Abstract
Objectives
To identify factors associated with length of stay (LOS) in chronic obstructive pulmonary disease (COPD) hospitalised patients, which may help shorten LOS and reduce economic burden accrued over hospital stay.
Design
A retrospective cohort study.
Setting
This study was performed in a tertiary hospital in China.
Participants
Patients with COPD who were aged ≥40 years and newly admitted between 2016 and 2017.
Primary and secondary outcome measures
LOS at initial admission was the primary outcome and health expenditures were the secondary outcome. To identify factors associated with LOS, we collected information at index hospitalisation and constructed a conceptual model using directed acyclic graph. Potential factors were classified into five groups: demographic information, disease severity, comorbidities, hospital admission and environmental factors. Negative binomial regression model was fitted for each block of factors and a parsimonious analysis was performed.
Results
In total, we analysed 565 patients with COPD. The mean age was 69±11 years old and 69.4% were men. The median LOS was 10 (interquartile range 8–14) days. LOS was significantly longer in patients with venous thromboembolism (VTE) (16 vs 10 days, p=0.0002) or with osteoporosis (15 vs 10 days, p=0.0228). VTE ((rate ratio) RR 1.38, 95% CI 1.07 to 1.76), hypoxic–hypercarbic encephalopathy (RR 1.53, 95% CI 1.06 to 2.20), respiratory infection (RR 1.12, 95% CI 1.01 to 1.24), osteoporosis (RR 1.45, 95% CI 1.07 to 1.96) and emergence admission (RR 1.08, 95% CI 1.01 to 1.16) were associated with longer LOS. In parsimonious analysis, all these factors remained significant except emergency admission, highlighting the important role of concomitant morbidities in patients’ hospital stay. Total hospitalisation cost and patients’ out-of-pocket cost increased monotonically with LOS (both ptrend <0.0001).
Conclusion
Patients’ concomitant morbidities predicted excessive LOS in patients with COPD. Healthcare cost increased over the LOS. Quality improvement initiatives may need to identify patients at high risk for lengthy stay and implement early interventions to reduce COPD economic burden.
Keywords: chronic airways disease, epidemiology, risk management
Strengths and limitations of this study.
Length of stay (LOS) and economic burden were analysed in newly admitted patients with chronic obstructive pulmonary disease in developing countries.
The primary data analysis was based on the conceptual model and theoretical model driven.
Real experiences of hospitalised patients in the real-world clinical setting were analysed.
Analysis in this single centre study may not reflect the comprehensive profile of LOS in Chinese patients.
Indirect hospitalisation costs were not analysed due to unavailability of data on patients’ indirect costs.
Introduction
Chronic obstructive pulmonary disease (COPD) is a burdensome chronic respiratory disease. It is estimated to cause 2.6% disability-adjusted life years (DALYs) globally and ranked as the third top cause of DALYs in China.1 The prevalence of COPD in Chinese adults is 8.6%, with approximately 100 million patients with COPD.2 People aged over 40 years are particularly at high risk due to its onset in later life. During COPD progress, acute worsening respiratory symptoms can be easily triggered,3 4 leading to hospitalisations and increased use of health service.5 Spending on hospital-based care constitutes the major healthcare cost in patients with COPD, accounting for 65.9%–77% of medical costs in China.6 7 The direct medical cost of COPD was estimated at US$1732.24 per patient annually in 20068 9 and hospitalisation cost per admission increased to US$3669.33 in 2016.10 COPD is also a costly disease in developed countries. In the USA, it consumes US$72 billion in direct healthcare cost each year11 and the tremendous cost is projected to be on the rise.11 12 Given the large prevalent population and substantial economic burden, interventions targeted to COPD hospitalisation are needed. Hospital stay is an important outcome for healthcare systems13 and indicates the acute impact of exacerbation on patients.14 Length of stay (LOS) correlates with hospitalisation cost.15 Shortening LOS might be one way to slow down the escalating healthcare cost.
Owing to disparities in healthcare systems or service use, there exists a high heterogeneity of LOS across countries.12 16 17 In China, some patients stay longer under certain circumstances, for example, discharged and readmitted on the same day due to complicated conditions, prolonging their actual stay for the same hospitalisation. Two prior studies in Chinese COPD patients demonstrated that respiratory coinfection and eosinopenia were associated with longer stay.14 18 However, these studies focused more on single exposure–outcome relationship between one risk factor and LOS. The lengthy stay is multifactorial and a diversity of factors can contribute to prolonged LOS, such as poor patients’ health status, complicated disease conditions and organisation operation.17 19–22 Some of these factors are related to health system and some are patient-level factors. To effectively reduce LOS and COPD disease burden, it is of paramount importance to identify important factors associated with LOS.
Thus, we conducted a retrospective longitudinal study to analyse factors associated with hospital stay. Study population was patients with COPD who were newly admitted to National Clinical Research Center for Respiratory Diseases (NCRCRD), a 354-bed, medical and clinical research centre in a tertiary hospital in Beijing, China.
