Abstract
Background:
High anterior knee laxity (AKL) has been prospectively identified as a risk factor for anterior cruciate ligament (ACL) injuries. Given that ACL morphometry and structural composition have the potential to influence ligamentous strength, understanding how these factors are associated with greater AKL is warranted.
Hypothesis:
Smaller ACL volumes combined with longer T2* relaxation times would collectively predict greater AKL.
Study Design:
Cross-sectional study; Level of evidence, 3.
Methods:
College-aged active male (n = 20) and female (n = 30) participants underwent magnetic resonance imaging (MRI) and AKL testing. T2-weighted MRI scans were used to assess ACL volumes, and T2* relaxation times were used to assess ACL structural composition. AKL was measured via a commercial knee arthrometer. Forward stepwise linear regression with sex and weight (first step; suppressor variables) as well as ACL volume and T2* relaxation time (second step; independent variables) was used to predict AKL (dependent variable).
Results:
After initially adjusting for sex and weight (R 2 = 0.19; P = .006), smaller ACL volumes combined with longer T2* relaxation times collectively predicted greater AKL (R 2 = 0.52; P < .001; R 2 Δ = 0.32; P Δ < .001). A smaller ACL volume was the primary predictor of greater AKL (R 2 Δ = 0.28; P < .001), with a longer T2* relaxation time trending toward a significant contribution to greater AKL (R 2 Δ = 0.04; P = .062). After adjusting for ACL volume and T2* relaxation time, sex (partial r = 0.05; P = .735) and weight (partial r = 0.05; P = .725) were no longer significant predictors.
Conclusion:
AKL was largely predicted by ACL volume and to a lesser extent by T2* relaxation time (and not a person’s sex and weight). These findings enhance our understanding of how AKL may be associated with a structurally weaker ACL. The current study presents initial evidence that AKL is a cost-effective and clinically accessible measure that shows us something about the structural composition of the ACL. As AKL has been consistently shown to be a risk factor for ACL injuries, work should be done to continue to investigate what AKL may tell a clinician about the structure and composition of the ACL.
Keywords: ACL size, MRI, relaxation times, knee
An anterior cruciate ligament (ACL) injury frequently occurs in active populations, with approximately 70% of ACL injuries resulting from noncontact mechanisms.8,18,24 Female patients younger than 20 years have been reported as having a higher incidence of ACL injuries as well as greater anterior knee laxity (AKL) than that of male patients.9,23,30 Knee stability during functional activity is provided both passively by the ligaments and actively by the muscles around the knee.38 It is possible that when there is a delay or an error in the neuromuscular control system, active restraint is insufficient, and greater relative demand is placed on passive restraint.20,22 During this situation, the capability of the ligament to resist external loads is critical in maintaining ACL integrity.
ACL function is most commonly assessed clinically using AKL testing.11 AKL is defined as anterior displacement of the tibia relative to the femur under a fixed load. This measure is clinically relevant, as high AKL has been prospectively identified as a risk factor for ACL injuries.34,48,54 The mechanisms underpinning this increased risk of injuries with greater AKL are not well understood. Greater anterior-posterior knee laxity has been associated with lower failure loads at 1 year after ACL reconstruction in canines at high risk for ACL ruptures.4 Additionally, 3-dimensional finite element modeling has demonstrated that greater posterior cruciate ligament graft laxity was associated with lower graft strength.26 While these studies were limited to ligamentous grafts and not healthy ACL tissue, they suggest that greater knee laxity may be indicative of structural insufficiency in native tissue. Conversely, factors associated with lesser laxity have the potential to be related to ligamentous strength and thus resiliency against injuries. A better understanding of the factors that contribute to a structurally stronger and less lax ligament may benefit our prevention efforts in ultimately reducing the incidence of ACL injuries.
It is well established that a greater structural size is positively related to the ability to resist external loads, thus resulting in less displacement of tissue when loaded.37 With regard to the ACL, this would indicate that greater ACL morphometry (ie, larger size) would be associated with less deformation of the ligament under fixed anterior loads and thus less AKL. This theory is supported by total anterior-posterior translation of animal knees being associated with the cross-sectional area of the reconstructed ACL (R 2 = 0.86).19 We are aware of only 1 study in humans in which a significant but weaker relationship was observed between ACL width and AKL (R 2 = 0.22).51 Thus, the size of the ligament may not be the only determinant of ligamentous behavior under loading.
Intrinsic factors, such as collagen fiber orientation,53 collagen density,1 and collagen fiber diameter,15,29 have the potential to affect ligamentous function beyond ligament size. These intrinsic factors could help to stabilize the extracellular matrix, thus increasing resistance to deformation under loading.
