Abstract
In this study, chemical profiling, phenolic and flavonoid contents, as well as the antibacterial and antioxidant activity of 17 Iranian Chrysanthemum morifolium cultivars were evaluated. The high performance liquid chromatography analysis revealed the presence of eight compounds with the major constituents including chlorogenic acid (0–934.7 mg/100 g), ferulic acid (12.7–171.6 mg/100 g), rutin (0–225.8 mg/100 g) and luteolin (2.83–213.5 mg/100 g). The cultivar “Ashna” with 63.6 mg tannic acid equivalents g−1 DW had the highest amount of total phenol, while the highest flavonoid content (13.52 mg quercetin equivalents g−1DW) was observed in cultivar “Shokoh”. The antioxidant activities of the samples were determined using 1,1-diphenyl-2-picrylhydrazyl (DPPH) and the reducing power assay. The results showed that the cultivars “Poya3” (IC50 = 385.7) and “Dorna2” (IC50 = 489.4) possessed a higher antioxidant activity than the others in DPPH model system. Antimicrobial activity was also evaluated based on minimal inhibitory concentration (MIC) and minimal bactericidal concentration values. MIC values were in the range of 5–10 mg ml−1 against Salmonella enterica and Bacillus cereus and 10–20 mg ml−1 against Staphylococcus aureus and Escherichia coli. Finally, Chrysanthemum cultivars with high bioactive compounds were introduced for beneficial usage in food and industrial applications.
Keywords: Chrysanthemum, Flavonoid, Phenol, Antioxidant, Antibacterial activity
Introduction
Natural antioxidants such as phenolic compounds and flavonoids contribute to protecting biological systems as free radical scavengers to reduce the risk of cancer and other chronic diseases (Tenorio-Rodriguez et al. 2017; Kumar and Singh 2015), which have also been used in many formulations of food and pharmaceutical. Medicinal herbs have been considered as the most important targets to obtain natural antioxidants (Prathapan et al. 2011). Chrysanthemum (Chrysanthemum morifolium Ramat.) is an important medicinal herb that belongs to the family of Asteraceae (Park et al. 2015). The flower extract of this medicinal herb has been used for centuries in many Asian countries as healthy food, beverage and traditional Chinese medicine (TCM) (Ye et al. 2007) because of its extensive biological characteristics including antioxidant, anticancer, antimutagenic, anti-inflammatory, antibacterial and antiviral activities (Xie et al. 2012). The Chrysanthemum flower extract can be used as a raw material in tea production, which is a very common beverage in China and Korea. Chrysanthemum leaves are also steamed or boiled and used as vegetables in Chinese food (Khaing et al. 2013).
The problem of microbial resistance and lower effectiveness of commercial antibiotics is growing, and the use of natural sources of medicinal plant extracts is increasing in food industries (Salami et al. 2016) Several types of research showed that C. morifolium contains a significant antioxidant and antibacterial activity (Yeasmin et al. 2016). The health benefits of C. morifolium flowers are closely related to its bioactive compound (Sun et al. 2010) such as polyphenols, which include phenolic acids, flavonoids and anthocyanins (Rezaei et al. 2017).
Various types of flavonoids have been identified in plant kingdom including anthocyanins, flavonols (quercetin and rutin), flavones (luteolin and apigenin), flavanone (naringenin, hesperetin), and isoflavones (genistein, daidzein). Previous studies identified some major phenolic acids and flavonoids in the flower extract of the genus Chrysanthemum with different bioactivity including anti-HIV (Lin and Harnly 2010) and antiallergic effects (Xie et al. 2012). According to Chinese pharmacopoeia, chlorogenic acid and luteolin are used as representative compounds for the quality control of Chrysanthemum flowers. Luteolin is one of the most common flavonoids that has been isolated from the C. morifolium flowers. Moreover, antioxidant activities also exhibited cytotoxic activity against human colon cancer cells (Xie et al. 2009). These considerations warrant the introduction of new cultivars with high chlorogenic acid and luteolin for useful applications by the food industry. Identification of the chemical composition of C. morifolium flowers not only is valuable for quality control but also would enhance the knowledge of the health benefits of this plant. Therefore, a comparison of the C. morifolium cultivars can provide new insights for the introduction of the potent cultivars of C. morifolium for further applications.
Previous studies reported some phenolic and flavonoid compounds in C. morfolium originated from different countries including China (Wang et al. 2008), Japan (Xie et al. 2012) and Korea (Park et al. 2015). However, no studies have been conducted to determine the quality and quantity of polyphenols in Iranian Chrysanthemum cultivars as well as their antioxidant and antibacterial activity. Moreover, different flower colors and their relationship with bioactive components provide another motivation for the present research to investigate the diversity among Iranian C. morfolium cultivars with different flower colors to introduce the elite ones for further food and pharmaceutical purposes.
Therefore, the purpose of this study was to assess the antioxidant and antibacterial activity, phenolic and flavonoid content and determine the major phenolic and flavonoid compounds in 17 Iranian C. morifolium.
Materials and methods
Plant materials
Seventeen cultivars of Chrysanthemum (Chrysanthemum morifolium Ramat.) including Shokoh, Atashgoon, Atash2, Sahar, Marmar, Sahand2, Dorna2, Ashna, Hour, Erica, Poya3, Bolor, Romina, Farhood, Khalijfars2, Taraneh, and Mehrnoosh2 originated from the Iranian Research Center for Ornamental Plants, Mahallat, Iran was used in this study. Stock plants were cultivated at the research farm of Isfahan University of Technology in April 2015, using a randomized complete block design in three replicates. The experimental samples were collected in October to November based on different studied cultivars. The flower samples were evaluated in the experimental farm in each replicate when ray florets were completely open and the pollens did not appear in disk florets.
Methanolic extraction
Dried ground flowers (2.5 g) was extracted with methanol–water (50 ml, 80:20, v/v) and orbital shaker (150 rpm) at 25 °C for 24 h. The extract was filtered through three layers of cheesecloth to remove the solid debris.
Total phenolic and flavonoid content of the extracts
Total phenolic contents (TPC) were determined by the modified Folin–Ciocalteau colorimetric method as described by Gharibi et al. (2013). The amounts of phenolics were stated in mg tannic acid equivalent (TAE) per gram dry weight of the sample. The methanolic extract (0.5 ml) was mixed with Folin–Ciocalteau reagent (2.5 ml, 1:10 diluted with distilled water) and 7.5% sodium carbonate (2 ml). The mixture heated at 45 °C for 15 min. The absorbance was measured at 765 nm against a blank.