Methods
We retrospectively analysed patients consecutively admitted between 1 January 2016 and 31 December 2017. The eligible patients were Chinese inpatients with primary diagnosis of COPD at initial admission in respiratory units, who were aged ≥40 years and had pulmonary function testing. Considering the aim to identify patients at high risk of prolonged hospital stay, patients discharged against medical advice were excluded. Patients who had been hospitalised for COPD in 2015 were also excluded to remove the influence of COPD admissions within prior 12 months on the first admission during the study period.20 Patients with LOS longer than 30 days at the first admission were excluded as well.
All diagnoses, including primary and five secondary diagnoses, were determined by International Classification of Diseases, 10th Revision (ICD10) coding system. COPD was defined as J40–J44 in ICD-10 codes. Unique individuals with primary diagnosis of COPD at index admission were identified. To guarantee the accuracy of ICD10 coding, diagnoses were ascertained by checking details in medical records.
Hospital stays
LOS was calculated as days by subtracting the admission date from discharge date at the index admission, which was the initial hospital stay. For patients readmitted frequently during the study period, their initial hospital stays were included for analyses. If patients were discharged and readmitted on the same day, the readmissions were recorded as the same hospitalisation as the initial one. LOS for the same-day discharge and readmission were calculated as the summed days of hospital stay in each consecutive admission.
Direct economic burden
Given the unavailability of data about days off from work due to COPD disabling effect, we only analysed direct healthcare expenditures during hospitalisation. Information on total costs and patients’ out-of-pocket costs was obtained and converted to US dollars using annual currency exchange rate in 2017 (1 dollar=6.7518 yuan).23
Potential factors
COPD is a complex disease involving airflow obstruction and multisystem diseases,24 We combined secondary diagnoses to characterise COPD’s concomitant diseases, including comorbidities and complications according to guidelines25 and researches.24 26–28 Comorbidities were cardio/cerebrovascular diseases, diabetes, respiratory infection, bronchiectasis, gastro-oesophageal reflux, obstructive sleep apnea syndrome, lung cancer, depression or anxiety and osteoporosis. Complications included respiratory failure, pulmonary heart diseases, hypoxemia, venous thromboembolism (VTE), pneumothorax and hypoxic–hypercarbic encephalopathy. As particulates in the air can trigger respiratory symptoms and lead to emergency visiting or hospitalisation,29–31 we analysed ambient air pollutants on admission. Data on daily concentrations of particulate matter with a diameter of <2.5 µm (PM2.5) and ozone were obtained from the Environmental Monitoring Station nearest to the study site. Air pollutant concentrations change across seasons. Given the potential impact of seasonal changes (temperatures, humidity),32 we also took seasons into consideration. Emergency admission and day of week of admission were analysed as hospital factors. The weekday of admission was dichotomised into Thursday to Sunday and Monday to Wednesday.
Patient and public involvement
Patients were not involved in this retrospective study.
Statistics
Data were summarised as number (percentage) for categorical variables and mean±SD or median (interquartile ranges (IQR)) for continuous variables as appropriate. The Wilcoxon rank sum test was adopted to compare LOS between patients with coexisting morbidities and those without the morbid condition. To comprehensively analyse important risk factors for longer LOS, we first developed a conceptual model using directed acyclic graph (DAG). According to the model, baseline covariates were grouped into five blocks: patient demographic characteristics, COPD complications, comorbidities, hospital and environmental factors. Given the overdispersion in distribution of LOS, negative binomial regression was modelled to estimate the effect of potential covariates on hospital stay in each block. Rate ratios (RR, also known as relative risk) with 95% CI were reported for the changes in per cent of association between covariates and LOS. Then we fitted a final parsimonious model with significant variables obtained in the aforementioned models. Hospitalisation costs among patients with different LOS were compared using Kruskal-Wallis test. All statistical analyses were performed using SAS V.9.4 (SAS Institute, Cary, North Carolina, USA) with two-tailed p<0.05.
This study was approved by China-Japan Friendship Hospital Clinical Research Ethics Committee (approval no. 2018163 K119). Privacy and confidentiality of all patient’s information were maintained. Patient informed consent was not required.
Results
Altogether, 565 new patients with COPD were analysed (online supplemental figure S1). The cohort had a mean age of 69±11 years and 69.4% were men. Table 1 summarised the general information of eligible patients. Respiratory failure (11.4%) and pulmonary heart diseases (10.7%) were the top complications of COPD while cardiovascular or cerebrovascular diseases (61.7%) were the most common comorbidity. Among the index admissions, 252 (44.6%) admissions occurred on Thursday to Sunday. Outpatient departments were the main source for COPD admissions, with 30.7% admissions from emergency. Inpatient numbers were similar across seasons. Median exposure to ambient O3 and PM2.5 was 92 ug/m3 and 52 ug/m3, respectively. LOS was 10 (IQR: 8–14) days and total hospitalisation cost was US$2080.7 (1501.6, 2877.17).
Table 1.
