Abstract
The role of self-expandable metallic stents is gradually evolving for a diverse group of benign and malignant gastrointestinal tract problems, with luminal obstruction being by far the most common. Although its role in refractory variceal bleeding is well established, it has rarely been tried for tumor-related bleeding, with only a few case reports in this regard. We share our experience of successfully controlling esophageal tumor–related bleeding with the use of a fully covered self-expandable metallic stent. A 58-year-old woman with irresectable distal esophageal cancer, presented with hematemesis. Esophago-gastro-duodenoscopy revealed an obstructing esophageal tumor with diffuse oozing of blood. This was unamenable to local injection of adrenaline and hemospray; therefore, a temporary self-expandable metallic stent was parked to create a tamponade effect. This successfully stopped bleeding and the patient remained asymptomatic till discharge. However, she was lost to follow-up, and therefore, the stent was removed after a period of 5 months instead of 2 weeks.
Keywords: Bleeding esophageal tumor, esophageal stent, malignant gastrointestinal tract bleeding
Introduction
The placement of metallic stents has become a standard therapy for benign and malignant strictures of the gastrointestinal (GI) tract.1,2 They not only help to alleviate obstruction but also appear to be useful in managing post-operative esophageal leaks, tracheo-esophageal fistulas and esophageal perforations.2–4 Similarly, fully covered self-expandable metallic stents (FCSEMSs) are now frequently used as a bridge to surgery in obstructing colorectal tumors.5 Although used as a salvage therapy for refractory variceal bleeding, their role is yet to be established in the management of malignant GI tract bleeding. We hardly came across a few case reports and a case series regarding use of temporary metallic stents to control malignant GI tract bleeding, with favorable results. Our experience of using self-expandable metallic stent (SEMS) for esophageal tumor–related bleeding could be a way forward toward its innovative use with a good safety and efficacy.
Case report
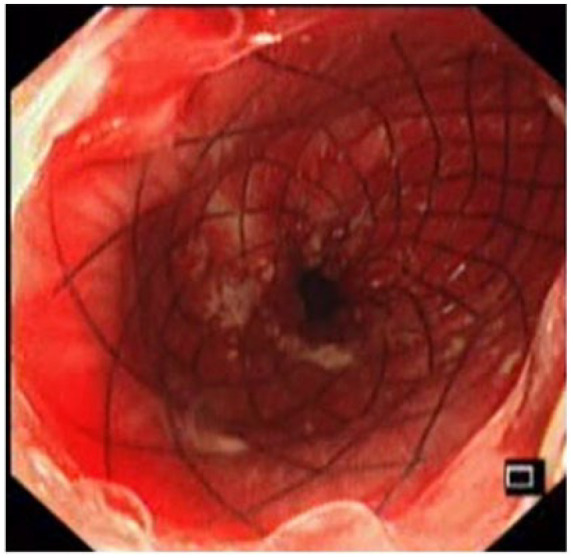
A 58-year-old woman was newly diagnosed with advance (T3N1M0) moderately differentiated squamous cell carcinoma of distal esophagus involving gastroesophageal junction. She received her first cycle of neoadjuvant chemotherapy Carboplatin and Paclitaxel. The next day, she presented to the emergency room with two episodes of hematemesis containing fresh blood. In addition to that she had progressive dysphagia for solids. She remained hemodynamically stable. The Rockall et al.6 score was 2, owing to the disseminated esophageal malignancy. Her blood reports showed a hemoglobin drop of 2.6 g from 13.1 to 10.5 mg/dL. The platelet count and coagulation profile were within the normal range along with the liver and renal functions also being unremarkable. After initial resuscitation, patient was made nil by mouth and started on proton pump inhibitor (PPI) infusion. After taking informed written consent, an upper GI endoscopy was done. The findings revealed a stenosing non-traversable tumor, starting at 33 cm from the incisors, containing old clotted blood as well as mild oozing, as shown in Image 1. Initially, an attempt was made to maintain hemostasis using conventional methods including adrenaline injection and hemospray, but this was unsuccessful for this purpose. In view of the stenosing tumor and ongoing bleed despite using the aforementioned measures, it was decided there and then to place a temporary 20 mm × 130 mm FCSEMS (nitinol). This would not only help to achieve luminal patency but would also aid in bleeding control due to the tamponade effect. Using a catheter, a guidewire was passed through the stricture and its position confirmed fluoroscopically, as shown in Image 2. An FCSEMS was deployed over the guidewire under both direct vision and fluoroscopic guidance, which is shown in Images 3 and 4, respectively. No further episodes of hematemesis or melena were observed along with no further hemoglobin drop. Patient stayed for 72 h in the hospital and was later discharged with a follow-up plan for stent removal at 2 weeks. Patient, however, was lost to follow-up with the GI team but continued to have chemotherapy and radiation therapy. She was sent back for esophageal stent removal after 5 months. Esophago-gastro-duodenoscopy (OGD) revealed no bleed from tumor, stent was removed, and scope was passed freely across the tumor, as shown in Image 5. Patient followed up notes of radiation oncology clinics confirmed no episodes of upper GI bleed between the time of stent insertion and removal. No stent-related early and late complications were seen.
