Abstract
Background:
Chronic lung allograft dysfunction (CLAD), a complication affecting the survival of lung transplanted patients, includes two clinical phenotypes: bronchiolitis obliterans syndrome (BOS) and restrictive allograft syndrome (RAS). Everolimus is used in CLAD because of its antiproliferative mechanism. In lung transplant patients treated with everolimus, the clinical course of renal and lung function has not yet been assessed systematically in CLAD, BOS and RAS patients for more than 6 months.
Methods:
We retrospectively evaluated the 12-month follow-up of renal and lung function of lung-transplanted patients switched to everolimus and evaluated the reduction in immunosuppressant dosage (ISD) and mortality. Subgroups were based on indication for everolimus treatment: CLAD and non-CLAD patients, BOS and RAS among CLAD patients.
Results:
We included 26 patients, 17 with CLAD (10 BOS, seven RAS). After 1 year from the everolimus switch, we observed renal function improvement (serum creatinine −17%, estimated glomerular filtration rate +24%) and stable pulmonary function [forced expiratory volume in the first second (FEV1) −0.5%, forced vital capacity (FVC) +0.05%]. RAS patients had progressive functional loss, whereas BOS patients had FEV1 improvement and FVC stability. All-cause mortality was higher in the CLAD versus non-CLAD group (41% versus 11%), without differences between BOS and RAS patients (p > 0.05). All patients had significant and persistent ISD reduction.
Conclusion:
Lung transplant patients treated with everolimus had improvements in renal function and reduced ISD. We observed sustained improvements in lung function for CLAD related to BOS subgroup results, whereas RAS confirmed the 1-year worsening functional trend. Data seem to suggest one more piece of the puzzle in CLAD phenotyping.
Keywords: bronchiolitis obliterans syndrome, chronic lung allograft dysfunction, lung transplantation, phenotypes, prognosis, restrictive allograft syndrome
Introduction
Long-term survival following lung transplantation remains poor despite advancements in surgical techniques and clinical expertise. Two of the most important factors that reduce survival are graft failure and chronic lung allograft dysfunction (CLAD).1,2 Recently, a consensus report standardized the nomenclature of CLAD, which is defined as a substantial (⩾20%) and persistent decline in measured forced expiratory volume in the first second (FEV1) value from the baseline value.3 Two clinical entities have been described within CLAD: bronchiolitis obliterans syndrome (BOS) and restrictive allograft syndrome (RAS).4 Initially, BOS was widely used as a synonym for chronic post-transplant rejection, but after the study by Gerhardt et al.5 which found a group of patients with BOS responsive to azithromycin, the CLAD nomenclature was introduced. Sato et al.6 identified and then established the concept of RAS to indicate patients who presented with a decline in total lung capacity and a worse prognosis compared with BOS patients.
BOS and RAS are two different phenotypes of CLAD, and some consensus reports were recently published to standardize nomenclature.3,7 In these documents, the authors confirmed that RAS patients generally have a worse prognosis than BOS patients.8,9 Changes in lung function after CLAD onset could be used as a surrogate of graft failure, in particular when the decline in FEV1 or forced vital capacity (FVC) is greater than 10% of basal values.10 Together with parenchymal fibrosis at computed tomography scan, FVC loss was initially used as a criterion for RAS diagnosis and then validated to predict the outcome of CLAD.8,11 FVC is easier to measure than total lung capacity (TLC), but many concerns are still present about its sensitivity to diagnose restriction.4 Similarly, in BOS patients, Belloli et al.12 demonstrated that although both FEV1 and FVC decline after the onset of BOS, only FVC loss was associated with a worse prognosis in these patients.
Everolimus is one of the most recent maintenance immunosuppressants introduced in lung transplantation.13 This medication is used to prevent CLAD,14 as well as calcineurin inhibitor (CNI) nephrotoxicity,15 repeated cytomegalovirus infections,16,17 development of post-transplant solid organ tumors, and intolerance of other immunosuppressors.13 It has been shown that the strong antifibrotic effect of everolimus may attenuate the clinical course for both BOS and RAS.13,18 However, the reno-pulmonary function and mortality of transplant patients treated with everolimus have not yet been compared systematically for more than 6 months in patients with and without CLAD, and in patients with CLAD subtypes.10 Describing the clinical course of these complex patients may be useful to suggest better positioning of everolimus in immunosuppressive treatment algorithms.
In this study, we aim to (i) describe the 12-month clinical course of lung transplant patients treated with everolimus, in terms of lung and renal function, immunosuppressive dosage (ISD) reduction and all-cause mortality; (ii) to compare the 12-month lung function and all-cause mortality in CLAD, non-CLAD, BOS and RAS patients.