Characteristics of patients hospitalised for COPD
| Study patients N=565 | Values* | |
| Demographic characteristics | Age, years | 69±11 |
| Men | 392 (69.4) | |
| Married | 544 (96.3) | |
| Local resident | 361 (63.9) | |
| Lung function and complications† | Pre-FEV1 | 1.16 (0.84, 1.73) |
| Respiratory failure | 63 (11.4) | |
| Pulmonary heart diseases | 59 (10.7) | |
| Hypoxemia | 10 (1.8) | |
| VTE | 9 (1.6) | |
| Pneumothorax | 2 (0.4) | |
| Hypxic-hypercarbic encephalopathy | 4 (0.7) | |
| Comorbidities† |
Cardio/cerebrovascular diseases | 340 (61.7) |
| Diabetes | 102 (18.5) | |
| Respiratory infection | 67 (12.2) | |
| Bronchiectasis | 47 (8.5) | |
| Reflux oesophagitis | 38 (6.9) | |
| OSAS | 18 (3.3) | |
| Lung cancer | 5 (0.9) | |
| Anxiety depression | 12 (2.2) | |
| Osteoporosis | 6 (1.1) | |
| Obesity | 56 (9.9) | |
| Admission |
Admitted on Thursday–Sunday | 252 (44.6) |
| Admitted from emergency | 173 (30.7) | |
| Seasons at admission | March–May | 164 (29.0) |
| June–August | 145 (25.7) | |
| September–November | 94 (16.6) | |
| December–February | 162 (28.7) | |
| Air pollution at admission | O3, ug/m3 | 92 (63, 150) |
| PM2.5, ug/m3 | 52 (25, 88) | |
| Hospital stay and healthcare cost | Length of stay, days | 10 (8, 14) |
| Direct cost of hospitalisation, US$ | 2080.7 (1501.6, 2877.17) | |
*Data were represented as mean±SD or median (interquartile) for continuous variables where appropriate and n (%) for categorical variables.
†Fourteen patients’ complications and comorbidities were missing due to their unavailable data on secondary diagnoses.
‡
COPD, chronic obstructive pulmonary disease; OSAS, obstructive sleep apnea syndrome; Pre-FEV1, Pre-bronchodilator forced expiratory volume in one second.; VTE, venous thromboembolism.
bmjopen-2020-040560supp001.pdf (105.5KB, pdf)
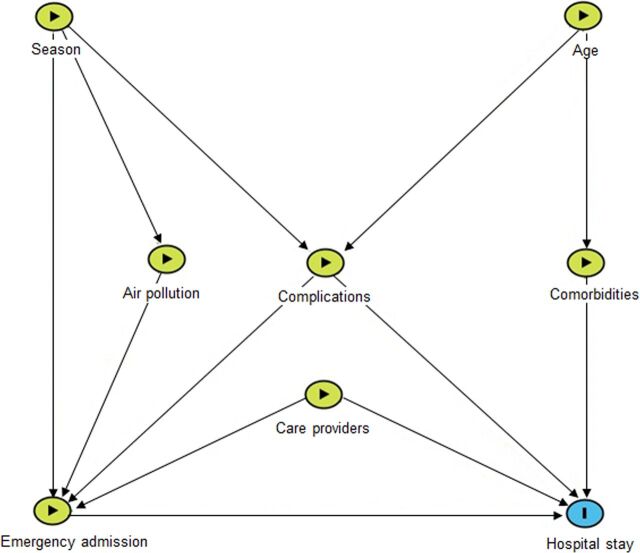
Conceptual model for LOS
A conceptual model was developed based on existing knowledge about COPD and its LOS (online supplemental table S1).17 19 20 29–35 Figure 1 presents the links from individual level characteristics, health system-related factors and environmental factors to LOS.
Figure 1.
Conceptual model using directed acyclic graph (DAG) for factors associated with hospital stay. Based on current knowledge on chronic obstructive pulmonary disease (COPD) risk factors, we developed a conceptual model using DAG. Potential covariates at baseline were grouped into five blocks: patient demographic characteristics (age, gender), COPD complications, comorbidities, hospital factors (emergency admission, weekday of admission) and environmental factors (season, ozone and PM2.5 at admission).
Factors associated with LOS
Based on the conceptual model, we grouped potential factors into five blocks: patient demographic characteristics (age, gender), lung function and COPD complications, comorbidities, hospital factors (emergency admission, weekday) and environmental factors (season, ozone and PM2.5 at admission).
In single model with each block of factors, concomitant VTE (RR 1.38, 95% CI 1.07 to 1.76) and hypoxic–hypercarbic encephalopathy (RR 1.53, 95% CI 1.06 to 2.20) were associated with an increased risk of longer stay. Lung function was negatively associated with LOS with marginal insignificance. Respiratory infection (RR 1.12, 95% CI 1.01 to 1.24), osteoporosis (RR 1.45, 95% CI 1.07 to 1.96) and emergence admission (RR 1.08, 95% CI 1.01 to 1.16) emerged as significant risk factors for longer LOS. No significant associations were observed regarding demographic and environmental factors (table 2).
Table 2.