To that end, the microstructural composition of ligaments, such as collagen content and collagen fiber orientation, may be examined in vivo via magnetic resonance imaging (MRI) through the derivation of T2* relaxation times.3 T2* relaxation times have been utilized to evaluate the subregional differences of the ACL in vivo.41 Lower T2* relaxation times have been shown to be related to greater yield loads on healing ACL grafts7 as well as characteristic of more mature ACL grafts from 1 to 2 years after ACL reconstruction.14 Thus, while T2* relaxation time could be associated with AKL, a better understanding of this quantitative MRI measure and how it predicts AKL in the healthy human knee would further advance our understanding of ACL injuries.
While greater AKL is an established ACL injury risk factor,34,48,54 there is much to be understood about the factors that contribute to greater AKL. Therefore, the purpose of this study was to determine the extent of ACL volume and T2* relaxation time in predicting AKL. We hypothesized that smaller ACL volumes combined with longer T2* relaxation times would collectively predict greater AKL.
Methods
Participants
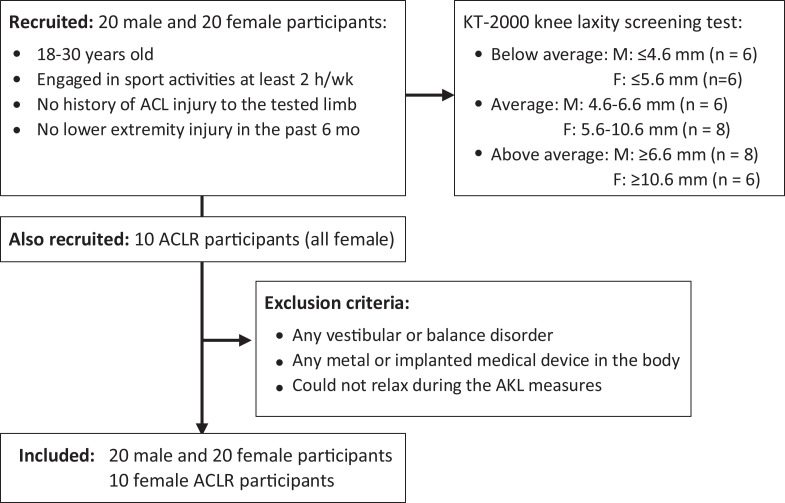
College-aged, recreationally active male (n = 20) and female (n = 30) participants were recruited from local universities to participate in the study between September 2015 and May 2016. Participants were part of a large study examining knee structure and function. As such, volume-related imaging data included in the current investigation have in part been previously reported.41,49,50 Potential participants were prescreened to obtain a wide distribution of AKL values in both sexes. This was done to ensure similar representations of average, above average, and below average laxity within each sex. Previously reported data43 were used to define average (male: 5.6 ± 1.0 mm; female: 8.1 ± 2.5 mm), above average (>1 SD; male: 6.6 mm; female: 10.6 mm), and below average (<1 SD; male: 4.6 mm; female: 5.6 mm) AKL. Overall, 10 female participants who had undergone ACL reconstruction were recruited to test their healthy limbs. This was done to sample a wide range of laxities. Inclusion criteria were (1) current participation in sport activities for at least 2 hours per week, (2) no history of ACL injuries to the tested limb, and (3) no lower extremity injury of any type in the last 6 months. Participants were excluded if they (1) had any vestibular or balance disorder, (2) had any metal or implanted medical device in the body, or (3) could not relax during AKL testing. An informed consent form approved by an institutional review board was signed by all participants. Each participant attended an AKL testing session as well as an MRI session consisting of structural T2 imaging along with T2* relaxation time mapping. Participants were instructed to avoid high-intensity activity for the 24 hours before testing. To control for potential hormonal effects on AKL, female participants were tested during a limited window of the menstrual cycle (3-8 days after menses onset).42 To quantify participant activity levels, the Marx activity rating scale, which included self-ranking in running, cutting, decelerating, and pivoting activities with a score between 0 and 16, was used31 (Figure 1).
Figure 1.
Flow chart of screened and included participants. ACL, anterior cruciate ligament; ACLR, ACL reconstruction; AKL, anterior knee laxity; F, female; M, male.
