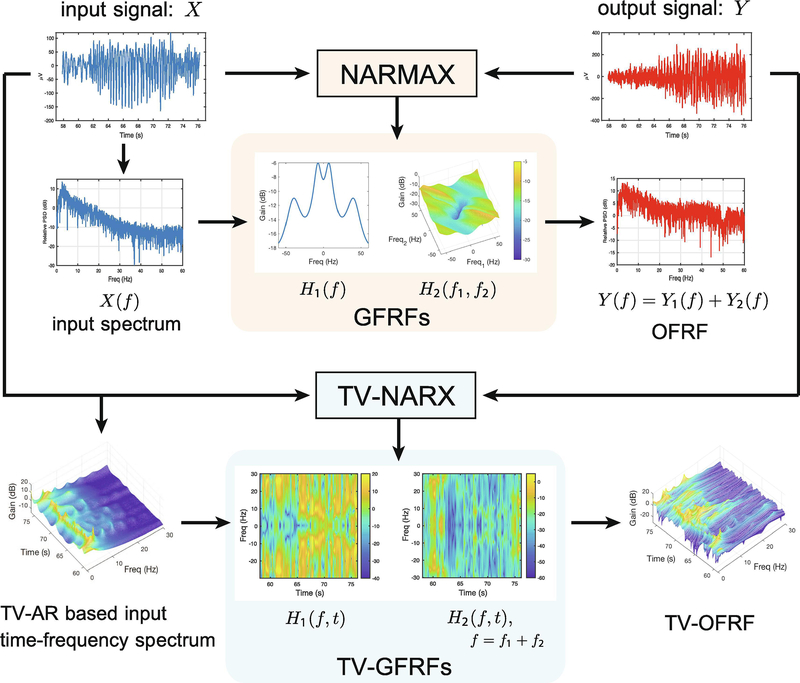
An overview of the NARMAX model-based system identification framework, including both time-invariant (NARMAX) and time-varying (TV-NARX) modelling along with corresponding frequency-domain analysis to neurophysiological signal analysis, is summarised in Figure 3.
Figure 3:
Analysing neurophysiological signals uses nonlinear time-invariant and time-varying system identification and corresponding frequency-domain analysis methods. The upper part of the diagram illustrates the nonlinear time-invariant modelling: first a NARMAX model is identified from the input and output neurophysiological signals (e.g. EEG, EMG, MEG, LFP); this time-domain model is then mapped to the frequency-domain with GFRFs (i.e. H1(f), H2(f), …), and the OFRF (Y (f) = Y1(f) + Y2(f), …) can be computed from the input spectrum and GFRFs. The lower part of the diagram shows the nonlinear time-varying system identification using a TV-NARX model, and the identified time-varying model can then be mapped to the time-frequency domain with (averaged) TV-GFRFs (i.e. H1(f, t), H2(f, t), …). The TV-OFRF can therefore be computed from a combination of the input time-frequency spectrum and the TV-GFRFs.
Nonlinear neural connectivity analysis
The communication between different neuronal populations which have very different firing behaviours can result in nonlinear neural connectivity, showing neural coupling across two or more different frequencies. To quantitatively study such a ‘cross-frequency coupling’, this section reviews recent advances in nonlinear neural functional and effective connectivity analysis.
High-order spectrum and nonlinear coherence
The power spectra and coherence discussed in the ‘Correlation and coherence’ section are Fourier transforms of the auto- and cross-correlations of signals, hence they are only linear frequency-domain measures. Practically these measures cannot detect certain nonlinear effects such as quadratic moments in or between signals that have a zero mean (Billings, 2013). Higher-order spectral analysis has been developed to detect nonlinear coupling between spectral components (Nikias and Mendel, 1993). For example, the most widely used bispectral analysis is the frequency-domain mapping of the third-order statistics. It can be used to quantify the quadratic nonlinear interactions, i.e. quadratic phase coupling. The bispectrum or bicoherence (and the bivariate cross-bispectrum or cross-bicoherence) analysis is well-known in engineering signal processing, whereas it has only relatively recently been applied to study the nonlinear interactions in neurophysiological signals (Sigl and Chamoun, 1994; Chella et al., 2014, 2016). For example, bispectral measures were used to detect long-range nonlinear coupling and synchronization in healthy subjects from human EEG (Isler et al., 2008; Chella et al., 2014), to characterise and predict epileptic seizures (Chua et al., 2009), and to study the nonlinear interactions between different frequency components related to Parkinson’s disease and tremor (Marceglia et al., 2006; Wang et al., 2014; He et al., 2016).
However, bispectrum or bicoherence cannot detect nonlinearity beyond second order, such as the higher-order harmonics and intermodulation effects, or the subharmonic coupling. A generalised nonlinear coherence analysis framework has therefore been proposed, based on two different nonlinear mappings from the input to the output of an ‘open-loop’ system in the frequency domain (Yang et al., 2016a):
1) n:m Mapping: to measure harmonic or subharmonic coupling related to individual input frequencies. , , where the output frequencies (fY ) are related to the input frequencies (fX) by the ratio n/m (n and m are co-prime positive integers), and H(n : m) is the n:m mapping function. The n:m mapping can generate cross-frequency (e.g. harmonic m = 1 or subharmonic m > 1) coupling between the input and the output.
2) Integer Multiplication Mapping: to quantify intermodulation coupling among multiple (≥ 2) input frequencies.
| (11) |
where fY = a1f1 + a2f2 + · · · + aNfN. The M is the corresponding multinomial coefficient, and H(f1, f2, · · ·, fN; a1, a2, · · ·, aN; fY ) indicates amplitude scaling and phase shift from the input to the output.
According to these two different types of nonlinear mapping, Yang and colleagues proposed two basic metrics for quantifying nonlinear coherence: (i) n:m coherence and (ii) multi-spectral coherence (Yang et al., 2016a).
n:m coherence
The n:m coherence is a generalized coherence measure for quantifying nonlinear coherence between two frequency components of the input X(f) and the output Y (f) (Yang et al., 2016a):
| (12) |
where n : m = fY : fX. SXY (fX, fY ) =< Xn(fX)(Ym(fY ))* > is the n:m cross-spectrum, with < · > represents the averaging over repetitions. is the nth-order auto-spectra. According to Cauchy-Schwarz-inequality, we have:
| (13) |
Thus, n:m coherence is bounded by 0 and 1, where one indicates that two signals are perfectly coupled for the given frequency pair (fX, fY ).
A simplified version of n:m coherence that considers only the phase relation between the input and the output is known as n:m phase synchronization index (Wacker and Witte, 2010). The n:m coherence and n:m phase synchronization index has been widely applied to neuroscience research to investigate nonlinear functional connectivity in different brain regions (Scheffer-Teixeira and Tort, 2016; Darvas et al., 2009a), as well as the nonlinear connectivity between the brain and muscles (Yang et al., 2016b).
