Abstract
Introduction
High tibial osteotomy (HTO) is a treatment of choice for active adult with knee osteoarthritis. With advancement in CT imaging with three-dimensional (3D) model reconstruction, virtual planning and 3D printing, patient-specific instrumentation (PSI) in form of cutting jigs is employed to improve surgical accuracy and outcome of HTO. The aim of this randomised controlled trial (RCT) is to explore the surgical outcomes of HTO for the treatment of medial compartment knee osteoarthritis with or without a 3D printed patient-specific jig.
Methods and analysis
A double-blind RCT will be conducted with patients and outcome assessors blinded to treatment allocation. This meant that neither the patients nor the outcome assessors would know the actual treatment allocated during the trial. Thirty-six patients with symptomatic medial compartment knee osteoarthritis fulfilling our inclusion criteria will be invited to participate the study. Participants will be randomly allocated to one of two groups (1:1 ratio): operation with 3D printed patient-specific jig or operation without jig. Measurements will be taken before surgery (baseline) and at postoperatively (6, 12 and 24 months). The primary outcome includes radiological accuracy of osteotomy. Secondary outcomes include a change in knee function from baseline to postoperatively as measured by three questionnaires: Knee Society Scores (Knee Scores and Functional Scores), Oxford Knee Scores and pain visual analogue scale (VAS) score.
Ethics and dissemination
Ethical approval has been obtained from the Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee (CREC no. 2019.050), in accordance with the Declaration of Helsinki. The results will be presented at international scientific meetings and through publications in peer-reviewed journals.
Trial registration number
NCT04000672; Pre-results.
Keywords: orthopaedic & trauma surgery, sports medicine, knee
Strengths and limitations of this study.
The first randomised controlled trial designed to study the accuracy and clinical outcome on using three-dimensional (3D) patient-specific instrumentation (PSI) on patients with knee osteoarthritis requiring high tibial osteotomy.
Data will be collected longitudinally at baseline and during follow-up at 3, 6, 12 and 24 months.
Valuable evidence will be provided to surgeons and decision makers by highlighting the efficacy and benefits of using PSI instrumentation on osteotomy.
The results are expected to have an immediate substantial impact on clinical practice on the potential of 3D PSI on improving the surgical outcome for patients with knee osteoarthritis.
A limitation of the study is conducted in a single-centre design.
Introduction
Background
Knee osteoarthritis (OA) is a long-term chronic disease characterised by cartilage degeneration, creating knee pain and impairing movement. It is the single most common cause of disability in older adults according to the WHO. In recent Lancet review, OA is expected to be the fourth leading cause of disability globally by 2020, with knee OA accounts for approximately 85% of the burden of OA worldwide.1 The medical cost of OA has been estimated to be around 1%–2.5% of the gross domestic product in various high-income countries, with joint replacements representing the major proportion of the cost.1
Total knee arthroplasty (TKA) is a common and highly effective orthopaedic procedure for treating end-stage knee OA with good long-term results when conservative treatment fails. Although TKR has been a successful surgery, up to 20% of patients were unsatisfied with the result.2 Some of the causes of dissatisfaction have been attributed to the failure of artificial implant to reproduce a normal native knee feeling and also high functional demand activities after replacement surgery.2 This has fuelled increasing popularity of joint-preserving surgery like high tibial osteotomy (HTO) to preserve the native knee joint and allow better function. Moreover, TKA performed at middle age fails to outlast the patient and is commonly associated with significant bone loss at revision surgery. The functional outcome of revision TKA is worse than TKA after HTO, which has been reported to have excellent long-term survivorship and clinical outcome.3
HTO can relieve the symptoms and slow down structural damage by unloading the medial knee compartment. It redistributes mechanical load in the knee, hence extending the longevity of native knee joint in this group of moderate OA patients with high daily activity demand. It is also a well-established surgical procedure for medial compartment knee OA with the probability of survival between 85.4% and 91.6% at 10 years.4 In Asia, HTO is increasingly popular as treatment for knee OA with rising number of HTO performed in conjunction with the fell in number of TKA performed. For example, the annual number of HTO in Korea increased from 2649 cases in 2009 to 8207 cases in 2013, and the annual number of HTO in Japan increased from 261 cases in 2007 to 2152 cases in 2014.5 6 Recently, with the advancement of technology, we started employing patient-specific instrumentation (PSI) on HTO. PSI is a surgical advancement made possible by the advancement in CT imaging with three-dimensional (3D) model reconstruction, virtual planning and 3D printing. By virtue of close approximation of PSI onto patient’s bony surface, PSI HTO cutting jigs are designed to improve surgical accuracy and outcome of HTO. Several groups have reported their results of using PSI jigs on HTO in small case series without a control group. However, without a well-designed randomised trial type of study design, whether there exists scientific significant difference in accuracy and clinical outcome by using PSI on HTO is not known.