The colorimetric aluminium chloride method was used to determine the total flavonoid contents (TFC) of the extract (Hossain et al. 2011). In this assay, 0.5 ml solution of each plant extracts in methanol was mixed with water (4.5 ml) and sodium nitrate 5% (0.3 ml). Then, the test tubes were kept in the dark for 5 min. After that, 10% aluminium chloride (0.6 ml) was added. Finally, the solution of sodium hydroxide 4% (2 ml), and 2 ml distilled water were also mixed. The absorbance of the reaction mixture was measured at 510 nm. The total flavonoid content was presented in mg quercetin equivalents (QE) per gram dry weight of the sample.
DPPH radical scavenging assay
The DPPH-free radical scavenging capacity of plant extracts was evaluated according to the methodology described by Braca et al. (2002). The reaction mixture consisted of adding 0.1 ml of extract and 5 ml of DPPH radical solution 1 mM in methanol at the chosen concentrations (50, 100 and 300 ppm). The mixtures were kept in the dark for 30 min. The range of color change was measured at 517 nm from deep violet to light yellow. The results obtained were compared with Butylatedhydroxytoluene (BHT) as standard synthetic antioxidant. The IC50 values (the concentration of antioxidant which reduces the free radical DPPH about 50%) were calculated by plotting the extract concentration versus the corresponding scavenging activity.
Reducing power
Reducing power activity was performed according to the method of Ardestani and Yazdanparast (2007). 1 ml of the methanol extract of different concentrations (50, 100, and 300 ppm) was mixed with phosphate buffer (2.5 ml, 0.2 M, PH = 6.6) and 2.5 ml potassium ferricyanide [K3Fe(CN)6]. Then, the reaction was incubated in water bath at 50 °C for 20 min. Then, 2.5 ml of trichloroacetic acid (10%) was added and the solution was centrifuged at 4000 rpm for 10 min. The supernatant (2.5 ml) was mixed with 2.5 ml of deionized water and 0.5 ml of FeCl3 (0.1%). The absorbance of the mixture was read by a spectrophotometer at 700 nm against a blank. BHT was also applied as a standard antioxidant.
HPLC analysis
Dried ground flowers (1.25 g) were extracted as the way that previously reported. The extract was filtered through a 0.22 μm nylon acro disk filter, and a 20 μl of the extract was injected to the analytical column for the analysis. The standards were purchased from Sigma-Aldrich with high purities including chlorogenic acid (≥ 96% purity), p-coumaric acid (98% purity), rutin (≥ 95% purity), ferulic acid (≥ 95% purity), quercetin (≥ 95% purity), gallicacid (97% purity), apigenin (95% purity) and luteolin (≥ 95% purity). The 1000 mg l−1 stock solutions were prepared in HPLC grade methanol. The UV spectra of standards were obtained at the range of 200–400 nm with HPLC system consisting a model Agilent 1090. A 250 mm × 4.6 mm, 5 μm, symmetry C18 column (Waters Crop., Milford, MA, USA) with 10 mm × 4 mm i.d., 5 μm, sentry guard column at flow rate 0.8 ml min−1. The column temperature was set at 25 °C. 0.1% of water-formic acid was used as the solvent A, while 0.1% of formic acid in acetonitrile was applied as solvent B in the mobile phase. The gradient conditions were set as follows: a linear step from 10 to 26% solvent B (v/v) in 40 min, 65% solvent B at 70 min, and finally to 100% solvent B to 75 min. The phenolic compounds of sample extracts were identified by the comparison of the peak area and retention time with those of pure standards. The results were expressed as mg per 100 g of sample dry weight.
Antibacterial activity of Chrysanthemum extracts
The flower extracts were individually tested against S. aureus (PTCC 1337), B. cereus (PTCC 1665), S. enterica (PTCC 1709), and E. coli (O157:H7). Bacterial strains were prepared in the Microbiology Laboratory of Isfahan University of Technology, Iran.
The minimum inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) were determined for each extract, using a serial dilution method. 1 ml of different doses of the flower extracts (0.15, 0.3, 0.6, 1.25, 2.5, 5, 10, 20 and 40 mg ml−1) were added to Mueller-Hinton broth (1 ml) and then 10 µl of the bacterial culture (diluted to 0.5 McFarland standards) was introduced. Tetracycline was used as a positive control. Stock solutions of the extracts and antibiotic standards were then incubated at 37 °C for 24 h. The microbial growth was examined after incubation the tubes by observing the turbidity. A loop-full of broth was collected from those tubes to determine the MBC, for each set of test tubes in the MIC determination, which did not show any growth and inoculated onto sterile nutrient agar by streaking. Nutrient agar plates only were streaked with the respective test organisms to serve as controls. All the plates were then incubated at 37 °C for 24 h. After incubation, the Minimum Bactericidal Concentration (MBC) was noted as the concentration at which no visible growth was observed.
Data analysis
The data represent mean ± SE from triplicate experiments. One-way analysis of variance (ANOVA) was carried out and Fisher’s (protected) least significant difference (LSD) was applied to compare means at the 95% confidence level using SAS ver. 9 software. The correlation coefficients were calculated using SPSS (version 16; SPSS Inc., Chicago, IL., USA). The principle component analysis (PCA) was performed to explore the relationships among the cultivars, using the Stat Graphics software (version 6).
Results
Total phenolic (TPC) and flavonoid content (TFC)
The present study revealed a significant variation in TPC values among Chrysanthemum cultivars. The results are presented in Table 1. TPC varied from 18.54 to 63.61 mg TAE/g DW of the samples. The highest phenolic content was in methanolic extract of the cultivar “Ashna”, whereas the cultivar “Poya3” contained the lowest value.
Table 1.
