Abstract
Purpose
To identify cytokines in plasma that may predict objective response and progression-free survival (PFS) in patients with locally advanced non-small cell lung cancer (NSCLC) treated with chemoradiotherapy.
Materials and Methods
From April 2016 to May 2017, thirty-one patients with locally advanced inoperable/unresectable NSCLC were included, and treated with concurrent chemoradiotherapy (CCRT). No immune checkpoint inhibitors were administered after CCRT. Plasma from each patient was collected before radiotherapy, and 25 cytokines in the plasma were measured by Luminex or U-PLEX assays. Logistic regression and COX regression were performed to identify the predictive factors for objective response and PFS, respectively. Kaplan-Meier survival analysis was used to compare the PFS between the groups.
Results
High levels of IL-13 and TNF-α, and low levels of ICAM-1, IFN-γ, and soluble PD-L1 (sPD-L1) were significantly associated with objective response (P <0.05). High levels of IL-8, CCL5, and CXCL3 also showed a trend toward association with objective response (P <0.1). The combination of cytokines (IL-8 and ICAM-1, or TNF-α and sPD-L1) improved predictive accuracy. Univariate analysis identified IL-8 and ICAM-1 as potential markers to predict PFS. Multivariate analysis suggested that high level of IL-8 (P =0.010) and low level of ICAM-1 (P =0.011) correlated significantly with a longer PFS.
Conclusion
IL-8 and ICAM-1 in plasma have the potential to predict objective response and PFS in patients with locally advanced NSCLC underwent chemoradiotherapy.
Keywords: non-small cell lung cancer, chemoradiotherapy, cytokines, IL-8, intercellular adhesion molecule 1 (ICAM-1), biomarkers
Introduction
Locally advanced non-small cell lung cancer (NSCLC) accounts for approximately 30% of NSCLC cases. Definitive concurrent chemoradiotherapy (CCRT) is the standard treatment for patients with unresectable or inoperable locally advanced NSCLC (1, 2). Concurrent regimen with once-daily thoracic radiation improves the 5-year survival compared with sequential chemoradiotherapy or concurrent regimen with twice-daily hyperfractionated radiotherapy (2). However, the outcome of radiotherapy varies among patients. The response rate varies between different chemotherapy regimens, from approximately 38% to 80% (3–5). The median progression-free survival (PFS) of concurrent chemoradiotherapy in locally advanced NSCLC is about 8–15 months (3–5). For patients whose tumor relapse or metastasis are likely to occur, more aggressive treatment or more frequent follow-up may be needed. Therefore, prediction of the outcome of radiotherapy is urgently needed for the selection of an optimal treatment strategy.
Cytokines and other soluble factors participate in carcinogenesis and treatment response. Factors that are involved in inflammatory and immune responses may contribute to tumor control and survival (6). Suwinski et al. measured six serum proteins in NSCLC patients treated with curative or palliative thoracic radiotherapy, and demonstrated potential utilities of osteopontin (OPN), vascular endothelial growth factor (VEGF), and erythropoietin (EPO) as prognostic factors (7). Using blood-biomarkers related to hypoxia, inflammation and tumor load, Carvalho et al. developed a model to predict survival of NSCLC patients receiving radiotherapy (8). These studies provided valuable information for noninvasively predicting clinical outcome for NSCLC patient treated with radiotherapy. However, the populations were relatively heterogeneous, including patients with stage I inoperable tumor, or patients with metastatic receiving palliative radiotherapy. Therefore the results may not be directly translated into the prediction of response to CCRT for patients with locally advanced NSCLC.
To identify peripheral blood-based biomarkers that may predict the objective response and PFS in patients with locally advanced NSCLC treated with chemoradiotherapy, we collected plasma from these patients prior to radiotherapy, and detected a panel of cytokines or soluble factors, which involve in inflammation, immune status, angiogenesis and other biological processes. We identified candidate biomarkers which have the potential to predict the objective response and PFS in patients with locally advanced NSCLC underwent chemoradiotherapy.
Materials and Methods
Patients and Treatment
From April 2016 to May 2017, 31 patients with locally advanced inoperable/unresectable NSCLC were continuously included in the study, and treated with CCRT in the Department of Radiation Oncology, Peking University Cancer Hospital and Institute, China. Radiation was delivered using the intensity-modulated radiotherapy (IMRT) technique, and a total dose of 60–66 Gy was administered in 2.0–2.2 Gy daily fractions over 6 weeks using 6 MV photons. Concurrent chemotherapy included pemetrexed/carboplatin (for adenocarcinoma), docetaxel/carboplatin, paclitaxel/carboplatin, etoposide/carboplatin, or paclitaxel. The majority of patients received platinum-based induction chemotherapy before CCRT. No immune checkpoint inhibitors were administered after CCRT. Patients were followed up approximately every 3 months during the first year after radiotherapy, every 6 months during the second year, and annually thereafter. The study protocol was approved by the Ethics Committee of Beijing Cancer Hospital (Peking University Cancer Hospital and Institute). Each patient provided written informed consent.
