Abstract
In the present investigation, essential oil (EO) of Ocimum tenuiflorum and its principal constituent (eugenol) was evaluated for its toxicity and mode of action against Callosobruchus maculatus. Furthermore, fumigant toxicity and germination studies on the application of O. tenuiflorum EO and eugenol against C. maculatus on different pulses was also studied. Fumigant activity studies revealed that EO toxicity was significantly (p < 0.05) influenced by concentration and exposure time. In fumigant toxicity assay without food, O. tenuiflorum EO and eugenol showed LC50 value of 278.6 and 256.5 µL/L air, respectively, at one hour exposure. Further, O. tenuiflorum EO displayed fumigant toxicity via inhibiting acetylcholinesterase activity. Pulses treated with O. tenuiflorum EO showed 70% of C. maculatus mortality at 250 µL/L air concentration after 24 h. Furthermore, these treatments didn’t affect the seed viability of the pulses tested. Hence, the application of O. tenuiflorum EO has potential scope as a botanical insecticide.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04871-y) contains supplementary material, which is available to authorized users.
Keywords: Acetylcholinesterase activity, Biopesticide, Bruchid, Callosobruchus maculatus, Leguminous seeds, Germination
Introduction
Pulses have excellent nutritional value, but the occurrence of insect pests during storage, leads to severe qualitative and quantitative loss such as weight loss, decreased nutritional and aesthetic value, increased mould growth and failure of seed/grain germination (Iturralde-García et al. 2016). Major threatening insects infesting the pulses, known as pulse beetles are the genus Callosobruchus of which, C. maculatus is the most pestilential (Gbaye et al. 2012). Synthetic chemical insecticides like organophosphates, pyrethroids, and fumigants such as phosphine, methyl bromide are apparently available for effective control of stored product insects (Bomzan et al. 2018). However, the best approach for pest management is the application of biopesticides that are eco-friendly and relatively safe alternatives (Isman 2000; Bhavya et al. 2020). Most plant extracts are characterized by low mammalian toxicity, selectivity, rapid degradation, and minimal influence on the environment, and seed germination (Nenaah 2014). Plant essential oils (EOs) are potential alternative to synthetic pesticides, used as contact/fumigant pesticides against various stored product insects (Isman 2000), because of its rich source of bioactive compounds. Numerous plant species used in the form of powder or EO have exhibited protection against pulse beetle with toxic, antifeeding, ovicidal, repellence, larvicidal, and growth inhibitory activities (Kedia et al. 2015). The bioactives of some plants with insecticidal property lead to different mode of action through GABA, octopamine synapses, and by inhibiting acetylcholinesterase (AChE), which are generally due to the synergistic effects of various active compounds (Koul et al. 2008).
Promising control of Callosobruchus species has been reported by the application of EO and their components (monoterpenoids, cyanohydrins, sulphur compounds, thiocyanates) as fumigants (Kedia et al. 2015; Sanon et al. 2018). Though there are a few reports on insecticidal activities of Ocimum species against Callosobruchus species, comprehensive assessment on the efficacy of Ocimum tenuiflorum EO as a biopesticide in stored pulses has not been studied. In this regard, the present investigation focuses on the evaluation of insecticidal activity of O. tenuiflorum EO and its major bioactive component (eugenol) against the major pulse beetle, C. maculatus in four stored pulses using fumigation procedure. Further, studies on the mode/mechanism of action of O. tenuiflorum EO and its constituent, against C. maculatus were carried out. Additionally, effect of the application of these EO on the seed germination was also investigated.
Materials and methods
Materials and chemicals
O. tenuiflorum EO was purchased from Falcon Essential oils, Bengaluru, India. Pulses: green gram (Vigna radia), Bengal gram (Cicer aritinum L), cowpea (Vigna unguicalata) and lablab bean (Lablab purpureus) were procured from local market in Mysuru, India. Eugenol standard was purchased from Himedia, Mumbai, India. Acetylthiocholine iodide (ATCI), bovine serum albumin (BSA) and 5,5′-dithio-bis(2-nitrobenzoic) acid (DTNB) were procured from Sigma–Aldrich Chemical Co., USA.
Rearing of stored product insect
C. maculatus (cowpea seed beetle) was cultured at Department of Food Protection and Infestation Control, CSIR-CFTRI, Mysuru, India using conditioned green gram (12–14% moisture content). The cultures were maintained under the laboratory conditions set at 28 ± 2 °C, 70 ± 5% RH and 16:8 light: dark photoperiod without any exposure to insecticides.
