Abstract
The effects of feeding different levels of poultry by-product meal (PBM) replacing fishmeal (FM) protein, supplemented with tuna hydrolysate (TH) and Hermetia illucens (HI) larvae, on the growth, fillet quality, histological traits, immune status, oxidative biomarker levels and gut microbiota of juvenile barramundi, Lates calcarifer were investigated for six weeks. Barramundi were fed four isonitrogenous and isolipidic diets in which a FM based diet was used as the Control diet (Diet1) and compared with other non-FM diets containing 80%, 85% and 90% PBM along with the concurrent supplementation of 5% and/or 10% TH and HI larvae meal. These treatment diets were designated as 80PBM10TH+10HI (Diet2), 85PBM5TH+10HI (Diet3) and 90PBM5TH+5HI (Diet4). The growth and condition factor of fish fed 80PBM10TH+10HI and 85PBM5TH+10HI were significantly higher than the Control. Total saturated, monounsaturated and polyunsaturated fatty acid retention in the fish muscle increased in fish fed PBM-based diets, supplemented with TH and HI larvae meal, with no adverse effect on post-harvest characteristics such as texture and colour of fish fillets. Improvement in serum total bilirubin and total protein content was found in all fish fed TH and HI larvae supplemented PBM. Similarly, immune response showed a significant increase in fish fed non-FM test diets than the Control. In the distal intestine, supplementation of any quantities of TH and HI larvae to PBM led to an increase in the microvilli density and neutral mucins while the number of goblet cells in the skin were unchanged. Liver, kidney, and spleen histology demonstrated a normal structure with no obvious changes in response to all test diets. Bacterial diversity increased in fish fed Diets 2 and 3 with a high abundance of Proteobacteria in Diets 1 and 4 and Firmicutes in Diets 2 and 3. The fish on test diets showed a lower abundance of genus Vibrio. Fish fed TH and HI larvae supplemented PBM diets showed lower infection rate to V. harveyi than the Control. Collectively, concurrent supplementation of TH and HI larvae could improve the quality of PBM diets with positive effects on growth, fillet quality, intestinal health, immunity, and disease resistance.
Subject terms: Immunology, Microbiology, Microscopy, Animal physiology
Introduction
Due to a favourable nutritional profile, compatible with the nutritional requirement of most aquaculture species1, the aquaculture industry has traditionally relied on fishmeal (FM) as the main protein source2. Since this dietary protein source is considered environmentally and ecologically unsustainable, there is societal and economical pressure on the aquaculture industry to search for alternative protein ingredients. Efforts have been dedicated over the past few decades to utilize plant-feedstuffs to replace FM1 however the resulting growth performance for carnivorous fish is inferior when compared to FM-based diets, likely due to the lower protein and imbalanced amino acids, anti-nutritional factors, and no taurine and hydroxyproline contents relative to FM in plant ingredients3–6.
Rendered animal by-products, particularly poultry by-product (PBM) could be a promising alternative protein source for carnivorous fish due to a higher protein content and lack of anti-nutritional factors7,8. The incorporation of 100% PBM replacing FM protein did not affect the welfare of a number of carnivorous fish such as humpback grouper9, Nile tilapia, Oreochromis niloticus10,11 and hybrid striped bass, Morone chrysops × Morone saxatilis12. However, feeding PBM at replacement levels more than 50% impacted the growth performance in barramundi, L. calcarifer13, Florida pompano, Trachinotus carolinus L tench14, juvenile tench, Tinca tinca15, black sea turbot, Psetta maeoticus16 and cobia, Rachycentron canadum17. In addition, impairment of immunity and serum biochemistry were reported in largemouth bass, Micropterus salmoides18,19 and barramundi fed PBM13,20 and hybrid grouper, Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂ fed an animal protein blend8. In our previous studies13,20,21, we have also shown that the exclusive inclusion of PBM protein at the expense of FM protein caused histopathological changes in the liver and impaired the integrity of intestinal micromorphology of barramundi. Though a number of studies have been conducted on re-formulating aquadiets supplementing fish protein hydrolysates (FPH)22–25 and other supplements26–28 that complimented animal proteins and helped to satisfy the needs of barramundi, developing functional sustainable feeds that remain cost-efficient, while promoting fish welfare and maximising growth potential, is a foremost challenge for the aquaculture industry.
FPH due to presence of low molecular weight peptides and free amino acids, have been supplemented in aquafeed as immunostimulants, palatability enhancers and attractants29–31. Promising results in terms of growth performance, immune response and disease resistance have been reported for many carnivorous fish species such as barramundi22,23,32, Atlantic salmon, Salmo salar33, European sea bass, Dicentrarchus labrax34 and Persian sturgeon, Acipenser persicus L.35 when fed FPH at an appropriate level. Moreover, supplementation of FM and PBM with FPH modulated the intestinal microbiome of barramundi by increasing the number of Bacillus, Lactococcus, and Cetobacterium under phylum of Firmicutes and Fusobacteria and also regulated the inflammatory response22.
Recently, insect have received much attention as an aquafeed ingredient due to rapid growth, and ease of reproduction36. Insects may be grown on low-value products and the final waste (frass) can be used as organic fertilizer, and insect production also has a lower risk of transmitting zoonotic infections, less greenhouse gas and ammonia emissions and less requirement for land and water than plant-feedstuffs production6,37–39. Among all insects investigated so far, the larvae of Hermetia illucens (HI), commonly known as black soldier fly has been identified as a potential aquafeed ingredient due to the ability to assimilate nutrients from low-value wastes and by-products and transform such ingredients into high-quality animal biomass, representing a sustainable way to produce edible proteins for livestock production2,40,41. It is also noteworthy that the lipid content of HI larvae is highly variable, depending on the substrate used for their culture42 and easy to manipulate. Also, HI larvae as an ecological decomposer, dwelling in a harsh environment, infested with a high concentration of harmful microorganisms, can produce novel antimicrobial peptides (AMP) to gain protection from microbial infection43,44. Indeed, a new defensin-like peptide4 AMP of 40 amino acids purified from immunized haemolymph of HI larvae has exhibited bactericidal activity to Gram-positive bacterial strains45. Hence, the dietary supplementation of HI larvae in the aquadiets could also play an important role in modulating immune response and defensive mechanisms against microbial pathogens.
Among all fish microbiota, bacteria are dominant in the fish intestine46. However, the bacterial composition is diverse in different parts of the intestine47 and is influenced by diet, life stage, sex, habitat and season48–52. Dietary manipulation of protein and lipid also has a selective pressure on the intestinal bacterial diversity in the digestive tract of fish46,53. Over the past few decades, many conventional methods have been applied to analyse the gut microbiota of fish54 but recently with the development of molecular techniques such as next-generation sequencing (NGS), a rapid and low-cost technique, research on the gut microbiota of fish has evolved rapidly46. Like humans and other mammals, there is little doubt concerning the importance of the intestinal microbiota for fish health22,55,56, however, dysbiosis of the intestinal microbiota could also be related to growth impairment, dysregulation of immune functions and disease development in fish57,58. A recent study22, has reported the modulatory effects of alternative protein sources on the intestinal microbiota in barramundi but possible links between diets, growth, and gut functions is fragmentary and incomplete. Hence, the profiling of gut microbiota is expected to be an important endpoint measurement to assess the effects of aquadiet in relation to growth and other health aspects.
As alternative protein ingredients could adversely affect the marketability of fish by altering fillet characteristics, a research evaluating the potential effects of extensive replacement of FM with other animal by-products on the post-harvest quality of fish flesh is highly desirable. Animal by-products particularly meat meal did not alter the organoleptic quality of barramundi fillet59, however, excessive inclusion of PBM negatively influenced the fillet quality of female tenches, Tinca tinca60. Nonetheless, the effects of animal based-diets at the exclusion of FM on the post-harvest quality of barramundi fillet have rarely been investigated with mixed results.
Barramundi is a commercially important euryhaline species, popularly cultured in South East Asian countries and Australia due to economic value and favourable flesh texture. The disease particularly Vibriosis, caused by V. harveyi is one of the common problems, causing mortalities in net-cages culture. A number of earlier studies have been conducted so far supplementing plant herbs27, FPH22,24 and HI larvae13 to improve the health status of barramundi. However, concurrent effects of TH and HI larvae meal have not been reported to date. Hence, the present study was designated to investigate the effects of total substitution of dietary FM with three different protein ingredients PBM and, TH and HI larvae meal on gut microbiota, health indices and fillet quality of barramundi. Protein from PBM was the main source of protein whereas TH and HI were supplemented in three different proportions to evaluate the replacement efficiency of PBM protein.
Materials and methods
Ethical statement
All experimental protocols involving handling and treatment of animals described in the present study were approved by the Animal Ethics Committee at Curtin University under Permit no. ARE2018-37 in full compliance with relevant guidelines and regulations in Australia for the care and use of animals. To minimize pain and discomfort during handling and culling of fish, anaesthesia (AQUI-S, 8 mg/l) and euthanasia (AQUI-S, 175 mg/l) were applied following standard operating procedure (SOP) of the Curtin Aquatic Research Laboratory (CARL).
Experimental diets
All the ingredients (Table 1) excluding PBM, TH, and HI larvae were bought from Specialty Feeds, Glen Forrest Stockfeeders, 3150 Great Eastern Highway, Glen Forrest, Western Australia 6071. PBM was provided by Derby Industries Pty Ltd T/A, Talloman Lot Lakes Rd, Hazelmere WA 6055 and liquid TH was provided by SAMPI, Port Lincoln, Australia. Four animal protein based-test diets with similar proximate compositions were formulated (Table 1). FM protein is substituted mainly by PBM followed by supplementing TH and/or HI. The control diet was formulated using FM as a protein source and the other three diets were formulated to replace 80%, 85% and 90% of FM protein with PBM along with the supplementation of 10% TH + 10% HI, 5% TH + 10% HI, and 5% TH + 5% HI, respectively. The four diets were designated as Control (Diet1), 80PBM10TH+10HI (Diet2), 85PBM5TH+10HI (Diet3) and 90PBM5TH+5HI (Diet4). The diets were formulated, packed and stored according to the common standard protocol in our laboratory13. Fatty acid and amino acid composition of experimental diets and different ingredients are presented in Tables 2 and 3, respectively.
Table 1.
Ingredients and nutritional composition of four experimental diets (% dry matter basis).
| Ingredients (g/100 g) | Diet1 (Control) | Diet2 (80PBM10TH+10HI) | Diet3 (85PBM5TH+10 HI) | Diet4 (90PBM5TH+ 5HI) |
|---|---|---|---|---|
| †FMa | 72.6 | 0.00 | 0.00 | 0.00 |
| ††PBMb | 0.00 | 57.00 | 60.50 | 63.50 |
| Canola oila | 2.20 | 4.00 | 4.00 | 4.00 |
| Cod Liver Oila | 1.40 | 2.50 | 2.50 | 3.60 |
| Corn/wheat starcha | 6.7 | 8.00 | 10.00 | 10.80 |
| wheat (10 CP)a | 13.5 | 7.90 | 5.90 | 6.00 |
| Lecithin – Soy (70%)a | 2.00 | 2.00 | 2.00 | 2.00 |
| Vitamin Ca | 0.05 | 0.05 | 0.05 | 0.05 |
| Dicalcium Phosphatea | 0.05 | 0.05 | 0.05 | 0.05 |
| Vitamin and mineral premix a | 0.50 | 0.50 | 0.50 | 0.50 |
| Salt (NaCl)a | 1.00 | 1.00 | 1.00 | 1.00 |
| †††HI full-fat larvaec | 0.00 | 10.00 | 10.00 | 5.00 |
| ‡THd | 0.00 | 7.00 | 3.50 | 3.50 |
| Total | 100 | 100 | 100 | 100 |
| Nutritional composition (%) | ||||
| Dry matter | 92.90 | 90.35 | 91.35 | 92.14 |
| Crude protein | 46.20 | 45.92 | 46.20 | 46.72 |
| Crude lipid | 12.93 | 13.01 | 12.88 | 13.00 |
| Ash | 12.78 | 12.64 | 11.89 | 12.44 |
| Gross energy (MJ kg-1) | 20.02 | 19.98 | 19.88 | 19.46 |
aPurchased from Specialty Feeds, Glen Forrest Stockfeeders, 3150 Great Eastern Highway, Glen Forrest, Western Australia 6071.
†Fishmeal (FM): 64.0% crude protein, 10.76% crude lipid and 19.12% ash.
††Poultry by-product meal (PBM): 67.13% crude protein, 13.52% crude lipid and 13.34% ash.
††††HI (Hermetia illucens) larvae: 40.04% crude protein, 28.30% crude lipid, 7.81% moisture and 8.91% ash.
‡Tuna hydrolysate (TH): 37.91% crude protein, 5.50% crude lipid, and 11.05% ash.
Table 2.
Amino acid composition (g/100 g DM) of PBM-based diets, supplemented with TH and HI larvae meal and different ingredients including PBM, TH and HI larvae meal.
| Diet1 (Control) | Diet2 (80PBM10TH+10HI) | Diet3 (85PBM5TH+10 HI) | Diet4 (90PBM5TH+ 5HI) | PBM | TH | HI | |
|---|---|---|---|---|---|---|---|
| Hydroxyproline | 1.79 | 3.14 | 3.16 | 3.37 | 3.48 | 1.81 | 0.24 |
| Histidine | 3.01 | 2.33 | 2.36 | 2.27 | 2.30 | 2.96 | 3.27 |
| Taurine | 0.48 | 0.59 | 0.56 | 0.53 | 0.44 | 2.84 | 1.08 |
| Serine | 4.50 | 4.22 | 4.26 | 4.19 | 4.34 | 4.55 | 4.48 |
| Arginine | 6.31 | 6.89 | 6.95 | 6.98 | 7.32 | 5.55 | 5.45 |
| Glycine | 7.99 | 9.62 | 9.74 | 9.98 | 10.13 | 8.79 | 5.86 |
| Aspartic acid | 9.45 | 8.84 | 8.71 | 8.81 | 8.50 | 9.07 | 10.42 |
| Glutamic acid | 13.90 | 14.55 | 14.31 | 14.49 | 13.83 | 11.69 | 13.42 |
| Threonine | 4.74 | 4.13 | 4.17 | 4.10 | 4.19 | 4.74 | 4.35 |
| Alanine | 6.72 | 6.65 | 6.65 | 6.66 | 6.61 | 7.01 | 6.59 |
| Proline | 5.67 | 6.70 | 6.70 | 6.71 | 6.63 | 5.64 | 6.24 |
| Lysine | 7.32 | 6.63 | 6.56 | 6.55 | 6.58 | 6.98 | 7.10 |
| Tyrosine | 2.87 | 2.90 | 2.86 | 2.79 | 2.97 | 2.90 | 5.97 |
| Methionine | 2.92 | 2.15 | 2.13 | 2.15 | 2.23 | 2.71 | 2.02 |
| Valine | 5.33 | 5.02 | 5.08 | 4.95 | 4.92 | 5.80 | 6.40 |
| Isoleucine | 4.54 | 4.20 | 4.24 | 4.14 | 4.07 | 4.61 | 4.86 |
| Leucine | 8.04 | 7.36 | 7.45 | 7.30 | 7.37 | 7.88 | 7.59 |
| Phenylalanine | 4.42 | 4.08 | 4.12 | 4.03 | 4.09 | 4.49 | 4.67 |
Table 3.
