Abstract
O-GlcNAcylation is an atypical, reversible, and dynamic glycosylation that plays a critical role in maintaining the normal physiological functions of cells by regulating various biological processes such as signal transduction, proteasome activity, apoptosis, autophagy, transcription, and translation. It can also respond to environmental changes and physiological signals to play the role of “stress receptor” and “nutrition sensor” in a variety of stress responses and biological processes. Even, a homeostatic disorder of O-GlcNAcylation may cause many diseases. Therefore, O-GlcNAcylation and its regulatory role in stress response are reviewed in this paper.
Keywords: O-GlcNAcylation, Stress, Homeostasis, Metabolism, Pathogenesis
Introduction
In the past few decades, the role of post-translational modifications (PTMs) such as phosphorylation, ubiquitination, and methylation in various biological processes has been extensively studied. However, like many other emerging PTMs, the understanding of O-GlcNAcylation in physiology is in its infancy. O-GlcNAcylation, also known as O-linked N-acylglucosamine modification, is an atypical, reversible, and dynamic glycosylation reaction (Hart et al. 2007; Yang and Qian 2017) of connecting a single N-acetylglucosamine (GlcNAc) to serine and threonine residues of the target protein through an O-glycosidic bond of β-configuration. However, O-GlcNAcylation is different from the classical glycosylation in many aspects: (1) O-GlcNAcylation involves only a single GlcNAc that is not further modified, while the classical glycosylation is more complex; (2) O-GlcNAcylation is a highly dynamic process like phosphorylation, while the classical glycosylation is more stable. (3) Most of the O-GlcNAcylated proteins are distributed in the cytoplasm and nucleus, while the classical glycosylated proteins are mainly distributed on the surface of the cell membrane.
O-GlcNAcylation can directly and dynamically control the activity and function of the target protein so that the body can quickly respond to internal and external physiological stimuli. This characteristic allows O-GlcNAcylation to act as “stress receptor” and “nutrition sensor” in response to environmental changes and physiological signals (Hanover et al. 2010; Hart et al. 2011). In other words, O-GlcNAcylation regulates an array of biological processes to maintain the normal physiological functions of cells, such as metabolic reprogramming (Yang and Qian 2017), immune regulation (Banerjee et al. 2016), apoptosis (Chen et al. 2017), autophagy (Ruan et al. 2017; Wani et al. 2015; Wani et al. 2017), transcription, and translation (Draime et al. 2018). Thus, disruption of O-GlcNAcylation homeostasis may cause many diseases (Lefebvre et al. 2010), such as cancer (Hanover et al. 2018; Qian et al. 2018), diabetes (Bond and Hanover 2013; Ruan et al. 2013b), and neurodegeneration (Ruan et al. 2013a; Wani et al. 2017; Zhu et al. 2014). In addition, O-GlcNAcylation occurs dynamically in tandem with some PTMs, such as phosphorylation (Hart et al. 2011; Leney et al. 2017; Mercier et al. 2018), ubiquitination (Guinez et al. 2008; Li et al. 2013; Ruan et al. 2013a), acetylation (Allison et al. 2012), and methylation (Deplus et al. 2013).
Since Torres first discovered O-GlcNAcylation in 1984 (Torres and Hart 1984), the research of O-GlcNAcylation has gained immense popularity and progress, and more than 4000 proteins modified by O-GlcNAcylation have been identified (Ma and Hart 2014). Understanding O-GlcNAcylation is crucial in the view of cell biology, disease treatment, and prevention. Therefore, in this review, we mainly explore how O-GlcNAcylation regulates the response process of cells to stress and nutrition, and elucidate the physiological significance of O-GlcNAcylation homeostasis, and discuss the potential relationship between O-GlcNAcylation and metabolism and diseases, in order to guide future research in the field of O-GlcNAcylation and stress.
The process of protein O-GlcNAcylation
O-GlcNAc transferase and O-GlcNAcase are antagonistic enzymes which maintain O-GlcNAcylation homeostasis
Other PTMs are under diverse complicated modulations, yet O-GlcNAcylation is totally different. The synergy of O-GlcNAc transferase (OGT) with O-GlcNAcase (OGA) plays a critical role in maintaining O-GlcNAcylation homeostasis in cells (Hanover et al. 2010). OGT can transfer GlcNAc to hydroxyls in target protein threonine and serine residues from uridine diphosphate-GlcNAc (UDP-GlcNAc), the donor substrate. On the contrary, OGA contributes to GlcNAc hydrolysis based on the O-GlcNAcylated proteins. O-GlcNAcylation shows high dynamics that allows for the addition and removal of reversible as GlcNAc monosaccharides in the protein lifetime. As a result, O-GlcNAcylation responds to a variety of physiological signals as well as environmental conditions, in particular to nutritional availability and stress (Hanover et al. 2010).
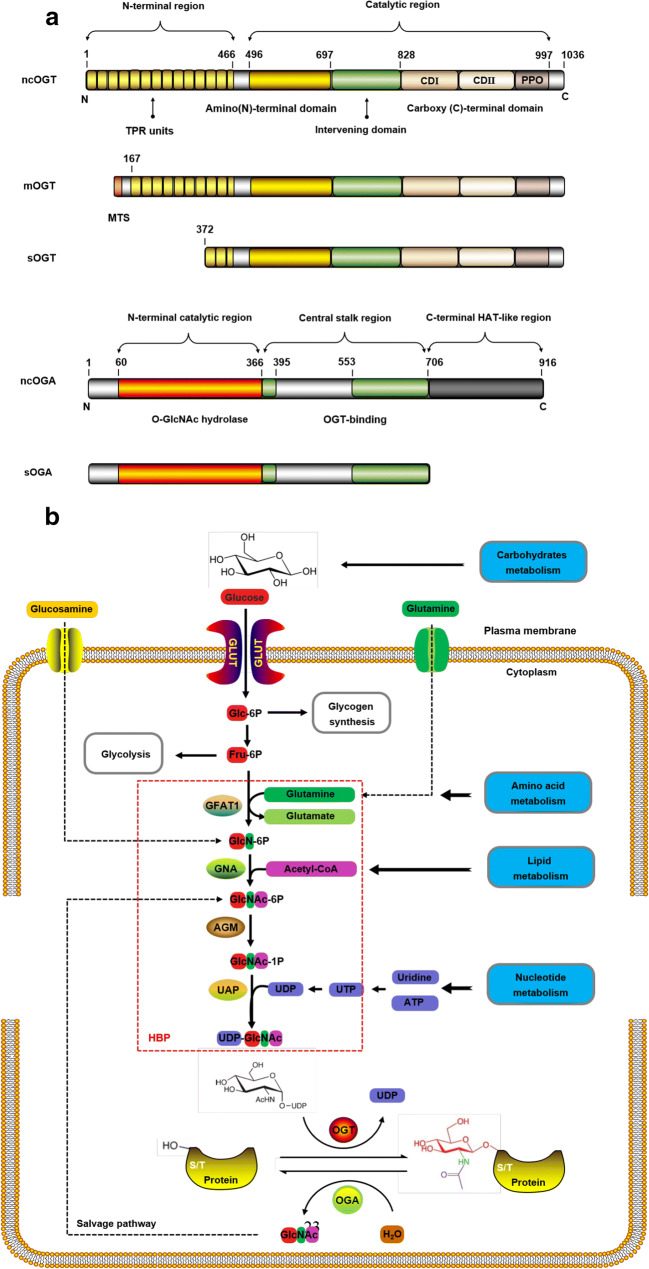
The OGT and OGA isoforms are shown in Fig. 1a, and these isoforms can be obtained from OGT and MGEA5, respectively (Hanover et al. 2003; Hrit et al. 2018; Keembiyehetty 2015). OGT activity has been correlated with nutrient availability and UDP-GlcNAc concentration. OGT has only three isoforms: nucleocytoplasmic OGT, mitochondrial OGT, and short OGT. They have the same carboxyl terminal catalytic and phosphoinositol-binding domain. However, the number of amino terminal tetrapeptide repeats is different, and so are their lengths (Joiner et al. 2019; Lazarus et al. 2011). It is worth noting that mitochondrial OGT has a unique mitochondrial targeting sequence (Janetzko and Walker 2014). In addition, they are also different in cellular localization: mitochondrial OGT exists in the mitochondria, while nucleocytoplasmic OGT and short OGT exist in the cytoplasm and nucleus (Joiner et al. 2019; Trapannone et al. 2016). The research of nucleocytoplasmic OGT function has been more extensive and detailed than the other two.
Fig. 1.