Objectives
This trial will explore the surgical outcomes of HTO for the treatment of medial compartment knee OA with or without the 3D printed patient-specific jig (PSI jig). The primary outcomes will be the radiological differences reflecting difference in surgical accuracy with or without PSI jig, and the secondary outcomes will be the postoperative change in knee function from baseline using four questionnaires: Knee Society Scores (KSS) (Knee Scores and Functional Scores), Oxford Knee Scores (OKS), Lysholm Knee Scoring Scale and pain visual analogue scale (VAS) score.7–10
Trial design
The study is a randomised, double-blind controlled study to compare the surgical outcomes for the treatment of medial compartment knee OA with or without the 3D printed patient-specific jig, in terms of radiological outcomes, knee scores, range of motion and pain score with a 24-month follow-up.
Methods and analysis
This clinical trial protocol follows the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines (see SPIRIT checklist in online supplemental file). The underlying protocol also follows the Consolidated Standards of Reporting Trials (CONSORT) guidelines (see CONSORT checklist in online supplemental files. The trial was registered on clinicaltrials.gov.).
bmjopen-2020-041129supp001.pdf (61.2KB, pdf)
Participants, interventions and outcomes
Participants and setting
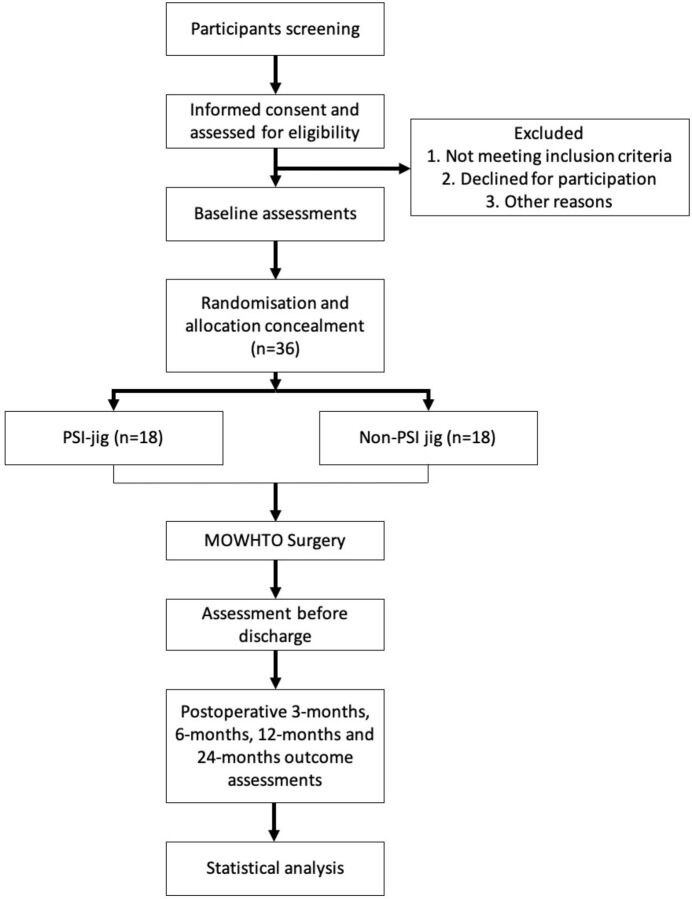
Participants will be primarily recruited from the outpatient clinic of the Department of Orthopaedics and Traumatology at the Alice Ho Miu Ling Nethersole Hospital. Additionally, the Prince of Wales Hospital (affiliated with the Chinese University of Hong Kong) in the same New Territories East Cluster, will also help to refer suitable patients for the trial. Figure 1 shows the overall flow chart of the study.