Fatty acids composition (g/100 g DM) of PBM-based diets, combinedly supplemented with TH and HI larvae meal and different ingredients including PBM, TH and HI.
| Diet1 (Control) | Diet2 (80PBM10TH+10HI) | Diet3 (85PBM5TH+10 HI) | Diet4 (90PBM5TH+ 5HI) | PBM | TH | HI | |
|---|---|---|---|---|---|---|---|
| C12:0 | 0.03 | 5.02 | 5.23 | 2.44 | 0.09 | 0.04 | 53.81 |
| C14:0 | 1.32 | 2.10 | 1.96 | 1.66 | 0.74 | 4.75 | 8.87 |
| C16:0 | 11.61 | 18.13 | 17.73 | 17.01 | 23.36 | 28.56 | 20.47 |
| C18:0 | 4.49 | 5.73 | 5.55 | 5.34 | 7.62 | 10.92 | 4.65 |
| ∑SFA | 19.81 | 33.03 | 32.41 | 28.13 | 33.45 | 48.41 | 93.05 |
| C14:1n5 | 0.02 | 0.10 | 0.10 | 0.09 | 0.16 | 0.04 | 0.15 |
| C16:1n7 | 1.65 | 3.93 | 3.90 | 3.71 | 5.40 | 5.70 | 6.21 |
| C18:1cis + trans | 11.59 | 43.54 | 43.93 | 42.49 | 44.11 | 33.34 | 23.37 |
| C20:1 | 0.80 | 2.03 | 1.96 | 2.34 | 0.61 | 2.56 | 0.29 |
| C22:1n9 | 0.09 | 0.20 | 0.19 | 0.25 | 0.06 | 0.31 | 0.00 |
| ∑MUFA | 14.82 | 50.41 | 50.64 | 49.47 | 50.42 | 43.01 | 30.67 |
| C18:3n3 | 1.20 | 4.28 | 4.37 | 3.99 | 2.60 | 1.53 | 5.17 |
| C18:4n3# | 0.30 | 0.55 | 0.47 | 0.42 | 0.13 | 2.25 | 1.84 |
| C20:3n3 | 0.08 | 0.08 | 0.07 | 0.07 | 0.04 | 0.25 | 0.11 |
| C20:5n3 | 1.78 | 2.15 | 1.68 | 1.77 | 0.17 | 12.12 | 3.67 |
| C22:5n3# | 0.63 | 0.66 | 0.53 | 0.60 | 0.37 | 3.41 | 0.28 |
| C22:6n3 | 9.09 | 4.25 | 2.95 | 3.49 | 0.27 | 32.32 | 0.78 |
| ∑n-3 PUFA | 13.09 | 11.97 | 10.06 | 10.34 | 3.59 | 51.88 | 11.85 |
| ∑n-3 LC PUFA | 11.59 | 7.14 | 5.22 | 5.93 | 0.01 | 48.10 | 4.83 |
| C20:2 | 0.15 | 0.18 | 0.17 | 0.17 | 0.20 | 0.35 | 0.12 |
| C18:3n6 | 0.09 | 0.09 | 0.09 | 0.13 | 0.23 | 0.21 | 0.15 |
| C20:3n6 | 0.15 | 0.30 | 0.30 | 0.30 | 0.57 | 0.47 | 0.23 |
| C20:4n6 | 1.13 | 1.01 | 0.98 | 0.93 | 1.80 | 1.99 | 1.31 |
| C22:4n6# | 0.91 | 0.17 | 0.12 | 0.13 | 0.05 | 1.18 | 0.20 |
| ∑n-6 PUFA | 2.28 | 1.58 | 1.50 | 1.49 | 2.65 | 3.86 | 1.88 |
| ∑PUFA | 21.84 | 31.24 | 29.86 | 29.00 | 23.87 | 58.32 | 27.38 |
Experimental fish and feeding
The feeding trial was conducted in a recirculating aquaculture system at CARL, Curtin University, Australia. A total of 450 juvenile barramundi (mean weight, 3.33 g) were provided by the Australian Centre for Applied Aquaculture Research, Fremantle, Australia and shipped to CARL in double-layered plastic bags filled with oxygenated 30 ppt saline water. Fish were housed in three 300L fiberglass tanks containing seawater for two weeks to adapt to the CARL conditions. Throughout the acclimation period, one-third of the water was changed daily and fish were hand-fed with a commercial diet. Fish were fasted for 24 h before commencing the trial and then 300 fish with almost similar weights were distributed randomly into 12 tanks (4 dietary treatments × 3 replicated tanks), with 25 individuals in each tank. Water quality parameters including temperature, dissolved oxygen, nitrite, nitrate, and temperature controlled by an electric heater, aerator and external bio-filter (ASTRO 2212, China), were maintained as described in our earlier study13. 14:10 h light: dark photoperiod was maintained throughout the study period using an automatic indoor timer (Clipsal, Australia). Fish were hand-fed the experimental diets until apparent satiation, twice daily at 8.00 am and 6.00 pm for 6 weeks. After each feeding session, provided feed and uneaten feed weights were recorded to calculate FI on a daily basis. Fish health and mortality, if any, were monitored regularly to calculate the survival rate.
Sampling procedure
After the six weeks of feeding trial, fish were starved for 24 h and anesthetized with 8 mg/l AQUI-S prior to taking total biomass, and fish were also counted individually to estimate survival rate. Viscera and liver weight were recorded from randomly chosen ten fish from each treatment to determine viscerosomatic (VSI) and hepatosomatic (HSI) indices. For serum collection, four fish from each tank (n = 12) were anesthetized with 8 mg/l AQUI-S and blood was collected from the caudal vein using 1 mL syringes fitted with 22G needles. Serum was collected and preserved according to the protocols of our earlier study13. Fish were then culled with a sharp blow to the head and dissected on trays to collect muscle, liver, and intestine samples. Pooled “fillet” muscle was collected and dried in a freeze dryer and stored at -80◦ C for further fatty acid analysis. Also, four fish/replicate were filleted to analyse texture and colour. The liver and intestine were also preserved immediately in -80◦ C until later analysis of antioxidant activity and gut microbiota. Four fish per tank were also euthanized to collect intestine, muscle with skin, liver, kidney, and spleen and immediately preserved in 10% buffered formalin for fixation for later histological examination.
Fillet fatty acids compositions and quality index
Fatty acids profile of four pooled dried muscle/replicate and experimental diets were performed according to the procedures of O'Fallon, et al.61 and Siddik, et al.21.
Texture profile in terms of hardness, cohesiveness, adhesiveness, springiness, gumminess, chewiness of fish fillet (three fish/treatment) were analysed by texture analyser TVT 6700 (PerkinElmer, Inc., Waltham, Middx, USA) and TexCal texture analyser software (version 5.0)62. Each sample was run in triplicate and expressed as force per g. Fillet colour coordinates (L*, a*, b*) were determined by calibrated BYK spectro-guide sphere gloss (BYK-Gardener USA, Columbia, MD, USA)63. 4 g of fillet were homogenized with 40 mL of distilled water and pH was determined in triplicate by Aqua-pH meter (TPS Pty Ltd, Brendale, QLD, AU) after calibrating at three-scale pH (pH 4, 7, 10)64.
Histological and scanning electron microscopy analysis
After fixation with 10% buffered formalin, fragments of intestine, liver, kidney, spleen, and skin tissue samples were dehydrated with a series of ethanol concentrations. Dehydrated samples were subsequently cleared in xylene, embedded in paraffin blocks, cut into around 5 µm slices, and stained with haematoxylin and eosin (H&E) for histopathological assessment. Intestine and liver samples were stained with Periodic Acid-Schiff (PAS) to visualize neutral mucins and glycogen, respectively. Skin tissue was stained with AB-PAS stain to visualize goblet cells. Microphotographs of all histological slides were taken with a light imaging microscope (BX40F4, Olympus, Tokyo, Japan). The number of neutral mucins in intestine and skin was counted as described in our earlier study13.
Scanning electron microscopy of distal intestine from four biological replicates were analysed according to the earlier study in our laboratory24. Intestinal samples (5 mm) were washed for 30 s with 1% S-carboxymethyl-L-cysteine to remove mucus and then fixed in 2.5% glutaraldehyde in sodium cacodylate buffer (0.1 M pH 7.2). Samples were processed as described elsewhere, screened with JSM 6610 LV (Jeol, Tokyo, Japan) SEM and analysed with Image J 1.46r (National Institute of Health, USA).
Serum immunity and biochemical assays
Serum immunity including lysozyme and bactericidal activity were analysed as described in our earlier study13.
The plasma clinical chemistry panel was processed on a AU480 Clinical Chemistry Analyser (Beckman Coulter Australia Pty Ltd, Lane Cove West, NSW). Beckman Coulter clinical chemistry kits were used for the following panel components: alanine transaminase (ALT OSR6107) gamma-glutamyl transferase (GGT OSR6219) total bilirubin (TB OSR6112), urea (OSR6134), creatinine (OSR6178), cholesterol (OSR6116), total protein (TP OSR6132) while Randox kits (Randox Australia Pty Ltd, Parramatta, NSW) were used for glutamate dehydrogenase (GLDH GL441).
Serum and liver antioxidant activity
For each replicate/treatment, approximately 0.20 mg of liver tissues was weighed and homogenized with 2 mL of chilled PBS. Immediately homogenized tissue was centrifuged at 10,000 × g for 15 min at 4◦ C and supernatant was collected and stored at 80◦ C till analysis.
Catalase activity (CAT) in serum and liver homogenate were performed according to the manufacturer company instruction (Bockit, BIOQUOCHEM SL, 33,428 Llanera-Asturias, Spain).
Microbiome analysis
Amplicon sequencing
After the trial, two randomly selected fish per tank (n = 6) were used for gut microbiota analysis. Processing of samples comprised of a collection of the whole gut, separation of the hindgut, homogenization of gut contents in tissue lyserII (Qiagen, Hilden, Germany), and pooling of homogenized samples from the respective tanks (n = 3). Bacterial DNA from pooled the hindgut samples was extracted using Blood and Tissue Kit (Qiagen, Hilden, Germany). DNA concentration was measured in NanoDrop (Thermo Fisher Scientific, USA). PCR master mixture was prepared as 50 µl final concentration containing 25 µl Hot Start 2X Master mix (New England BioLabs Inc., USA), 2 µl of sample DNA, 1 µl of each V3V4 primers65 and 21 µl of nuclease-free water. A total of 40 cycles of amplification reactions was performed in a thermal cycler (Bio-Rad Laboratories, Inc., USA). The library for 16S rRNA amplicons was prepared according to Illumina standard protocol (Part # 15,044,223 Rev. B). Samples were then sequenced on an Illumina MiSeq platforms (Illumina Inc., San Diego, California, USA using a v3 kit (600 cycles).
Sequence data processing and analysis
Low quality reads (phred score ≤ 20, > 6 homopolymers), adaptors, short bases (< 200 bp) were trimmed and cleaned using TrimGalore (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/). The quality of sequences was checked before and after trimming in FastQC and MultiQC pipelines66,67. MeFiT program was used for the merging of overlapping pair-end reads68. De novo assembly, picking of OTUs at 97% similarity threshold level and removing of singleton OTUs were performed in micca (v1.6.1)-qiime (v2.0) pipelines69,70. Phylogenetic assignment of OTUs at different taxa levels was performed against SILVA 1.32 release71. PASTA-aligned sequences were used for phylogenetic tree constructions under FastTree (version 2.1.8) GTR + CAT72,73. The rarefaction depth was set to 38,296 bp and subsequent measurements of alpha–beta diversity were performed using qiime (v1.9.1) and R packages. Alpha diversity was calculated in terms of observed OTUs and Shannon indices. Beta diversity was measured based on Bray–Curtis dissimilarity of Weighted UniFrac matric. Relative abundance (≥ 1% of read abundance) of phyla and genera was calculated phyloseq R package74. Differential abundance was calculated using the linear discriminant analysis effect size (LEfSe) at LDA ≥ 2.0 and 0.05 level of significance75.
Challenge test
After the feeding trial, 10 fish/tank (30 fish/treatment) with the same rearing condition were challenged with V. harveyi, provided by Diagnostic and Laboratory Services, Department of Primary Industries and Regional Development (DPIRD), 3 Baron-Hay Court, South Perth WA 6151. Fish were intraperitoneally injected with 0.1 mL of phosphate-buffered saline (PBS) containing 1.1 × 10^8 cfu/ml of a pathogenic strain of V. harveyi as elucidated in our earlier study25. Symptoms of vibriosis such as thick layer of mucous on the body surface, congestion of the fins, and haemorrhages and ulceration of the skin and muscle tissue were monitored every 8 h and lasted for 96 h. Fish with vibriosis were culled according to the protocol of the CARL SOP for fish euthanization.