The process of protein O-GlcNAcylation. a Isoforms of OGT and OGA. The main difference between the three isoforms of OGT is the number of TRPs. These TRP units are stacked to form a super spiral structure, which is related to the recognition of the protein substrate. However, they share common carboxy-terminal catalytic regions, where CD I is related to UDP recognition and catalysis, and CD II is related to GlcNAc binding. The PPO can interact strongly with PI(3,4,5)P3 and participate in the insulin signaling pathway. The MTS is unique to mOGT. The intervening domain is a flexible connecting domain, which seems not conservative, only exists in metazoa. It is also one of the reasons why it is very difficult to crystallize OGT in higher metazoa. b Nutrient flux through HBP regulates protein O-GlcNAcylation. The characteristics of HBP pathway and UDP-GlcNAc connect the metabolism of glucose, lipid, amino acid, and nucleotide in a series. Coupled with the reversible and highly dynamic nature of protein O-GlcNAcylation, O-GlcNAcylation can function well as a “stress receptor” and “nutrition sensor” in many physiological processes. ncOGT, nucleocytoplasmic OGT; mOGT, mitochondrial OGT; sOGT, short OGT; ncOGA, nucleocytoplasmic OGA; sOGA, short OGA; TRPs, tetrapeptide repeats; CD, catalytic domain; PPO, phosphoinositide-binding domains; MTS, mitochondrial-targeting sequence; HAT-like, histone acetyltransferase-like; GLUT, glucose transporter; Glc-6P, glucose-6-phosphate; Fru-6P, fructose-6-phosphate; GFAT1, glutamine-fructose-6-phosphate amidotransferase 1; GlcN-6P, glucosamine-6-phosphate; GNA, GlcN-6-P acetyltransferase; GlcNAc-6-P, acetylglucosamine-6-phosphate; AGM, N-acetylglucosamine-phosphate mutase; GlcNAc-1-P, acetylglucosamine-1-phosphate; UAP, UDP-GlcNAc pyrophosphorylase; HBP, hexosamine biosymthesis pathway
The research on OGT recognizing the target protein substrate accurately has always been critical and challenging in the field of O-GlcNAcylation. Due to the lack of a crystal structure and the limitations of technology, understanding of the molecular mechanism, substrate recognition, and inhibitors of OGT is not substantial. Concisely, the detailed mechanism of precise recognition of protein substrates by OGT remains unknown. Although, elementary studies suggest that the restriction of active sites independently contributes to the site-specific and preferred recognition sequence of O-GlcNAcylation (Pathak et al. 2015). Moderate sequence restriction imposed by active sites may affect the selection of OGT substrates (Lazarus et al. 2011; Pathak et al. 2015). Also, tetrapeptide repeats acts like a “gatekeeper” that can generate unique binding sites to participate in substrate recognition in the absence of strictly consistent sequences (Clarke et al. 2008; Jínek et al. 2004; Pathak et al. 2015). Moreover, the structural characteristics of OGT isoforms are also helpful for substrate recognition (Lazarus et al. 2006; Sacoman et al. 2017).
OGA has only two isoforms: the nucleocytoplasmic OGA and short OGA. The only difference in their structure is that nucleocytoplasmic OGA has a histone acetyltransferase-like domain (Elsen et al. 2017; Li et al. 2017). In addition, the two isoforms are different in localization: nucleocytoplasmic OGA is located in the cytoplasm and nucleus, and short OGA is located in the endoplasmic reticulum and lipid droplets (Gao et al. 2001). These two isoforms can hydrolyze GlcNAc from O-GlcNAcylated proteins in the nucleus and cytoplasm, but the efficiency of short OGT is lower. Nucleocytoplasmic OGA and short OGA are only dependent on the presence of sugar components, and the kinetics is contrasting to that of OGT. No matter what the target protein is, OGA is removed with stable kinetics, while the kinetics of OGT varies greatly according to different protein targets (Shen et al. 2012). Interestingly, OGA is considered as a biomarker for some cancers and has potential clinical application (Dahl et al. 2005; Luanpitpong et al. 2018). It is noteworthy that OGA can interact with OGT in the presence of high glucose to form the “O-GlcNAczyme” complex (Whisenhunt et al. 2006). The clear understanding of the molecular mechanisms of OGA substrate specificity faces many limitations (Alonso et al. 2014). However, the sequence near O-GlcNAcylation site has some influence on substrate recognition, but it usually lacks sequence sensitivity (Martin et al. 2014). Also, structural features and binding proteins also play a role in substrate recognition. Additionally, intrinsic substrate promiscuity may contribute to OGA identification of O-GlcNAcylated proteins that can be referred to the regulation of phosphorylation.
O-GlcNAcylation of protein is regulated by the nutritional availability
The GlcNAc required for O-GlcNAcylation was only provided by UDP-GlcNAc (Hardivillé and Hart 2014). In vivo, the main pathway of UDP-GlcNAc synthesis is hexosamine biosynthesis pathway (HBP) (Rodriguez et al. 2015). HBP flux reflects the availability of nutrients and energy (Wellen et al. 2010). HBP depends not only on the availability of glucose and carbohydrate but also on lipids, amino acids, nucleotides, and ATP (Aquino-Gil et al. 2017). Therefore, HBP links the metabolism of glucose, amino acids, fatty acids, and nucleotides (Wellen et al. 2010; Yang and Qian 2017). Protein O-GlcNAcylation process is regulated by nutrient flux through HBP as shown in Fig. 1b. The glucose transporter is responsible for the uptake of glucose from the extracellular environment. Under physiological conditions, most of the glucose enters the pathway of glycogen synthesis, glycolysis and fat synthesis, and only about 2–5% of glucose enters the HBP (Marshall et al. 1991).
Hexokinase catalyzes glucose to glucose-6-phosphate. Then glucose-6-phosphate is further converted to fructose-6-phosphate by the action of isomerase. Fructose-6-phosphate enters both the glycolytic pathway and the HBP. Glutamine-fructose-6-phosphate amidotransferase 1 catalyzes the amino group transfer from glucosamine to fructose-6-phosphate to synthesize glucosamine-6-phosphate and glutamic acid. This is the first rate–limiting step in HBP. Thus, glutamine-fructose-6-phosphate amidotransferase 1 is regulated by the concentration of its product glucosamine-6-phosphate and the final product UDP-GlcNAc. Then, glucosamine-6-phosphate acetyltransferase catalyzes the acetylation of glucosamine-6-phosphate into acetylglucosamine-6-phosphate. Subsequently, N-acetylglucosamine-phosphate mutase catalyzes the formation of acetylglucosamine-1-phosphate from acetylglucosamine-6-phosphate. Furthermore, UDP-GlcNAc pyrophosphorylase catalyzes the synthesis of UDP-GlcNAc from acetylglucosamine-1-phosphate (Cork et al. 2018). Finally, the GlcNAc of UDP-GlcNAc is transferred to the serine and threonine residues of the target protein catalyzed by OGT (Yang and Qian 2017). The free GlcNAc hydrolyzed by OGA is recovered through the salvage pathway to convert GlcNAc to acetylglucosamine-6-phosphate (Lima et al. 2012). The intracellular concentration of UDP-GlcNAc also affects the activity of OGT. UDP-GlcNAc, a biosynthetic precursor involved almost in each metabolic pathway, which can respond to the metabolism of glucose, amino acid, fatty acid, and nucleoside, and is particularly suitable as a glucose sensor (Lucena et al. 2016). As a nutrition sensor, O-GlcNAcylation depends on the concentration of UDP-GlcNAc. The change in extracellular nutrition leads to the slight change in UDP-GlcNAc concentration that significantly changes the level of O-GlcNAcylation, so that cells can make appropriate stress response to changes in external conditions.
O-GlcNAcylation and the various cellular stress responses
O-GlcNAcylation promotes cell survival during cellular stress responses by regulating cell metabolism, transcription, translation, and signal transduction (Bond and Hanover 2015; Groves et al. 2013; Guinez et al. 2007). These studies show that O-GlcNAcylation can promote cell survival in various types of stress and injury viz.: cold and heat shock (Kazemi et al. 2010; Zhang et al. 2018), oxidative stress (Groves et al. 2017), ethanol stress (Groves et al. 2013), genotoxic stress (Han et al. 2017), reductive stress (Groves et al. 2013), endoplasmic reticulum stress (Xu et al. 2017), hypoxia reoxygenation (Ngoh et al. 2011; Zafir et al. 2013), osmotic stress (Reeves et al. 2014), ATP depletion (Hong and Hagen 2015), ischemia-reperfusion injury (Ducheix et al. 2018; Jensen et al. 2019), and trauma hemorrhage (Nöt et al. 2010). Moderate increase of O-GlcNAcylation level can significantly improve the survival rate. The signal pathway of O-GlcNAcylation regulating cell stress response is shown in Fig. 2. The regulation mechanism of common stress by O-GlcNAcylation is mainly described below.
Fig. 2.
O-GlcNAcylation can maintain intracellular homeostasis and promote survival during stress. The main pathways of O-GlcNAcylation protecting cell survival include: (1) HSP expression, (2) PI3K/Akt, (3) Ca2+ homeostasis, (4) inflammation, (5) PTM interplay, (6) ROS regulation, and (7) mitochondrial dynamics. Meanwhile, O-GlcNAcylation stabilize the structure of the protein and improve the solubility of protein, and trigger various signaling pathways, such as calpain, P38 MAP, TNF-α, Bcl-2, and IL-6, to protect cells. IR, insulin receptor; IRS, insulin receptor substrate; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; PDK1, 3-phosphoinositide-dependent protein kinase-1; CHOP, C/EBP-homologous protein; eIF2ɑ, eukaryotic translation initiation factor 2α; Bcl-2, B cell lymphoma-2; ROS, reactive oxygen species; VDAC, voltage-dependent anion-selective channel; GSK3β, glycogen synthase kinase 3β; FoxO1, forkhead transcription factor 1; 14-3-3, 14-3-3 protein; TAK1, TGFβ-activated kinase 1; IL-1, Interleukin-1; IL-6, Interleukin-6; HSF1, heat shock factors 1; SP1, specificity protein 1; HSP72, heat hock protein 72; TNF-α, tumor necrosis factor-α; UPR, unfolded protein response; ER, endoplasmic reticulum; SOD, superoxide dismutase; GPX1, glutathione peroxidase 1
Environmental stress
Heat stress
Heat shock proteins (HSPs) and heat shock factors (HSFs) are the central mediators for maintaining protein homeostasis in the process of the cell stress response and are crucial for O-GlcNAcylation regulation (Hoter et al. 2018). When OGT knockout leads to the decrease of O-GlcNAcylation level, the expression of various chaperone proteins such as HSP60, HSP72, HSP110, and other heat shock proteins is inhibited (Kazemi et al. 2010). HSP expression is regulated by two key transcription factors: HSF-1 and specificity protein 1. Moreover, O-GlcNAcylation can directly or indirectly regulate these two transcription factors, thereby regulating the expression of molecular chaperones (Gong and Jing 2011; Lee et al. 2016). O-GlcNAcylation affects glycogen synthase kinase 3β (GSK3β) phosphorylation at serine9 site to regulate HSP expression (Kazemi et al. 2010). When the level of O-GlcNAcylation is reduced, the inhibitory phosphorylation of the serine9 site of GSK3β in the nucleus is also reduced, and phosphorylation of GSK3β serine and threonine residues inhibits HSF-1 (Rusai et al. 2013). Thus, the regulatory effect of O-GlcNAcylation on chaperone protein expression has been reproduced in a variety of cellular stress and injury models, and its regulation of HSP expression through the GSK3β/protein kinase B (AKT) pathway has been further confirmed.
