Abstract
The drying process used to obtain active food additives is critical to ensure its functionality. In this study, freeze- and spray-drying techniques were evaluated for encapsulation of extracts with antioxidant activity from yerba mate (Ilex paraguariensis), using maltodextrin (MD) as wall material. Additionally, the oxidative stability in a real food matrix (mayonnaise) was assessed. Both MD addition and drying methods affected the physical properties [moisture content, water activity (aW)] and oxidative stability. MD addition diminished moisture content and prevented polyphenol compounds from degradation. The spray-dried powders displayed the lowest moisture content (1.6 ± 0.3% bs), the highest polyphenol content (135.4 mg GAE/g pure extract), and oxidative stability than the freeze-dried samples. The antioxidant capacity of the encapsulated powder subjected to spray-drying increased the oxidative stability of the mayonnaise (258 ± 32 min) more than the other assayed system (165 ± 5 min). Therefore, a natural spray-dried antioxidant food additive was obtained with potential use in the food industry.
Keywords: Yerba mate, Maltodextrin, Encapsulation, Drying process, Antioxidant additive, Mayonnaise
Introduction
Yerba mate (Ilex paraguariensis, YM) is highly consumed as an infusion in various countries of South America. YM industrial processing involves blanching, drying, grinding, classification, and seasoning operations (Valerga et al. 2012). Harvested green yerba mate leaves are subjected to blanching followed by two drying stages. Aged-canchada YM corresponds to one step of the industrialization step of yerba mate production. Once dried, leaves are coarsely ground and submitted to aging (aged canchada YM leaves) for the product to acquire adequate flavor, aroma, and color (Valerga et al. 2012).
There is evidence to suggest that YM is a rich source of antioxidant compounds, minerals, and vitamins (Heck and De Mejia 2007). Bioactive compounds from YM have proven to provide different therapeutic properties like preventing DNA oxidation, low-density lipoprotein (LDL) lipoperoxidation, and promoting weight loss (Heck and De Mejia 2007; Harris et al. 2011; Plaza et al. 2018). Additionally, they showed antioxidant and antimicrobial properties against Gram-positive and Gram-negative bacteria (Plaza et al. 2018; Gutiérrez del Río et al. 2018).
Food products with natural and easily recognized additives are currently highly demanded by consumers. This trend has driven food producers to develop safe and effective substitutes for synthetic preservatives. Therefore, the high concentration of active compounds found in YM makes it ideal for obtaining extracts which could be useful in food preservation and the development of novel nutraceutical food products (Heck and De Mejia 2007; Ferrario et al. 2018).
Conventional methods for extraction of functional compounds from natural sources such as heating, boiling, or agitation at room temperature, display some disadvantages encompassing hydrolysis and oxidation during extraction, low yield and poor performance due to the required long extraction times (Huang et al. 2009). Consequently, some alternative methods have been developed in order to replace conventional solid–liquid extraction techniques. In particular, ultrasound-assisted (UA) extraction is a simple and effective method (Picó 2013). It allows better solvent penetration, shorter extraction time, higher mass transfer and extraction yield, even at lower temperatures compared to other conventional methods (Rodríguez Chanfrau and López Armas 2014; Wang et al. 2008). During the ultrasound process (US), a cavitation phenomenon occurs, in which microbubbles of gas and/or vapor formed within a liquid, undergo violent collapse during the compression cycle of the wave (Guerrero et al. 2017), releasing large amounts of energy. It creates hotspots, which can dramatically improve solvent penetration into cell components, and even may induce cell breakdown, thus provoking a release of the cell’s inner content (Picó 2013). Besides, US treatment randomly breaks the cell wall, which is more effective than enzymatic breakdown (Khan et al. 2018), leading to an increase of the total phenolic content in the obtained extracts. The concentration of phenolic compounds, such as flavonoids, may also increase due to hydroxylation of molecules because of the radicals OH* formed during ultrasound (Ashokkumar et al. 2008; Khan et al. 2018).
Nonetheless, the addition of phenolic compounds from natural sources to food has some drawbacks as these compounds are susceptible to environmental and processing conditions, leading to a considerable loss of their activity (Fang and Bhandari 2010; Plaza et al. 2018). Another disadvantage is related to an undesirable taste that they can display due to its herbal nature (Fang and Bhandari 2010). For instance, Ferrario et al. (2018) developed a carrot-orange juice blend processed by UV-C with YM extract addition; and they reported that consumers would have expected a weaker herbal taste than that they perceived in the samples. Therefore, the encapsulation technique might be a good alternative for the preservation of YM phenolic compounds as well as the overlapping of undesirable flavor notes and/or for preventing undesirable color changes caused by its incorporation into a real food product.
It is documented in the literature that YM extracts have been successfully encapsulated in chitosan and alginate beads (Deladino et al. 2008, 2013; López Córdoba et al. 2013) or chitosan hydrochloride nanoparticles and microspheres (Harris et al. 2011). Regarding the drying step, freeze-drying has proven to be a very suitable method for drying thermo-sensitive substances, minimizing thermal degradation reactions (Orjuela-Palacio et al. 2014; Ballesteros et al. 2017). However, it requires high energy input and long processing times (Nedovic et al. 2011). Whereas, spray drying is an economical, flexible, and continuous operation, which produces powder particles of good quality (Nedovic et al. 2011). Extracts from coffee, another natural source of polyphenols, were successfully encapsulated with maltodextrin as wall material by freeze-drying (Ballesteros et al. 2017). Nunes et al. (2015) reported that coffee extracts, produced with MD by spray drying showed freeze concentrated YM better retention and stability than freeze concentrates without MD addition. Therefore, the antioxidant activity of these compounds could be protected using MD. Maltodextrin is an inexpensive hydrolyzed starch with high water solubility (> 75%) and low viscosity in aqueous solutions, which forms a coating film that minimizes oxygen contact with the encapsulated material (Pourashouri et al. 2014).
Accordingly, the present study evaluates the encapsulation of ethanolic YM extracts, using MD as wall material and two different drying techniques (spray and freeze-drying) to obtain a dried natural antioxidant additive. Additionally, its impact on the oxidative stability of a real food matrix (mayonnaise) was evaluated.
Materials and methods
Raw materials and chemicals
YM dried-aged canchada leaves (dried and aged for 7 months) were gently provided by La Cachuera (Apóstoles, Misiones, Argentina). The aged-canchada industrialization stage of YM leaves was chosen as it displayed in a previous study the highest polyphenol content and antioxidant activity (Guerrero et al. 2019) compared to the other stages assayed (unprocessed leaves and unaged canchada leaves). MD (dextrose equivalent 20, DE20), used as wall material, was purchased from Quimatic S.A. (Santiago, Chile). Purified water (conductivity: 26 μs/cm) from an inverse osmosis system (Vigaflow S.A., Santiago-Chile) was used as the dispersant. All chemicals used were of analytical grade.