Multi-spectral coherence
Multi-spectral coherence measures the multi-frequency nonlinear coupling generated by the integer multiplication mapping (Yang et al., 2016a). It is defined as:
| (14) |
where fY = a1f1 + · · · + aNfN; SXY (f1, · · ·, fN; a1, · · ·, aN; fY ) is the high-order cross-spectrum between X and Y, and equal to . Here ‘*’ indicates the complex conjugate. When there are only two input frequencies, the multi-spectral coherence is degraded to the bicoherence (Elgar and Guza, 1988). The multi-spectral coherence or bicoherence has been applied to study the nonlinear behaviours in visual (Shils et al., 1996), auditory (Chandran, 2012) and somatosensory systems (Yang et al., 2016a), which are thought to be associated with neural coding and functional integration of various sensory inputs (Gundlach and Müller, 2013).
A simplified version of multi-spectral coherence that considers only the phase relation between the input and the output is known as multi-spectral phase coherence (Yang et al., 2016c). Similarly, there is a degraded measure, named bi-phase locking value (Darvas et al., 2009b), for the case involving only two input frequencies. The advantage of multi-spectral phase coherence or bi-phase locking value is that it allows precise estimation of time delay in the nervous system based on the relative phase relationship between the input and output (Yang et al., 2016c; Tian et al., 2020). The multi-spectral phase coherence or bi-phase locking value has been previously used to determine neural transmission delays in the human visual system (Shils et al., 1996) and the stretch reflex loops (Yang et al., 2016b).
Nonlinear causality analysis: system identification based approaches
Time-domain analysis
In terms of effective connectivity, classical linear Granger causality analysis (GCA) (as discussed in the ‘System identification and causality’ section) may provide misleading results when used to analyse EEG/MEG or EMG signals, as the possible nonlinear interactions within a neural system are not modelled explicitly by simply using linear regression models. The Granger causality definition has been extended to nonlinear cases in the time domain, based on nonparametric methods (Diks and Panchenko, 2006; Hiemstra and Jones, 1994), radial basis functions (Ancona et al., 2004), kernel methods (Marinazzo et al., 2008), local linear models (Chen et al., 2004). DCM (Friston et al., 2003) (see the ‘Dynamic causal modelling’ section) was developed to accommodate both linear and nonlinear causal effects using a dynamic state-space model, and the effective connectivity among hidden states (unobserved neuronal dynamics) can be identified via Bayesian inference. Information-theoretical effective connectivity measures have also been proposed, such as the bivariate transfer entropy (TE) (Liu and Aviyente, 2012; Shovon et al., 2017) and phase transfer entropy (PTE). TE is a model-free measure, which compares two conditional probabilities using the Kullback-Leibler divergence - the amount of uncertainty in the future of target signal Y conditioned only on the target’s past and the future of Y conditioned on the past of both its own Y and the source X, in a conceptually similar way as the GCA. A more recent work (Harmah et al., 2019) generalised the TE method by using multivariate transfer entropy, which can overcome the problems of inferring spurious or redundant causality and missing synergistic interactions between multiple sources and target.
Another strategy to implement nonlinear granger causality under a system identification framework is to use NARX models (Faes et al., 2008; Zhao et al., 2013), by calculating the relative predictability improvement obtained from the NARX model compared to the corresponding NAR model. More importantly, compared to other nonlinear causality measures (e.g. nonparametric or information-theoretic measures), the advantage of using NARX-based causal inference (Zhao et al., 2013) is that one can easily separate the linear and nonlinear causal influence, for example from an input X to an output Y. After fitting a polynomial NARX model with the form (9), the linear causality can still be calculated from (4) based on the linear part of the NARX model, while the nonlinear causal influence from X to Y can be defined as:
| (15) |
Here , and denote the sets of all nonlinear delayed terms of Y, X and nonlinear product terms XY. This nonlinear causality measure can also be generalised to nonlinear time-varying systems, by computing similar linear and nonlinear causality indices based on the identified TV-NARX models (as described in the ‘Time-varying nonlinear system identification’ section), as proposed in (Zhao et al., 2013; Li et al., 2012).
Frequency-domain analysis
In the frequency domain, linear Granger causality measures, such as PDC, DTF and spectral Granger causality, can all be expressed as a function of the elements in the coefficient matrix or its inverse the transfer function matrix of the corresponding linear ARX models (3). By identifying the link between the PDC and the FRFs of the corresponding linear ARX models (as described in the the ‘System identification and causality’ section), a new nonlinear PDC (NPDC) measure has been proposed (He et al., 2014a) by generalising the spectrum decomposition with respect to a nonlinear NARX model in a similar way as the linear case. The NPDC from X to Y can then be expressed as a direct generalization of the linear PDC:
| (16) |
Here, the HX→Y (f) is the ‘nonlinear FRF’ which replaces the FRF in the linear PDC (7), and and are the error-driven GFRFs correspond to the restricted NAR models with respect to Y and X as discussed in (He et al., 2014a). The NPDC measures both linear and nonlinear causal influences from X to Y. The linear causal effects can be interpreted as a special case of (16) by only considering the 1st-order nonlinear FRFs of NARX (i.e. H1,X→Y (f)) and NAR (i.e. and ) models.
This new NPDC measure has recently been applied to predict epileptic seizures from EEG data (Zhang et al., 2020) by advancing the construction of functional brain networks, nonlinear feature selection and classification. This new nonlinear causality measure helps to provide better prediction accuracy compared to other standard graph theory or nonlinear classification based methods. A nonlinear generalization of Geweke’s spectral Granger causality has also been proposed (He et al., 2014b) using the NARX methodology.
Neurological and clinical applications
Movement, sensation and cognition arise from the cumulative activity of neurons within neural circuits and across distant, macroscale networks in the nervous system. Although the behaviour of an individual neuron has been investigated and well understood for decades, the mechanisms underlying neural communications between macroscale neural networks are still yet to better understand. Newly developed nonlinear system identification approaches allow us to investigate neural communications from large-scale neural activities measured by EEG, MEG and EMG, with the most recent application examples discussed below.
Nonlinear cortical response to somatosensory inputs
The human somatosensory system is highly nonlinear (Yang et al., 2018). Previous studies applied periodic sinusoid tactile stimulations to the index finger and measured the cortical response, where they found harmonic and subharmonic patterns in the response (Langdon et al., 2011; Tobimatsu et al., 1999). Several recent studies used sum-of-sinusoid stimulations to the wrist joint and found not only harmonics and subharmonics but also intermodulation patterns (Vlaar et al., 2016; Yang et al., 2016a). The majority of intermodulation responses presented the second-order nonlinearity, which is the sum or the difference between input frequencies (Yang et al., 2020b). These findings indicate that the nonlinearities in the somatosensory system allow the functional integration of input signals at different frequencies, and they can be transmitted in different somatosensory ascending pathways.
Yang and colleagues recently built a hierarchical neural network based on known neuroanatomical connections and corresponding transmission delays in neural pathways to model the cortical response to somatosensory input (Tian et al., 2018). The proposed computational model contains a neural layer at the thalamus that integrates the inputs from different ascending pathways, including Ia and II afferents. The computational model well captured the majority of the cortical response to the given somatosensory inputs, indicating the functional integration of different somatosensory input signals may occur at the thalamus and is then transmitted to the cortex via the thalamocortical radiation.
