Abstract
Aims:
To examine whether moderate adolescent cannabis use has neurocognitive effects that are unexplained by familial confounds, which prior family-controlled studies may not have identified.
Design:
A quasi-experimental, sibling-comparison design was applied to a prospective, observational study of adolescents with moderate cannabis use. Participants were recruited from 2001–2006 (M age=17). A second wave of data was collected from 2008–2013 (M age=24).
Setting:
Two United States metropolitan communities.
Participants:
1,192 adolescents from 596 families participated in this study. Participants were primarily male (64%) and racially and ethnically diverse (Non-Hispanic White=45%). A sibling in each family was a clinical proband identified due to delinquent behaviors. Whereas prior family-controlled studies have used samples of primarily infrequent cannabis users (M=1–2 days/month), participants here endorsed levels of cannabis use comparable to findings from epidemiological cohort studies (M=7–9 days/month).
Measurements:
Semi-structured clinical interviews assessed drug use, and a neuropsychological battery assessed cognitive abilities. Covariates included age at assessment, gender, and alcohol use.
Findings:
After correcting for multiple testing, a greater frequency and earlier onset of regular cannabis use were associated with poorer cognitive performance, specifically on tests of verbal memory. Further, after accounting for familial factors shared by siblings and alcohol use, poorer verbal memory performance was still associated with greater lifetime frequency of cannabis use at Wave 1 (b=−0.007 [−0.002, −0.012], adjusted-p=.036); earlier cannabis use at Wave 2 (b=−0.12 [−0.05, −0.19], adjusted-p=.006; b=−0.14 [−0.06, −0.23], adjusted-p=.006); and greater frequency of past-six-months use at Wave 2 (b=−0.02 [−0.01, −0.03], adjusted-p=.002; b=−0.02 [−0.01, −0.03], adjusted-p=.008).
Conclusions:
Moderate adolescent cannabis use may have adverse effects on cognitive functioning, specifically verbal memory, that cannot be explained by familial factors.
Keywords: adolescents, marijuana, cannabis, sibling, family, cognitive
Familial factors may not explain the effect of moderate-to-heavy cannabis use on cognitive functioning in adolescents: A sibling-comparison study
Cannabis use is broadly associated with adverse outcomes, including poorer cognitive functioning and academic performance.1–3 Quasi-experimental, family-controlled studies suggest that familial factors, not direct cannabis exposure, explain the association between cannabis use and cognitive outcomes.4–6 Specifically, among twin pairs, cotwins who use more cannabis do not tend to have worse cognitive functioning. Although these studies are inconsistent with cannabis use causing cognitive deficits, their authors have noted that they may not address effects due to “long-term” or “intense” cannabis use.4,5 For example, previous family-controlled studies have examined adolescent samples that used cannabis, on average, one to two days per month,5,6 compared to five to nine days per month in the U.S. nationally-representative National Longitudinal Survey of Youth (assessed at approximately the same time as prior family-controlled studies and the current study).7 Thus, prior family-controlled studies are most informative with regard to the effects of infrequent cannabis use, rather than levels of use that are more common in adolescents. The current study extends this literature by examining the effects of cannabis use on cognitive functioning via sibling-comparison analysis in a sample of adolescents with early initiation and moderate-to-heavy use (M=13.6 years-old at onset of regular use; M=7–9 days/month at age 17).
Ongoing shifts in the U.S. cannabis landscape highlight the need for more research on the effects of cannabis. In the last 25 years, the Monitoring the Future Study suggests that cannabis use has almost doubled (22% to 36%) and perceived risk from regular use has dropped by more than half among 12th graders (77% to 31%).8 Further, 11 states (including the District of Columbia) have legalized recreational cannabis. Cannabis products have also become increasingly potent, which may exacerbate any neuropsychological consequences.9 The increasing THC potency of confiscated, black-market cannabis is well documented (4% to 12% THC, 1995–2012),10,11 and legal-market products are now many times more potent (20+% flower, 80+% concentrates). Thus, there is a clear and pressing need to understand the effects of cannabis.
Several studies suggest that initiating cannabis use earlier in life may be especially detrimental to cognitive functioning, including IQ,3 visual search,12 executive functioning,13,14 sustained attention,15 impulse control,15 and verbal memory.16 These neuropsychological deficits may, in part, occur via THC activating the cannabinoid-1 receptor, which is particularly abundant in the adolescent brain17and in substrates involved in cognitive functioning (e.g., prefrontal cortex).18 Additionally, the acute and long-term effects of cannabis are THC-dose dependent,19,20 suggesting that high-potency products or persistent use beginning in adolescence may pose substantial consequences.21
Although studies on the consequences of cannabis use have primarily focused on adolescents, cannabis may also have adverse effects into adulthood. Neurodevelopment continues through emerging adulthood, including in prefrontal substrates involved in executive functioning and emotion regulation.22,23 Further, meta-analytic studies have identified adverse cognitive effects of cannabis use through at least age 26.24 The cognitive effects of cannabis use should, therefore, be considered beyond adolescence.
Despite a large body of evidence linking cannabis use to cognitive deficits, plausible alternative explanations must be ruled out to support a causal relationship.25 Cannabis use and lower cognitive functioning share many environmental risk factors, including peer group deviance, parental psychopathology, parental drug use, parental marital instability, and lower parental socioeconomic status.26–28 Additionally, genetic factors may explain shared risk for worse cognitive functioning and earlier or heavier cannabis use.29 Thus, family-controlled studies of high-risk adolescents can fill a critical gap by rigorously testing the effects of moderate-to-heavy THC exposure.
The current study used a sibling-comparison design in a sample of high-risk adolescents, in which one sibling was a clinical proband identified due to delinquent behaviors. First, analyses examined the association between cannabis use and cognitive functioning. Second, multilevel models partitioned phenotypic associations into between-family (i.e., genetic and environmental factors shared by siblings) and within-family effects (i.e., the effects of differential cannabis use among siblings). A final multilevel model accounted for alcohol use. If within-family, sibling comparisons suggest that differential levels of cannabis use are associated with poorer cognitive functioning, this would support, but not definitively prove, a causal relationship of cannabis use on neurocognitive outcomes. A failure to find any effect in this study would raise skepticism that adolescent cannabis use, even at higher levels, has adverse effects.
Methods
Participants
From 2001 to 2006, 245 probands/257 siblings from San Diego and 351 probands/373 siblings from Denver were recruited via substance abuse treatment programs, alternative schools, and juvenile probation departments (see30 for ascertainment details). A Wave 2 assessment was conducted between 2008–2013, collecting data from 206 probands/219 siblings from the original San Diego sample and 225 probands/241 siblings from the original Denver sample. Participants were primarily male (63%) and racially and ethnically diverse (Hispanic=33%, Black=9%, Non-Hispanic White=45%; see Table 1 for descriptive statistics). Participants were tested at both waves on an array of assessments, including neurocognitive measures and history of substance use. All research protocols were reviewed and approved by Institutional Review Boards at the University of Colorado, Denver and the University of California, San Diego.
Table 1.