Preparation of the real food matrix: mayonnaise
Mayonnaise was elaborated in the laboratory by mixing 250 mL of commercial vegetable oil, fresh egg (one unit), a spoonful of vinegar, and a pinch of salt, following the guidelines recommended in the instruction manual for Thermomix equipment (Vorwerk, Wuppertal, Germany). Mayonnaise, without any chemical preservative, was prepared the same day that oxidative stability assays were performed.
Preparation of ethanolic extracts from yerba mate
YM leaves (20 g) were coarsely ground, mixed with 150 mL absolute ethanol (Merck S.A. Santiago, Chile) and subjected to two successive UA (600 W, 20 kHz, 95.2 µm, 25 °C, 10 min) extractions as previously optimized by Ferrario et al. (2018). UA extractions were carried out in a 600 mL-double wall cylindrical vessel connected to a thermostatically controlled water bath (HAAKE, Model Rotovisco RV12, Germany). The extract was vacuum filtered, and then the solvent was evaporated under low-pressure conditions in a rotary evaporator (Buchi R-100, Flawil, Switzerland) at 50 °C and resuspended in 25 mL of water per extraction to obtain an aqueous extract (E).
Encapsulation of yerba mate extracts
Encapsulation of YM extract was performed using MD as wall material. For the assays, MD was mixed with YM extracts in the 2:1 proportion (MD: extract) for 60 min at 35 °C in agitation and darkness until complete homogenization. Afterward, the samples were subjected to freeze-drying or spray-drying. Previous results obtained in the laboratory showed that the selected conditions for encapsulation resulted in achieving the highest yield values (higher than 50%), thus considering the procedure successful, as stated by Bhandari et al. (1997).
For freeze-drying, the samples were previously frozen and placed in the freeze-dryer chamber collector at − 60 °C and a shelf at 30 °C under a pressure of 0.05 bar for 48 h (Ilshin FD5508, Gyeonggi-do, Korea).
Spray-drying was conducted in laboratory-scale equipment (Mini Spray Dryer Buchi model B-290, Flawil, Switzerland) using a liquid feed volumetric flow rate of 2 mL/min, drying air inlet temperature of 120 °C, nozzle air flow-rate of 600 NL (litters at normal conditions/h) and aspiration of 80% (32 m3/h).
Therefore, the following systems were studied: control samples corresponding to non-encapsulated YM extract obtained by freeze-drying (PL) and spray drying (PS) and samples corresponding to encapsulated extracts with MD subjected to lyophilization (PEL) and spray-drying (PES). All systems were stored in an incubator (Velp, Usmate Velate, Italy) at refrigeration temperature (4 °C) using amber glass bottles hermetically sealed and protected from light until further studies.
Extraction yield (%)
YM extracts were freeze- or spray-dried and total solids obtained in each case were weighed in an analytical balance (AND HR-120, Japan). The extraction yield (EY%) was calculated using Eq. 1:
| 1 |
Powder physicochemical characterization
Moisture content and water activity
The moisture content of the dry powders was determined gravimetrically by the difference in mass before and after drying the samples in an oven (Shel Lab 1410-2E, USA) at 105 °C until constant weight (AOAC 1998). Results were expressed as dry basis percentage (% db; g water/100 g solids). Water activity (aw) was measured using an Aqualab Serie 3 TE (USA) apparatus (AOAC 1998) at ~ 25 °C.
Optical properties
The UV–Vis absorption spectra of dried YM powders were obtained and characterized using a spectrophotometer (Shimadzu UV mini-1240, Japan). Samples (1%v/v) were suspended in water, and the corresponding spectra were obtained in the range of 240–380 nm.
The color of the powders was determined through image analysis using a computer vision system (previously calibrated). It consists of a black box with four natural lights D65 (18 W, Phillips) and a digital camera (Canon EOS Rebel XS) at a distance of 22.5 cm from the sample (camera lens angle and lights at 45°) (Matiacevich et al. 2012). Samples were measured as pellets by pressing powders with a Quick Press hand press (Perkin-Elmer, USA). The digital color parameters were obtained in the RGB space using software Adobe Photoshop 7.0 (Adobe Systems Incorporated, 2007), which was subsequently converted to the CIELAB space in which L* indicates lightness, a* the red-green axis and b* the blue-yellow axis.
Color variations between each sample and its corresponding control (PEL and PL, PES, and PS) were calculated using the CIEDE2000 equation (Luo et al. 2001).
Structural characterization
The chemical groups and bonding arrangement of components present in the samples were determined by Fourier transform infrared spectroscopy (FTIR) (Perkin-Elmer Spectrum Two spectrometer, Massachusetts, USA). It was equipped with a diamond-composite universal attenuated total reflectance (ATR) unit with an incident angle of 45° (Perkin-Elmer, USA). The samples were prepared as pellets by pressing powders with a Quick Press hand press (Perkin-Elmer, USA). Measurements were performed in a spectral range from 4000 to 400 cm−1, resolution of 4 cm−1, an applied force of 20 N, and 16 scans per sample.
Scanning electron microscopy (SEM) was performed to examine the microstructure of encapsulated or non-encapsulated (control) YM powders, using a field emission scanning electron microscope (Supra 40, Carl Zeiss NTA, Oberkohen, Germany). The samples were mounted on stubs using a two-sided adhesive tape, then coated with a layer of gold (40–50 nm). Images were obtained by applying an acceleration voltage of 10 kV.
Stability test of rehydrated powders
A suspension (1%w/v) of each sample (PS, PES, PL, and PEL) was prepared, thoroughly homogenized, and placed in flat bottom transparent glass tubes. Separation of phases or sedimentation was visually observed during storage (30 min, 1 h, 24 h).
Total phenolic content
Total phenolic content (TPC) was determined using the Folin–Ciocalteu method (Singleton et al. 1999). A calibration curve with Gallic acid (Merck, Darmstadt, Germany) (ranging from 0.0025 to 0.125 mg/mL) was prepared. Sample concentrations were determined from the calibration curve (y = 1.2013x + 0.0081, R2 = 0.999) and expressed as milligrams of Gallic acid equivalent per milliliter of the sample (mg GAE/mL). Briefly, 0.5 mg of YM powder samples were diluted 10 times in distilled water, 0.1 mL of this solution was mixed in a 10 mL volumetric flask with 4.9 mL of distilled water, 0.5 mL of Folin–Ciocalteu (Merck, Darmstadt, Germany) reagent (10%v/v), followed by 1.7 mL of Na2CO3 (20% w/v, Merck, Darmstadt, Germany) addition. Then, distilled water was added until reaching 10 mL. The reactive mixture was allowed to stand for 2 h in darkness, and the formation of blue color, as an indicator of TPC, was quantified at 740 nm using a UV–Vis spectrophotometer (V-630, Jasco, Tokyo, Japan). A blank was performed to amend the final value of polyphenol content in the sample, according to the MD dilution factor. TPC was also expressed as mg GAE/g pure extract. All assays were performed in triplicate.