Tremor: nonlinearity in the thalamocortical loop
Essential tremor is a common neurological movement disorder widely considered to have a centrally-driven origin. There is both neurophysiological and clinical evidence of thalamic involvement in the central oscillatory network generating essential tremor (Hua and Lenz, 2005; Marsden et al., 2000; Deuschl et al., 2011). Local field potential (LFP) recordings of thalamic ventralis intermedius (Vim) nucleus show a strong linear correlation with the contralateral EMG during tremor (Marsden et al., 2000). Some studies using EEG and MEG suggest that the sensorimotor cortex is also part of the central tremor-related oscillatory network, with significant coupling in some cases with the contralateral tremorgenic EMG (Hellwig et al., 2001; Raethjen and Deuschl, 2012; Buijink et al., 2015). Despite a well-established reciprocal anatomical connection between the thalamus and cortex, the functional association between the two structures during ‘tremor-on’ periods had not been extensively defined.
He and co-authors (He et al., 2016) investigated the functional connectivity among cortical EEG, thalamic (Vim) LFPs and contralateral EMG signals over both ‘tremor-on’ and ‘tremor-off’ states, using linear coherence and nonlinear bispectral analysis methods. In addition to expected strong coherence between EMG and thalamic LFP, nonlinear interactions (i.e. quadratic phase coupling) at different frequencies, i.e. low frequency during tremor on and higher frequency during tremor off, in LFPs have been reported. More importantly, by using the NARX-based nonlinear system identification and frequency-domain analysis (as described in the ‘Frequency-domain nonlinear system analysis: nonlinear frequency response functions’ section), two distinct and non-overlapping frequency ‘channels’ of communication between thalamic Vim and the ipsilateral motor cortex were identified, which robustly defined the ‘tremor-on’ versus ‘tremor-off’ states. Longer corticothalamic nonlinear phase lags in the tremor active state were also uncovered, suggesting the possible involvement of an indirect multi-synaptic loop. This work demonstrates, for the first time, the importance of cross-frequency nonlinear interactions between the cortex and the thalamus in characterising the essential tremor.
Nonlinear analysis for determining motor impairment in stroke
After a stroke, damage to the brain increases the reliance on indirect motor pathways resulting in motor impairments and changes in neural connectivity between the brain and muscles. A hallmark of impairments post-stroke is a loss of independent joint control that leads to abnormal co-activation between shoulder, arm and hand muscles, known as the upper limb synergy (Roh et al., 2013). The upper limb synergy is thought to be caused by progressive recruitment of indirect motor pathways via the brainstem following a stroke-induced loss of corticospinal projections (McPherson et al., 2018). Thus, a neural connectivity measure that quantifies the recruitment of these indirect motor pathways would be crucial to evaluate post-stroke motor impairments. Recent model-based simulation and clinical studies indicate that the increased usage of indirect motor pathways enhances nonlinear distortion of motor command transmissions, which leads to stronger nonlinear interaction between the brain and muscles (Sinha et al., 2020a; Yang et al., 2020a). The ratio of nonlinear interaction over linear interaction, known as the nonlinear-over-linear index (N-L Index), has been reported to be associated with the relative ratio of the recruitment of indirect versus direct motor pathways (Yang et al., 2020a). This new measure may facilitate the future determination of the effect of new therapeutic interventions that aim to optimise the usage of motor pathways, and thus facilitate the stroke recovery.
Epilepsy
It has been widely recognised that epileptic seizures are highly nonlinear phenomena, due to low dimensional chaos during epileptic seizure or transitions between ordered and disordered stats (Iasemidis and Sackellares, 1996). Currently, the treatment mainly relies on long-term medication with antiepileptic drugs or neurosurgery, which can cause cognitive or other neurological deficits. New treatment strategies such as on-demand therapies during the pre-seizure (preictal) state or electrical stimulation are therefore needed. A vital part of this new on-demand strategy is the accurate and timely detection of the preictal state, even seconds before seizure onset (Lehnertz, 2008). A range of univariate, bivariate and multivariate linear and nonlinear measures have been developed for the characterisation and detection or prediction of epileptic brain states and achieving a better understanding of the spatial and temporal dynamics of the epileptic process (Carney et al., 2011; Lehnertz, 2008). There is a comprehensive review of using different parametric and nonparametric nonlinear features (in time, frequency and time-frequency domains) for the automated epilepsy stage detection and classification (Acharya et al., 2013).
Given the current challenges in epilepsy detection and diagnostics (Elger and Hoppe, 2018; Lehnertz, 2008), e.g. to improve the understanding of brain dynamics and mechanisms during the seizure-free interval and seizure initiation and termination, there is a great need to develop more accurate nonlinear methods to improve the detectability of directional interactions in underlying functional and anatomical networks. Developing new nonlinear system identification and nonlinear causality measures are therefore crucial. A nonlinear causality measure, partial transfer entropy (Papana et al., 2012), has been applied to analyse the EEG of epileptic patients during preictal, ictal and postictal states. It can provide better detection of causality strength variations compared to linear PDC. An adaptive nonlinear Granger causality measure was also proposed (Sysoeva et al., 2014) and applied to LFP data (intracranial EEG in cortex and thalamus) in rats. It was reported to provide more sensitive detection of changes in the dynamics of network properties compared to linear Granger causality. The recently proposed nonlinear frequency-domain causality measure NPDC (He et al., 2014a) (as reviewed in the ‘Frequency-domain analysis’ section) has been applied to analyse EEG recordings of two bipolar channels from a patient with childhood absence epilepsy. It shows this nonlinear measure can detect extra frequency-domain causal interactions compared to standard linear PDC.
Discussion
The complexity and nonlinearity of neural systems require advanced system identification techniques to understand their properties and mechanisms better. This review investigated the links between connectivity analysis and system identification, as well as recent progress of nonlinear system identification of neural systems. With the state-of-the-art examples of clinical applications, we argued that nonlinear dynamic modelling and corresponding connectivity analysis allows new insights into the underlying neural functions and neuropathological mechanisms of the abnormality caused by various neurological disorders. These novel approaches may well facilitate the development of new precision diagnostic tools and brain-computer interface (BCI) techniques (Nicolas-Alonso and Gomez-Gil, 2012; Chaudhary et al., 2016; Abiri et al., 2019), and therefore improve the diagnosis and treatment of neurological disorders as well as restore communication and movement for people with motor disabilities.