Demographics for participants recruited at the Denver and San Diego sites at Wave 1 and Wave 2.
| Wave 1 (n=1,192) | Wave 2 (n = 875) | ||||
|---|---|---|---|---|---|
| Denver (n=710) | San Diego (n=482) | Denver (n=459) | San Diego (n=416) | ||
| Probands/Siblings | 351/359 | 245/237 | 225/234 | 206/210 | |
| M Age (SD) | 17.1 (2.3) | 17.6 (2.1) | 23.5 (2.7) | 23.8 (2.6) | |
| % Male | 69.3% | 55.5% | 68.2% | 54.9% | |
| % Hispanic | 24.4% | 45.0% | 23.1% | 42.8% | |
| White (Non-Hispanic) | 53.0% | 33.6% | 55.8% | 36.1% | |
| Black/African-American | 8.5% | 10.0% | 7.2% | 9.6% | |
| American Indian | 1.8% | 0.8% | 1.1% | 1.0% | |
| Asian/Pacific Islander | 0.4% | 3.1% | 0.4% | 3.1% | |
| Multiracial | 14.5% | 6.4% | 14.4% | 6.2% | |
| Other (Non-Hispanic) | 0.8% | 2.3% | 0.9% | 2.4% | |
| % Ever Used Cannabis | 88.5% | 87.1% | 94.5% | 92.8% | |
| Age of Onset of Monthly Cannabis Use [Minimum, Maximum] | 13.2 (2.0) [9, 20] | 14.2 (1.8) [9, 22] | 13.9 (2.2) [9, 25] | 14.9 (2.2) [9, 26] | |
| Monthly Frequency of Past Six Month Cannabis Usea | 9.2 (10.8) | 7.3 (10.7) | 8.3 (11.7) | 7.8 (11.7) | |
| Lifetime Frequency of Cannabis Useb | 42.0 (25.5) | 39.1 (26.2) | 50.4 (20.6) | 48.3 (21.5) | |
| % Ever Used Alcohol | 92.3% | 94.8% | 99.3% | 99.5% | |
| Age of Onset of Monthly Alcohol Use [Minimum, Maximum] | 14.7 (2.2) [9, 22] | 14.9 (2.1) [9, 22] | 15.5 (2.5) [9, 23] | 15.7 (2.5) [9, 28] | |
| Monthly Frequency of Past Six Month Alcohol Usea | 3.7 (6.0) | 4.0 (5.5) | 5.2 (8.3) | 7.3 (8.3) | |
| Lifetime Frequency of Alcohol Useb | 33.7 (24.4) | 40.3 (23.4) | 53.6 (15.0) | 55.6 (13.3) | |
| IQ | 93.8 (15.2) | 93.8 (15.7) | NA | NA | |
Note:
At both waves/sites, the minimum and maximum of monthly frequency of past six-month use were 0 and 30 days, respectively.
At both waves/sites, the minimum and maximum for lifetime frequency were 0 and 60, respectively. NA = not available.
Measures
Substance Use.
Trained interviewers administered the Composite International Diagnostic Interview (CIDI), a well-validated structured clinical interview, as well as the CIDI Substance Abuse Module.31,32
Participants who endorsed the having “ever used (cannabis/alcohol)” were administered additional questions designed to supplement the CIDI to quantify use patterns.33 Age of onset was assessed as the age when participants began using regularly (at least monthly). Participants who denied ever using regularly were coded as missing. Recent frequency was assessed as “How many days have you used (cannabis/alcohol) in the past six months (180 Days)?” (recoded to represent monthly frequency). Lifetime frequency was also assessed for cannabis/alcohol use (responses=recoding: “1–2 times”=1.5, “3–5 times”=4, “6–9 times”=7.5, “10–19 times”=15, “20–39 times”=30, and “more than 40 times”=60). Frequencies were coded as zero for participants who denied ever using cannabis/alcohol.
Cognitive Functioning.
Participants completed a battery assessing response inhibition (Stroop), learning and memory (California Verbal Learning Test, Second Edition [CVLT-II]), attention and working memory (digit span), cognitive flexibility (Trail Making Test parts A and B), and intelligence (block design, vocabulary, and full-scale IQ on age-appropriate Wechsler scales). The CVLT and digit span tests were only administered to participants at the San Diego site (cognitive tasks are described in detail in supplementary material).
Analytic Procedures
Models testing the association between each measure of cannabis use and each measure of cognitive functioning were conducted in Mplus version 7.4.34 All models included age and gender as covariates and accounted for the clustering of data (i.e., siblings from the same family). Missing data were handled using full-information maximum likelihood. Importantly, the assumption of multivariate normality in multilevel modeling is flexible, and violating this assumption underestimates standard errors only with small samples.35 We applied Hochberg’s correction for multiple testing using the p.adjust function from the stats package in R, which provides adjusted p-values to a set of estimated p-values.36 Model-derived 95% confidence intervals (CIs) are also presented but do not indicate statistical significance. Scripts used for data management and analyses are available at: https://github.com/jme6f4/GADD_MJ_COG_RR. This project was not preregistered, and findings may be considered exploratory.
Model 1: Phenotypic analyses examined the general association between cannabis use and cognitive outcomes.
Model 1 estimated the association between each measure of cannabis use and each measure of cognitive performance, without accounting for familial confounds. Standard errors were estimated with a sandwich estimator to correct for within-family correlations (i.e., siblings from the same family).
Models 2–3: Multilevel analyses decomposed the association between cannabis use and cognitive outcomes into factors shared by, and distinct among, siblings.
Models 2–3 estimated the association between cannabis use and cognitive performance within families (i.e., differential sibling exposure in each family).37 This multilevel approach accounts for unmeasured familial factors that make siblings alike. To index general familial risk, we included the mean cannabis use (MCan) for each sibling set at the between-family level. To index sibling-specific risk, we included the deviation of cannabis use (DCan) from the general familial risk (MCan) for each individual. Independent variables and covariates were grand-mean centered, and DCan was group-centered by family.
Of the 596 families in the current study, there were sibling differences in the age of onset in 271 families (45%). That is, 271 families contributed to the within-family effect estimates (DCan) for age of onset of regular use. Among families in which siblings differed in age of onset, 56% began regularly using within two years of age of each other (M=2.7, SD=1.8). Further, at Wave 2, there were sibling differences in the past-six-month frequency of use in 265 families (44%). Among families in which siblings differed in the frequency of use at Wave 2, siblings differed in use by, on average, two days per week (M=3.1, SD=2.8). Thus, within-family parameter estimates were based sibling pairs from over 250 families.
Given that the nature of cannabis use and exposure may vary across wide age gaps between siblings, we excluded 10 participants who were 10+ years older than their youngest sibling in the study. An additional 14 participants were over the age of 25 at Wave 1 and were excluded from these analyses. Of the remaining siblings in the current sample, 66% were within three years (M=2.8, median=2.3 years apart). Age was included as a covariate to account for age differences in the analyzed sample.