Methods
We conducted a retrospective, single-center, observational study reported in accordance with the Strengthening the Reporting of Observational studies in Epidemiology statement.19 We included all patients who received everolimus after lung transplantation at the Turin Lung Transplantation Center, Italy, from 1 January 2012 to 31 December 2015. This investigation is compliant with the principles outlined in the Declaration of Helsinki and was approved by the Ethics Committee of the Città della Salute e della Scienza University Hospital, Turin (Protocol No. 0004577—CS/416). All patients were informed about the purposes of the study and consequently provided their written consent. We collected measurements at baseline and first, third, sixth and 12th month after in-hospital everolimus initiation.
We extracted data regarding renal function [serum creatinine levels (mg/dL), estimated glomerular filtration rate (eGFR, in mL/min per 1.73 m2) according to the Cockcroft–Gault formula], pulmonary function (% of predicted FEV1 and FVC) and demographics (age at everolimus start, gender, indication for lung transplantation, single/bilateral transplantation, ongoing immunosuppressive scheme, everolimus indication); we calculated: (i) mean difference from baseline at each observation (first, third, sixth, 12th month) for renal (serum creatinine and eGFR), pulmonary function (FEV1 and FVC), calcineurin inhibitors (cyclosporine and tacrolimus); (ii) percentage difference from baseline values (represented as %∆) of each measured renal (serum creatinine and eGFR) and pulmonary function (FEV1 and FVC) and ISD. ISD was the dosage of the concomitant immunosuppressant course (cyclosporin A or tacrolimus). We calculated them as: [(parameter at Tx × 100)/parameter at T0] − 100, where T is time in months (0 = basal, 1 = first month, 3 = third month, 6 = sixth month and 12 = 12th month).
For renal function analysis at each evaluation, we used the average values of creatinine and eGFR, or the most recent value in the case of creatinine stability for at least 2 months.
We guided immunosuppression conversion to everolimus in patients after more than 3 months following lung transplantation. In particular, CNI dosages were halved (with stable basal trough levels of 300–350 ng/mL and 3–5 ng/mL for cyclosporine and tacrolimus respectively) and everolimus was added (with stable trough levels of 3–5 ng/mL). Due to different dosage regimens of the immunosuppressant, we decided to calculate the %∆ISD in order to standardize the percentage variation of these drugs.
Indications for everolimus initiation were: deterioration of renal function, chronic lung allograft dysfunction (CLAD, both obstructive, BOS, and restrictive, RAS) and presence of tumors (in pre-transplant history and after lung transplant).
Diagnosis of CLAD, BOS and RAS was made according to the most updated international guidelines and literature available when the patient was enrolled.6,8
In the descriptive analysis, we included sample size and frequency for categorical data, means with standard deviation and standard error for variables which were approximately normally distributed, and medians with interquartile range for variables that did not appear to be normally distributed. We applied the paired t-test or non-parametric signed-rank test to test differences between baseline assessment and all the post-baseline assessments for quantitative parameters. We performed ordinary one-way ANOVA for comparison of three or more means, and Tukey test for multiple comparisons when appropriate. We compared survival curves using the log-rank test. All p-values were two-tailed and a p-value <0.05 was considered statistically significant. We performed statistical analysis using GraphPad Prism 7 (GraphPad Software, San Diego, CA, USA).
Results
The analysis sample included 26 patients undergoing maintenance immunosuppression therapy with everolimus. Demographic data are summarized in Table 1. The most frequent indication for starting everolimus was the development of CLAD (n = 17, 65%) and most patients were CLAD stage 2 (52.9%);3 CLAD patients underwent everolimus switch after a mean 5.7 (± 0.8) years from lung transplantation. Most patients concluded 1 year of follow-up (n = 18, 69%); among those who did not complete the 12-month follow-up, five died because of pulmonary deterioration, and three died owing to infectious diseases. None of them discontinued the treatment due to the occurrence of progressive renal dysfunction or developed everolimus-induced cytopenia.
Table 1.
Demographics of patients included in the study.