Compared to the linear system identification and iso-frequency connectivity analysis, nonlinear dynamic modelling and cross-frequency analysis are much more complicated. Such complexity brings challenges but also research opportunities. Potential future work includes: i) further developing multivariate system identification techniques and corresponding multivariate nonlinear frequency-domain analysis and causality analysis measures. Most existing nonlinear system identification based (time or frequency-domain) analysis or causality analysis are primarily bivariate, which limits nonlinear analysis to the only pre-defined local brain or neural regions. New multivariate system identification (e.g. multivariate nonlinear regression modelling) or inference approaches would generalise the existing nonlinear connectivity analysis to larger neural networks, although developing efficient model selection and reducing the computational cost would be challenging tasks; ii) many neuronal systems or interactions are in nature nonstationary and nonlinear, how to automatically distinguish the nonlinearity and time-varying effects (nonstationarity) via novel system identification technique is still an open and important research topic, although significant progress has been made so far (as reviewed in the ‘Time-varying nonlinear system identification’ section); iii) machine learning and deep learning techniques have recently been applied to Granger causality analysis (Tank et al., 2018; Chivukula et al., 2018; Peng, 2020), an interesting future work is to further explore and combine the advantages of deep learning, e.g. accurate quantification of complex and long-range nonlinear interactions, and nonlinear system identification approaches to study the nonlinear causal interactions in complex neural networks; iv) using nonlinear system identification techniques to extract new nonlinear features for the BCI; v) apart from those clinical applications described in the ‘Neurological and clinical applications’ section, the importance of nonlinearity in understanding and characterising other important neurological disorders, e.g. Parkinson’s disease and Alzheimer’s disease (Stam, 2005; Stam et al., 1995; Blackburn et al., 2018; Gunawardena et al., 2020), has been reported recently. Therefore nonlinear system identification approaches will have great potential in developing new diagnostic tools for those primary neurological disorders that affect a large population worldwide.
Acknowledgment
This work was supported by NIH NICHD R21HD099710.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abiri R, Borhani S, Sellers EW, Jiang Y, Zhao X, 2019. A comprehensive review of eeg-based brain–computer interface paradigms. J Neural Eng 16, 011001. [DOI] [PubMed] [Google Scholar]
- Acharya UR, Sree SV, Swapna G, Martis RJ, Suri JS, 2013. Automated eeg analysis of epilepsy: a review. Knowl Based Syst 45, 147–165. [Google Scholar]
- Ancona N, Marinazzo D, Stramaglia S, 2004. Radial basis function approach to nonlinear granger causality of time series. Phys Rev E 70, 056221. [DOI] [PubMed] [Google Scholar]
- Aru J, Aru J, Priesemann V, Wibral M, Lana L, Pipa G, Singer W, Vicente R, 2015. Untangling cross-frequency coupling in neuroscience. Curr Opin Neurol 31, 51–61. [DOI] [PubMed] [Google Scholar]
- Baccalá LA, Sameshima K, 2001. Partial directed coherence: a new concept in neural structure determination. Biol Cybern 84, 463–474. [DOI] [PubMed] [Google Scholar]
- Barton MJ, Robinson PA, Kumar S, Galka A, Durrant-Whyte HF, Guivant J, Ozaki T, 2008. Evaluating the performance of kalman-filter-based eeg source localization. IEEE Trans Biomed Eng 56, 122–136. [DOI] [PubMed] [Google Scholar]
- Bastos AM, Schoffelen JM, 2016. A tutorial review of functional connectivity analysis methods and their interpretational pitfalls. Front Syst Neurosci 9, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty E, Merel J, Brackbill N, Heitman A, Sher A, Litke A, Chichilnisky E, Paninski L, 2016. Multilayer recurrent network models of primate retinal ganglion cell responses.
- Billings S, Tsang K, 1989. Spectral analysis for non-linear systems, part ii: Interpretation of non-linear frequency response functions. Mech Syst Sig Process 3, 341–359. [Google Scholar]
- Billings S, Voon W, 1983. Structure detection and model validity tests in the identification of nonlinear systems, in: IEE Proc-D, IET. pp. 193–199. [Google Scholar]
- Billings SA, 2013. Nonlinear system identification: NARMAX methods in the time, frequency, and spatio-temporal domains. John Wiley & Sons. [Google Scholar]
- Billings SA, Wei HL, 2005. The wavelet-narmax representation: A hybrid model structure combining polynomial models with multiresolution wavelet decompositions. Int J Syst Sci 36, 137–152. [Google Scholar]
- Birpoutsoukis G, Marconato A, Lataire J, Schoukens J, 2017. Regularized nonparametric volterra kernel estimation. Automatica 82, 324–327. [Google Scholar]
- Blackburn DJ, Zhao Y, De Marco M, Bell SM, He F, Wei HL, Lawrence S, Unwin ZC, Blyth M, Angel J, et al. , 2018. A pilot study investigating a novel non-linear measure of eyes open versus eyes closed eeg synchronization in people with alzheimer’s disease and healthy controls. Brain Sci 8, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinowska KJ, 2011. Review of the methods of determination of directed connectivity from multichannel data. Med Biol Eng Comput 49, 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakspear M, 2017. Dynamic models of large-scale brain activity. Nat Neurosci 20, 340. [DOI] [PubMed] [Google Scholar]
- Brillinger DR, Bryant HL, Segundo JP, 1976. Identification of synaptic interactions. Biol Cybern 22, 213–228. [DOI] [PubMed] [Google Scholar]
- Buijink AW, van der Stouwe AM, Broersma M, Sharifi S, Groot PF, Speelman JD, Maurits NM, van Rootselaar AF, 2015. Motor network disruption in essential tremor: a functional and effective connectivity study. Brain 138, 2934–2947. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Knight RT, 2010. The functional role of cross-frequency coupling. Trends Cogn Sci 14, 506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney PR, Myers S, Geyer JD, 2011. Seizure prediction: methods. Epilepsy Behav 22, S94–S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran V, 2012. Time-varying bispectral analysis of auditory evoked multi-channel scalp eeg, in: 11th Int Conf on Information Science, Signal Processing and their Applications (ISSPA), IEEE. pp. 205–212. [Google Scholar]
- Chaudhary U, Birbaumer N, Ramos-Murguialday A, 2016. Brain–computer interfaces for communication and rehabilitation. Nat Rev Neurosci 12, 513. [DOI] [PubMed] [Google Scholar]
- Chella F, Marzetti L, Pizzella V, Zappasodi F, Nolte G, 2014. Third order spectral analysis robust to mixing artifacts for mapping cross-frequency interactions in eeg/meg. Neuroimage 91, 146–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chella F, Pizzella V, Zappasodi F, Nolte G, Marzetti L, 2016. Bispectral pairwise interacting source analysis for identifying systems of cross-frequency interacting brain sources from electroencephalographic or magnetoencephalographic signals. Phys Rev E 93, 052420. [DOI] [PubMed] [Google Scholar]