In Model 3, alcohol involvement was added as a covariate. At the between-family level, alcohol involvement can account for general familial risk factors for alcohol use. At the within-family family level (group-centered by sibling pair), alcohol involvement accounts for confounding due to the potential neurotoxic effects that may be specific to alcohol. Specifically, alcohol variables were modeled to correspond to each cannabis variable (e.g., lifetime frequency of alcohol use was a covariate in models of lifetime frequency of cannabis use).
Results
Descriptive Statistics
A vast majority of participants endorsed cannabis use at Waves 1 (88%) and 2 (94%), with a mean age of onset of monthly use of 13.7 years. Further, participants reported using, on average, 8.4 (SD=10.8) days per month in the previous six months at Wave 1.
Model 1: Phenotypic Analyses (Table 2)
Table 2.
Unstandardized path coefficients of cannabis use measures on cognitive outcomes in clinical probands and siblings.
| Cognitive Outcome | Ever Used | Onset of Monthly Use | Lifetime Frequency | Past-Six-Month Frequency |
|---|---|---|---|---|
| Wave 1 (M age = 17) | ||||
| Stroop Word (n=1,188) | 0.02 (−1.44,1.49) | 0.11 (−0.23,0.44) | 0.00 (−0.03,0.03) | 0.00 (−0.04,0.05) |
| Block Design (n=1,106) | −0.73 (−2.73,1.27) | 0.32 (−0.07,0.70) | 0.00 (−0.03,0.03) | 0.03 (−0.03,0.08) |
| Digit Span (n=481) | 0.87 (0.21,1.53) | 0.15 (0.01,0.30) | 0.00 (−0.01,0.01) | 0.02 (0.00,0.05) |
| Vocabulary (n=1,105) | −0.64 (−2.79,1.52) | 0.23 (−0.17,0.63) | 0.00 (−0.03,0.02) | 0.04 (−0.01,0.09) |
| IQ (n=1,103) | −1.49 (−4.79,1.81) | 0.55 (−0.10,1.19) | 0.00 (−0.05,0.04) | 0.07 (−0.02,0.15) |
| Trails A (n=1,191) | 1.84 (−0.31,3.99) | −0.13 (−0.52,0.26) | 0.01 (−0.01,0.04) | −0.01 (−0.06,0.05) |
| Trails B (n=1,169) | 0.98 (−1.41,3.37) | 0.11 (−0.28,0.50) | 0.01 (−0.02,0.04) | 0.03 (−0.03,0.09) |
| CVLT Long Delay Free (n=479) | −0.09 (−0.35,0.17) | 0.06 (0.00,0.12) | −0.01 *(−0.01,−0.00) | −0.01 (−0.02,0.00) |
| CVLT Composite (n=479) | −0.10 (−0.34,0.14) | 0.06 (0.00,0.12) | 0.00 (−0.01,0.00) | −0.01 (−0.02,0.00) |
| Wave 2 (M age = 23) | ||||
| Stroop Word (n=874) | −0.76 (−2.98,1.45) | −0.09 (−0.39,0.21) | 0.00 (−0.03,0.03) | −0.03 (−0.08,0.02) |
| Block Design (n=790) | 0.66 (−3.20,4.52) | 0.37 (−0.02,0.76) | 0.01 (−0.04,0.05) | 0.02 (−0.04,0.09) |
| Digit Span (n=416) | 0.58 (−0.62,1.79) | −0.03 (−0.17,0.11) | 0.01 (−0.01,0.02) | 0.02 (−0.01,0.05) |
| Trails A (n=875) | 4.01 (0.37,7.65) | −0.14 (−0.55,0.27) | 0.04 (0.00,0.08) | 0.03 (−0.04,0.10) |
| Trails B (n=867) | −0.70 (−3.74,2.35) | −0.08 (−0.48,0.31) | 0.01 (−0.03,0.04) | 0.05 (−0.02,0.11) |
| CVLT Long Delay Recall (n=415) | −0.23 (−0.71,0.26) | 0.09 *** (0.04,0.14) | 0.00 (−0.01,0.01) | 0.00 (−0.01,0.01) |
| CVLT Composite (n=415) | −0.09 (−0.48,0.30) | 0.09 *** (0.05,0.14) | 0.00 (−0.01,0.01) | 0.00 (−0.01,0.00) |
Note: CVLT = California Verbal Learning Test.
p < .001,
p < .01,
p < .05.
Statistical significance is based on p-values adjusted for multiple testing, using Hochberg’s correction. Model-estimated 95% confidence intervals are in parentheses. Due to the use of model-estimated confidence intervals and adjusted significance thresholds, some estimates are not statistically significant despite confidence intervals that do not span zero. Models included age and gender as covariate and accounted for the correlation of participants from the same family.
Across Waves 1 and 2, there were consistent associations for performance on the CVLT, both as previously examined in the literature (i.e., long delay free-recall) and a composite of all four recall tasks. At Wave 1, poorer performance on CVLT long-delay free-recall task was associated with greater lifetime frequency (b=−0.006 [95% CI=−0.002,−0.009], adjusted-p=.010). Additionally, at Wave 2, an earlier age of onset of regular use was associated with poorer performance on CVLT long-delay free-recall (b=0.09 [0.04,0.14], adjusted-p<.001) and the CVLT Composite (b=0.09 [0.05,0.14], adjusted-p<.001). There were no other statistically significant effects indicating that cannabis has adverse effects on cognitive performance.
Model 2: Multilevel Analyses (Table 3)
Table 3.
Unstandardized path coefficients of between- and within-family effects of cannabis use on cognitive outcomes in clinical probands and siblings.