Cold stress
Cold stress can affect a variety of biochemical regulatory systems in the body and has a major impact on thermogenesis, immune system, and metabolism. O-GlcNAcylation is involved in the regulation of these physiological processes. However, less attention has been paid to the cell protection effect of O-GlcNAcylation under cold stress, while studies are more focused on the regulation of body energy metabolism by O-GlcNAcylation under cold stress (Chen et al. 2019; Li et al. 2018). For example, O-GlcNAcylation is essential for thermogenesis in brown adipose tissue under cold stress (Ohashi et al. 2017). Acute cold stress increases liver O-GlcNAcylation levels that perturbs glucose transport, glycogen synthesis, and glycolysis; and reduces apoptosis and autophagy to promote cell survival (Yao et al. 2018). Similar results have been shown in the skeletal muscles of mice and piglets, which further demonstrated that O-GlcNAcylation could protect cells from acute cold stress by inducing AKT activation (Yao 2018). In addition, OGT promote the expression of certain cold shock proteins in mouse skeletal muscle to protect cells and regulate glycolysis under cold stress, such as cold-inducible RNA-binding protein and RNA-binding motif protein 3 (Liu et al. 2019a; Shi et al. 2019).
Oxidative stress
In addition to several common mechanisms of defense against oxidative damage, O-GlcNAcylation level also responds to oxidative stress (Chen et al. 2018). Enhancement of O-GlcNAcylation signal transduction by glucosamine can reduce oxidative stress and apoptosis after acute kidney injury induced by contrast agent (Hu et al. 2018). Furthermore, adenovirus overexpression of OGA to inhibit O-GlcNAcylation can increase reactive oxygen species levels (Ngoh et al. 2011). One of the mechanisms of O-GlcNAcylation to inhibit reactive oxygen species is by up-regulating the expression of anti-oxidase, thus affecting the activity of forkhead transcription factor 1 (FoxO1) and peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) (Chen et al. 2018). However, O-GlcNAcylation can promote the production of reactive oxygen species in hyperglycemia or glucose toxicity models (Goldberg et al. 2011). Therefore, the specific molecular mechanism of O-GlcNAcylation in oxidative stress needs further study.
Endoplasmic reticulum stress
The protective effects of O-GlcNAcylation include the rescue of endoplasmic reticulum (ER) stress-induced lesions (Cui et al. 2019; Ngoh et al. 2009). The application of Tunicamycin or Brefeldin A induces ER stress in neonatal rat cardiomyocytes, while overexpression of OGT or inhibition of OGA by adenovirus that increased O-GlcNAcylation levels significantly decreased cardiomyocyte death and activated unfolded protein response, and the reduction of cell death is related to the reduction of C/EBP-homologous protein (CHOP) activation (Ngoh et al. 2009). Similarly, glucosamine administration–mediated elevation of O-GlcNAcylation also inhibited CHOP levels in rabbit renal ischemia-reperfusion injury models (Suh et al. 2014). The O-GlcNAcylation of eukaryotic translation initiation factor 2α at serine219, Threonine239, and Threonine241 site might hinder its phosphorylation, thereby inhibiting CHOP activation and reducing cell death (Jang et al. 2015). Meanwhile, O-GlcNAcylation can reduce ER stress by forming stress granules and processing bodies, which mainly regulate mRNA translation and degradation. OGT and HBP are necessary for stress-induced stress granules and processing bodies assembly (Ohn et al. 2008). These data indicate that OGT and O-GlcNAcylation can reduce ER stress and prevent apoptosis.
O-GlcNAcylation and diseases
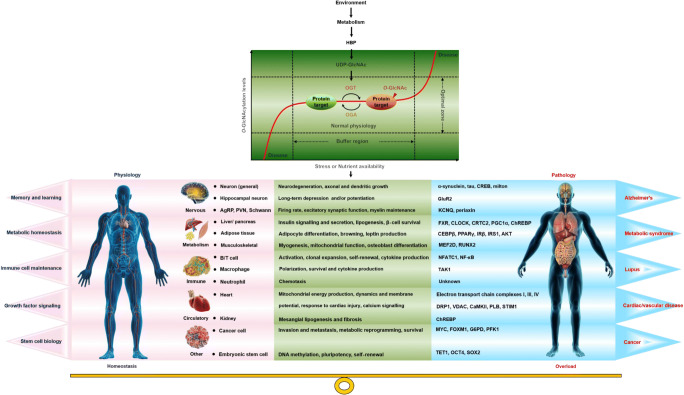
The increase of O-GlcNAcylation induced by cellular stress is an adaptive process of injury, and its failure may lead to some diseases (Martinez et al. 2017). OGT and OGA regulate each other at the transcription and translation levels to form a “buffer” system that accurately controls the global O-GlcNAcylation level and maintains it in the “optimal zone” (Fig. 3). Moderate disturbance results in slight changes of O-GlcNAcylation level in the “optimal zone”. In this state, O-GlcNAcylation helps to prevent cell stress and protect cells (Yang and Qian 2017). However, high intensity or long-term stress may cause O-GlcNAcylation level to exceed the “optimal zone”, causing overload and harmful effects on cell function, further contributing to certain diseases. For example, O-GlcNAcylation can reduce myocardial cell death caused by ER stress, and can also activate ER stress to promote fatty liver associated liver cancer (Ngoh et al. 2009; Xu et al. 2017). O-GlcNAcylation regulates cell stress response and injury that is involved in the pathogenesis of cancer, type II diabetes, and neurodegeneration (Chen et al. 2019; Kátai et al. 2016; Reeves et al. 2014; Xu et al. 2017).
Fig. 3.
O-GlcNAcylation overload is the pathogenesis of some diseases. O-GlcNAcylation integrates environmental and genetic information to dynamically regulate biological homeostasis and disease pathology, and its homeostasis is essential for health. In the face of mild stress, OGT and OGA jointly regulate the O-GlcNAcylation level so that it only fluctuates slightly in the “optimal zone,” thereby maintaining normal cell function and promoting cell survival. However, high intensity or long-term stress may cause O-GlcNAcylation level to exceed the “optimal zone,” causing overload and harmful effects on cell function. In severe cases, certain diseases might be induced. tau, microtubule-associated protein tau; CREB, cAMP-responsive element–binding protein; GluR2, glutamate receptor 2; KCNQ3, potassium voltage-gated channel subfamily Q member 3; CLOCK, circadian locomoter output cycles protein kaput; CRTC2, CREB-regulated transcription coactivator 2; PGC1α, peroxisome proliferator–activated receptor gamma coactivator-1α; ChREBP, carbohydrate response element–binding protein; CEBPβ, CCAAT/enhancer–binding protein-β; IRS1, insulin receptor substrate-1; AKT, protein kinase B; MEF2D, myocyte-specific enhancer factor 2D; NF-κB, nuclear factor kappa-B; TAK1, TGFβ-activated kinase 1; G6PD, glucose-6-phosphate 1-dehydrogenase
Cancer
O-GlcNAcylation modification may be closely related to the pathogenesis and progress of cancer (Efimova et al. 2019; Hanover et al. 2018; Slawson and Hart 2011; Trinca and Hagan 2018), such as prostate cancer (Itkonen et al. 2016), breast cancer (Akella et al. 2020; Barkovskaya et al. 2019; Trinca et al. 2018), colorectal cancer (Jiang et al. 2019; Zhu et al. 2019), liver cancer (Duan et al. 2018; Lee et al. 2019; Xu et al. 2017; Zhang et al. 2017), and pancreatic cancer (Ma et al. 2013; Sharma et al. 2019). O-GlcNAcylation combines various nutritional signal transductions with growth signal transductions in cancer; thus, the increase of its level has been identified as a common cancer feature (Ma and Vosseller 2014). One of the characteristics of cancer is the remarkable change in metabolism. Dynamic O-GlcNAcylation is involved in a variety of metabolic regulation as a nutritional sensor (Hanover et al. 2018; Shi et al. 2018).