Calculation and statistical analysis
Weight gain (WG), specific growth rate (SGR), feed intake (FI), feed conversion ratio (FCR), condition factor (CF), viscerosomatic index (VSI) and hepatosomatic index (HSI) were estimated using the following formulae:
All experimental results are presented as mean ± standard error (SE). Groups of fish per tank were used as experimental units for growth data. Individual fish were used as experimental units for organo-somatic assessment, biochemical assays, immune response, fatty acids, antioxidant activity, and histological analysis. One-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons test was performed to the significant differences between treatments when data met the normality, checked by Shapiro–Wilk's and Levene's tests. Infection data from the challenge trial were analysed by the Kaplan–Meier method based on the pairwise multiple comparison Log-Rank (Mantel-Cox) test.
Results
Growth performance and organo-somatic indices
The effects of different levels of PBM concurrently supplemented with TH and HI on the growth performance and organo-somatic indices are shown in Table 4. Fish fed Diet2 (80PBM10TH+10HI) and Diet3 (85PBM5TH+10HI) recorded significantly higher final body weight (FBW), weight gain (WG) and specific growth rate (SGR) after six weeks of feeding whereas fish fed Diet 4 (80PBM5TH+5HI) was similar to Control. No significant variation was observed in feed intake (FI) and feed conversion ratio (FCR) between Control and test diets. The survival rate, ranging from 89.33 to 93.33%, was not influenced by the test diets. Condition factor (CF) increased significantly in fish fed supplemented TH and HI larvae diets, although hepatosomatic (HSI) and viscerosomatic (VSI) indices were not influenced by any test diets.
Table 4.
Growth performance, feed utilization, survival and organo-somatic indices of barramundi fed Control (Diet1), 80PBM10TH+10HI (Diet2), 85PBM5TH+10HI (Diet3), and 90PBM5TH+5HI (Diet4) for six weeks.
| Diet1 (Control) | Diet2 (80PBM10TH+10HI) | Diet3 (85PBM5TH+10 HI) | Diet4 (90PBM5TH+ 5HI) | |
|---|---|---|---|---|
| FBW (g) | 50.05 ± 2.05c | 67.60 ± 1.93a | 60.49 ± 2.37ab | 54.24 ± 2.57bc |
| WG (g) | 42.30 ± 2.05c | 59.84 ± 1.93a | 52.73 ± 2.37ab | 46.48 ± 2.57bc |
| SGR (%/d) | 4.28 ± 0.11c | 5.07 ± 0.08a | 4.73 ± 0.11ab | 4.42 ± 0.13bc |
| FI (g/fish/d) | 1.48 ± 0.10 | 1.60 ± 0.11 | 1.40 ± 0.19 | 1.40 ± 0.17 |
| FCR | 1.49 ± 0.16 | 1.14 ± 0.07 | 1.11 ± 0.05 | 1.28 ± 0.08 |
| SR (%) | 90.67 ± 3.53 | 89.33 ± 1.33 | 93.33 ± 1.33 | 92.00 ± 2.31 |
| CF (%) | 1.27 ± 0.01b | 1.38 ± 0.01a | 1.35 ± 0.01a | 1.37 ± 0.01a |
| VSI (%) | 9.86 ± 0.46 | 10.69 ± 0.40 | 11.06 ± 0.30 | 11.08 ± 0.30 |
| HSI (%) | 1.70 ± 0.07 | 1.75 ± 0.08 | 1.67 ± 0.07 | 1.77 ± 0.08 |
Multiple comparisons were performed by one-way ANOVA analysis followed by Dunnett’s multiple comparisons test.
Results are expressed as mean ± SE (standard error) (n = 3). Means with different superscripts letters in the same row indicates significant difference at P < 0.05.
Muscle chemical and fatty acid composition
Moisture and ash were unchanged among the test diets at the end of the trial. Lipid in muscle of fish fed PBM based-diets supplemented with TH and HI larvae increased significantly while protein levels decreased significantly in PBM based diets (Table 5). The concentration of C12:0, C14:0, C16:0, and C18:0 led to an increase in the total SFA in the muscle of fish fed PBM-based diets, concurrently supplemented with TH and HI larvae. Total MUFA content increased in the same diets and it was mainly due to the higher concentration of C14:1n5, C16:1n7, C20:1, C18:1cis + trans, and C22:1n9. TH and HI larvae supplemented PBM substantially improved the total PUFA, manifested by an elevated level of C18:3n3, C18:4n3, C20:3n3, C22:5n3, C18:3n6, C20:3n6 and C20:4n6. However, C22:6n3 and C22:4n6 decreased significantly in TH and HI larvae supplemented PBM fed fish than the Control diet.
Table 5.
Chemical composition and fatty acids composition (mg g-1 DM) of barramundi muscle (skin less) after six weeks feeding with a gradient PBM diets, concurrently supplemented with TH and HI larvae meal.
| Diet1 (Control) | Diet2 (80PBM10TH+10HI) | Diet3 (85PBM5TH+10 HI) | Diet4 (90PBM5TH+ 5HI) | |
|---|---|---|---|---|
| Chemical composition (%) | ||||
| Moisture (WW) | 75.22 ± 0.37 | 74.88 ± 0.44 | 75.67 ± 0.15 | 75.12 ± 0.33 |
| CP (DM) | 75.27 ± 0.18a | 70.07 ± 0.62b | 70.72 ± 0.60b | 71.16 ± 0.42b |
| CL (DM) | 5.70 ± 0.13b | 10.70 ± 0.38a | 10.58 ± 0.53a | 10.74 ± 0.25a |
| Ash (DM) | 5.69 ± 0.02 | 5.61 ± 0.13 | 5.67 ± 0.08 | 5.65 ± 0.10 |
| Fatty acid composition, mg g−1 DM | ||||
| C12:0 | 0.03 ± 0.01c | 1.55 ± 0.09a | 1.63 ± 0.10a | 0.74 ± 0.04b |
| C14:0 | 0.52 ± 0.06b | 1.18 ± 0.06a | 1.17 ± 0.06a | 0.96 ± 0.06a |
| C16:0 | 5.81 ± 0.31b | 10.99 ± 0.53a | 11.13 ± 0.41a | 10.92 ± 0.40a |
| C18:0 | 2.00 ± 0.11b | 3.70 ± 0.15a | 3.69 ± 0.09a | 3.63 ± 0.13a |
| ∑SFA | 9.21 ± 0.51b | 18.46 ± 0.88a | 18.60 ± 0.68a | 17.21 ± 0.66a |
| C14:1n5 | 0.01 ± 0.00b | 0.04 ± 0.00a | 0.05 ± 0.00a | 0.04 ± 0.00a |
| C16:1n7 | 0.86 ± 0.05b | 2.27 ± 0.14a | 2.32 ± 0.10a | 2.20 ± 0.11a |
| C20:1 | 0.48 ± 0.04b | 1.06 ± 0.07a | 1.06 ± 0.05a | 1.25 ± 0.05a |
| C18:1cis + trans | 7.43 ± 0.45b | 22.85 ± 1.35a | 23.61 ± 1.09a | 23.59 ± 0.85a |
| C22:1n9 | 0.05 ± 0.01a | 0.10 ± 0.01b | 0.10 ± 0.00b | 0.12 ± 0.01a |
| ∑MUFA | 9.11 ± 0.56b | 26.65 ± 1.58a | 27.44 ± 1.26a | 27.53 ± 1.00a |
| C18:3n3 | 0.72 ± 0.05b | 2.03 ± 0.12a | 2.11 ± 0.10a | 1.91 ± 0.08a |
| C18:4n3# | 0.11 ± 0.01b | 0.31 ± 0.02a | 0.28 ± 0.02a | 0.26 ± 0.02a |
| C20:3n3 | 0.03 ± 0.00b | 0.06 ± 0.00a | 0.07 ± 0.00a | 0.06 ± 0.00a |
| C20:5n3 | 0.80 ± 0.03b | 1.24 ± 0.02a | 1.06 ± 0.04a | 1.10 ± 0.07a |
| C22:5n3# | 0.47 ± 0.04b | 0.80 ± 0.04a | 0.74 ± 0.02a | 0.69 ± 0.03a |
| C22:6n3 | 6.26 ± 0.34a | 4.36 ± 0.09b | 3.32 ± 0.06c | 3.73 ± 0.12bc |
| ∑n-3 PUFA | 8.39 ± 0.48 | 8.78 ± 0.29 | 7.57 ± 0.23 | 7.74 ± 0.32 |
| ∑n-3 LC PUFA | 7.56 ± 0.42a | 6.45 ± 0.16ab | 5.18 ± 0.12c | 5.58 ± 0.23bc |
| C18:3n6 | 0.08 ± 0.01b | 0.21 ± 0.02a | 0.26 ± 0.01a | 0.21 ± 0.01a |
| C20:3n6 | 0.13 ± 0.01b | 0.28 ± 0.02a | 0.33 ± 0.01a | 0.28 ± 0.01a |
| C20:4n6 | 0.64 ± 0.03b | 0.94 ± 0.01a | 0.93 ± 0.01a | 0.91 ± 0.02a |
| C22:4n6# | 0.52 ± 0.03a | 0.17 ± 0.00b | 0.13 ± 0.00b | 0.13 ± 0.00b |
| ∑n-6 PUFA | 1.37 ± 0.07b | 1.60 ± 0.04a | 1.65 ± 0.04a | 1.53 ± 0.04ab |
| ∑PUFA | 6.73 ± 0.41b | 15.19 ± 0.65a | 15.61 ± 0.63a | 14.68 ± 0.68a |
Results are expressed as mean ± SE (standard error) (n = 12). Means with different superscripts letters in the same row indicates significant difference at P < 0.05.
pH, Texture and colour of fish fillet
At the end of the trial, pH, texture profile (springiness, cohesiveness, gumminess, chewiness, adhesiveness and hardness) and colour (L*, a*, b* and chroma) in skin and fillet flesh were similar in fish fed any diet (Table 6).
Table 6.
Texture profile and colour of barramundi fillet after six weeks feeding with a gradient PBM diets, concurrently supplemented with TH and HI larvae meal.
| Diet1 (Control) | Diet2 (80PBM10TH+10HI) | Diet3 (85PBM5TH+10 HI) | Diet4 (90PBM5TH+ 5HI) | |
|---|---|---|---|---|
| PH | 6.90 ± 0.05 | 6.91 ± 0.04 | 6.90 ± 0.02 | 6.91 ± 0.02 |
| Texture parameter | ||||
| Springiness | 0.99 ± 0.01 | 1.00 ± 0.00 | 0.98 ± 0.01 | 0.98 ± 0.01 |
| Cohesiveness | 0.36 ± 0.03 | 0.33 ± 0.00 | 0.35 ± 0.03 | 0.32 ± 0.01 |
| Gumminess (g) | 957.76 ± 82.30 | 1433.56 ± 165.93 | 1147.32 ± 149.53 | 1431.16 ± 115.31 |
| Chewiness (g) | 957.53 ± | 1430.60 ± | 1144.52 ± | 1426.03 ± |
| Adhesiveness (g.mm) | − 13.51 ± 2.17 | − 15.82 ± 4.24 | − 16.07 ± 2.42 | − 21.30 ± 6.93 |
| Hardness | 2780.83 ± 462.31 | 4427.33 ± 477.80 | 3369.83 ± 525.92 | 4453.17 ± 494.24 |
| Skin colour | ||||
| L* | 60.93 ± 1.81 | 59.30 ± 3.58 | 59.99 ± 1.75 | 59.99 ± 1.57 |
| a* | − 1.37 ± 0.10 | − 1.30 ± 0.24 | − 1.20 ± 0.12 | − 1.45 ± 0.07 |
| b* | − 4.06 ± 0.26 | − 4.03 ± 0.43 | − 4.76 ± 0.39 | − 3.60 ± 0.25 |
| Chroma | 4.30 ± 0.23 | 4.26 ± 0.44 | 4.93 ± 0.36 | 3.90 ± 0.22 |
| Fillet muscle colour | ||||
| L* | 56.55 ± 1.12 | 57.11 ± 1.03 | 60.25 ± 1.03 | 58.61 ± 1.52 |
| a* | 0.37 ± 1.07 | − 1.04 ± 0.32 | − 1.36 ± 0.29 | − 1.21 ± 0.56 |
| b* | 3.90 ± 1.13 | 3.72 ± 0.41 | 4.80 ± 0.59 | 4.04 ± 0.32 |
| Chroma | 4.36 ± 1.32 | 4.00 ± 0.28 | 5.08 ± 0.52 | 4.40 ± 0.34 |
L*, light/dark; a*, red/green and b*, yellow/blue.
Histological analysis of liver, kidney, and spleen
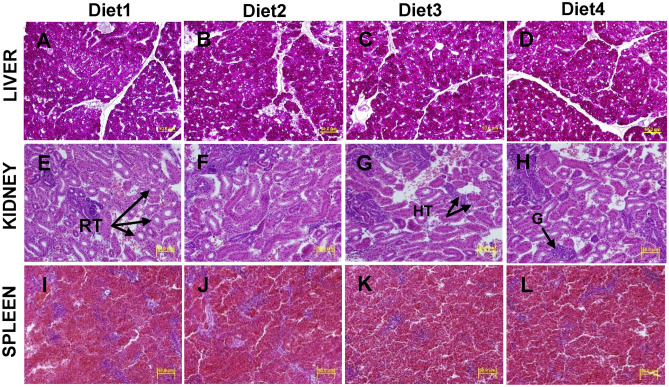
Hitopathological analysis did not demonstrate any substantial changes in the liver, kidney and spleen between diets at the end of the trial (Fig. 1A–L). Higher pigmented hepatic cytoplasm indicating higher amount of glycogen was examined in the liver of fish fed all test diets (Fig. 1A–D). In all dietary group, normal renal glomeruli and renal tubules in the kidney (Fig. 1E–H) and normal white and red pulp in the spleen (Fig. 1I–L) were observed.
Figure 1.