| Between-Family Effects | Within-Family Effects | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cognitive Outcome | Ever Used | Onset of Regular Use | Lifetime Frequency | Six-Month Frequency | Ever Used | Onset of Regular Use | Lifetime Frequency | Six-Month Frequency | |
| Wave 1 (M age = 17) | |||||||||
| Stroop Word (n = 1187) | 0.07 (−2.28,2.41) | 0.10 (−0.24,0.44) | 0.00 (−0.03,0.03) | −0.01 (−0.07,0.06) | 0.18 (−1.67,2.02) | 0.14 (−0.43,0.71) | 0.01 (−0.02,0.03) | 0.02 (−0.05,0.09) | |
| Block Design (n = 1105) | −1.19 (−4.68,2.30) | 0.32 (−0.10,0.74) | −0.02 (−0.05,0.02) | 0.01 (−0.06,0.09) | 0.23 (−1.72,2.18) | 0.04 (−0.44,0.52) | 0.02 (−0.01,0.05) | 0.06 (0.00,0.13) | |
| Digit Span (n = 481) | 1.30 (0.16,2.44) | 0.18 (0.00,0.35) | 0.01 (0.00,0.02) | 0.04 (0.01,0.07) | 0.53 (−0.11,1.17) | 0.13 (−0.06,0.33) | −0.01 (−0.02,0.01) | 0.00 (−0.02,0.03) | |
| Vocabulary (n = 1104) | −0.92 (−4.22,2.37) | 0.22 (−0.23,0.67) | −0.01 (−0.05,0.02) | 0.05 −0.02,0.13) | −0.49 (−2.40,1.43) | 0.09 (−0.33,0.50) | −0.01 (−0.04,0.02) | 0.02 (−0.04,0.08) | |
| IQ (n = 1102) | −2.15 (−7.66,3.36) | 0.54 (−0.20,1.27) | −0.03 (−0.09,0.03) | 0.07 (−0.05,0.18) | −0.31 (−3.25,2.63) | 0.12 (−0.57,0.82) | 0.01 (−0.04,0.06) | 0.09 (−0.01,0.18) | |
| Trails A (n = 1190) | 1.59 (−1.32,4.50) | −0.05 −0.48,0.38) | 0.01 (−0.02,0.04) | −0.03 (−0.11,0.05) | 1.81 (−0.71,4.33) | −0.19 (−0.79,0.41) | 0.00 (−0.04,0.04) | 0.02 (−0.05,0.10) | |
| Trails B (n = 1168) | 0.03 (−3.30,3.36) | −0.03 (−0.49,0.42) | 0.00 (−0.04,0.04) | 0.05 (−0.04,0.13) | 1.89 (−0.81,4.59) | 0.08 (−0.49,0.66) | 0.00 (−0.04,0.04) | −0.01 (−0.09,0.08) | |
| CVLT Long Delay Recall (n = 479) | −0.03 (−0.43,0.37) | 0.06 (−0.02,0.13) | −0.01 (−0.01,0.00) | −0.01 (−0.02,0.00) | −0.16 (−0.50,0.19) | 0.07 (0.00,0.15) | −0.01 (−0.01,0.00) | −0.01 (−0.02,0.00) | |
| CVLT Composite (n = 479) | 0.00 (−0.39,0.39) | 0.07 (0.00,0.13) | 0.00 (−0.01,0.00) | −0.01 (−0.02,0.00) | −0.21 (−0.52,0.10) | 0.05 (−0.02,0.13) | −0.01 (−0.01,0.00) | −0.01 (−0.02,0.00) | |
| Wave 2 (M age = 23) | |||||||||
| Stroop Word (n = 874) | −2.34 (−5.00,0.31) | 0.15 (−0.23,0.52) | −0.02 (−0.07,0.02) | −0.08 (−0.14,−0.02) | 0.89 (−2.51,4.29) | −0.52 (−1.00,−0.04) | 0.03 (−0.02,0.07) | 0.06 (−0.03,0.16) | |
| Block Design (n = 790) | 2.58 (−3.07,8.22) | 0.17 (−0.33,0.68) | 0.03 (−0.04,0.09) | 0.08 (−0.02,0.18) | −0.48 (−4.11,3.16) | 0.54 (−0.03,1.11) | 0.01 (−0.04,0.06) | −0.06 (−0.13,0.02) | |
| Digit Span (n = 416) | 0.97 (−0.77,2.70) | 0.02 (−0.18,0.21) | 0.02 (0.00,0.04) | 0.04 (0.00,0.07) | 0.52 (−0.65,1.69) | −0.04 (−0.25,0.17) | −0.01 (−0.02,0.01) | −0.01 (−0.04,0.03) | |
| Trails A (n = 875) | 5.55 (0.59,10.51) | 0.12 (−0.32,0.57) | 0.04 (−0.01,0.10) | 0.03 (−0.06,0.12) | 1.90 (−2.61,6.41) | −0.40 (−1.01,0.22) | 0.04 (−0.01,0.09) | 0.04 (−0.07,0.16) | |
| Trails B (n = 867) | 2.84 (−1.51,7.18) | 0.21 (−0.20,0.63) | 0.04 (−0.02,0.09) | 0.05 (−0.03,0.13) | −5.15 (−9.48,−0.81) | −0.44 (−1.00,0.12) | −0.03 (−0.07,0.03) | 0.03 (−0.08,0.14) | |
| CVLT Long Delay Recall (n = 415) | 0.22 (−0.45,0.89) | 0.04 (−0.03,0.10) | 0.00 (0.00,0.01) | 0.01 (−0.01,0.02) | −0.70 * (−1.21,−0.19) | 0.14 *** (0.08,0.21) | 0.00 (−0.01,0.00) | −0.02 * (−0.03,−0.01) | |
| CVLT Composite (n = 415) | 0.33 (−0.23,0.88) | 0.06 (0.00,0.11) | 0.00 (0.00,0.01) | 0.01 (0.00,0.02) | −0.52 (−0.94,−0.09) | 0.12 *** (0.07,0.18) | 0.00 (−0.01,0.00) | −0.02 ** (−0.03,−0.01) | |
Note: CVLT = California Verbal Learning Test.
p < .001,
p < .01,
p < .05.
Statistical significance is based on p-values adjusted for multiple testing, using Hochberg’s correction. 95% confidence intervals are in parentheses. Due to the use of model-derived confidence intervals and adjusted significance thresholds, some estimates are not statistically significant despite confidence intervals that do not span zero. Covariates were age and gender. Models accounted for the correlation of participants from the same family.
Multilevel level models disaggregated the associations between cannabis use and cognitive performance measures into common sibling effects (i.e., between-family effects) and differential use among siblings (i.e., within-family effects). After correcting for multiple testing, only poorer delayed verbal memory (CVLT) was associated with cannabis use. At Wave 1, there were no statistically significant effects after correcting for multiple testing. At Wave 2, poorer performance on the CVLT was associated with ever using cannabis (long-delay free-recall: b=−0.70 [−0.19,−1.21], adjusted-p=.049), an earlier onset of regular use (long-delay free-recall: b=0.14 [0.08,0.21], adjusted-p<.001; CVLT composite: b=0.12 [0.07,0.18], adjusted-p<.001), and six-month frequency (long-delay free-recall: b=−0.02 [−0.01,−0.03], adjusted-p=.012; CVLT composite: b=−0.02 [−0.01,−0.03], adjusted-p=.007).
Models 3: Controlling for Alcohol Use (Table 4)
Table 4.
Unstandardized path coefficients of between- and within-family effects of cannabis use on cognitive outcomes in clinical probands and siblings, after controlling for alcohol involvement.