Sample Collection and Cytokine Measurement
Blood samples were collected into EDTA-containing collection tubes from each patient within 3 days before the beginning of radiotherapy, and from 17 patients in this cohort at the end of radiotherapy. Blood samples were centrifuged within 1 h of collection at 1000 × g for 20 min at 4°C. The supernatant was collected as plasma and was stored at -80°C until analysis.
Interleukin-2 (IL-2), IL-10, IL-12p70, vascular endothelial growth factor (VEGF), tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), chemokine (C-X-C motif) ligand 3 (CXCL3), chemokine (C-C motif) ligand 5 (CCL5), colony-stimulating factor (M-CSF), granulocyte colony-stimulating factor (G-CSF), thrombopoietin (TPO), erythropoietin (EPO), WNT3A, soluble programmed death-ligand 1 (sPD-L1), interferon gamma (IFN-γ), and intercellular adhesion molecule 1 (ICAM-1) were measured by Luminex assay (R&D Systems, Minneapolis, MN). Eotaxin, FMS-related tyrosine kinase 3 ligand (FLT3L), granulocyte-macrophage colony stimulating factor (GM-CSF), IL-1α, IL-1β, IL-6, IL-8, IL-13, IP-10, and tumor necrosis factor-alpha (TNF-α) were measured by U-PLEX assay (Meso Scale Diagnostics, Rockville, MD, USA).
Statistics Analysis
Objective response rate (ORR) was defined as the percentage of patients with the best overall response of complete remission (CR) and partial remission (PR) according to Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST1.1). PFS was defined as the time from the first day of radiotherapy to the date of any progression, death, and loss to follow-up. Patients were censored at the last follow-up if no progression or death occurred. Logistic regression was performed to identify the predictive factors for objective response. The area under the curve (AUC) of the receiver operating characteristic (ROC) curves was calculated for the prediction of objective response. COX regression was performed to identify the predictive factors for PFS. ROC analysis was applied to optimize the cut-off. Kaplan-Meier survival curves were applied to compare the PFS between the groups. Variables with P<0.1 under univariate analysis were selected for multivariate analysis. P values <0.05 (two-sided) were considered statistically significant. All statistical analyses were performed using SPSS Statistics 21.0 (IBM SPSS Statistics, Chicago, IL).
Results
Patient Characteristics
From April 2016 to May 2017, there were 31 patients included in the analysis. Patient characteristics are shown in Table 1 . The median age was 58.4 years (range: 43.3–69.5). The median follow-up time was 33.9 months (95% CI: 28.6–39.2). The percentage of patients with partial remission (PR), stable disease (SD), and progression disease (PD) was 58.1%, 32.3%, and 9.7%, respectively. The ORR was 58.1%, and 23 patients (74.2%) experienced disease progression at the last follow-up. Median PFS was 10.5 months (95% CI: 4.4–16.7), and the 1-year and 2-year PFS were 48.4% and 25.8% respectively. Median overall survival (OS) was not reached, and the 1-year and 2-year OS were 87.1% and 70.1% respectively.
Table 1.
Patient characteristics (N=31).
| Characteristic | N | % |
|---|---|---|
| Age, median years (range) | 58.4 (43.3–69.5) | |
| Gender | ||
| Male | 26 | 83.9 |
| Female | 5 | 16.1 |
| Smoking | ||
| Never | 6 | 19.4 |
| Ever | 25 | 80.6 |
| Histology | ||
| Adenocarcinoma (ADC) | 7 | 22.6 |
| Squamous cell carcinoma (SCC) | 24 | 77.4 |
| Stage | ||
| IIB | 1 | 3.2 |
| IIIA | 10 | 32.3 |
| IIIB | 20 | 64.5 |
| EGFR mutation | ||
| Wildtype | 28 | 90.3 |
| Mutant (Del19/L858R/T2573G) | 3 (1/1/1) | 9.7 (3.2/3.2/3.2) |
| Induction chemotherapy | ||
| No | 5 | 16.1 |
| Yes | 26 | 83.9 |
Correlation Between Objective Response and Cytokines in Plasma
We analyzed the association between objective response and clinical factors or cytokines in plasma collected prior to CCRT by logistic regression ( Table 2 ). Pathology, EGFR mutation status and induction chemotherapy were not associated with objective response. Patients with objective response to CCRT had high levels of IL-13 and TNF-α, and low levels of ICAM-1, IFN-γ, and sPD-L1. High levels of IL-8, CCL5, and CXCL3 also showed a trend toward association with objective response.
Table 2.
