Abstract
Prunus serotine oil, was extracted from the seeds without shells, resulting in an oil yield of 23.41 ± 3.62%. Through GC it was shown that 52.38% of the total fatty acids present in the oil were polyunsaturated fatty acids. The fatty acids profile presented in the P. serotine oil were oleic (41.42%), linoleic (26.97%) and α-eleostearic acid (25.33%). It had a high concentration of total phenols (221 ± 15.85 mg as gallic acid equivalents/kg oil) and flavonoids (0.77 ± 0.01 mg catechin equivalents/kg oil). The antiradical activity was 31.52 ± 2.71% and 12.94 ± 0.67% of radical inhibition for colorimetric methods using ABTS [2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid)] and DPPH (2,2-diphenyl-1-picrylhydrazyl), respectively. The activity inhibition was 2.3 (ABTS) and 1.8 (DPPH) times higher, respectively, than the ones of Prunus dulcis oil. Lipid oxidation showed that at day nine, P. serotine oil has it maximum hydroperoxide production through two methods (hydroperoxide and MDA). Three oregano fractions were added (code: 642, 655 and A01) as natural antioxidants at four different concentrations (3000, 300, 30 and 3 ppm) each one, to extend its shelf life. Fraction 642 managed to extend its shelf life until day 30 (30 °C ± 2 °C), in both methodologies. The fraction 642 at 3 ppm, controls the production of hydroperoxide formation. Resulting in values of 3.65 µM equivalents of cumene hydroperoxide/kg of oil and 10.29 µM equivalents of 1,1,3,3-Tetraethoxypropane/kg of oil, decreasing by 3.2 times the peroxide formation with respect to P. serotine oil without leaving a Poliomintha longiflora fraction.
Keywords: Prunus serotine seed oil, α-Eleostearic acid, Hydroperoxide, Poliomintha longiflora fractions oils, Malondialdehyde
Introduction
Fat and polyunsaturated oils are essential to the human diet due to the established relationship between serum cholesterol level and cardiovascular diseases (CV). It is well known that regular consumption or dietary supplementation with polyunsaturated fatty acids (PUFAs) have effects on LDL cholesterol. Fats, nonetheless, also affect HDL cholesterol, and the ratio of total to HDL cholesterol is a more specific marker of CV (Mensink et al. 2003). Fatty acids improve insulin resistance and reduce systemic inflammation (Mozaffarian et al. 2010).
The α-eleostearic acid is an unusual PUFA that provides health benefits as decrease hepatic cholesterol level, and suppressing growth in cancer cells, in addition to being a chemotherapeutic agent against breast cancer (Aguerrebere et al. 2011; Sbihi et al. 2014). The beneficial effects are due mostly to its molecular structure, which contains triene conjugated bonds (c9, t11, t13-18:3) (Sbihi et al. 2014). It has been proposed as a PUFA with therapeutic potential (Aguerrebere et al. 2011). This fatty acid can be found in seed oils like bitter gourds (Momordica charantia) in a 60% concentration, white mahlab (Prunus mahaleb) with 38.32% and Tung tree nut (Aleurites fordii) at 67.7% (Sbihi et al. 2014). Nevertheless, these sources are not reported in Mexico.
Prunus serotine var. capuli is an almond widely distributed in Mexico and especially in the central region of the country. It is also known as capulin or black cherry seed. Its fruit and leaves have been used in traditional Mexican medicine since colonial times (Aguerrebere et al. 2011). Meanwhile, the seeds can be considered a forgotten byproduct that is only occasionally consumed in roasted form and salted as a snack. The seed contains protein and antioxidants, as well as being a source of PUFAs like oleic, linoleic and α-eleostearic acid (García Aguilar et al. 2015).
Despite these characteristics, these oils are very susceptible to lipid oxidation because of their polyunsaturated structure (Yamamoto et al. 2014; McClements and Decker 2000), and many factors, such as UV light, enzymes, metallic ions, and temperature, can initiate or accelerate the oxidation process. Factors that end up affecting physicochemical attributes such a like color, flavor, nutritional profile, and shelf life (McClements and Decker 2000).
In the food industry, butylhydroxyanisole (BHA), butylhydroxytoluene (BHT), tert-butylhydroquinone (TBHQ) and propyl gallate (PG) are synthetic antioxidants commonly used to prevent or delay oxidation, but their innocuousness has been questioned. Nowadays, natural antioxidants found in isolated compounds of cereals, mushrooms, herbs, citrus fruit, especially spices such as oregano, have become the alternative (Aranha and Jorge 2012).
Studies have demonstrated that the specie Poliomintha longiflora has higher antioxidant activity than α-tocopherol and is comparable to that of BHA against linoleic acid oxidation. These properties are attributed due to the presence of carvacrol, β-caryophyllene oxide, ρ-cymene, β-caryophyllene, 6-methyl-3,4-xylenol, carvacrol acetate, cyclohexanol 4- (1-methylethenyl), rosmarinic acid, protocatechuic acid, quercetin and ρ-coumaric acid, which can be found in the essential oils of the spice (Méndez Zamora et al. 2016; Herrera Rodríguez et al. 2019). The aim of this study was to evaluate the physicochemical characteristics of P. serotine seed oil extracted by manual cold press and extend its life by delaying lipid oxidation with Poliomintha longiflora fraction oil as a natural antioxidant.
Materials and methods
Prunus serotine seeds were obtained from the Xochimilco's market in Mexico City, Mexico. Prunus dulcis was purchased from a local food store in Monterrey, Nuevo Leon, Mexico. Poliomintha longiflora was collected from a mountain range where the states of Coahuila, Durango and Zacatecas convergence, under the coordinates 24.9278, − 103.178 west, 24.9278, − 103.1782 south, 25.277, − 103.1215 east and 25.2999, − 103.1124 north. Samples were stored at − 20 °C until used. Solvents: ethanol, methanol, 1-butanol, glacial acetic acid, isooctane, propanol, boron trifluoride, chloride sodium, n-heptane, potassium persulfate, ammonium thiocyanate, chloride barium, and sulfate ferrous anhydrous were analytical grade (JT Baker reagents, Mexico). The reagents such as: Folin and Ciocalteu, gallic acid, catechin hydrate, ABTS, DPPH, trolox, 1,1,3,3-Tetraethoxypropane, thiobarbituric acid, external standard, Supelco 37 mix components, tung oil and cumene hydroperoxide were purchased from Sigma-Aldrich (Sigma-Aldrich, Mexico).