| Between-Family Effects | Within-Family Effects | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cognitive Outcome | Ever Used | Onset of Regular Use | Lifetime Frequency | Six-Month Frequency | Ever Used | Onset of Regular Use | Lifetime Frequency | Six-Month Frequency | |
| Wave 1 (M age = 17) | |||||||||
| Stroop Word (n = 1188) | 0.64 (−1.91,3.18) | 0.27 (−0.12,0.66) | 0.00 (−0.03,0.03) | 0.00 (−0.06,0.07) | 0.23 (−1.95,2.42) | 0.09 (−0.58,0.76) | −0.01 (−0.04,0.03) | 0.04 (−0.03,0.12) | |
| Block Design (n = 1106) | −1.82 (−5.81,2.17) | 0.38 (−0.09,0.86) | −0.03 (−0.07,0.01) | 0.01 (−0.07,0.09) | −0.11 (−2.20,1.97) | −0.08 (−0.60,0.44) | 0.01 (−0.03,0.04) | 0.07 (0.00,0.14) | |
| Digit Span (n = 481) | 1.21 (−0.01,2.42) | 0.24 (0.06,0.42) | 0.01 (0.00,0.03) | 0.04 (0.01,0.07) | 0.43 (−0.31,1.16) | 0.10 (−0.12,0.31) | 0.00 (−0.02,0.01) | 0.00 (−0.03,0.03) | |
| Vocabulary (n = 1105) | −2.60 (−6.12,0.91) | 0.10 (−0.43,0.62) | −0.02 (−0.06,0.01) | 0.07 (0.00,0.14) | −1.01 (−3.27,1.24) | 0.09 (−0.40,0.58) | −0.01 (−0.04,0.03) | 0.01 (−0.05,0.08) | |
| IQ (n = 1103) | −4.41 (−10.48,1.66) | 0.48 (−0.35,1.31) | −0.05 (−0.12,0.01) | 0.08 (−0.05,0.20) | −1.27 (−4.47,1.93) | 0.01 (−0.73,0.74) | 0.00 (−0.05,0.05) | 0.09 (−0.02,0.19) | |
| Trails A (n = 1191) | 1.61 (−1.61,4.83) | −0.13 (−0.60,0.34) | 0.01 (−0.03,0.04) | −0.03 (−0.11,0.05) | 1.17 (−1.64,3.97) | −0.21 (−0.90,0.49) | −0.01 (−0.05,0.03) | 0.01 (−0.06,0.09) | |
| Trails B (n = 1169) | −1.19 (−4.93,2.54) | −0.03 (−0.53,0.46) | −0.01 (−0.05,0.04) | 0.06 (−0.04,0.15) | 0.59 (−2.54,3.73) | 0.22 (−0.47,0.91) | −0.01 (−0.06,0.04) | −0.01 (−0.09,0.08) | |
| CVLT Long Delay Recall (n = 479) | −0.04 (−0.49,0.41) | 0.05 (−0.02,0.13) | −0.01 (−0.01,0.00) | −0.01 (−0.02,0.01) | −0.20 (−0.54,0.15) | 0.08 (−0.01,0.16) | −0.01 (−0.01,0.00) | −0.01 (−0.02,0.00) | |
| CVLT Composite (n = 479) | −0.01 (−0.44,0.42) | 0.06 (0.00,0.12) | −0.01 (−0.01,0.00) | −0.01 (−0.02,0.01) | −0.30 (−0.61,0.02) | 0.04 (−0.04,0.12) | −0.01* (−0.01,−0.00) | −0.01 (−0.03,0.00) | |
| Wave 2 (M age = 23) | |||||||||
| Stroop Word (n = 874) | −2.47 (−5.23,0.29) | 0.15 (−0.25,0.54) | −0.02 (−0.06,0.03) | −0.09 * (−0.15,−0.03) | 0.69 (−2.89,4.28) | −0.29 (−0.82,0.25) | 0.02 (−0.03,0.06) | 0.07 (−0.03,0.16) | |
| Block Design (n = 790) | 2.77 (−2.85,8.40) | 0.52 (−0.04,1.08) | 0.00 (−0.07,0.07) | 0.06 (−0.04,0.16) | −0.23 (−3.94,3.49) | 0.79 (0.17,1.42) | 0.03 (−0.02,0.08) | −0.05 (−0.13,0.03) | |
| Digit Span (n = 416) | 0.91 (−0.84,2.66) | 0.04 (−0.15,0.23) | 0.02 (0.00,0.04) | 0.04 (0.00,0.08) | 0.57 (−0.63,1.77) | −0.04 (−0.27,0.19) | −0.01 (−0.03,0.01) | −0.01 (−0.05,0.02) | |
| Trails A (n = 875) | 5.05 (−0.07,10.16) | 0.12 (−0.38,0.63) | 0.02 (−0.04,0.07) | 0.05 (−0.05,0.14) | 2.13 (−2.44,6.71) | −0.31 (−1.01,0.40) | 0.03 (−0.03,0.08) | 0.03 (−0.08,0.14) | |
| Trails B (n = 867) | 2.45 (−2.03,6.94) | 0.11 (−0.36,0.57) | 0.02 (−0.04,0.08) | 0.07 (−0.01,0.15) | −5.48 (−10.02,−0.94) | −0.39 (−1.03,0.26) | −0.05 (−0.10,0.01) | 0.03 (−0.08,0.14) | |
| CVLT Long Delay Recall (n = 415) | 0.28 (−0.38,0.93) | 0.06 (−0.02,0.13) | 0.00 (−0.01,0.01) | 0.00 (−0.01,0.02) | −0.69 (−1.21,−0.17) | 0.14 ** (0.06,0.23) | 0.00 (−0.01,0.01) | −0.02 * (−0.03,−0.01) | |
| CVLT Composite (n = 415) | 0.37 (−0.17,0.90) | 0.07 (0.01,0.13) | 0.00 (−0.01,0.01) | 0.00 (−0.01,0.01) | −0.51 (−0.95,−0.08) | 0.12 ** (0.05,0.19) | 0.00 (−0.01,0.00) | −0.02 * (−0.03,−0.01) | |
Note: CVLT = California Verbal Learning Test.
p < .001,
p < .01,
p < .05.
Statistical significance is based on p-values adjusted for multiple testing, using Hochberg’s correction. 95% confidence intervals are in parentheses. Due to the use of model-derived confidence intervals and adjusted significance thresholds, some estimates are not statistically significant despite confidence intervals that do not span zero. Covariates were age, gender, and measures of alcohol involvement that correspond with each cannabis measure (e.g., ever used alcohol was included as a covariate for models of ever used cannabis). Models accounted for the correlation of participants from the same family.
Controlling for alcohol use was unable to explain the observed effects of cannabis use on delayed verbal recall (CVLT performance). At Wave 1, there was a significant effect of lifetime frequency on poorer CVLT composite score (b=−0.007 [−0.002,−0.012], adjusted-p=.027 on). At Wave 2 there were significant effects of an earlier onset of regular use (long-delay free-recall: b=0.14 [0.06,0.23], adjusted-p=.006; CVLT composite: b=0.12 [0.05,0.19], adjusted-p=.006) and six-month frequency long-delay free-recall: b=−0.02 [−0.01,−0.03], adjusted-p=.048; CVLT composite: b=−0.02 [−0.01,−0.03], adjusted-p=.014). There were no other statistically significant within-family effects, after correcting for multiple testing. At the between-family level, there was an effect of greater Wave 2 six-month frequency on poorer Stroop performance (b=−0.09 [−0.03,−0.15], adjusted-p=.035), suggesting that familial factors associated with greater cannabis use are associated with poorer inhibitory control at Wave 2.
Post-Hoc Analyses: Persistent Use (Figure 1)
Figure 1.