Phosphofructokinase 1 activity is inhibited by O-GlcNAcylation at serine529 that changes glucose flow through the pentose phosphate pathway (PPP), thus giving cancer cells the advantage of selective growth (Yi et al. 2012). In addition, the PPP is essential for macromolecular biosynthesis and maintenance of redox homogeneity in rapidly proliferating cells. PPP was upregulated in various types of cancer. Glucose-6-phosphate dehydrogenase, as the key rate-limiting enzyme of PPP, demonstrates dynamic O-GlcNAcylation in response to hypoxia (Rao et al. 2015). O-GlcNAcylation activates glucose-6-phosphate dehydrogenase activity and increases glucose flux through the PPP, thereby conferring a selective growth advantage to the tumor. Furthermore, O-GlcNAcylation of phosphoglycerate kinase 1 coordinates glycolysis and tricarboxylic acid cycle to promote tumor growth (Nie et al. 2020).
The phosphoinositide 3-kinase (PI3K)/AKT/mammalian target of rapamycin pathway modulates the growth of cells and the metabolism of glucose. In addition, it plays a critical part in the pivotal tumor marker metabolic reprogramming; in this way, cancer cells acquire the energy together with macromolecular precursors necessary for the rapid cancer development (Masui et al. 2014). The PI3K/Akt pathway displays high sensitivity to the glucose level outside the cells while modulating the absorption and use of glucose. Glucose deprivation in a short time remarkably suppresses the activation of AKT triggered by insulin and delays U2OS tumor cell growth (Jones et al. 2014). Nonetheless, O-GlcNAcylation together with HBP maintains the phosphorylation of PI3K induced by insulin and the growth of cancer cells following glucose deprivation for a short time (Jones et al. 2014). HBP, OGT, and nuclear factor kappa-B are directly associated with each other in the human pancreatic ductal adenocarcinoma cells (Ma et al. 2013). The elevated O-GlcNAcylation level activates nuclear factor kappa-B and suppresses apoptosis (Ma et al. 2013). OGT is demonstrated to be located in chromatin that triggers the p300-regulated p65 acetylation at the lysine310 position to respond to stimulation by tumor necrosis factor (Allison et al. 2012). These pieces of evidence suggest that HBP and O-GlcNAcylation may play a regulatory role in the metabolism of cancer cells. OGT can regulate lipid metabolism in cancer. In order to maintain growth, cancer cells usually adopt de novo synthesis of fat, which involves the activation of the key enzymes, such as fatty acid synthase and sterol regulatory element–binding proteins-1 (Sodi et al. 2018). Inhibition of OGT can lead to fat formation defects that can be remedied by excessive production of sterol regulatory element–binding proteins-1 (Sodi et al. 2018). In addition, fatty acid synthase binds and inhibits OGA, thereby increasing O-GlcNAcylation level in cells, especially under oxidative stress (Groves et al. 2017).
Type II diabetes
There is increasing evidence of a complex association between abnormal levels of O-GlcNAcylation and diabetes. Abnormal O-GlcNAcylation is the basis of the molecular mechanism of glucose toxicity, insulin resistance, mitochondrial dysfunction, and abnormal insulin secretion from β cells (Banerjee et al. 2016; Hardivillé and Hart 2014; Peterson and Hart 2016). For example, islet β cells express a large amount of OGT and OGA that indicates that O-GlcNAc cycle is important for islet β cell function. Some evidence indicates a link between hyperglycemia-induced O-GlcNAcylation in pancreatic β cells (D'Alessandris et al. 2004). After long-term hyperglycemia, O-GlcNAcylation will increase, thus increasing the apoptosis of β cells (D'Alessandris et al. 2004). The negative effect on the survival of beta cells seems to be due to the imbalance of key elements in the insulin signaling pathway. Increased O-GlcNAcylation also leads to an increase in pancreatic β cell apoptosis in mice by blocking the phosphorylation of Akt1 at serine473 (Kang et al. 2008). In insulin-sensitive tissues, O-GlcNAcylation induced by hyperglycemia is one of the important causes of insulin resistance (Shi et al. 2018; Wang et al. 2016b; Whelan et al. 2010). O-GlcNAcylation occurs in many participants of insulin signaling, viz. insulin receptor, insulin receptor substrate-1, insulin receptor substrate-2, 3-phosphoinositide-dependent protein kinase-1, Akt, PIK, and FoxO1 (Ball et al. 2006; Yang et al. 2008). Glucosamine-induced O-GlcNAcylation inhibits tyrosine residue phosphorylation of insulin receptor, insulin receptor substrate-1, insulin receptor substrate-2, and subsequent PI3K/Akt inactivation (Very et al. 2018). Inactivation of PI3K/Akt resulted in the inactivation of GSK3β and FoxO1 and downregulation of Bcl-2 cell death interaction medium (Liu et al. 2019b). In addition, the inhibition of tyrosine residue phosphorylation of insulin receptor substrate-1, insulin receptor substrate-2, and glucosamine-induced apoptosis of β cells have shown to be prevented by reducing O-GlcNAcylation with specific OGT inhibitors (D'Alessandris et al. 2004). Increased O-GlcNAcylation by HBP results in impaired insulin signaling protein PTMs in insulin-sensitive tissues and inhibits glucose uptake mediated by the glucose transporter 4 (Buse et al. 2002; Whelan et al. 2010). Knockdown or inhibition of OGA can promote the degradation of PGC-1α in C2C12 cells, leading to inhibition of mitochondrial biogenesis and myogenesis; and inhibition of myotubular glucose uptake and insulin signal has been observed, indicating that OGA influence the development of myogenesis and skeletal muscle insulin resistance (Buse et al. 2002; Shi et al. 2018; Wang et al. 2016b). Conclusively, an increase in O-GlcNAcylation leads to a deterioration of hyperglycemia during diabetes.
Neurodegeneration
O-GlcNAcylated proteins frequently occur in the brain (Rexach et al. 2008). The expression and activity of OGT and OGA in the brain were several times higher than that in peripheral tissue (Akan et al. 2018; Rexach et al. 2008). O-GlcNAcylation is associated with normal brain function and the etiology of neurodegeneration (Choi et al. 2019). O-GlcNAcylation is associated with the pathogenesis of neurodegeneration, such as Parkinson’s disease, Alzheimer’s disease (AD), and stroke (Akan et al. 2018; He et al. 2017; Jiang et al. 2017; Wani et al. 2017).
AD is characterized by a local decrease in glucose metabolism (An et al. 2018). Considering that O-GlcNAcylation is dependent on nutritional availability, the reduction in glucose uptake, and metabolism in the brain of AD patients is a contributing factor to the reduction in O-GlcNAcylation (Rexach et al. 2008). Some scholars applied proteomics to analyze 530 proteins in the human brain and identified 1850 peptides modified by O-GlcNAcylation, among which 128 peptides (78 proteins) showed significant changes in the brain of AD patients, suggesting disorder of O-GlcNAcylation of various proteins in the brain must be related to the pathogenesis of sporadic AD (Wang et al. 2017). In addition, reduction in O-GlcNAylation in OGT knockout mice was found to induce progressive neurodegenerative phenotypes similar to AD, such as amyloid β-protein production, tau hyperphosphorylation, and memory deficits (Wang et al. 2016a). Reduction of O-GlcNAcylation of protein kinase A leads to memory and learning deficits, and ultimately to inhibition of the protein kinase A/cAMP-response element–binding protein signaling pathway (Xie et al. 2016). Together, these studies support O-GlcNAcylization as a key regulator that contributes to the health and survival of neurons and can play a key role in the pathogenesis, development, and treatment of neurodegenerative diseases.
Conclusions and perspectives
Over the past decade, a deep understanding of O-GlcNAcylation in biochemistry, molecular and cell biology, and physiology has been developed. The potential mechanisms of OGT and OGA for correct substrate recognition and maintenance of O-GlcNAcylation homeostasis are still insufficient. The research on these mechanisms will be a long-term research focus and challenge. Our understanding of O-GlcNAcylation in pathophysiology was obtained from the research on diseases such as aging, cancer, neurodegenerative diseases, diabetes, and diabetic complications, but these understandings are not comprehensive and in-depth. Therefore, the relationship between the abnormal O-GlcNAcylation level and the pathogenesis of these diseases will also be an important research direction in this field. The research on O-GlcNAcylation in inflammation, immunity, and cardiovascular diseases is in its initial stage, and needs further development. Furthermore, the same is true for the interaction between O-GlcNAcylation and other PTMs. On the other hand, the benefit of O-GlcNAcylation in mediating cell survival has been increasingly recognized, and has been regarded as a “stress receptor” and “nutrition sensor” involved in biological processes. Despite our extensive and growing knowledge regarding the influence of O-GlcNAcylation signaling on cellular function, our understanding of how O-GlcNAcylation acts as “stress receptor” and “nutrition sensor” in response to a variety of physiological signals is still limited. Therefore, more efforts are needed to better elucidate and understand the cytoprotective mechanism of O-GlcNAcylation in various stress responses. In the future, exploring how O-GlcNAcylation responds and coordinates a variety of molecular signals to play a regulatory role in stress response will be the research focus in this field.
Authors’ contributions
Yang Liu had the idea for the manuscript and wrote the manuscript. Rui-Zhi Yao performed the literature search. Shuai Lian and Peng Liu performed complementary literature searches. Ya-Jie Hu, Hong-Zhao Shi, and Hong-Ming Lv composed figures. Yu-Ying Yang corrected the manuscript for publication. Bin Xu and Shi-Ze Li supervised the work, evaluated the data, and approved the final version. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the General Project of National Natural Science Foundation of China (31972637, 31772695) and Key Project of Heilongjiang Natural Science Foundation (ZD2019C004).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rui-Zhi Yao contributed equally to this work.
Contributor Information
Bin Xu, Email: xubin@byau.edu.cn.
Shi-Ze Li, Email: byndlsz@163.com.
