Abstract
Pseudoaneurysm (PSA) or false aneurysm is a vascular lesion resulting from a focal and incomplete rupture of the arterial wall (intimate and/or elastic lamina), that allows blood to escape into the arterial wall; this small contained break causes a contained collection of blood and the creation of a “new” less resistant vessel wall, consisting of adventitia and perivascular tissues. Intrasplenic pseudoaneurysms are rare and more frequently recognize traumatic origin, sometimes are also unexpected lesions due to non-recent trauma. In contrast, non-traumatic intrasplenic pseudoaneurysms are rare complications usually due to splenic infarction, infiltration by malignant systemic disorders, infectious process, chronic pancreatitis, and arteritis. Both traumatic and non-traumatic PSA are potentially life threatening, known to cause spontaneous rupture of the spleen with massive hemoperitoneum. Contrast-enhanced CT is the gold standard technique to detect splenic PSA; however, it is important to know how to recognize it also with other imaging methods such as with ultrasound (US) and contrast-enhanced ultrasound (CEUS). US and CEUS can be often the first-line diagnostic techniques and allow to detect these lesions; they are also very useful in the follow-up. Our case report can be a reminder of the utility of the US and CEUS in detecting splenic pseudoaneurysms, which are potentially a life-threatening complication; we also recall the semiotics of these lesions with baseline ultrasound (US), color Doppler US and contrast-enhanced ultrasound (CEUS). Then, we highlight the role of contrast-enhanced CT in confirming the diagnosis and we report about the diagnostic and therapeutic value of angiography. We have to think about the possibility of a pseudoaneurysm even in the absence of a recent trauma, associated with other conditions such as a lymphoproliferative disease.
Keywords: Ultrasound, Contrast-enhanced ultrasound, CEUS, Emergency radiology, Splenic pseudoaneurysms, Spleen embolization
Introduction
Different from a true aneurysm that develops when all three layers of arterial wall dilate, pseudoaneurysm or false aneurysm involves a focal and incomplete disruption of the arterial wall (intima and/or elastic lamina). In this case, blood flow passes through the arterial wall; such contained rupture causes the creation of a “new” vessel wall consisting of the adventitia and the perivascular tissues [1].
These lesions can originate following a traumatic injury [2–6] and in the absence of trauma can be caused by neoplastic diseases, inflammatory pathologies, drugs, etc.; the mortality rate is high [7–9].
Contrast-enhanced computed tomography (CECT) exam is the gold standard to detect vascular lesions and their effects on the surrounding parenchyma, in case of parenchymal organs injuries [1]. Moreover, it is important to know how to recognize them also with other imaging methods such as with ultrasound (US), color Doppler (CD) ultrasound and contrast-enhanced ultrasound (CEUS). US, especially if supplemented with the CD evaluation, and CEUS have a valid semeiotic suggestive for this type of lesions. These techniques are mainly useful in the pediatric age [10–14].
Case report
We present a case of an eighteen-year-old male patient who came to the emergency department with 20 days history of fever and weight loss with altered blood tests, without previous medical history of pathology and without history of traumatic events. Because of the symptoms and abnormalities in the blood tests, the patient was submitted to an ultrasound (US) exam for the evaluation of the spleen in the suspicion of a lymphoproliferative disease.
At the time of the US evaluation, the patient had pale skin, was sweaty and had a fever of 38.5 °C.