Total phenolic (TPC) and flavonoid (TFC) content of 17 Chrysanthemum cultivars
| Cultivars | TPC (mg tannic acid/g DW) | TFC (mg quercetin/g DW) |
|---|---|---|
| Shokoh | 46.04e ± 0.0009 | 13.52a ± 0.09 |
| Atashgoon | 47.82d ± 0.008 | 8.65ij ± 0.36 |
| Atash2 | 35.12k ± 0.0004 | 11.88cde ± 1.41 |
| Erica | 25.24p ± 0.016 | 9.46hi ± 0.43 |
| Sahar | 30.70n ± 0.012 | 9.35hi ± 0.49 |
| Marmar | 29.73o ± 0.021 | 6.71l ± 0.42 |
| Poya3 | 18.54q ± 0.020 | 7.76jk ± 0.02 |
| Sahand2 | 31.21m ± 0.01 | 10.03gh ± 0.45 |
| Dorna2 | 32.17l ± 0.67 | 12.04cd ± 0.13 |
| Ashna | 63.61a ± 0.008 | 13.23ab ± 0.13 |
| Hour | 43.26f ± 0.019 | 7.29kl ± 0.52 |
| Bolor | 36.52j ± 0.007 | 11.44efd ± 0.27 |
| Romina | 39.24g ± 0.012 | 11.02ef ± 0.22 |
| Farhood | 37.53h ± 0.009 | 10.56fg ± 0.06 |
| Khalijfars2 | 52.34b ± 0.011 | 11.13def ± 0.11 |
| Taraneh | 36.69i ± 0.010 | 12.54bc ± 0.20 |
| Mehrnoosh2 | 50.40c ± 0.012 | 9.18hi ± 0.02 |
TPC: total phenolic content, TFC: total flavonoid content
Means with different letter are statistically significant at 5% level probability
Regarding the total flavonoid content (TFC), the differences between the cultivars were statistically significant (P < 0.05). The flavonoid contents ranged between 6.71 mg QE/gDW in cultivar “Marmar” and 13.52 mg QE/g DW in cultivar “Shokoh”, respectively (Table 1).
DPPH radical scavenging assay
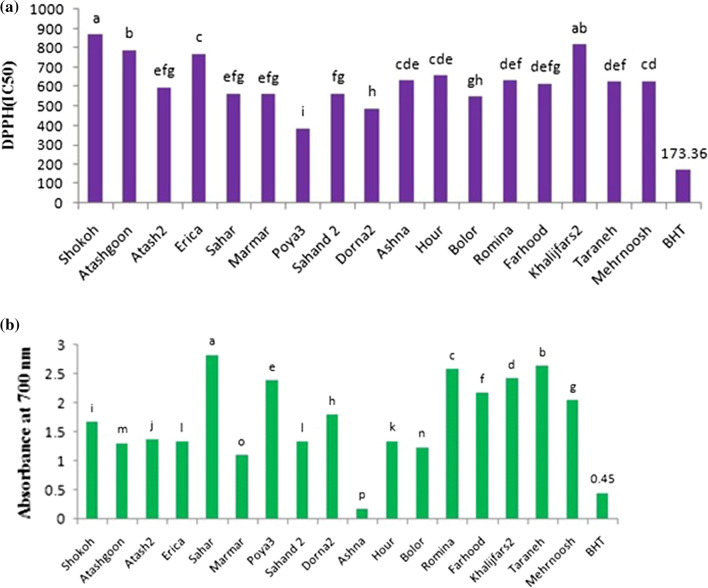
The DPPH radical scavenging activity of the cultivars was recorded according to their IC50 values (Fig. 1a). The IC50 values were found in the range of 385.72–868.6 μg ml−1. Basically, the higher DPPH radical scavenging activity is associated with a lower IC50 value. In the present research, “Poya3” revealed the highest antioxidant activity, while “Shokoh” exhibited the lowest value. The cultivar “Poya3” exhibited the highest total antioxidant activity with yellow petals, but it showed lower DPPH radical scavenging activity than BHT with an IC50 value as much as 173.36 μg ml−1.
Fig. 1.
a IC50 values of Chrysanthemum cultivars extracts compared to BHT, b reducing power of Chrysanthemum extracts compared to BHT. Means with different letter are statistically significant at 5% level probability
Reducing power
Figure 1b presents the ferric reducing antioxidant powers of Chrysanthemum extracts at the concentration of 300 ppm. The highest activity was recorded by the cultivars “Sahar” and “Taraneh” with an absorbance value as much as 2.812 and 2.624, respectively. In this model system, the cultivar “Ashna” showed a lower reducing power than BHT.
Chemical profiling of Chrysanthemum cultivars
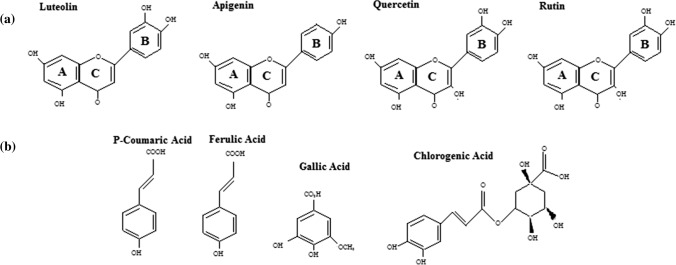
Determination of total phenolic content does not give a full picture of the quality and quantity of phenolic constituents in the extracts. Therefore, the chemical profiling and quantification of some important compounds from 17 cultivars of C. morifolium flower were carried out using the HPLC method. The HPLC analysis revealed a high chemical polymorphism between the studied cultivars. Generally, four phenolic acids (chlorogenic acid, p-coumaric acid, gallic acid and ferulic acid) and four flavonoids (rutin, apigenin, quercetin and luteolin) were identified. The concentrations of compounds in the studied cultivars are illustrated in Table 2. The structure of the studied compounds is presented in Fig. 2.
Table 2.