AKL Assessment
AKL was assessed using a KT-2000 knee arthrometer (MedMetric) as previously described51 and defined as anterior displacement of the tibia relative to the femur at a 130-N load. The participant was placed in a supine position with the knee flexed to a mean of 25° ± 5° over a thigh bolster. The foot/ankle rested in the foot cradle, while a hook-and-loop strap was placed around both thighs. This method was used to prevent rotation of the lower extremities during testing. The examiner first applied a 90-N posterior-directed force and then a 130-N anterior-directed force to the tibia, while displacement (mm) of the tibia with respect to the femur was recorded using computer software (MedMetric). In total, 3 continuous measurement trials were performed, and the last 2 trials were averaged for analyses. AKL was defined as the average anterior displacement of the tibia relative to the femur over the last 2 trials. A single research team member (H.-M.W.) who had established between-day measurement consistency and precision performed all measurements (intraclass correlation coefficient [SEM] = 0.87 [0.5] mm).45
MRI Examination
The examination of MRI data has been described in previous studies.41,49,50 For ACL morphometry, T2-weighted, multiplanar MRI was used with a repetition time of 1300 milliseconds, echo time (TE) of 39 milliseconds, flip angle of 160°, field of view of 150 × 150 mm, and voxel size of 0.5 × 0.5 × 0.5 mm.
T2* relaxation time mapping was described in a previous study.41 Gradient echo data sets were used for T2* relaxation time mapping with the following parameters: repetition time of 1000 milliseconds; TE of 8.26, 10.28, 12.3, 14.32, 16.34, 18.36, 20.38, 22.4, 24.42, 26.44, 28.46, and 30.48 milliseconds; flip angle of 90°; field of view of 280 × 280 mm; and voxel size of 0.5 × 0.5 × 3.0 mm.5,6
MRI Morphologic Data Reduction
ACL volume was measured as previously reported by Chaudhari et al.13 ACL contouring from a sagittal slice was performed manually using a digitizing tablet (DTK1300; Wacom) and ITK-SNAP software (http://www.itksnap.org/pmwiki/pmwiki.php). All contoured sagittal slices were utilized in the calculation of ACL volume (Figure 2). For the establishment of intratester reliability and precision, ACL volume was measured in 10 pilot participants twice at least a week apart (intraclass correlation coefficient (3,1) [SEM] = 0.97 [36.1] mm3).50
Figure 2.
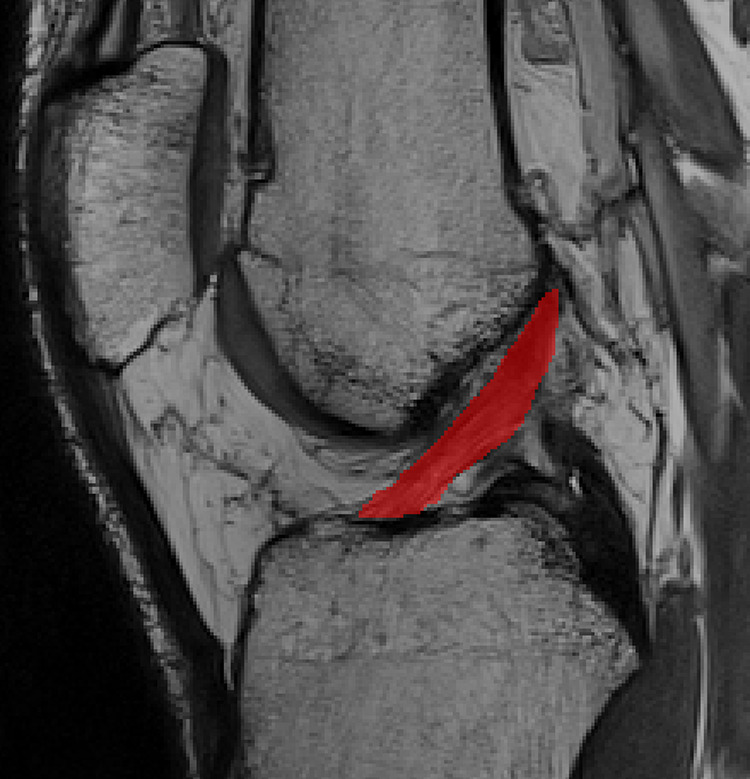
Anterior cruciate ligament volume manual segmentation on sagittal image. Red area represents the anterior cruciate ligament segmentation.
MRI Structural Composition Data Reduction
Following the methods of previous work reporting regional T2* values of the ACL,41 voxel-wise T2* relaxation time maps were obtained using the signal intensity (SI) from all 12 TEs of the T2* relaxation time mapping sequence via customized MATLAB codes (MathWorks). The calculation equation is the following:
where SI(TE) represents the voxel-specific SIs for the various TEs and S 0 is the SI at the initial TE.5,6,17 To isolate specific ACL T2* values, the measured T2* relaxation time map (Figure 3) was registered to the ACL structural imaging sequence using 3D Slicer software (https://www.slicer.org), and the mean T2* relaxation time value of the voxels included in 3-dimensional ACL volume modeling (Figure 2) described above was calculated using ITK-SNAP software and included in analyses.