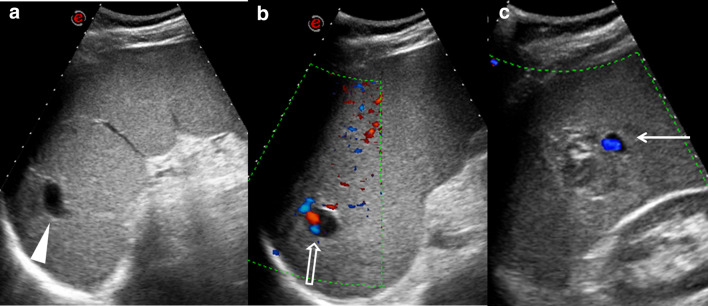
The US exam (Fig. 1a), performed with a convex probe, showed the condition of splenomegaly (long diameter 19 cm); an accessory spleen was seen at the lower pole of the spleen. The splenic echostructure was homogeneous, except for the presence in the parenchyma of two anechoic, rounded areas, about the size of 25 mm, located in the upper and in the middle zone of the spleen. These lesions did not accentuate posteriorly and no distinct walls were noted in association with the lesions. Due to the lack of these two characteristics, they did not look like cystic formations. There was no free fluid around the spleen.
Fig. 1.
a Ultrasound shows the condition of splenomegaly; at the upper pole a rounded anechoic area is present (arrowhead). b Color Doppler ultrasound demonstrates the presence of blood flow signals within the upper pole anechoic lesion (empty arrow) c Another hypoechoic lesion is shown at the middle of the spleen; in this case vascular flow is also appreciable (arrow)
A color Doppler US evaluation of the spleen showed flow signal in these anechoic areas (Fig. 1b, c).
For this reason, suspecting the presence of contained vascular lesions, precisely pseudoaneurysms, CEUS exam was performed. Before performing the CEUS, the patient was informed about the examination and agreed to signing the informed consent.
From a peripheral venous access, a single infusion of 2.4 ml of contrast agent SonoVue (Bracco Imaging S.p.A., Milan, Italy) was applied followed by 5 ml of the physiological saline solution.
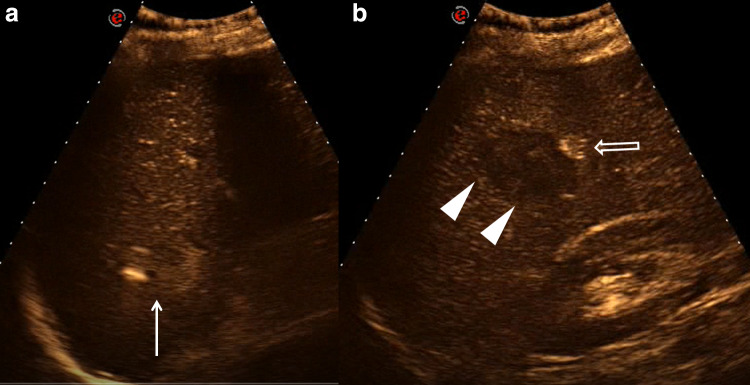
CEUS evaluation (Fig. 2) showed poorly enhanced spleen parenchyma except in the two anechoic visualized areas in which the contrast medium was collected. These images confirmed the suspicion of vascular contained lesions.
Fig. 2.
a–b CEUS shows the presence of enhanced area both in the lesion at the upper pole (arrow) and in the lesion in the middle of the spleen (empty arrow); in this lesion there is also an inhomogeneous hypoechoic area, without enhancement, suggestive of the clot (arrowheads)
For definitive confirmation, the patient was submitted to a contrast-enhanced CT.
Before examination, we again asked the patient if he had any trauma and he confirmed he had not had any recent trauma but reported that he practiced, up to about a month before, contact sport: kickboxing.