Oxidative stability in a real food matrix
The oxidative stability of the mayonnaise prepared in the laboratory with or without (control) the addition of the four dried YM extracts in the 1:1 (w/w) proportion was measured using an accelerated oxidative stability test (Rapidoxy, Anton Paar, Germany) by increasing oxygen pressure and temperature. Oxidation conditions for temperature and oxygen pressure were 120 °C and 700 kPa, respectively. Induction time was recorded when a 10% oxygen pressure drop was detected (Difonzo et al. 2018). Oxidative stability was expressed in time units as the difference in the time for oxidizing the mayonnaise in the presence or absence of YM extract.
Statistical analysis
All experiments were run by triplicate. Data were reported as mean with their corresponding standard deviation. A MANOVA test was performed to determine the differences in color parameters, aw, moisture, TPC, and oxidative stability between the processing stages: drying process and addition of MD. Significant differences were analyzed using the Hotelling test with Bonferroni correction. The significance of the differences was determined at a 95% confidence level (p < 0.05).
Additionally, multivariate statistical treatment considering the parameters (aw, color, moisture, polyphenol content, and oxidative stability) was made by Principal component analysis (PCA). Statistical analyses were performed using InfoStat 2009 (InfoStat Group, FCA-UNC, Córdoba, Argentina).
Results and discussion
Physical characterization of powders
Moisture content and water activity
The moisture content and water activity (aw) are critical physical parameters of powdered additives since they strongly influence their storage stability and safety. Both parameters of the YM powders obtained in this study showed significant differences (p < 0.05) as a consequence of the drying step (Table 1). The moisture content values were lower for spray-dried powders than freeze-dried ones when comparing PS to PL or PES to PEL. The differences in the moisture content ranged from 1.5% up to 9.2%.
Table 1.
Physical properties of yerba mate powders
| Samples | Moisture content (%db) | Water activity | Color parameters | CIEDE2000 | Images | ||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||
| PL | 9.2 ± 1.5a | 0.05 ± 0.03a | 39.1 ± 1.8a | − 8.8 ± 0.1a | 28.2 ± 0.9a |

|
|
| PS | 8.8 ± 0.9b | 0.32 ± 0.02b | 58.5 ± 1.0b | − 7.5 ± 0.7b | 23.3 ± 0.9b | ||
| PEL | 4.0 ± 0.3c | 0.07 ± 0.02a | 46.1 ± 1.1c | − 7.9 ± 0.9c | 27.3 ± 1.3c | 6.4 ± 1.1c | |
| PES | 1.6 ± 0.3d | 0.32 ± 0.01b | 66.3 ± 0.5d | − 3.8 ± 0.1d | 16.7 ± 0.2d | 7.9 ± 0.4d | |
a,bDifferent letters indicate significant differences (p < 0.05) between samples according to MANOVA
In the present work, all the samples exhibited aw values varying from 0.05 to 0.32. It is well-known that aw values below 0.6 can be considered microbiologically and/or chemically stable because the amount of free water available for microorganisms, as well as the development of biochemical reactions, is low to negligible (Tang and Yang 2004). Results showed that aw was significantly lower (p < 0.05) in the freeze-dried samples (PL and PEL) compared to the spray-dried samples (PS and PES), independently if the YM extract was protected or not by MD. Therefore, the water activity was more dependent on the type of drying than the encapsulation process. This result is in accordance with the results reported by Quintana et al. (2018), who analyzed the moisture content and aw of Lactobacillus plantarum CIDCA 83114 coated with okara oil-caseinate. Likewise, they observed lower aw in the freeze-dried samples compared to the spray-dried ones. They attributed these differences to the higher surface contact of the spray-dried samples compared to the freeze-dried emulsions, which enables better contact with water.
Meanwhile, moisture content was dependent on the encapsulation process, due to the addition of MD. The encapsulated spray-dried samples exhibited lower moisture content (PS: 8.8 ± 0.9, PES: 1.6 ± 0.3, (%d.b.) than the freeze-dried (PL: 9.2 ± 1.5, PEL: 4.0 ± 0.3, (%d.b.) samples. These results are in accordance with those reported by Catelam et al. (2011), who demonstrated for maltodextrin-encapsulated powdered additives, that differences in moisture content were observed between freeze and spray-drying methods at lower aw values (< 0.35). It shows the highest water content in the freeze-dried samples and differences in isotherms were observed when maltodextrin-encapsulated powdered additives were dried using freeze and spray-drying, corresponding the highest water content to the freeze-dried samples. These authors attributed it to the higher water-binding capacity of carbohydrates (such as maltodextrin) at low temperatures compared to those corresponding to high temperatures, through hydroxyl groups of carbohydrates. In addition, it was explained by the fast contact between the hot drying air and the microparticles (Wilkowska et al. 2016). Besides, Palipane and Driscoll (1993) suggested that at increased temperatures, water molecules get activated to higher energy levels, causing them to become less stable and breakdown away from the water-binding sites of the substrate. They were thus decreasing the water content (as observed in spray-dried samples).
It is also important to remark that encapsulated samples (PEL and PES) displayed moisture contents lower than 5% (Table 1), which prevents particle agglomeration and caking of powders. Thus, increasing the retention of active components (Daza et al. 2016) comparing to not encapsulated samples (PL and PS).
Powder optical properties
One absorbance peak at 324 nm was observed by the UV wavelength spectra of YM extracts (data not shown). It is commonly attributed to chlorogenic acid and its derivatives, which are present in ethanolic extracts (Belay and Gholap 2009). This peak was also observed in the powder samples containing MD, indicating that these compounds obtained in the extraction procedure were preserved after the drying step (PES and PEL).
Color is a relevant feature for consumer acceptability since it creates visual attractiveness. Color parameters of the YM dried powders and significant differences obtained by the MANOVA test are displayed in Table 1. Significant differences (p < 0.05) were recorded between all YM powders, indicating that color parameters were affected by the drying method applied and MD addition. Since YM extract was naturally green, the green color of the powder samples was, as expected, linked to a negative value of the a* parameter, which ranged from − 3.8 to − 8.8. A slight change in the greenness of the studied samples was observed when comparing the PL and PEL systems, which suggests that homogenization between the YM extract and the MD was not favored during the drying step, thus implying that encapsulation was only partially achieved. Assuming that the added MD was not completely incorporated in the system, the adequate protection of the bioactive compounds in the extracts may be altered, leaving them exposed to environmental factors such as light and oxygen, with detrimental consequences.