Oil extraction
The seeds were cracked open with a sterilized metal squeezer and selected. One hundred grams of P. serotine seeds were placed in the feed container of the cold press (Henan Wecare Industry Co. Ltd, China) and by a simultaneous process of milling and pressing, the fraction oil was cold obtained at 30 °C. The Prunus dulcis oil extraction was carried out using the same process.
Fatty acid composition
The P. serotine oil previously obtained manually by cold pressing, as well as P. dulcis oil (control) was analyzed by gas chromatography (GC). The method was based on Mexican standard NMX-F-490-1999-NORMEX. -Food-oils-fats- (Diario Oficial de la Federación 1999). The method consists of analysis of methyl esters of fatty acids. The fatty acids were saponified and then the boron trifluoride was aggregate. In the cold sample, n-heptane was added, then mixed with chloride sodium resulting in two phases. The organic phase was separate and, then was mixed with sodium sulfate anhydrous and the separate organic phase. Both samples were dissolved at a ratio of 1:100 n-heptane, and 1 µL from each sample was injected into GC three times with a flame ionization detector (Agilent Technologies, 7890A (GC)/7693(ALS), CA, EUA). The analysis was performed using fused silica capillary column (30 m × 0.25 mm, df 0.20 μm film thickness, Agilent GC, EUA). The run was under an optimized temperature with electronic baseline compensation as follows: initial column temperature 100 °C, programmed to increase at a rate of 10 °C up 160 °C and then at 5 °C min−1 up 220 °C. This temperature was raised to 10 °C min−1. Finally, to 260 °C and held at that temperature for 5 min. Injector and detector temperatures were at 260 °C and 280 °C, respectively. Helium was used as it carries gas at flow rate of 1 mL min−1 with a split ratio of 30:1. The peaks were identified with external standards (Supelco 37 mix components; tung oil, Sigma–Aldrich, Mexico). The results were reported as the relative percentage of each component, considering that the total area under the peaks represents one hundred percent of the total elution.
Dilution solutions (Ds)
The oils samples, were diluted with ethanol to extract the antioxidant compounds and increase the miscibility with the test radicals. For total phenols and flavonoid analyses, 1 g of almond oil with 5 mL of ethanol 96% (1:5, w/v), were mixed in vortex for 4 min, followed by centrifugation at 3000 rpm for 5 min at 25 °C. For antiradical capacity analysis (ABTS and DPPH), the 1 g of sample was mixed and centrifuged under the same conditions but with 25 mL of ethanol 96% (1:25, w/v). In both cases, aqueous solutions were used for the analysis (Bail et al. 2008).
Total phenols (TP)
The phenolic content was quantified from the Ds according to Gutiérrez Avella et al. (2008) with little modifications. The volume of 100 µL of Ds was mixed into a cuvette with 600 µL of distilled water and 100 µL 1 N Folin and Ciocalteu's. After 5 min, 300 µL 20% sodium carbonate was added. The solution was mixed and allowed to stand for 90 min, protected from light. Absorbance was measured at 760 nm (Spectronic UV visible-Genesys 5, Thermo Fisher Scientific, Inc., Waltham, MA, USA) against a blank sample. Gallic acid was used as the standard (0.66–5.3 mg/kg). The TP content was expressed as gallic acid equivalents (GAE) in milligrams per kilogram of oil (mg GAE/kg oil).
Total flavonoid (TF)
The TF content was assayed through the colorimetric method, previously reported by Jahanban Esfahlan and Jamei (2012) with little modifications. The Ds (250 µL) was mixed with 1525 µL of distilled water in a test tube followed by the addition of 75 µL of NaNO2 (5% w/v) solution. After 5 min, 150 µL 10% AlCl3.6H2O (w/v) solution was mixed. Allowed to stand 5 min, 500 µL 1 M NaOH was added and mixed with vortex. Absorbance was measured against the blank at 510 nm (Spectronic UV visible-Genesys 5, Thermo Fisher Scientific, Inc., Waltham, MA, USA). Catechin hydrate was used as standard curve (0.0016 a 0.016 mg/g). The TF result was expressed as milligrams of catechin equivalents per kg of polar extract (mg CE/kg polar extract).
Antiradical activity
The antiradical activity was determined using ABTS [2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid)] according to Fernandes et al. (2013) with some modifications. The ABTS radical was prepared by mixing potassium persulfate (2.45 mM) and ABTS (7 mM) solutions (1:1, v/v), and storing it in amber flask for at least 16 h protected from light. The preparation free radical was diluted (200 µL of the radical being diluted in 10 mL of ethanol). The optical density obtained (0.700 ± 0.05), was the working solution. The volume of 100 µL of the Ds with 2000 µL of ABTS* working solution was mixed and the absorbance was measured 7 min after mixing at 734 nm (Spectronic UV visible-Genesys 5, Thermo Fisher Scientific, Inc., Waltham, MA, USA).
The antiradical capacity was also determined utilizing the DPPH (2,2-diphenyl-1-picrylhydrazyl) decoloration test, according to Moosavi Dolatabadi et al. (2015) method. A stock solution 0.1 mM of DPPH was prepared and adjusted to an optical density of 1.000 with ethanol. The volume 50 µL of Ds was mixed with 2000 µL of DPPH* and, after 30 min the absorbance was determined at 517 nm. Trolox solution was used as a standard curve (25–505 µmol) for both methods and, the results were expressed as µM Trolox equivalent/g oil (Pinheiro do Prado et al. 2009).