References
- Akan I, Olivier-Van Stichelen S, Bond MR, Hanover JA. Nutrient-driven O-GlcNAc in proteostasis and neurodegeneration. J Neurochem. 2018;144:7–34. doi: 10.1111/jnc.14242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akella NM, le Minh G, Ciraku L, Mukherjee A, Bacigalupa ZA, Mukhopadhyay D, Sodi VL, Reginato MJ. O-GlcNAc transferase regulates cancer stem-like potential of breast cancer cells. Mol Cancer Res. 2020;18:585–598. doi: 10.1158/1541-7786.Mcr-19-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DF, Wamsley JJ, Kumar M, Li D, Gray LG, Hart GW, Jones DR, Mayo MW. Modification of RelA by O-linked N-acetylglucosamine links glucose metabolism to NF-κB acetylation and transcription. Proc Natl Acad Sci U S A. 2012;109:16888–16893. doi: 10.1073/pnas.1208468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso J, Schimpl M, van Aalten DM. O-GlcNAcase: promiscuous hexosaminidase or key regulator of O-GlcNAc signaling? J Biol Chem. 2014;289:34433–34439. doi: 10.1074/jbc.R114.609198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Y, Varma VR, Varma S, Casanova R, Dammer E, Pletnikova O, Chia CW, Egan JM, Ferrucci L, Troncoso J, Levey AI, Lah J, Seyfried NT, Legido-Quigley C, O'Brien R, Thambisetty M. Evidence for brain glucose dysregulation in Alzheimer's disease. Alzheimers Dement. 2018;14:318–329. doi: 10.1016/j.jalz.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino-Gil M, Pierce A, Perez-Cervera Y, Zenteno E, Lefebvre T. OGT: a short overview of an enzyme standing out from usual glycosyltransferases. Biochem Soc Trans. 2017;45:365–370. doi: 10.1042/bst20160404. [DOI] [PubMed] [Google Scholar]
- Ball LE, Berkaw MN, Buse MG. Identification of the major site of O-linked beta-N-acetylglucosamine modification in the C terminus of insulin receptor substrate-1. Mol Cell Proteomics. 2006;5:313–323. doi: 10.1074/mcp.M500314-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee PS, Lagerlöf O, Hart GW. Roles of O-GlcNAc in chronic diseases of aging. Mol Asp Med. 2016;51:1–15. doi: 10.1016/j.mam.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Barkovskaya A, Seip K, Hilmarsdottir B, Maelandsmo GM, Moestue SA, Itkonen HM. O-GlcNAc Transferase inhibition differentially affects breast cancer subtypes. Sci Rep. 2019;9:5670. doi: 10.1038/s41598-019-42153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond MR, Hanover JA. O-GlcNAc cycling: a link between metabolism and chronic disease. Annu Rev Nutr. 2013;33:205–229. doi: 10.1146/annurev-nutr-071812-161240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond MR, Hanover JA. A little sugar goes a long way: the cell biology of O-GlcNAc. J Cell Biol. 2015;208:869–880. doi: 10.1083/jcb.201501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse MG, Robinson KA, Marshall BA, Hresko RC, Mueckler MM. Enhanced O-GlcNAc protein modification is associated with insulin resistance in GLUT1-overexpressing muscles. Am J Physiol Endocrinol Metab. 2002;283:E241–E250. doi: 10.1152/ajpendo.00060.2002. [DOI] [PubMed] [Google Scholar]
- Chen PH, Chi JT, Boyce M. Functional crosstalk among oxidative stress and O-GlcNAc signaling pathways. Glycobiology. 2018;28:556–564. doi: 10.1093/glycob/cwy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Gong P, Tao T, Gao Y, Shen J, Yan Y, Duan C, Wang J, Liu X. O-GlcNAc glycosylation of nNOS promotes neuronal apoptosis following glutamate excitotoxicity. Cell Mol Neurobiol. 2017;37:1465–1475. doi: 10.1007/s10571-017-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhao X, Wu H. Metabolic stress and cardiovascular disease in diabetes mellitus: the role of protein O-GlcNAc modification. Arterioscler Thromb Vasc Biol. 2019;39:1911–1924. doi: 10.1161/atvbaha.119.312192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Kim C, Song H, Cha MY, Cho HJ, Son SM, Kim HJ, Mook-Jung I. Amyloid β-induced elevation of O-GlcNAcylated c-Fos promotes neuronal cell death. Aging Cell. 2019;18:e12872. doi: 10.1111/acel.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AJ, Hurtado-Guerrero R, Pathak S, Schüttelkopf AW, Borodkin V, Shepherd SM, Ibrahim AFM, van Aalten DMF. Structural insights into mechanism and specificity of O-GlcNAc transferase. EMBO J. 2008;27:2780–2788. doi: 10.1038/emboj.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork GK, Thompson J, Slawson C. Real talk: the inter-play between the mTOR, AMPK, and hexosamine biosynthetic pathways in cell signaling. Front Endocrinol (Lausanne) 2018;9:522. doi: 10.3389/fendo.2018.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui YL, Xue RQ, Xi He, Ming Zhao, Yu XJ, Liu LZ, Wu Q, Si Yang, Li DL, Zang WJ. Cholinergic drugs ameliorate endothelial dysfunction by decreasing O-GlcNAcylation via M3 AChR-AMPK-ER stress signaling. Life Sci. 2019;222:1–12. doi: 10.1016/j.lfs.2019.02.036. [DOI] [PubMed] [Google Scholar]
- D'Alessandris C, Andreozzi F, Federici M, Cardellini M, Brunetti A, Ranalli M, del Guerra S, Lauro D, del Prato S, Marchetti P, Lauro R, Sesti G. Increased O-glycosylation of insulin signaling proteins results in their impaired activation and enhanced susceptibility to apoptosis in pancreatic beta-cells. FASEB J. 2004;18:959–961. doi: 10.1096/fj.03-0725fje. [DOI] [PubMed] [Google Scholar]
- Dahl E, Sadr-Nabavi A, Klopocki E, Betz B, Grube S, Kreutzfeld R, Himmelfarb M, An HX, Gelling S, Klaman I, Hinzmann B, Kristiansen G, Grützmann R, Kuner R, Petschke B, Rhiem K, Wiechen K, Sers C, Wiestler O, Schneider A, Höfler H, Nährig J, Dietel M, Schäfer R, Rosenthal A, Schmutzler R, Dürst M, Meindl A, Niederacher D. Systematic identification and molecular characterization of genes differentially expressed in breast and ovarian cancer. J Pathol. 2005;205:21–28. doi: 10.1002/path.1687. [DOI] [PubMed] [Google Scholar]
- Deplus R, Delatte B, Schwinn MK, Defrance M, Méndez J, Murphy N, Dawson MA, Volkmar M, Putmans P, Calonne E, Shih AH, Levine RL, Bernard O, Mercher T, Solary E, Urh M, Daniels DL, Fuks F. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. EMBO J. 2013;32:645–655. doi: 10.1038/emboj.2012.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draime A, Bridoux L, Belpaire M, Pringels T, Degand H, Morsomme P, Rezsohazy R. The O-GlcNAc transferase OGT interacts with and post-translationally modifies the transcription factor HOXA1. FEBS Lett. 2018;592:1185–1201. doi: 10.1002/1873-3468.13015. [DOI] [PubMed] [Google Scholar]
- Duan F, Wu H, Jia D, Wu W, Ren S, Wang L, Song S, Guo X, Liu F, Ruan Y, Gu J. O-GlcNAcylation of RACK1 promotes hepatocellular carcinogenesis. J Hepatol. 2018;68:1191–1202. doi: 10.1016/j.jhep.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Ducheix S, Magré J, Cariou B, Prieur X. Chronic O-GlcNAcylation and diabetic cardiomyopathy: the bitterness of glucose. Front Endocrinol (Lausanne) 2018;9:642. doi: 10.3389/fendo.2018.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova EV, Appelbe OK, Ricco N, Lee SSY, Liu Y, Wolfgeher DJ, Collins TN, Flor AC, Ramamurthy A, Warrington S, Bindokas VP, Kron SJ. O-GlcNAcylation enhances double-Strand break repair, promotes cancer cell proliferation, and prevents therapy-induced senescence in irradiated tumors. Mol Cancer Res. 2019;17:1338–1350. doi: 10.1158/1541-7786.Mcr-18-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsen NL, Patel SB, Ford RE, Hall DL, Hess F, Kandula H, Kornienko M, Reid J, Selnick H, Shipman JM, Sharma S, Lumb KJ, Soisson SM, Klein DJ. Insights into activity and inhibition from the crystal structure of human O-GlcNAcase. Nat Chem Biol. 2017;13:613–615. doi: 10.1038/nchembio.2357. [DOI] [PubMed] [Google Scholar]
- Gao Y, Wells L, Comer FI, Parker GJ, Hart GW. Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain. J Biol Chem. 2001;276:9838–9845. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- Goldberg H, Whiteside C, Fantus IG. O-linked β-N-acetylglucosamine supports p38 MAPK activation by high glucose in glomerular mesangial cells. Am J Physiol Endocrinol Metab. 2011;301:E713–E726. doi: 10.1152/ajpendo.00108.2011. [DOI] [PubMed] [Google Scholar]
- Gong J, Jing L. Glutamine induces heat shock protein 70 expression via O-GlcNAc modification and subsequent increased expression and transcriptional activity of heat shock factor-1. Minerva Anestesiol. 2011;77:488–495. [PubMed] [Google Scholar]