Single model analysis for factors associated with hospital stay at index admission based on DAG
| Block | Variable | RR 95% CI | P value |
| Demographic characteristics | Age, per 5 years | 1.02 (1.00 to 1.03) | 0.032 |
| Male | 1.04 (0.96 to 1.12) | 0.312 | |
| Lung function and complications | FEV1 | 0.97 (0.95 to 1.00) | 0.055 |
| Respiratory failure | 1.05 (0.94 to 1.18) | 0.396 | |
| Pulmonary heart diseases | 1.03 (0.92 to 1.16) | 0.624 | |
| Hypoxemia | 0.96 (0.74 to 1.24) | 0.757 | |
| VTE | 1.38 (1.07 to 1.76) | 0.012* | |
| Pneumothorax | 1.63 (0.98 to 2.70) | 0.057 | |
| Hypoxic to hypercarbic encephalopathy | 1.53 (1.06 to 2.20) | 0.023* | |
| COPD comorbidities | Cardio/cerebrovascular diseases | 1.05 (0.98 to 1.13) | 0.184 |
| Diabetes | 0.98 (0.90 to 1.07) | 0.648 | |
| Respiratory infection | 1.12 (1.01 to 1.24) | 0.035* | |
| Bronchiectasis | 1.00 (0.88 to 1.13) | 0.967 | |
| Reflux oesophagitis | 1.00 (0.88 to 1.15) | 0.959 | |
| OSAS | 1.17 (0.97 to 1.42) | 0.094 | |
| Lung cancer | 1.09 (0.77 to 1.57) | 0.62 | |
| Anxiety depression | 0.89 (0.69 to 1.13) | 0.324 | |
| Osteoporosis | 1.45 (1.07 to 1.96) | 0.018* | |
| Obesity | 0.97 (0.86 to 1.08) | 0.559 | |
| Hospital factors | Admitted on Thursday–Sunday | 1.04 (0.97 to 1.11) | 0.319 |
| Admitted from emergency | 1.08 (1.01 to 1.16) | 0.033* | |
| Environmental factors | Season at admission, March–May | 1.01 (0.91 to 1.12) | 0.85 |
| Season at admission, June–August | 1.08 (0.96 to 1.22) | 0.176 | |
| Season at admission, September–November | 0.98 (0.88 to 1.09) | 0.752 | |
| O3, per 10 ug/m3 | 1.00 (0.995 to 1.01) | 0.573 | |
| PM2.5, per 10 ug/m3 | 1.00 (0.998 to 1.01) | 0.217 |
*P<0.05.
†
COPD, chronic obstructive pulmonary disease; DAG, directed acyclic graph; OSAS, obstructive sleep apnoea syndrome; Pre-FEV1, Pre-bronchodilator forced expiratory volume in one second; RR, rate ratio; VTE, venous thromboembolism.
When comparing LOS in patients with COPD with coexisting morbidities and those without the morbid conditions, longer LOS was observed in patients with VTE than those without VTE (16 vs 10 days, p=0.0002). In patients with osteoporosis, LOS was longer compared with those without osteoporosis (15 vs 10 days, p=0.0228).
In final parsimonious analysis, concomitant VTE, hypoxic–hypercarbic encephalopathy, respiratory infection and osteoporosis remained significant for increased risk in lengthy stay (all p<0.05) (table 3).
Table 3.
Parsimonious analysis for factors associated with hospital stay at index admission
| Variable | RR 95% CI | P value |
| Age, per 5 years | 1.01 (0.997 to 1.03) | 0.126 |
| Pre-FEV1 | 0.97 (0.95 to 1.00) | 0.055 |
| VTE | 1.40 (1.09 to 1.78) | 0.007* |
| Hypoxic-hypercarbic encephalopathy | 1.53 (1.07 to 2.18) | 0.020* |
| Respiratory infection | 1.11 (1.01 to 1.23) | 0.040* |
| Osteoporosis | 1.42 (1.05 to 1.91) | 0.021* |
| Emergency admission | 1.06 (0.98 to 1.14) | 0.147 |
*P<0.05.
†
Pre-FEV1, Pre-bronchodilator forced expiratory volume in one second.; VTE, venous thromboembolism.
COPD economic burden
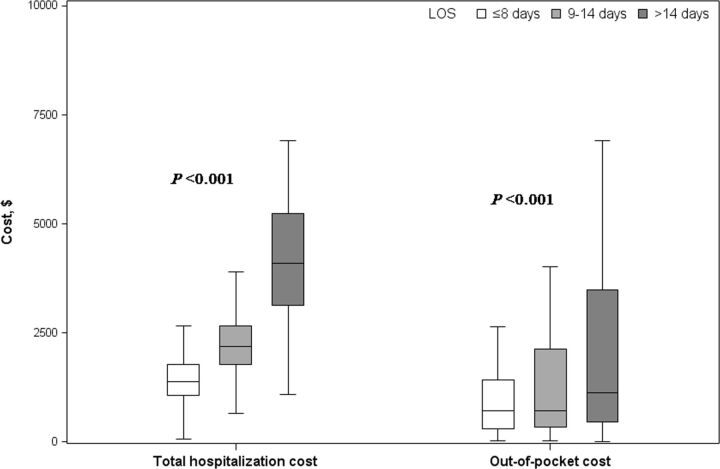
Patients having prolonged LOS were supposed to consume greater health resources. We further analysed the changes in COPD healthcare cost over LOS and observed an upward trend in both total hospitalisation cost and patients’ out-of-pocket cost (both ptrend<0.0001). Figure 2 depicted the increase in costs among patients with LOS ≤8 days, 9–14 days and >14 days divided by quantiles. Total costs in the three subgroups were US$1385.72 (1066.28, 1781.46), US$2177.73 (1779.58, 2650.42) and US$4104.61 (3124.56, 5233.09), respectively (p<0.0001). The corresponding figures for patients’ out-of-pocket cost were US$701.16 (291.69, 1426.55), US$708.13 (329.97, 2130.87) and US$1124.53 (461.49, 3491.55) (p<0.0001).