Association between objective response and clinical factors or cytokines in plasma by univariate analysis.
| Factor | Median | Odds Ratio (95% CI) | P |
|---|---|---|---|
| Age (years) | 58.3 | 1.129 (0.987–1.291) | 0.076 |
| Gender (Male vs. Female) | NA | 0.417 (0.059–2.946) | 0.38 |
| Smoking (Never vs. Ever) | NA | 1.5 (0.251–8.977) | 0.657 |
| Pathology (ADC vs. SCC) | NA | 2.222 (0.402–12.285) | 0.36 |
| Stage* (IIB vs. IIIA vs. IIIB) |
NA | 0.371 (0.084–1.638) | 0.191 |
| EGFR mutation (wildtype vs. mutant) |
NA | 0.000 (NA) | 0.999 |
| Induction chemotherapy (No vs. Yes) |
NA | 0.909 (0.129–6.396) | 0.924 |
| CCL5 (ng/ml) | 14.863 | 1.051 (0.998–1.107) | 0.062 |
| CXCL3 | 314.12 | 1.004 (0.999–1.008) | 0.096 |
| Eotaxin | 139.09 | 1.005 (0.992–019) | 0.445 |
| EPO | 15.455 | 1.042 (0.956–1.136) | 0.345 |
| FLT3L | 175.11 | 1 (0.994–1.005) | 0.942 |
| G-CSF | 23.59 | 0.984 (0.954–1.015) | 0.321 |
| GM-CSF | 0.18 | 0.307 (0.000–3465.908) | 0.804 |
| ICAM-1 (ng/ml) | 553.397 | 0.997 (0.995–1.000) | 0.03 |
| IFN-γ | 25.36 | 0.963 (0.928–0.998) | 0.038 |
| IL-1α | 0.51 | 1.084 (0.677–1.736) | 0.737 |
| IL-1β | 0.2 | 1.533 (0.007–350.442) | 0.878 |
| IL-10 | 1.3 | 1.378 (0.056–33.828) | 0.844 |
| IL-12p70 | 40.9 | 0.933 (0.804–1.083) | 0.362 |
| IL-13 | 3.2 | 1.421 (1.039–1.941) | 0.028 |
| IL-2 | 5.7 | 0.496 (0.155–1.593) | 0.239 |
| IL-6 | 1.73 | 1.114 (0.832–1.491) | 0.469 |
| IL-8 | 4.12 | 1.507 (0.964–2.355) | 0.072 |
| IP-10 | 414.65 | 1.002 (0.997–1.006) | 0.474 |
| M-CSF | 50.11 | 0.993 (0.971–1.016) | 0.57 |
| sPD-L1 | 68.27 | 0.997 (0.995–1.000) | 0.042 |
| TNF-α | 4.59 | 2.985 (1.230–7.242) | 0.016 |
| TPO | 28.54 | 0.999 (0.994–1.004) | 0.661 |
| TRAIL | 31.57 | 1.007 (0.971–1.044) | 0.702 |
| VEGF | 16.1 | 1.061 (0.987–1.141) | 0.108 |
| WNT3A | 0.751 | 0.641 (0.172–2.396) | 0.509 |
The unit of the cytokines is pg/ml if not otherwise specified. Cytokines with P<0.1 are shown in bold.
*According to American Joint Commission on Cancer (AJCC) staging system 7th edition.
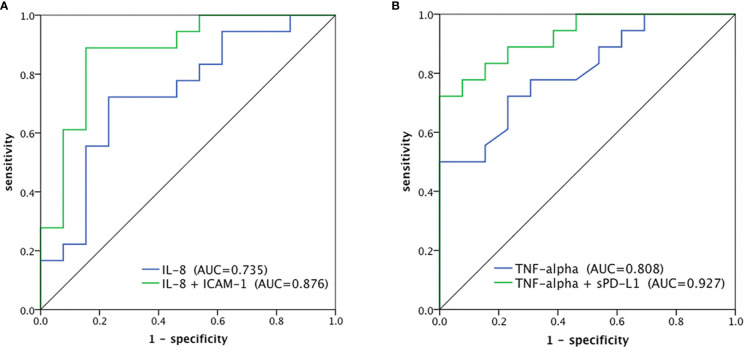
Multivariate logistic analysis showed that the combination of IL-8 and ICAM-1, or sPD-L1 and TNF-α, might predict the objective response to CCRT ( Table 3 ). ROC curve analysis was performed to assess whether the levels of cytokines would predict objective response to chemoradiotherapy. The AUC was 0.744 (95% CI: 0.567–0.920; P=0.022) with ICAM-1, 0.735 (95% CI: 0.551–0.919; P=0.028) with IL-8, 0.808 (95% CI: 0.658–0.958; P=0.004) with TNF-α, and 0.774 (95% CI: 0.598–0.949; P=0.010) with sPD-L1. The combination of cytokines improved the prediction accuracy. The combination of IL-8 and ICAM-1 enhanced the AUC to 0.876 (95% CI: 0.745–1.000; P=0.000) ( Figure 1A ). The combination of sPD-L1 and TNF-α provided a higher AUC of 0.927 (95% CI: 0.842–1.000; P=0.000) ( Figure 1B ). The AUC was not improved by the other combinations of cytokines.
Table 3.
Association between objective response and cytokines in plasma by multivariate analysis.
| Cytokine | Odds Ratio (95% CI) | P | |
|---|---|---|---|
| Combination 1 | IL-8 | 1.975 (1.004–885) | 0.049 |
| ICAM-1 | 0.996 (0.994–0.999) | 0.017 | |
| Combination 2 | sPD-L1 | 0.996 (0.992–0.999) | 0.014 |
| TNF-α | 5.060 (1.380–18.553) | 0.014 |
Figure 1.