Representative micrographs of staining liver (A–D), kidney (E–H) and spleen (I–L) sections of barramundi fed control (Diet1), 80PBM10TH+10HI (Diet2), 85PBM5TH+10HI (Diet3), and 90PBM5TH+5HI (Diet4) for six weeks using PAS and H&E (40 × magnification). RT, renal tubule; HT, hematopoietic tissue and G, glomerulus
Intestinal mucosal morphology and histochemistry
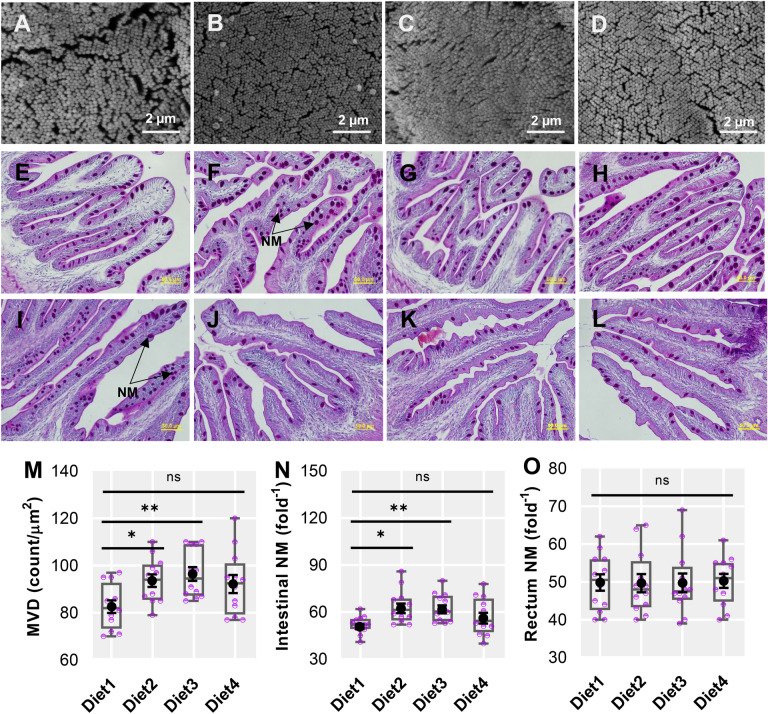
The intestinal mucosal micromorphology of barramundi fed Control and test diets were examined by SEM (Fig. 2A–D) and light microscopy (Fig. 2E–L). Microvilli count (density) (Fig. 2M) and neutral mucins (Fig. 2N) were significantly higher in barramundi fed TH and HI larvae supplemented at different levels of PBM when compared with those fed the Control diet. However, neutral mucins producing goblet cells in rectum were unchanged (Fig. 2O).
Figure 2.
Scanning electron micrographs (A–D) to visualize the microvilli density and goblet cells containing neutral mucins (E–H, PAS stain, 40 × magnification) in the distal intestine (E–H) and rectum (I–L) of barramundi fed Control (Diet1), 80PBM10TH+10HI (Diet2), 85PBM5TH+10HI (Diet3), and 90PBM5TH+5HI (Diet4) for six weeks. Variation in the microvilli density (M) and neutral mucins in the distal intestine (N) and rectum (O) of barramundi fed test diets. Multiple comparisons were performed by one-way ANOVA analysis followed by Dunnett’s multiple comparisons test. NM, neutral mucins and ns, not significant.
Skin histochemistry
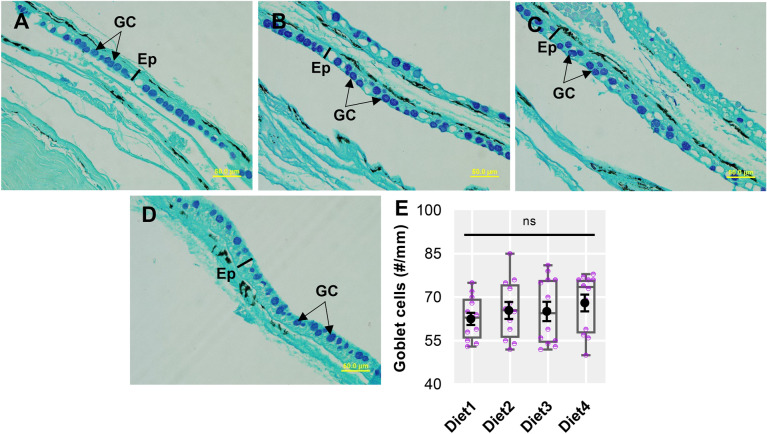
The results of skin morphology in response to different levels of PBM, supplemented with TH and HI larvae are shown in Fig. 3A–E. Mixed types of goblet cells were unchanged in fish fed Control and test diets (Fig. 3E).
Figure 3.
Representative skin micrographs (A–D, AB-PAS stain, 40 × magnification) and number of mixed goblet cells of barramundi fed Control (Diet1), 80PBM10TH+10HI (Diet2), 85PBM5TH+10HI (Diet3), and 90PBM5TH+5HI (Diet4) for six weeks. Variation in the mixed types of goblet cells in the skin (E) of barramundi fed test diets. Multiple comparisons were performed by one-way ANOVA analysis followed by Dunnett’s multiple comparisons test. ns denotes not significant. GC, goblet cell and Ep, epidermis.
Biochemical assays, immune response and antioxidant activity
A significant decrease in the total bilirubin activity of fish fed PBM diets supplemented with TH and HI larvae meal was observed, whilst total protein content in fish fed 85PBM5TH+10 HI increased significantly when compared to the Control (Table 7). The remaining biochemical assays including AST, GGT, GLDH, urea, creatinine, and cholesterol were not affected by any of the test diets. Concurrent supplementation of TH and HI larvae meal to PBM diets also modulated the serum lysozyme and bactericidal activity than Control. CAT activity both in serum and liver was not affected by different levels of PBM supplemented with TH and HI larvae meal with respect to the Control. Liver protein content did not differ significantly among the test diets.
Table 7.
Biochemical assays, immune response and antioxidant activity of barramundi fed with a gradient PBM diets, concurrently supplemented with TH and HI larvae meal for six weeks.
| Diet1 (Control) | Diet2 (80PBM10TH+10HI) | Diet3 (85PBM5TH+10 HI) | Diet4 (90PBM5TH+ 5HI) | |
|---|---|---|---|---|
| Serum biochemical assays | ||||
| ALT (U/L) | 2.00 ± 1.00 | 3.67 ± 0.88 | 3.33 ± 1.33 | 8.33 ± 1.67 |
| GGT (U/L) | 0.00 ± 0.00 | 0.67 ± 0.33 | 0.67 ± 0.33 | 0.67 ± 0.33 |
| GLDH (U/L) | 8.00 ± 0.57 | 6.00 ± 0.00 | 6.67 ± 0.33 | 6.33 ± 0.88 |
| Total Bilirubin (umol/L) | 3.33 ± 0.33a | 2.00 ± 0.00b | 2.00 ± 0.00b | 2.00 ± 0.00b |
| Urea (mmol/L) | 4.07 ± 0.47 | 5.47 ± 0.59 | 5.50 ± 0.12 | 5.90 ± 0.28 |
| Creatinine (umol/L) | 17.33 ± 0.88 | 21.00 ± 1.00 | 19.00 ± 0.00 | 18.67 ± 0.33 |
| Cholesterol (mmol/L) | 8.08 ± 0.16 | 13.08 ± 1.24 | 10.77 ± 0.37 | 11.38 ± 1.43 |
| Total Protein (g/L) | 43.47 ± 0.52b | 49.07 ± 1.47ab | 47.60 ± 0.90a | 45.87 ± 0.22b |
| Serum immune response and antioxidant activity | ||||
| Lysozyme (U/mL) | 765.56 ± 85.69bc | 1025.56 ± 76.60a | 940.22 ± 20.76ab | 668.89 ± 66.45c |
| BA (log10) | 4.60 ± 0.26a | 2.50 ± 0.30b | 2.45 ± 0.64b | 3.76 ± 0.68ab |
| CAT (U mg.prot-1) | 0.82 ± 0.32 | 0.74 ± 0.12 | 0.72 ± 0.22 | 0.56 ± 0.06 |
| Liver protein and antioxidant activity | ||||
| Total protein (mg/mL) | 3.87 ± 0.59 | 2.69 ± 0.07 | 2.34 ± 0.32 | 3.04 ± 0.17 |
| CAT (U mg.prot-1) | 786.33 ± 72.76 | 692.67 ± 146.68 | 917.00 ± 178.18 | 757.33 ± 196.17 |
Results are expressed as mean ± SE (standard error) (n = 3). Means with different superscripts letters in the same row indicates significant difference at P < 0.05.
Alanine transaminase, ALT; gamma-glutamyl transferase, GGT; glutamate dehydrogenase, GLDH, bactericidal activity, BA and catalase activity, CAT.
Sequence stats and alpha–beta diversity
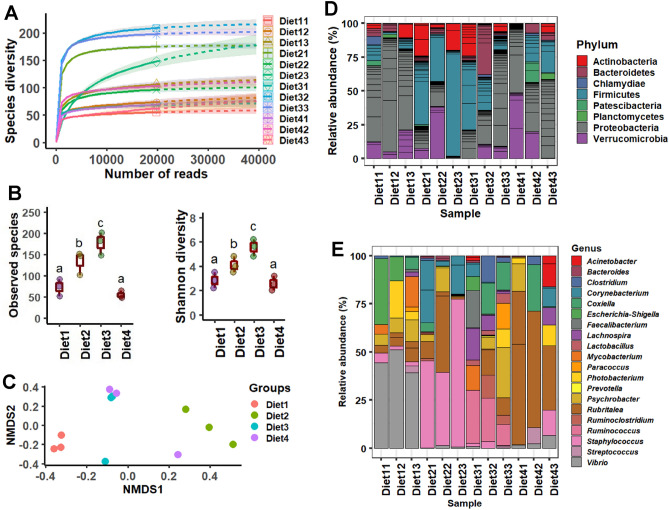
Quality trimming yielded total of 537,070 reads, ranging from 38,180 to 57,975 (44,755.8 ± 28,444.2), extracted from amplicon sequence data that assigned into 415 OTUs (286.8 ± 48.2), 19 phyla and 176 genera. The highest average OTUs (328.6 ± 32.4) and genera (132.8 ± 28.2) were obtained from the Diet3 group, in relation to other diets. The rarefaction plot indicated that each sample was sequenced at enough depth and up to saturation to capture most of the bacterial diversity (Fig. 4A). All samples also had high good’s coverage index, ranging from 0.998 to 0.999, implying satisfactory bacterial coverage. Diets 2 and 3 had the highest (P < 0.05) alpha diversity indices in term of observed OTUs and Shannon (Fig. 4B), indicating that this group had a different spectrum of bacterial diversity when compared to other diets. Beta ordination showed distinct clustering of bacterial OTUs and PERMANOVA R-value of 0.46 and P-value of 0.032 revealed that feeding had significant impacts on gut microbial diversity (Fig. 4C).
Figure 4.
Rarefaction curve (A) showing the depth of sequencing in terms of species diversity, observed species and Shannon diversity (B), Non-metric multidimensional scaling (nMDS) plot demonstrating clustering (C) and relative abundance of bacteria at phylum (D) and genus (E) level of different test diets fed to barramundi for 6 weeks.
Microbial communities
Relative abundance at the phyla level showed that Proteobacteria was most dominant in Diet1 and Diet4 while Firmicutes richness was observed for Diet2 and Diet3 (Fig. 4D). At the genus level, Diet3 found to influence the colonization of more bacteria than other diets and therefore, a diverse range of genera was identified, including Ruminococcus (58%), Psychrobacter (12%), Lachnospira (11%), Clostridium sensu stricto (8%) and Ruminoclostridium (8%). Diet1, Diet2, and Diet4 were dominated by Vibrio (71%), Staphylococcus (44%), and Rubritelea (57%), respectively (Fig. 4E). Differential abundance with LDA 2.0 and above and at 0.05 level of significance identified Ruminococcus and Lactobacillus as significantly abundant genera in Diet3, and Vibrio, Staphylococcus, and Rubritelea in Diet1, Diet2 and Diet4 groups, respectively (Table 8).
Table 8.
Distinguishing bacterial genera in different diets after feeding trial.
| Genus | Diet1 (Control) | Diet2 (80PBM10TH+10HI) | Diet3 (85PBM5TH+10 HI) | Diet4 (90PBM5TH+ 5HI) | P value |
|---|---|---|---|---|---|
| Vibrio | 43.08 ± 3.09 | 1.62 ± 0.63 | 1.02 ± 0.68 | 5.44 ± 2.2 | < 0.001 |
| Ruminococcus | 1.89 ± 0.92 | 0.68 ± 0.61 | 16.52 ± 4.22 | 0.81 ± 0.07 | < 0.001 |
| Staphylococcus | 2.34 ± 0.38 | 48.86 ± 9.98 | 2.33 ± 0.67 | 2.58 ± 1.78 | < 0.001 |
| Rubritalea | 10.05 ± 1.61 | 11.55 ± 4.18 | 3.68 ± 1.08 | 47.02 ± 13.01 | < 0.01 |
Each value displayed under the columns diet represents mean relative abundance with standard deviation.
Bacterial challenge
Infection rate in fish fed 80PBM and 85PBM supplemented with TH and HI larvae meal was significantly lower than the control (Fig. 5). Meanwhile, infection rate in fish fed TH and HI larvae meal supplemented 90PBM showed no significant variation than control.
Figure 5.
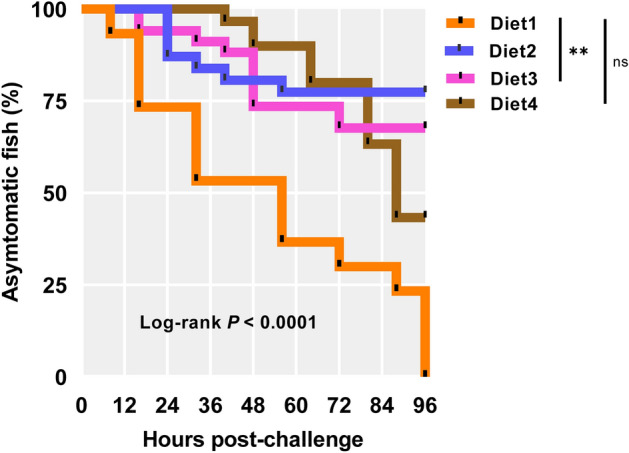
Variation in the infection rate in fish fed different levels of PBM concurrently supplemented with TH and HI larvae after 96 h post-challenge by injection with V. harveyi. Infection started in Control at 8 h post challenge (hpc) and while infection started at 24 hpc in 80PBM10TH+10HI, 16 hpc in 85PBM5TH+10HI and 40 hpc in 90PBM5TH+5HI, respectively. Asterisks demonstrated significant variation between Control- vs 80PBM10TH+10HI, and 85PBM5TH+10H—fed fish at P < 0.01 (Kaplan Meyer survival method, followed by Log-rank test).
