Abstract
In previous years, the role of gastroesophageal (GE) ultrasound as a diagnostic tool in gastroesophageal reflux disease (GERD) has been disputed. Most authors believe that it is difficult to diagnose GERD without correlation studies between esophageal pathology and ultrasonographic signs. Indeed, there are many anatomic descriptions of the normal GE junction. The fact that GERD diagnosis was made by morphological studies was believed to be an incorrect deduction. We revisit the pathophysiologic data concerning the gastroesophageal junction and gastric function and review the data in the literature of the past 30 years.
Keywords: Gastroesophageal reflux disease, Lower esophageal sphincter, Gastric emptying time, Ultrasound
Introduction
Gastroesophageal reflux disease (GERD) is complex and involves changes that lead to the failure of normal anti-reflux mechanisms. Esophageal damage is a consequence of delayed gastric emptying time (GET) and hiatal hernia, but it is considered a motor defect. The anti-reflux barrier is a well-defined anatomic region that is the result of a delicate balance between the lower esophageal sphincter and the crural diaphragm. The phrenoesophageal ligament and the gastric sling fibers of the gastric cardia also contribute to the functioning of the barrier. In case of anatomic laxity or increased intragastric pressure, the barrier does not guarantee its function, and reflux occurs [1].
Ultrasonographic aspects
The gastroesophageal region and gastric motor function can be studied by means of ultrasonography. The main ultrasound parameters that are considered in the literature are the following:
The length of the abdominal tract of the esophagus was measured only on the longitudinal scans from the point at which the esophagus traversed the diaphragm to the gastroesophageal region identified on the sonogram by a small triangular pad of gastric folds radiating from the cardia [2] (Fig. 1).
The esophageal wall thickness was measured on the anterior wall at the midpoint of the abdominal esophagus [2] (Fig. 2).
The gastroesophageal angle (angle’s His) was delimited within the tangent line passing from the left fornix of the stomach and the line of the esophageal wall [3] (Fig. 3).
The esophageal diameter was measured from serosa to serosa in longitudinal scans [4] (Fig. 4).
Fig. 1.
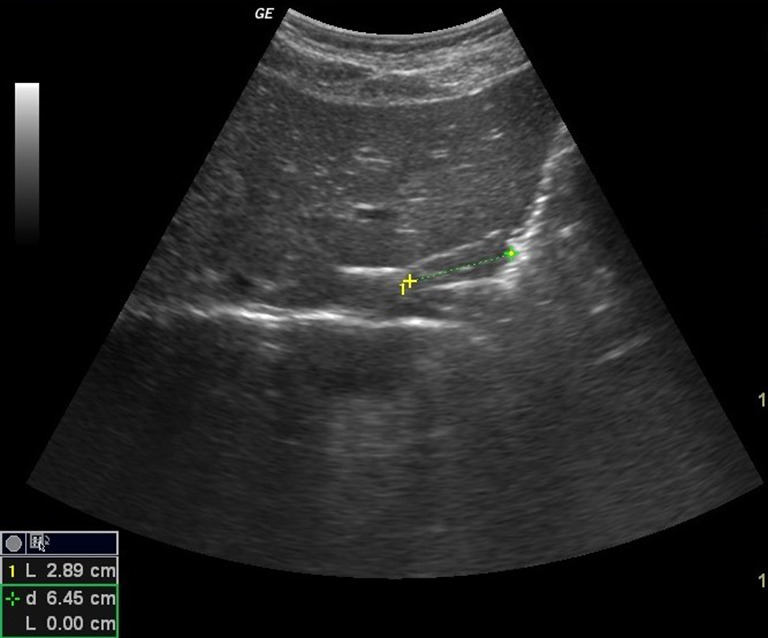
Longitudinal scan showing the length of the abdominal tract of the esophagus
Fig. 2.