Figure 3.
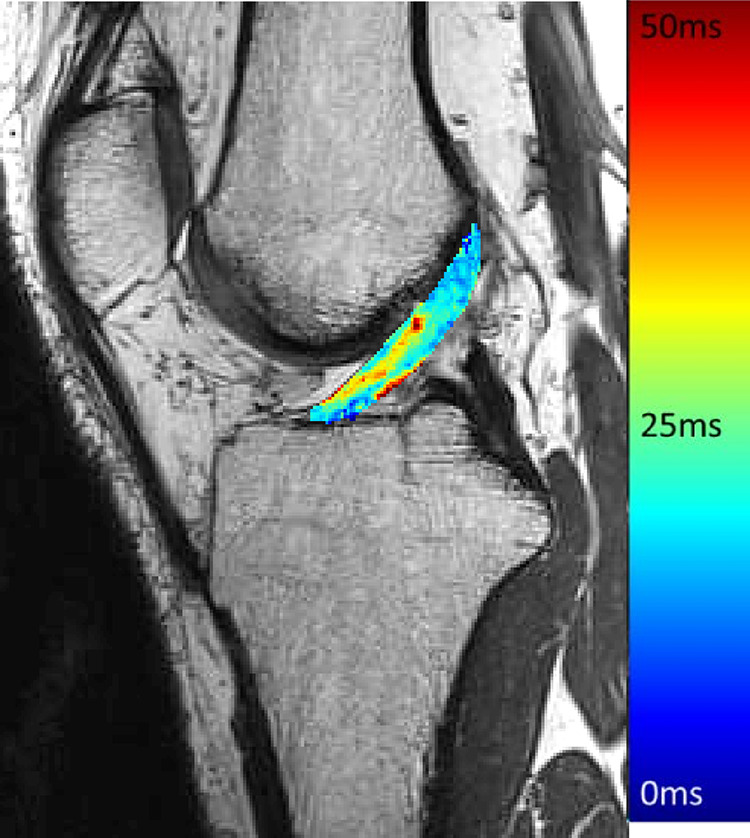
T2* relaxation time map.
Statistical Analysis
Power analysis revealed that a sample size of 36 would achieve 80% power to detect a large effect size of 0.30 attributed to the 4 independent variables of interest (sex, weight, ACL volume, and T2* relaxation time) using the F test. Independent-samples t tests were used to examine the difference in demographics between male and female participants. Stepwise linear regression analyses were used to test the hypothesis that T2* relaxation time and ACL volume would each have unique contributions in explaining the variance in AKL in a multivariate model. Because female patients are often reported to have greater AKL as well as smaller ACL morphometry and body sizes,2,25 sex was entered to adjust for these differences and any other confounding factors associated with sex. In the next step, weight was entered to adjust for the effect of varying weights of the different participants. Finally, the stepwise method was used to assess the unique contributions of ACL volume and T2* relaxation time. Sex and weight were removed in the final step to ascertain the influence of only T2* relaxation time and ACL volume. The alpha level for all analyses was set a priori at ≤.05. All calculations were performed using SPSS statistical software (version 21.0; IBM Corp).
Results
Descriptive statistics for AKL, ACL volume, and T2* relaxation time are shown in Table 1. Independent-samples t tests indicated that the female participants were younger, shorter, and lighter than were the male participants and had greater AKL and a smaller ACL volume. After adjusting for sex and weight (R 2 = 0.19; P = .006), smaller ACL volumes (R 2 Δ = 0.28; P < .001) combined with longer T2* relaxation times (R 2 Δ = 0.04; P = .062) predicted greater AKL (R 2 = 0.52; P < .001; R 2 Δ = 0.32; P Δ < .001). Once ACL volume and T2* relaxation time were entered into the model, sex (partial r = 0.05; P = .735) and weight (partial r = 0.05; P = .725) no longer contributed predictivity to the model. The regression model and regression coefficients can be found in Tables 2 and 3, respectively.
Table 1.