Figure 1.
The study flow diagram, including participants' recruitment, eligibility, screening, randomisation, allocation concealment and outcome assessments. MOWHTO, medial open-wedge high tibial osteotomy; PSI, patient specific instrumentation.
Eligibility criteria
To be enrolled in this trial, the following eligibility criteria, assessed at screening, will be met:
Inclusion criteria
The inclusion criteria are as follows:
Aged ≥18 years and ≤70 years.
Symptomatic patient with medial compartment knee OA.
Clinical diagnosis of knee OA (American College of Rheumatology criteria) with radiographic changes (Kellgren-Lawrence grades 2 or 3).
Body mass index (BMI) ≤35 kg/m2.
Informed consent obtained.
Exclusion criteria
The exclusion criteria are as follows:
Lateral compartment OA.
Symptomatic patellofemoral compartment OA.
Inflammatory arthritis.
Significant loss of knee joint range in flexion (less than 100°) or in extension (less than − 10°).
Ligamentous instability.
Obesity with BMI >35 kg/m2.
Significant psychological disorder.
Inability to communicate in Chinese or English language.
Recruitment
Eligible patients will be recruited from the outpatient clinic with written consent in the Prince of Wales Hospital & Alice Ho Miu Ling Nethersole Hospital based on the inclusion and exclusion criteria. Basic patient demographics, including age, gender, ethnicity, occupation, BMI, and smoking and drinking habits, will be recorded. Medical history will also be confirmed and recorded from the Clinical Management System, Hospital Authority, which is the central electronic database for public hospitals in Hong Kong. Before signing the consent form, each patient will be explained the objectives, benefits and risks of the study and their rights and responsibilities, as well as privacy and confidentiality information. An information sheet will be distributed, and all patients are asked for their understanding of the trial and encouraged to ask questions at any time.
Sample size calculation
Radiological assessment of accuracy will serve as the study primary outcome. Specifically the average osteotomy cut from joint line will be used as a determinant outcome of this study. As no previous reports guide the expected results, our preliminary pilot data have guided our calculations. Based on our previous cases of HTO, we noted the average osteotomy plane entry point deviation from planning with PSI jig is 0 cm±0.3 cm and without PSI jig is 0.76 cm±1.2 cm. Therefore, a sample size of 15 per group can achieve an 95% power to detect the difference between the two groups, with an alpha level of 0.05 and effect size of 0.95 using a two-sided two sample t-test. To account for attrition, we have increased our sample by 20%. Our sample size of 18 participants per treatment arm (total n=36) will be sufficient to address our primary objective. Our secondary objectives will be considered hypothesis-generating information to guide future work. The sample size was calculated using G*Power 3.0 software.
Randomisation and allocation concealment
Randomisation will be accomplished by computer-generated randomisation sequence using serially numbered opaque, sealed envelopes with patients assigned either to intervention or control groups. All investigators, research staff and patients will be blinded to the group assignment of the subjects, nor will they be aware of the allocation during the study and evaluation periods. However, blinding the surgeon performing the HTO is not feasible because they shall perform surgery either with or without using the jig, but the subsequent assessment and analysis shall be done by blinded research staff and investigators. A randomisation code will be allocated to each included subject to maintain blindness. Randomisation code will be broken only after the database had been locked. Patient rehabilitation, postoperative assessment and data analysis are conducted by personnel blinded to the patients’ randomisation assignment.