ROC curves to predict objective response of CCRT. (A) IL8 alone and IL-8 in combination with ICAM-1, (B) TNF-α alone and TNF-α in combination with sPD-L1.
Cytokines in plasma collected from 17 patients at the end of radiotherapy were also measured and compared with pre-CCRT levels with paired t-test. Levels of CXCL3, EPO, FLT3L, GM-CSF, IL-1α, IL-1β, IL-6, IP-10, TNF-α, TRAIL, and TPO significantly increased after CCRT. Other cytokines showed no significant difference between pre- and post-CCRT. Neither cytokine levels post CCRT nor the post-/pre-CCRT ratios of cytokines were associated with objective response. However, the sample size was too small to reach a conclusion.
Correlation Between PFS and Cytokines in Plasma
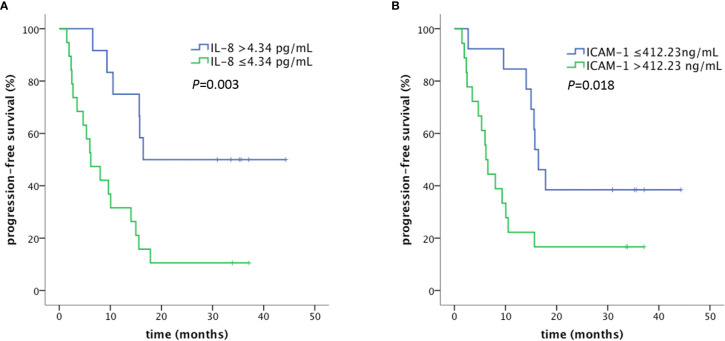
The association between PFS and clinical factors or cytokines in plasma in prior to CCRT was analyzed by univariate Cox regression ( Table 4 ). Patients who achieved objective response after chemoradiotherapy had longer PFS (P=0.006). The median PFS was 15.6 months (95% CI: 14.0–17.1) and 5.3 months (95% CI: 1.5–9.2) in patients with and without objective response, respectively. Pathology, EGFR mutation status and induction chemotherapy were not correlated with PFS. The level of IL-8 was correlated with PFS (HR=0.788, P=0.036). Using the cut-off optimized by ROC analysis, patients with high levels of plasma IL-8 (>4.34 pg/ml) had a longer median PFS (16.4 months, 95% CI: NA) compared with 6.2 months (95% CI: 2.4–10.0) in the low IL-8 (≤4.34 pg/ml) group ( Figure 2A ). Although not significant, the level of ICAM-1 also showed a trend toward association between lower concentration and longer PFS (HR=1.001, P=0.07). The median PFS of patients with high level of ICAM-1 (>412.23 ng/ml) and low level of ICAM-1 (≤412.23 ng/ml) was 6.2 months (95% CI: 4.9–7.3) and 16.4 months (95% CI: 13.8–19.1), respectively ( Figure 2B ).
Table 4.
Association between PFS and clinical factors or cytokines in plasma by univariate analysis.
| Factor | Hazard Ratio (95% CI) | P |
|---|---|---|
| Age (years) | 1.021 (0.960–1.085) | 0.513 |
| Gender (Male vs. Female) | 2.511 (0.908–6.948) | 0.076 |
| Smoking (Never vs. Ever) | 1.138 (0.428–3.022) | 0.796 |
| Pathology (ADC vs. SCC) | 0.596 (0.245–1.453) | 0.255 |
| Stage* (IIB vs. IIIA vs. IIIB) | 1.679 (0.750–3.755) | 0.207 |
| EGFR mutation (wildtype vs. mutant) | 2.212 (0.622–7.861) | 0.220 |
| Induction chemotherapy (No vs. Yes) | 0.606 (0.238–1.542) | 0.293 |
| CCL5 | 0.987 (0.961–1.015) | 0.357 |
| CXCL3 | 0.999 (0.997–1.000) | 0.121 |
| Eotaxin | 0.999 (0.991–1.007) | 0.812 |
| EPO | 1.005 (0.984–1.027) | 0.651 |
| FLT3L | 0.997 (0.993–1.002) | 0.218 |
| G-CSF | 1.009 (0.993–1.026) | 0.284 |
| GM-CSF | 0.465 (0.002–109.226) | 0.783 |
| ICAM-1 | 1.001 (1.000–1.001) | 0.070 |
| IFN-γ | 1.011 (0.996–1.027) | 0.143 |
| IL-1α | 1.001 (0.766–1.310) | 0.992 |
| IL-1β | 4.388 (0.165–117.024) | 0.377 |
| IL-10 | 0.225 (0.036–1.395) | 0.109 |
| IL-12p70 | 0.952 (0.879–1.032) | 0.235 |
| IL-13 | 0.979 (0.855–1.121) | 0.759 |
| IL-2 | 1.149 (0.680–1.940) | 0.605 |
| IL-6 | 0.985 (0.845–1.147) | 0.845 |
| IL-8 | 0.788 (0.630–0.985) | 0.036 |
| IP-10 | 0.998 (0.995–1.001) | 0.152 |
| M-CSF | 1 (0.989–1.010) | 0.953 |
| sPD-L1 | 1.001 (1.000–1.002) | 0.117 |
| TNF-α | 0.974 (0.710–1.336) | 0.869 |
| TPO | 0.999 (0.996–1.002) | 0.550 |
| TRAIL | 0.984 (0.963–1.006) | 0.155 |
| VEGF | 0.978 (0.947–1.009) | 0.155 |
| WNT3A | 1.333 (0.589–3.020) | 0.491 |
Cytokines with P<0.1 are shown in bold. *According to American Joint Commission on Cancer (AJCC) staging system 7th edition.