In contrast, crucial differences in the a* values were observed between PS and PES. In this case, MD successfully coated the YM extract and led to a more homogenous powder, suggesting that the extract may be less exposed to the surface than in the one obtained by freeze-drying. It is important to highlight that the significant increase in a* and decrease in b* parameters of PES samples compared to PS, is an indicator of slight pigment oxidation in these samples during thermal exposure. The addition of MD increase lightness, through a higher L* value. This effect can be observed in Table 1. It is evident that the L* parameter was affected only by the presence of MD. Significant differences (p < 0.05) in CIEDE2000 of 6.4 and 7.9 were observed for PL and PS comparing to PEL and PES, respectively, confirming that the variations in the L* parameter were attributed to the presence of MD. These differences in color were in the same range of color perception (6–12 “much difference”), according to Yang et al. (2012). It is essential to highlight that the decrease in the greenness of the powders, as a consequence of MD addition and spray-drying steps, maybe a desirable feature when planning to add it to a real food matrix without altering its natural color. It could due to as consumers would perceive the product as “less natural” or “more processed” if changes in color were detected. For instance, as an alternative application for YM, in previous studies, Ferrario et al. (2018) elaborated a natural carrot-orange juice blend enriched with a non-encapsulated YM lyophilized extract. They reported that juice samples were instrumentally measured as greener and more bluish and less red and yellow compared to the controls without additives. Even though, in a sensory test, the assessors described the juice as having “intense color,” one group of consumers expressed a strong interest in the product and assigned a score of 7 points in a 9-point hedonic scale (“like it moderately”).
Powder structural characterization
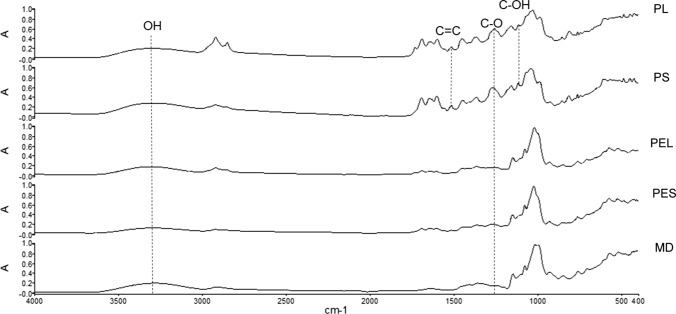
FT-IR studies were conducted to assess the interaction between the YM extract and the wall material. Figure 1 illustrates the FT-IR spectra of freeze- or spray-dried samples with or without MD as wall material. Non-encapsulated YM extract showed characteristic peaks of functional groups in the ethanolic YM extracts (Chang-Bravo et al. 2014), such as hydroxyl-related compounds with a peak between 1160 and 1070 cm−1, and one peak at 1520 cm−1 attributed to the C=C vibration of an aromatic ring, as well as the C–O stretching band at 1270 cm−1 (Arrieta et al. 2018). On the other hand, all the FTIR- spectra showed a typical absorption band at a wavelength of = 3300 cm−1, which is characteristic of the hydroxyl group (–OH) of the water.
Fig. 1.
Fourier transform infrared spectrophotometry spectra (FTIR) comparing freeze-dried and spray-dried powders: PL (freeze-dried powder); PS (spray-dried powder); PEL (encapsulated powder by freeze-dried); PES (encapsulated powder by spray-dried); MD (maltodextrin)
The protective effect of MD on the bioactive compounds present in the YM extracts was evidenced by a decrease or disappearance of the typical signals of YM bands, especially in the range from 1700 to 1100 cm−1. The disappearance of the signal corresponding to the aromatic rings stretching from phenolic compounds was observed at 1520 cm−1. This signal has been related to phenolic acids, including the caffeic and chlorogenic acids and their isomers (Heck et al. 2008), which are highly present in YM. In this sense, another evidence of the interaction between the polyphenols of YM extract and MD is the disappearance of the signal at 1160 cm−1 related to C–OH (hydroxyl) bonds, which are part of the structural characteristics of polyphenols (Chang-Bravo et al. 2014). Therefore, several interactions between compounds from YM extracts and MD were detected by FT-IR, which implies that encapsulation was achieved.
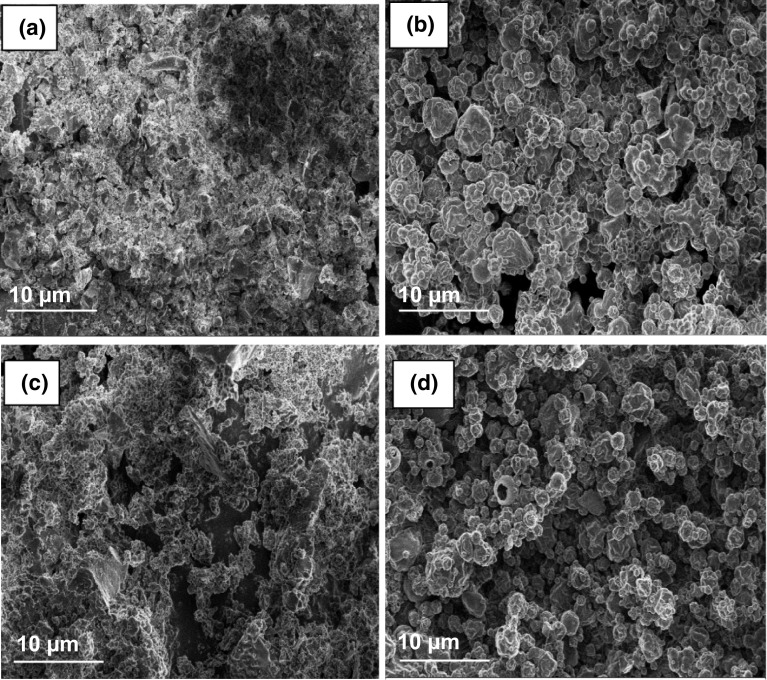
Scanning electron microscopy (SEM) was conducted in order to evaluate the morphology of the YM powders. Micrographs corresponding to freeze-dried systems with MD or not (PL and PEL) resulted in inhomogeneous rough surfaces, with visible pores on the surface (Fig. 2a, c). In contrast, systems subjected to spray-drying (PS and PES) resulted in hemispherical structures with shriveled surfaces with cavities and structural cracks (Fig. 2b, d).
Fig. 2.