- Chen S, Billings SA, Luo W, 1989. Orthogonal least squares methods and their application to non-linear system identification. Int J Control 50, 1873–1896. [Google Scholar]
- Chen Y, Rangarajan G, Feng J, Ding M, 2004. Analyzing multiple nonlinear time series with extended granger causality. Phys Lett A 324, 26–35. [Google Scholar]
- Chicharro D, 2011. On the spectral formulation of granger causality. Biol Cybern 105, 331–347. [DOI] [PubMed] [Google Scholar]
- Chivukula AS, Li J, Liu W, 2018. Discovering granger-causal features from deep learning networks, in: Australasian Joint Conference on Artificial Intelligence, Springer. pp. 692–705. [Google Scholar]
- Chua K, Chandran V, Rajendra Acharya U, Lim C, 2009. Analysis of epileptic eeg signals using higher order spectra. J Med Eng Technol 33, 42–50. [DOI] [PubMed] [Google Scholar]
- Darvas F, Miller KJ, Rao RP, Ojemann JG, 2009a. Nonlinear phase–phase cross-frequency coupling mediates communication between distant sites in human neocortex. J Neurosci 29, 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas F, Ojemann JG, Sorensen LB, 2009b. Bi-phase locking—a tool for probing non-linear interaction in the human brain. Neuroimage 46, 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David O, Kiebel SJ, Harrison LM, Mattout J, Kilner JM, Friston KJ, 2006. Dynamic causal modeling of evoked responses in eeg and meg. NeuroImage 30, 1255–1272. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Raethjen J, Hellriegel H, Elble R, 2011. Treatment of patients with essential tremor. Lancet Neurol 10, 148–161. [DOI] [PubMed] [Google Scholar]
- Diks C, Panchenko V, 2006. A new statistic and practical guidelines for nonparametric granger causality testing. J Econ Dyn Control 30, 1647–1669. [Google Scholar]
- Dimitrov AG, Lazar AA, Victor JD, 2011. Information theory in neuroscience. J Comput Neurosci 30, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgar S, Guza RT, 1988. Statistics of bicoherence. IEEE Trans Acoust 36, 1667–1668. [Google Scholar]
- Elger CE, Hoppe C, 2018. Diagnostic challenges in epilepsy: seizure under-reporting and seizure detection. Lancet Neurol 17, 279–288. [DOI] [PubMed] [Google Scholar]
- Emami A, Kunii N, Matsuo T, Shinozaki T, Kawai K, Takahashi H, 2019. Seizure detection by convolutional neural network-based analysis of scalp electroencephalography plot images. NeuroImage Clin 22, 101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faes L, Nollo G, Chon KH, 2008. Assessment of granger causality by nonlinear model identification: application to short-term cardiovascular variability. Ann Biomed Eng 36, 381–395. [DOI] [PubMed] [Google Scholar]
- Faul S, Gregorcic G, Boylan G, Marnane W, Lightbody G, Connolly S, 2007. Gaussian process modeling of eeg for the detection of neonatal seizures. IEEE Trans Biomed Eng 54, 2151–2162. [DOI] [PubMed] [Google Scholar]
- Fell J, Axmacher N, 2011. The role of phase synchronization in memory processes. Nat Rev Neurosci 12, 105–118. [DOI] [PubMed] [Google Scholar]
- Fetz EE, 1997. Temporal coding in neural populations? Science 278, 1901–1902. [DOI] [PubMed] [Google Scholar]
- Florescu D, Coca D, 2018. Identification of linear and nonlinear sensory processing circuits from spiking neuron data. Neural Comput 30, 670–707. [DOI] [PubMed] [Google Scholar]
- French AS, Korenberg M, 1989. A nonlinear cascade model for action potential encoding in an insect sensory neuron. Biophys J 55, 655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, 2015. Rhythms for cognition: communication through coherence. Neuron 88, 220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, 1994. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp 2, 56–78. [Google Scholar]
- Friston KJ, 2001. Book review: brain function, nonlinear coupling, and neuronal transients. Neuroscientist 7, 406–418. [DOI] [PubMed] [Google Scholar]
- Friston KJ, 2011. Functional and effective connectivity: a review. Brain Connect 1, 13–36. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W, 2003. Dynamic causal modelling. Neuroimage 19, 1273–1302. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Preller KH, Mathys C, Cagnan H, Heinzle J, Razi A, Zeidman P, 2019. Dynamic causal modelling revisited. Neuroimage 199, 730–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima M, Yamashita O, Kanemura A, Ishii S, Kawato M, Sato M.a., 2012. A state-space modeling approach for localization of focal current sources from meg. IEEE Trans Biomed Eng 59, 1561–1571. [DOI] [PubMed] [Google Scholar]
- Fyshe A, Fox E, Dunson D, Mitchell T, 2012. Hierarchical latent dictionaries for models of brain activation, in: Artificial Intelligence and Statistics, pp. 409–421. [Google Scholar]
- Gourévitch B, Le Bouquin-Jeannés R, Faucon G, 2006. Linear and nonlinear causality between signals: methods, examples and neurophysiological applications. Biol Cybern 95, 349–369. [DOI] [PubMed] [Google Scholar]
- Gregorčič G, Lightbody G, 2008. Nonlinear system identification: From multiple-model networks to gaussian processes. Eng Appl Artif Intell 21, 1035–1055. [Google Scholar]
- Gregorčič G, Lightbody G, 2009. Gaussian process approach for modelling of nonlinear systems. Eng Appl Artif Intell 22, 522–533. [Google Scholar]
- Gu Y, Yang Y, Dewald J, Van der Helm FC, Schouten AC, Wei LH, 2020. Nonlinear modeling of cortical responses to mechanical wrist perturbations using the narmax method. IEEE Trans Biomed Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena SR, He F, Sarrigiannis P, Blackburn DJ, 2020. Nonlinear classification of eeg recordings from patients with alzheimer’s disease using gaussian process latent variable model. medRxiv. [Google Scholar]
- Gundlach C, Müller MM, 2013. Perception of illusory contours forms intermodulation responses of steady state visual evoked potentials as a neural signature of spatial integration. Biol Psychol 94, 55–60. [DOI] [PubMed] [Google Scholar]
- Guo L, Rivero D, Dorado J, Rabunal JR, Pazos A, 2010. Automatic epileptic seizure detection in eegs based on line length feature and artificial neural networks. J Neurosci Methods 191, 101–109. [DOI] [PubMed] [Google Scholar]
- Guo Y, Wang L, Li Y, Luo J, Wang K, Billings S, Guo L, 2019. Neural activity inspired asymmetric basis function tv-narx model for the identification of time-varying dynamic systems. Neurocomputing 357, 188–202. [Google Scholar]
- Harmah DJ, Li C, Li F, Liao Y, Wang J, Ayedh WM, Bore JC, Yao D, Dong W, Xu P, 2019. Measuring the non-linear directed information flow in schizophrenia by multivariate transfer entropy. Front Comput Neurosci 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Billings SA, Wei HL, Sarrigiannis PG, 2014a. A nonlinear causality measure in the frequency domain: Nonlinear partial directed coherence with applications to eeg. J Neurosci Methods 225, 71–80. [DOI] [PubMed] [Google Scholar]