Figure 2.
PFS in patients with high and low levels of plasma IL-8 (A) and ICAM-1 (B). PFS was defined as the time from the first day of radiotherapy to the date of any progression, death, and loss to follow-up. Patients were censored at the last follow-up if no progression or death occurred.
IL-8 and ICAM-1 were selected for multivariate analysis ( Table 5 ). High level of IL-8 and low level of ICAM-1 correlated significantly with longer PFS. Patients were stratified into three groups according to the levels of IL-8 and ICAM-1 concentration in plasma: Group 1 with high IL-8 and low ICAM-1; Group 2 with high IL-8 and high ICAM-1, or low IL-8 and low ICAM-1; and Group 3 with low IL-8 and high ICAM-1 ( Figure 3 ). The median PFS in Group 1 was not reached, and all six patients in this group were alive at the last follow-up. The median PFS was 15.0 months (95% CI: 9.1-21.0) in Group 2, and 4.7 months (95% CI: 1.6-7.8) in Group 3 (P=0.000).
Table 5.
Association between PFS and cytokines in plasma by multivariate analysis.
| Cytokine | Hazard Ratio (95% CI) | P |
|---|---|---|
| IL-8 | 0.716 (0.555–0.925) | 0.010 |
| ICAM-1 | 1.001 (1.000–1.002) | 0.011 |
Figure 3.
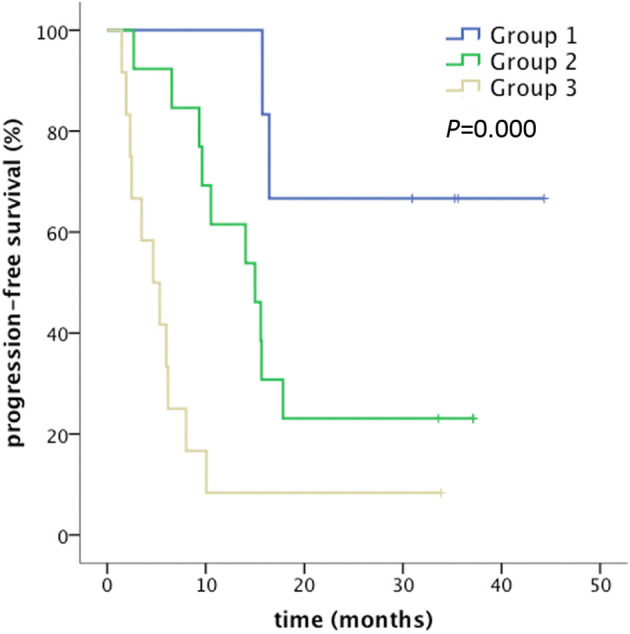
Combination effect of IL-8 and ICAM-1 for PFS prediction. Group 1: IL-8 >4.34 pg/ml, ICAM-1 ≤412.23 ng/ml; Group 2: IL-8 >4.34 pg/ml, ICAM-1 >412.23 ng/ml or IL-8 ≤4.34 pg/ml, ICAM-1 ≤412.23 ng/ml; Group 3: IL-8 ≤4.34 pg/ml, ICAM-1 >412.23 ng/ml. PFS was defined as the time from the first day of radiotherapy to the date of any progression, death, and loss to follow-up. Patients were censored at the last follow-up if no progression or death occurred.
Discussion
This study systemically investigated the value of cytokines in plasma as a predictive marker for clinical outcome in patients with locally advanced NSCLC treated with chemoradiotherapy. High levels of IL-13, TNF-α, IL-8, CCL5, and CXCL3, and low levels of ICAM-1, IFN-γ, and sPD-L1 before radiotherapy were associated (or showed a trend toward association) with objective response after chemoradiotherapy. The combination of IL-8 and ICAM-1, or sPD-L1 and TNF-α, improved the prediction accuracy of objective response with a higher AUC. Only the levels of IL-8 and ICAM-1 translated into the prediction of PFS. Patients with high IL-8 and low ICAM-1 had longer PFS. This study provided candidate markers for the prediction of response and PFS of chemoradiotherapy in patients with locally advanced NSCLC.