Images of YM freeze-dried powder (PL) (a); spray-dried powder (PS) (b); freeze-dried encapsulated powder (PEL) (c); Spray-dried encapsulated powder (PES) (d) obtained by scanning electron microscopy (SEM)
Saikia et al. (2015) and Kuck and Norena (2016) studied the effect of spray and freeze-drying on the surface morphology of the powders obtained from Averrhoacarambola pomace and grape (Vitislabrusca var. Bordo) peel, respectively. They reported that the spray-dried particles had a spherical shape with different concavities and sizes. At the same time, the freeze-dried powders showed irregular, porous, and brittle structures, which were similar to those obtained in this study. The spherical shape of the atomized powders is based on the principle of generation of droplets by spraying and conversion of these droplets into particles by evaporation of the solvent (Stunda-Zujeva et al. 2017). According to Tonon et al. (2011), the presence of roughness in these spray-dried microparticles occurs when the temperature condition during the process is not very drastic, as softer surfaces can be obtained by using higher temperatures. Freeze-dried powder displayed high porosity, which may be a consequence of the formation of ice in the material during the freeze-drying process. Additionally, the presence of ice may have prevented shrinkage and collapse while preserving the structure volume (Kuck and Norena 2016). Hence, the structural difference observed between samples was attributed to the drying process more than the addition of MD.
Powder rehydration
Suspension stability
All the suspensions of the rehydrated powdered samples were stable, as no sedimentation nor separation of phases were recorded (data not shown).
Total phenolic content and oxidative stability of a real food matrix
Figure 3 illustrates the TPC corresponding to each YM extract, and the oxidative stability of the mayonnaise when the YM extract (encapsulated or not) was incorporated. Non-encapsulated YM extracts (PS and PL) showed the lowest TPC values (50.6–57.0 mg GAE/g pure extract), and accordingly, they displayed the lowest mayonnaise oxidative stability. No significant differences (p > 0.001) between samples were observed, indicating that both drying methods decreased TPC to the same extent when the extract was non-encapsulated. On the other hand, encapsulated freeze- and spray-dried systems (PEL and PES) exhibited higher TPC values (87.63–135.38 mg GAE/g pure extract) than the non-encapsulated ones. This result suggests that MD protected these bioactive compounds from drying; however, better performance was achieved in the case of spray-drying. It is in agreement with those shown in Sect. 3.2.1, which suggested that encapsulation by freeze-drying was only partially achieved as the presence of MD coating was not able to mask the greenness of the YM as much as in PES samples.
Fig. 3.
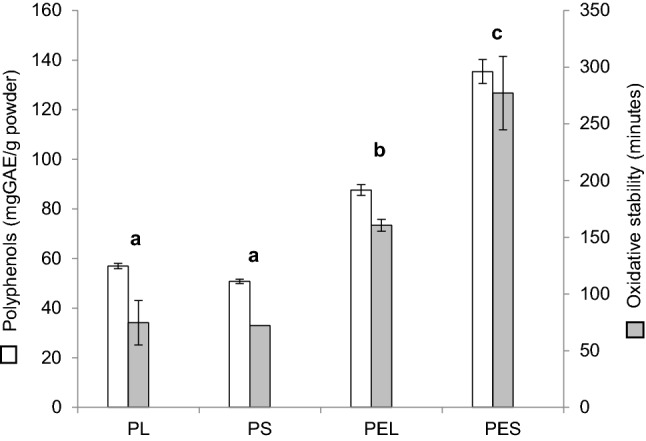
Polyphenol content and oxidative stability of powdered extracts incorporated in mayonnaise (1:1 w/w). PL: freeze-dried powder; PS: spray-dried powder; PEL: freeze-dried encapsulated powder; PES: Spray-dried encapsulated powder. Different letters (a, b, and c) indicate significant differences (p < 0.001) between samples. Error bars indicate the mean standard deviation of triplicates
Ramírez et al. (2015) studied the freeze (0.3–0.7 °C/min, 300–500 mTorr) and spray-drying (1.2–2.4 mL/h, 80–120 °C) conditions that better retained total polyphenol content of a model fruit juice (0.1% citrus pectin, 10% sucrose and 0.5% Gallic acid) encapsulated with different concentrations of maltodextrin/gum arabic (10–30%). They observed that the polyphenol retention depended on the selected conditions of the applied drying technology, as certain spray-drying conditions yielded higher polyphenol content compared to lyophilized samples. Moreover, on the contrary, certain freeze-drying conditions resulted in higher polyphenol content compared to spray-drying. Therefore, these authors concluded that spray drying is the most suitable technique for the encapsulation of antioxidant biocompounds.
These results could be attributed to the fact that MD is a wall material that tends to form spheres (Ballesteros et al. 2017), which will entrap the extract during the homogenization process previous to the drying process. Thus, MD successfully protected the extracts from the high temperature applied during the spray-drying process. The high temperature and short time of the process may have enhanced the mass and heat transfer, thus obtaining a better encapsulation yield (Sablania and Bosco 2018). According to the results shown in the present work, MD was not able to mask the greenness of the YM extract in the PEL samples as much as it was observed in the case of PES samples (Sect. 3.1.2). Additionally, a more porous surface was recorded in these samples, as commented on Sect. 3.1.3. These results suggested that encapsulation was only partially achieved in the lyophilized samples, thus promoting the degradation of the bioactive compounds to a much greater extent than in the spray-dried samples.
The methodology used in the present work involving an ultrasound-assisted extraction allowed to obtain noticeable higher TPC values (50.6–135.38 mg GAE/g pure extract) than those reported by traditional extraction methods. For instance, Prado Martin et al. (2013) reported a low polyphenol content (0.19 mg GAE/g) by the percolation of a yerba mate’s hydroethanolic (40:60) extract. On the same fashion, Bassani et al. (2014) also reported a relatively low polyphenol content, in the range from 0.34 to 0.48 mg GAE/mL obtained in a traditional extraction of 2 g of grounded roasted yerba mate leaves by heating at 60–90 °C during 5–10 min in 100 mL of distilled water. Moreover, Deladino et al. (2013) examined the polyphenol content of encapsulated yerba mate extracted by a traditional extraction procedure (100 °C, 40 min) and encapsulated in alginate or alginate and lyophilized chitosan beads. These authors reported a loading capacity of 4.8 × 10−3 to 8.7 × 10−3 mg GAE/g. Exceptionally, López Córdoba et al. (2013) studied the polyphenol content of yerba mate extract obtained by traditional extraction (100 °C, 40 min) and encapsulated in calcium-alginate-starch hydrogel beads, and reported a high polyphenol content of 127 ± 1.16 mg chlorogenic acid/g yerba mate dried leaves.