| No. of patients | 26 |
| Gender, male/female | 13/13 |
| Age, mean years (SD) | 51.07 (17.1) |
| Time of everolimus introduction from transplantation, mean years (SD) | 5.6 (0.9) |
| Indication for lung transplantation, n (%) | |
| COPD | 9 (35) |
| IPF | 7 (27) |
| Cystic fibrosis | 6 (23) |
| Others | 4 (15) |
| Single/bilateral transplantation | 11/15 |
| Ongoing immunosuppressive scheme, n (%) | |
| CyA + MMF + steroids | 9 (35) |
| TAC + MMF + steroids | 17 (65) |
| Indication for everolimus treatment, n (%) | |
| CLAD | 17 (65) |
| BOS | 10 (59) |
| RAS | 7 (41) |
| CKD | 3 (12) |
| Antiproliferative | 6 (23) |
| CLAD staging | |
| 0 | 0 (0) |
| 1 | 2 (11.8) |
| 2 | 9 (52.9) |
| 3 | 2 (11.8) |
| 4 | 4 (23.5) |
| Mean (SD) basal | |
| Serum creatinine, mg/dL | 1.6 (0.9) |
| eGFR, mL/min per 1.73 m2 | 53.8 (22.1) |
| % Predicted FEV1 | 59.8 (21.1) |
| % Predicted FVC | 77.0 (14.8) |
BOS, bronchiolitis obliterans syndrome; CKD, chronic kidney disease; CLAD, chronic lung allograft dysfunction; COPD, chronic obstructive pulmonary disease; CyA, cyclosporine A; eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume in first second; FVC, forced vital capacity; IPF, idiopathic pulmonary fibrosis; MMF, mycophenolate mofetil; RAS, restrictive allograft syndrome; SD, standard deviation; TAC, tacrolimus.
All transplanted patients: reno-pulmonary function, ISD change and mortality
Creatinine serum levels significantly decreased after the introduction of everolimus; this reduction was maintained for the whole observation period and increased during the follow-up in comparison with basal values (Table 2). This improvement in renal function was stable during the whole observation period, as evidenced by the lack of statistical significance in %∆creatinine and %∆eGFR (Supplemental Material Table S1 online). In particular, serum creatinine had a mean %∆ reduction of −15.29% at the first month, which was maintained at 12 months (−17.34%), and there was a persistent improvement in EGFR, with mean %∆eGFR always greater than 20%.
Table 2.
Renal, pulmonary function, calcineurin inhibitors dosages in all transplanted patients (N = 26).
| Baseline | 1 month | 3 months | 6 months | 12 months | p | |
|---|---|---|---|---|---|---|
| Creatinine, mg/dL | ||||||
| Mean (SD) | 1.6 (0.9) | 1.2 (0.7) | 1.2 (0.5) | 1.21 (0.5) | 1.2 (0.5) | 0.263 |
| Mean difference from baseline (SE) | – | −0.3 (0.5) | −0.3 (0.6) | −0.4 (0.7) | −0.4 (0.7) | – |
| p = 0.007 | p = 0.005 | p = 0.009 | p = 0.025 | |||
| eGFR, mL/min per 1.73 m2 | ||||||
| Mean (SD) | 53.8 (22.1) | 64.2 (27.1) | 66.4 (29.6) | 67.9 (31.3) | 66.7 (29.4) | 0.380 |
| Mean difference from baseline (SE) | – | 9.8 (13.4) | 12.6 (17.3) | 14.7 (18.6) | 12.3 (17.6) | – |
| p = 0.001 | p = 0.001 | p = 0.001 | p = 0.008 | |||
| FEV1, % of predicted value | ||||||
| Mean (SD) | 59.8 (21.1) | 62.1 (21.2) | 58.5 (24) | 60.0 (26.3) | 61.4 (23.6) | 0.986 |
| Mean difference from baseline (SE) | – | 1.7 (1.4) | −1.2 (2.4) | −0.6 (3.1) | −0.5 (3.8) | – |
| p = 0.261 | p = 0.608 | p = 0.824 | p = 0.898 | |||
| FVC, % of predicted value | ||||||
| Mean (SD) | 77.0 (14.8) | 79.8 (17.1) | 77.6 (17.3) | 75.6 (22.2) | 76.9 (15.5) | 0.875 |
| Mean difference from baseline (SE) | – | 3.5 (1.5) | 0.6 (2.1) | 0.6 (2.8) | 0.05 (2.6) | – |
| p = 0.031 | p = 0.764 | p = 0.823 | p = 0.983 | |||
| Cyclosporine, ng/mL | ||||||
| Mean (SD) | 180.0 (71.1) | 132.5 (49.7) | 96.2 (46.8) | 108.3 (59.8) | 78.0 (21.6) | 0.013 |
| Mean difference from baseline (SE) | – | −47.5 (23.6) | −83.7 (30.8) | −56.6 (29.5) | −100.0 (29.4) | – |
| p = 0.144 | p = 0.014 | p = 0.069 | p = 0.011 | |||
| Tacrolimus, ng/mL | ||||||
| Mean (SD) | 6.3 (3.3) | 3.7 (3.4) | 3.8 (3.8) | 4.3 (4.2) | 3.3 (3.0) | 0.134 |
| Mean difference from baseline (SE) | – | −2.6 (0.3) | −2.4 (0.4) | −2.1 (0.5) | −2.5 (0.6) | – |
| p = 0.024 | p = 0.041 | p = 0.122 | p = 0.011 | |||
eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume in first second; FVC, forced vital capacity; SD, standard deviation; SE, standard error.