Study interventions
Current standard practice (routine HTO surgery)
The controlled arm would be standard medial open-wedge HTO using current standard practice. In brief, an incision is made in the midway between posteromedial border of the tibia and medial aspect of the tibial tuberosity. Sartorius fascia is cut and retracted medially to expose the medial collateral ligament (MCL). Two to three 2.5 mm K-wires are placed 4 cm below the medial joint line towards the proximal tibiofibular joint (PTFJ) over lateral tibial cortex under fluoroscopy, and osteotomy is done below and parallel to the k-wires using an oscillating saw (blade thickness 0.9 mm) leaving the lateral 5 mm intact. Thin osteotomes are used to gradually open the osteotomy, and finally, the desired correction is achieved with the use of computer navigation (Orthomap ASM, Stryker, Michigan, USA) checking overall lower limb alignment.
Intervention group
Three-dimensional printed patient-specific jigs (PSI jig) (figure 2) are created based on the preoperative CT image. Before operation, lower limb from hip to ankle centre were scanned by CT with slice thickness ≤1 mm covering the proximal tibia and knee joint. CT image data were made available in Digital Imaging and Communications in Medicine format and transferred to a standard desktop computer and loaded to Mimics software (Materialise, Louvain, Belgium) for segmentation. Virtual planning of osteotomy plane and the associated jig was performed on Materialise 3-matic 13.0 (Materialise) according to TomoFix plate (Synthes Medical, Oberdorf, Switzerland) surgical technique manual. PSI jigs were printed in stainless steel by 3D metal printing machine (LUMEX Avance-25, Matsuura, Japan). Standard medial open wedge osteotomy similar as described previously is performed with modification. Incision is made in the midway between posteromedial border of the tibia and medial aspect of the tibial tuberosity. Sartorius fascia is cut and retracted medially to expose the MCL. Then the PSI jig is positioned onto the tibia. Due to the patient-specific design (individually based on each patient’s CT image), it can fit closely to the proximal tibia. The slot opening on the PSI jig corresponds to 4 cm below the medial joint line, and the slot design allows the sawblade (blade thickness 0.9 mm) to cut direction towards PTFJ over lateral tibial cortex under fluoroscopy. The PSI jig is removed after the bone cut completed and would not retain in patient’s body. Thin osteotomes are used to gradually open the osteotomy. A 3D printed wedge that corresponds to opening gap size of osteotomy is used to achieve the desired correction and supersede the computer navigation (set-up also as part of blinding) values for alignment in case of discrepancy. The rehabilitation and follow-up of the intervention group is the same as the routine patients (control group) undergoing medial open-wedge HTO for knee OA.
Figure 2.