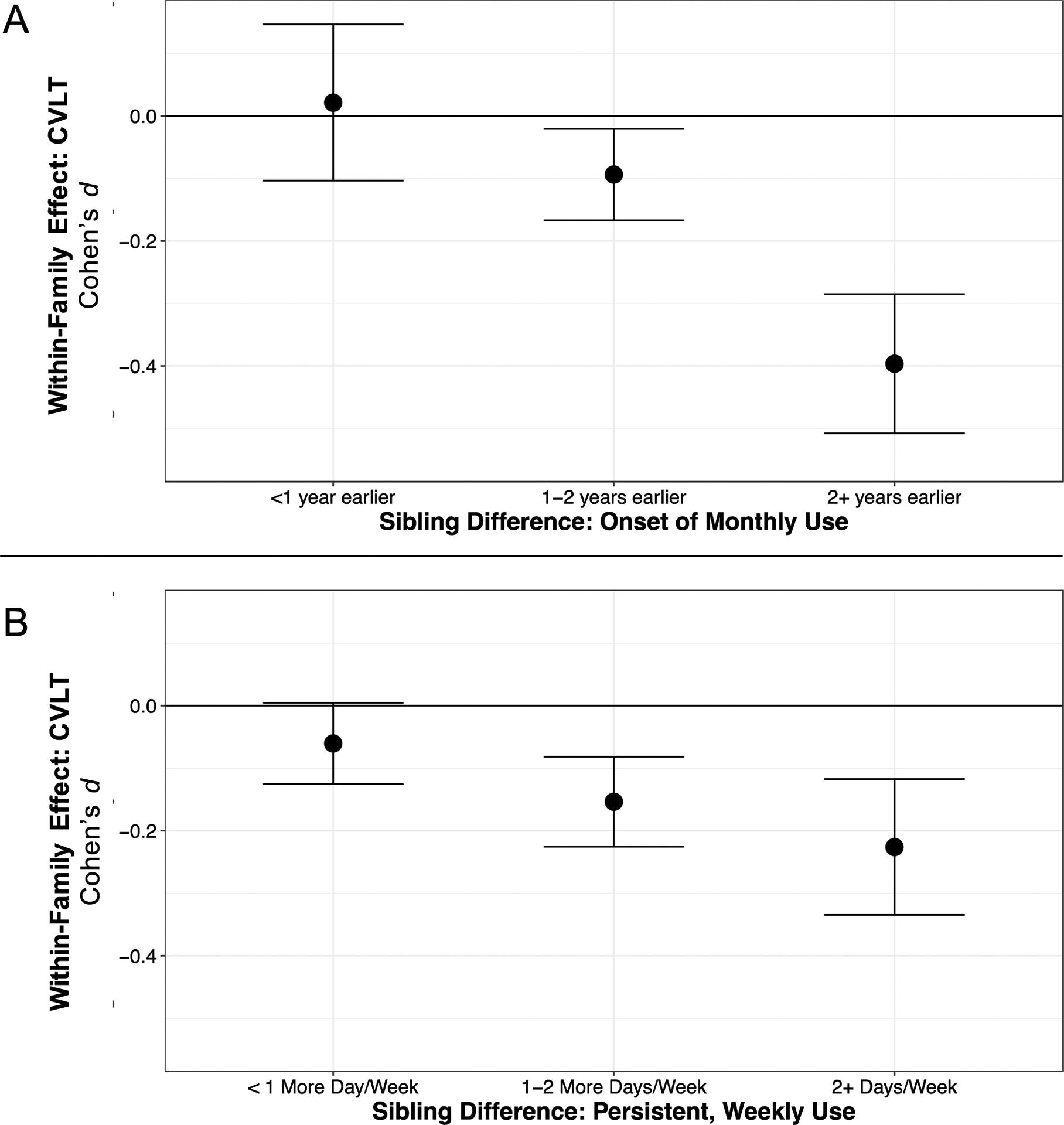
The sibling difference in delayed verbal memory, based on sibling differences in (A) age of onset of monthly use and (B) frequency of use in the past six months. Error bars represent standard errors around the point estimate. For age of onset (A), using cannabis two or more years earlier than one’s sibling was associated with a 0.40 standard-deviation decrease in delayed verbal memory performance, relative to the mean performance of participants from the same family (i.e., after accounting for familial factors shared by siblings).For frequency of use (B), using cannabis two or more days per week than one’s sibling was associated with a 0.23 standard-deviation decrase in delayed veriabl memory performance, relative to the mean performance of participants from the same family (i.e., after accounting for familial factors).
There was a pattern in which the effects of regular use onset and six-month frequency were most prominent at Wave 2, compared to Wave 1. Post-hoc analyses examined the effects of persistent use on CVLT performance, based on other findings in the literature.3 We created a variable to measure persistent frequency by averaging the level of six-month frequency of use across both waves. Persistent use across Waves 1 and 2 was associated with poorer CVLT performance (b=−0.03 [−0.01,−0.04], adjusted-p=.001). These findings are consistent with the possibility that heavy, persistent cannabis use may adversely affect cognitive functioning. Figure 1 displays the effects of using cannabis earlier or more frequently than one’s sibling on CVLT composite performance at Wave 2. Initiating monthly cannabis use two or more years earlier than one’s sibling is associated with scoring 0.40 standard deviations lower on the CVLT composite than one’s sibling (1A). Similarly, using cannabis two or more times per week than one’s sibling is associated with scoring 0.23 standard deviations lower on the CVLT composite than one’s sibling (1B).
Discussion
The current study examined a sample of at-risk adolescents and their siblings, finding that having an earlier initiation and higher frequency of cannabis use than one’s sibling is associated with poorer delayed verbal memory. That is, the adverse effects of cannabis use could not be explained by environmental or genetic factors shared by siblings or by alcohol use. These findings differ from other family-controlled studies that have not found evidence suggestive of a causal effect of early-life cannabis use on cognitive functioning. These findings are consistent, however, with work suggesting that persistent cannabis use may have adverse effects.3 While prior studies suggest that low levels of cannabis use (M=0.3 days/week) may not cause cognitive deficits, moderate use (M=1.9 days/week in the current sample) may have adverse effects.
These findings should be interpreted with the caveat that sibling-controlled designs can rigorously test effects by controlling for all confounds shared by siblings. However, this design does not exhaustively control for every potential confound. On average, siblings share only 50% of genetic factors and likely much less than 100% of environmental factors. Therefore, while these results support a potential causal association between moderate-to-heavy cannabis use and poorer delayed verbal memory, this association may yet be explained by important confounds that were not controlled by the current study design.
The strongest effect in the current study was on the CVLT, which assesses short-term verbal memory. Notably, recent meta-analytic work has found strong links between cannabis use and adverse effects on learning and delayed memory (d>.20, most frequently assessed by CVLT across the literature).24 Importantly, this meta-analyses also implicated other cognitive domains that were not associated with cannabis use after controlling for familial factors in the current study, such as cognitive flexibility (Trail Making) and working memory (digit span). Thus, cognitive deficits linked to adolescent cannabis use, such as cognitive flexibility and working memory, may precede use (e.g., via genetic propensity or stressful environment), whereas effects on learning and delayed memory may be the result of moderate-to-heavy cannabis exposure. It must be noted, however, that effects on verbal memory (CVLT) were found only at Wave 2 (emerging adulthood) and not at Wave 1 (adolescence). These findings could indicate effects of prolonged exposure that manifest in emerging adulthood (e.g., starting in adolescence and continuing for several years), as well as the potential vulnerability of the still-developing brain to moderate-heavy cannabis use in emerging adulthood.