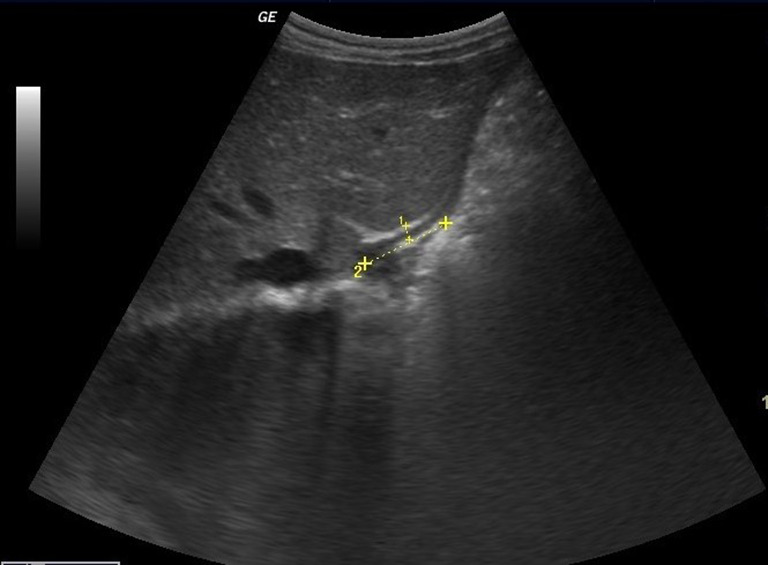
Longitudinal scan showing that the esophageal wall thickness was measured on the anterior wall at the midpoint of the abdominal esophagus
Fig. 3.
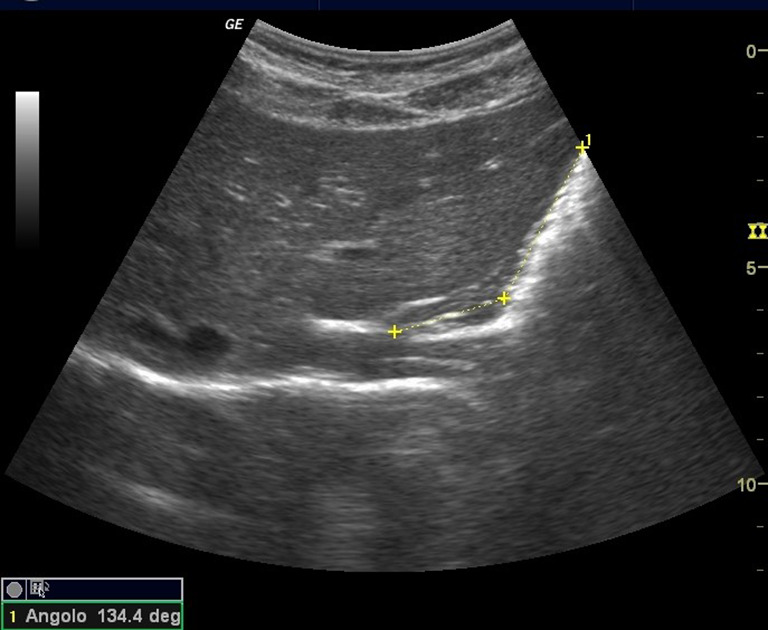
Longitudinal scan showing the gastroesophageal angle
Fig. 4.
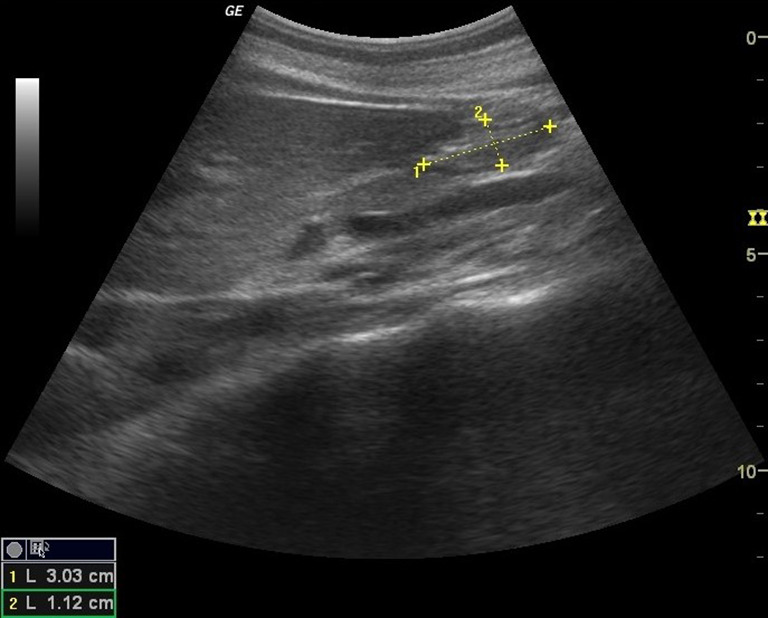
Longitudinal scan from serosa to serosa
The reference values of these parameters are not well established, despite many articles and reviews [5–7]. In effect, the design of each study lacks randomization or pathological controls, and is conducted in only a few patients, or is statistically incorrect. The results are expert opinions because of the low level and quality of the evidence. The stomach, too, can be studied by ultrasonography, but at present only functional studies are performed regarding motor activity. The GET was calculated by measuring the cross-section of the gastric antrum corresponding to the sagittal plane passing through the superior mesenteric vein (as a point of reference) to obtain scanning consistency. The cross-section of the gastric antrum shows an elliptic shape at this level, and its area in cubic centimeters was measured by the following formula: π a × b /4 (a: longitudinal diameter, b: anteroposterior diameter). The stomach was considered empty when the section area of the antrum returned to the baseline value and persisted unchanged for at least 30 min. The final GET was calculated in relation to the start of the meal. In controls, the final GET of a standard meal is 180 min [8]. Another intriguing feature of functional ultrasound of the stomach is the study of fundus accommodation. Gastric accommodation, in fact, is an important mechanism in comprehending the pathophysiology of functional disorders of the stomach. In 2000, Olafsdottir et al. demonstrated that gastric accommodation ultrasonography is a noninvasive imaging test [9] during the ingestion of a meal, with the proximal stomach acting as a reservoir by increasing its volume without a significant change in intragastric pressure. For proximal stomach measurements, two standardized sonographic sections were chosen by placing the transducer at the left subcostal margin and tilting it cranially. First, a sagittal section of the stomach was obtained, with the left renal pelvis in a longitudinal projection and the left lobe of the liver and the tail of the pancreas serving as internal landmarks. The stomach area was outlined by tracing the inner echogenic layer, corresponding to the interface between the soup and the mucosa of the gastric wall, from the top margin of the fundus and 7 cm downward along the axis of the stomach (sagittal section, sa). Then, the transducer was rotated 90° clockwise to obtain an oblique frontal gastric section. In this case, the left hemidiaphragm, the top margin of the fundus, and the liver parenchyma served as landmarks. In this way, it is feasible to