Image 1.
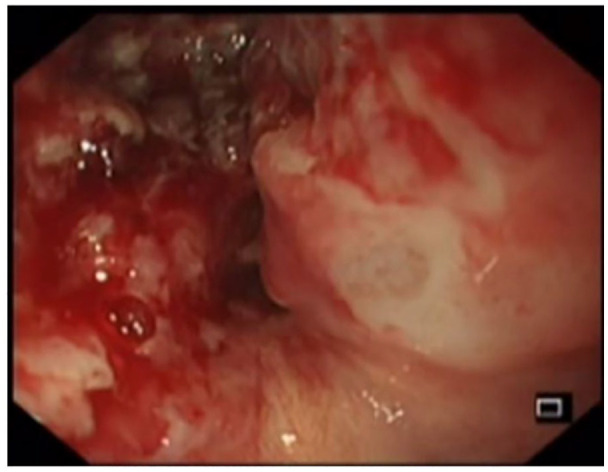
OGD showing bleeding esophageal tumor.
Image 2.
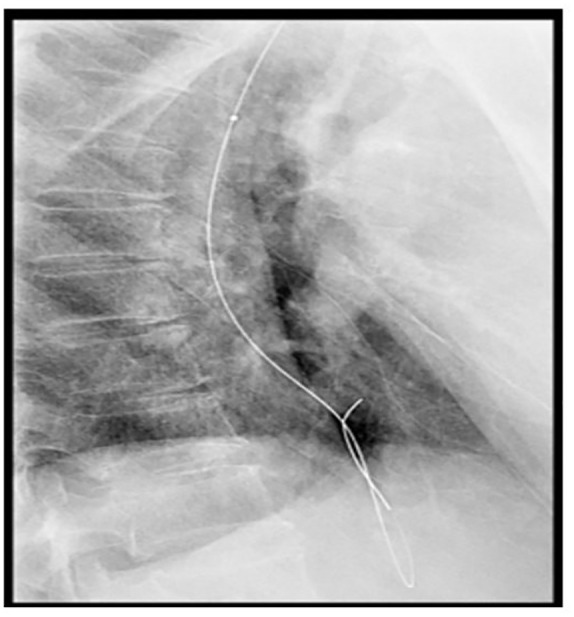
Fluoroscopic image of guidewire crossing the obstructing tumor.
Image 3.
Fluoroscopic image of fully expanded SEMS.
Image 4.
Placement of fully covered stent for bleeding esophageal tumor.
Image 5.
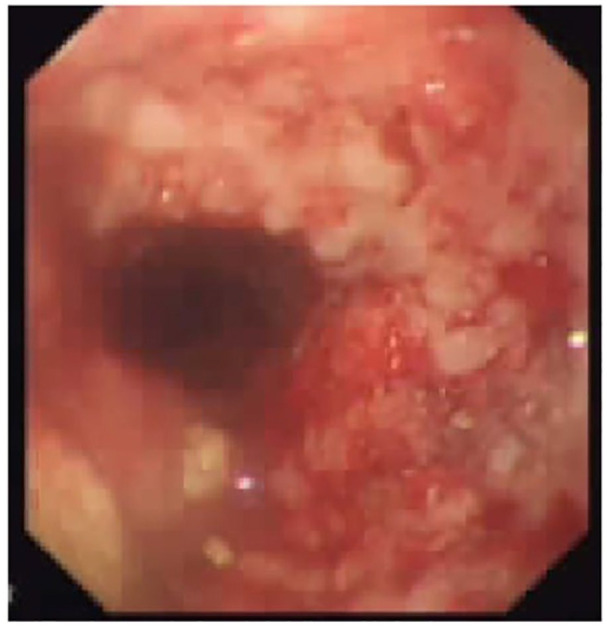
Follow-up endoscopy showing no bleeding from esophageal tumor on stent removal.