With regard to pulmonary function, FEV1 did not change significantly during the follow-up (p = 0.986) (Table 2). FVC significantly improved at 1 month compared with basal values (p = 0.031), but this effect was transient because the comparison with the other follow-up values was not significant (p = 0.875). FEV1 and FVC trends of general, CLAD, Non-CLAD, BOS and RAS populations are illustrated in Figure 1 and 2.
Figure 1.
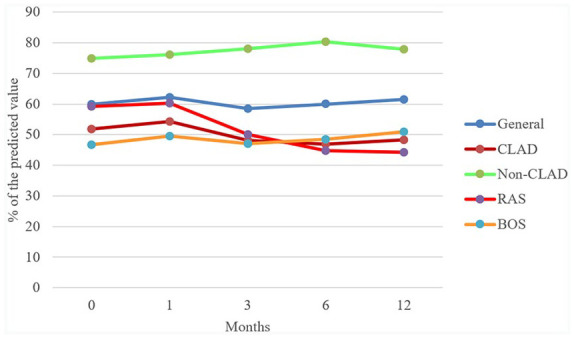
FEV1: percentage of the predicted value trend in general, CLAD, non-CLAD, RAS and BOS populations.
BOS, bronchiolitis obliterans syndrome; CLAD, chronic lung allograft dysfunction; FEV1, forced expiratory volume in the first second; RAS, restrictive allograft syndrome.
Figure 2.
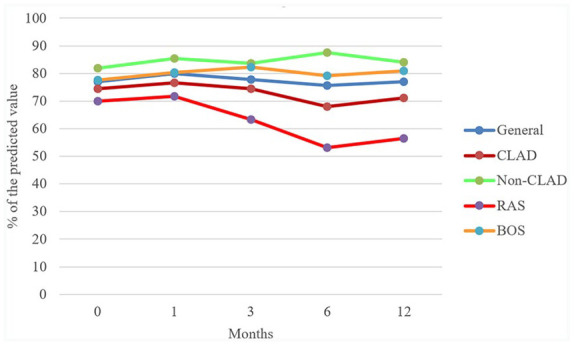
FVC: percentage of the predicted value trend in general, CLAD, non-CLAD, RAS and BOS populations.
BOS, bronchiolitis obliterans syndrome; CLAD, chronic lung allograft dysfunction; FVC, forced vital capacity; RAS, restrictive allograft syndrome.
The introduction of everolimus was associated with a reduction of dosages of CNIs (mean, mean difference with basal levels) (Table 2). At the first month of follow-up, we recorded a mean ISD reduction of 37.7% compared with baseline. This reduction was maintained for the whole observation period, as shown by the lack of significance in comparison of percentage mean differences from baseline (p = 0.375) (Supplemental Table S1).
Out of 26 patients, eight (30.7%) died before the end of the follow-up.
CLAD and non-CLAD patients: pulmonary function, ISD change and mortality
We found evidence of a higher mean FEV1 among non-CLAD patients, compared with CLAD patients, during the whole period of observation (Table 3 and Supplemental Table S2). Mean FVC was also higher in non-CLAD patients, but the difference did not achieve statistical significance. In the non-CLAD group, the mean difference from baseline increased over time for both FEV1 and FVC (Table 3 and Supplemental Table S2), and this difference approached statistical significance after 12 months for FVC (+5.87 from baseline, p = 0.058) but not for FEV1 (p = 0.166). In CLAD patients, we observed a loss of FEV1 and FVC as measured with the mean difference from baseline after a transient gain at the first month (Table 3 and Supplemental Table S2).
Table 3.
Pulmonary function in CLAD (n = 17) and non-CLAD (n = 9) patients.
| Group | Baseline | 1 month | 3 months | 6 months | 12 months |
|---|---|---|---|---|---|
| FEV1, % of predicted value | |||||
| CLAD, mean (SD) | 51.8 (16.4) | 54.2 (16.7) | 48.2 (18.2) | 46.9 (20.8) | 48.3 (21.2) |
| Non-CLAD, mean (SD) | 74.8 (21.5) | 76.1 (22.0) | 78.0 (22.0) | 80.3 (20.9) | 77.8 (14.8) |
| p, difference CLAD versus non-CLAD | 0.005 | 0.010 | 0.001 | 0.001 | 0.004 |
| CLAD, mean difference (SE) | 2.0 (2.0) | −3.5 (3.1) | −4.6 (3.7) | −7.0 (5.0) | |
| p, difference from baseline | 0.339 | 0.274 | 0.233 | 0.196 | |
| Non-CLAD, mean difference (SE) | 1.2 (2.2) | 3.1 (3.5) | 4.2 (3.6) | 7.6 (4.9) | |
| p, difference from baseline | 0.597 | 0.402 | 0.283 | 0.166 | |
| FVC, % of predicted value | |||||
| CLAD, mean (SD) | 74.4 (14.8) | 76.6 (17.4) | 74.5 (18.3) | 68.0 (22.3) | 71.2 (16.5) |
| Non-CLAD (SD) | 81.8 (14.1) | 85.4 (15.7) | 83.6 (14.4) | 87.5 (16.8) | 84.1 (11.2) |
| p, difference CLAD versus non-CLAD | 0.231 | 0.225 | 0.207 | 0.036 | 0.078 |
| CLAD, mean difference from baseline (SE) | 3.5 (2.1) | 0.05 (3.1) | −2.5 (4.1) | −4.6 (3.8) | |
| p, difference from baseline | 0.128 | 0.985 | 0.544 | 0.266 | |
| Non-CLAD, mean difference from baseline (SE) | 3.5 (1.9) | 1.7 (2.2) | 5.6 (3.2) | 5.8 (2.6) | |
| p, difference from baseline | 0.112 | 0.458 | 0.119 | 0.058 | |
CLAD, chronic lung allograft dysfunction; FEV1, forced expiratory volume in first second; FVC, forced vital capacity; SD, standard deviation; SE, standard error.