Poliomintha longiflora oil fractions
Poliomintha longiflora fractions were obtained by the steam entrainment and obtained by the Or-Lag Company. The Poliomintha longiflora fractions were obtained through a fractional distillation system with a distillation column equivalent to 20 theoretical plates at a pressure of 5 Torr. The distillation temperature range was 82–140 °C (Frutech International corporation, NL, Mexico). The collection period between each fraction was approximately 20 min. The fractions 642 (2.5 ×), 655 (10 ×) and natural oil (AO1) were obtained by steam entrainment. The Poliomintha longiflora fractions were analyzed using the methodology proposed by Rostro Alanís et al. (2019) with a slight modification. GC was conducted on a HP-5 MS (30 m × 0.25 mm × 0.25 μm) capillary column. The injection temperature for the GC was 200 °C. The oven was controlled at 70 °C for 5 min with the heating rate from 10 °C/min to 200 °C occurring over a period of 2 min, and finally heating from 5 °C/min to 300 °C over a 5 min period. Helium gas was used as a carrier gas at a constant flow rate of 1 mL/min, sample size being 1 μL. The parameters for MS analysis 5973 N were with an EI ion source, electron energy 70 eV, a temperature of 150 °C for quadrupoles, interface temperature 230 °C, m/z = 30–400 amu. Identification of compounds was carried out by comparing their mass spectra with those of Wiley 7 n.L library, considering a quality match > 95%.
Lipid oxidation
Thirteen amber flasks (7 mL) were filled with the P. serotine oil (cold press extracted). One exclusively contained P. serotine as control, while four of them also contained Poliomintha longiflora fraction labeled as 642. Another four flasks had a different Poliomintha longiflora fraction labeled as 655, and the last four had the Poliomintha longiflora fraction labeled as A01. The three different fractions had the same four concentrations (3000, 300, 30 and 3 ppm). These fractions were added as antioxidant. A fourteenth flask filled only with P. dulcis oil was also cold press extracted. All flasks were analyzed every 3 days at 30 °C ± 2 °C for 30 days.
The samples were monitored by the quantification of hydroperoxides formation, which are the primary oxidation products, using a method described by Shantha and Decker (1994) with minor modifications. Briefly, the oil sample (100 µL) was mixed with 1.5 mL of isooctane/2-propanol (3:1, v/v), then 2.8 mL of methanol/1-butanol (2:1, v/v), 15 µL of 3.94 M ammonium thiocyanate and 15 µL of ferrous solution (prepared by mixing 0.132 M BaCl2 and 0.144 M FeSO4; 1:1, v/v) were added. The absorbance was measured after 20 min at 510 nm (Spectronic UV visible-Genesys 5, Thermo Fisher Scientific, Inc., Waltham, MA, USA). The standard curve of cumene hydroperoxide was used (0.128–1.984 mg/kg). Hydroperoxide concentration was expressed as µmol equivalent of cumene hydroperoxide/kg oil (µM Eq CH/kg oil) (Tong et al. 2000).
Malondialdehyde (MDA) formation (a secondary oxidation product), was determined using the TBARs method described by Papastergiadis et al. (2012) with some modifications. In a centrifuge tube 220 µL of oil with 1 mL of water was aggregate, them mixed in vortex for 2 min and centrifuged (Hermle Z326, Labortechnik GmbH, Wehingen, Germany) at 5000 rpm for 5 min at 25 °C. The aqueous phase was collected and, the procedure was repeated twice. For the MDA analysis, 1 mL of the aqueous extract and 1 mL of thiobarbituric acid (TBA 46 mM in 99% glacial acetic acid) were mixed in test tubes and heated at 100 °C for 35 min. The reaction was quickly cooled. The absorbance was measured at 532 nm (Spectronic UV visible-Genesys 5, Thermo Fisher Scientific, Inc., Waltham, MA, USA) and quantified with the standard curve. The reagent 1,1,3,3-Tetraethoxypropane (TEP) was used as MDA standard (0.043–2.197 mg/kg). Results were expressed as µmol equivalent of TEP/kg oil (µM Eq TEP/kg oil). Finally, the monitoring of hydroperoxides and MDA were monitored during the storage time.
Statistical analysis
All experiments were analyzed statistically and the results expressed as the mean ± standard deviation (SD). Lipid oxidation results were analyzed through one-way analysis of variance (ANOVA). The significant differences were determined using Tukey’s test in Statgraphics centurion XVII. All analyses were carried out in triplicate. Results were accepted as statistically significant at p < 0.05.
Results and discussion
The oil yield of P. serotine extracted by cold pressed was 23.4 ± 3.6% in dry weight. Other methods report yields of 21.3% and 30.9% with supercritical CO2 and solvent, respectively (Aguerrebere et al. 2011). Results from the cold pressed method exceeds the supercritical extraction by 2%. The former is an attractive and economical method, despite solvent extraction having a greater yield.
Fatty acid composition
The fatty acid profile of P. serotine, analyzed through GC was 41.42% oleic, 26.97% linoleic, 25.33% α-eleostearic, 4.33% palmitic, 1.33% stearic and 0.08% (dry weight) linolenic. It was found that 51.88% of the total fatty acids present in the oil were polyunsaturated fatty acids. Among the fatty acids, what stands out is the presence of α-eleostearic acid in the P. serotine variety, which unlike P. dulcis (which contains 93.46% of unsaturated fatty acids) was not detected by GC. Profile fatty acids in P. serotine were similar to the ones reported by other researchers (hexane extracted) (Aguerrebere et al. 2011); 35.3% oleic, 26.7% α-eleostearic, linoleic 26.6%, palmitic 4.3%, stearic 4%, and 0.2% linolenic. The α-eleostearic acid increases the potential of the oil as a functional food or nutraceutical ingredient due to its effects against common diseases and chronic degenerative that have been reported (Sbihi et al. 2014; Aguerrebere et al. 2011). The quantity of oleic acid on Prunus serotine was 0.62:1 compared with P. dulcis. It has been reported that oleic acid reduces blood pressure, increase the burning of fat to help with weight loss, protects cells from radical damage, prevents type 2 diabetes and ulcerative colitis, generates brain myelin and reduces cardiovascular risk by reducing blood lipids, mainly cholesterol (Rustaivar et al. 2016). Similar effects have been reported by α-eleostearic, an isomer of α-linolenic acid. It has been reported that α-eleostearic acid shows antioxidant and oxygen scavenging properties; exhibits protective effects against lipid oxidative damage in plasma, reduces plasma lipid peroxidation and prevents chronic inflammatory diseases (Chattopadhyay et al. 2012; Saha and Ghosh 2012). However, in the seeds of Prunus serotine, both fatty acids (oleic and α-eleostearic acid) were found, the ratio being 1.6:1. Vidrih et al. (2009) both fatty acids having been determined in cherry seed. The ratio of fatty acids is reported to be 4:1. It was mentioned that α-eleostearic fatty acid had been studied due to their health benefits, especially, for the prevention of cardiovascular diseases and antitumoral.