The cannabis available at the time when data were collected should be considered when interpreting the current findings. At Wave 1 (2001–2006), when no U.S. states had legalized recreational cannabis, the average THC potency of confiscated cannabis was 6.1–8.8%.11 It is unclear whether the current findings generalize to adolescent use of high-potency oils/waxes (80–95% THC) available on state-regulated markets. For example, how might weekly use of 80% THC concentrates affect the developing brain, relative to weekly use of 8% THC flower? Recent empirical studies suggest that higher potency products have more adverse, mental health effects; however, these studies have not rigorously controlled for familial confounds, which may very well explain some effects of high-potency cannabis.9
Although our findings suggest that familial factors shared by siblings do not explain the link between moderate-to-heavy cannabis use and learning and delayed memory, it is still possible that differences in verbal memory preceded cannabis use. However, contrary to this possibility, recent longitudinal work suggests that earlier episodic memory does not predict subsequent changes in cannabis use.38 Additionally, given that participants here used, on average, two days per week, some observed effects could have been the residual effects of recent use. Thus, additional work is needed to examine the residual effects of adolescent cannabis use. Finally, while a majority of discordant twin studies have found little evidence that cannabis use causes poorer cognitive functioning,4–6 this study was the first among family-controlled studies to assess verbal memory or to examine a sample primarily comprised of moderate-to-heavy cannabis users. However, the current study design only controls familial factors shared by siblings, and siblings may differ on important confounds that underlie the observed effects of cannabis use on poorer verbal memory.
Future Directions
Given the contrast between findings from the current study and other family-controlled studies examining cannabis use, this study points to a clear need for additional family-controlled studies of samples with moderate-to-heavy cannabis use. Cannabis use lies on a continuum, and previous family-controlled studies have used samples primarily comprising individuals who exhibit few externalizing problems and use cannabis infrequently. From these studies, many have concluded that cannabis use does not have direct, adverse effects on cognitive functioning. These studies do not, however, inform how moderate-to-heavy cannabis exposure affects cognitive functioning. Further, sample selection is known to affect how drug-related problems relate to each other,39 and the observed relationships between drug use and its consequences may also vary based on sample selection criteria (e.g., characteristics self-selecting into studies).
Future studies may also include polygenic scores in multilevel approaches to control for important risk factors, such as genetic factors that may underlie sibling differences in cognitive functioning. Importantly, however, the appropriate summary statistics (from large-scale genome-wide association studies [GWAS]) are not yet available to infer valid polygenic scores in individuals with non-European ancestry. For example, polygenic scores were originally included in the current study but were removed, given the ethnic diversity of participants and concerns about under- or overestimating the variance explained in cognitive measures by polygenic scores.40 Clearly, GWAS studies are needed on more diverse samples to help move this and other public health research forward.
NIDA’s Adolescent Brain Cognitive Development (ABCD) study is also intended to address many of the aforementioned pitfalls by recruiting a large national sample of youth before initiating drug use (Wave 1, ages 9–10), oversampling underrepresented segments of the U.S. population (e.g., African-Americans, children in rural/non-urban school districts), and including a subsample of twins.41 The ABCD study will be an invaluable resource for examining the risk factors and consequences of drug use in a population-based sample. In addition, the ABCD study included a brief screening to identify and recruit children at–risk for early cannabis use to ensure that a portion of the participants was at-risk youth. This screener includes items about child externalizing behavior (e.g., property destruction, stealing, lying/cheating, and disobedience at school) and parental smoking.42 These factors are linked to early cannabis exposure among youth, which is associated with numerous adverse outcomes (e.g., poorer educational achievement, substance use disorders) that are less prominent in population-based samples.43,44 Thus, including diverse and at-risk samples of youth, such those in the current study and those recruited for the ABCD study, may help elucidate the range of consequences of cannabis use on the developing brain.
Clinical and Public Health Implications
Due to changes in the legality of recreational and medical cannabis and widespread access in many states, valid empirical data must be available to inform policy and public health decisions, including how cannabis use may affect the developing brain. The current findings, along with the broader literature, suggest that there may be incentive for delaying cannabis use and that adverse effects may increase with the intensity of use. By extension, the current findings suggest that legal-market, high-potency products may be particularly harmful, especially to the developing brain. Finally, it is critical to understand whether specific individuals (e.g., at-risk youth) are particularly susceptible to the adverse effects of cannabis use, thereby informing how to effectively target prevention efforts.
Summary
The current study used a quasi-experimental, family-controlled design to examine the effects of cannabis use in a high-risk sample of adolescent sibling pairs. In contrast to previous cotwin-controlled designs, findings suggest that an earlier onset of regular use and persistent use may adversely affect cognitive functioning. Thus, recruiting high-risk genotyped samples for family-controlled studies may be a critical step forward for understanding the potential effects of drug use.
Supplementary Material
Acknowledgments
We thank the participants of the clinical studies of the Center for the Genetics and Treatment of Antisocial Drug Dependence, as well as the many research staff who made this study possible. We greatly appreciate reviewer and editor feedback that highlighted important limitations to polygenic scores when based on genome-wide association studies and applied to target samples with different ancestries. Further, we also thank Matthew Keller for his helpful input regarding the biases of polygenic scores in these instances.
Financial Support: This research was supported by the National Institutes of Health Grants AA026635 (JME), DA032555 (CJH), DA035804 (CJH), DA042755 (CJH), and DA017637 (JMR).