Discussion
The ability of FPH supplementation to enhance growth performance has been reported in many species23,76–78, however, the concurrent supplementation of TH and HI larvae meal to non-FM based in the diet of barramundi has been reported for the first time in the present study. The total substitution of FM with PBM concomitantly supplemented with TH and HI larvae improved the growth and condition factor in barramundi. Our earlier20 and other study by Siddik, et al.21 evaluating the total FM substitution with PBM resulted in poor growth performance in barramundi . Also, many other studies reported negative outcomes in the growth of a number of fish when dietary inclusion of PBM exceeded > 50%15–17. The ameliorative effects on growth and condition factor in the present study could be explained by the presence of greater amounts of low molecular weight peptides in TH and bioactive peptides in HI larvae. The molecular weight of peptides in TH in our earlier study showed that more than 90% of the peptides were less than 10 kda25 which have been reported as biologically active peptides acting as growth promoters79–82. The hydrolysis process has been reported to elevate chemical and functional properties of feed as well as produce free amino acids, di- and try-peptides which reach the intestine faster than intact proteins and are easily absorbed by enterocytes76,83. Also, inclusion of insect meals at lower levels has been recommended by many researchers to promote the growth and health. For instance, Chaklader, et al.13 reported a significantly higher growth performance in barramundi fed a 10% full-fat HI larvae supplemented PBM diet. Caimi, et al.40 recommended to include 18.5% HI larvae meal in the diet of Siberian sturgeon, Acipenser baerii without affecting antioxidant response, liver and gut health.
The taste and appearance of cooked flesh for the consumer is influenced by the lipid composition in the edible part of fish fillets84,85. The higher lipid levels in PBM fed fish in the current study was similar to the reported lipid content in rainbow trout86 and tench15, however, contradict the results of24 who found lower levels of lipid in barramundi fed PBM based diets supplemented with TH. The higher fillet lipid content in this study could be due to higher levels of lipid content in the added full-fat HI larvae meal. The unchanged moisture, protein and ash content is similar to previous studies examining barramundi fed PBM based diets21,24.
In this study, total SFA content increased in fish fed all inclusion levels of PBM concurrently supplemented with TH and HI larvae due to a rise in lauric acid (C12:0), myristic acid (C14:0), palmitic acid (C16:0), and stearic acid (C18:0), and confirming that fatty acid composition of fish is generally a reflection of the fatty acids composition in the diets87. HI larvae meal contain higher levels of lauric acid, causing an increase in lauric acid content in rainbow trout, Oncorhynchus mykiss Walbaum87 and juvenile jian carp, Cyprinus carpio var. Jian88 when fed HI larvae based-diets. Also, a rise in palmitic acid and stearic acid levels led to an elevated total SFA in the muscle of barramundi fed PBM based diets supplemented with various FPH25. However, lauric acid, palmitic acid, and stearic acid were relatively low in the muscle of fish fed TH and HI supplemented PBM than the dietary concentrations, perhaps indicating the utilization of these SFA for energy production. Similar to total SFA content, total MUFA content improved in the muscle of fish fed PBM diets, supplemented with TH and HI, similar to the muscle fatty acids of barramundi fed FPH supplemented PBM based diets25. This increase is mainly due to the higher proportion of MUFA content in the PBM based diets. Meanwhile, concurrent TH and HI supplemented PBM based-diets contained higher PUFAs content than the control, causing a corresponding augmentation in total PUFA content in the muscle of fish. However, feeding barramundi with exclusive levels of PBM reduced the total PUFA particularly n-3 PUFA in the muscle of barramundi21. The amelioration of PUFA content in the present study could be due to the supplementation of TH and HI as they contain a higher amount of PUFA than PBM. Similarly, the modulatory effect of FPH on lipid accumulation, lipid metabolism, and fatty acid composition has been reported in barramundi25, turbot, Scophthalmus maximus89 and some other animals (mice/rats)90,91. The FA results suggest that total replacement of FM with PBM supplemented with TH and HI improved the fillet FA composition, manifested by an increased level of MUFA and PUFA content. Such changes suggest that barramundi produced from feeds examined in this study would be beneficial to human health since MUFA and PUFA content are highly associated with reducing the risk of cardiovascular and neurological disease92.
Besides the fatty acid profile, post-harvest fillet characteristics such as texture and colour are affected by dietary modification which are subsequently reflected in consumer acceptability and thus market demand. However, previous no information was available on the effects of total substitution of FM with PBM on the post-harvest quality of barramundi fillets. High inclusion of meat meal (40% or more) at the expense of FM did not adversely affect the organoleptic quality of 66 days post-feeding pond-reared barramundi muscle59 which was similar to the current study. Whereas, 100% substitution of FM with PBM negatively influenced the sensory characteristics of female tenches, Tinca tinca60.
Histological damages of immune-sensitive organs including liver, kidney, and spleen could be easily assessed when host species are fed non-FM diets. For example, total substitution of FM with PBM resulted in lipid droplets and multifocal necrosis in the liver of barramundi13,20. Similarly, higher replacement of FM with PBM (50–70%) and animal protein blend containing PBM, shrimp meal and spray-dried blood meal (20–80%) induced hepatocytes steatosis in juvenile hybrid grouper, Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂8,93. However, supplementation of TH and HI larvae meal appear to prevent such organ damage in the current study. FPH supplementation ameliorates the alternative protein quality and reduces the lipid accumulation coupled with increasing lipid metabolism in fish89 and other animals. Also, the presence of chitin and its derivatives in HI larvae have been reported to boost the hydrolysis of lipoproteins and triglycerides, coupled with reducing the synthesis of fatty acids in the liver of fish and other animals 88,94,95. Besides chitin, lauric acid (C12:0), one of the main fatty acids in HI larvae could also prevent liver damage induced by PBM in the current study since animals can quickly oxidize lauric acid after consumption rather than being stored in liver96. A similar effect was observed by Kumar, et al.97 who reported biliary duct hyperplasia in the liver of rainbow trout fed HI larvae oil, indicating more release of bile to facilitate lipid digestion.
Evaluating intestinal morphological structure is also important to understand the potential effects of alternative protein on any fish health, as such evaluation is reported to be highly correlated with nutrient assimilation and immunological function98. An improvement in microvilli density intestine indicates elevated enterocyte absorptive surface area99 and neutral mucins number in the intestine have been linked to the protection of the intestinal epithelium by lubricating, trapping and eradicating opportunistic pathogens100,101, prevent proteolytic damages and promoting enzymatic digestion and functionality of the gastric glands102–104. In this study, barramundi fed different levels of PBM with TH and HI larvae meal demonstrated a higher microvilli density and higher levels of neutral mucins than the Control, indicating more uptake and transportation of amino acids and free fatty acids105. Such effects might modulate the growth performance and disease resistance via improving the serum immune response. This could be due to the concurrent effect of TH and HI to confer a relatively higher benefit than when TH was used alone with 75 and 90% PBM in barramundi24. Similarly, HI larvae meal supplementation alone with 90% PBM did not influence villus height and neutral mucins in barramundi13. Regardless of TH supplementation, antimicrobial peptides, chitin, and lauric acid reported in HI larvae might have played a further key role in improving intestinal health since a number of recent studies have reported probiotic effects of HI larvae meal in rainbow trout106–108.
Goblet cells synthesize biologically active substances and other numerous defensive molecules, generally known as mucus, which have been reported to exert an important role in both the innate and acquired immune systems in fish. Goblet cells enumeration in the skin revealed by AB-PAS showed that total substitution of FM with PBM supplemented with TH and HI larvae did not affect the number of goblet cells. However, supplementation of various hydrolysates with PBM improved the number of goblet cells in terms of acidic mucins in barramundi25.
Valuable information on internal organs, nutritional status, and metabolic state can be achieved through the investigation via a panel of serum biochemical assays. Dietary incorporation of exclusive levels of alternative animal protein, in particular PBM, has been reported to have negative impacts on the activity of fish liver enzymes such AST, ALT and GLDH8,13, which leak into the blood at abnormal levels when there is liver cell damage. In the present study, such a negative impact was not observed in the measured activity of ALT, GGT, and GLDH, indicating that supplementation of TH and HI larvae meal could prevent liver damage caused by higher inclusion levels of PBM. Total bilirubin, which at high levels can be associated with kidney damage, was significantly lower in fish fed TH and HI larvae supplemented PBM diets, suggesting no adverse effects of total substitution of FM with PBM on kidney function. Similar effects were observed in barramundi fed a TH supplemented PBM diet25. Serum TP is usually more stable in fish in well-nourished conditions109 and elevation in levels is indicative of stronger innate immunity in fish110. A significant increase in the TP levels in fish fed concomitant TH and HI larvae supplemented PBM diets indicates an improvement in the immune system. However, TH and HI larvae meal supplementation separately with PBM did not influence the serum TP in barramundi25. Other unchanged biochemical assays including urea, creatinine, and cholesterol, were within the reported healthy ranges for barramundi25.
It is well established that FPH supplementation has been reported to improve diet quality24,111, coupled with immunostimulating properties due to the presence of biologically active di- and tripeptides and other oligopeptides112,113. Separate supplementation of FPH or insect larvae with PBM improved the immune response such as lysozyme and bactericidal activity in barramundi13,24 but combined supplemental effects on barramundi immunity is reported here for the first time. In the present study, different levels of PBM supplemented with TH and HI larvae modulated the lysozyme and bactericidal activity of barramundi. Similarly, supplementation of FPH and crustacean hydrolysate have been reported to improve the nutritional quality of alternative protein source including PBM and plant protein with concomitant modulatory effects on immune responses such as lysozyme and complement activity in barramundi24 and European sea bass, Dicentrarchus labrax114. The modulatory effects of TH supplemented PBM diets could be due to a higher proportion of low molecular weight peptides in TH as earlier studies confirmed immune-stimulating properties of medium size-bioactive peptides (3000 Da > Mw > 500 Da)115,116. Besides the beneficial effects of TH, HI larvae supplementation could further boost the immunity of barramundi since earlier reports showed that HI larvae contain antibacterial peptides and some polysaccharides such as silkose or dipterose which, along with chitin, play an important role in modulating the immune response. Elevated immune response was reported in gilthead seabream, Sparus aurata L.117 and common carp, Cyprinus carpio118 when fed low levels of crustacean derived chitin which was not analysed in HI larvae meal in the present study. Similar with the present findings, an upsurge in lysozyme and bactericidal activity was reported in barramundi fed 10% HI larvae with a PBM based diet13 and also enhanced lysozyme, complement 3 and complement 3 was found in black carp, Mylopharyngodon piceus fed 25 g kg−1 maggot meal in a FM-based diet119.
Reactive oxygen species (ROS) including superoxide anion (O2−), hydrogen peroxide (H2O2) and hydroxyl radical (OH) are essential by-products of phagocytic processes, causing impairment of DNA and injury to lipids, proteins and nucleic acids membranes, or even death if there is no equilibrium between ROS production and antioxidant defence mechanisms. Like other terrestrial animals, fish have a similar manner of enzymatic and non-enzymatic antioxidant defence mechanisms to protect themselves from oxidative damage120. Radical scavenging enzymes particularly CAT protect cells from oxidative damage by converting hydrogen peroxide into water121. In the present study, CAT activity both in serum and the liver was not affected by PBM inclusion supplemented with TH and HI larvae, suggesting that total replacement of FM with PBM supplemented with TH and HI larvae did not cause potential damage in the liver of barramundi. The present results agree with the findings of Siddik, et al.22 who reported no alteration of CAT activity in the serum of barramundi when fed TH supplemented PBM diets. However, feeding animal protein blends including PBM, shrimp meal, and spray-dried blood meal impaired the liver health of hybrid grouper, Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂8. No impairment in the liver of barramundi fed PBM based diets reported here can be explained by the supplementation of TH and HI larvae since many studies have been reported the presence of antioxidative capacity of FPH and insect larvae.
Due to the advent of NGS technologies, knowledge of intestinal microbial ecosystem alteration in response to dietary modification has improved. High bacterial diversity in the intestine of fish fed 80PBM10TH+10HI and 85PBM5TH+10HI than the control may indicate a healthier gut since several studies have reported a number of beneficial effects of the rich bacterial community such as out-competition pathogens for nutrients and colonization and consequently resisting pathogen invasion and intestinal infection108,122,123. The possible reason for the highest bacterial richness may be the supplementation of HI larvae rich in chitin and lauric acid content which are similar to the bacterial diversity of rainbow trout, Oncorhynchus mykiss106–108, and other animals such as laying hens124 fed HI larvae meal-based diets. The unchanged bacterial diversity in 90PBM5TH+5HI with respect to Control could be due to the highest replacement of FM protein with PBM. The same effect is supported by an earlier study in our laboratory with no changes in intestinal bacteria diversity of barramundi fed 90PBM supplemented with FPH22. In agreement with the earlier studies on barramundi, metagenomics results revealed that Proteobacteria, Firmicutes, and Bacteroidetes were the dominant phyla in all groups22,125,126. Indeed these phyla constitute the “core gut microbiota” constituting up to 90% in the intestine of different marine water fish species127–129. There is little information concerning the effects of dietary replacement of FM with alternative protein sources on the intestinal gut microbiota of barramundi. However, the dietary substitution of FM with a mix of terrestrial animal and plant proteins did not influence the intestinal microbiota of barramundi126 whereas a recent study22 found a modulatory effect of fermented PBM along with supplementation of TH on the intestinal microbial community. In this study, Proteobacteria abundance was improved in the intestine of fish fed Control and 90PBM5TH+5HI whilst Firmicutes were enriched in 80PBM10TH+10HI and 85PBM5TH+10HI fed groups. At the genus level, Ruminococcus and Lactobacillus, under Firmicutes phylum, were significantly more abundant in fish fed 85PBM5TH+10HI. The Ruminococcus genus is highly associated with the degradation of indigestible carbohydrate ingredients particularly resistant starch and dietary fibres, thereby contributing to a more efficient energy utilization of feed and this genus also plays an important role in the fermentation of dietary fibre, the subsequent butyrate end product is reported to influence the intestinal health of the host130–133. Also the genera of lactic acid bacteria (LAB), in particular Lactobacillus, are beneficial microorganisms commonly used as probiotics for fish and other vertebrates134 due to an ability to create biofilms by producing bacterial compounds (lactic acid hydrogen peroxide, and bacteri-ocins or biosurfactants) that can prevent the adherence of pathogens to the intestinal surface135. Similar to the present study, dietary inclusion of partially defatted HI larvae was reported to enrich the LAB in the intestine of rainbow trout, Oncorhynchus mykiss106,107. The presence of chitin and MCFA, particularly lauric acid, in HI larvae meal were reported to have a modulatory effect on LAB, as reported by the same authors. In addition, TH might have influenced the LAB abundance since FPH is a good source of nitrogen serving as a good medium for bacterial growth76. However, the lower abundance of Vibrio in test diets in the present study could be due to the inclusion of HI larvae irrespective of TH supplementation. Similarly, the abundance of Vibrio decreased in the intestine of zebrafish when fed various levels of HI larvae meal136. The presence of chitin, lauric acid, and recently extracted novel antimicrobial peptides of HI larvae meal are active against Gram-negative and Gram-positive bacteria13,44,106 which could have reasonably negatively influenced the abundance of Vibrio in the current study. The Staphylococcus, and Rubritelea were abundant in control, 80PBM10TH+10HI and 90PBM5TH+5HI groups. The presence of Staphylococcus is common in the gastrointestinal tract of fish, have been documented in several studies over the last decade137,138.