Both CLAD and non-CLAD had a reduction of ISD, and we did not find a difference among groups for the whole analyzed period; we found a greater reduction of %∆ISD in the non-CLAD group (Supplemental Table S4).
Twelve-month mortality was nearly fourfold higher in the CLAD group (7/17, 41.1%) than in the non-CLAD group (1/9, 11.1%) but this difference was not statistically significant (p = 0.115).
RAS and BOS patients: pulmonary function, ISD change and mortality
Among the 17 patients with CLAD who were treated with everolimus, seven were diagnosed with RAS and 10 with BOS.
In the RAS group, we observed a progressive loss of pulmonary function after the first month (both FEV1 and FVC, p > 0.05) (Table 4). In contrast, in the BOS group, we found a progressive increase in FEV1 while FVC remained stable (p > 0.05).
Table 4.
Pulmonary function in RAS (n = 7) and BOS (n = 10) patients.
| Group | Baseline | 1 month | 3 months | 6 months | 12 months |
|---|---|---|---|---|---|
| FEV1, % of predicted value | |||||
| RAS, mean (SD) | 59.2 (5.3) | 60.2 (7.1) | 50.0 (13.8) | 44.8 (15.6) | 44.2 (19.1) |
| BOS, mean (SD) | 46.7 (19.7) | 49.5 (20.7) | 47.1 (21.5) | 48.5 (25.1) | 51.0 (23.9) |
| p, difference RAS versus BOS | 0.124 | 0.214 | 0.758 | 0.759 | 0.650 |
| RAS, mean difference from baseline (SE) | 1.0 (3.9) | −9.2 (5.9) | −13.6 (5.9) | −15.2 (9.2) | |
| p, difference from baseline | 0.810 | 0.170 | 0.070 | 0.198 | |
| BOS, mean difference from baseline (SE) | 2.7 (2.0) | 0.4 (3.0) | 2.1 (3.2) | −1.5 (5.1) | |
| p, difference from baseline | 0.214 | 0.899 | 0.534 | 0.780 | |
| FVC, % of predicted value | |||||
| RAS, mean (SD) | 70.0 (15.1) | 71.8 (18.3) | 63.4 (11.1) | 53.1 (7.9) | 56.5 (5.9) |
| BOS, mean (SD) | 77.6 (14.6) | 80.4 (16.8) | 82.3 (18.7) | 79.1 (23.5) | 81.0 (13.5) |
| p, difference RAS versus BOS | 0.315 | 0.345 | 0.031 | 0.024 | 0.010 |
| RAS, mean difference from baseline (SE) | 1.8 (1.5) | −6.5 (2.9) | −11.3 (4.8) | −6.0 (4.0) | |
| p, difference from baseline | 0.271 | 0.068 | 0.065 | 0.236 | |
| BOS, mean difference from baseline (SE) | 4.7 (3.7) | 4.7 (4.3) | 4.0 (5.3) | −3.6 (6.1) | |
| p, difference from baseline | 0.236 | 0.312 | 0.478 | 0.578 | |
BOS, bronchiolitis obliterans syndrome; FEV1, forced expiratory volume in first second; FVC, forced vital capacity; RAS, restrictive allograft syndrome; SD, standard deviation; SE, standard error.
About FVC, we found a progressive difference in the mean of the two groups with a divergent trend. FVC was stable in the BOS group but progressively decreased in the RAS group (p < 0.05).
Comparing the mean difference from baseline of the two different groups, we did not observe remarkable differences in FEV1 and FVC values. In the RAS group, this difference was progressively more negative, bordering on significance for FVC from the third month onwards, but not for FEV1. On the contrary, in the BOS group, the differences in FEV1 and FVC were mildly positive until the sixth month, when they became slightly negative (Table 4).