- Groves JA, Lee A, Yildirir G, Zachara NE. Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis. Cell Stress Chaperones. 2013;18:535–558. doi: 10.1007/s12192-013-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves JA, Maduka AO, O'Meally RN, Cole RN, Zachara NE. Fatty acid synthase inhibits the O-GlcNAcase during oxidative stress. J Biol Chem. 2017;292:6493–6511. doi: 10.1074/jbc.M116.760785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinez C, Mir AM, Dehennaut V, Cacan R, Harduin-Lepers A, Michalski JC, Lefebvre T. Protein ubiquitination is modulated by O-GlcNAc glycosylation. FASEB J. 2008;22:2901–2911. doi: 10.1096/fj.07-102509. [DOI] [PubMed] [Google Scholar]
- Guinez C, Mir AM, Leroy Y, Cacan R, Michalski JC, Lefebvre T. Hsp70-GlcNAc-binding activity is released by stress, proteasome inhibition, and protein misfolding. Biochem Biophys Res Commun. 2007;361:414–420. doi: 10.1016/j.bbrc.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Han C, Gu Y, Shan H, Mi W, Sun J, Shi M, Zhang X, Lu X, Han F, Gong Q, Yu W. O-GlcNAcylation of SIRT1 enhances its deacetylase activity and promotes cytoprotection under stress. Nat Commun. 2017;8:1491. doi: 10.1038/s41467-017-01654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JA, Chen W, Bond MR. O-GlcNAc in cancer: An Oncometabolism-fueled vicious cycle. J Bioenerg Biomembr. 2018;50:155–173. doi: 10.1007/s10863-018-9751-2. [DOI] [PubMed] [Google Scholar]
- Hanover JA, Krause MW, Love DC. The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim Biophys Acta. 2010;1800:80–95. doi: 10.1016/j.bbagen.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover JA, Yu S, Lubas WB, Shin SH, Ragano-Caracciola M, Kochran J, Love DC. Mitochondrial and nucleocytoplasmic isoforms of O-linked GlcNAc transferase encoded by a single mammalian gene. Arch Biochem Biophys. 2003;409:287–297. doi: 10.1016/s0003-9861(02)00578-7. [DOI] [PubMed] [Google Scholar]
- Hardivillé S, Hart GW. Nutrient regulation of signaling, transcription, and cell physiology by O-GlcNAcylation. Cell Metab. 2014;20:208–213. doi: 10.1016/j.cmet.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:825–858. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Ma X, Li D, Hao J. Thiamet G mediates neuroprotection in experimental stroke by modulating microglia/macrophage polarization and inhibiting NF-κB p65 signaling. J Cereb Blood Flow Metab. 2017;37:2938–2951. doi: 10.1177/0271678x16679671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SY, Hagen T. 2-Deoxyglucose induces the expression of thioredoxin interacting protein (TXNIP) by increasing O-GlcNAcylation - implications for targeting the Warburg effect in cancer cells. Biochem Biophys Res Commun. 2015;465:838–844. doi: 10.1016/j.bbrc.2015.08.097. [DOI] [PubMed] [Google Scholar]
- Hoter A, Amiri M, Prince A, Amer H, Warda M, Naim HY (2018) Differential glycosylation and modulation of camel and human HSP isoforms in response to thermal and hypoxic stresses. Int J Mol Sci 19. 10.3390/ijms19020402 [DOI] [PMC free article] [PubMed]
- Hrit J et al (2018) OGT binds a conserved C-terminal domain of TET1 to regulate TET1 activity and function in development. eLife 7. 10.7554/eLife.34870 [DOI] [PMC free article] [PubMed]
- Hu J, Wang Y, Zhao S, Chen J, Jin S, Jia P, Ding X. Remote ischemic preconditioning ameliorates acute kidney injury due to contrast exposure in rats through augmented O-GlcNAcylation. Oxidative Med Cell Longev. 2018;2018:4895913–4895915. doi: 10.1155/2018/4895913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itkonen HM, et al. Inhibition of O-GlcNAc transferase activity reprograms prostate cancer cell metabolism. Oncotarget. 2016;7:12464–12476. doi: 10.18632/oncotarget.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetzko J, Walker S. The making of a sweet modification: structure and function of O-GlcNAc transferase. J Biol Chem. 2014;289:34424–34432. doi: 10.1074/jbc.R114.604405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang I, Kim HB, Seo H, Kim JY, Choi H, Yoo JS, Kim JW, Cho JW. O-GlcNAcylation of eIF2α regulates the phospho-eIF2α-mediated ER stress response. Biochim Biophys Acta. 2015;1853:1860–1869. doi: 10.1016/j.bbamcr.2015.04.017. [DOI] [PubMed] [Google Scholar]
- Jensen RV, Andreadou I, Hausenloy DJ, Bøtker HE (2019) The role of O-GlcNAcylation for protection against ischemia-reperfusion injury. Int J Mol Sci 20. 10.3390/ijms20020404 [DOI] [PMC free article] [PubMed]
- Jiang M, Xu B, Li X, Shang Y, Chu Y, Wang W, Chen D, Wu N, Hu S, Zhang S, Li M, Wu K, Yang X, Liang J, Nie Y, Fan D. O-GlcNAcylation promotes colorectal cancer metastasis via the miR-101-O-GlcNAc/EZH2 regulatory feedback circuit. Oncogene. 2019;38:301–316. doi: 10.1038/s41388-018-0435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Yu S, Yu Z, Sheng H, Li Y, Liu S, Warner DS, Paschen W, Yang W. XBP1 (X-box-binding protein-1)-dependent O-GlcNAcylation is neuroprotective in ischemic stroke in young mice and its impairment in aged mice is rescued by thiamet-G. Stroke. 2017;48:1646–1654. doi: 10.1161/strokeaha.117.016579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jínek M, Rehwinkel J, Lazarus BD, Izaurralde E, Hanover JA, Conti E. The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin alpha. Nat Struct Mol Biol. 2004;11:1001–1007. doi: 10.1038/nsmb833. [DOI] [PubMed] [Google Scholar]
- Joiner CM, Li H, Jiang J, Walker S. Structural characterization of the O-GlcNAc cycling enzymes: insights into substrate recognition and catalytic mechanisms. Curr Opin Struct Biol. 2019;56:97–106. doi: 10.1016/j.sbi.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DR, Keune WJ, Anderson KE, Stephens LR, Hawkins PT, Divecha N. The hexosamine biosynthesis pathway and O-GlcNAcylation maintain insulin-stimulated PI3K-PKB phosphorylation and tumour cell growth after short-term glucose deprivation. FEBS J. 2014;281:3591–3608. doi: 10.1111/febs.12879. [DOI] [PubMed] [Google Scholar]
- Kang ES, Han D, Park J, Kwak TK, Oh MA, Lee SA, Choi S, Park ZY, Kim Y, Lee JW. O-GlcNAc modulation at Akt1 Ser473 correlates with apoptosis of murine pancreatic beta cells. Exp Cell Res. 2008;314:2238–2248. doi: 10.1016/j.yexcr.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Kátai E, Pál J, Poór VS, Purewal R, Miseta A, Nagy T. Oxidative stress induces transient O-GlcNAc elevation and tau dephosphorylation in SH-SY5Y cells. J Cell Mol Med. 2016;20:2269–2277. doi: 10.1111/jcmm.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi Z, Chang H, Haserodt S, McKen C, Zachara NE. O-linked beta-N-acetylglucosamine (O-GlcNAc) regulates stress-induced heat shock protein expression in a GSK-3beta-dependent manner. J Biol Chem. 2010;285:39096–39107. doi: 10.1074/jbc.M110.131102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keembiyehetty C. Disruption of O-GlcNAc cycling by deletion of O-GlcNAcase (Oga/Mgea5) changed gene expression pattern in mouse embryonic fibroblast (MEF) cells. Genomics Data. 2015;5:30–33. doi: 10.1016/j.gdata.2015.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus BD, Love DC, Hanover JA. Recombinant O-GlcNAc transferase isoforms: identification of O-GlcNAcase, yes tyrosine kinase, and tau as isoform-specific substrates. Glycobiology. 2006;16:415–421. doi: 10.1093/glycob/cwj078. [DOI] [PubMed] [Google Scholar]
- Lazarus MB, Nam Y, Jiang J, Sliz P, Walker S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature. 2011;469:564–567. doi: 10.1038/nature09638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DE, Lee SJ, Kim SJ, Lee HS, Kwon OS (2019) Curcumin ameliorates nonalcoholic fatty liver disease through inhibition of O-GlcNAcylation. Nutrients 11. 10.3390/nu11112702 [DOI] [PMC free article] [PubMed]