The mean of major phenolic and flavonoid compounds of the studied cultivars based on HPLC analysis (The values are expressed in mg/100 g of sample dry weight; each value is represented as mean ± SEa)
| Cultivar | Gallic acid | Chlorogenic acid | P-coumaric acid | Rutin | Ferulic acid | Luteolin | Quercetin | Apigenin |
|---|---|---|---|---|---|---|---|---|
| Shokoh | 2.55 ± 0.10 | 242.49 ± 1.05 | 0.05 ± 0.007 | 36.30 ± 0.21 | 15.72 ± 0.15 | 77.42 ± 0.08 | 22.39 ± 0.06 | 11.46 ± 0.11 |
| Atashgoon | nd | 395.28 ± 1.61 | 0.03 ± 0.003 | 114.32 ± 0.93 | 58.73 ± 0.09 | 10.46 ± 0.11 | 20.73 ± 0.09 | 9.26 ± 0.11 |
| Atash2 | 1.01 ± 0.007 | 295.37 ± 0.97 | 0.01 ± 0.0007 | 94.47 ± 1.04 | 64.72 ± 0.44 | 2.83 ± 0.16 | 8.45 ± 0.03 | 9.24 ± 0.17 |
| Sahar | nd | 264.33 ± 0.46 | 0.08 ± 0.012 | 40.21 ± 0.43 | 14.94 ± 0.52 | 29.58 ± 0.34 | 30.90 ± 0.28 | 9.23 ± 0.02 |
| Marmar | 2.74 ± 0.10 | 241.68 ± 1.19 | 0.04 ± 0.002 | 43.65 ± 0.46 | 12.72 ± 0.50 | 46.58 ± 0.12 | 9.86 ± 0.11 | 9.28 ± 0.05 |
| Sahand 2 | 3.20 ± 0.07 | 552.89 ± 1.34 | 0.07 ± 0.004 | 96.77 ± 0.75 | 22.38 ± 0.20 | 10.19 ± 0.13 | 24.01 ± 0.01 | 9.57 ± 0.26 |
| Dorna2 | 4.13 ± 0.09 | 365.59 ± 1.83 | 0.09 ± 0.007 | 62.72 ± 0.44 | 22.16 ± 0.11 | 81.66 ± 0.25 | 16.82 ± 0.08 | 11.14 ± 0.10 |
| Ashna | 1.89 ± 0.10 | 199.61 ± 1.14 | 0.052 ± 0.003 | 42.54 ± 0.38 | 17.59 ± 0.42 | 19.82 ± 0.29 | 38.69 ± 0.13 | 10.16 ± 0.11 |
| Hour | 4.22 ± 0.07 | 472.78 ± 1.26 | 0.02 ± 0.0007 | 61.41 ± 0.29 | 20.64 ± 0.38 | 19.83 ± 0.30 | 36.80 ± 0.21 | 10.37 ± 0.19 |
| Erica | nd | 3.63 ± 0.09 | 9.27 ± 0.47 | nd | 79.68 ± 0.76 | 38.20 ± 0.14 | 41.28 ± 0.06 | 14.65 ± 0.46 |
| Poya3 | 0.98 ± 0.03 | 279.26 ± 1.59 | 0.06 ± 0.007 | 26.48 ± 0.34 | 17.82 ± 0.43 | 37.38 ± 0.20 | 39.62 ± 0.15 | 8.48 ± 0.27 |
| Bolor | 1.48 ± 0.05 | 934.74 ± 1.02 | nd | 77.34 ± 0.52 | 13.34 ± 0.24 | 121.23 ± 0.16 | 8.02 ± 0.01 | 17.09 ± 0.20 |
| Romina | nd | 3.27 ± 0.12 | 0.03 ± 0.0007 | 100.70 ± 0.28 | 17.22 ± 0.15 | 213.48 ± 0.55 | 24.26 ± 0.18 | 10.81 ± 0.36 |
| Farhood | 4.96 ± 0.06 | 353.36 ± 1.67 | 0.09 ± 0.001 | 225.82 ± 0.86 | 24.29 ± 0.20 | 5.39 ± 0.28 | 14.51 ± 0.15 | 71.37 ± 0.26 |
| Khalijfars2 | 3.507 ± 0.04 | 741.61 ± 1.13 | 3.31 ± 0.14 | 154.51 ± 0.36 | 24.19 ± 0.14 | 78.14 ± 0.17 | 17.74 ± 0.10 | 23.34 ± 0.24 |
| Taraneh | nd | nd | nd | 103.24 ± 0.38 | 171.56 ± 0.32 | 40.76 ± 0.40 | 43.82 ± 0.50 | 20.37 ± 0.26 |
| Mehrnoosh2 | 1.31 ± 0.01 | 504.08 ± 0.62 | 0.09 ± 0.0007 | 73.10 ± 0.21 | 14.88 ± 0.48 | 190.89 ± 0.49 | nd | 69.00 ± 0.28 |
| RTb | 5.15 | 13.44 | 25.32 | 27.91 | 28.66 | 49.79 | 50.09 | 55.95 |
aData are mean ± SE (standard error), bretention time (Min)
nd none detected
Fig. 2.
Structure of studied a flavonoid and b phenolic acid compounds
Rutin and luteolin amounted to 76.68% of the total concentration of detected flavonoids. Rutin showed a high variation among different studied samples. The highest level of rutin was detected in cultivar “Farhood” (225.82 mg/100 g DW) and the lowest one observed in “Poya3” (26.48 mg/100 g DW) and “Erica” (0 mg/100 g DW) (Table 2). Luteolin is a flavone, which has been as an antioxidant, free radical scavenger, immune system modulator, anti-inflammatory and cancer inhibitor agent (Ghasemzadeh and Ghasemzadeh 2011) that found in various food plants. Significant differences were found among the luteolin levels in different cultivars. The cultivar “Romina” showed the highest level of luteolin (213.48 mg/100 g DW) followed by “Bolor” (121.23 mg/100 g DW) and “Atash2” contained the least amount of luteolin (2 mg/100 g DW). Quercetin and apigenin were also detected in the present study. Quercetin ranged from 8.02 mg/100 g DW in “Bolor” to 43.82 mg/100 g DW in cultivar “Taraneh”. The highest amount of apigenin was obtained in “Farhood” (71.37 mg/100 g DW) followed by the cultivar “Mehrnoosh2” (69.00 mg/100 g DW) and the lowest one was obtained in “Poya3” (8.48 mg/100 g DW).
Like flavonoids, phenolic acids are secondary metabolites to confer special taste, flavor and medicinal properties to the plants. The total concentration of the detected phenolic acids was more than flavonoids in all studied cultivars except for the cultivars “Erica”, “Bolor” and “Taraneh” (Table 2). In the present research, the major phenolic acids were chlorogenic acid, followed by ferulic acid. The chlorogenic acid has been considered as a suitable component for the quality control of C. morifolium (Xie et al. 2012), which represents up to 60% of total polyphenols in the present study. It ranged from 3.63 mg/100 g DW in cultivar “Erica” to 934.74 mg/100 g in “Bolor”. The chlorogenic acid was not detected in cultivar “Taraneh”. Moreover, ferulic acid varied from 12.72 mg/100 g DW in “Marmar” to 171.56 mg/100 g DW in “Taraneh”. Gallic and p-coumaric acid had the lowest amount in the present study among identified phenolic compounds. The cultivar “Farhood” (4.96 mg/100 g) possessed the highest amount of gallic acid (Table 2), which was not detected in cultivars “Atashgoon”, “Sahar”, “Romina”, “Taraneh” and “Erica”.