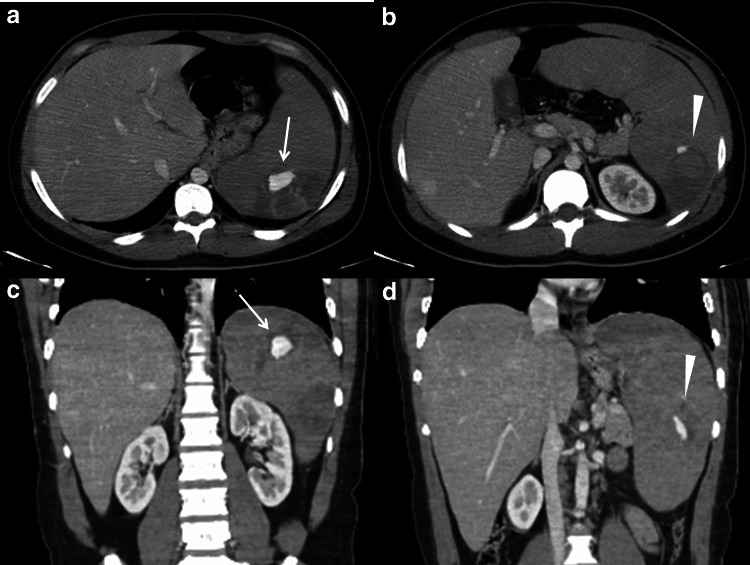
The CECT scan (Fig. 3) confirmed all the findings showed in the previous exams: the condition of splenomegaly and the presence of two contained vascular lesions, that enhanced same to the aorta, suggestive of pseudoaneurysms.
Fig. 3.
Contrast-enhanced CT. a–b axial scans and c–d coronal reconstructions confirm the splenomegaly, and the two contained vascular lesions, located in the upper pole (arrows in a and c) and in the middle of the spleen (arrowheads in b and d). These are pseudoaneurysms and enhance same to the aorta
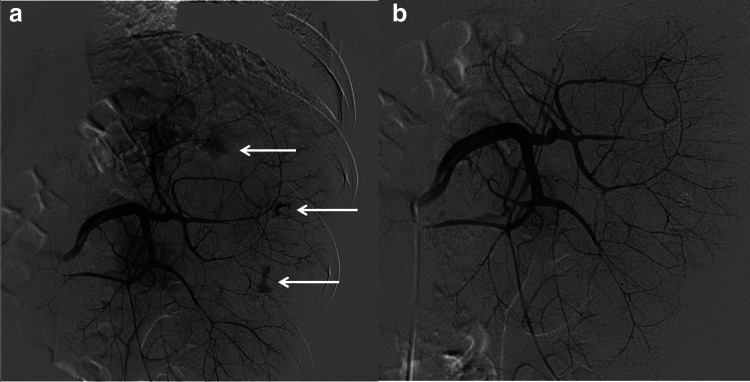
Due to the high risk of open splenectomy, caused by the underlying hematologic disease and the coagulation disorders, we decided to treat the patient with endovascular techniques according to the recent trauma guidelines. Therefore, a superselective angiography of splenic artery was performed, followed by selective embolization of the two pseudoaneurysms (Fig. 4).
Fig. 4.
Superselective angiography of splenic artery. a Angiographic study of the spleen detects three small pseudoaneurysms (arrows). b After selective embolization with cyano-acrylate, there are no residual vascular lesions
The right common femoral artery was accessed under US guidance to avoid any vascular access problem due to the low platelet count. Through a 5-F sheath (Terumo), we catheterized the celiac trunk and splenic artery (catheter Simons 1). The angiogram showed high-grade lesions of the spleen, with several intraparenchymal pseudoaneurysms. Every vessel responsible for bleeding was selectively reached with a microcatheter (Terumo 2,7 FR); embolization with a mixture 1:3 of n-BCA (n-butyl cyano-acrylate) glue and lipiodol (Guerbet) was performed. The final angiogram confirmed a good morphological result, with no more evidence of bleeding lesion and good perfusion of the remnant parenchyma.
The result was confirmed after 48 h by the follow-up CT exam, and the patient was able to start the chemotherapy, because the results of blood tests confirmed acute T-cell lymphoblastic leukemia.
Discussion
Pseudoaneurysm or false aneurysm involves a focal and incomplete disruption of the arterial wall (intima and/or elastic lamina) that allows blood to pass into the arterial wall; such contained rupture causes the creation of a “new” vessel wall consisting of the adventitia and the perivascular tissues. Differently, a true aneurysm develops when all three layers of arterial wall dilate and thin but remain intact [1].