- Lee HJ, Ryu JM, Jung YH, Lee KH, Kim DI, Han HJ. Glycerol-3-phosphate acyltransferase-1 upregulation by O-GlcNAcylation of Sp1 protects against hypoxia-induced mouse embryonic stem cell apoptosis via mTOR activation. Cell Death Dis. 2016;7:e2158. doi: 10.1038/cddis.2015.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre T, Dehennaut V, Guinez C, Olivier S, Drougat L, Mir AM, Mortuaire M, Vercoutter-Edouart AS, Michalski JC. Dysregulation of the nutrient/stress sensor O-GlcNAcylation is involved in the etiology of cardiovascular disorders, type-2 diabetes and Alzheimer's disease. Biochim Biophys Acta. 2010;1800:67–79. doi: 10.1016/j.bbagen.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Leney AC, El Atmioui D, Wu W, Ovaa H, Heck AJR. Elucidating crosstalk mechanisms between phosphorylation and O-GlcNAcylation. Proc Natl Acad Sci U S A. 2017;114:E7255–e7261. doi: 10.1073/pnas.1620529114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Li H, Lu L, Jiang J. Structures of human O-GlcNAcase and its complexes reveal a new substrate recognition mode. Nat Struct Mol Biol. 2017;24:362–369. doi: 10.1038/nsmb.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Ruan HB, Hughes ME, Lee JS, Singh JP, Jones SP, Nitabach MN, Yang X. O-GlcNAc signaling entrains the circadian clock by inhibiting BMAL1/CLOCK ubiquitination. Cell Metab. 2013;17:303–310. doi: 10.1016/j.cmet.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Vera NB, Yang Y, Zhang B, Ni W, Ziso-Qejvanaj E, Ding S, Zhang K, Yin R, Wang S, Zhou X, Fang EX, Xu T, Erion DM, Yang X. Adipocyte OGT governs diet-induced hyperphagia and obesity. Nat Commun. 2018;9:5103. doi: 10.1038/s41467-018-07461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima VV, Spitler K, Choi H, Webb RC, Tostes RC. O-GlcNAcylation and oxidation of proteins: is signalling in the cardiovascular system becoming sweeter? Clin Sci (Lond) 2012;123:473–486. doi: 10.1042/cs20110638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Yao R, Shi H, Liu Y, Lian S, Yang Y, Yang H, Li S (2019a) Effects of cold-inducible RNA-binding protein (CIRP) on liver glycolysis during acute cold exposure in C57BL/6 mice. Int J Mol Sci 20. 10.3390/ijms20061470 [DOI] [PMC free article] [PubMed]
- Liu Y, Wang X, Zhu T, Zhang N, Wang L, Huang T, Cao Y, Li W, Zhang J. Resistance to bortezomib in breast cancer cells that downregulate Bim through FOXA1 O-GlcNAcylation. J Cell Physiol. 2019;234:17527–17537. doi: 10.1002/jcp.28376. [DOI] [PubMed] [Google Scholar]
- Luanpitpong S, Chanthra N, Janan M, Poohadsuan J, Samart P, U-Pratya Y, Rojanasakul Y, Issaragrisil S. Inhibition of O-GlcNAcase sensitizes apoptosis and reverses bortezomib resistance in mantle cell lymphoma through modification of truncated bid. Mol Cancer Ther. 2018;17:484–496. doi: 10.1158/1535-7163.Mct-17-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucena MC, Carvalho-Cruz P, Donadio JL, Oliveira IA, de Queiroz RM, Marinho-Carvalho MM, Sola-Penna M, de Paula IF, Gondim KC, McComb ME, Costello CE, Whelan SA, Todeschini AR, Dias WB. Epithelial mesenchymal transition induces aberrant glycosylation through hexosamine biosynthetic pathway activation. J Biol Chem. 2016;291:12917–12929. doi: 10.1074/jbc.M116.729236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Hart GW. O-GlcNAc profiling: from proteins to proteomes. Clin Proteomics. 2014;11:8. doi: 10.1186/1559-0275-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Vocadlo DJ, Vosseller K. Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-κB activity in pancreatic cancer cells. J Biol Chem. 2013;288:15121–15130. doi: 10.1074/jbc.M113.470047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Vosseller K. Cancer metabolism and elevated O-GlcNAc in oncogenic signaling. J Biol Chem. 2014;289:34457–34465. doi: 10.1074/jbc.R114.577718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–4712. doi: 10.1016/S0021-9258(19)67706-9. [DOI] [PubMed] [Google Scholar]
- Martin JC, Fadda E, Ito K, Woods RJ. Defining the structural origin of the substrate sequence independence of O-GlcNAcase using a combination of molecular docking and dynamics simulation. Glycobiology. 2014;24:85–96. doi: 10.1093/glycob/cwt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez MR, Dias TB, Natov PS, Zachara NE. Stress-induced O-GlcNAcylation: an adaptive process of injured cells. Biochem Soc Trans. 2017;45:237–249. doi: 10.1042/bst20160153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui K, Cavenee WK, Mischel PS. mTORC2 in the center of cancer metabolic reprogramming. Trends Endocrinol Metab. 2014;25:364–373. doi: 10.1016/j.tem.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier T, Bouvet M, Dubois-Deruy E, Dechaumes A, Beseme O, Richard V, Mulder P, Pinet F. Interplay between phosphorylation and O-GlcNAcylation of sarcomeric proteins in ischemic heart failure. Front Endocrinol (Lausanne) 2018;9:598. doi: 10.3389/fendo.2018.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngoh GA, Hamid T, Prabhu SD, Jones SP. O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death. Am J Physiol Heart Circ Physiol. 2009;297:H1711–H1719. doi: 10.1152/ajpheart.00553.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngoh GA, Watson LJ, Facundo HT, Jones SP. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids. 2011;40:895–911. doi: 10.1007/s00726-010-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Ju H, Fan J, Shi X, Cheng Y, Cang X, Zheng Z, Duan X, Yi W. O-GlcNAcylation of PGK1 coordinates glycolysis and TCA cycle to promote tumor growth. Nat Commun. 2020;11:36. doi: 10.1038/s41467-019-13601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nöt LG, Brocks CA, Vámhidy L, Marchase RB, Chatham JC. Increased O-linked beta-N-acetylglucosamine levels on proteins improves survival, reduces inflammation and organ damage 24 hours after trauma-hemorrhage in rats. Crit Care Med. 2010;38:562–571. doi: 10.1097/CCM.0b013e3181cb10b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi N, Morino K, Ida S, Sekine O, Lemecha M, Kume S, Park SY, Choi CS, Ugi S, Maegawa H. Pivotal role of O-GlcNAc modification in cold-induced thermogenesis by brown adipose tissue through mitochondrial biogenesis. Diabetes. 2017;66:2351–2362. doi: 10.2337/db16-1427. [DOI] [PubMed] [Google Scholar]
- Ohn T, Kedersha N, Hickman T, Tisdale S, Anderson P. A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly. Nat Cell Biol. 2008;10:1224–1231. doi: 10.1038/ncb1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak S, Alonso J, Schimpl M, Rafie K, Blair DE, Borodkin VS, Schüttelkopf AW, Albarbarawi O, van Aalten DMF. The active site of O-GlcNAc transferase imposes constraints on substrate sequence. Nat Struct Mol Biol. 2015;22:744–750. doi: 10.1038/nsmb.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SB, Hart GW. New insights: a role for O-GlcNAcylation in diabetic complications. Crit Rev Biochem Mol Biol. 2016;51:150–161. doi: 10.3109/10409238.2015.1135102. [DOI] [PubMed] [Google Scholar]
- Qian K, Wang S, Fu M, Zhou J, Singh JP, Li MD, Yang Y, Zhang K, Wu J, Nie Y, Ruan HB, Yang X. Transcriptional regulation of O-GlcNAc homeostasis is disrupted in pancreatic cancer. J Biol Chem. 2018;293:13989–14000. doi: 10.1074/jbc.RA118.004709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao X, Duan X, Mao W, Li X, Li Z, Li Q, Zheng Z, Xu H, Chen M, Wang PG, Wang Y, Shen B, Yi W. O-GlcNAcylation of G6PD promotes the pentose phosphate pathway and tumor growth. Nat Commun. 2015;6:8468. doi: 10.1038/ncomms9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RA, Lee A, Henry R, Zachara NE. Characterization of the specificity of O-GlcNAc reactive antibodies under conditions of starvation and stress. Anal Biochem. 2014;457:8–18. doi: 10.1016/j.ab.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach JE, Clark PM, Hsieh-Wilson LC. Chemical approaches to understanding O-GlcNAc glycosylation in the brain. Nat Chem Biol. 2008;4:97–106. doi: 10.1038/nchembio.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AC, Yu SH, Li B, Zegzouti H, Kohler JJ. Enhanced transfer of a photocross-linking N-acetylglucosamine (GlcNAc) analog by an O-GlcNAc transferase mutant with converted substrate specificity. J Biol Chem. 2015;290:22638–22648. doi: 10.1074/jbc.M115.667006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan HB, Ma Y, Torres S, Zhang B, Feriod C, Heck RM, Qian K, Fu M, Li X, Nathanson MH, Bennett AM, Nie Y, Ehrlich BE, Yang X. Calcium-dependent O-GlcNAc signaling drives liver autophagy in adaptation to starvation. Genes Dev. 2017;31:1655–1665. doi: 10.1101/gad.305441.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan HB, Nie Y, Yang X. Regulation of protein degradation by O-GlcNAcylation: crosstalk with ubiquitination. Mol Cell Proteomics. 2013;12:3489–3497. doi: 10.1074/mcp.R113.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan HB, Singh JP, Li MD, Wu J, Yang X. Cracking the O-GlcNAc code in metabolism. Trends Endocrinol Metab. 2013;24:301–309. doi: 10.1016/j.tem.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusai K, Herzog R, Kuster L, Kratochwill K, Aufricht C. GSK-3β inhibition protects mesothelial cells during experimental peritoneal dialysis through upregulation of the heat shock response. Cell Stress Chaperones. 