Antibacterial activity
The results showed that the range of MIC values in Chrysanthemum cultivars was 5–10 mg ml−1 against S. enterica and B. cereus and 10–20 mg ml−1 against S. aureus and E. coli (Table 3). The methanol extract of cultivars “Shokoh”, “Bolor”, “Romina”, “Erica”, “Sahar”, “Marmar” and “Poya3” possessed higher antibacterial activity than other samples for B. cereus (MIC = 5 mg ml−1). Cultivars “Bolor”, “Poya3” and “Khalijfars2” showed the lowest MIC (5 mg ml−1) and MBC (10 mg ml−1) values against S. enterica. For E. coli all flower extracts were effective except the cultivar “Atash2”. Probably, more concentration of the extract could be effective against this bacterium.
Table 3.
MIC and MBC (mg ml−1) values of 17 C. morifolium cultivars
| Samples | Microorganisms | |||||||
|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | Bacillus cereus | Salmonella enterica | Escherichia coli | |||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Shokoh | 10 | 20 | 5 | 10 | 10 | 20 | 10 | 20 |
| Atashgoon | 20 | 40 | 10 | 20 | 10 | 20 | 10 | 20 |
| Atash2 | 10 | 20 | 10 | 20 | 10 | 20 | – | – |
| Sahar | 10 | 20 | 5 | 10 | 10 | 20 | 10 | 20 |
| Marmar | 10 | 20 | 5 | 10 | 10 | 20 | 20 | 40 |
| Sahand 2 | 10 | 20 | – | – | 10 | 20 | 10 | 20 |
| Dorna2 | 20 | 40 | – | – | 10 | 20 | 20 | 40 |
| Ashna | 10 | 20 | – | – | 10 | 20 | 10 | 20 |
| Hour | 20 | 40 | – | – | 10 | 20 | 20 | 40 |
| Erica | 10 | 20 | 5 | 10 | 10 | 20 | 10 | 20 |
| Poya3 | 10 | 20 | 5 | 10 | 5 | 10 | 10 | 20 |
| Bolor | 10 | 20 | 5 | 10 | 5 | 10 | 10 | 20 |
| Romina | 10 | 20 | 5 | 10 | 10 | 20 | 10 | 20 |
| Farhood | 10 | 20 | – | – | 10 | 20 | 10 | 20 |
| Khalijfars2 | 10 | 20 | – | – | 5 | 10 | 10 | 20 |
| Taraneh | 20 | 40 | – | – | 10 | 20 | 20 | 40 |
| Mehrnoosh2 | 10 | 20 | – | – | 10 | 20 | 10 | 20 |
| Tetracycline | < 0.15 | 0.15 | < 0.15 | 0.15 | < 0.15 | 0.15 | < 0.15 | 0.15 |
According to Sanchez et al. (2014) the lack of significant correlation between the antibacterial activity and the total phenolics suggest that the antibacterial activity could be related to a specific or a mixture of compounds. The results of the present study established a good correlation between these phytochemicals and antimicrobial activity. According to the correlation analysis the contents of apigenin (r = − 0.558*) and gallic acid (r = − 0.541*) were negatively correlated to the MIC values of B. cereus.
Cluster and principal component analysis
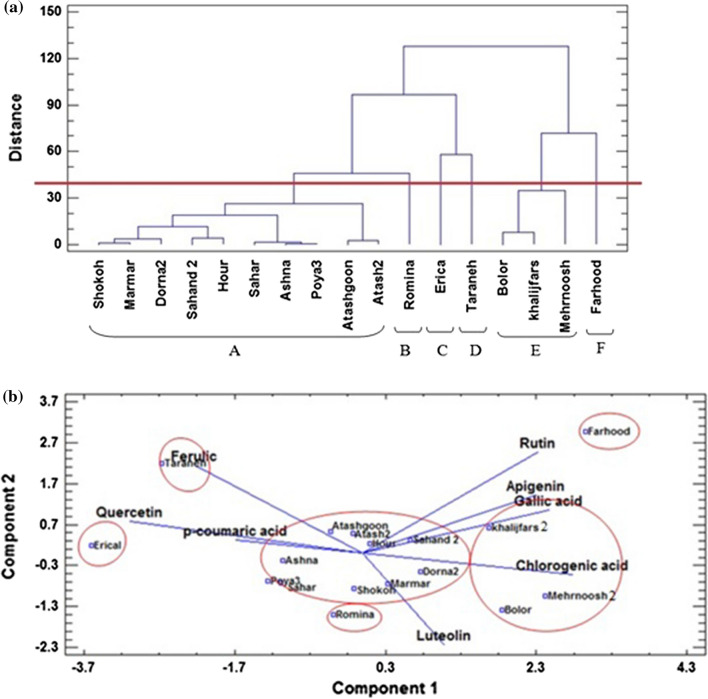
According to cluster analysis the chemical profiles of cultivars were compared and divided into six distinct groups namely A, B, C, D, E, and F (Fig. 3). The cultivars “Romina”, “Erica”, “Taraneh” and “Farhood” formed single groups (B, C, D, and F), because of their highest amount of p-coumaric acid, ferulic acid, luteolin and rutin, respectively. Cultivars “Mehrnoosh2”, “Khalijfars2” and “Bolor” were classified in group E, characterized by higher quantities of chlorogenic acid and luteolin. These cultivars also possessed high amounts of TPC and TFC values. Other cultivars were categorized in group A with moderate or high levels of these compounds.
Fig. 3.
a Dendrogram of 17 C. morifolium cultivars using ward clustering method, b PCA analysis to classification of studied Chrysanthemum cultivars based on bioactive compounds
The principal component analysis (PCA) was also performed on chromatographic data to identify relationships among the cultivars and all measured bioactive compounds. The PCA classification confirmed the results of cluster analysis (Fig. 3). Most of the variation (69.67%) was explained by the first three principal components. The results showed that the first and second components explained 53.44% of the total variation. The first PC (PC1) showed 36.02% of the total variation, which had a positive correlation with chlorogenic acid (+ 0.42), but a negative correlation with quercetin (− 0.47). PC2 explained 17.41% of the total variance. The PC2 results showed a high correlation by rutin (+ 0.55), ferulic acid (+ 0.0.48), and luteolin (− 0.49).