Discussion
Historically, plastic stents were first introduced for relieving obstruction in esophageal tumors. Although they achieved a success rate of 80% but were also associated with significant complications including stent migration, obstruction and perforation. Then, in early 1990, partially covered metallic stents and later on, uncovered metallic stents were introduced, showing less complication rate, but the ingrowth of tumor and difficult removability were the main issues. Recently, this favored introduction of the new generation FCSEMS, predominantly used in different GI tract malignancies.3,4 Colonic stenting as a bridge to surgery during radical colectomy is now a part of clinical practice guidelines.1,5
Another challenge associated with upper GI tract tumors is hemorrhage, which accounts for almost 1%–5%.7 Endoscopic intervention is considered as a first-line treatment in achieving hemostasis, which comprises local injections of adrenaline, application of heater probe, argon plasma coagulation, radiofrequency ablation, cryotherapy and hemospray. Despite the variety of treatment modalities, re-bleeding is common.8 The use of SEMS in refractory esophageal variceal bleeding has been studied in a randomized controlled setting.8–10 In contrary to this, only a few case reports and a case series described the use of SEMS to achieve hemostasis in malignant upper GI tumors. Three case reports showed failure to stop bleeding from ulcerated esophageal and duodenal tumors, using the conventional endoscopic interventions, but resulted in immediate cessation of bleeding after placement of FCSEMS.1,11,12 A case series described the successful use of SEMS in four patients with bleeding esophageal tumors and only one of these re-bled following removal of first SEMS after 4 days but achieved hemostasis after second SEMS.9 Caution should be taken in placing SEMS for either obstruction or bleeding for patients having hepatocellular carcinoma (HCC) with gastroduodenal invasion due to high vascularity of tumor and deranged coagulation secondary to cirrhosis of liver as compared to other types of malignancies.13 In our case, we preferred to deploy stent in bleeding esophageal tumor because it was diffusely bleeding and causing a high degree of luminal obstruction. This resulted in complete resolution of symptoms without re-bleeding and patient was discharged after 72 h.
Conclusion
In our case report and a few others, FCSEMS placement was both a safe and effective utility for the management of recurrent or refractory malignant GI hemorrhage. This, however, requires more data and larger studies for statistical validation.
Footnotes
Author contributions: S.M. performed the endoscopy. S.B. identified the case and discussed the concept. S.M.S., M.Z.S. and M.S. reviewed the literature and co-wrote the report. M.A.Y. reviewed, analyzed and approved the final report.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: Ethical approval to report this case was obtained from the Institutional Review Board (IRB) of Shaukat Khanum Memorial Cancer Hospital & Research Centre (SKMCH & RC), Lahore (IRB no. EX-26-05-20-01).
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
ORCID iD: Sundus Bilal  https://orcid.org/0000-0003-1739-5056
https://orcid.org/0000-0003-1739-5056
References
- 1. Orii T, Karasawa Y, Kitahara H, et al. Efficacy of self-expandable metallic stent inserted for refractory hemorrhage of duodenal cancer. Case Rep Gastroenterol 2016; 10(1): 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spaander Manon CW, Baron TH, Siersema PD, et al. Esophageal stenting for benign and malignant disease: ESGE clinical guideline. Endosc 2016; 48: 939–948. [DOI] [PubMed] [Google Scholar]
- 3. Rabenstein T. Palliative endoscopic therapy of esophageal cancer. Viszeralmedizin 2015; 31: 354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martinez JC, Puc MM, Quiros RM. Esophageal stenting in the setting of malignancy. ISRN Gastroenterol 2011; 2011: 719575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gallo G, Sammarco G, Chiriatti AP, et al. The role of self-expandable metallic stents as “bridge to surgery” for the treatment of acute malignant colorectal obstruction. Ann Ital Chir 2017; 6: 418–424. [PubMed] [Google Scholar]
- 6. Rockall TA, Logan RF, Devlin HB, et al. Risk assessment after acute upper gastrointestinal haemorrhage. Gut 1996; 38(3): 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ofosu A, Ramai D, Latson W, et al. Endoscopic management of bleeding gastrointestinal tumors. Ann Gastroenterol 2019; 32(4): 346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parsi MA, Schulman AR, Aslanian HR, et al. Devices for endoscopic hemostasis of nonvariceal GI bleeding (with videos). VideoGIE 2019; 4(7): 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. YuQian Z, JiRong H, XueHong W, et al. Covered self-expanding metal stents for the treatment of refractory esophageal non-variceal bleeding: a case series. J Laparoendosc Adv Surg Tech A 2014; 14(10): 713–717. [DOI] [PubMed] [Google Scholar]
- 10. Escorsell A, Bosch J. Self-expandable metal stents in the treatment of acute esophageal variceal bleeding. Gastroenterol Res Pract 2011; 2011: 910986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yen HH, Chen YY, Su PY. Successful use of a fully covered metal stent for refractory bleeding from a duodenal cancer. Endosc 2015; 47(Suppl. 1): E34–E35. [DOI] [PubMed] [Google Scholar]
- 12. Isono Y, Saito T, Tochio T, et al. [Hemostasis by esophageal stent placement for management of esophageal tumor bleeding: a case report]. Nihon Shokakibyo Gakkai Zasshi 2016; 113(12): 2029–2034. [DOI] [PubMed] [Google Scholar]
- 13. Lee YJ, Kim JH, Song H-Y, et al. Hepatocellular carcinoma complicated by gastroduodenal obstruction: palliative treatment with metallic stent placement. Cardiovasc Intervent Radiol 2012; 35(5): 1129–1135. [DOI] [PubMed] [Google Scholar]