2013;18:569–579. doi: 10.1007/s12192-013-0410-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacoman JL, Dagda RY, Burnham-Marusich AR, Dagda RK, Berninsone PM. Mitochondrial O-GlcNAc Transferase (mOGT) regulates mitochondrial structure, function, and survival in HeLa cells. J Biol Chem. 2017;292:4499–4518. doi: 10.1074/jbc.M116.726752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma NS, Gupta VK, Dauer P, Kesh K, Hadad R, Giri B, Chandra A, Dudeja V, Slawson C, Banerjee S, Vickers SM, Saluja A, Banerjee S. O-GlcNAc modification of Sox2 regulates self-renewal in pancreatic cancer by promoting its stability. Theranostics. 2019;9:3410–3424. doi: 10.7150/thno.32615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen DL, Gloster TM, Yuzwa SA, Vocadlo DJ. Insights into O-linked N-acetylglucosamine ([0-9]O-GlcNAc) processing and dynamics through kinetic analysis of O-GlcNAc transferase and O-GlcNAcase activity on protein substrates. J Biol Chem. 2012;287:15395–15408. doi: 10.1074/jbc.M111.310664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Munk A, Nielsen TS, Daughtry MR, Larsson L, Li S, Høyer KF, Geisler HW, Sulek K, Kjøbsted R, Fisher T, Andersen MM, Shen Z, Hansen UK, England EM, Cheng Z, Højlund K, Wojtaszewski JFP, Yang X, Hulver MW, Helm RF, Treebak JT, Gerrard DE. Skeletal muscle O-GlcNAc transferase is important for muscle energy homeostasis and whole-body insulin sensitivity. Mol Metab. 2018;11:160–177. doi: 10.1016/j.molmet.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Yao R, Lian S, Liu P, Liu Y, Yang YY, Yang H, Li S. Regulating glycolysis, the TLR4 signal pathway and expression of RBM3 in mouse liver in response to acute cold exposure. Stress. 2019;22:366–376. doi: 10.1080/10253890.2019.1568987. [DOI] [PubMed] [Google Scholar]
- Slawson C, Hart GW. O-GlcNAc signalling: implications for cancer cell biology. Nat Rev Cancer. 2011;11:678–684. doi: 10.1038/nrc3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodi VL, Bacigalupa ZA, Ferrer CM, Lee JV, Gocal WA, Mukhopadhyay D, Wellen KE, Ivan M, Reginato MJ. Nutrient sensor O-GlcNAc transferase controls cancer lipid metabolism via SREBP-1 regulation. Oncogene. 2018;37:924–934. doi: 10.1038/onc.2017.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh HN, Lee YJ, Kim MO, Ryu JM, Han HJ. Glucosamine-induced Sp1 O-GlcNAcylation ameliorates hypoxia-induced SGLT dysfunction in primary cultured renal proximal tubule cells. J Cell Physiol. 2014;229:1557–1568. doi: 10.1002/jcp.24599. [DOI] [PubMed] [Google Scholar]
- Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259:3308–3317. doi: 10.1016/S0021-9258(17)43295-9. [DOI] [PubMed] [Google Scholar]
- Trapannone R, Mariappa D, Ferenbach AT, van Aalten DM. Nucleocytoplasmic human O-GlcNAc transferase is sufficient for O-GlcNAcylation of mitochondrial proteins. Biochem J. 2016;473:1693–1702. doi: 10.1042/bcj20160092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinca GM, Goodman ML, Papachristou EK, D’Santos CS, Chalise P, Madan R, Slawson C, Hagan CR. O-GlcNAc-dependent regulation of progesterone receptor function in breast cancer. Horm Cancer. 2018;9:12–21. doi: 10.1007/s12672-017-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinca GM, Hagan CR. O-GlcNAcylation in women's cancers: breast, endometrial and ovarian. J Bioenerg Biomembr. 2018;50:199–204. doi: 10.1007/s10863-017-9730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Very N, Vercoutter-Edouart AS, Lefebvre T, Hardivillé S, El Yazidi-Belkoura I. Cross-dysregulation of O-GlcNAcylation and PI3K/AKT/mTOR Axis in human chronic diseases. Front Endocrinol (Lausanne) 2018;9:602. doi: 10.3389/fendo.2018.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AC, Jensen EH, Rexach JE, Vinters HV, Hsieh-Wilson LC. Loss of O-GlcNAc glycosylation in forebrain excitatory neurons induces neurodegeneration. Proc Natl Acad Sci U S A. 2016;113:15120–15125. doi: 10.1073/pnas.1606899113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Yang F, Petyuk VA, Shukla AK, Monroe ME, Gritsenko MA, Rodland KD, Smith RD, Qian WJ, Gong CX, Liu T. Quantitative proteomics identifies altered O-GlcNAcylation of structural, synaptic and memory-associated proteins in Alzheimer's disease. J Pathol. 2017;243:78–88. doi: 10.1002/path.4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Feng Z, Wang X, Yang L, Han S, Cao K, Xu J, Zhao L, Zhang Y, Liu J. O-GlcNAcase deficiency suppresses skeletal myogenesis and insulin sensitivity in mice through the modulation of mitochondrial homeostasis. Diabetologia. 2016;59:1287–1296. doi: 10.1007/s00125-016-3919-2. [DOI] [PubMed] [Google Scholar]
- Wani WY, Boyer-Guittaut M, Dodson M, Chatham J, Darley-Usmar V, Zhang J. Regulation of autophagy by protein post-translational modification. Lab Investig. 2015;95:14–25. doi: 10.1038/labinvest.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani WY, Ouyang X, Benavides GA, Redmann M, Cofield SS, Shacka JJ, Chatham JC, Darley-Usmar V, Zhang J. O-GlcNAc regulation of autophagy and α-synuclein homeostasis; implications for Parkinson's disease. Mol Brain. 2017;10:32. doi: 10.1186/s13041-017-0311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Lu C, Mancuso A, Lemons JMS, Ryczko M, Dennis JW, Rabinowitz JD, Coller HA, Thompson CB. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 2010;24:2784–2799. doi: 10.1101/gad.1985910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan SA, Dias WB, Thiruneelakantapillai L, Lane MD, Hart GW. Regulation of insulin receptor substrate 1 (IRS-1)/AKT kinase-mediated insulin signaling by O-linked beta-N-acetylglucosamine in 3T3-L1 adipocytes. J Biol Chem. 2010;285:5204–5211. doi: 10.1074/jbc.M109.077818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisenhunt TR, Yang X, Bowe DB, Paterson AJ, Van Tine BA, Kudlow JE. Disrupting the enzyme complex regulating O-GlcNAcylation blocks signaling and development. Glycobiology. 2006;16:551–563. doi: 10.1093/glycob/cwj096. [DOI] [PubMed] [Google Scholar]
- Xie S, Jin N, Gu J, Shi J, Sun J, Chu D, Zhang L, Dai CL, Gu JH, Gong CX, Iqbal K, Liu F. O-GlcNAcylation of protein kinase a catalytic subunits enhances its activity: a mechanism linked to learning and memory deficits in Alzheimer's disease. Aging Cell. 2016;15:455–464. doi: 10.1111/acel.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Zhang X, Wu JL, Fu L, Liu K, Liu D, Chen GG, Lai PBS, Wong N, Yu J. O-GlcNAc transferase promotes fatty liver-associated liver cancer through inducing palmitic acid and activating endoplasmic reticulum stress. J Hepatol. 2017;67:310–320. doi: 10.1016/j.jhep.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- Yang X, Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat Rev Mol Cell Biol. 2017;18:452–465. doi: 10.1038/nrm.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao R. Effects of O-GlcNAcylation modification on glucose metabolism in gastrocnemius and liver of acute cold-exposed mice and piglets. Daqing: Heilongjiang Bayi Agricultural University; 2018. [Google Scholar]
- Yao R, Yang Y, Lian S, Shi H, Liu P, Liu Y, Yang H, Li S (2018) Effects of acute cold stress on liver O-GlcNAcylation and Glycometabolism in mice. Int J Mol Sci 19. 10.3390/ijms19092815 [DOI] [PMC free article] [PubMed]
- Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA, Peters EC, Driggers EM, Hsieh-Wilson LC. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–980. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafir A, Readnower R, Long BW, McCracken J, Aird A, Alvarez A, Cummins TD, Li Q, Hill BG, Bhatnagar A, Prabhu SD, Bolli R, Jones SP. Protein O-GlcNAcylation is a novel cytoprotective signal in cardiac stem cells. Stem Cells. 2013;31:765–775. doi: 10.1002/stem.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Qiao Y, Wu Q, Chen Y, Zou S, Liu X, Zhu G, Zhao Y, Chen Y, Yu Y, Pan Q, Wang J, Sun F. The essential role of YAP O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nat Commun. 2017;8:15280. doi: 10.1038/ncomms15280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Shu XE, Qian SB. O-GlcNAc modification of eIF4GI acts as a translational switch in heat shock response. Nat Chem Biol. 2018;14:909–916. doi: 10.1038/s41589-018-0120-6. [DOI] [PubMed] [Google Scholar]
- Zhu G, Qian M, Lu L, Chen Y, Zhang X, Wu Q, Liu Y, Bian Z, Yang Y, Guo S, Wang J, Pan Q, Sun F (2019) O-GlcNAcylation of YY1 stimulates tumorigenesis in colorectal cancer cells by targeting SLC22A15 and AANAT. Carcinogenesis. 10.1093/carcin/bgz010 [DOI] [PubMed]
- Zhu Y, Shan X, Yuzwa SA, Vocadlo DJ. The emerging link between O-GlcNAc and Alzheimer disease. J Biol Chem. 2014;289:34472–34481. doi: 10.1074/jbc.R114.601351. [DOI] [PMC free article] [PubMed] [Google Scholar]