Footnotes
Declarations of Interest: None
References
- 1.Fergusson DM, Horwood LJ, Swain-Campbell N. Cannabis use and psychosocial adjustment in adolescence and young adulthood. Addiction. 2002;97(9):1123–1135. [DOI] [PubMed] [Google Scholar]
- 2.Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: A review. Addiction. 2000;95(11):1621–1630. [DOI] [PubMed] [Google Scholar]
- 3.Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci. 2012;109(40):E2657–E2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson NJ, Isen JD, Khoddam R, et al. Impact of adolescent marijuana use on intelligence: Results from two longitudinal twin studies. Proc Natl Acad Sci. 2016;113(5):E500–E508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meier MH, Caspi A, Danese A, et al. Associations between adolescent cannabis use and neuropsychological decline: A longitudinal co-twin control study. Addiction. Published online 2017. doi: 10.1111/add.13946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross JM, Ellingson JM, Rhee SH, et al. Investigating the causal effects of cannabis use on cognitive function with a quasi-experimental co-twin design. Drug Alcohol Depend. Published online In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore W, Pedlow S, Krishnamurty P, Wolter K. National Longitudinal Survey of Youth 1997. (NLSY97; ).:257. [Google Scholar]
- 8.Miech RA, Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the Future National Results on Drug Use 1975–2017: Secondary School Students. Institute for Social Research; 2018. https://eric.ed.gov/?id=ED529133 [Google Scholar]
- 9.Di Forti M, Sallis H, Allegri F, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2013;40(6):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ElSohly MA, Ross SA, Mehmedic Z, Arafat R, Yi B, Banahan BF. Potency trends of Δ9-THC and other cannabinoids in confiscated marijuana from 1980–1997. J Forensic Sci. 2000;45(1):24–30. [PubMed] [Google Scholar]
- 11.ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995–2014): Analysis of current data in the United States. Biol Psychiatry. 2016;79(7):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huestegge L, Kunert H-J, Radach R. Long-term effects of cannabis on eye movement control in reading. Psychopharmacology (Berl). 2010;209(1):77–84. doi: 10.1007/s00213-009-1769-z [DOI] [PubMed] [Google Scholar]
- 13.Solowij N, Jones KA, Rozman ME, et al. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology (Berl). 2011;216(1):131–144. doi: 10.1007/s00213-011-2203-x [DOI] [PubMed] [Google Scholar]
- 14.Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of Onset of Marijuana Use and Executive Function. Psychol Addict Behav J Soc Psychol Addict Behav. 2012;26(3):496–506. doi: 10.1037/a0026269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontes MA, Bolla KI, Cunha PJ, et al. Cannabis use before age 15 and subsequent executive functioning. Br J Psychiatry J Ment Sci. 2011;198(6):442–447. doi: 10.1192/bjp.bp.110.077479 [DOI] [PubMed] [Google Scholar]
- 16.Solowij A, Battisti R. The chronic effects of cannabis on memory in humans: A review. Curr Drug Abuse Rev. 2008;1(1):81–98. [DOI] [PubMed] [Google Scholar]
- 17.Rubino T, Parolaro D. The impact of exposure to cannabinoids in adolescence: Insights from animal models. Biol Psychiatry. 2016;79(7):578–585. [DOI] [PubMed] [Google Scholar]
- 18.Jacobus J, Tapert SF. Effects of cannabis on the adolescent brain. Curr Pharm Des. 2014;20(13):2186–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curran VH, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Δ9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl). 2002;164(1):61–70. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez R Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychol Rev. 2007;17(3):347–361. [DOI] [PubMed] [Google Scholar]
- 21.Lubman DI, Cheetham A, Yücel M. Cannabis and adolescent brain development. Pharmacol Ther. 2015;148:1–16. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Dev Psychol. 2008;44(6):1764–1778. doi: 10.1037/a0012955 [DOI] [PubMed] [Google Scholar]
- 23.Harden KP, Tucker-Drob EM. Individual differences in the development of sensation seeking and impulsivity during adolescence: Further evidence for a dual systems model. Dev Psychol. 2011;47(3):739–746. doi: 10.1037/a0023279 [DOI] [PubMed] [Google Scholar]
- 24.Scott JC, Slomiak ST, Jones JD, Rosen AFG, Moore TM, Gur RC. Association of Cannabis With Cognitive Functioning in Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2018;75(6):585–595. doi: 10.1001/jamapsychiatry.2018.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutter M, Pickles A, Murray R, Eaves L. Testing hypotheses on specific environmental causal effects on behavior. Psychol Bull. 2001;127(3):291–324. [DOI] [PubMed] [Google Scholar]
- 26.Hayatbakhsh R, Williams GM, Bor W, Najman JM. Early childhood predictors of age of initiation to use of cannabis: A birth prospective study. Drug Alcohol Rev. 2013;32(3):232–240. [DOI] [PubMed] [Google Scholar]
- 27.Rogeberg O Correlations between cannabis use and IQ change in the Dunedin cohort are consistent with confounding from socioeconomic status. Proc Natl Acad Sci. 2013;110(11):4251–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillespie NA, Neale MC, Jacobson K, Kendler KS. Modeling the genetic and environmental association between peer group deviance and cannabis use in male twins. Addiction. 2009;104(3):420–429. doi: 10.1111/j.1360-0443.2008.02457.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehlers CL, Gizer IR, Vieten C, et al. Cannabis dependence in the San Francisco Family Study: Age of onset of use, DSM-IV symptoms, withdrawal, and heritability. Addict Behav. 2010;35(2):102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelhorn H, Hartman C, Sakai J, et al. Toward DSM-V: An item response theory analysis of the diagnostic process for DSM-IV alcohol abuse and dependence in adolescents. J Am Acad Child Adolesc Psychiatry. 2008;47(11):1329–1339. doi: 10.1097/CHI.0b013e318184ff2e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robins LN, Wing J, Wittchen HU, et al. The Composite International Diagnostic Interview: An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45(12):1069–1077. [DOI] [PubMed] [Google Scholar]
- 32.Cottler LB, Robins LN, Helzer JE. The reliability of the CIDI-SAM: A comprehensive substance abuse interview. Br J Addict. 1989;84(7):801–814. [DOI] [PubMed] [Google Scholar]
- 33.Salomonsen-Sautel S, Sakai JT, Thurstone C, Corley R, Hopfer C. Medical marijuana use among adolescents in substance abuse treatment. J Am Acad Child Adolesc Psychiatry. 2012;51(7):694–702. doi: 10.1016/j.jaac.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muthén LK, Muthén BO. Mplus User’s Guide. 7th ed. Muthén & Muthén; 1998. [Google Scholar]
- 35.van der Leeden R, Busing FMTA, Meijer E. Bootstrap methods for two-level models. In: ; 1997. [Google Scholar]
- 36.Hochberg Y A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800–802. [Google Scholar]
- 37.Neuhaus JM, McCulloch CE. Separating between-and within-cluster covariate effects by using conditional and partitioning methods. J R Stat Soc Ser B Stat Methodol. 2006;68(5):859–872. [Google Scholar]
- 38.Duperrouzel JC, Hawes SW, Lopez-Quintero C, et al. Adolescent cannabis use and its associations with decision-making and episodic memory: Preliminary results from a longitudinal study. Neuropsychology. 2019;33(5):701–710. doi: 10.1037/neu0000538 [DOI] [PubMed] [Google Scholar]
- 39.Hoffman M, Steinley D, Trull TJ, Lane SP, Wood PK, Sher KJ. The influence of sample selection on the structure of psychopathology symptom networks: An example with alcohol use disorder. J Abnorm Psychol. 10.1037/abn0000438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin AR, Gignoux CR, Walters RK, et al. Human demographic history impacts genetic risk prediction across diverse populations. Am J Hum Genet. 2017;100(4):635–649. doi: 10.1016/j.ajhg.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lisdahl KM, Sher KJ, Conway KP, et al. Adolescent brain cognitive development (ABCD) study: Overview of substance use assessment methods. Dev Cogn Neurosci. 2018;32:80–96. doi: 10.1016/j.dcn.2018.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loeber R, Clark DB, Ahonen L, FitzGerald D, Trucco EM, Zucker RA. A brief validated screen to identify boys and girls at risk for early marijuana use. Dev Cogn Neurosci. 2018;32:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agrawal A, Neale MC, Prescott CA, Kendler KS. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychol Med. 2004;34(7):1227–1237. [DOI] [PubMed] [Google Scholar]
- 44.Fergusson DM, Horwood LJ, Beautrais AL. Cannabis and educational achievement. Addiction. 2003;98(12):1681–1692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