- He F, Billings SA, Wei HL, Sarrigiannis PG, Zhao Y, 2013. Spectral analysis for nonstationary and nonlinear systems: A discrete-time-model-based approach. IEEE Trans Biomed Eng 60, 2233–2241. [DOI] [PubMed] [Google Scholar]
- He F, Sarrigiannis PG, Billings SA, Wei H, Rowe J, Romanowski C, Hoggard N, Hadjivassilliou M, Rao DG, Grünewald R, et al. , 2016. Nonlinear interactions in the thalamocortical loop in essential tremor: a model-based frequency domain analysis. Neurosci 324, 377–389. [DOI] [PubMed] [Google Scholar]
- He F, Wei HL, Billings SA, 2015. Identification and frequency domain analysis of non-stationary and nonlinear systems using time-varying narmax models. Int J Syst Sci 46, 2087–2100. [Google Scholar]
- He F, Wei HL, Billings SA, Sarrigiannis PG, 2014b. A nonlinear generalization of spectral granger causality. IEEE Trans Biomed Eng 61, 1693–1701. [DOI] [PubMed] [Google Scholar]
- Hellwig B, Häußler S, Schelter B, Lauk M, Guschlbauer B, Timmer J, Lücking C, 2001. Tremor-correlated cortical activity in essential tremor. Lancet 357, 519–523. [DOI] [PubMed] [Google Scholar]
- Hiemstra C, Jones JD, 1994. Testing for linear and nonlinear granger causality in the stock price-volume relation. J Finance 49, 1639–1664. [Google Scholar]
- Honey CJ, Kötter R, Breakspear M, Sporns O, 2007. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci 104, 10240–10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O, 1988. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol 405, 345–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua SE, Lenz FA, 2005. Posture-related oscillations in human cerebellar thalamus in essential tremor are enabled by voluntary motor circuits. J Neurophysiol 93, 117–127. [DOI] [PubMed] [Google Scholar]
- Hyafil A, Giraud AL, Fontolan L, Gutkin B, 2015. Neural cross-frequency coupling: connecting architectures, mechanisms, and functions. Trends Neurosci 38, 725–740. [DOI] [PubMed] [Google Scholar]
- Iasemidis LD, Sackellares JC, 1996. Review: Chaos theory and epilepsy. Neuroscientist 2, 118–126. [Google Scholar]
- Iatrou M, Berger TW, Marmarelis VZ, 1999. Modeling of nonlinear nonstationary dynamic systems with a novel class of artificial neural networks. IEEE Trans Neural Netw 10, 327–339. [DOI] [PubMed] [Google Scholar]
- Isler JR, Grieve PG, Czernochowski D, Stark RI, Friedman D, 2008. Cross-frequency phase coupling of brain rhythms during the orienting response. Brain Res 1232, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Colgin LL, 2007. Cross-frequency coupling between neuronal oscillations. Trends Cogn Sci 11, 267–269. [DOI] [PubMed] [Google Scholar]
- Jeong J, Shi WX, Hoffman R, Oh J, Gore JC, Bunney BS, Peterson BS, 2012. Bursting as a source of non-linear determinism in the firing patterns of nigral dopamine neurons. Eur J Neurosci 36, 3214–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan AY, Fingelkurts AA, Fingelkurts AA, Borisov SV, Darkhovsky BS, 2005. Nonstationary nature of the brain activity as revealed by eeg/meg: methodological, practical and conceptual challenges. Signal Process 85, 2190–2212. [Google Scholar]
- Kearney RE, Hunter IW, 1988. Nonlinear identification of stretch reflex dynamics. Ann Biomed Eng 16, 79–94. [DOI] [PubMed] [Google Scholar]
- Kell AJ, McDermott JH, 2019. Deep neural network models of sensory systems: windows onto the role of task constraints. Curr Opin Neurol 55, 121–132. [DOI] [PubMed] [Google Scholar]
- Keshishian M, Akbari H, Khalighinejad B, Herrero JL, Mehta AD, Mesgarani N, 2020. Estimating and interpreting nonlinear receptive field of sensory neural responses with deep neural network models. Elife 9, e53445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebel SJ, Garrido MI, Moran RJ, Friston KJ, 2008. Dynamic causal modelling for eeg and meg. Cogn Neurodyn 2, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klindt D, Ecker AS, Euler T, Bethge M, 2017. Neural system identification for large populations separating “what” and “where”, in: Adv Neural Inf Process Syst, pp. 3506–3516. [Google Scholar]
- Koch C, Segev I, 2000. The role of single neurons in information processing. Nat Neurosci 3, 1171–1177. [DOI] [PubMed] [Google Scholar]
- Kocijan J, Girard A, Banko B, Murray-Smith R, 2005. Dynamic systems identification with gaussian processes. Math Comput Modell Dyn Syst 11, 411–424. [Google Scholar]
- Lang ZQ, Billings S, 1996. Output frequency characteristics of nonlinear systems. Int J Control 64, 1049–1067. [Google Scholar]
- Langdon AJ, Boonstra TW, Breakspear M, 2011. Multi-frequency phase locking in human somatosensory cortex. Prog Biophys Mol Biol 105, 58–66. [DOI] [PubMed] [Google Scholar]
- Lau B, Stanley GB, Dan Y, 2002. Computational subunits of visual cortical neurons revealed by artificial neural networks. Proc Natl Acad Sci 99, 8974–8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar AA, Slutskiy YB, 2015. Spiking neural circuits with dendritic stimulus processors. J Comput Neurosci 38, 1–24. [DOI] [PubMed] [Google Scholar]
- Lehky SR, Sejnowski TJ, Desimone R, 1992. Predicting responses of nonlinear neurons in monkey striate cortex to complex patterns. J Neurosci 12, 3568–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnertz K, 2008. Epilepsy and nonlinear dynamics. J Biol Phys 34, 253–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnertz K, Geier C, Rings T, Stahn K, 2017. Capturing time-varying brain dynamics. EPJ Nonlinear Biomedical Physics 5, 2. [Google Scholar]
- Leontaritis I, Billings SA, 1985. Input-output parametric models for nonlinear systems part i: deterministic non-linear systems. Int J Control 41, 303–328. [Google Scholar]
- Li S, Chen YT, Francisco GE, Zhou P, Rymer WZ, 2019. A unifying pathophysiological account for post-stroke spasticity and disordered motor control. Front Neurol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cui WG, Guo YZ, Huang T, Yang XF, Wei HL, 2018. Time-varying system identification using an ultra-orthogonal forward regression and multiwavelet basis functions with applications to eeg. IEEE Trans Neural Netw Learn Syst 29, 2960–2972. [DOI] [PubMed] [Google Scholar]
- Li Y, Wei HL, Billings SA, Liao XF, 2012. Time-varying linear and nonlinear parametric model for granger causality analysis. Phys Rev E 85, 041906. [DOI] [PubMed] [Google Scholar]
- Li Y, Wei HL, Billings SA, Sarrigiannis PG, 2016. Identification of nonlinear time-varying systems using an online sliding-window and common model structure selection (cmss) approach with applications to eeg. Int J Syst Sci 47, 2671–2681. [Google Scholar]
- Liu Y, Aviyente S, 2012. Quantification of effective connectivity in the brain using a measure of directed information. Comput Math Methods Med 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung L, 1999. System identification. Wiley encyclopedia of electrical and electronics engineering, 1–19. [Google Scholar]
- Ljung L, Gunnarsson S, 1990. Adaptation and tracking in system identification—a survey. Automatica 26, 7–21. [Google Scholar]