Total phenols (TP)
Phenols and polyphenols are polycyclic compounds, an integral part of the human diet. Polyphenols as free radical scavenging and metal chelating agents, called antioxidants. Hence, the phenolic content in oilseed Prunus serotine obtained by cold pressing was determine. Phenolic compounds may exert their antioxidant activity directly as chain-breaking (scavenging reactive species such as hydroxyl, peroxyl and superoxide radicals), suppressing lipid peroxidation or preventing the formation of free radicals binding pro-oxidant metals, such as iron and copper and performing antimicrobial activity (Walter and Marchesan 2011; Taghvaei and Jafari 2015). These compounds are of great importance because they help to fight against diseases and prolong the stability of food products.
The content of TP was quantified using Eq. 1:
| 1 |
where was mg equivalents of gallic acid/kg polar extract.
TP content in serotine oil was 243.85 ± 0.02 mg GAE/kg oil extracted by cold press. In P. dulcis oil extracted with the solvent hexane and chloroform/methanol. Miraliakbari and Shahidi (2008) reported 124.0 ± 11.0 and 168.0 ± 15.0 mg GAE/kg oil, respectively. The relevance of the extraction P serotine oil, was not used solvents on extraction process.
Total flavonoid (TF)
Flavonoids have antioxidant and chelating properties, with which the consumer can prevent chronic diseases. Depending on the nature, extent, and position of the substituents and number of the hydroxyl groups, it can act as an antioxidant or modulator of enzyme activity. The flavonoids can be effective inhibitors of lipid peroxidation and chelate redox-active metals and the inhibitor effect depends on the degree of OH substitution on the structure (Belge Kurutas 2016).
The content of TF was quantified using Eq. 2:
| 2 |
where was mg equivalent of catechin hydrate/kg polar extract.
The TF content in P. serotine was 330 ± 0.01 mg CE/kg polar extract, which differs from other vegetable oils, such as extra virgin olive oil (destoned extra virgin olive oil: 110 ± 0.01 and extra virgin olive oil 26 ± 0.003 mg CE/kg polar extract) (Restuccia et al. 2011) having up to 12 times more TF content in P. serotine oil cold press. Compared with TF in the seeds of other tree nuts such as almonds, cashews, hazelnuts, pecans, pine nuts, pistachios and walnuts (150, 20, 120, 340, 5, 180 and 30 mg of flavonoids/kg, respectively). P. serotine contains a higher concentration of TF in the oil fraction than in the entire seed of other tree nuts (Bolling et al. 2011).
Antiradical activity
The phenols and flavonoid (soluble and insoluble in water) are molecules that frequently present in vegetable products and by-products. The oil of P. serotine seed exhibited the high antiradical effect equivalent to trolox. The value was determined using two colorimetric reactions (ABTS and DPPH) compared with P. dulcis oil. P. serotine oil had the most antiradical activity in both methods, which was also reflected in the % radical inhibition.
Using the ABTS method, P. serotine oil (17,888.89 ± 1537.34 μM Trolox equivalent/g oil, 31.52% ± 2.71% radical inhibition) was 2.36 times more antiradical when compare to P. dulcis (7555.56 ± 1131.41 μM Trolox equivalent/g oil, 11.31% ± 1.99% radical inhibition).
The percent antiradical activity was estimated using the Eq. 3:
| 3 |
where was μM Trolox equivalent/g on function of ABTS*+.
The percent radical inhibition obtained with DPPH (4,178.57 ± 217.24 μM Trolox equivalent/g oil, 12.94% ± 0.67% radical inhibition) was 1.82 times compared to P. dulcis (2297.62 ± 359.52 μM Trolox equivalent/g oil, 7.12% ± 1.11% radical inhibition).
The percent antiradical activity DPPH*+ was estimated using Eq. 4:
| 4 |
where was μM Trolox equivalent/g on function of DPPH*+
Poliomintha fractions oils
We prepared two fractions of concentrated oil (642 and 655) by fractional distillation and, then compared the effectivity antioxidant on P. serotine. Poliomintha longiflora oil A01 appeared in 13 different chemical compounds. Quantitative analysis showed hydrocarbons monoterpene at 77.65%, monoterpene oxygenated at 15.84% and sesquiterpene hydrocarbons at 5.03%. Monoterpenes and sesquiterpenes were quantified to contain 98.52% of total natural oil. With six compounds were determined in the natural oil with mayor greater concentration at 3%; trans-caryophyllene, carvacrol, γ-terpinene, α-cymene, α-terpinene and β-myrcene. The most abundant hydrocarbon monoterpenes were; 39.13% o-cymene, 22.34% γ-terpinene, 5.5% β-mircene and 5.57% α-terpinene. Similar relative abundance of hydrocarbon monoterpenes has been reported by Herrera Rodríguez et al. (2019) and Cid Pérez et al. (2019). However, the relative abundance of hydrocarbon monoterpenes reported was lower (23.38–29.05%). Evidently, abundance of compounds depends on location, harvest season and oil extraction process. Oregano essential oil has been used as a potential antioxidant and, effective antimicrobial against pathogenic microorganisms (Herrera Rodríguez et al. 2019). Notwithstanding, the antioxidant effect depends on composition and diverse monoterpenes, oxygenate and hydrocarbon compounds on natural oil A01 (Table 1).