Furthermore, Fig. 3 shows the increase in the time (min) required to oxidize mayonnaise when adding different YM extracts to this matrix. In particular, PL and PS increased time by 60.57 min (139.7%) and 72.29 min (138.8%), respectively, when compared to control (data not shown), while the addition of PEL and PES retarded oxidation time by 164.45 min (206.44%) and 257.69 min (250.75%), respectively. In every case, the addition of YM extract showed antioxidant capacity following the high TPC present in the additives. Nonetheless, the encapsulated systems presented a higher increase in the oxidation time than the non-encapsulated ones, being PES the one with the highest antioxidant capacity.
The results obtained from TPC and oxidative stability tests suggested that spray-drying is an exciting alternative for encapsulation of YM extracts with MD, as it preserved the bioactive compounds in the extract during the drying step and prevented oxidation when used in a real food matrix.
Multivariate analysis by principal components analysis
A Principal component analysis (PCA) showed the spatial relationships between color parameters, aw, moisture, total polyphenol content, and oxidative stability and the evaluated YM samples (Fig. 4). The first principal components (PC 1and PC 2) explained 79.3%, and 19.4% of the variance, respectively. The first axis, PC 1 contrasted aw, L*, a*, TPC and oxidative stability, positively, and moisture content and b*, negatively. The second axis (PC 2) was defined positively by aw. PES sample was associated with the highest TPC, oxidative stability, L* and a* values, indicating that encapsulation with MD protected polyphenolic compounds from its degradation. Moreover, spray-drying preserved TPC noticeably better than freeze-drying, probably due to the porosity of lyophilized microstructures revealed by SEM.
Fig. 4.
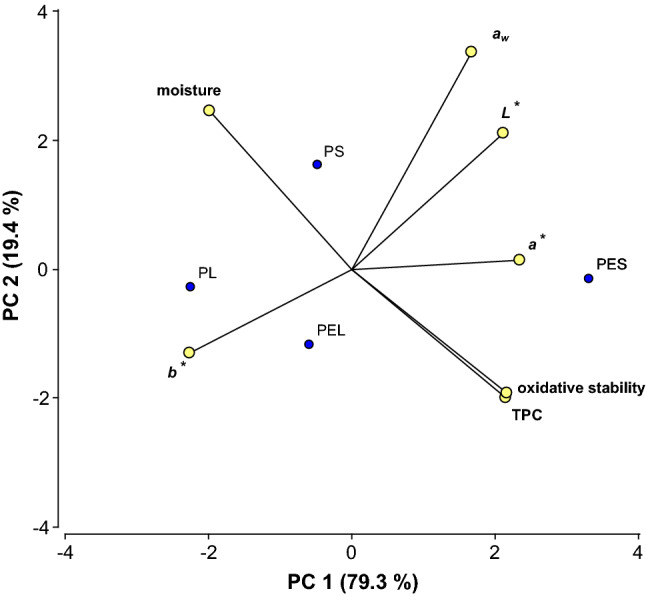
Principal component analysis (PCA) bi-plot of total polyphenol content, oxidative stability, color, moisture, and aw corresponding to freeze-dried (PL), spray-dried (PS), encapsulated powder by freeze-dried (PEL) and encapsulated powder by spray-dried (PES) yerba mate extract
Encapsulated samples (PEL and PES) displayed higher L* and a* values than the non-encapsulated systems (PL and PS), indicating that presence of MD increased lightness, while decreased greenness of YM (Fig. 4). L* and a* increased while b* decreased, being more evident when the samples were spray-dried instead of using freeze-drying, suggesting that they could have suffered slight pigment oxidation during thermal exposure.
PL and PEL samples displayed higher moisture content than PS and PES samples, respectively. As explained above the lower aw and higher moisture content observed in the freeze-dried samples compared to the spray-dried ones, may be attributed to the higher water-binding capacity of carbohydrates (such as maltodextrin) at low temperatures than at high temperatures, through hydroxyl groups of carbohydrates (Catelam et al. 2011). Besides, Palipane and Driscoll (1993) suggested that at increased temperatures, water molecules are activated to higher energy levels, causing them to become less stable and break away from the water-binding sites of the substrate. Thus, decreasing the water content (as observed in spray-dried samples).
Conclusion
US-assisted extraction resulted in a fast, reproducible, and convenient method for extracting bioactive compounds from aged canchada yerba mate leaves at low temperatures. Moreover, the addition of MD prevented degradation of these biocompounds when used as a wall material. Spray drying was the most convenient process to obtain YM extract, as spray-dried powders displayed the highest polyphenol content and proper oxidative stability, also achieving the lowest moisture content compared to the lyophilized samples. Additionally, MD addition and the effect of spray drying reduced the greenness of the powders, which may be a desirable feature when planning to add the active ingredient to a real food matrix without altering its natural color. It is essential to highlight that the antioxidant capacity of the additives assayed was proven in a real food matrix, where a noticeable increase in oxidative stability was obtained by the addition of this active additive in a mayonnaise. These results suggest that the coated YM extract could be used as a functional additive for novel foods and beverages.
Further studies should be performed shortly to improve shelf life and organoleptic properties of this real food matrix in order to obtain a mayonnaise with natural additives.
Acknowledgements
Authors acknowledge the financial support from Agencia Nacional de Promoción Científica y Tecnológica de la República Argentina (ANPCyT) together with Banco InterAmericano de Desarrollo (BID) [2015-0401 Project], Instituto Nacional de la Yerba Mate (INYM) [69- 310/2015 Project] and Fondecyt Project 1160463. Author Silvia Matiacevich also acknowledge Proyecto Fortalecimiento Usach USA1799_MS172228, DICYT-VRIDEI project 531 and Dicyt USA1899 from University of Santiago de Chile. Author Daniela Soto Madrid and Jessica Alarcon acknowledge Conicyt Ph.D. Grant 2019 N° 21190731 and 21190697, respectively.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Daniela Fenoglio and Daniela Soto Madrid: Co-First/Equal authorship.
Contributor Information
Daniela Fenoglio, Email: fenoglio.daniela86@gmail.com.
Daniela Soto Madrid, Email: daniela.sotom@usach.cl.
Jessica Alarcón Moyano, Email: jessica.alarcon@usach.cl.
Mariana Ferrario, Email: mariana_ferrario@di.fcen.uba.ar.
Sandra Guerrero, Email: sguerrero@di.fcen.uba.ar.
Silvia Matiacevich, Email: silvia.matiacevich@usach.cl.