An intrasplenic pseudoaneurysm of segmental arteries is defined as a special form of parenchymal splenic rupture due to a secondary laceration of the arterial wall. With respect to the underlying cause, traumatic pseudoaneurysms are distinguished from non-traumatic or spontaneous pseudoaneurysms [2–9].
In patients with traumatic rupture of the spleen, the reported frequency of traumatic intrasplenic pseudoaneurysms is 14% [1]. In contrast, non-traumatic intrasplenic pseudoaneurysms are rare complications of splenic infarction, infiltration by malignant systemic disorders, infectious process, chronic pancreatitis, and arteritis and they are potentially life threatening, known to cause spontaneous non-traumatic splenic rupture with hemoperitoneum. They can be single or multiple, more common in men and are extremely fragile [3–10].
These lesions are usually asymptomatic, discovered incidentally during other procedures such as ultrasound or other imaging techniques. If the symptoms are present, they may be nonspecific vague pain in the left hypochondrium, nausea and vomiting. Incidences of rupture reported range from 8 to 46% and mortality rates are very high [10].
In our case, the patient did not report having had any trauma, but the practice in the past of a contact sport cannot exclude with certainty the traumatic cause.
Multi-detector row CT by multiphase acquisitions (precontrast, arterial phase, venous phase, and delayed phase images) is the best and most frequently used modality to correctly assess vascular anatomy and its disease, in particular for detecting intrasplenic pseudoaneurysms or extravasation of contrast medium suggestive of active hemorrhage [8, 9, 11–14].
In contrast-enhanced CT, pseudoaneurysms appear as well-defined focal intrasplenic areas of high attenuation similar to the adjacent contrast-enhanced arteries and greater than that of the normally enhancing splenic parenchyma (contained vascular injury) and besides a delayed “washout” of enhanced blood is seen with an attenuation value similar to that of adjacent intact arteries [1, 15].
The rate of enlargement of the pseudoaneurysm depends not only on the integrity of the adventitial layer of the artery but also on the strength of the surrounding tissues that resists the expansion of the pseudoaneurysm [16].
Although large pseudoaneurysms can readily be detected on contrast-enhanced CT, small lesions are easily overlooked in the initial study [17]. As reported in a review, their diagnostic timing is between 5 days and 120 days from a traumatic event [18].
Delayed splenic rupture is defined as an injury occurring after 48 h after the initial insult, this period is known as the “latent period of Baudet” and it is probably related to a temporary tamponade of a minor laceration or the presence of a slowly enlarging subcapsular hematoma that eventually ruptures with hemoperitoneum [19].
Also, the formation of a pseudoaneurysm can occur in two stages, at the beginning the splenic lesion causes little damage to the arterial vessel wall with the formation of a small clot (sentinel clot sign) and then the arterial pressure and the lysis of the clot result in the pseudoaneurysm being visible on the follow-up CT [20, 21].
The evolution of splenic pseudoaneurysm is not clearly known. Although some splenic pseudoaneurysms solve spontaneously by thrombosis without intervention, recent studies have shown that up to 67% of these lesions ultimately may rupture and therefore represent a strong risk of delayed splenic rupture and a life-threatening condition [15].
In the literature, there is no reference to a certain association between grade of parenchymal injury and presence of PSA or the association between its size and symptoms [9].
In our case, the patient underwent an ultrasound examination for the evaluation of the spleen in the suspicion of a lymphoproliferative disease.
Ultrasound is a fast noninvasive imaging technique, which gave us the opportunity to evaluate not only the morphology and size of the organ, confirming the condition of splenomegaly, but also its echostructure clearly detecting the two focal anechoic areas within the spleen, that, in the lack of posterior accentuation and of a capsule, were not suggestive of the cystic nature.
However, the baseline US was not able to understand the nature of the two anechoic areas, so the evaluation with color Doppler was helpful in demonstrating the pulsatile flow of those formations and their vascular nature.