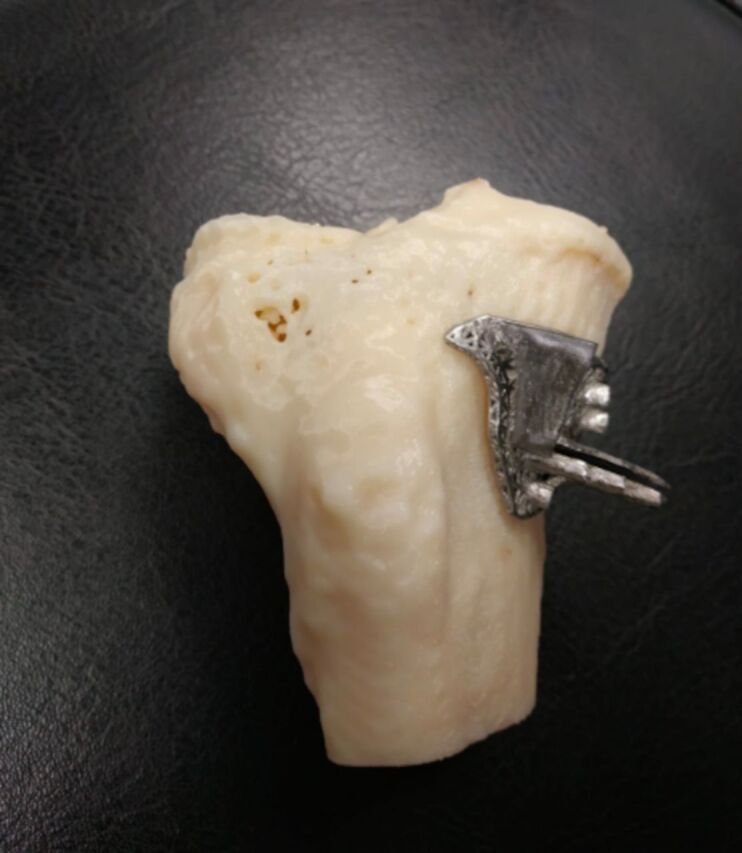
Image of PSI jig. PSI, patient-specific instrumentation.
Outcomes and outcome assessments
Outcome assessments of the patients will be performed at baseline (0 month), immediately before discharge, at 3 months, 6 months, 12 months and 24 months timepoints. Table 1 shows the overall assessments needed for each timepoint.
Table 1.
Study timeline of assessment
| Enrolment | Assessment period | |||||
| Preop | Immediate before discharge | 3 months | 6 months | 12 months | 24 months | |
| Enrolment | ||||||
| Informed consent | ✓ | |||||
| Assessment of eligibility | ✓ | |||||
| Randomisation | ✓ | |||||
| Assessments | ||||||
| Anatomical | ||||||
| CT scan | ✓ | ✓ | ✓ | |||
| Scanogram | ✓ | ✓ | ||||
| Knee radiographs | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Functional | ||||||
| Knee Society Knee Score | ✓ | ✓ | ✓ | ✓ | ||
| Knee Society Function Score | ✓ | ✓ | ✓ | ✓ | ||
| Oxford Knee Score | ✓ | ✓ | ✓ | ✓ | ||
| Lysholm Knee Scoring Scale | ✓ | ✓ | ✓ | ✓ | ||
| ROM | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| VAS score | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Others | ||||||
| Additional use of analgesics | ✓ | |||||
| Postoperative complications and adverse events | ✓ | ✓ | ✓ | ✓ | ✓ | |
ROM, range of motion; VAS, visual analogue scale.
Primary outcome
Radiographic assessment on surgical outcome
The primary outcome is obtained by postoperative radiological assessment of radiographs and CT images to compare the accuracy of PSI jig with freehand bone cut in achieving preoperative planned bone cut. The planned bone cut is from 4 cm below the medial joint line towards PTFJ near the lateral tibial cortex. Accuracy is measured by comparing the planned versus final position of: the blade entrance point (proximal/distal translation on CT images), osteotomy plane (towards PTFJ) angulation and osteotomy gap opening angle (two-dimensional angles in coronal and sagittal plane on CT images). It also includes comparison with navigation on overall alignment correction. Anteroposterior full-length lower limb radiographs are taken with patients in the standing position to assess postoperative lower limb alignment correction, which is compared with the preoperative planning based on Miniaci method calculation to achieve target alignment passing through the Fujisawa point.11 12
Secondary outcome
Knee function and pain score
Secondary outcomes include the clinical outcome on knee score and knee function. The quality of knee function and pain will be assessed by the previously reported and validated Knee Society Knee Score and Function Score. The KSS was designed to provide a simple and objective scoring system to rate the knee and patient’s functional abilities before and after TKA and also employed to assess HTO as well.13 14 The KSS has a Knee Score section and a Functional Score section, covering on pain, symptom and activities of daily living. Both sections are scored from 0 to 100 with lower scores being indicative of worse knee conditions and higher scores being indicative of better knee conditions.