References
- AOAC . Official methods of analysis. 16. Gaithersburg: AOAC; 1998. [Google Scholar]
- Arrieta MP, Peponi L, López D, Fernández-García M. Recovery of yerba mate (Ilex paraguariensis) residue for the development of PLA-based bionanocomposite films. Ind Crops Prod. 2018;111:317–328. doi: 10.1016/j.indcrop.2017.10.042. [DOI] [Google Scholar]
- Ashokkumar M, Sunartio D, Kentish S, Mawson R, Simons L, Vilkhu K, Versteeg CK. Modification of food ingredients by ultrasound to improve functionality: a preliminary study on a model system. Innov Food Sci Emerg Technol. 2008;9(2):155–160. doi: 10.1016/j.ifset.2007.05.005. [DOI] [Google Scholar]
- Ballesteros LF, Ramirez MJ, Orrego CE, Texeira JA, Mussatto SI. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray drying using different coating materials. Food Chem. 2017;237:623–631. doi: 10.1016/j.foodchem.2017.05.142. [DOI] [PubMed] [Google Scholar]
- Bassani DC, Nunes DS, Granato D. Optimization of phenolics and flavonoids extraction conditions and antioxidant activity of roasted yerba-mate leaves (Ilex paraguariensis Saint Hilaire, Aquifoliaceae) using response surface methodology. An Acad Bras Ciênc. 2014;86(2):923–934. doi: 10.1590/0001-3765201420130019. [DOI] [PubMed] [Google Scholar]
- Belay A, Gholap AV. Characterization and determination of chlorogenic acids (CGA) in coffee beans by UV–Vis spectroscopy. Afr J Pure Appl Chem. 2009;3(11):34–240. [Google Scholar]
- Bhandari BR, Datta N, Howes T. Problems associated with spray drying of sugar-rich foods. Dry Technol. 1997;15(2):671–684. doi: 10.1080/07373939708917253. [DOI] [Google Scholar]
- Catelam KT, Trindade CSF, Romero JT. Water adsorption isotherms and isosteric sorption heat of spray-dried and freeze-dried dehydrated passion fruit pulp with additives and skimmed milk. Ciênc Agrotecnol. 2011;35(6):1196–1203. doi: 10.1590/S1413-70542011000600021. [DOI] [Google Scholar]
- Chang-Bravo L, López-Córdoba A, Martino M. Biopolymeric matrices made of carrageenan and corn starch for the antioxidant extracts delivery of Cuban red propolis and yerba mate. React Funct Polym. 2014;85:11–19. doi: 10.1016/j.reactfunctpolym.2014.09.025. [DOI] [Google Scholar]
- Daza LD, Fujita A, Fávaro-Trindade CS, Rodrigues-Ract JN, Granato D, Genovese MI. Effect of spray drying conditions on the physical properties of Cagaita (Eugenia dysenterica DC.) fruit extracts. Food Bioprod Process. 2016;97:20–29. doi: 10.1016/j.fbp.2015.10.001. [DOI] [Google Scholar]
- Deladino L, Anbinder PS, Navarro AS, Martino MN. Encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydr Polym. 2008;71(1):126–134. doi: 10.1016/j.carbpol.2007.05.030. [DOI] [Google Scholar]
- Deladino L, Navarro AS, Martino MN. Carrier systems for yerba mate extract (Ilex paraguariensis) to enrich instant soups. Release mechanisms under different pH conditions. LWT Food Sci Technol. 2013;53(1):163–169. doi: 10.1016/j.lwt.2013.01.030. [DOI] [Google Scholar]
- Difonzo G, Pasqualone A, Silletti R, Cosmai L, Summo C, Paradiso VM, Caponio F. Use of olive leaf extract to reduce lipid oxidation of baked snacks. Food Res Int. 2018;108:48–56. doi: 10.1016/j.foodres.2018.03.034. [DOI] [PubMed] [Google Scholar]
- Fang Z, Bhandari B. Encapsulation of polyphenols—a review. Trends Food Sci Technol. 2010;21(10):510–523. doi: 10.1016/j.tifs.2010.08.003. [DOI] [Google Scholar]
- Ferrario M, Schenk M, Garcia Carrillo M, Guerrero S. Development and quality assessment of a turbid carrot-orange juice blend processed by UV-C light assisted by mild heat and addition of Yerba Mate (Ilex paraguariensis) extract. Food Chem. 2018;269:567–576. doi: 10.1016/j.foodchem.2018.06.149. [DOI] [PubMed] [Google Scholar]
- Guerrero SN, Ferrario M, Schenk M, Carrillo MG. Hurdle technology using ultrasound for food preservation. In: Bermudez-Aguirre D, editor. Ultrasound: advances for food processing and preservation. New York: Academic Press; 2017. pp. 39–99. [Google Scholar]
- Guerrero S, Ferrario M, Schenk M, Fenoglio D. Development of Tangerine-orange juice added with yerba mate (Ilex paraguariensis) extract and processed by UV-C light. Proceedings of 22nd Euro-global summit on food and beverages, 2019. J Food Process Technol. 2019;10:36–37. doi: 10.4172/2157-7110-C1-110. [DOI] [Google Scholar]
- Gutiérrez del Río I, Fernández J, Lombó F. Plant nutraceuticals as antimicrobial agents in food preservation: terpenoids, polyphenols and thiols. Int J Antimicrob Agents. 2018;52:309–315. doi: 10.1016/j.ijantimicag.2018.04.024. [DOI] [PubMed] [Google Scholar]
- Harris R, Lecumberri E, Mateos-Aparicio I, Mengíbar M, Heras A. Chitosan nanoparticles and microspheres for the encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydr Polym. 2011;84(2):803–806. doi: 10.1016/j.carbpol.2010.07.003. [DOI] [Google Scholar]
- Heck CI, De Mejia EG. Yerba mate tea (Ilex paraguariensis): a comprehensive review on chemistry, health implications, and technological considerations. J Food Sci. 2007;72(9):R138–R151. doi: 10.1111/j.1750-3841.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- Heck C, Schmalko M, González de Mejia E. Effect of growing and drying conditions on the phenolic composition of mate teas (Ilex paraguariensis) J Agric Food Chem. 2008;56(18):8394–8403. doi: 10.1021/jf801748s. [DOI] [PubMed] [Google Scholar]
- Huang W, Xue A, Niu H, Jia Z, Wang J. Optimised ultrasonic-assisted extraction of flavonoids from Folium eucommiae and evaluation of antioxidant activity in multi-test systems in vitro. Food Chem. 2009;114(3):1147–1154. doi: 10.1016/j.foodchem.2008.10.079. [DOI] [Google Scholar]
- Khan MK, Ahmad K, Hassan S, Imran M, Ahmad N, Xu C. Effect of novel technologies on polyphenols during food processing. Innov Food Sci Emerg Technol. 2018;45:361–381. doi: 10.1016/j.ifset.2017.12.006. [DOI] [Google Scholar]