CEUS is a relatively new radiation-free technique alternative to contrast-enhanced CT with the potential to identify any kind of vascular and parenchymal lesions [2–6, 11–15]. In our case, CEUS confirmed without any doubt the vascular nature of the lesion showing the flow of contrast medium in those formations. The morphology of those areas made them compatible with contained vascular lesions, suggesting the diagnosis of pseudoaneurysm and not arteriovenous fistula that has a more branched and less lacunar aspect.
In the literature, CEUS has an high sensitivity at detection of PSA, about 83%, with specificity of 92% (PPV = 71%, NPV = 96%) [9].
Contrast-enhanced CT is useful to identify the contained vascular injury, providing information on surrounding soft-tissue relationships as well, but sometimes the CT differentiation between pseudoaneurysms with respect to arteriovenous fistulas is not easy; often the two conditions can be differentiated only with angiography, therefore an angiography is important for further diagnosis and treatment [1, 22].
In our case, the spleen was involved with a hematologic disorder and this condition could explain damage of the vascular wall with formation of a pseudoaneurysm by means of two possible mechanisms, mechanical or traumatic and “spontaneous” or non-traumatic.
An enlarged spleen is also more susceptible to rupture after minor trauma, but our patient did not remember any trauma and any abdominal symptoms either, although he had practiced a potentially traumatic sporting activity (kickboxing) up to a month before the diagnosis of T-cell acute lymphoblastic leukemia.
A second supposed mechanism is a direct damage to the vessel wall by the lymphoproliferative disease invading the spleen.
We found two reported cases of “spontaneous” pseudoaneurysms, splenic and renal, developed during a B-cell lymphoma [23, 24].
In advanced phases of that disease, splenic architecture is diffusely obliterated by sheets of neoplastic cells and it is supposed that neoplastic invasion could injure the artery wall and cause pseudoaneurysm.
As with most pseudoaneurysms, there is a high risk of rupture without treatment, and all splenic lesions should be treated regardless of size or clinical manifestation. Endovascular treatment is often the first-line therapy and may be performed with a variety of techniques [8].
Angiography remains the gold standard to localize the lesions and determine the presence of pseudoaneurysms; it has a greater sensitivity than the CEUS and the CECT in the detection of pseudoaneurysms. Furthermore, angiography is very useful in the endovascular management of the patient, allowing to embolize these contained vascular lesions [25–28].
Regardless of the approach, the leading principle in treatment is “exclusion” of the vascular lesion from the circulation and initially the first thought goes to embolization [6]. In case of intrasplenic pseudoaneurysm, embolization is performed as distally as possible, in a small arterial branch that supplies the lesion to preserve perfusion to the remaining splenic parenchyma. Common embolizing agents include simple and complex metallic structures (coils), particulate materials (PVA, microspheres, Gelfoam) and liquid agents (n-butyl cyano-acrylate, onyx) [8].
As the case in our study, distal embolization was performed using acrylic glue and no residual filling of the lesion was seen on following control.
Although most endovascular procedures are technically successful, with typically only a small degree of splenic infarction due to collateral flow maintaining end-organ perfusion, the splenic infarction risks increase with more distal embolizations as was the result in our case.