We compared %∆FEV1 and %∆FVC means of BOS and RAS: we observed a significant difference at the sixth month for %∆FEV1 and close to the significant level for %∆FVC means; this trend was lost at the subsequent observation, when the number of patients decreased (Table S3).
Both BOS and RAS had a reduction of ISD, but we did not find a difference among groups during observation for the whole analyzed period; we found a greater reduction of %∆ISD in the RAS group (Supplemental Table S4).
At 12 months, the mortality rate was similar (p = 0.877) in the RAS group (3/7, 42%) compared with BOS (4/10, 40%).
Non-CLAD, RAS and BOS patients: pulmonary function and mortality
For completeness, we compared the non-CLAD group’s FEV1 and FVC with the BOS and RAS groups. We performed this analysis to evaluate and compare functional trends among three groups of patients with different indications: non-CLAD did not have a functional indication for everolimus introduction, while the RAS and BOS patients did, as specified above. Results are presented in the Supplemental Material (Tables S4 and S5). FEV1 in BOS patients was lower during the whole 12 months of follow-up while FVC was only in the late period of observation; RAS had a higher functional loss than BOS group. In particular, %∆FEV1 rapidly dropped in the RAS group, whereas it was stable in the BOS group and raised in non-CLAD patients. We observed a similar pattern for %∆FVC, which dropped in the RAS group, then leveled off after the sixth month, whereas in BOS and non-CLAD groups %∆FVC was stable.
We did not find statistically significant differences in 12-month mortality among the three groups of patients (p = 0.288).
Discussion
Our study showed the stability of pulmonary function, measured with FEV1 and FVC, after the introduction of everolimus in lung-transplanted patients with CLAD, but we observed different functional responses to everolimus among RAS and BOS groups: RAS patients had a progressive functional loss whereas BOS ones had a gradual increase of FEV1 and stability of FVC. To date, this is the first published study that shows different pulmonary functional trends in different phenotypes of patients who underwent an everolimus switch for CLAD.
When we compared the functional trend in the CLAD and in the non-CLAD groups, the only statistically significant difference was found in the 12th month: %∆FVC; FEV1 and FVC progressively increased in the non-CLAD group, probably due to ameliorative effects of everolimus on renal function; indeed it has been demonstrated that there is a non-linear dose-relationship between eGFR and FEV1 and FVC.20 In patients with chronic kidney disease, fluid overload together with increased pulmonary capillary permeability are closely associated with restrictive and obstructive respiratory abnormalities, as demonstrated in patients who underwent hemodialysis.21 Even in the CLAD group, we observed a non-statistically significant and transient improvement of both FEV1 and FVC after 1 month, but, subsequently, these parameters worsened. We assume that these transient increases were most likely due to the effect of everolimus on renal function and not directly pulmonary effects. Moreover, we observed a trend in differences of %∆FEV1, as for %∆FVC, among CLAD and non-CLAD groups; %∆FEV1 decreased too during the year of observation but statistical significance at 12 months was not achieved, likely due to the small size of this group.
In our cohort, seven patients underwent an everolimus switch for RAS and 10 for BOS: RAS had lower basal FVC and BOS lower basal FEV1. In the RAS group, we observed that both FEV1 and FVC and mean differences from baseline rapidly fell; on the other hand, in BOS group FEV1 and FVC slightly increased during the follow-up even if the trend of mean difference from baseline, after 1 year, was negative.
Even if we observed these different variations among two different groups, we observed a statistical significance only at the sixth-month observation when BOS’ mean %∆FEV1 (and almost %∆FVC) was higher; this difference was not confirmed at the 12th-month observation, probably due to the decreased number of patients at that observation. Indeed, in our sample, both BOS and RAS patients died during the follow-up and the mortality rate was slightly higher in the RAS group (42% versus 40%).
Interestingly, after the drop of FEV1 and FVC in the RAS group, we observed a relative stabilization of functional loss at the 12th month with respect to the sixth month. These results could be due to the tardive antiproliferative and antifibrotic effects of everolimus on transplanted lung with RAS in contrast to BOS.
These results could identify the BOS group as an “early everolimus responsive phenotype” in contrast to the RAS one. This response is probably due to the strict link between the antifibrotic activity of everolimus, as the inhibition of fibroblastic growth factor, platelet-derived growth factor, vascular endothelial growth factor22 and chronic fibroblast activation present in BOS.23 This is an interesting result because in the literature only azithromycin and montelukast were demonstrated to have a significant impact on CLAD incidence and long-term survival.2
One of the indications for switching to everolimus is the reduction of ISD, in particular, CNIs.22 The switch allows ISD minimization with the preservation of renal function without significant changes in the patients’ survival or graft rejection rates.24 Our results confirm these findings, demonstrating a stable reduction of ISD during the whole observation period with a mean reduction of 49.9% after 1 year. This reduction was cross-sectional because we did not find a difference when we compared non-CLAD, BOS and RAS groups.