Figure 2.
COPD costs during hospitalisation. During hospitalisation, total cost and the out-of-pocket cost were compared among patients with LOS ≤8 days, 9–14 days and>14 days according to quartiles of LOS. The costs increased with longer LOS (ptrend<0.0001 for both total cost and the out-of-pocket cost). COPD, chronic obstructive pulmonary disease; LOS, length of stay.
Discussion
COPD is costly and shortening hospital stay is one way to reduce its high economic burden. In this retrospective study involving nearly 600 patients with COPD admitted between 2016 and 2017, we analysed factors associated with LOS from multiple aspects, aiming to identify risk factors that could characterise high-risk population or determine early interventions that should be promptly delivered. COPD complications (VTE, hypoxic–hypercarbic encephalopathy) and comorbidities (respiratory infection and osteoporosis) were identified as important factors for prolonged LOS. Direct healthcare cost was in a graded increase with longer LOS.
Existing evidence on LOS in patients with COPD showed a high heterogeneity and geographical variation.17 19 20 36–40 The analysis of COPD audit data across 13 European countries demonstrated an average LOS of 7 days in patients with COPD 17 In a study of COPD comorbidity and LOS in the USA, mean LOS exceeded 1 week.40 A real-life study in Norway showed a much longer LOS among patients admitted to rehabilitation unit, which were longer than 30 days.39 In our study, the median LOS was 11 days. Risk factors for longer LOS also varied across different studies. Such variance is probably due to disparities in health systems in which patients are managed,17 diverse study population defined by different in/exclusion criteria and various ways to deal with data, for example, outcome definitions.19 39 In this study, there was a subgroup of patients who were discharged and readmitted immediately on the same day for the same cause of hospitalisation. To reflect their real-life experience, we took the same-day discharge and readmission into consideration.
Coexisting morbidities have been mentioned as predictors of LOS.20 21 As shown in our data, VTE predicted longer LOS. It can be provoked by immobilisation, heightened systemic inflammation, venous stasis or other factors that place patients with COPD at risk of thrombosis.40 Patients presenting lower limb swelling, dyspnoea or other symptoms usually undergo ultrasound or CT pulmonary angiogram for diagnosis. The additional examinations and antithrombotic therapy can prolong their LOS. Likewise, hypoxic–hypercarbic encephalopathy may contribute to the prolonged stay. It occurs when neuronal damage is induced by oxygen and CO2 retention and acidosis,41 resulting in physical impairment and coma, subsequently prolonging hospital stay. Respiratory infections are a common trigger for COPD exacerbation. In our study, it was significantly associated with LOS. The greater bacterial load and inflammation could delay patients’ recovery, which, in return, placed patients at greater likelihood of lengthy stay. In an AECOPD study on infectious phenotypes,14 patients with COPD with virus and/or bacteria infection had longer LOS than non-infectious patients. Osteoporosis was another contributor to lengthy stay. In GOLD 2020, osteoporosis is mentioned as a concomitant chronic disease that influences COPD patients’ hospitalisations.42 Some COPD-related factors precipitate osteoporosis development, for example, long-term inhaled corticosteroids use for maintenance therapy40 43 44 and lung function deterioration in disease progress.45 Systematic inflammation and oxidative stress through sarcopenia in COPD can lead to bone metabolic abnormalities and COPD-associated osteoporosis,45 placing patients with COPD at risk of fractures and longer stay.46 The prolonged LOS in patients with aforementioned concomitant diseases underscores the need for effective management of COPD comorbidities and early prevention.
In addition, we observed a monotonically increasing trend in both total hospitalisation cost and out-of-pocket cost over LOS, indicating longer hospital stay cost more in patients with COPD. The domestic and international studies concordantly showed that COPD is a highly expensive disease and hospital-based care cost represents the major cost driver.7 12 In 2016, COPD hospitalisation cost was US$3669.33 per Chinese patient per admission.10 In the USA, the cost accounted for over 70% of total COPD healthcare costs.19 The direct cost is estimated at US$72 billion annually11 and is projected to be on the rise.11 12 The substantial economic burden may be addressed through shortening hospital stay with tailored interventions taken.