- Kuck LS, Norena CPZ. Microencapsulation of grape (Vitis labrusca var. Bordo) skin phenolic extract using gum arabic, polydextrose, and partially hydrolyzed guar gum as encapsulating agents. Food Chem. 2016;194:569–576. doi: 10.1016/j.foodchem.2015.08.066. [DOI] [PubMed] [Google Scholar]
- López Córdoba A, Deladino L, Martino M. Effect of starch filler on calcium-alginate hydrogels loaded with yerba mate antioxidants. Carbohydr Polym. 2013;95(1):315–323. doi: 10.1016/j.carbpol.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Luo MR, Cui G, Rigg B. The development of the CIE2000 color-difference formula: CIEDE2000. Color Res Appl. 2001;26(5):340–350. doi: 10.1002/col.1049. [DOI] [Google Scholar]
- Matiacevich S, Mery D, Pedreschi F. Prediction of mechanical properties of corn and tortillas chips using computer vision. Food Bioprocess Technol. 2012;5(5):2025–2030. doi: 10.1007/s11947-011-0662-z. [DOI] [Google Scholar]
- Nedovic V, Kalusevic A, Manojlovic V, Levic S, Bugarski B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011;1:1806–1815. doi: 10.1016/j.profoo.2011.09.265. [DOI] [Google Scholar]
- Nunes GL, Boaventura BCB, Pinto SS, Verruck S, Murakami FS, Prudêncio ES, Amboni RDDMC. Microencapsulation of freeze concentrated Ilex paraguariensis extract by spray drying. J Food Eng. 2015;151:60–68. doi: 10.1016/j.jfoodeng.2014.10.031. [DOI] [Google Scholar]
- Orjuela-Palacio JM, Zamora MC, Lanari MC. Consumers’ acceptance of a high-polyphenol yerba mate/black currant beverage: effect of repeated tasting. Food Res Int. 2014;57:26–33. doi: 10.1016/j.foodres.2014.01.017. [DOI] [Google Scholar]
- Palipane KB, Driscoll RH. Moisture sorption characteristics of in-shell macadamia nuts. J Food Eng. 1993;18(1):63–76. doi: 10.1016/0260-8774(93)90075-U. [DOI] [Google Scholar]
- Picó Y. Ultrasound-assisted extraction for food and environmental samples. Trends Anal Chem. 2013;43:84–99. doi: 10.1016/j.trac.2012.12.005. [DOI] [Google Scholar]
- Plaza M, Dominguez-Rodriguez G, Castro-Puyana M, Marina ML. Polyphenols analysis and related challenges. In: Galanakis CM, editor. Polyphenols: properties, recovery, and applications. Cambridge: Woodhead Publishing Inc.; 2018. pp. 117–220. [Google Scholar]
- Pourashouri P, Shabanpour B, Razavi SH, Jafari SM, Shabani A, Aubourg SP. Oxidative stability of spray-dried microencapsulated fish oils with different wall materials. J Aquat Food Prod Technol. 2014;23(6):567–578. doi: 10.1080/10498850.2012.738357. [DOI] [Google Scholar]
- Prado Martin J, Porto E, de Alencar S, da Glória E, Corrêa C, Ribeiro Cabral S. Antimicrobial activity of yerba mate (Ilex paraguariensis St. Hil.) against food pathogens. Rev Argent Microbiol. 2013;45:93–98. doi: 10.1016/S0325-7541(13)70006-3. [DOI] [PubMed] [Google Scholar]
- Quintana G, Gerbino E, Gómez-Zavaglia A. Valorization of okara oil for the encapsulation of Lactobacillus plantarum. Food Res Int. 2018;106:81–89. doi: 10.1016/j.foodres.2017.12.053. [DOI] [PubMed] [Google Scholar]
- Ramírez MJ, Giraldo GI, Orrego CE. Modeling and stability of polyphenol in spray-dried and freeze-dried fruit encapsulates. Powder Technol. 2015;277:89–96. doi: 10.1016/j.powtec.2015.02.060. [DOI] [Google Scholar]
- Rodríguez Chanfrau J, López Armas M (2014) Ultrasound assisted extraction of polyphenols from Punicagranatum (Grenada) fruit. Rev Cuba Farmacol 48:1–8. http://scielo.sld.cu/pdf/far/v48n3/far12314.pdf
- Sablania V, Bosco SJD. Optimization of spray drying parameters for Murraya koenigii (Linn) leaves extract using response surface methodology. Powder Technol. 2018;335:35–41. doi: 10.1016/j.powtec.2018.05.009. [DOI] [Google Scholar]
- Saikia S, Mahnot NK, Mahanta CL. Optimisation of phenolic extraction from Averrhoa carambola pomace by response surface methodology and its microencapsulation by spray and freeze drying. Food Chem. 2015;171:144–152. doi: 10.1016/j.foodchem.2014.08.064. [DOI] [PubMed] [Google Scholar]
- Singleton V, Orthofer R, Lamuela-Raventós R, Lester P. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Stunda-Zujeva A, Irbe Z, Berzina-Cimdina L. Controlling the morphology of ceramic and composite powders obtained via spray drying—a review. Ceram Int. 2017;43(15):11543–11551. doi: 10.1016/j.ceramint.2017.05.023. [DOI] [Google Scholar]
- Tang J, Yang T. Dehydrated vegetables: principles and systems. In: Hui YH, Ghazala S, Graham DM, Murrell KD, Nip W-K, editors. Handbook of vegetable preservation and processing. New York: Marcel Dekker; 2004. [Google Scholar]
- Tonon RV, Freitas SS, Hubinger MD. Spray drying of açai (Euterpe oleraceae Mart.) juice: effect of inlet air temperature and type of carrier agent. J Food Process Preserv. 2011;35(5):691–700. doi: 10.1111/j.1745-4549.2011.00518.x. [DOI] [Google Scholar]
- Valerga J, Reta M, Lanari MC. Polyphenol input to the antioxidant activity of yerba mate (Ilex paraguariensis) extracts. LWT Food Sci Technol. 2012;45(1):28–35. doi: 10.1016/j.lwt.2011.07.022. [DOI] [Google Scholar]
- Wang J, Sun B, Cao Y, Tian Y, Li X. Optimization of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008;106:804–810. doi: 10.1016/j.foodchem.2007.06.062. [DOI] [Google Scholar]
- Wilkowska A, Ambroziak W, Czyżowska A, Adamiec J. Effect of microencapsulation by spray-drying and freeze-drying technique on the antioxidant properties of blueberry (Vaccinium myrtillus) juice polyphenolic compounds. Pol J Food Nutr Sci. 2016;66(1):11–16. doi: 10.1515/pjfns-2015-0015. [DOI] [Google Scholar]
- Yang Y, Ming J, Yu N. Color image quality assessment based on CIEDE2000. Adv Multimed. 2012;2012:1–6. doi: 10.1155/2012/273723. [DOI] [Google Scholar]