IL-8, also known as CXCL8, has been reported as a marker for poor outcomes in hormone-dependent breast cancer (9), colorectal cancer (10, 11), and lung cancer (12, 13). Xiao et al. reported that high CXCL8 immunohistochemical staining score was associated with shorter OS and disease-free survival (DFS) in patients with colorectal cancer (8). Liu et al. summarized the expression of CXCL8 in different stages of NSCLC. High CXCL8 mRNA levels were associated with advanced stage and poor survival in NSCLC (12). Ryan et al. reported that high levels of serum IL-8 were associated with an increased risk of lung cancer mortality in patients with Stage I lung cancer (13). In our dataset, plasma IL-8 prior to radiotherapy was not correlated with OS (P=0.832, data not shown), but correlated with objective response and PFS of chemoradiotherapy. Sanmamed et al. reported that the dynamics of serum IL-8 levels predict the response to anti-programmed cell death protein 1 (PD-1) treatment. In responders, IL-8 levels decreased between baseline and best response, and increased upon progression (14). In our cohort, the change in IL-8 level was not associated with objective response in 17 patients whose blood samples were collected before and after CCRT. However, the population was too limited to draw conclusions. IL-8 binds to C-X-C motif chemokine receptor 1 and 2 (CXCR1 and CXCR2), and activates the phosphatidyl-inositol-3-kinase (PI3K) signaling pathway, leading to the activation of downstream signals including Akt, protein kinase C (PKC), mitogen-activated protein kinase (MAPK), etc. (15). It has been reported that IL-8 could enhance the production of matrix metalloproteinases in CXCR1- and CXCR2-expressing endothelial cells and regulate angiogenesis (16). It is possible that IL-8 promoted angiogenesis and reduced hypoxia in tumors, thereby increasing the radiosensitivity of tumor cells.
ICAM-1 belongs to the immunoglobulin gene superfamily. It is involved in the inflammatory process and tumor metastasis. High levels of ICAM-1 were correlated with advanced stage and poor prognosis in multiple types of cancer (17–21). Qian et al. reported that advanced NSCLC patients with lower levels of soluble ICAM-1 had longer survival and a higher objective response rate of chemotherapy (17). Zhou et al. collected exhaled breath condensate (EBC) and peripheral blood samples from patients with NSCLC, patients with chronic obstructive pulmonary disease (COPD) and healthy controls, and detected the level of soluble ICAM-1. sICAM-1 was significantly elevated in both EBC and serum in patients with NSCLC compared to that in patients with COPD and healthy controls, and high levels of both exhaled and serum sICAM-1 were associated with distant metastasis (18). This suggested the role of ICAM-1 in diagnosis and prognosis was both local and systemic. In our study, lower plasma ICAM-1 before chemoradiotherapy was correlated with objective response and longer PFS, which was in accordance with the prognostic value of ICAM-1 in previous studies.
We also identified high level of TNF-α and low level of sPD-L1 as plasma markers for predicting objective response to chemoradiotherapy in locally advanced NSCLC. TNF-α is an inflammatory cytokine, and its role in cancer is complicated. Endogenous TNF-α has tumor-promoting activity. The level of TNF-α was elevated in cancers compared with normal tissues or healthy controls (22). Meanwhile, other studies have reported that TNF-α also has anti-tumor activity by destroying tumor vasculature and facilitating antitumor immune response (23). Boldrini et al. reported that TNF-α mRNA levels in surgically resected cancer tissues was associated with better prognosis in NSCLC (24). PD-L1 binds to PD-1, resulting in T cell dysfunction. Blockade of PD-1/PD-L1 is applied to many types of malignancies (25). Meta-analysis showed that NSCLC patients with positive immunohistochemical PD-L1 staining had poor OS (26). Soluble PD-L1 has been reported to be associated with poor prognosis in lung cancer and hepatocellular carcinoma (27–29). Our results suggested that plasma TNF-α was positively correlated with objective response, while sPD-L1 was negatively correlated with objective response to chemoradiotherapy. The combination of these two factors enhanced the predictive accuracy (AUC=0.927). However, the predictive value of the objective response did not translate into the prediction of PFS.
Our study had several limitations. First, the sample size was limited. The identified potential markers need to be validated in a larger population. Despite the limited population, patients in our cohort received concurrent definitive CCRT, and the radiotherapy is delivered using IMRT technique. The modality of treatment was relatively homogenous, which enhanced the stringency of this study. Second, the rationale for prediction of objective response and PFS needs to be explored. The dynamics of cytokines during treatment may provide clues to understand the relationship between cytokines and clinical outcomes. However, some post-treatment blood samples were not available in this study. We will compare the levels of cytokines before and after radiotherapy in further studies. Third, although adding induction chemotherapy to CCRT could not bring benefits according to a randomized phase 3 trial (30), most of the patients in our study received induction chemotherapy prior to CCRT due to the pattern of referrals in our region. Blood samples were collected before CCRT, and PFS was calculated from the first day of radiotherapy. There was no significant difference in objective response or PFS between patients with or without induction chemotherapy. Fourth, patients were enrolled from April 2016 to May 2017, prior to the publication of PACIFIC results (31), which established the consolidation therapy with durvalumab after CCRT as standard treatment in NCCN guideline. Therefore in this study, no immune checkpoint inhibitors were used after CCRT. Considering durvalumab is used as consolidative rather than concurrent regimen, we speculated the results obtained in this study might provide clues to predict the outcome of CCRT in combination with consolidative PD-L1 blockade. Moreover, due to the medical insurance reasons, durvalumab is not broadly used after CCRT for locally advanced NSCLC currently in China. Therefore, the results still showed some significances under current clinical practices.