Vapour toxicity assay (without food) against C. maculatus
The vapour toxicity/fumigant activity of O. tenuiflorum EO and its major component (eugenol) without food against C. maculatus was determined according to Negahban et al. (2007) with slight modifications. Briefly, in 30 mL screw cap glass vial (lined with Insect-A-slip, 1 cm below the stopper rim), nine different concentrations of the EO and eugenol (20–500 µL/L of air) were impregnated on Whatman filter paper strips (1 cm × 5 cm) and pasted to the lower side of stopper. Then, fifteen beetle adults (0–1 day old) were released into each vial including untreated control and triplicates were kept for each experiment according to Tripathi et al. (2009). After 1 and 3 h of treatment, adult mortality was recorded. Percentage of beetle mortality was calculated using Abbott’s correction method (Abbott 1925) and LC50, LC90 values for the tested concentration were calculated using Probit analysis (Finney 1971).
Evaluation of in vivo acetylcholinesterase (AChE) activity
The in vivo AChE inhibitory action of O. tenuiflorum EO and its major compound, eugenol against C. maculatus was estimated as described by Bhavya et al. (2018). In brief, beetles were exposed to different concentrations (LC25, LC50 and LC75) of O. tenuiflorum EO and eugenol for 1 h. Further, live beetles were homogenized in sodium phosphate buffer (pH 8, 100 mM) in a glass-Teflon homogenizer. Then, homogenate was centrifuged at 10,000 rpm at 4 °C for 10 min and the supernatant was further used. For the assay, sodium phosphate buffer (5.6 mL), enzyme supernatant (40 μL) were mixed with 100 μL of DTNB reagent (10 mM) and further, by the addition of substrate (100 μL ATCI), the reaction was initiated. The coloured product formed, 5-thio-2-nitrobenzoate anion, was measured at 412 nm using UV spectrophotometer (UV-1700, Pharma Spec, SHIMADZU). Protein content in the supernatant was determined by Lowry’s method (Lowry et al., 1951) using BSA as the standard.
Vapour toxicity bioassay (with food) against C. maculatus
The vapour toxicity of O. tenuiflorum EO and eugenol was also studied in pulses (green gram, Bengal gram, cowpea and lablab bean) according to Vendan et al. (2017) with few modifications against C. maculatus, at five different concentrations (50–250 µL/L of air). In 50 mL glass screw cap vials, 15 g of conditioned pulses were taken separately and fifteen C. maculatus adults were introduced including untreated control. Later, the known concentration of the EO and eugenol were added onto the filter paper strip and immediately introduced into the glass vials as mentioned above. At 24 and 48 h post exposure, mortality of the beetles were noted down and percentage mortality was calculated.
Seed germination studies
After 60 days of treatment, the viability of control and treated pulses were tested according to Dalvi et al. (1972) with slight modifications. For germination assay, 25 seeds from each replicate of treated and control group were placed separately on glass Petriplates containing moist filter paper. The germination of the seeds was assessed for each treatment by keeping the Petriplates in a dark incubator maintained at 27 ± 2 °C. The plates were observed for the germination of seeds for one week and later the percentage germination, root and shoot length were estimated.
Statistical analysis
All the experiments were conducted in triplicates and values were presented as mean ± SD. Further, statistical analysis was performed using SPSS statistical software (SPSS version 20.0). One-way ANOVA with Tukey's HSD posthoc test was used to find significance between the groups and value of p < 0.05 was considered as significant. Probit analysis was also performed using SPSS statistical package to calculate the lethal concentrations.
Results and discussion
Vapour toxicity against C. maculatus without food
In the fumigation study, O. tenuiflorum EO and eugenol showed toxicity against C. maculatus in dose dependent manner (Fig. 1). The response of C. maculatus to both the EO (Fig. 1a) and eugenol (Fig. 1b) in fumigant toxicity assay showed significant (p < 0.05) lethality at 1 h, leading to 93.33 and 96.67% mortality at 500 µL/L air respectively. Ke´ita et al. (2001) observed ~ 70 and 80% fumigant toxicity with O. gratissimum and O. basilicum EO against C. maculatus, respectively, at 25 µL concentration in 8 mL vial after 12 h treatment. While, O. gratissimum EO and eugenol (1 µL/L air) showed 100% mortality of C. chinensis after 24 h (Ogendo et al. 2008). Additionally, EO and its components showed strong species specific toxicity, dependent on the concentration (dosage) and exposure period (Ogendo et al. 2008). Abd El-Salam (2010) observed 100% mortality of C. chinensis after 3 days of exposure to O. basilicum EO at 1.0 mL/38.5 mL air. Mann (2012) also mentioned that hexane fraction of O. gratissimum EO containing eugenol has shown grain protectant action against C. maculatus. However, the differences in the bruchid mortality among different studies can be attributed to the variation in Callosobruchus species as well as basil species tested, which are of different compositions.