Correlation analysis among bioactive compounds
The correlation analysis represented a positive correlation coefficient between rutin and apigenin (r = + 0.562*). There was a negative correlation between the chlorogenic acid and quercetin (r = − 0.539*). Furthermore, a positive and significant correlation (r = + 0.530*) was observed between the antioxidant activity (measured by reducing power model system) and total flavonoids, measured by HPLC analysis.
Discussion
This was the first report on the total phenolic content of Iranian C. morifolium cultivars. Previous studies in other countries, reported different ranges based on various standards and solvents for TPC value in Chrysanthemum cultivars. For instance, in Chinese C. morifolium TPC ranged from 1.070 to 96.20 mg catechin/g (Zeng et al. 2014). Duh et al. (1999) reported the range of 32.3–45.7 mg g−1 for TPC in the water extract of Chinese C. morifolium cultivars. Hung et al. (2012) also compared the total phenolic content of Chrysanthemum species. They showed a higher amount of TPC in the flower part of C. morifolium in comparison with Chrysanthemum indicum. Moreover, the previous studies on C. morifolium have been reported different amounts of flavonoids, which might be attributed to the application of different standards, solvents, phenological stages and genotypes. Jin and Wen (2015) revealed the content of flavonoids in the ethanolic extract of C. morifolium based on rutin as a standard (12.46 mg rutin/g) while, TFC of the aqueous extract of Chinese C. morifolium cultivars varied from 10.83 to 83.79 mg catechin/g DW (Zeng et al. 2014).
Lim (2014) reported that the highest antioxidant activity was obtained in yellow petal cultivars of Chrysanthemum such as “Mottenohoka” followed by “Kotobuki” and “Iwakaze” with purple petals. Higher antioxidant activity in methanolic extract of C. morifolium has also been reported compared to the ethyl acetate or petroleum ether extract. Scavenging effects of the water extracts from Chinese C. morifolium cultivars were in the range of 71.7–95.1% (Duh et al. 1999), while Zeng et al. (2014) reported lower radical scavenging activity (11.97%) for ethanolic extract of Chinese C. morifolium. Debnath et al. (2013) reported the reducing power activities from 0.07 to 0.55 and 0.09 to 0.68 for the water and ethanol extract of C. indicum, respectively at the concentration range from 0.125 to 2 ppm. In the present study, the reducing power of methanolic extracts, was higher than those reported in C. indicum. Duh et al. (1999) evaluated the antioxidant activity of water extracts from four varieties of C. morifolium. The results showed that all varieties had effective activities as metal chelators. In the present research, the reducing power of the examined cultivars suggests that most of the cultivars have high chelating power for the transition metals.
Earlier studies identified gallic acid, chlorogenic acid, caffeic acid, ferulic acid and p-coumaric acid as the major phenolic acids in the flower extract of the genus Chrysanthemum. Furthermore, the flowers also contain significant amounts of flavonoids (Lin and Harnly 2010) such as luteolin, luteolin-7-O-β-d-glucoside, acacetin-7-O-β-d-glucoside and apigenin-7-O-β-d-glucoside (Ye et al. 2007). Rutin and luteolin were the most abundant flavonoid compounds in C. morifolium cultivars used in this research. Hajimehdipoor et al. (2014) showed that a combination of rutin with other phenolic and flavonoid compounds did not change their antioxidant activity. Previous studies reported 7.14 mg/100 g FW (Sugawara and Igarashi 2009) and 5.22 mg/g DW (Sun et al. 2010) luteolin in Japanese and Chinese C. morifolium flowers, respectively. Hence, luteolin concentration in Iranian C. morifolium cultivars was much higher than those reported in Japanese ones. Shen et al. (2010) determined quercetin and apigenin concentration, in the range of 1.02–20.48 mg l−1 and 1.12–22.40 mg l−1, in Chinese Chrysanthemum flowers. In the present study, quercetin concentration in Iranian Chrysanthemum cultivars (4.01–21.91 mg l−1) was similar to Chinese flowers, but the range of apigenin (4.24–34.50 mg l−1) was higher than those reported in Chinese Chrysanthemum.
Antioxidant activity of flavonoids depends on the structure and substitution pattern of hydroxyl groups (Fig. 2). Two hydroxyl groups are required in B ring for stronger antioxidant potential. The lack of one of these hydroxyl groups significantly reduces this activity. For example, the B ring has one hydroxyl group in apigenin that decreases its antioxidant activity (Wojdylo et al. 2007). Similar results were also obtained by Firuzi et al. (2005). They reported the antioxidant properties of flavonoids using ferric reducing antioxidant power (FRAP) assay. In their study, the order of FRAP values for flavonoids was reported as quercetin > rutin > apigenin.
The present results showed that the cultivars “Romina”, “Khalijfars2” and “Taraneh” were rich in these active compounds (quercetin, luteolin and rutin), which could be used as potential sources of antioxidants for the food and drug industries. It should be considered that a combination of these compounds in different cultivars may also affect their antioxidant properties. Yagi et al. (2012) reported the chemical profile of edible purple Chrysanthemum variety “Enmeiraku” that contains 330 mg/100 g chlorogenic acid. Also, Du et al. (2014) showed the highest chlorogenic acid content in C. morifolium cultivar “Tai Ju” (859 mg/100 g DW). The chlorogenic acid concentration in cultivar “Bolor” was strongly higher than that detected in Chinese one (“Tai Ju”).
The high amount of p-coumaric acid has been reported in biomass with low lignin content such as rye, barley and wheat, while Chrysanthemum flower residues were rich in lignin, could be a cost-effective raw material to produce enzymes for lignin degradation (Quevedo-Hidalgo et al. 2015). So, no remarkable peak was found for p-coumaric acid in studied cultivars, except for cultivar “Erica” (9.27 mg/100 g). Furthermore, no measurable peaks in terms of p-coumaric acid were found for the cultivars “Taraneh” and “Bolor”.
Chlorogenic acids are family of esters formed between certain hydroxyl cinnamates and quinic acid, and comprise sub-groups the most frequent of which are caffeoylquinic acids, feruloylquinic acids and p-coumaroylquinic acids (Stalmach et al. 2006). Thus, it can be suggested that cultivars might have higher amounts of ferulic acid and p-coumaric acid with low amounts of chlorogenic acid. A similar trend was also obtained in this research for Taraneh and Erica cultivars.