measure the maximal oblique frontal diameter (ofd), which is located within 7 cm along the long axis of the proximal stomach. An approximate volume of the proximal stomach (av) was calculated by combining these two measures (av = sa × ofd). The av values were used to compute the individual emptying fraction of the proximal stomach, defined as (av1 min-av actual)/av1 min. Time zero was defined as the end of the ingestion period of 5 min. The field of application of gastric proximal ultrasound examination is currently confined to the study of functional disorders of the gastrointestinal (GI) tract in accordance with Rome IV Pediatric, but it is an important prospective tool for the complex evaluation of patients with gastroesophageal reflux.
Historical review
The 2009 ESPGHAN guidelines [10] suggest that these tests are not recommended for the routine evaluation of GERD in children. The guidelines of 2018 [11] modify in part these recommendations, suggesting that at present, ultrasound has no role as a routine diagnostic tool for GERD in children, but this test may be useful for evaluating other conditions that might mimic GERD, including, most importantly, pyloric stenosis in the infant population. Abdominal ultrasound may also pick up other diagnoses that may trigger symptoms of discomfort and vomiting, including diagnoses such as hydronephrosis, ureteropelvic obstruction, gallstones, and ovarian torsion. Like barium swallow X-ray, ultrasound can detect hiatal hernia and the length and position of the lower esophageal sphincter (les) relative to the diaphragm and magnitude of the gastroesophageal angle of His. It has also been proposed as a diagnostic test for gastric dysmotility, which may have implications from a reflux perspective. In 1993, Gomes et al. demonstrated that the length of the abdominal esophagus is fundamental in reflux studies [13]. In 2003, the same authors validated the sonographic method. In 1994, Westra affirmed the ability of the ultrasonographic measurement to diagnose symptomatic gastroesophageal reflux [14]. In 2001, Esposito et al. [2] concluded that the visualization of the GE junction and the measurement of the abdominal esophagus are achievable with real-time sonography. Many studies were performed in the subsequent years to demonstrate the feasibility of ultrasonography in diagnosing GERD, but none was performed with the correct design; therefore, each contribution must be considered an expert opinion. Cucchiara et al. demonstrated that the GET in patients with functional dyspepsia [8–12] and cystic fibrosis [15] was delayed. The field of application of gastric proximal ultrasound examination is at present confined to the study of functional disorders of the GI tract in accordance with Rome IV Pediatric, or in evaluating rehabilitation in anorexia, but it is an important prospective tool for the complex evaluation of patients with gastroesophageal reflux [6, 16–18].