Whereas, the OKS is a 12-item patient-reported outcome measures (PROMs) originally designed and developed to measure subjective outcome after TKA but later have also been used to assess outcome of HTO.8 15 16 Each question is scored from 0 to 4 (0 being the worst outcome and 4 being the best). The overall score is the sum of all items and can range from 0 to 48, with higher scores corresponding to better outcomes. The Lysholm Knee Scoring Scale is a patient-reported instrument that consists of subscales for pain, instability, locking, swelling, limp, stair climbing, squatting and the need for support. Scores range from 0 (worse disability) to 100 (less disability).10
The pain VAS is an unidimensional measure of pain intensity, which has been widely used in diverse adult populations, including those with degenerative knee diseases.
Adverse events, safety and compliance assessment
Any postoperative pain, complications and other complaints from the participants will be monitored and taken care of by medical officers. Any adverse event or problems arise during the study will be reported directly to the ethnic committee in the institution. In addition, participants are allowed to quit the study at any time for any reason; if so, they will be asked whether they wish to be followed up according to the trial schedule.
Data management and confidentiality
A research assistant will be trained to ensure accuracy of outcome assessments and data collection. The ethics committee will oversee any issues disturbing quality of research, and corresponding measures will be taken if necessary. Patients are free to withdraw from the study at any time without giving any reasons, and their medical care or legal rights will not be affected. The study will comply with the good clinical practice guideline according to the International Council for Harmonisation. Each patient will be assigned an identification code. The patient identification code list and database will be safeguarded.
Data statement
Data and resources will be shared with other eligible investigators through academically established means. The protocol and datasets used and/or analysed in this study will be available from the corresponding author on reasonable request.
Statistical analysis
Data in this study will be analysed according to the intention-to-treat principle. Only full analysis set and per-protocol set will be used for primary analysis. Any missing data will not be input for calculation. Quantitative variables will be expressed as mean±SD. Normality tests will be performed to determine whether the data are normally distributed. Analysis of variance tests with Bonferroni correction are used for multiple testing of continuous variables. Whereas, χ2 test will be used to compare proportions of categorical variables and to calculate the differences in the count data. Mixed effects models will be used to analyse the trend of changes in the scores with two factors of groups and time. In addition, a survival analysis on the surgical approach will be shown as a Kaplan-Meier curve. The statistical analysis will be performed using a commercialised statistical software (SPSS, V.25). All statistical significance is defined as p<0.05.
Patient and public involvement
This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Ethics and dissemination
Ethics approval and consent to participate have been obtained from the Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee (CREC no. 2019.050), in accordance with the declaration of Helsinki. The results will be presented at international scientific meetings and through publications in peer-reviewed journals.
Protocol version
This study protocol was approved on 13 March 2019 as detailed in this manuscript.
Study participant consent (see online supplemental file)
Surgeon consent: the principal investigator(PI) and coinvestigators met with potential surgeons (with ≥5 year of experience in performing HTO) individually or as part of faculty meetings to discuss the study and to answer any questions. The surgeons were given a copy of the proposal detailing the assessments to review. Surgeons provided verbal and email consent to the PI to indicate their willingness to participate.
Patient consent: informed written consents for participation into this PROTECTED HTO trial will be obtained from every patient before their operation. Detailed risks and benefits will be explained when obtaining the consent from the patients.