Our study has some limitations. First, the observational nature of the study, the lack of a control group with people unexposed to everolimus and the small sample size do not enable us to draw definitive conclusions. Second, the definition of CLAD, BOS and RAS used in our study refers to the definitions present at the enrollment time. At that time, Sato et al.6 conducted the largest study identifying RAS subgroups and then Todd et al.8 defined the prognostic value on the survival rate of functional loss. Recently, definitions of CLAD, BOS and RAS were defined by consensus reports.3,7 However, even using different diagnostic criteria, we found functional trends that are in line with those previously reported in the literature.10 Although TLC is actually the main functional parameter used to define a diagnosis of restriction, TLC monitoring is not always available. Moreover, hyperinflation present in BOS patients may counteract TLC decline in the RAS group when these two conditions are present at the same time in a patient.6 These reasons, in addition to the availability and reproducibility of spirometric measurements, led us to use FEV1 and FVC to evaluate functional trends in different populations (CLAD versus non-CLAD and BOS versus RAS).
For the first time in the literature, we reported functional trends in lung-transplanted patients who underwent an everolimus switch for different indications. Our results confirm the renal preservation with reduction of ISDs. Moreover, we found that, despite the introduction of everolimus, CLAD patients had a loss both of FEV1 and FVC. These results are sustained by the worst trend obtained in the RAS group, identifying the BOS subgroup as an “early everolimus responsive phenotype”, but further studies with a higher number of patients are needed.
Supplemental Material
Supplemental material, sj-pdf-1-taj-10.1177_2040622321993441 for Twelve-month effects of everolimus on renal and lung function in lung transplantation: differences in chronic lung allograft dysfunction phenotypes by Filippo Patrucco, Elias Allara, Massimo Boffini, Mauro Rinaldi, Cristina Costa, Carlo Albera and Paolo Solidoro in Therapeutic Advances in Chronic Disease
Acknowledgments
We thank Ray Hill, an independent medical writer, who provided English-language editing and journal styling prior to submission on behalf of Health Publishing & Services Srl. These services were supported by Novartis Farma SpA.
Footnotes
Author contributions: Conceptualization: FP, PS, EA; Methodology: FP, PS, EA; Investigation: FP, PS; Writing-Original Draft: FP, PS, EA; Writing-Review and Editing: FP, PS, EA, MB, MR, CC, CA; Supervision: PS, MR.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Filippo Patrucco  https://orcid.org/0000-0002-4794-8734
https://orcid.org/0000-0002-4794-8734
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Filippo Patrucco, Division of Respiratory Diseases, Cardiovascular and Thoracic Department, University of Turin, Città della Salute e della Scienza di Torino, C.so Bramante 88/90, 10100 Torino, Italy; Department of Translational Medicine, Università del Piemonte Orientale, Novara, Italy.
Elias Allara, Cardiovascular Epidemiology Unit, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK.
Massimo Boffini, Division of Cardiac Surgery, Cardiovascular and Thoracic Department, University of Turin, Città della Salute e della Scienza di Torino, Torino, Italy.
Mauro Rinaldi, Division of Cardiac Surgery, Cardiovascular and Thoracic Department, University of Turin, Città della Salute e della Scienza di Torino, Torino, Italy.
Cristina Costa, Division of Virology, Department of Public Health and Pediatrics, University of Turin, Città della Salute e della Scienza di Torino, Torino, Italy.
Carlo Albera, Division of Respiratory Diseases, Medical Sciences Department University of Turin and Cardiovascular and Thoracic Department, AOU Città della Salute e della Scienza di Torino, Torino, Italy.
Paolo Solidoro, Division of Respiratory Diseases, Medical Sciences Department University of Turin and Cardiovascular and Thoracic Department, AOU Città della Salute e della Scienza di Torino, Torino, Italy.