As shown in our study, COPD complications, (VTE and hypoxic–hypercarbic encephalopathy) and comorbidities (respiratory infection and osteoporosis) increased risk of prolonged hospital stay. These comorbid conditions are potentially preventable and treatable. For instance, early thromboprophylaxis like anticoagulation or mechanical prophylaxis is adopted when patients with COPD are admitted to prevent thrombotic events during hospitalisation; provide respiratory support promptly when patients present hypoxic–hypercarbic syndrome and recommend patients pneumococcal and influenza vaccination to avoid respiratory infection. These tailored interventions are expected to shorten LOS and save healthcare cost of patients with COPD.
There were several strengths in our study. First, we provided clinical epidemiological data on LOS and its risk factors in newly admitted patients for COPD. Currently, studies on hospital stay are mainly restricted to developed countries.14 17–20 22 36 47 48 Our study demonstrated healthcare for Chinese patients with COPD, including a comprehensive risk factor analysis for LOS and an increase in economic burden along with longer stay. Our study, together with the few prior studies in Chinese people,14,18 would help fill the gap in knowledge of COPD inpatients’ LOS in developing countries. Second, we took the same-day discharge and readmission into consideration when analysing the collected routinely data in daily clinical practice, which reflected real experiences of hospitalised patients in the real-world clinical setting. The identified risk factors could help determine early interventions to prevent excessive stay, which further reduce economic burden accrued over hospital stay. Hence, the findings are clinically relevant and have clinical implications. In addition, as the analysed data were from electronic medical records, data collection process was standardised and all data were under scrutiny before entering into electronic system in hospital. Diagnoses of COPD and other diseases were ascertained by reviewing medical records to ensure reliable data. Fourth, the primary data analysis was based on conceptual model by the use of DAG, which visually represented models in a graph with unidirectional arrows and made assumptions about exposures’ effects on LOS transparent and explicit.49 Thus, the findings were theoretical model driven.
Hospital stay is a complex issue that is subject to patient, hospital and heath system factors. The underlying mechanisms have not been completely disentangled and merit further investigations. The monotonically increasing hospitalisation cost with LOS underscores the great importance of shortening hospital stay in reducing COPD economic burden, which may keep rising with the growing and ageing population.
There were several limitations. First, the study was performed in NCRCRD, one of the top hospitals in respiratory field in China. LOS may differ in patients admitted to secondary hospitals or other health institutes that provide suboptimal care for respiratory disease. This single-centre study may not reflect the overall scenario of COPD hospitalised patients. We could not analyse the average LOS among patients with multiple rehospitalisations, which could reflect long-term disease burden of COPD. To obtain a more comprehensive profile of hospital stay in Chinese patients with COPD, it is necessary to conduct a multicentre study with hospitals across different grades and regions involved. A nation-wide network of health information system is recommended to capture all hospitalisations and track the real-time dynamic admissions in patients with COPD. Second, we used hospitalisation costs as a proxy for COPD economic burden because information about patients’ indirect costs was not available in this study. To perform a profound analysis of COPD economic burden, indirect costs also need to be evaluated. Further study on COPD economic burden should take both direct and indirect costs into consideration in the future.
Conclusion
This study underscores the significance of concomitant morbidities and disease severity in predicting COPD hospital stay. Healthcare cost increased over hospital stay. Identification of high-risk patients for excess stay and delivery of early interventions such as thromboprophylaxis may offer one way to shorten LOS in patients with COPD, which would help reduce the economic burden accrued over the stay.
Supplementary Material
Acknowledgments
We thank hospital’s administrative and finance departments for their help with data collection.
Footnotes
TY and JJ contributed equally.
Contributors: TY, JJ, CW were involved in conceptualisation, funding acquisition and supervision. FD, YW, YL, XL collected data. FD, XR, KH analysed and interpreted the data. FD wrote the draft of manuscript: TY, JJ, FD, CW, KH, XR, SQ, HN, YW, YL, ML, XL contributed to writing review and approved the final manuscript.