The results indicated that S. enterica and B. cereus were the most sensitive tested microorganisms and in the case of these bacteria methanol extracts were more effective. Yeasmin et al. (2016) evaluated the antibacterial activity of flower extracts of three flower colors of C. morifolium in Bangladesh. They showed that MIC and MBC values of the extracts have ranged from 150–250 mg ml−1 and 200–300 mg ml−1, respectively. Also, Indira et al. (2015) found a significantly effective antibacterial property of Indian C. morifolium at 400 μl concentration against Salmonella typhi, S. aureus and Pseudomonas aeruginosa. Therefore, the antibacterial activity of Iranian cultivars was much higher than the cultivars from other countries.
Methanolic extracts of cultivars “Bolor” and “Poya3” showed the highest antibacterial activity with the lowest MIC and MBC values against all tested bacterial strains. The cultivar “Bolor” contained the highest amounts of luteolin and chlorogenic acid, while, the cultivar “Poya3” had the highest antioxidant activity (in terms of DPPH assay) than the others. Therefore, variation in antibacterial properties can be referred to as the presence of various polyphenolic compounds in these cultivars. The polyphenolic compounds could be attributed to their interaction with the cell membrane and inhibition of the bacterial functions (Salami et al. 2016).
Free radicals can participate in the formation of advanced glycation end-products (AGEs) (Salami et al. 2016). Food products with a high concentration of polyphenols, such as luteolin, and chlorogenic acid are likely to inhibit AGEs formation. Accumulation of AGEs in tissues is referred to as glycation stress (Yagi et al. 2012). Therefore, the cultivars” Bolor” and “Mehrnoosh2”and “Khalijfars2” with high amounts of these polyphenols, might have a similar effect. The significant correlation between antioxidant activity and total flavonoids, measured by HPLC analysis, can suggested that flavonoid compounds were dominant antioxidant components for these cultivars.
Conclusion
The chemical compositions analysis of flowers in Chrysanthemum cultivars could provide new insights into the exploration of inherent and dynamic changes in antioxidants and health-promoting components. High antioxidant activities including the chlorogenic acid, rutin and luteolin which constituted as the major compounds of the examined cultivars would explain the health benefits of C. morifolium flower. In the present study, the Iranian cultivars including “Mehrnoosh2”, “Bolor”, and “Khalijfars2” possessed the highest amounts of chlorogenic acid and luteolin, which may be considered as valuable natural sources of antioxidants. According to the results obtained, it was evident that the metabolites derived from the flowers could effectively inhibit the growth of bacteria. In this respect, cultivar “Bolor” which contains the highest amounts of luteolin and chlorogenic acid showed the lowest MIC and MBC values against all tested bacterial strains. The results of this study may be useful for food or medical purposes. Finally, future research could be directed toward the development of new cultivars with a higher content of these major compounds.
Acknowledgements
The authors would like to express their appreciation to Mr. Mohammad Reza Shafie and the National Research Center of Ornamental Plants, Mahallat, Iran for providing plant materials for this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ardestani A, Yazdanparast R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007;104:21–29. doi: 10.1016/j.foodchem.2006.10.066. [DOI] [Google Scholar]
- Braca A, Sortino C, Politi M, Morelli I, Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J Ethnopharmacol. 2002;79:379–381. doi: 10.1016/S0378-8741(01)00413-5. [DOI] [PubMed] [Google Scholar]
- Debnath T, Jin HL, Hasnat MA, Kim Y, Samad NB, Park PJ, Lim BO. Antioxidant potential and oxidative DNA damage preventive activity of Chrysanthemum indicum extracts. J Food Biochem. 2013;37(4):440–448. doi: 10.1111/j.1745-4514.2011.00644.x. [DOI] [Google Scholar]
- Du H, Li SS, Wu Q, Ji KX, Wu J, Liu Y, Wang LS. Analysis of active compounds and antioxidant activity assessment of six popular Chinese Juhua teas. Nat Prod Commun. 2014;10:495–498. [PubMed] [Google Scholar]
- Duh PD, Tu YY, Yen GC. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat) Lebensm Wiss Technol. 1999;32:269–277. doi: 10.1006/fstl.1999.0548. [DOI] [Google Scholar]
- Firuzi O, Lacanna A, Petrocci R, Marrosu G, Saso L. Evaluation of the antioxidant, activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim Biophys Acta. 2005;1721:174–184. doi: 10.1016/j.bbagen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Gharibi S, Sayed Tabatabaei BE, Saeidi G, Goli SAH, Talebi M. Total phenolic content and antioxidant activity of three endemic Achillea species. Ind Crops Prod. 2013;50:154–158. doi: 10.1016/j.indcrop.2013.07.038. [DOI] [Google Scholar]
- Ghasemzadeh A, Ghasemzadeh N. Flavonoids and phenolic acids; role and biochemical activity in plants and human. J Med Plants Res. 2011;5:6697–6703. [Google Scholar]
- Hajimehdipoor H, Shahrestani R, Shekarchi M. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Res J Pharmacogn. 2014;1:35–40. [Google Scholar]
- Hossain M, Muhammad MD, Charles G, Mahammad I. In vitro total phenolics, flavonoids contents and antioxidant activity of essential oil, various organic extracts from the leaves of tropical medicinal plant Tetrastigma from Sabah. Asian Pac J Trop Med. 2011;4:717–721. doi: 10.1016/S1995-7645(11)60180-6. [DOI] [PubMed] [Google Scholar]
- Hung CY, Tsai YC, Li KY. Phenolic antioxidants isolated from the flowers of Osmanthus fragrans. Molecules. 2012;17:10724–10737. doi: 10.3390/molecules170910724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indira TK, Shubha G, Deepak K, Anil K. Study of antimicrobial effect of Chrysanthemum morifolium Ramat. (Asteraceae) against some human pathogens. J Pharm Biol Sci. 2015;9:179–188. [Google Scholar]