Table 1.
Organic compounds determined on 642 (2.5 ×) and 655 (10 ×) and natural oil Poliomintha longiflora
| Compound | BP (°C)a | 642 | 655a | AO1a |
|---|---|---|---|---|
| % Peak area | ||||
| α-Thujene | 150–152 | 1.74 | ||
| α-Pinene | 156 | 1.07 | ||
| β-Myrcene | 166–168 | 5.50 | ||
| Phellandrene | 172 | 0.72 | ||
| α-Terpinene | 174 | 5.57 | ||
| o-Cymene | 174 | 11.45 | 0.97 | 39.13 |
| d-Limonene | 175 | 1.58 | ||
| γ-Terpinene | 181–183 | 26.76 | 0.94 | 22.34 |
| Thymol | 232 | 2.23 | 3.77 | 1.71 |
| 1,8-Cineole | 177 | 1.53 | ||
| Carvacrol | 237–238 | 38.98 | 64.31 | 12.60 |
| Trans-caryophyllene | 268 | 9.80 | 13.78 | 3.47 |
| α-Humelene | 276 | 8.36 | 1.56 | |
| Monoterpene hydrocarbons | 38.21 | 1.91 | 77.65 | |
| Monoterpene oxygenated | 41.21 | 68.08 | 15.84 | |
| Sesquiterpene hydrocarbons | 9.80 | 22.14 | 5.03 | |
| Total identified components | 89.22 | 92.13 | 98.52 | |
Monoterpenes, oxygenated and sesquiterpenes compounds with 99.9% coincidence are mentioned. PB was consulted on https://pubchem.ncbi.nlm.nih.gov/
aRostro Alanís et al. (2019)
We prepared two concentrated fractions, 642 (2.5 ×) and 655 (10 ×), from the Poliomintha longiflora oil, in order to estimate the relative concentration of components and their antioxidant effects. Fraction 642 was enriched, maintaining the following average values of components on average; 11.5% hydrocarbons monoterpenes, 48.8% oxygenated monoterpenes and 9.8% sesquiterpenes. The concentrated fraction 655, reduced the concentration of hydrocarbon monoterpenes, almost down to 2% (o-cymene and γ-terpinene), while the concentration of oxygenated compounds increased to 68%, especially in thymol and carvacrol and sesquiterpenes increased to 22%. That is to say, 10 × concentration, changes the polarity of components (Table 1), and possibility the antioxidant effects. The concentration, types terpenes and oxygenated compounds were separated by fractional distillation using boiling point and pressure for estimate the antioxidant effect. The A01 oil and fractions 642 and 655, were used to prolong the storage time of P. serotine oil.
Lipid oxidation
Hydroperoxide and their breakdown products leads to the formation of off-flavors, reduce bioactive compounds, decrease quality and affect safety (Bakkali et al. 2008). The results presented demonstrate the inhibition of hydroperoxide formation and, to some extent the formation of malondialdehyde.
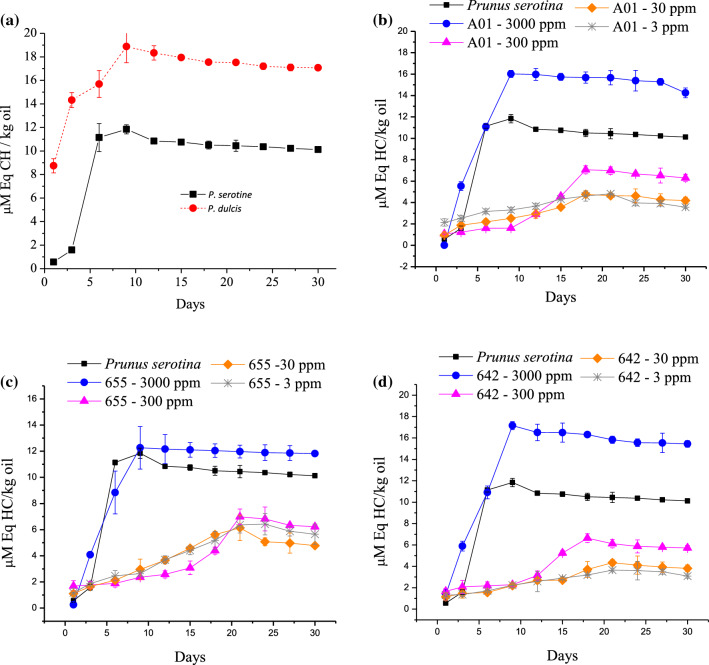
The oxidation of P. serotine oil obtained by cold pressing was observed over a period of 30 days. The concentration of hydroperoxides was progressive increase until day 9 of storage at 30 °C, when of 3000 ppm of anyone P. longiflora concentrate in P. serotine oil was added. The same effect was observed without antioxidant concentrate. Was observed maximum hydroperoxides 11.00 ± 0.23 μM and 19.00 ± 0.51 μM/kg in P. serotine and P. dulcis, respectively was observed (Fig. 1a). On the other hand, production time of hydroperoxides was displaced in six days for P. dulcis. After the maximum production of hydroperoxides, the values were nearly constant.
Fig. 1.
a Hydroperoxide formation on Prunus serotine and Prunus dulcis oils obtained by cold pressing. b, c Hydroperoxide formation on serotine oil obtained by cold pressing with Poliomintha longiflora fractions (A01, 655 and 642) at four concentrations (3000, 300, 30 and 3 ppm)
The unsaturated fatty acids present in P. serotine oil, were sensitive to the oxidative processes. Tsuzuki et al. (2004) mentioned that α-eleostearic acid has a faster rate of oxidation than linoleic acid, even more that conjugated linoleic acid, linoleic and linolenic acids. However, the relative abundance of unsaturated fatty acids with respect to saturated fatty acids was 13.58/1, compared to 11.77/1, P. dulcis and P. serotine, respectively. It is known that the formation of hydroperoxides and MDA in food oil can be inhibited by using an antioxidant of natural origin.