In conclusion, cytokines in plasma may predict the response and PFS in patients with locally advanced NSCLC underwent chemoradiotherapy. High levels of TNF-α and IL-8, and low levels of ICAM-1 and sPD-L1 before radiotherapy were associated (or showed a trend toward association) with objective response after chemoradiotherapy. Patients with high IL-8 and low ICAM-1 had longer PFS. Our results provide a potential model to predict the response and PFS of CCRT for patients with NSCLC.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Peking University Cancer Hospital and Institute. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XS, LJ, HY, and WW: conceptualization. XS, LJ, HT, and LM: methodology. XS, LJ, BL, XD, and DY: investigation. AS, RY, DL, and HY: resources. XS and LJ: writing-original draft preparation. HT, LM, HY, and WW: writing-review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by funding from National Natural Science Foundation of China (81672969, 81472814, 82073333, 32071156), Beijing Municipal Science & Technology Commission (Z181100001718192), and Science Foundation of Peking University Cancer Hospital (18-03).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol (2010) 28:2181–90. 10.1200/JCO.2009.26.2543 [DOI] [PubMed] [Google Scholar]
- 2. Curran WJ, Jr., Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst (2011) 103:1452–60. 10.1093/jnci/djr325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carter DL, Garfield D, Hathorn J, Mundis R, Boehm KA, Ilegbodu D, et al. A randomized phase III trial of combined paclitaxel, carboplatin, and radiation therapy followed by weekly paclitaxel or observation for patients with locally advanced inoperable non-small-cell lung cancer. Clin Lung Cancer (2012) 13:205–13. 10.1016/j.cllc.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 4. Ahn JS, Ahn YC, Kim JH, Lee CG, Cho EK, Lee KC, et al. Multinational Randomized Phase III Trial With or Without Consolidation Chemotherapy Using Docetaxel and Cisplatin After Concurrent Chemoradiation in Inoperable Stage III Non-Small-Cell Lung Cancer: KCSG-LU05-04. J Clin Oncol (2015) 33:2660–6. 10.1200/JCO.2014.60.0130 [DOI] [PubMed] [Google Scholar]
- 5. Lin H, Chen Y, Shi A, Pandya KJ, Yu R, Yuan Y, et al. Phase 3 Randomized Low-Dose Paclitaxel Chemoradiotherapy Study for Locally Advanced Non-Small Cell Lung Cancer. Front Oncol (2016) 6:260. 10.3389/fonc.2016.00260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Ruysscher D, Jin J, Lautenschlaeger T, She J-X, Liao Z, Kong FMS. Blood-based biomarkers for precision medicine in lung cancer: precision radiation therapy. Transl Lung Cancer Res (2017) 6:661–9. 10.21037/tlcr.2017.09.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suwinski R, Giglok M, Galwas-Kliber K, Idasiak A, Jochymek B, Deja R, et al. Blood serum proteins as biomarkers for prediction of survival, locoregional control and distant metastasis rate in radiotherapy and radio-chemotherapy for non-small cell lung cancer. BMC Cancer (2019) 19:427. 10.1186/s12885-019-5617-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carvalho S, Troost EGC, Bons J, Menheere P, Lambin P, Oberije C. Prognostic value of blood-biomarkers related to hypoxia, inflammation, immune response and tumor load in non-small cell lung cancer – a survival model with external validation. Radiother Oncol (2016) 119:487–94. 10.1016/j.radonc.2016.04.024 [DOI] [PubMed] [Google Scholar]
- 9. Milovanovic J, Todorovic-Rakovic N, Radulovic M. Interleukin-6 and interleukin-8 serum levels in prognosis of hormone-dependent breast cancer. Cytokine (2019) 118:93–8. 10.1016/j.cyto.2018.02.019 [DOI] [PubMed] [Google Scholar]
- 10. Xiao YC, Yang ZB, Cheng XS, Fang XB, Shen T, Xia CF, et al. CXCL8, overexpressed in colorectal cancer, enhances the resistance of colorectal cancer cells to anoikis. Cancer Lett (2015) 361:22–2. 10.1016/j.canlet.2015.02.021 [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Zhao M, He S, Luo Y, Zhao Y, Cheng J, et al. Necroptosis regulates tumor repopulation after radiotherapy via RIP1/RIP3/MLKL/JNK/IL8 pathway. J Exp Clin Cancer Res (2019) 38:461. 10.1186/s13046-019-1423-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Q, Li A, Yu S, Qin S, Han N, Pestell RG, et al. DACH1 antagonizes CXCL8 to repress tumorigenesis of lung adenocarcinoma and improve prognosis. J Hematol Oncol (2018) 11:53. 