Supplementation of 3% anchovy and giant squid hydrolysates in the diet of European seabass, Dicentrarchus labrax showed bactericidal and bacteriostatic activities against a number of fish pathogenic bacteria including V. parahaemolyticus, V. harveyi and P. damselae subsp. piscicida, and V. anguillarum139. A number of previous studies supplementing FPH have also proven the modulation of disease resistance against pathogenic bacteria in barramundi23,24, red sea bream, Pagrus major80,82,140 and European sea bass, Dicentrarchus labrax141. In the present study, the improved infection rate against V. harveyi in the test diets could be due to the presence of bioactive peptides in FPH. Also improved infection rate may be due to the presence of low molecular weight peptides (< 6500 Da) described in our earlier study in TH25 with possible antibacterial properties142, as supported by the improved lysozyme and bactericidal activity of barramundi fed TH supplemented PBM. Some peptides, in particular lactoferrin-derived peptides, have the ability to damage the bacterial membrane of different species and strains of Vibrio143, and this remains to be further studied. Besides the role of TH, the presence of antibacterial peptides in HI larvae meal has been demonstrated to have an inhibitory response against Gram-positive bacteria, Gram-negative bacteria and fungus44 and this may have also influenced the infection rate. In our earlier study, supplementation of 10% HI larvae meal with 45% PBM modulated the disease resistance in response to a two-week challenge with V. harveyi13. However, the specific role of antibacterial peptides in TH and HI larvae in boosting the immune response of fish need to be further explored.
In summary, concurrent supplementation of 10 and/or 5% TH and HI larvae can facilitate the total replacement of FM with PBM in barramundi, including an improved growth performance and biometry indices, and improved microvilli density and neutral mucin levels in intestine. Fatty acid profiles such as MUFA and PUFA improved in TH and HI supplemented PBM fed barramundi with no significant effects on all tested post-harvest fillet quality characteristics. No histopathological changes in liver, kidney and spleen further proved the ability of TH and HI supplementation to hamper the negative effects previously reported to be caused by dietary animal protein ingredients. Whilst serum biochemical assays, particularly total bilirubin and total protein, improved in TH and HI larvae supplemented PBM groups, antioxidant activities in the serum and liver were unaffected. Concurrent supplementation of TH and HI larvae demonstrated bioactivity via modulating lysozyme and bactericidal activity which may elucidate higher survival rates in response to V. harveyi infection. Also, bacterial diversity on 80PBM10TH+10HI and 85PBM5TH+10HI increased but the presence of Vibrio decreased in barramundi fed PBM based diets concurrently supplemented with TH and/or HI larvae meal.
Acknowledgements
The authors are thankfully acknowledge Australian Centre for Applied Aquaculture Research (ACAAR), Fremantle, Australia for providing fish and Alexis Wing Huen Chung for technical assistant during texture and colour analysis of fish fillets.
Author contributions
R.F. and J.H. conceptualized the experimental design and reviewed the manuscript. M.R.C. was involved in experimental design, conducting experiment, collecting samples, analysing data, making table and figures, and writing the paper. M.J.F. analysed bacterial data and proofread the manuscript. M.A.B.S. helped in formal analysis and proofreading the manuscript.
Funding
The PhD grant held by Curtin University is funded the Research Training Program (RTP) Stipend Scholarship, funded by Australian Government to Md Reaz Chaklader (No. 19061054-Curtin).
Data availability
The amplicon sequence data in fastq format has been deposited to National Centre for Biotechnology Information (NCBI) under the BioProject accession number PRJNA660066.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fawole FJ, et al. Substituting fishmeal with Hermetia illucens in the diets of African catfish (Clarias gariepinus): Effects on growth, nutrient utilization, haematophysiological response, and oxidative stress biomarker. Aquaculture. 2020;518:734849. doi: 10.1016/j.aquaculture.2019.734849. [DOI] [Google Scholar]
- 2.Henry M, Gasco L, Piccolo G, Fountoulaki E. Review on the use of insects in the diet of farmed fish: past and future. Anim. Feed Sci. Technol. 2015;203:1–22. doi: 10.1016/j.anifeedsci.2015.03.001. [DOI] [Google Scholar]
- 3.Gatlin DM, et al. Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac. Res. 2007;38:551–579. doi: 10.1111/j.1365-2109.2007.01704.x. [DOI] [Google Scholar]
- 4.Hassaan M. Responsible fishmeal consumption and alternatives in the face of climate changes. International Journal of Marine Science. 2017 doi: 10.5376/ijms.2017.07.0015. [DOI] [Google Scholar]
- 5.Aksnes, A., Mundheim, H., Toppe, J. & Albrektsen, S. The effect of dietary hydroxyproline supplementation on salmon (Salmo salar L.) fed high plant protein diets. Aquaculture275, 242–249. 10.1016/j.aquaculture.2007.12.031 (2008).
- 6.Wang, G. et al. Evaluation of defatted black soldier fly (Hermetia illucens L.) larvae meal as an alternative protein ingredient for juvenile Japanese seabass (Lateolabrax japonicus) diets. Aquaculture507, 144–154. 10.1016/j.aquaculture.2019.04.023 (2019).
- 7.Allan GL, et al. Replacement of fish meal in diets for Australian silver perch, Bidyanus bidyanus: V Least-cost formulation of practical diets. Aquaculture. 2000;186:327–340. doi: 10.1016/S0044-8486(99)00382-8. [DOI] [Google Scholar]
- 8.Ye H, et al. Effects of replacing fish meal with rendered animal protein blend on growth performance, hepatic steatosis and immune status in hybrid grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂) Aquaculture. 2019;511:734203. doi: 10.1016/j.aquaculture.2019.734203. [DOI] [Google Scholar]
- 9.Shapawi R, Ng W-K, Mustafa S. Replacement of fish meal with poultry by-product meal in diets formulated for the humpback grouper Cromileptes altivelis. Aquaculture. 2007;273:118–126. doi: 10.1016/j.aquaculture.2007.09.014. [DOI] [Google Scholar]
- 10.El-Sayed AFM. Total replacement of fish meal with animal protein sources in Nile tilapia, Oreochromis niloticus (L.), feeds. Aquac. Res. 1998;29:275–280. doi: 10.1046/j.1365-2109.1998.00199.x. [DOI] [Google Scholar]
- 11.Hernández C, et al. Complete replacement of fish meal by porcine and poultry by-product meals in practical diets for fingerling Nile tilapia Oreochromis niloticus : digestibility and growth performance. Aquac. Nutr. 2010;16:44–53. doi: 10.1111/j.1365-2095.2008.00639.x. [DOI] [Google Scholar]
- 12.Rawles S, et al. Effects of replacing fish meal with poultry by product meal and soybean meal and reduced protein level on the performance and immune status of pondagrown sunshine bass (Morone chrysops M. saxatilis) Aquac. Nutr. 2011;17:708–721. doi: 10.1111/j.1365-2095.2010.00831.x. [DOI] [Google Scholar]
- 13.Chaklader MR, Siddik MAB, Fotedar R, Howieson J. Insect larvae, Hermetia illucens in poultry by-product meal for barramundi, Lates calcarifer modulates histomorphology, immunity and resistance to Vibrio harveyi. Sci. Rep. 2019;9:16703. doi: 10.1038/s41598-019-53018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossi W, Davis DA. Replacement of fishmeal with poultry by-product meal in the diet of Florida pompano Trachinotus carolinus L. Aquaculture. 2012;338–341:160–166. doi: 10.1016/j.aquaculture.2012.01.026. [DOI] [Google Scholar]
- 15.González-Rodríguez Á, et al. Evaluation of poultry by-product meal as partial replacement of fish meal in practical diets for juvenile tench (Tinca tinca L) Aquac. Res. 2016;47:1612–1621. doi: 10.1111/are.12622. [DOI] [Google Scholar]
- 16.Yigit M, et al. Substituting fish meal with poultry by-product meal in diets for black Sea turbot Psetta maeotica. Aquac. Nutr. 2006;12:340–347. doi: 10.1111/j.1365-2095.2006.00409.x. [DOI] [Google Scholar]
- 17.Zhou Q-C, Zhao J, Li P, Wang H-L, Wang L-G. Evaluation of poultry by-product meal in commercial diets for juvenile cobia (Rachycentron canadum) Aquaculture. 2011;322:122–127. doi: 10.1016/j.aquaculture.2011.09.042. [DOI] [Google Scholar]
- 18.Subhadra B, Lochmann R, Rawles S, Chen R. Effect of fish-meal replacement with poultry by-product meal on the growth, tissue composition and hematological parameters of largemouth bass (Micropterus salmoides) fed diets containing different lipids. Aquaculture. 2006;260:221–231. doi: 10.1016/j.aquaculture.2006.06.029. [DOI] [Google Scholar]
- 19.Subhadra B, Lochmann R, Rawles S, Chen R. Effect of dietary lipid source on the growth, tissue composition and hematological parameters of largemouth bass (Micropterus salmoides) Aquaculture. 2006;255:210–222. doi: 10.1016/j.aquaculture.2005.11.043. [DOI] [Google Scholar]
- 20.Chaklader MR, Siddik MA, Fotedar R. Total replacement of fishmeal with poultry by-product meal affected the growth, muscle quality, histological structure, antioxidant capacity and immune response of juvenile barramundi Lates calcarifer. Plos one. 2020;15:e0242079. doi: 10.1371/journal.pone.0242079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddik M, Chungu P, Fotedar R, Howieson J. Bioprocessed poultry by-product meals on growth, gut health and fatty acid synthesis of juvenile barramundi, Lates calcarifer (Bloch) PLoS ONE. 2019;14:e0215025. doi: 10.1371/journal.pone.0215025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siddik MAB, et al. Influence of fish protein hydrolysate produced from industrial residues on antioxidant activity, cytokine expression and gut microbial communities in juvenile barramundi Lates calcarifer. Fish Shellfish Immunol. 2019;97:465–473. doi: 10.1016/j.fsi.2019.12.057. [DOI] [PubMed] [Google Scholar]
- 23.Siddik MAB, Howieson J, Partridge GJ, Fotedar R, Gholipourkanani H. Dietary tuna hydrolysate modulates growth performance, immune response, intestinal morphology and resistance to Streptococcus iniae in juvenile barramundi Lates calcarifer. Sci. Rep. 2018;8:15942. doi: 10.1038/s41598-018-34182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siddik MAB, Howieson J, Fotedar R. Beneficial effects of tuna hydrolysate in poultry by-product meal diets on growth, immune response, intestinal health and disease resistance to Vibrio harveyi in juvenile barramundi Lates calcarifer. Fish Shellfish Immunol. 2019;89:61–70. doi: 10.1016/j.fsi.2019.03.042. [DOI] [PubMed] [Google Scholar]
- 25.Chaklader MR, Fotedar R, Howieson J, Siddik MA, Foysal J. The ameliorative effects of various fish protein hydrolysates in poultry by-product meal based diets on muscle quality, serum biochemistry and immunity in juvenile barramundi Lates calcarifer. Fish Shellfish Immunol. 2020;104:567–578. doi: 10.1016/j.fsi.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Talpur AD. Mentha piperita (Peppermint) as feed additive enhanced growth performance, survival, immune response and disease resistance of Asian seabass, Lates calcarifer (Bloch) against Vibrio harveyi infection. Aquaculture. 2014;420–421:71–78. doi: 10.1016/j.aquaculture.2013.10.039. [DOI] [Google Scholar]
- 27.Talpur AD, Ikhwanuddin M. Azadirachta indica (neem) leaf dietary effects on the immunity response and disease resistance of Asian seabass, Lates calcarifer challenged with Vibrio harveyi. Fish Shellfish Immunol. 2013;34:254–264. doi: 10.1016/j.fsi.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Talpur AD, Ikhwanuddin M. Dietary effects of garlic (Allium sativum) on haemato-immunological parameters, survival, growth, and disease resistance against Vibrio harveyi infection in Asian sea bass, Lates calcarifer (Bloch) Aquaculture. 2012;364:6–12. doi: 10.1016/j.aquaculture.2012.07.035. [DOI] [Google Scholar]
- 29.Önal U, Langdon C. Potential delivery of water-soluble protein hydrolysates to marine suspension feeders by three different microbound particle types. Aquacuture. 2009;296:174–178. doi: 10.1016/j.aquaculture.2009.07.002. [DOI] [Google Scholar]
- 30.Ospina-Salazar GH, Ríos-Durán MG, Toledo-Cuevas EM, Martínez-Palacios CA. The effects of fish hydrolysate and soy protein isolate on the growth performance, body composition and digestibility of juvenile pike silverside, Chirostoma estor. Anim. Feed Sci. Technol. 2016;220:168–179. doi: 10.1016/j.anifeedsci.2016.08.011. [DOI] [Google Scholar]
- 31.Kasumyan, A. O. & Døving, K. B. Vol. 4 289–347 (Oxford, 2003).