Conclusion
The evaluation of children with gastroesophageal reflux disease requires an accurate ultrasonographic approach, but ultrasound requires more and accurate studies for complete validation.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki declaration of 1975, and its late amendments. Additional informed consent was obtained from all patients for which identifying information is not included in this article.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tack J, et al. Pathophysiology of gastroesophageal reflux disease. Gastroenterology. 2018;154:277–288. doi: 10.1053/j.gastro.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 2.Esposito F, et al. Transabdominal sonography of the normal gastroesophageal junction in children. J Clin Ultrasound. 2001;29:326–331. doi: 10.1002/jcu.1043. [DOI] [PubMed] [Google Scholar]
- 3.Halkiewicz F, et al. Ultrasound picture of gastroesophageal junction in children with reflux disease. Med Sci Monit. 2000;6:96–99. [PubMed] [Google Scholar]
- 4.Hashemi H, et al. Diagnostic efficacy of distal esophagus ultrasonography in diagnosis of gastroesophageal reflux disease in children. Res J Biol Sci. 2009;4:71–76. [Google Scholar]
- 5.Savino A, et al. Us in the diagnosis of gastroesophageal reflux in children. Pediatr Radiol. 2012;42:515–524. doi: 10.1007/s00247-012-2344-z. [DOI] [PubMed] [Google Scholar]
- 6.Di Serafino M, Severino R, Lisanti L, Rocca R, Scarano E. Dysphagia lusoria: an uncommon cause of dysphagia. J Hepatol Gastroint Dis. 2016;2(134):2. doi: 10.4172/2475-3181.1000134. [DOI] [Google Scholar]
- 7.Di Serafino M, Mercogliano C, Vallone G. Ultrasound evaluation of the enteric duplication cyst: the gut signature. J Ultrasound. 2015;19(2):131–133. doi: 10.1007/s40477-015-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cucchiara S, et al. Real time ultrasound reveals gastric motor abnormalitiesin children investigated for dyspeptic symptoms. Pediatr Gastroenterol Nutr. 1995;21:446–453. doi: 10.1097/00005176-199511000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Olafsdottir E, et al. Impaired accommodation of the proximal stomach in children with recurrent abdominal pain. J Pediatr Gastroenterol Nutr. 2000;30:157–163. doi: 10.1097/00005176-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Vandenplas Y, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommandations of the north american society for pediatric gastroenterology, hepatology and nutrition (naspghan) and the european society for pediatric gastroenterology, hepatology and nutrition (espghan) J Pediatr Gastronetrol Nutr. 2009;49:498–547. doi: 10.1097/MPG.0b013e3181b7f563. [DOI] [PubMed] [Google Scholar]
- 11.Rosen N, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommandations of the north american society for pediatric gastroenterology, hepatology and nutrition (naspghan) and the european society for pediatric gastroenterology, hepatology and nutrition (espghan) J Pediatr Gastronetrol Nutr. 2018;66:516–554. doi: 10.1097/MPG.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tufano A, Minelli R, Rossi E, et al. Inferior epigastric artery pseudoaneurysm secondary to port placement during a robot-assisted laparoscopic radical cystectomy. J Ultrasound. 2020 doi: 10.1007/s40477-020-00442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes H, et al. Ultrasonography and gastric emptiyng in children: valuidation of a sonographic method and determination of physiological and pathological patterns. Pediatr Radiol. 2003;33:522–529. doi: 10.1007/s00247-003-0954-1. [DOI] [PubMed] [Google Scholar]
- 14.Westra SJ, et al. Symptomatic gastroesophageal reflux: diagnosis with ultrasound. J Pediatr Gastroenterol Nutr. 1994;19:58–64. doi: 10.1097/00005176-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Cucchiara S, et al. Us measurement of gastric emptying time in aptients with cystic fibrosis and effect of ranitidine on delayed gastric emptying. J Pediatr Gastroenterol Nutr. 1996;128:485–488. doi: 10.1016/s0022-3476(96)70358-x. [DOI] [PubMed] [Google Scholar]
- 16.Corvino A, Sandomenico F, Setola SV, et al. Added value of contrast-enhanced ultrasound (CEUS) with Sonovue® in the diagnosis of inferior epigastric artery pseudoaneurysm: report of a case and review of literature. J Ultrasound. 2019;22:485–489. doi: 10.1007/s40477-019-00398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Serafino M, et al. A case of esophageal perforation: clinical and diagnostic management in emergency medicine. Emerg Care J. 2017;13:6848. doi: 10.4081/ecj.2017.6848. [DOI] [Google Scholar]
- 18.Esposito F, Di Serafino M, Mercogliano C, et al. The pediatric gastrointestinal tract: ultrasound findings in acute diseases. J Ultrasound. 2019;22:409–422. doi: 10.1007/s40477-018-00355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