The oil of P. serotine can be a product of great interest, due to its anti-carcinogenic properties. The same concentrations of initial fatty acids must be maintained in the oil. Three Polimintha longiflora oil fractions were added (655, A01 and 642) at four concentrations (3000, 300, 30 and 3 ppm) respectively, in P. serotine oil, to extend shelf life. The hydroperoxides had a constant increase until day 9, despite aggregate antioxidants (655, A01 and 642) even at different concentrations, especially at 3000 ppm. When 3000 ppm was aggregated, the oxidation was higher than the control. The maximum oxidation, in increasing order were: 12.26 ± 1.63 µM Eq CH/kg oil, 16.02 ± 0.33 µM Eq CH/kg oil and 17.16 ± 1.37 µM Eq CH/kg oil, respectively. The values shown in Fig. 1b–d were higher than in the control sample (P. serotine) 11.84 ± 2.45 µM Eq CH/kg oil. The value of hydroperoxide without aggregate fraction in P. dulcis was higher (18.87 ± 1.63 µM Eq CH/kg oil) than in all samples with antioxidant fractions (Fig. 1a). Using high concentrations of antioxidants in P. serotine oil, promotes oxidation with effects similar to P. serotine at 30 °C. After the prolonged increase, the hydroperoxides values, maintained the maximum concentration during the monitoring.
The antioxidant reduction fractions of Poliomintha longiflora in P. serotine oil cold pressing at one order of magnitude (3000–300 ppm), reduces the hydroperoxide rate between 2.70, 2.26 and 1.90 times for 642, A01 and 655, respectively. By reducing the amount fractions to two orders of magnitude (3000–30 ppm), the hydroperoxide rate was reduced at 4, 3.4 and 2 times using 642, A01 and 655. By adding antioxidant fractions, minor at three orders of magnitude with respect to 3000 ppm, the rate of hydroperoxide formation was reduced to 5, 4 and 2.5 times using 642, A01 and 655. We suggest that 3 ppm of the fraction 642, efficiently reduces the rate of hydroperoxides. The hydroperoxide formation of cold pressed P. serotine during a period of 30 days at 30 °C was 2.5 µM Eq CH/kg oil. The value obtained using 642, was 3.3 times lower than the amount of hydroperoxide with respect to the oil of P. serotine without treatment.
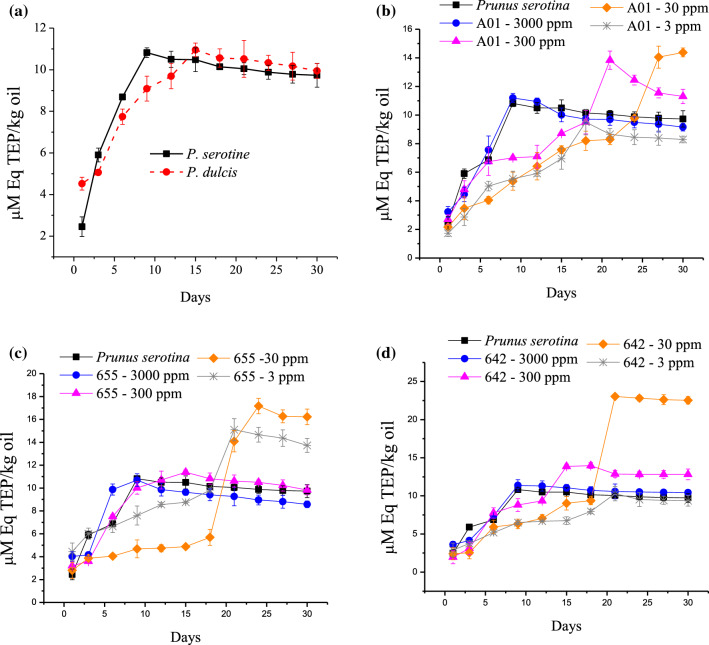
Malondialdehyde was used for the estimation of oxidation of P. serotine oil by reactive oxygen species. The concentration of malondialdehyde in comparison of aldehydes fatty acid oxidation products has been controversial, mainly with respect to health (Gentile et al. 2017). It has been suggested that MDA in food and edible oils has harmful effects on health.
Even so, we must emphasize that the fractions added to the oil of P. serotine, do not interfere efficiently with the formation of MDA in all cases. The balance between pro-oxidant and antioxidant is important to avoid the oxidation of oils (Shahidi and Zhong 2010). Excess antioxidant fractions do not control the formation of MDA and low concentration partially reduces the quantity of MDA. The maximum development of MDA in P. serotine occurred in 9 days. For P. dulcis, on the other hand, the development of MDA was similar, but occurred during the first 15 days of monitoring. The malondialdehyde concentration was 10.82 ± 0.15 µM Eq TEP/kg oil and 10.95 ± 0.24 µM Eq TEP/kg oil (Fig. 2a), respectively. The oxidative propagation in P. serotine oil, using 3000 and 300 ppm of 655 was similar to the oil without the addition of fraction, during the MDA monitoring. The concentration of 30 and 3 ppm of the fraction 655, partially reduced the formation of MDA until day 18. Afterwards, the concentration of MDA increased rapidly, between 41 and 66.8% more than the P. serotine without fraction 655, during MDA analysis (Fig. 2c).
Fig. 2.
a Malondialdehyde formation on Prunus serotine and Prunus dulcis oils obtained by cold pressing. b, c Malondialdehyde formation on serotine oil obtained by cold pressing with Poliomintha longiflora fractions (A01, 655 and 642) at four concentrations (3000, 300, 30 and 3 ppm)
The oil obtained by steam entrainment (A01) at 3000 ppm, aggregate to oil P. serotine did not reduce formation of MDA. The increase of MDA was similar to that in oil P. serotine cold pressing without A01. The values were 10.82 µM Eq TEP/kg oil as a maximum MDA concentration, and 9.12 µM Eq TEP/kg oil at time of monitoring. The oil A01 (300 and 30) slightly prolongs the stability of the oil of P. serotine until some point between day 18 and day 24, with respect to the control sample. However, the MDA was higher when compared to the oil sample of P. serotine. The increase in MDA was 16.1% and 47.8% respectively. The minimum concentration of A01 (3 ppm.) appears to partially reduce the production of MDA (by 17.3%) during the P. serotine oil stability test, compared to the other concentrations used (Fig. 2b).