References
- 1. Yusen RD, Edwards LB, Dipchand AI, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-third adult lung and heart-lung transplant report—2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant 2016; 35: 1170–1184. [DOI] [PubMed] [Google Scholar]
- 2. Verleden SE, Vos R, Vanaudenaerde BM, et al. Chronic lung allograft dysfunction phenotypes and treatment. J Thorac Dis 2017; 9: 2650–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verleden GM, Glanville AR, Lease ED, et al. Chronic lung allograft dysfunction: definition, diagnostic criteria, and approaches to treatment—a consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant 2019; 38: 493–503. [DOI] [PubMed] [Google Scholar]
- 4. Verleden SE, Vos R, Verleden GM. Chronic lung allograft dysfunction: light at the end of the tunnel? Curr Opin Organ Transplant 2019; 24: 318–323. [DOI] [PubMed] [Google Scholar]
- 5. Gerhardt SG, McDyer JF, Girgis RE, et al. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. Am J Respir Crit Care Med 2003; 168: 121–125. [DOI] [PubMed] [Google Scholar]
- 6. Sato M, Waddell TK, Wagnetz U, et al. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant 2011; 30: 735–742. [DOI] [PubMed] [Google Scholar]
- 7. Glanville AR, Verleden GM, Todd JL, et al. Chronic lung allograft dysfunction: definition and update of restrictive allograft syndrome—a consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant 2019; 38: 483–492. [DOI] [PubMed] [Google Scholar]
- 8. Todd JL, Jain R, Pavlisko EN, et al. Impact of forced vital capacity loss on survival after the onset of chronic lung allograft dysfunction. Am J Respir Crit Care Med 2014; 189: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verleden SE, Ruttens D, Vandermeulen E, et al. Predictors of survival in restrictive chronic lung allograft dysfunction after lung transplantation. J Heart Lung Transplant 2016; 35: 1078–1084. [DOI] [PubMed] [Google Scholar]
- 10. Todd JL, Neely ML, Copeland CAF, et al. Prognostic significance of early pulmonary function changes after onset of chronic lung allograft dysfunction. J Heart Lung Transplant 2019; 38: 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DerHovanessian A, Todd JL, Zhang A, et al. Validation and refinement of chronic lung allograft dysfunction phenotypes in bilateral and single lung recipients. Ann Am Thorac Soc 2016; 13: 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Belloli EA, Wang X, Murray S, et al. Longitudinal forced vital capacity monitoring as a prognostic adjunct after lung transplantation. Am J Respir Crit Care Med 2015; 192: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Pablo A, Santos F, Solé A, et al. Recommendations on the use of everolimus in lung transplantation. Transplant Rev (Orlando) 2013; 27: 9–16. [DOI] [PubMed] [Google Scholar]
- 14. Snell GI, Valentine VG, Vitulo P, et al. Everolimus versus azathioprine in maintenance lung transplant recipients: an international, randomized, double-blind clinical trial. Am J Transplant 2006; 6: 169–177. [DOI] [PubMed] [Google Scholar]
- 15. Gullestad L, Iversen M, Mortensen SA, et al. Everolimus with reduced calcineurin inhibitor in thoracic transplant recipients with renal dysfunction: a multicenter, randomized trial. Transplantation 2010; 89: 864–872. [DOI] [PubMed] [Google Scholar]
- 16. De la Torre-Cisneros J, Farinas MC, Caston JJ, et al. GESITRA-SEIMC/REIPI recommendations for the management of cytomegalovirus infection in solid-organ transplant patients. Enferm Infecc Microbiol Clin 2011; 29: 735–758. [DOI] [PubMed] [Google Scholar]
- 17. Rittà M, Costa C, Solidoro P, et al. Everolimus-based immunosuppressive regimens in lung transplant recipients: impact on CMV infection. Antiviral Res 2015; 113: 19–26. [DOI] [PubMed] [Google Scholar]
- 18. Roman A, Ussetti P, Zurbano F, et al. A retrospective 12-month study of conversion to everolimus in lung transplant recipients. Transplant Proc 2011; 43: 2693–2698. [DOI] [PubMed] [Google Scholar]
- 19. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 20. Yu D, Chen T, Cai Y, et al. Association between pulmonary function and renal function: findings from China and Australia. BMC Nephrol 2017; 18: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yilmaz S, Yildirim Y, Yilmaz Z, et al. Pulmonary function in patients with end-stage renal disease: effects of hemodialysis and fluid overload. Med Sci Monit 2016; 22: 2779–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fine NM, Kushwaha SS. Recent advances in mammalian target of rapamycin inhibitor use in heart and lung transplantation. Transplantation 2016; 100: 2558–2568. [DOI] [PubMed] [Google Scholar]
- 23. Ensor CR, Doligalski CT. Proliferation signal inhibitor toxicities after thoracic transplantation. Expert Opin Drug Metab Toxicol 2013; 9: 63–77. [DOI] [PubMed] [Google Scholar]
- 24. Peddi VR, Wiseman A, Chavin K, et al. Review of combination therapy with mTOR inhibitors and tacrolimus minimization after transplantation. Transplant Rev (Orlando) 2013; 27: 97–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-taj-10.1177_2040622321993441 for Twelve-month effects of everolimus on renal and lung function in lung transplantation: differences in chronic lung allograft dysfunction phenotypes by Filippo Patrucco, Elias Allara, Massimo Boffini, Mauro Rinaldi, Cristina Costa, Carlo Albera and Paolo Solidoro in Therapeutic Advances in Chronic Disease