Conclusion
The occurrence of non-traumatic intrasplenic pseudoaneurysm is rare. This potentially life-threatening complication either in case of a lymphoproliferative disease or in a patient with a history of possible trauma, must be known, considered and investigated. The gold standard technique is contrast-enhanced CT, but it is very important to be able to also make the diagnosis with ultrasound exam. CEUS exam is a new radiation-free alternative to contrast-enhanced CT with the potential to identify pseudoaneurysm; angiography is fundamental for treatment with beneficial effects by a non-operative management.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Formal consent has been acquired.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Agrawal GA, Johnson PT, Fishman EK. Splenic artery aneurysms and pseudoaneurysms: clinical distinctions and CT appearances. AJR Am J Roentgenol. 2007;188:992–999. doi: 10.2214/AJR.06.0794. [DOI] [PubMed] [Google Scholar]
- 2.Piccolo CL, Trinci M, Pinto A, et al. Role of contrast-enhanced Ultrasound (CEUS) in the diagnosis and management of traumatic splenic injuries. J Ultrasound. 2018;21:215–227. doi: 10.1007/s40477-018-0327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sessa B, Trinci M, Ianniello S, et al. Blunt abdominal trauma: role of contrast-enhanced ultrasound in the detection and staging of abdominal traumatic lesions compared with US and CE-MDCT. Radiol Med. 2015;120:180–189. doi: 10.1007/s11547-014-0425-9. [DOI] [PubMed] [Google Scholar]
- 4.Miele V, Piccolo CL, Galluzzo M, et al. Contrast enhanced ultrasound (CEUS) in blunt abdominal trauma. Br J Radiol. 2016;89(1061):20150823. doi: 10.1259/bjr.20150823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tagliati C, Argalia G, Polonara GGM, et al. Contrast-enhanced ultrasound in delayed splenic vascular injury and active extravasation diagnosis. Radiol Med. 2019;124:170–175. doi: 10.1007/s11547-018-0961-9. [DOI] [PubMed] [Google Scholar]
- 6.Tagliati C, Argalia G, Graziani B, et al. Contrast-enhanced ultrasound in the evaluation of splenic injury healing time and grade. Radiol Med. 2019;124:163–169. doi: 10.1007/s11547-018-0954-8. [DOI] [PubMed] [Google Scholar]
- 7.Görg C, Cölle J, Wied M, et al. Spontaneous nontraumatic intrasplenic pseudoaneurysm: causes, sonographic, diagnosis, and prognosis. J Clin Ultrasound. 2003;31:129–134. doi: 10.1002/jcu.10145. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Khalaf MM, Al-Ameer SM, Smadi MM, et al. Intrasplenic arterial aneurysms during pregnancy. Case Rep Obstet Gynecol. 2015;2015:248141. doi: 10.1155/2015/248141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durkin N, Deganello A, Sellars ME, et al. Liver and splenic pseudoaneurysms in children: diagnosis, management and follow-up screening using contrast enhanced Ultrasound (CEUS) J Pediatr Surg. 2016;51:289–292. doi: 10.1016/j.jpedsurg.2015.10.074. [DOI] [PubMed] [Google Scholar]
- 10.Derchi LE, Biggi E, Cicio GR, et al. Aneurysms of the splenic artery: noninvasive diagnosis by pulsed Doppler sonography. J Ultrasound Med. 1984;3:41–44. doi: 10.7863/jum.1984.3.1.41. [DOI] [PubMed] [Google Scholar]
- 11.Trinci M, Piccolo CL, Ferrari R, et al. Contrast-enhanced Ultrasound (CEUS) in pediatric blunt abdominal trauma. J Ultrasound. 2019;22:27–40. doi: 10.1007/s40477-018-0346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menichini G, Sessa B, Trinci M, et al. Accuracy of Contrast-Enhanced Ultrasound (CEUS) in the identification and characterization of traumatic solid organ lesions in children: a retrospective comparison with baseline-US and CE-MDCT. Radiol Med. 2015;120:989–1001. doi: 10.1007/s11547-015-0535-z. [DOI] [PubMed] [Google Scholar]