- 32.Chotikachinda R, Tantikitti C, Benjakul S, Rustad T, Kumarnsit E. Production of protein hydrolysates from skipjack tuna (Katsuwonus pelamis) viscera as feeding attractants for Asian seabass (Lates calcarifer) Aquac. Nutr. 2013;19:773–784. doi: 10.1111/anu.12024. [DOI] [Google Scholar]
- 33.Kousoulaki K, et al. High growth rates in Atlantic salmon (Salmo salar L.) fed 7.5% fish meal in the diet. Micro-, ultra- and nano-filtration of stickwater and effects of different fractions and compounds on pellet quality and fish performance. Aquaculture. 2012;338–341:134–146. doi: 10.1016/j.aquaculture.2012.01.017. [DOI] [Google Scholar]
- 34.Delcroix J, et al. The effects of dietary marine protein hydrolysates on the development of sea bass larvae, Dicentrarchus labrax, and associated microbiota. Aquac. Nutr. 2015;21:98–104. doi: 10.1111/anu.12139. [DOI] [Google Scholar]
- 35.Ovissipour M, AbedianKenari A, Nazari R, Motamedzadegan A, Rasco B. Tuna viscera protein hydrolysate: nutritive and disease resistance properties for Persian sturgeon (Acipenser persicus L.) larvae. Aquac. Res. 2014;45:591–601. doi: 10.1111/j.1365-2109.2012.03257.x. [DOI] [Google Scholar]
- 36.Guerreiro I, et al. Catching black soldier fly for meagre: growth, whole-body fatty acid profile and metabolic responses. Aquaculture. 2020 doi: 10.1016/j.aquaculture.2019.734613. [DOI] [Google Scholar]
- 37.van Huis A, Dicke M, van Loon JJA. Insects to feed the world. J. Insects Food Feed. 2015;1:3–5. doi: 10.3920/JIFF2015.x002. [DOI] [Google Scholar]
- 38.Mertenat A, Diener S, Zurbrügg C. Black soldier fly biowaste treatment—assessment of global warming potential. Waste Manage. 2019;84:173–181. doi: 10.1016/j.wasman.2018.11.040. [DOI] [PubMed] [Google Scholar]
- 39.Zarantoniello M, et al. A six-months study on Black Soldier Fly (Hermetia illucens) based diets in zebrafish. Sci. Rep. 2019;9:8598–8598. doi: 10.1038/s41598-019-45172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caimi C, et al. First insights on black soldier fly (Hermetia illucens L.) larvae meal dietary administration in Siberian sturgeon (Acipenser baerii Brandt) juveniles. Aquaculture. 2020 doi: 10.1016/j.aquaculture.2019.734539. [DOI] [Google Scholar]
- 41.Makkar HPS, Tran G, Heuzé V, Ankers P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014;197:1–33. doi: 10.1016/j.anifeedsci.2014.07.008. [DOI] [Google Scholar]
- 42.Zarantoniello M, et al. Zebrafish (Danio rerio) physiological and behavioural responses to insect-based diets: a multidisciplinary approach. Sci. Rep. 2020;10:1–16. doi: 10.1038/s41598-020-67740-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park SI, Chang BS, Yoe SM. Detection of antimicrobial substances from larvae of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae) Entomol. Res. 2014;44:58–64. doi: 10.1111/1748-5967.12050. [DOI] [Google Scholar]
- 44.Elhag O, et al. Screening, expression, purification and functional characterization of novel antimicrobial peptide genes from Hermetia illucens (L.) PLoS One. 2017;12:e0169582. doi: 10.1371/journal.pone.0169582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park S-I, Kim J-W, Yoe SM. Purification and characterization of a novel antibacterial peptide from black soldier fly (Hermetia illucens) larvae. Dev. Comp. Immunol. 2015;52:98–106. doi: 10.1016/j.dci.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Egerton S, Culloty S, Whooley J, Stanton C, Ross RP. The gut microbiota of marine fish. Front. Microbiol. 2018;9:873–873. doi: 10.3389/fmicb.2018.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merrifield, D. L., & Rodiles, A. The fish microbiome and its interactions with mucosal tissues. In Mucosal Health in Aquaculture (ed. E. Peatman) 273–295 (San Diego, CA: Academic Press, 2015).
- 48.Cordero H, et al. Modulation of immunity and gut microbiota after dietary administration of alginate encapsulated Shewanella putrefaciens Pdp11 to gilthead seabream (Sparus aurata L.) Fish Shellfish Immunol. 2015;45:608–618. doi: 10.1016/j.fsi.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Hansen GH, Olafsen JA. Bacterial interactions in early life stages of marine cold water fish. Int. J. 1999;38:1–26. doi: 10.1007/s002489900158. [DOI] [PubMed] [Google Scholar]
- 50.Dhanasiri A, et al. Changes in the intestinal microbiota of wild atlantic cod Gadus morhua L. upon captive rearing. Microbial. Ecol. 2011;61:20–30. doi: 10.1007/s00248-010-9673-y. [DOI] [PubMed] [Google Scholar]
- 51.Bano N, DeraeSmith A, Bennett W, Vasquez L, Hollibaugh JT. Dominance of Mycoplasma in the guts of the long-jawed Mudsucker, Gillichthys mirabilis, from five California salt marshes. Environ. Microbiol. 2007;9:2636–2641. doi: 10.1111/j.1462-2920.2007.01381.x. [DOI] [PubMed] [Google Scholar]
- 52.Hovda MB, Fontanillas R, McGurk C, Obach A, Rosnes JT. Seasonal variations in the intestinal microbiota of farmed Atlantic salmon (Salmo salar L.) Aquac. Res. 2012;43:154–159. doi: 10.1111/j.1365-2109.2011.02805.x. [DOI] [Google Scholar]
- 53.Kim D-H, Kim D-Y. Microbial diversity in the intestine of olive flounder (Paralichthys olivaceus) Aquaculture. 2013;414–415:103–108. doi: 10.1016/j.aquaculture.2013.06.008. [DOI] [Google Scholar]
- 54.Wang AR, Ran C, Ringø E, Zhou ZG. Progress in fish gastrointestinal microbiota research. Rev. Aquac. 2018;10:626–640. doi: 10.1111/raq.12191. [DOI] [Google Scholar]
- 55.Huyben D, et al. Effects of dietary yeast inclusion and acute stress on post-prandial whole blood profiles of dorsal aorta-cannulated rainbow trout. Fish Physiol. Biochem. 2017;43:421–434. doi: 10.1007/s10695-016-0297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Semova I, et al. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe. 2012;12:277–288. doi: 10.1016/j.chom.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pérez T, et al. Host–microbiota interactions within the fish intestinal ecosystem. Mucosal Immunol. 2010;3:355. doi: 10.1038/mi.2010.12. [DOI] [PubMed] [Google Scholar]
- 58.Estruch G, Collado M, Peñaranda D, Martinez-Llorens S. Impact of fishmeal replacement in diets for gilthead sea bream (Sparus aurata) on the gastrointestinal microbiota determined by pyrosequencing the 16S rRNA gene. PLoS ONE. 2015;10:e0136389. doi: 10.1371/journal.pone.0136389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams KC, Paterson BD, Barlow CG, Ford A, Roberts R. Potential of meat meal to replace fish meal in extruded dry diets for barramundi, Lates calcarifer (Bloch)—II: organoleptic characteristics and fatty acid composition. Aquac. Res. 2003;34:33–42. doi: 10.1046/j.1365-2109.2003.00786.x. [DOI] [Google Scholar]
- 60.Panicz R, Żochowska-Kujawska J, Sadowski J, Sobczak M. Effect of feeding various levels of poultry by-product meal on the blood parameters, filet composition and structure of female tenches (Tinca tinca) Aquac. Res. 2017;48:5373–5384. doi: 10.1111/are.13351. [DOI] [Google Scholar]
- 61.O'Fallon JV, Busboom JR, Nelson ML, Gaskins CT. direct method for fatty acid methyl ester synthesis: application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007;85:1511–1521. doi: 10.2527/jas.2006-491. [DOI] [PubMed] [Google Scholar]
- 62.Ayala MD, et al. Muscle tissue structural changes and texture development in sea bream, Sparus aurata L., during post-mortem storage. LWT Food Sci. Technol. 2010;43:465–475. doi: 10.1016/j.lwt.2009.08.023. [DOI] [Google Scholar]
- 63.Ozbay G, et al. Delaware inland bays and market oyster (Crassostrea virginica) quality for consumption. J. Food Q. 2018;8:1511–1521. [Google Scholar]
- 64.AOAC. Official Methods of Analysis, 16th Ed, Association of Official Analytical Chemists, Washington DC, USA (1995).
- 65.Klindworth A, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1–e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andrew S. A quality control tool for high throughput sequence data. Fast QC. 2010;532:1. [Google Scholar]
- 67.Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32:3047–3048. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parikh HI, Koparde VN, Bradley SP, Buck GA, Sheth NU. MeFiT: merging and filtering tool for illumina paired-end reads for 16S rRNA amplicon sequencing. BMC Bioinform. 2016;17:491. doi: 10.1186/s12859-016-1358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Albanese D, Fontana P, De Filippo C, Cavalieri D, Donati C. MICCA: a complete and accurate software for taxonomic profiling of metagenomic data. Sci. Rep. 2015;5:1–7. doi: 10.1038/srep09743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quast C, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2012;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mirarab S, et al. PASTA: ultra-large multiple sequence alignment for nucleotide and amino-acid sequences. J. Comput. Biol. 2015;22:377–386. doi: 10.1089/cmb.2014.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Price MN, Dehal PS, Arkin AP. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS ONE. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Segata N, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:1–18. doi: 10.1186/1465-6906-12-S1-P1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ha N, et al. Sardine (Sardinella spp.) protein hydrolysate as growth promoter in South American catfish (Rhamdia quelen) feeding: productive performance, digestive enzymes activity, morphometry and intestinal microbiology. Aquaculture. 2019;500:99–106. doi: 10.1016/j.aquaculture.2018.10.004. [DOI] [Google Scholar]
- 77.Tang H-G, Wu T-X, Zhao Z-Y, Pan X-D. Effects of fish protein hydrolysate on growth performance and humoral immune response in large yellow croaker (Pseudosciaena crocea R.) Int. Biomed. Biotechnol. J. 2008;9:684–690. doi: 10.1631/jzus.B0820088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hevrøy E, et al. Nutrient utilization in Atlantic salmon (Salmo salar L.) fed increased levels of fish protein hydrolysate during a period of fast growth. Aquac. Nutr. 2005;11:301–313. doi: 10.1111/j.1365-2095.2005.00357.x. [DOI] [Google Scholar]
- 79.Aksnes A, Hope B, Hostmark O, Albrektsen S. Inclusion of size fractionated fish hydrolysate in high plant protein diets for Atlantic cod Gadus morhua. Aquaculture. 2006;261:1102–1110. doi: 10.1016/j.aquaculture.2006.07.038. [DOI] [Google Scholar]
- 80.Khosravi S, et al. Effects of protein hydrolysates supplementation in low fish meal diets on growth performance, innate immunity and disease resistance of red sea bream Pagrus major. Fish Shellfish Immunol. 2015;45:858–868. doi: 10.1016/j.fsi.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 81.Robert M, et al. Molecular characterization of peptide fractions of a Tilapia (Oreochromis niloticus) by-product hydrolysate and in vitro evaluation of antibacterial activity. Process Biochem. 2015;50:487–492. doi: 10.1016/j.procbio.2014.12.022. [DOI] [Google Scholar]
- 82.Bui HTD, Khosravi S, Fournier V, Herault M, Lee K-J. Growth performance, feed utilization, innate immunity, digestibility and disease resistance of juvenile red seabream (Pagrus major) fed diets supplemented with protein hydrolysates. Aquaculture. 2014;418:11–16. doi: 10.1016/j.aquaculture.2013.09.046. [DOI] [Google Scholar]
- 83.Ganapathy V. Intestinal transport of amino acids and peptides. Physiol. Gastrointest. Tract. 1994;1994:1773. [Google Scholar]
- 84.Grigorakis K. Compositional and organoleptic quality of farmed and wild gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) and factors affecting it: a review. Aquaculture. 2007;272:55–75. doi: 10.1016/j.aquaculture.2007.04.062. [DOI] [Google Scholar]
- 85.Sabbagh M, et al. Poultry by-product meal as an alternative to fish meal in the juvenile gilthead seabream (Sparus aurata) diet. Aquaculture. 2019;511:734220. doi: 10.1016/j.aquaculture.2019.734220. [DOI] [Google Scholar]
- 86.Baboli MJ, Dawodi M, Gorjipor A. Effect of replacement fish meal by poultry meal on growth, survival and body composition of rainbow trout (Oncorhynchos mykiss) Int. Res. J. Appl. Basic Sci. 2013;5:296–300. [Google Scholar]
- 87.Renna M, et al. Evaluation of the suitability of a partially defatted black soldier fly (L.) larvae meal as ingredient for rainbow trout (Walbaum) diets. J. Anim. Sci. Biotechnol. 2017;8:57. doi: 10.1186/s40104-017-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li S, et al. Influence of black soldier fly (Hermetia illucens) larvae oil on growth performance, body composition, tissue fatty acid composition and lipid deposition in juvenile Jian carp (Cyprinus carpio var Jian) Aquaculture. 2016;465:43–52. doi: 10.1016/j.aquaculture.2016.08.020. [DOI] [Google Scholar]
- 89.Xu H, et al. Graded levels of fish protein hydrolysate in high plant diets for turbot (Scophthalmus maximus): effects on growth performance and lipid accumulation. Aquaculture. 2016;454:140–147. doi: 10.1016/j.aquaculture.2015.12.006. [DOI] [Google Scholar]
- 90.Hosomi R, et al. Fish protein decreases serum cholesterol in rats by inhibition of cholesterol and bile acid absorption. J. Food Sci. 2011;76:H116–H121. doi: 10.1111/j.1750-3841.2011.02130.x. [DOI] [PubMed] [Google Scholar]