Funding: This study was supported by National Key R&D Program of China (2016YFC0206502), National Key R&D Program of China (2016YFC1303900), CAMS Innovation Fund for Medical Sciences (CIFMS) (2018-I2M-1-001), Medical record-based study for respiratory diseases in hospitalized patients [grant number Q[2017]001] and Science Foundation of China-Japan Friendship Hospital for junior researchers (2017-1-QN-3).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: We obtained approval from the China-Japan Friendship Hospital Clinical Research Ethics Committee (approval number 2018-163-K119). Privacy and confidentiality of all patient's information were maintained. Patient informed consent was not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data may be obtained from a third party and are not publicly available. No data are available. Data sharing statement: No additional data are available.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2019;394:1145–58. 10.1016/S0140-6736(19)30427-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018;391:1706–17. 10.1016/S0140-6736(18)30841-9 [DOI] [PubMed] [Google Scholar]
- 3.Viniol C, Vogelmeier CF. Exacerbations of COPD. Eur Respir Rev 2018;27:1701031 10.1183/16000617.0103-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lareau S, Moseson E, Slatore CG. Exacerbation of COPD. Am J Respir Crit Care Med 2018;198:P21–2. 10.1164/rccm.19811P21 [DOI] [PubMed] [Google Scholar]
- 5.Donaldson GC, Wedzicha JA. COPD exacerbations 1: Epidemiology. Thorax 2006;61:164–8. 10.1136/thx.2005.041806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Wang N, Chen Y, et al. Costs of chronic obstructive pulmonary disease in urban areas of China: a cross-sectional study in four cities. Int J Chron Obstruct Pulmon Dis 2016;11:2625–32. 10.2147/COPD.S118523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu B, Wang Y, Ming J, et al. Disease burden of COPD in China: a systematic review. Int J Chron Obstruct Pulmon Dis 2018;13:1353–64. 10.2147/COPD.S161555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang X, Wang X, Bai C. Copd in China: the burden and importance of proper management. Chest 2011;139:920–9. 10.1378/chest.10-1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Q-Y, Zhou X, Xie C-M, et al. [Impact of chronic obstructive pulmonary disease on quality of life and economic burden in Chinese urban areas]. Zhonghua Jie He He Hu Xi Za Zhi 2009;32:253–7. [PubMed] [Google Scholar]
- 10.Li M, Wang F, Chen R, et al. Factors contributing to hospitalization costs for patients with COPD in China: a retrospective analysis of medical record data. Int J Chron Obstruct Pulmon Dis 2018;13:3349–57. 10.2147/COPD.S175143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khakban A, Sin DD, FitzGerald JM, et al. The projected epidemic of chronic obstructive pulmonary disease hospitalizations over the next 15 years. A population-based perspective. Am J Respir Crit Care Med 2017;195:287–91. 10.1164/rccm.201606-1162PP [DOI] [PubMed] [Google Scholar]
- 12.Guarascio AJ, Ray SM, Finch CK, et al. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clinicoecon Outcomes Res 2013;5:235–45. 10.2147/CEOR.S34321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsey SD, Hobbs FDR. Chronic obstructive pulmonary disease, risk factors, and outcome trials: comparisons with cardiovascular disease. Proc Am Thorac Soc 2006;3:635–40. 10.1513/pats.200603-094SS [DOI] [PubMed] [Google Scholar]
- 14.Dai M-Y, Qiao J-P, Xu Y-H, et al. Respiratory infectious phenotypes in acute exacerbation of COPD: an aid to length of stay and COPD assessment test. Int J Chron Obstruct Pulmon Dis 2015;10:2257–63. 10.2147/COPD.S92160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y-hong, Yao W-zhen, Cai B-qiang, et al. Economic analysis in admitted patients with acute exacerbation of chronic obstructive pulmonary disease. Chin Med J 2008;121:587–91. 10.1097/00029330-200804010-00003 [DOI] [PubMed] [Google Scholar]
- 16.Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and nationwide emergency department sample 2006-2011. Chest 2015;147:989–98. 10.1378/chest.14-2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruparel M, López-Campos JL, Castro-Acosta A, et al. Understanding variation in length of hospital stay for COPD exacerbation: European COPD audit. ERJ Open Res 2016;2:00034-2015 10.1183/23120541.00034-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko FWS, Chan KP, Ngai J, et al. Blood eosinophil count as a predictor of hospital length of stay in COPD exacerbations. Respirology 2020;25:259–66. 10.1111/resp.13660 [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Stavem K, Dahl FA, et al. Factors associated with a prolonged length of stay after acute exacerbation of chronic obstructive pulmonary disease (AECOPD). Int J Chron Obstruct Pulmon Dis 2014;9:99–105. 10.2147/COPD.S51467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harries TH, Thornton HV, Crichton S, et al. Length of stay of COPD hospital admissions between 2006 and 2010: a retrospective longitudinal study. Int J Chron Obstruct Pulmon Dis 2015;10:603–11. 10.2147/COPD.S77092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts CM, Stone RA, Lowe D, et al. Co-Morbidities and 90-day outcomes in hospitalized COPD exacerbations. COPD 2011;8:354–61. 10.3109/15412555.2011.600362 [DOI] [PubMed] [Google Scholar]
- 22.Limsuwat C, Mankongpaisarnrung C, Dumrongmongcolgul N, et al. Factors influencing the length of hospital stay in patients with acute exacerbations of chronic obstructive pulmonary disease admitted to intensive care units. Qual Manag Health Care 2014;23:86–93. 10.1097/QMH.0000000000000024 [DOI] [PubMed] [Google Scholar]
- 23.National Bureau of Statistics. Available: http://www.stats.gov.cn/tjsj/zxfb/201802/t20180228_1585631.html [Accessed 1 Dec 2019].