The development of MDA on the sample with 642 fraction (3000 ppm and 300 ppm) was similar to that observed with MDA monitoring without treatment. Saying that, the addition of fraction 642 in concentrations of 30 ppm and 3 ppm, reduces the concentration of MDA between 16 and 55% until day 15, when compared with the oil of P. serotine without fraction 642 (Fig. 2d). During the following 15 days of analysis, the development of MDA in the combination of 30 ppm of fraction 642 and P. serotine oil, increases the concentration of MDA to 2.3 times that of its former concentration in spite of keeping the oil under conditions of 30 °C in well-sealed amber containers. It is evident that the use of antioxidant fractions manages to reduce the production effect of hydroperoxides, but not the reaction of MDA formation.
Poliomintha longiflora fractions at their highest concentrations, showed high values of hydroperoxides formations in a shorter period of time in both analyses. Meanwhile, fractions with the lowest concentrations reduced hydroperoxide. MDA formation was inhibited partially using 30 ppm fraction 655.
Conclusion
The oil of P. serotine is a source of polyunsaturated fatty acid, especially alfa-eleostearic, an isomer of linoleic acid. Nonetheless, the natural oil is highly sensitive to process oxidation, diminishing the storage time due to the counterproductive effects of an unpleasant taste. The P. serotine oil must be consumed fresh to take advantage of its benefits.
The addition of antioxidant could retard oxidation of P. serotine of the oil, but should be regulated since high levels can have the opposite effect (≥ 3000 ppm) and cause a pro-oxidation. P. serotine oil with Poliomintha longiflora fractions as an antioxidant, at a concentration of 3 ppm controls hydroperoxides production. It was observed in particular that the use of fraction 642 reduces the primary reaction, but not the secondary one.
Acknowledgements
The authors would like to thank the “Consejo Nacional de Ciencia y Tecnología” (CONACyT) of Mexico for the partial financing of this project through the Grant 61120, also through the project Problemas Nacionales-2015-01-1470 and the Grant CONACyT complementary support for consolidation of SNI -119097.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aguerrebere IA, Molina AR, Oomah BD, Drover JCG. Characteristics of Prunus serotina seed oil. Food Chem. 2011;124:983–990. doi: 10.1016/j.foodchem.2010.07.040. [DOI] [Google Scholar]
- Aranha CPM, Jorge N. Antioxidant potential of oregano extract (Origanum vulgare L.) Br Food J. 2012;114:954–965. doi: 10.1108/00070701211241554. [DOI] [Google Scholar]
- Bail S, Stuebiger G, Krist S, Unterweger H, Buchbauer G. Characterisation of various grape seed oils by volatile compounds, triacylglycerol composition, total phenols and antioxidant capacity. Food Chem. 2008;108:1122–1132. doi: 10.1016/j.foodchem.2007.11.063. [DOI] [PubMed] [Google Scholar]
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils—a review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Belge Kurutas E. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2016;15:71. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling BW, Oliver Chen CY, McKay DL, Blumberg JB. Tree nut phytochemicals: composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr Res Rev. 2011;24:244–275. doi: 10.1017/S095442241100014X. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay K, Maity M, Banerjee S, Chattopadhyay B. Ameliorative role of dietary supplemented conjugated linolenic acid against nicotine-induced toxicity in rats. Pharm Anal Acta. 2012;3:8. doi: 10.4172/2153-2435.1000182. [DOI] [Google Scholar]
- Cid Pérez T, Ávila Sosa R, Ochoa Velasco CE, Rivera Chavira BE, Nevárez Moorillón GV. Antioxidant and antimicrobial activity of Mexican oregano (Poliomintha longiflora) essential oil, hydrosol and extracts from waste solid residues. Plants. 2019;8:22. doi: 10.3390/plants8010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diario Oficial de la Federación (1999) NMX-F-490-1999-NORMEX Alimentos. Aceites y grasas. Determinación de la composición de ácidos grasos a partir de C6 por cromatografía de gases. IOP Publishing Diario Oficial de la Federación. https://www.colpos.mx/bancodenormas/nmexicanas/NMX-F-490-1987.PDF. Accessed 24 Oct 2016
- Fernandes L, Casal S, Cruz R, Pereira JA, Ramalhosa E. Seed oils of ten traditional Portuguese grape varieties with interesting chemical and antioxidant properties. Food Res Int. 2013;50:161–166. doi: 10.1016/j.foodres.2012.09.039. [DOI] [Google Scholar]
- García Aguilar L, Rojas Molina A, Ibarra Alvarado C, Rojas Molina JI, Vázquez Landaverde PA, Luna Vázquez FJ, Zavala Sánchez MA. Nutritional value and volatile compounds of black cherry (Prunus serotina) seeds. Molecules. 2015;20:3479–3495. doi: 10.3390/molecules20023479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile F, Arcaro A, Pizzimenti S, Daga M, Cetrangolo GP, Dianzani C, Lepore A, Graf M, Ames PRJ, Barrera G. DNA damage by lipid peroxidation products: implications in cancer, inflammation and autoimmunity. AIMS Genet. 2017;4:103–137. doi: 10.3934/genet.2017.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez Avella DM, García Ortiz CA, Mendoza Cisneros A (2008) Medición de Fenoles y Actividad Antioxidante en Malezas Usadas para Alimentación Animal. IOP Publishing CENAM. https://www.cenam.mx/simposio2008/sm_2008/memorias/M2/SM2008-M220-1108.pdf. Accessed 1 Nov 2016