10.1186/s13045-018-0597-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ryan BM, Pine SR, Chaturvedi AK, Caporaso N, Harris CC. A combined prognostic serum interleukin-8 and interleukin-6 classifier for stage 1 lung cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. J Thorac Oncol (2014) 9:1494–503. 10.1097/JTO.0000000000000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol (2017) 28:1988–95. 10.1093/annonc/mdx190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raghuwanshi SK, Su Y, Singh V, Haynes K, Richmond A, Richardson RM. The chemokine receptors CXCR1 and CXCR2 couple to distinct G protein-coupled receptor kinases to mediate and regulate leukocyte functions. J Immunol (2012) 189:2824–32. 10.4049/jimmunol.1201114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol (2003) 170:3369–76. 10.4049/jimmunol.170.6.3369 [DOI] [PubMed] [Google Scholar]
- 17. Qian Q, Zhan P, Yu L, Shi Y, Cheng J, Wei S, et al. Baseline levels and decrease in serum soluble intercellular adhesion molecule-1 during chemotherapy predict objective response and survival in patients who have advanced non-small-cell lung cancer. Clin Lung Cancer (2011) 12:131–7. 10.1016/j.cllc.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 18. Zhou F, Chen J, Tao G, Zhu M, Xie W, Cao X. Increased levels of exhaled sICAM1, sVCAM1, and sE-selectin in patients with non-small cell lung cancer. Respir Med (2014) 108:1670–6. 10.1016/j.rmed.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 19. Shi X, Jiang J, Ye X, Liu Y, Wu Q, Wang L. Prognostic prediction and diagnostic role of intercellular adhesion molecule-1 (ICAM-1) expression in clear cell renal cell carcinoma. J Mol Hist (2014) 45:427–34. 10.1007/s10735-014-9568-1 [DOI] [PubMed] [Google Scholar]
- 20. Kotteas EA, Gkiozos I, Tsagkouli S, Bastas A, Ntanos I, Saif MW, et al. Soluble ICAM-1 levels in small-cell lung cancer: prognoistic value for survival and predictive significance for response during chemotherapy. Med Oncol (2013) 30:662. 10.1007/s12032-013-0662-0 [DOI] [PubMed] [Google Scholar]
- 21. Schroder C, Witzel I, Muller V, Krenkel S, Wirtz RM, Jänicke F, et al. Prognostic value of intercellular adhesion molecule (ICAM)-1 expression in breast cancer. J Cancer Res Clin Oncol (2011) 137:1193–201. 10.1007/s00432-011-0984-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Derin D, Soydinc HO, Guney N, Tas F, Camlica H, Duranyildiz D, et al. Serum levels of apoptosis biomarkers, survivin and TNF-alpha in nonsmall cell lung cancer. Lung Cancer (2008) 59:240–5. 10.1016/j.lungcan.2007.08.005 [DOI] [PubMed] [Google Scholar]
- 23. Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer (2009) 9:361–71. 10.1038/nrc2628 [DOI] [PubMed] [Google Scholar]
- 24. Boldrini L, Calcinai A, Samaritani E, Pistolesi F, Mussi A, Lucchi M, et al. Tumour necrosis factor-alpha and transforming growth factor-beta are significantly associated with better prognosis in non-small cell lung carcinoma: putative relation with BCL-2-mediated neovascularization. Br J Cancer (2000) 83:480–6. 10.1054/bjoc.2000.1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zou W, Wolchok J D, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med (2016) 8:328rv4. 10.1126/scitranslmed.aad7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang A, Wang HY, Liu Y, Zhao MC, Zhang HJ, Lu ZY, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol (2015) 41:450–6. 10.1016/j.ejso.2015.01.020 [DOI] [PubMed] [Google Scholar]
- 27. Okuma Y, Hosomi Y, Nakahara Y, Watanabe K, Sagawa Y, Homma S. High plasma levels of soluble programmed cell death ligand 1 are prognostic for reduced survival in advanced lung cancer. Lung Cancer (2017) 104:1–6. 10.1016/j.lungcan.2016.11.023 [DOI] [PubMed] [Google Scholar]
- 28. Chang B, Huang T, Wei H, Shen L, Zhu D, He W, et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother (2019) 68:353–63. 10.1007/s00262-018-2271-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim HJ, Park S, Kim K J, Seong J. Clinical significance of soluble programmed cell death ligand-1 (sPD-L1) in hepatocellular carcinoma patients treated with radiotherapy. Radiother Oncol (2018) 129:130–5. 10.1016/j.radonc.2017.11.027 [DOI] [PubMed] [Google Scholar]
- 30. Vokes EE, Herndon JE2, Kelley MJ, Cicchetti MG, Ramnath N, Neill H, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol (2007) 25:1698–704. 10.1200/JCO.2006.07.3569 [DOI] [PubMed] [Google Scholar]
- 31. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small cell lung cancer. N Engl J Med (2017) 377:1919–29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.