- Marceglia S, Foffani G, Bianchi A, Baselli G, Tamma F, Egidi M, Priori A, 2006. Dopamine-dependent non-linear correlation between subthalamic rhythms in parkinson’s disease. J Physiol 571, 579–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinazzo D, Pellicoro M, Stramaglia S, 2008. Kernel-granger causality and the analysis of dynamical networks. Phys Rev E 77, 056215. [DOI] [PubMed] [Google Scholar]
- Marmarelis PZ, Marmarelis VZ, 1978. The white-noise method in system identification, in: Analysis of physiological systems. Springer, pp. 131–180. [Google Scholar]
- Marmarelis PZ, Naka K, 1973a. Nonlinear analysis and synthesis of receptive-field responses in the catfish retina. i. horizontal cell leads to ganglion cell chain. J Neurophysiol 36, 605–618. [DOI] [PubMed] [Google Scholar]
- Marmarelis PZ, Naka K, 1973b. Nonlinear analysis and synthesis of receptive-field responses in the catfish retina. ii. one-input white-noise analysis. J Neurophysiol 36, 619–633. [DOI] [PubMed] [Google Scholar]
- Marmarelis PZ, Naka KI, 1972. White-noise analysis of a neuron chain: an application of the wiener theory. Science 175, 1276–1278. [DOI] [PubMed] [Google Scholar]
- Marmarelis V, 2012. Analysis of physiological systems: The white-noise approach. Springer Science & Business Media. [Google Scholar]
- Marsden J, Ashby P, Limousin-Dowsey P, Rothwell J, Brown P, 2000. Coherence between cerebellar thalamus, cortex and muscle in man: cerebellar thalamus interactions. Brain 123, 1459–1470. [DOI] [PubMed] [Google Scholar]
- McIntosh L, Maheswaranathan N, Nayebi A, Ganguli S, Baccus S, 2016. Deep learning models of the retinal response to natural scenes, in: Adv Neural Inf Process Syst, pp. 1369–1377. [PMC free article] [PubMed] [Google Scholar]
- McPherson JG, Chen A, Ellis MD, Yao J, Heckman C, Dewald JP, 2018. Progressive recruitment of contralesional cortico-reticulospinal pathways drives motor impairment post stroke. J Physiol 596, 1211–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruelo AC, Simpson DM, Veres SM, Newland PL, 2016. Improved system identification using artificial neural networks and analysis of individual differences in responses of an identified neuron. Neural Netw 75, 56–65. [DOI] [PubMed] [Google Scholar]
- Negro F, Farina D, 2011. Linear transmission of cortical oscillations to the neural drive to muscles is mediated by common projections to populations of motoneurons in humans. J Physiol 589, 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas-Alonso LF, Gomez-Gil J, 2012. Brain computer interfaces, a review. Sensors 12, 1211–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwiecki M, Klaput T, 2002. Fast recursive basis function estimators for identification of time-varying processes. IEEE Trans Signal Process 50, 1925–1934. [Google Scholar]
- Nikias CL, Mendel JM, 1993. Signal processing with higher-order spectra. IEEE Signal Process Mag 10, 10–37. [Google Scholar]
- Ocak H, 2009. Automatic detection of epileptic seizures in eeg using discrete wavelet transform and approximate entropy. Expert Syst Appl 36, 2027–2036. [Google Scholar]
- Paninski L, Ahmadian Y, Ferreira DG, Koyama S, Rad KR, Vidne M, Vogelstein J, Wu W, 2010. A new look at state-space models for neural data. J Comput Neurosci 29, 107–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papana A, Kugiumtzis D, Larsson PG, 2012. Detection of direct causal effects and application to epileptic electroencephalogram analysis. Int J Bifurcation Chaos 22, 1250222. [Google Scholar]
- Peng W, 2020. Dli: A deep learning-based granger causality inference. Complexity 2020. [Google Scholar]
- Pereda E, Quiroga RQ, Bhattacharya J, 2005. Nonlinear multivariate analysis of neurophysiological signals. Prog Neurobiol 77, 1–37. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Eggermont JJ, 2010. Nonlinear cross-frequency interactions in primary auditory cortex spectrotemporal receptive fields: a wiener–volterra analysis. J Comput Neurosci 28, 285–303. [DOI] [PubMed] [Google Scholar]
- Pintelon R, Schoukens J, 2012. System identification: a frequency domain approach. John Wiley & Sons. [Google Scholar]
- Raethjen J, Deuschl G, 2012. The oscillating central network of essential tremor. Clin Neurophysiol 123, 61–64. [DOI] [PubMed] [Google Scholar]
- Ren J, Xiang J, Chen Y, Li F, Wu T, Shi J, 2019. Abnormal functional connectivity under somatosensory stimulation in migraine: a multi-frequency magnetoencephalography study. J Headache Pain 20, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh J, Rymer WZ, Perreault EJ, Yoo SB, Beer RF, 2013. Alterations in upper limb muscle synergy structure in chronic stroke survivors. J Neurophysiol 109, 768–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros T, J Baars B, Lanius RA, Vuilleumier P, 2014. Tuning pathological brain oscillations with neurofeedback: a systems neuroscience framework. Front Hum Neurosci 8, 1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum R, Trousdale J, Josic K, 2010. Pooling and correlated neural activity. Front Comput Neurosci 4, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakkalis V, 2011. Review of advanced techniques for the estimation of brain connectivity measured with eeg/meg. Comput Biol Med 41, 1110–1117. [DOI] [PubMed] [Google Scholar]
- Scheffer-Teixeira R, Tort AB, 2016. On cross-frequency phase-phase coupling between theta and gamma oscillations in the hippocampus. Elife 5, e20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schön TB, Wills A, Ninness B, 2011. System identification of nonlinear state-space models. Automatica 47, 39–49. [Google Scholar]
- Schoukens J, Ljung L, 2019. Nonlinear system identification: A user-oriented road map. IEEE Control Syst Mag 39, 28–99. [Google Scholar]
- Sclabassi RJ, Eriksson JL, Port RL, Robinson GB, Berger TW, 1988a. Nonlinear systems analysis of the hippocampal perforant path-dentate projection. i. theoretical and interpretational considerations. J Neurophysiol 60, 1066–1076. [DOI] [PubMed] [Google Scholar]
- Sclabassi RJ, Krieger DN, Berger TW, 1988b. A systems theoretic approach to the study of cns function. Ann Biomed Eng 16, 17–34. [DOI] [PubMed] [Google Scholar]
- Shils J, Litt M, Skolnick B, Stecker M, 1996. Bispectral analysis of visual interactions in humans. Electroencephalogr Clin Neurophysiol 98, 113–125. [DOI] [PubMed] [Google Scholar]
- Shovon MHI, Nandagopal N, Vijayalakshmi R, Du JT, Cocks B, 2017. Directed connectivity analysis of functional brain networks during cognitive activity using transfer entropy. Neural Process Lett 45, 807–824. [Google Scholar]
- Sigl JC, Chamoun NG, 1994. An introduction to bispectral analysis for the electroencephalogram. J Clin Monit 10, 392–404. [DOI] [PubMed] [Google Scholar]
- Sinha N, Dewald J, Heckman CJ, Yang Y, 2020a. Cross-frequency coupling in descending motor pathways: Theory and simulation. Front Syst Neurosci 13, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N, Heckman C, Yang Y, 2020b. Slowly activating outward membrane currents generate input-output sub-harmonic cross frequency coupling in neurons. J Theor Biol, 110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Brown EN, 2003. Estimating a state-space model from point process observations. Neural Comput 15, 965–991. [DOI] [PubMed] [Google Scholar]