- 13.Miele V, Piccolo CL, Trinci M, et al. Diagnostic imaging of blunt abdominal trauma in pediatric patients. Radiol Med. 2016;121:409–430. doi: 10.1007/s11547-016-0637-2. [DOI] [PubMed] [Google Scholar]
- 14.Trinci M, Ianniello S, Galluzzo M, et al. A rare case of accessory spleen torsion in a child diagnosed by ultrasound (US) and contrast-enhanced ultrasound (CEUS) J Ultrasound. 2019;22:99–102. doi: 10.1007/s40477-019-00359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jesinger RA, Thoreson AA, Lamba R. Abdominal and pelvic aneurysms and pseudoaneurysms: imaging review with clinical, radiologic, and treatment correlation. Radiographics. 2013;33:E71–E96. doi: 10.1148/rg.333115036. [DOI] [PubMed] [Google Scholar]
- 16.Madoff DC, Denys A, Wallace MJ, et al. Splenic arterial interventions: anatomy, indications, technical considerations, and potential complications. Radiographics. 2005;25(Suppl 1):S191–S211. doi: 10.1148/rg.25si055504. [DOI] [PubMed] [Google Scholar]
- 17.Shanmuganathan K, Mirvis SE, Boyd-Kranis R, et al. Nonsurgical management of blunt splenic injury: use of CT Criteria to select patients for splenic arteriography and potential endovascular therapy. Radiology. 2000;217:75–82. doi: 10.1148/radiology.217.1.r00oc0875. [DOI] [PubMed] [Google Scholar]
- 18.Ackermann LV, Rosai J. Surgical pathology. St. Louis: C. V. Mosby Co; 1974. pp. 2205–2207. [Google Scholar]
- 19.Ikeda O, Nakasone Y, Tamura Y, et al. Endovascular management of visceral artery pseudoaneurysms: transcatheter coil embolization using the isolation technique. Cardiovasc Intervent Radiol. 2010;33:1128–1134. doi: 10.1007/s00270-010-9973-0. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Tomassetti Fernández E, Delgado-Plasencia L, Arteaga-Gonzalez I, et al. Posttraumatic intrasplenic pseudoaneurysm with high-flow arteriovenous fistula: new lessons to learn. Eur J Trauma Emerg Surg. 2008;34:305–308. doi: 10.1007/s00068-007-7106-5. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz SI, Shires GT, Spencer FC. Principles of surgery. 6. New York: McGraw-Hill; 1994. pp. 1437–1438. [Google Scholar]
- 22.Weinberg JA, Lockhart ME, Parmar AD, et al. Computed Tomography identification of latent pseudoaneurysm after blunt splenic injury: pathology or technology? J Trauma. 2010;68:1112–1116. doi: 10.1097/TA.0b013e3181d769fc. [DOI] [PubMed] [Google Scholar]
- 23.Huang YC, Xie ZY, Tseng HS, et al. Splenic artery pseudoaneurysm with rupture by transformed splenic marginal zone B cell lymphoma. Ann Hematol. 2010;89:639–640. doi: 10.1007/s00277-009-0851-2. [DOI] [PubMed] [Google Scholar]
- 24.Masamoto Y, Imai Y, Seo S, et al. A case report of non-traumatic renal artery pseudoaneurysm due to chemotherapy for diffuse large B-cell lymphoma. Ann Hematol. 2010;89:107–108. doi: 10.1007/s00277-009-0776-9. [DOI] [PubMed] [Google Scholar]
- 25.Sunagozaka H, Tsuji H, Mizukoshi E, et al. The development and clinical features of splenic aneurysm associated with liver cirrhosis. Liver Int. 2006;26:291–297. doi: 10.1111/j.1478-3231.2005.01231.x. [DOI] [PubMed] [Google Scholar]
- 26.Dave SP, Reis ED, Hossain A, et al. Splenic artery aneurysm in the 1990s. Ann Vasc Surg. 2000;14:223–229. doi: 10.1007/s100169910039. [DOI] [PubMed] [Google Scholar]
- 27.Barbiero G, Groff S, Battistel M, et al. Are iatrogenic renal artery pseudoaneurysms more challenging to embolize when associated with an arteriovenous fistula? Radiol Med. 2018;123:742–752. doi: 10.1007/s11547-018-0906-3. [DOI] [PubMed] [Google Scholar]
- 28.Mohan B, Singal S, Bawa AS. Endovascular management of traumatic pseudoaneurysm: short and long term outcomes. J Clin Orthop Trauma. 2017;8:276–280. doi: 10.1016/j.jcot.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]