Fig. 1.
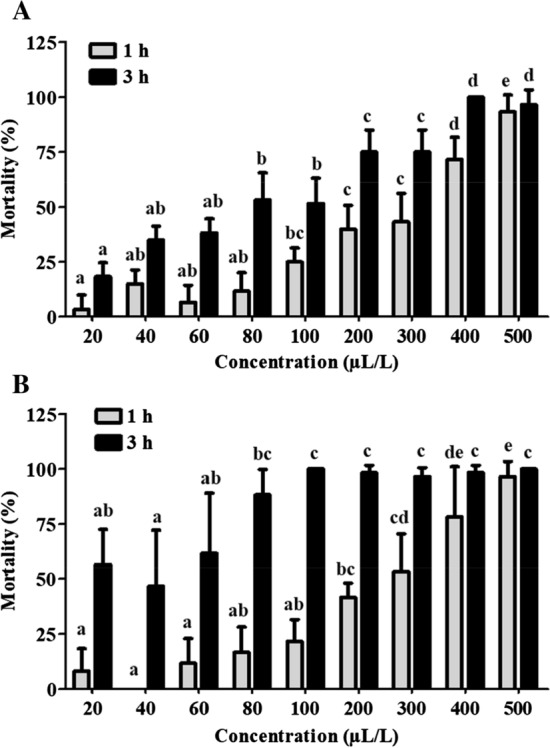
Fumigant action of O. tenuiflorum essential oil (a) and eugenol (b) against C. maculatus. Different letters on the bar chart indicate statistically significant values (ANOVA, Tukey HSD posthoc test, p < 0.05)
The GC-MS analysis of O. tenuiflorum EO used comprised of phenylpropenes (61.99%) like eugenol (50.39%), methyl eugenol (8.36%), estragole (2.99%); terpenes (5.87%) and sesquiterpenes (22.53%) like β-caryophyllene (20.07%), humulene (1.92%) as per our previous work (Bhavya et al., 2018). In the present study, the lethal concentrations, LC50 and LC90 values estimated for O. tenuiflorum EO and eugenol against C. maculatus are presented in supplementary Table 1. It was found that LC50 of O. tenuiflorum EO (consisting of 50.4% eugenol) and eugenol were 279 and 256 µL/L air, respectively at 1 h exposure. The strong fumigant toxicity of O. tenuiflorum EO against C. maculatus could be attributed to the distinct and synergistic effect of eugenol (50.39%) and β-caryophyllene (20.07%). Ke´ita et al. (2001) found the LC50 values (12 h) of O. basilicum and O. gratissimum EOs were 660 and 1060 µL/L, respectively, against C. maculatus. Contrastingly, Ogendo et al. (2008) reported LC50 values (24 h) of 0.20 and 0.01 µL/L air of O. gratissimum EO and eugenol, respectively against C. chinensis. Abd El-Salam (2010) calculated the LC50 values to be 1.88 µL/38.5 mL air for O. basilicum EO against C. chinensis after 24 h treatment.
In vivo acetylcholinesterase (AChE) activity against C. maculatus
Acetylcholinesterase inhibition is the potential, well explored mode of action of EOs and their bioactive compounds against the stored product insects (Isman 2000). Hence, in the current study, effect of O. tenuiflorum EO on in vivo AChE inhibition of C. maculatus was studied. Pulse beetle exposed to EO and eugenol at LC25, LC50 and LC75 for 1 h caused ~ 10, 19 and 48% AChE inhibition, respectively compared to control. Further, a direct correlation was also noticed, highlighting the dose-dependent inhibition, between the anti-AChE activity and the exposure dose (Fig. 2). It suggests that the tested EO and its bioactive compound targets the key site, AChE.
Fig. 2.