- Herrera Rodríguez SE, López Rivera RJ, García Márquez E, Estarrón Espinosa M, Espinosa Andrews H. Mexican oregano (Lippia graveolens) essential oil-in-water emulsions: impact of emulsifier type on the antifungal activity of Candida albicans. Food Sci Biotechnol. 2019;28:441–448. doi: 10.1007/s10068-018-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanban Esfahlan A, Jamei R. Properties of biological activity of ten wild almond (Prunus amygdalus L.) species. Turk J Biol. 2012;36:201–209. doi: 10.3906/biy-1101-174. [DOI] [Google Scholar]
- McClements D, Decker E. Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. J Food Sci. 2000;65:1270–1282. doi: 10.1111/j.1365-2621.2000.tb10596.x. [DOI] [Google Scholar]
- Méndez Zamora G, Durán Meléndez LA, Aquino López JL, Santellano Estrada E, Silva Vázquez R (2016) Effects of oregano oil (Polimintha longiflora Gray) on the productivity and quality of rabbit meat. IOP Publishing Ecosistemas recur agropecuarios. https://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-90282016000200259&lng=es&nrm=iso. Accessed 20 Nov 2018
- Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- Miraliakbari H, Shahidi F. Antioxidant activity of minor components of tree nut oils. Food Chem. 2008;111:421–427. doi: 10.1016/j.foodchem.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Moosavi Dolatabadi KS, Dehghan G, Hosseini S, Jahanban Esfahlan A (2015) Effect of five year storage on total phenolic content and antioxidant capacity of almond (Amygdalus communis L.) hull and shell from different genotypes. IOP Publishing Avicenna J Phytomed. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4352530/. Accessed 15 Dec 2016 [PMC free article] [PubMed]
- Mozaffarian D, Micha R, Wallace S. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLOS Med. 2010 doi: 10.1371/journal.pmed.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papastergiadis A, Mubiru E, Van Langenhove H, De Meulenaer B. Malondialdehyde measurement in oxidized foods: evaluation of the spectrophotometric thiobarbituric acid reactive substances (TBARS) test in various foods. J Agric Food Chem. 2012;60:9589–9594. doi: 10.1021/jf302451c. [DOI] [PubMed] [Google Scholar]
- Pinheiro do Prado AC, Monalise Aragão A, Fett R, Block JM. Antioxidant properties of pecan nut [Carya illinoinensis (Wangenh.) C. Koch] shell infusion. Grasas Aceites. 2009;60:330–335. doi: 10.3989/gya.107708. [DOI] [Google Scholar]
- Restuccia D, Spizzirri UG, Chiricosta S, Puoci F, Altimari I, Picci N (2011) Antioxidant properties of extra virgin olive oil from cerasuola cv olive fruit: effect of stone removal. IOP Publishing Researchgate. https://www.researchgate.net/publication/287589432. Accessed 14 Mar 2017
- Rostro Alanís MJ, Báez González J, Torres Álvarez C, Parra Saldívar R, José Rodriguez Rodriguez J, Castillo S. Chemical composition and biological activities of oregano essential oil and its fractions obtained by vacuum distillation. Molecules. 2019;24:1904. doi: 10.3390/molecules24101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustaivar A, Samiee K, Shahbazi S (2016) Identification of omega-3, 6 and 9 fatty acids composition and lipid content from muscle tissue of Ophionereis dubia (y-striped brittle star) in Qeshm island of the Persian gulf. IOP Publishing EJPMR. https://www.ejpmr.com/admin/assets/article_issue/1477895562.pdf. Accessed 22 Oct 2018
- Saha S, Ghosh M. Antioxidant and anti-inflammatory effect of conjugated linolenic acid isomers against streptozotocin-induced diabetes. Br J Nutr. 2012;108:974–983. doi: 10.1017/S0007114511006325. [DOI] [PubMed] [Google Scholar]
- Sbihi HM, Nehdi IA, Al Resayes SI. Characterization of white mahlab (Prunus mahaleb L.) seed oil: a rich source of α-eleostearic acid. J Food Sci. 2014;79:795–801. doi: 10.1111/1750-3841.12467. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Zhong Y. Lipid oxidation and improving the oxidative stability. Chem Soc Rev. 2010;39:4067–4079. doi: 10.1039/b922183m. [DOI] [PubMed] [Google Scholar]
- Shantha NC, Decker EA. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J AOAC Int. 1994;77:421–424. doi: 10.1093/jaoac/77.2.421. [DOI] [PubMed] [Google Scholar]
- Taghvaei M, Jafari SM. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J Food Sci Technol. 2015;55:1272–1282. doi: 10.1007/s13197-013-1080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Sasaki S, McClements DJ, Decker E. Antioxidant activity of whey in a salmon oil emulsion. J Food Sci. 2000;65:1325–1329. doi: 10.1111/j.1365-2621.2000.tb10606.x. [DOI] [Google Scholar]
- Tsuzuki T, Igarashi M, Iwata T, Yamauchi-Sato Y, Yamamoto T, Ogita K, Suzuki T, Miyazawa T. Oxidation rate of conjugated linoleic acid and conjugated linolenic acid is slowed by triacylglycerol esterification and α-tocopherol. Lipids. 2004;39:475–480. doi: 10.1007/s11745-004-1253-z. [DOI] [PubMed] [Google Scholar]
- Vidrih R, Hribar J, Sekse L. Cherry seeds as a source of nutritionally important fatty acids. Acta Hortic. 2009;1020:165–172. doi: 10.17660/ActaHortic.2014.1020.23. [DOI] [Google Scholar]
- Walter M, Marchesan E. Phenolic compounds and antioxidant activity of rice. Braz Arch Biol Technol. 2011;54:371–377. doi: 10.1590/S1516-89132011000200020. [DOI] [Google Scholar]
- Yamamoto Y, Imori Y, Hara S. Oxidation behavior of triacylglycerol containing conjugated linolenic acids in sn-1 (3) or sn-2 position. J Oleo Sci. 2014;63:31–37. doi: 10.5650/jos.ess13129. [DOI] [PubMed] [Google Scholar]