Discussion
As previously shown, HTO is a proven effective method to treat relative young and active adults with knee OA.17 In conventional method, HTO is performed using intraoperative fluoroscopy to judge the site and direction of osteotomy, degree of alignment correction and change of posterior slope. However, surgical accuracy with the conventional method is reported to be limited and hence computer navigation has been introduced to improve accuracy in performing HTO. In a recent publication on comparing between computer navigated HTO and conventional HTO, it reported that the risk of outlier in alignment was lower in computer navigated HTO than conventional method.18 In addition, the tibial slope maintenance was comparable, if not better, in navigated HTO than conventional HTO.18 Moreover, navigated HTO did not show a discrepancy with conventional HTO on the functional scores.18
PSI is a development in orthopaedic field made possible by the advancement in in CT imaging with 3D model reconstruction, virtual planning and 3D printing technology, in which an instrument that can couple closely to the targeting bony surface is virtually planned and later produced by 3D printing. The putative benefits of these PSI include increased surgical accuracy, decreased operation time and elimination of the need for extra devices or reference trackers.19 20 The application of PSI on HTO as a cutting jig is reported achieving precise osteotomy and accurate realignment of lower limb in case series.19 However, evidence in form of randomised controlled trial evaluating outcome of HTO performed with PSI is lacking. The current study described in this protocol can fill this gap in knowledge regarding the advantages of PSI use on HTO. A head-to-head comparison with computer navigated HTO was designed in this protocol given previously reported superiority of computer navigated HTO over conventional HTO.18 Radiological outcome, in terms of discrepancy to planned osteotomy and realignment, and clinical outcome, in terms of functioning score assessment, were reported. Various PROMs or clinical scoring system have been used to gauge the surgical outcome of HTO,21 and in this study, KSS (Knee Score and Function Score) and OKS will be used. These are also the most common PROMs and clinical scoring system for unicompartmental knee arthroplasty and TKA, with the former being a common alternative treatment for isolated medial compartment OA and the latter being the choice of conversion when HTO fails. Moreover, by using the same sets of PROMs and clinical scoring system as in other reports, this would allow seamless and meaningful comparison between different treatment modalities for the same clinical problem.21
Enrolment of this trial have commenced on late 2019, and completion is expected to take 36 months. The results from this trial may help to change the current clinical practice, as this will be the first randomised study to evaluate whether patient specific jigs can improve the surgical accuracy and clinical outcome for those requiring HTO. Importantly, we speculate that positive results would allow the incorporation of PSI into multiple orthopaedics surgeries to help to improve healthcare for our patients in the future.
bmjopen-2020-041129supp002.pdf (3.1MB, pdf)
bmjopen-2020-041129supp003.pdf (93.3KB, pdf)
Supplementary Material
Footnotes
Twitter: @LCMLau
LCML, ECSC and JCHF contributed equally.
Contributors: LCML and EC planned the study. LCML and GCWM planned the statistical analysis methods. LCML, YWH and EC designed the jig. All authors contributed to the design and development of the trial. LCML and GCWM drafted the manuscript. LCML, KYC, PS-HY and MB contributed to the revision of the manuscript. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019;393:1745–59. 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 2. Gunaratne R, Pratt DN, Banda J, et al. Patient Dissatisfaction following total knee arthroplasty: a systematic review of the literature. J Arthroplasty 2017;32:3854–60. 10.1016/j.arth.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 3. Chalmers BP, Limberg AK, Tibbo ME, et al. Total knee arthroplasty after high tibial osteotomy results in excellent long-term survivorship and clinical outcomes. J Bone Joint Surg Am 2019;101:970–8. 10.2106/JBJS.18.01060 [DOI] [PubMed] [Google Scholar]