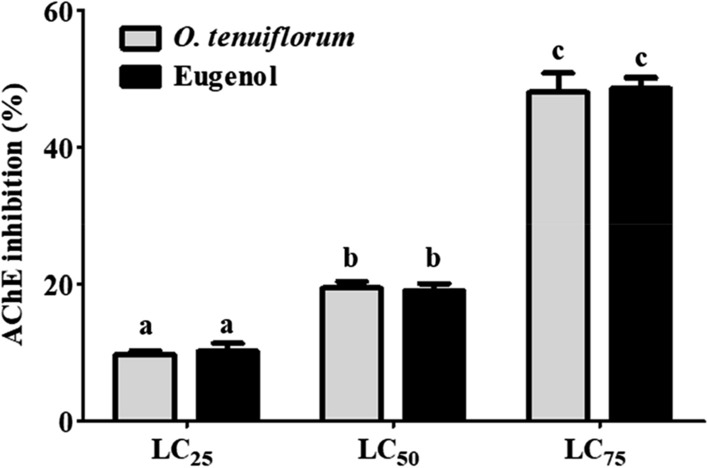
In vivo acetylcholinesterase activity of O. tenuiflorum essential oil and eugenol. Different letters on the bar chart indicate statistically significant values (ANOVA, Tukey HSD posthoc test, p < 0.05)
Eugenol (50.4% in O. tenuiflorum EO) was found to possess potential anti-AChE activity even, when tested individually. However, the EO showed the same inhibitory activity to that of eugenol, even though it constituted only for ~ 50% of the total composition. Hence, the AChE inhibitory activity of the O. tenuiflorum EO may be attributed to the synergistic effect of the major bioactive compounds such as eugenol (50.39%), and β-caryophyllene (20.07%). Similarly, Dohi et al. (2009) also reported that eugenol being the major compound accounted for 25% of the observed AChE inhibitory activity (in vitro) of the O. sanctum EO. Even, our previous studies showed that the O. tenuiflorum EO exhibited anti-AChE activity against S. oryzae adults (Bhavya et al. 2018). Therefore, the O. tenuiflorum EO was responsible for the mortality of C. maculatus, possessing neurotoxic effect on pulse beetle, C. maculatus.
Vapour toxicity against C. maculatus with food (pulses)
The observed mortality caused by O. tenuiflorum EO was positively dependent on the concentration of oil/bioactive compound and the exposure time (Supplementary Fig. 1). The green gram treated with the EO and eugenol showed a range of 25–73.3% and 31.7–71.7% mortality, respectively at 50–250 µL/L air after 24 h of exposure (Supplementary Fig. 2). Similar trend was also observed for Bengal gram and lablab bean treated with the EO and eugenol. While, cowpea treated with O. tenuiflorum EO and eugenol showed 25–70% and 11.7–55% mortality, respectively after 24 h. In a previous study by Pascual-Villalobos and Ballesta-Acosta (2003), O. basilicum EO (50.5% linalool and 18.5% eugenol) showed 48% mortality of C. maculatus after 24 h treatment on kidney beans at 5 µL concentration.
The LC50 and LC90 values estimated for O. tenuiflorum EO and eugenol in presence of pulses against C. maculatus are listed in supplementary Table 2. It clearly shows that the fumigant toxicity and the lethal dose of the EO and eugenol decreased when treated with-pulses (with-food) compared to that of without-food. This difference can be due to the low vapour pressure and high sorption of the botanical components onto the food grains thereby reducing the efficacy of the test compounds (Vendan et al. 2017; Bhavya et al. 2018).