- 91.Bjørndal B, et al. A fish protein hydrolysate alters fatty acid composition in liver and adipose tissue and increases plasma carnitine levels in a mouse model of chronic inflammation. Lipids Health Dis. 2013;12:143. doi: 10.1186/1476-511X-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blondeau N, et al. Alpha-linolenic acid: an omega-3 fatty acid with neuroprotective properties—ready for use in the stroke clinic? BioMed Res. Int. 2015 doi: 10.1155/2015/519830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou Z, et al. Effects of replacing fishmeal protein with poultry by-product meal protein and soybean meal protein on growth, feed intake, feed utilization, gut and liver histology of hybrid grouper (Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂) juveniles. Aquaculture. 2020;516:734503. doi: 10.1016/j.aquaculture.2019.734503. [DOI] [Google Scholar]
- 94.Zhang J, Liu J, Li L, Xia W. Dietary chitosan improves hypercholesterolemia in rats fed high-fat diets. Nutr. Res. 2008;28:383–390. doi: 10.1016/j.nutres.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 95.Li S, Ji H, Zhang B, Zhou J, Yu H. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture. 2017;477:62–70. doi: 10.1016/j.aquaculture.2017.04.015. [DOI] [Google Scholar]
- 96.Belghit I, et al. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar) Aquaculture. 2019;503:609–619. doi: 10.1016/j.aquaculture.2018.12.032. [DOI] [Google Scholar]
- 97.Kumar V, et al. Insect (Black soldier fly, Hermetia illucens) meal supplementation prevents the soybean meal-induced intestinal enteritis in rainbow trout and health benefits of using insect oil. Fish Shellfish Immunol. 2020;109:116–124. doi: 10.1016/j.fsi.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 98.Nicholson JK, et al. Host-gut microbiota metabolic interactions. Science (New York, NY) 2012;336:1262. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 99.Adeoye AA, et al. Combined effects of exogenous enzymes and probiotic on Nile tilapia (Oreochromis niloticus) growth, intestinal morphology and microbiome. Aquaculture. 2016;463:61–70. doi: 10.1016/j.aquaculture.2016.05.028. [DOI] [Google Scholar]
- 100.Sklan D, Prag T, Lupatsch I. Structure and function of the small intestine of the tilapia Oreochromis niloticus × Oreochromis aureus (Teleostei Cichlidae) Aquac. Res. 2004;35:350–357. doi: 10.1111/j.1365-2109.2004.01020.x. [DOI] [Google Scholar]
- 101.Padra JT, et al. Aeromonas salmonicida binds differentially to mucins isolated from skin and intestinal regions of Atlantic salmon in an N-acetylneuraminic acid-dependent manner. Infect. Immun. 2014;82:5235. doi: 10.1128/IAI.01931-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Díaz A, Garcia A, Devincenti C, Goldemberg A. Morphological and histochemical characterization of the mucosa of the digestive tract in Engraulis anchoita (Hubbs and Marini, 1935) Anatomia Histol. Embryol. 2003;32:341–346. doi: 10.1111/j.1439-0264.2003.00490.x. [DOI] [PubMed] [Google Scholar]
- 103.Gisbert E, Piedrahita RH, Conklin DE. Ontogenetic development of the digestive system in California halibut (Paralichthys californicus) with notes on feeding practices. Aquaculture. 2004;232:455–470. doi: 10.1016/S0044-8486(03)00457-5. [DOI] [Google Scholar]
- 104.Ofelio C, Cohen S, Adriaens D, Radaelli G, Díaz AO. Histochemistry of goblet cells and micro-computed tomography to study the digestive system in the long-snouted seahorse Hippocampus guttulatus. Aquaculture. 2019;502:400–409. doi: 10.1016/j.aquaculture.2018.12.048. [DOI] [Google Scholar]
- 105.Grosell M, Farrell AP, Brauner CJ. Fish Physiology: The Multifunctional Gut of Fish. New York: Academic Press; 2010. [Google Scholar]
- 106.Terova G, et al. Rainbow trout (Oncorhynchus mykiss) gut microbiota is modulated by insect meal from Hermetia illucens prepupae in the diet. Rev. Fish Biol. Fish. 2019;29:465–486. doi: 10.1007/s11160-019-09558-y. [DOI] [Google Scholar]
- 107.Bruni L, Pastorelli R, Viti C, Gasco L, Parisi G. Characterisation of the intestinal microbial communities of rainbow trout (Oncorhynchus mykiss) fed with Hermetia illucens (black soldier fly) partially defatted larva meal as partial dietary protein source. Aquaculture. 2018;487:56–63. doi: 10.1016/j.aquaculture.2018.01.006. [DOI] [Google Scholar]
- 108.Huyben D, Vidaković A, Hallgren SW, Langeland M. High-throughput sequencing of gut microbiota in rainbow trout (Oncorhynchus mykiss) fed larval and pre-pupae stages of black soldier fly (Hermetia illucens) Aquaculture. 2019;500:485–491. doi: 10.1016/j.aquaculture.2018.10.034. [DOI] [Google Scholar]
- 109.Peres H, Santos S, Oliva-Teles A. Blood chemistry profile as indicator of nutritional status in European seabass (Dicentrarchus labrax) Fish Physiol. Biochem. 2014;40:1339–1347. doi: 10.1007/s10695-014-9928-5. [DOI] [PubMed] [Google Scholar]
- 110.Rebl A, Goldammer T. Under control: the innate immunity of fish from the inhibitors' perspective. Fish Shellfish Immunol. 2018;77:328–349. doi: 10.1016/j.fsi.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 111.Egerton S, et al. Replacing fishmeal with plant protein in Atlantic salmon (Salmo salar) diets by supplementation with fish protein hydrolysate. Sci. Rep. 2020;10:1–16. doi: 10.1038/s41598-020-60325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hou Y, Wu Z, Dai Z, Wang G, Wu G. Protein hydrolysates in animal nutrition: industrial production, bioactive peptides, and functional significance. J. Anim. Sci. Biotechnol. 2017;8:24. doi: 10.1186/s40104-017-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martínez-Alvarez O, Chamorro S, Brenes A. Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: a review. Food Res. Int. 2015;73:204–212. doi: 10.1016/j.foodres.2015.04.005. [DOI] [Google Scholar]
- 114.Gisbert E, Fournier V, Solovyev M, Skalli A, Andree KB. Diets containing shrimp protein hydrolysates provided protection to European sea bass (Dicentrarchus labrax) affected by a Vibrio pelagius natural infection outbreak. Aquaculture. 2018;495:136–143. doi: 10.1016/j.aquaculture.2018.04.051. [DOI] [Google Scholar]
- 115.Bøgwald J, Dalmo R, McQueenLeifson R, Stenberg E, Gildberg A. The stimulatory effect of a muscle protein hydrolysate from Atlantic cod, Gadus morhua L., on Atlantic salmon, Salmo salar L., head kidney leucocytes. Fish Shellfish Immunol. 1996;6:3–16. doi: 10.1006/fsim.1996.0002. [DOI] [Google Scholar]
- 116.Gildberg A, Bøgwald J, Johansen A, Stenberg E. Isolation of acid peptide fractions from a fish protein hydrolysate with strong stimulatory effect on Atlantic salmon (Salmo salar) head kidney leucocytes. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996;114:97–101. doi: 10.1016/0305-0491(96)00011-9. [DOI] [Google Scholar]
- 117.Esteban MA, Cuesta A, Ortuño J, Meseguer J. Immunomodulatory effects of dietary intake of chitin on gilthead seabream (Sparus aurata L.) innate immune system. Fish Shellfish Immunol. 2001;11:303–315. doi: 10.1006/fsim.2000.0315. [DOI] [PubMed] [Google Scholar]
- 118.Gopalakannan A, Arul V. Immunomodulatory effects of dietary intake of chitin, chitosan and levamisole on the immune system of Cyprinus carpio and control of Aeromonas hydrophila infection in ponds. Aquaculture. 2006;255:179–187. doi: 10.1016/j.aquaculture.2006.01.012. [DOI] [Google Scholar]
- 119.Ming, J. et al. The influence of maggot meal and l-carnitine on growth, immunity, antioxidant indices and disease resistance of black carp (Mylopharyngodon piceus). J. Chin. Cereals Oils Assoc.28, 80e86 (2013).
- 120.Martínez-Álvarez RM, Morales AE, Sanz A. Antioxidant defenses in fish: biotic and abiotic factors. Rev. Fish Biol. Fish. 2005;15:75–88. doi: 10.1007/s11160-005-7846-4. [DOI] [Google Scholar]
- 121.Imlay JA, Linn S. DNA damage and oxygen radical toxicity. Science. 1988;240:1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- 122.Cerezuela R, et al. Changes in intestinal morphology and microbiota caused by dietary administration of inulin and Bacillus subtilis in gilthead sea bream (Sparus aurata L.) specimens. Fish Shellfish Immunol. 2013;34:1063–1070. doi: 10.1016/j.fsi.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 123.Levine JM, Dantonio CM. Elton revisited: a review of evidence linking diversity and invasibility. Oikos. 1999;87:15–26. doi: 10.2307/3546992. [DOI] [Google Scholar]
- 124.Borrelli L, et al. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 2017;7:16269–16269. doi: 10.1038/s41598-017-16560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xia JH, et al. The intestinal microbiome of fish under starvation. BMC Genomics. 2014;15:266. doi: 10.1186/1471-2164-15-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Apper E, et al. Hydrolysed wheat gluten as part of a diet based on animal and plant proteins supports good growth performance of Asian seabass (Lates calcarifer), without impairing intestinal morphology or microbiota. Aquaculture. 2016;453:40–48. doi: 10.1016/j.aquaculture.2015.11.018. [DOI] [Google Scholar]
- 127.Ringø E, et al. Effect of dietary components on the gut microbiota of aquatic animals: a never-ending story? Aquac. Nutr. 2016;22:219–282. doi: 10.1111/anu.12346. [DOI] [Google Scholar]
- 128.Li J, et al. Comparative study on gastrointestinal microbiota of eight fish species with different feeding habits. J. Appl. Microbiol. 2014;117:1750–1760. doi: 10.1111/jam.12663. [DOI] [PubMed] [Google Scholar]
- 129.Givens CE, Ransom B, Bano N, Hollibaugh JT. Comparison of the gut microbiomes of 12 bony fish and 3 shark species. Mar. Ecol. Prog. Ser. 2015;518:209–223. doi: 10.3354/meps11034. [DOI] [Google Scholar]
- 130.Walker AW, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012;6:1535–1543. doi: 10.1038/ismej.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bonder MJ, et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016;8:45. doi: 10.1186/s13073-016-0295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rimoldi S, et al. Assessment of dietary supplementation with galactomannan oligosaccharides and phytogenics on gut microbiota of European sea bass (Dicentrarchus Labrax) fed low fishmeal and fish oil based diet. PLoS ONE. 2020;15:e0231494. doi: 10.1371/journal.pone.0231494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ringø E, Gatesoupe F-J. Lactic acid bacteria in fish: a review. Aquaculture. 1998;160:177–203. doi: 10.1016/S0044-8486(97)00299-8. [DOI] [Google Scholar]
- 135.Gudiña EJ, Fernandes EC, Rodrigues AI, Teixeira JA, Rodrigues LR. Biosurfactant production by Bacillus subtilis using corn steep liquor as culture medium. Front. Microbiol. 2015;6:59. doi: 10.3389/fmicb.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zarantoniello M, et al. Black soldier fly (Hermetia illucens) reared on roasted coffee by-product and Schizochytrium sp. as a sustainable terrestrial ingredient for aquafeeds production. Aquaculture. 2020;518:734659. doi: 10.1016/j.aquaculture.2019.734659. [DOI] [Google Scholar]
- 137.Ringø E, Sperstad S, Myklebust R, Mayhew TM, Olsen RE. The effect of dietary inulin on aerobic bacteria associated with hindgut of Arctic charr (Salvelinus alpinus L.) Aquac. Res. 2006;37:891–897. doi: 10.1111/j.1365-2109.2006.01509.x. [DOI] [Google Scholar]
- 138.Ringø E, Sperstad S, Myklebust R, Refstie S, Krogdahl Å. Characterisation of the microbiota associated with intestine of Atlantic cod (Gadus morhua L.): the effect of fish meal, standard soybean meal and a bioprocessed soybean meal. Aquaculture. 2006;261:829–841. doi: 10.1016/j.aquaculture.2006.06.030. [DOI] [Google Scholar]
- 139.Costa M, et al. Anchovy and giant squid hydrolysates can enhance growth and the immune response of European seabass (Dicentrarchus labrax) fed plant-protein-based diets. Aquaculture. 2020;523:735182. doi: 10.1016/j.aquaculture.2020.735182. [DOI] [Google Scholar]
- 140.Khosravi S, et al. Dietary supplementation of marine protein hydrolysates in fish-meal based diets for red sea bream (Pagrus major) and olive flounder (Paralichthys olivaceus) Aquaculture. 2015;435:371–376. doi: 10.1016/j.aquaculture.2014.10.019. [DOI] [Google Scholar]
- 141.Kotzamanis YP, Gisbert E, Gatesoupe FJ, ZamboninoInfante J, Cahu C. Effects of different dietary levels of fish protein hydrolysates on growth, digestive enzymes, gut microbiota, and resistance to Vibrio anguillarum in European sea bass (Dicentrarchus labrax) larvae. Comp. Biochem. Physiol. Part A. 2007;147:205–214. doi: 10.1016/j.cbpa.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 142.Offret C, Fliss I, Bazinet L, Marette A, Beaulieu L. Identification of a novel antibacterial peptide from Atlantic mackerel belonging to the GAPDH-related antimicrobial family and its in vitro digestibility. Mar. Drugs. 2019;17:413. doi: 10.3390/md17070413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Acosta-Smith E, et al. Bovine lactoferrin and lactoferrin-derived peptides inhibit the growth of Vibrio cholerae and other Vibrio species. Front. Microbiol. 2018;8:2633. doi: 10.3389/fmicb.2017.02633. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The amplicon sequence data in fastq format has been deposited to National Centre for Biotechnology Information (NCBI) under the BioProject accession number PRJNA660066.