- 24.Sethi S. Infection as a comorbidity of COPD. Eur Respir J 2010;35:1209–15. 10.1183/09031936.00081409 [DOI] [PubMed] [Google Scholar]
- 25.Celli BR, Decramer M, Wedzicha JA, et al. An official American thoracic Society/European respiratory Society statement: research questions in COPD. Eur Respir Rev 2015;24:159–72. 10.1183/16000617.00000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev 2013;22:454–75. 10.1183/09059180.00008612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Olmos L, Alberquilla A, Ayala V, et al. Comorbidity in patients with chronic obstructive pulmonary disease in family practice: a cross sectional study. BMC Fam Pract 2013;14:11. 10.1186/1471-2296-14-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerrero M, Crisafulli E, Liapikou A, et al. Readmission for acute exacerbation within 30 days of discharge is associated with a subsequent progressive increase in mortality risk in COPD patients: a long-term observational study. PLoS One 2016;11:e0150737. 10.1371/journal.pone.0150737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ling SH, van Eeden SF. Particulate matter air pollution exposure: role in the development and exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2009;4:233–43. 10.2147/COPD.S5098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Sun S, Tang R, et al. Major air pollutants and risk of COPD exacerbations: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis 2016;11:3079–91. 10.2147/COPD.S122282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: a review of the effects of particulate matter air pollution on human health. J Med Toxicol 2012;8:166–75. 10.1007/s13181-011-0203-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donaldson GC, Wedzicha JA. The causes and consequences of seasonal variation in COPD exacerbations. Int J Chron Obstruct Pulmon Dis 2014;9:1101–10. 10.2147/COPD.S54475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hillas G, Perlikos F, Tsiligianni I, et al. Managing comorbidities in COPD. Int J Chron Obstruct Pulmon Dis 2015;10:95–109. 10.2147/COPD.S54473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eccles R. An explanation for the seasonality of acute upper respiratory tract viral infections. Acta Otolaryngol 2002;122:183–91. 10.1080/00016480252814207 [DOI] [PubMed] [Google Scholar]
- 35.Parappil A, Depczynski B, Collett P, et al. Effect of comorbid diabetes on length of stay and risk of death in patients admitted with acute exacerbations of COPD. Respirology 2010;15:918–22. 10.1111/j.1440-1843.2010.01781.x [DOI] [PubMed] [Google Scholar]
- 36.Agboado G, Peters J, Donkin L. Factors influencing the length of hospital stay among patients resident in Blackpool admitted with COPD: a cross-sectional study. BMJ Open 2012;2 10.1136/bmjopen-2012-000869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holland M, Alkhalil M, Chandromouli S, et al. Eosinopenia as a marker of mortality and length of stay in patients admitted with exacerbations of chronic obstructive pulmonary disease. Respirology 2010;15:165–7. 10.1111/j.1440-1843.2009.01651.x [DOI] [PubMed] [Google Scholar]
- 38.Ingadottir AR, Beck AM, Baldwin C, et al. Association of energy and protein intakes with length of stay, readmission and mortality in hospitalised patients with chronic obstructive pulmonary disease. Br J Nutr 2018;119:543–51. 10.1017/S0007114517003919 [DOI] [PubMed] [Google Scholar]
- 39.Vitacca M, Marino S, Comini L, et al. Bacterial colonization in COPD patients admitted to a rehabilitation respiratory unit and impact on length of stay: a real-life study. COPD 2018;15:581–7. 10.1080/15412555.2019.1572731 [DOI] [PubMed] [Google Scholar]
- 40.Inabnit LS, Blanchette C, Ruban C. Comorbidities and length of stay in chronic obstructive pulmonary disease patients. COPD 2018;15:355–60. 10.1080/15412555.2018.1513470 [DOI] [PubMed] [Google Scholar]
- 41.Han J, Zeng F, Cheng S, et al. The role of Neurous autophagy in pulmonary encephalopathy and signaling pathways. West Indian Med J 2015. 10.7727/wimj.2015.208 [DOI] [Google Scholar]
- 42.Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease 2020 report [EB/OL], 2019. Available: https://goldcopd.org/gold-reports/ [Accessed 12 Apr 2020].
- 43.Waljee AK, Rogers MAM, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ 2017;357:j1415. 10.1136/bmj.j1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jørgensen NR, Schwarz P, Holme I, et al. The prevalence of osteoporosis in patients with chronic obstructive pulmonary disease: a cross sectional study. Respir Med 2007;101:177–85. 10.1016/j.rmed.2006.03.029 [DOI] [PubMed] [Google Scholar]
- 45.Okazaki R, Watanabe R, Inoue D. Osteoporosis associated with chronic obstructive pulmonary disease. J Bone Metab 2016;23:111–20. 10.11005/jbm.2016.23.3.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papaioannou A, Adachi JD, Parkinson W, et al. Lengthy hospitalization associated with vertebral fractures despite control for comorbid conditions. Osteoporos Int 2001;12:870–4. 10.1007/s001980170039 [DOI] [PubMed] [Google Scholar]
- 47.Rinne ST, Wong ES, Hebert PL, et al. Weekend discharges and length of stay among Veterans admitted for chronic obstructive pulmonary disease. Med Care 2015;53:753–7. 10.1097/MLR.0000000000000395 [DOI] [PubMed] [Google Scholar]
- 48.Quintana JM, Unzurrunzaga A, Garcia-Gutierrez S, et al. Predictors of hospital length of stay in patients with exacerbations of COPD: a cohort study. J Gen Intern Med 2015;30:824–31. 10.1007/s11606-014-3129-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lederer DJ, Bell SC, Branson RD, et al. Control of confounding and reporting of results in causal inference studies. guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc 2019;16:22–8. 10.1513/AnnalsATS.201808-564PS [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-040560supp001.pdf (105.5KB, pdf)