Germination studies of treated pulses
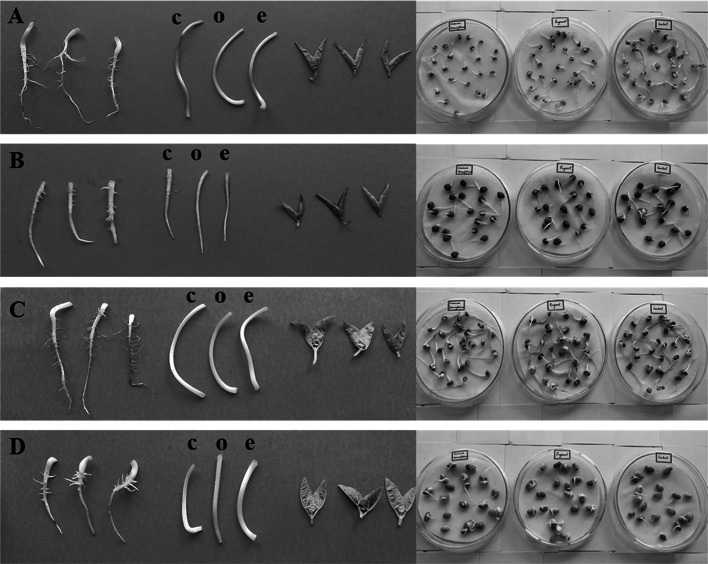
The pulses, green gram, Bengal gram, cowpea and lablab beans, treated with O. tenuiflorum EO and eugenol were subjected to germination studies to check its viability/toxicity on the propagation capacity. Tested EO and the major bioactive compound did not show any adverse effect on germination of these pulses compared to control (Fig. 3). Similarly, Ke´ita et al. (2001) also reported that cowpea seeds treated with O. basilicum and O. gratissimum aromatized powder exhibited 79% of germination, while control showed 97% of germination. Even, the effect of O. tenuiflorum EO and eugenol did not show any significant change on the length of the root and the shoot (Supplementary Fig. 3). These results implicate that O. tenuiflorum EO exhibiting significant fumigant toxicity without affecting the seed germination, can be applied for pulses stored for sowing purpose also.
Fig. 3.
Germination of pulses treated with O. tenuiflorum essential oil and eugenol. Green gram (a), Bengal gram (b), Cow pea (c), Lablab beans (d), (c—control untreated, o—O. tenuiflorum essential oil and e—eugenol)
Conclusion
To summarize, O. tenuiflorum EO and its major constituent, eugenol showed significant vapour toxicity (93–97% mortality at 500 µL/L air for 1 h) against C. maculatus via AChE inhibition (~ 48% at LC75). The application of O. tenuiflorum EO has shown insecticidal activity against C. maculatus in pulses, without affecting its germination ability. Hence, O. tenuiflorum EO, rich in eugenol can be exploited as a rational, safer fumigant substitute to conventional pesticide for the management of C. maculatus (pulse beetle) and other stored product insect-pests with an appropriate formulation and dose. However, scale-up studies are required further to test the efficacy of O. tenuiflorum EO at a larger extent to be used as stored product pest control agent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
All the authors thank the Director, CSIR—Central Food Technological Research Institute, Mysore for constant support and providing facilities to conduct the study. This research was financially supported by Council of Scientific and Industrial Research, Government of India.
Compliance with ethical standard
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
M. L. Bhavya and S. Obulaxmi have contributed equally to this work.
References
- Abbott WS. A method of computing the effectiveness of an insecticide. J Enon Entomol. 1925;18(2):265–267. [PubMed] [Google Scholar]
- Abd El-Salam AME Toxic and deterrent effects of two volatile oils against cowpea weevil, Callosobruchus chinensis (Coleoptera: Bruchidae) Arch Phytopathol Plant Prot. 2010;43(16):1596–1607. doi: 10.1080/03235400802677735. [DOI] [Google Scholar]
- Bhavya ML, Chandu AGS, Devi SS. Ocimum tenuiflorum oil, a potential insecticide against rice weevil with anti-acetylcholinesterase activity. Ind Crops Prod. 2018;126:434–439. doi: 10.1016/j.indcrop.2018.10.043. [DOI] [Google Scholar]
- Bhavya ML, Chandu AGS, Devi SS, Quirin KW, Pasha A, Vijayendra SVN. In-vitro evaluation of antimicrobial and insect repellent potential of supercritical-carbon dioxide (SCF-CO2) extracts of selected botanicals against stored product pests and foodborne pathogens. J Food Sci Technol. 2020;57(3):1071–1079. doi: 10.1007/s13197-019-04141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomzan DP, Bhavya ML, Chandu AGS, Manivannan S, Lavanya G, Ramasamy K, Pasha A. Potential of pyrethroid-synergised pyrethrum on stored product insects and implications for use as prophylactic sprays. J Food Sci Technol. 2018;55(6):2270–2278. doi: 10.1007/s13197-018-3144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvi RR, Singh B, Sulunkhe DK. Influence of selected pesticides on germination and associated metabolic changes in wheat and mung beans seeds. J Agric Food Chem. 1972;20(5):1000–1003. doi: 10.1021/jf60183a034. [DOI] [PubMed] [Google Scholar]