- Jin JZ, Wen M. Extraction of Chrysanthemum morifolium extractum by ethanol modified supercritical CO2. Asian J Chem. 2015;27:2389–2392. doi: 10.14233/ajchem.2015.17843. [DOI] [Google Scholar]
- Khaing AA, Moe KT, Hong WJ, Park CS, Yeon KH, Park HS, Kim DC, Choi BJ, Jung JY, Chae SC, Lee KM, Park YJ. Phylogenetic relationships of Chrysanthemums in Korea based on novel SSR markers. Genet Mol Res. 2013;12:5335–5347. doi: 10.4238/2013.November.7.8. [DOI] [PubMed] [Google Scholar]
- Kumar CM, Singh SA. Bioactive lignans from sesame (Sesamum indicum L.): evaluation of their antioxidant and antibacterial effects for food applications. J Food Sci Technol. 2015;52:2934–2941. doi: 10.1007/s13197-014-1334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim TK. Edible medicinal and non-medicinal plants. Berlin: Springer; 2014. [Google Scholar]
- Lin LZ, Harnly JM. Identification of the phenolic components of Chrysanthemum flower (Chrysanthemum morifolium Ramat) Food Chem. 2010;120:319–326. doi: 10.1016/j.foodchem.2009.09.083. [DOI] [Google Scholar]
- Park CH, Chae SC, Park SY, Kim JK, Kim YJ, Chung SO, Arasu MV, Al-Dhabi NA, Park SU. Anthocyanin and carotenoid contents in different cultivars of Chrysanthemum (Dendranthema grandiflorum Ramat.) flower. Molecules. 2015;20:11090–11102. doi: 10.3390/molecules200611090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prathapan A, Singh MK, Anusree SS, Kumar DRS, Sundaresan A, Raghu KG. Antiperoxidative, free radical scavenging and metal chelating activities of Boerhaavia Diffusa L. J Food Biochem. 2011;35:1548–1554. doi: 10.1111/j.1745-4514.2010.00477.x. [DOI] [Google Scholar]
- Quevedo-Hidalgo B, Narvaez-Rincon PC, Pedroza-Rodriguez AM, Velasquez-Lozano ME. Production of lignocellulolytic enzymes from floriculture residues using Pleurotus ostreatus. J Zhejiang Univ Sci. 2015;20:117–127. doi: 10.11144/Javeriana.SC20-1.eple. [DOI] [Google Scholar]
- Rezaei F, Jamei R, Heidari R, Maleki R. Chemical composition and antioxidant activity of oil from wild Achillea setacea and A. vermicularis. Int J Food Prop. 2017;20:1522–1531. doi: 10.1080/10942912.2016.1213281. [DOI] [Google Scholar]
- Salami M, Rahimmalek M, Ehtemam MH. Inhibitory effect of different fennel samples and their phenolic compounds on formation of advanced glycation products and comparison of antimicrobial and antioxidant activities. Food Chem. 2016;213:196–205. doi: 10.1016/j.foodchem.2016.06.070. [DOI] [PubMed] [Google Scholar]
- Sanchez E, Davila-Aviria J, Castillo L, Heredia N, Vazquez-Alvarado R, Garcia S. Antibacterial and antioxidant activities in extracts of fully grown Cladodes of 8 cultivars of Cactus pear. J Food Sci. 2014;79:659–664. doi: 10.1111/1750-3841.12416. [DOI] [PubMed] [Google Scholar]
- Shen H, Guo Q, Fang H, Wang Y, Jin M. Determination of quercetin, luteolin, apigenin, and acacetin in Flos Chrysanthemi indici by RP-HPLC. Zhongguo Zhong Yao Za Zhi. 2010;35:191–193. doi: 10.4268/cjcmm20100216. [DOI] [PubMed] [Google Scholar]
- Stalmach A, Mullen W, Nagai Ch, Crozier A. On-line HPLC analysis of the antioxidant activity of phenolic compounds in brewed, paper-filtered coffee. Braz J Plant Physiol. 2006;18:253–262. doi: 10.1590/S1677-04202006000100018. [DOI] [Google Scholar]
- Sugawara T, Igarashi K. Identification of major flavonoids in petals of edible Chrysanthemum flowers and their suppressive effect on carbon tetrachloride-induced liver injury in mice. Food Sci Technol Res. 2009;15:499–506. doi: 10.3136/fstr.15.499. [DOI] [Google Scholar]
- Sun QL, Hua S, Ye JH, Zheng XQ, Liang YR. Flavonoids and volatiles in Chrysanthemum morifolium Ramat. From Tongxiang country in China. Afr J Biotechnol. 2010;9:3817–3821. doi: 10.5897/AJB09.1253. [DOI] [Google Scholar]
- Tenorio-Rodriguez PA, Murillo-Álvarez JI, Campa-Cordova ÁI, Angulo C. Antioxidant screening and phenolic content of ethanol extracts of selected Baja California Peninsula macroalgae. J Food Sci Technol. 2017;54:422–429. doi: 10.1007/s13197-016-2478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Yang XW, Guo QS. Studies on chemical constituents in Huangjuhua (flowers of Chrysanthemum morifolium) Zhongguo Zhong Yao Za Zhi. 2008;33:526–530. [PubMed] [Google Scholar]
- Wojdylo A, Oszmianski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–949. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]
- Xie YY, Yuana D, Yang JY, Wang LH, Wub CF. Cytotoxic activity of flavonoids from the flowers of Chrysanthemum morifolium on human colon cancer 205 cells. J Asian Nat Prod Res. 2009;11:771–778. doi: 10.1080/10286020903128470. [DOI] [PubMed] [Google Scholar]
- Xie YY, Qu JL, Wang QL, Wang Y, Yoshikawa M, Yuan D. Comparative evaluation of cultivars of Chrysanthemum morifolium flowers by HPLC-DAD-ESI/MS analysis and antiallergic assay. J Agric Food Chem. 2012;60:12574–12583. doi: 10.1021/jf304080v. [DOI] [PubMed] [Google Scholar]
- Yagi M, Nomoto K, Hori M, Kitano T, Yabukita H, Ogura M, Hamada U, Yonei Y. The effect of edible purple Chrysanthemum extract on advanced glycation end product generation in skin, a randomized controlled clinical trial and in vitro study. J Anti Aging Med. 2012;9:61–74. [Google Scholar]
- Ye Q, Liang Y, Lu J. Effect of different extracting methods on quality of Chrysanthemum morifolium Ramat. infusion. Asia Pac J Clin Nutr. 2007;16:183–187. [PubMed] [Google Scholar]
- Yeasmin D, Swarna RJ, Nasrin MS, Parvez S, Alam MF. Evaluation of antibacterial activity of three flower colours Chrysanthemum morifolium Ramat. against multi-drug resistant human pathogenic bacteria. Int J Biosci. 2016;9:78–87. [Google Scholar]
- Zeng Y, Deng M, Lv Z, Peng Y. Evaluation of antioxidant activities of extract from 19 Chinese edible flowers. Springerplus. 2014;3:315. doi: 10.1186/2193-1801-3-315. [DOI] [PMC free article] [PubMed] [Google Scholar]