- 4. Kim J-H, Kim H-J, Lee D-H. Survival of opening versus closing wedge high tibial osteotomy: a meta-analysis. Sci Rep 2017;7:7296. 10.1038/s41598-017-07856-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koh IJ, Kim MW, Kim JH, et al. Trends in high tibial osteotomy and knee arthroplasty utilizations and demographics in Korea from 2009 to 2013. J Arthroplasty 2015;30:939–44. 10.1016/j.arth.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 6. Kawata M, Sasabuchi Y, Inui H, et al. Annual trends in knee arthroplasty and tibial osteotomy: analysis of a national database in Japan. Knee 2017;24:1198–205. 10.1016/j.knee.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 7. Insall JN, Dorr LD, Scott RD, et al. Rationale of the knee Society clinical rating system. Clin Orthop Relat Res 1989;248:13–14. 10.1097/00003086-198911000-00004 [DOI] [PubMed] [Google Scholar]
- 8. Dawson J, Fitzpatrick R, Murray D, et al. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br 1998;80:63–9. 10.1302/0301-620X.80B1.0800063 [DOI] [PubMed] [Google Scholar]
- 9. Boonstra AM, Schiphorst Preuper HR, Reneman MF, et al. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int J Rehabil Res 2008;31:165–9. 10.1097/MRR.0b013e3282fc0f93 [DOI] [PubMed] [Google Scholar]
- 10. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 1985;198:42–9. 10.1097/00003086-198509000-00007 [DOI] [PubMed] [Google Scholar]
- 11. Fujisawa Y, Masuhara K, Shiomi S. The effect of high tibial osteotomy on osteoarthritis of the knee. An arthroscopic study of 54 knee joints. Orthop Clin North Am 1979;10:585–608. [PubMed] [Google Scholar]
- 12. Miniaci A, Ballmer FT, Ballmer PM, et al. Proximal tibial osteotomy. A new fixation device. Clin Orthop Relat Res 1989;246:250–9. [PubMed] [Google Scholar]
- 13. Bonasia DE, Dettoni F, Sito G, et al. Medial opening wedge high tibial osteotomy for medial compartment overload/arthritis in the varus knee: prognostic factors. Am J Sports Med 2014;42:690–8. 10.1177/0363546513516577 [DOI] [PubMed] [Google Scholar]
- 14. Medalla GA, Moonot P, Peel T, et al. Cost-Benefit comparison of the Oxford knee score and the American knee Society score in measuring outcome of total knee arthroplasty. J Arthroplasty 2009;24:652–6. 10.1016/j.arth.2008.03.020 [DOI] [PubMed] [Google Scholar]
- 15. Floerkemeier S, Staubli AE, Schroeter S, et al. Does obesity and nicotine abuse influence the outcome and complication rate after open-wedge high tibial osteotomy? A retrospective evaluation of five hundred and thirty three patients. Int Orthop 2014;38:55–60. 10.1007/s00264-013-2082-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haviv B, Bronak S, Thein R, et al. Mid-term outcome of opening-wedge high tibial osteotomy for varus arthritic knees. Orthopedics 2012;35:e192–6. 10.3928/01477447-20120123-08 [DOI] [PubMed] [Google Scholar]
- 17. Lau CM, Fan JCH, Chung K-Y. Satisfactory long-term survival, functional and radiological outcomes of open-wedge high tibial osteotomy for managing knee osteoarthritis: minimum 10-year follow-up study. Journal of Orthopaedic Translation 2020;26:60–6. 10.1016/j.jot.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nha KW, Shin Y-S, Kwon HM, et al. Navigated versus conventional technique in high tibial osteotomy: a meta-analysis focusing on weight bearing effect. Knee Surg Relat Res 2019;31:81–102. 10.5792/ksrr.17.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang JC-S, Chen C-F, Luo C-A, et al. Clinical experience using a 3D-printed patient-specific instrument for medial opening wedge high tibial osteotomy. Biomed Res Int 2018;2018:1–9. 10.1155/2018/9246529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wong KC. 3D-Printed patient-specific applications in Orthopedics. Orthop Res Rev 2016;8:57–66. 10.2147/ORR.S99614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Webb M, Dewan V, Elson D. Functional results following high tibial osteotomy: a review of the literature. Eur J Orthop Surg Traumatol 2018;28:555–63. 10.1007/s00590-017-2112-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-041129supp001.pdf (61.2KB, pdf)
bmjopen-2020-041129supp002.pdf (3.1MB, pdf)
bmjopen-2020-041129supp003.pdf (93.3KB, pdf)