- Dohi S, Terasaki M, Makino M. Acetylcholinesterase inhibitory activity and chemical composition of commercial essential oils. J Agric Food Chem. 2009;57(10):4313–4318. doi: 10.1021/jf804013j. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Probit analysis. 3. Cambridge: University Press; 1971. [Google Scholar]
- Gbaye OA, Holloway GJ, Callaghan A. Variation in the sensitivity of Callosobruchus (Coleoptera: Bruchidae) acetylcholinesterase to the organophosphate insecticide malaoxon: effect of species, geographical strain and food type. Pest Manag Sci. 2012;68(9):1265–1271. doi: 10.1002/ps.3293. [DOI] [PubMed] [Google Scholar]
- Isman MB. Plant essential oils for pest and disease management. Crop Prot. 2000;19(8–10):603–608. doi: 10.1016/S0261-2194(00)00079-X. [DOI] [Google Scholar]
- Iturralde-García RD, Borboa-Flores J, Cinco-Moroyoqui FJ, Riudavets J, Del Toro-Sánchez CL, Rueda-Puente EO, Martínez-Cruz O, Wong-Corral FJ. Effect of controlled atmospheres on the insect Callosobruchus maculatus Fab. in stored chickpea. J Stored Prod Res. 2016;69:78–85. doi: 10.1016/j.jspr.2016.06.004. [DOI] [Google Scholar]
- Keita SM, Vincent C, Schmit JP, Arnason JT, Bélanger A. Efficacy of essential oil of Ocimum basilicum L. and O. gratissimum L. applied as an insecticidal fumigant and powder to control Callosobruchus maculatus (Fab.)[Coleoptera: Bruchidae] J Stored Prod Res. 2001;37(4):339–349. doi: 10.1016/S0022-474X(00)00034-5. [DOI] [PubMed] [Google Scholar]
- Kedia A, Prakash B, Mishra PK, Singh P, Dubey NK. Botanicals as eco-friendly biorational alternatives of synthetic pesticides against Callosobruchus spp. (Coleoptera: Bruchidae)—a review. J Food Sci Tech. 2015;52(3):1239–1257. doi: 10.1007/s13197-013-1167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul O, Walia S, Dhaliwal GS. Essential oils as green pesticides: potential and constraints. Biopestic Int. 2008;4(1):63–84. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193(1):265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- Mann A. Phytochemical constituents and antimicrobial and grain protectant activities of clove basil (Ocimum gratissimum L.) grown in Nigeria. Int J Plant Res. 2012;2(1):51–58. doi: 10.5923/j.plant.20120201.08. [DOI] [Google Scholar]
- Negahban M, Moharramipour S, Sefidkon F. Fumigant toxicity of essential oil from Artemisia sieberi Besser against three stored-product insects. J Stored Prod Res. 2007;43(2):123–128. doi: 10.1016/j.jspr.2006.02.002. [DOI] [Google Scholar]
- Nenaah GE. Bioactivity of powders and essential oils of three Asteraceae plants as post-harvest grain protectants against three major coleopteran pests. J Asia Pac Entomol. 2014;17(4):701–709. doi: 10.1016/j.aspen.2014.07.003. [DOI] [Google Scholar]
- Ogendo JO, Kostyukovsky M, Ravid U, Matasyoh JC, Deng AL, Omolo EO, Kariuki ST, Shaaya E. Bioactivity of Ocimum gratissimum L. oil and two of its constituents against five insect pests attacking stored food products. J Stored Prod Res. 2008;44(4):328–334. doi: 10.1016/j.jspr.2008.02.009. [DOI] [Google Scholar]
- Pascual-Villalobos MJ, Ballesta-Acosta MC. Chemical variation in an Ocimum basilicum germplasm collection and activity of the essential oils on Callosobruchus maculatus. Biochem Syst Ecol. 2003;31(7):673–679. doi: 10.1016/S0305-1978(02)00183-7. [DOI] [Google Scholar]
- Sanon A, Zakaria I, Clémentine LDB, Niango BM, Honora NR. Potential of botanicals to control Callosobruchus maculatus (Col.: Chrysomelidae, Bruchinae), a major pest of stored cowpeas in Burkina Faso: a review. Int J Insect Sci. 2018;10:1–8. doi: 10.1177/1179543318790260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendan SE, Manivannan S, Sunny AM, Murugesan R. Phytochemical residue profiles in rice grains fumigated with essential oils for the control of rice weevil. PLoS ONE. 2017;12(10):e0186020. doi: 10.1371/journal.pone.0186020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.