- Song D, Chan RH, Marmarelis VZ, Hampson RE, Deadwyler SA, Berger TW, 2007. Nonlinear dynamic modeling of spike train transformations for hippocampal-cortical prostheses. IEEE Trans Biomed Eng 54, 1053–1066. [DOI] [PubMed] [Google Scholar]
- Song D, Robinson BS, Hampson RE, Marmarelis VZ, Deadwyler SA, Berger TW, 2016. Sparse large-scale nonlinear dynamical modeling of human hippocampus for memory prostheses. IEEE Trans Neural Syst Rehabil Eng 26, 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan V, Eswaran C, Sriraam N, 2007. Approximate entropy-based epileptic eeg detection using artificial neural networks. IEEE Trans Inf Technol Biomed 11, 288–295. [DOI] [PubMed] [Google Scholar]
- Stam C, Jelles B, Achtereekte H, Rombouts S, Slaets J, Keunen R, 1995. Investigation of eeg non-linearity in dementia and parkinson’s disease. Electroencephalogr Clin Neurophysiol 95, 309–317. [DOI] [PubMed] [Google Scholar]
- Stam CJ, 2005. Nonlinear dynamical analysis of eeg and meg: review of an emerging field. Clin Neurophysiol 116, 2266–2301. [DOI] [PubMed] [Google Scholar]
- Stanley GB, 2005. Neural system identification, in: Neural Eng. Springer, pp. 367–388. [Google Scholar]
- Stephan KE, Harrison LM, Kiebel SJ, David O, Penny WD, Friston KJ, 2007. Dynamic causal models of neural system dynamics: current state and future extensions. J Biosci 32, 129–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Penny WD, Moran RJ, den Ouden HE, Daunizeau J, Friston KJ, 2010. Ten simple rules for dynamic causal modeling. Neuroimage 49, 3099–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subasi A, 2006. Automatic detection of epileptic seizure using dynamic fuzzy neural networks. Expert Syst Appl 31, 320–328. [Google Scholar]
- Sysoeva MV, Sitnikova E, Sysoev IV, Bezruchko BP, van Luijtelaar G, 2014. Application of adaptive nonlinear granger causality: Disclosing network changes before and after absence seizure onset in a genetic rat model. J Neurosci Methods 226, 33–41. [DOI] [PubMed] [Google Scholar]
- Tank A, Covert I, Foti N, Shojaie A, Fox E, 2018. Neural granger causality for nonlinear time series. arXiv:1802.05842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R, Dewald J, Yang Y, 2020. Assessing neural connectivity and associated time delays of muscle responses to continuous position perturbations. Ann Biomed Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R, Yang Y, van der Helm FC, Dewald J, 2018. A novel approach for modeling neural responses to joint perturbations using the narmax method and a hierarchical neural network. Front Comput Neurosci 12, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobimatsu S, Zhang YM, Kato M, 1999. Steady-state vibration somatosensory evoked potentials: physiological characteristics and tuning function. Clin Neurophysiol 110, 1953–1958. [DOI] [PubMed] [Google Scholar]
- Tsatsanis MK, Giannakis GB, 1993. Time-varying system identification and model validation using wavelets. IEEE Trans Signal Process 41, 3512–3523. [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J, 2001. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci 2, 229–239. [DOI] [PubMed] [Google Scholar]
- Verdult V, 2002. Non linear system identification: a state-space approach.
- Vlaar MP, Birpoutsoukis G, Lataire J, Schoukens M, Schouten AC, Schoukens J, van der Helm FC, 2017. Modeling the nonlinear cortical response in eeg evoked by wrist joint manipulation. IEEE Trans Neural Syst Rehabil Eng 26, 205–215. [DOI] [PubMed] [Google Scholar]
- Vlaar MP, Solis-Escalante T, Vardy AN, Van Der Helm FC, Schouten AC, 2016. Quantifying nonlinear contributions to cortical responses evoked by continuous wrist manipulation. IEEE Trans Neural Netw Learn Syst 25, 481–491. [DOI] [PubMed] [Google Scholar]
- Wacker M, Witte H, 2010. On the stability of the n: m phase synchronization index. IEEE Trans Biomed Eng 58, 332–338. [DOI] [PubMed] [Google Scholar]
- Wang Z, Huang Y, Wang S, Green A, Aziz T, Stein J, 2014. Tremor dependant nonlinear interaction in deep brain local field potentials of parkinson’s disease, in: Int Conf Biomed, IEEE. pp. 399–404. [Google Scholar]
- Wu W, Nagarajan S, Chen Z, 2015. Bayesian machine learning: Eeg\/meg signal processing measurements. IEEE Signal Process Mag 33, 14–36. [Google Scholar]
- Yang Y, Dewald JP, van der Helm FC, Schouten AC, 2018. Unveiling neural coupling within the sensorimotor system: directionality and nonlinearity. Eur J Neurosci 48, 2407–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Sinha N, Tian R, Gurari N, Drogos J, Dewald J, 2020a. Quantifying altered neural connectivity of the stretch reflex in chronic hemiparetic stroke. IEEE Trans Neural Syst Rehabil Eng 28, 1436–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Solis-Escalante T, van der Helm FC, Schouten AC, 2016a. A generalized coherence framework for detecting and characterizing nonlinear interactions in the nervous system. IEEE Trans Biomed Eng 63, 2629–2637. [DOI] [PubMed] [Google Scholar]
- Yang Y, Solis-Escalante T, van de Ruit M, van der Helm FC, Schouten AC, 2016b. Nonlinear coupling between cortical oscillations and muscle activity during isotonic wrist flexion. Front Comput Neurosci 10, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Solis-Escalante T, Yao J, Daffertshofer A, Schouten AC, Van Der Helm FC, 2016c. A general approach for quantifying nonlinear connectivity in the nervous system based on phase coupling. INT J NEURAL SYST 26, 1550031. [DOI] [PubMed] [Google Scholar]
- Yang Y, Yao J, Dewald J, Van der Helm FC, Schouten AC, 2020b. Quantifying the nonlinear interaction in the nervous system based on phase-locked amplitude relationship. IEEE Trans Biomed Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Hu Y, Potter T, Li R, Quach M, Zhang Y, 2020. Establishing functional brain networks using a nonlinear partial directed coherence method to predict epileptic seizures. J Neurosci Methods 329, 108447. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Billings SA, Wei H, He F, Sarrigiannis PG, 2013. A new narx-based granger linear and nonlinear casual influence detection method with applications to eeg data. J Neurosci Methods 212, 79–86. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Lin Z, Tay DBH, 2001. Time-varying parametric system multiresolution identification by wavelets. Int J Syst Sci 32, 775–793. [Google Scholar]
- Zhong Y, Jan KM, Ju KH, Chon KH, 2007. Representation of time-varying nonlinear systems with time-varying principal dynamic modes. IEEE Trans Biomed Eng 54, 1983–1992. [DOI] [PubMed] [Google Scholar]
- Zhou M, Tian C, Cao R, Wang B, Niu Y, Hu T, Guo H, Xiang J, 2018. Epileptic seizure detection based on eeg signals and cnn. Front Neuroinform 12, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou R, Wang H, Chon KH, 2003. A robust time-varying identification algorithm using basis functions. Ann Biomed Eng 31, 840–853. [DOI] [PubMed] [Google Scholar]