Descriptive Statistics of Participantsa
| Male (n = 20) | Female (n = 20) | Female With ACL Reconstruction (n = 10) | |
|---|---|---|---|
| Age, y | 23.2 ± 2.9 | 21.3 ± 2.3b | 21.9 ± 2.7 |
| Height, cm | 180.4 ± 6.7 | 166.9 ± 7.7b | 170.3 ± 5.5b |
| Weight, kg | 84.0 ± 10.9 | 61.9 ± 7.2b | 67.5 ± 7.0b |
| AKL, mm | 6.3 ± 1.9 | 8.1 ± 2.7b | 8.8 ± 1.8b |
| ACL volume, mm3 | 1712.2 ± 356.3 | 1200.1 ± 337.8b | 1012.8 ± 175.5b |
| T2* relaxation time, ms | 19.1 ± 2.5 | 18.5 ± 2.2 | 19.1 ± 2.4 |
| Marx activity rating score | 9.2 ± 4.1 | 10.7 ± 3.9 | 13.9 ± 2.2b |
aData are reported as mean ± SD. ACL, anterior cruciate ligament; AKL, anterior knee laxity.
bStatistically significant difference (P < .05) between male and female participants.
Table 2.
Regression Model of Sex, Weight, ACL Volume, and T2* Relaxation Time Predicting AKLa
| Model | R 2 Value | R 2 Δ Value | Sig. FΔ | P Value |
|---|---|---|---|---|
| 1 (sex) | 0.16 | 0.16 | .004 | .004 |
| 2 (sex and weight) | 0.19 | 0.03 | .178 | .006 |
| 2 (sex, weight, and ACL volume) | 0.48 | 0.28 | <.001 | <.001 |
| 3 (sex, weight, ACL volume, and T2* relaxation time) | 0.52 | 0.04 | .062 | <.001 |
| 4 (ACL volume and T2* relaxation time) | 0.51 | –0.01 | .513 | <.001 |
aACL, anterior cruciate ligament; AKL, anterior knee laxity.
Table 3.
Regression Coefficients and Correlations of Models for Predicting AKLa
| Beta Coefficient | t Coefficient | P Value | Correlation | |||
|---|---|---|---|---|---|---|
| Zero Order | Partial | Part | ||||
| Constant | 8.483 | 3.295 | .002 | |||
| Sex | 0.277 | 0.340 | .735 | –0.404 | 0.051 | 0.035 |
| Weight | 0.011 | 0.354 | .725 | –0.421 | 0.053 | 0.037 |
| ACL volume | –0.004 | –5.348 | <.001 | –0.688 | –0.623 | –0.554 |
| T2* relaxation time | 0.210 | 1.914 | .062 | 0.100 | 0.274 | 0.198 |
aACL, anterior cruciate ligament; AKL, anterior knee laxity.
Discussion
Greater AKL has been identified as a prospective risk factor for noncontact ACL injuries.34,48,54 However, little is understood of the factors that contribute to greater inherent knee laxity. The current investigation assessed both ACL size and structural composition as contributing factors. The primary findings were that after adjusting for sex and weight, the combination of smaller ACL volume and longer T2* relaxation time predicted greater AKL. While these associations were consistent with the directional hypothesis, ACL volume was a strong predictor of AKL, while T2* relaxation time contributed to a lesser extent (∼5% of the variance; P = .062). It is of note in the final model that the partial correlation of sex decreased from –0.404 (P = .004) to 0.051 (P = .735) and that the partial correlation of weight decreased from –0.421 (P = .178) to 0.053 (P = .725) after the inclusion of ACL volume and T2* relaxation time in the regression model. The results indicated that after adjusting for sex and weight, smaller ACL volumes with longer T2* relaxation times predicted greater AKL. To the best of our knowledge, the current study is the first in vivo report of the relationship of AKL to ACL volumes and quantitative tissue relaxation times in healthy ACL tissue. Such findings may indicate that ACL volume combined with T2* relaxation time contributes to AKL when considering sex-related differences.
The current finding that a smaller ACL volume was associated with greater AKL is consistent with previous animal and human studies reporting that a smaller ACL size was associated with greater AKL.19,51 A previous computational study indicated that smaller ACL graft sizes led to greater strain on the ligament during loading,52 with an animal study also reporting that smaller ACL graft volumes had lower failure loads.17 These findings collectively suggest that a smaller in vivo ACL volume is less capable of resisting external forces, thus likely leading to greater AKL and potentially lower failure loads.
Given that ligaments are composed of diverse materials, the various intrinsic properties of these materials could potentially affect function of the ligament.15,29,36,37,39 As determined in articular cartilage, shorter relaxation times in ligaments could reflect greater collagen density, a more organized collagen structure, and less water content, while longer relaxation times could reflect less collagen density, a less organized collagen structure, and more free water content.32 Given that lower ligamentous fibril density has been related to weaker cadaveric ACLs,21 these findings indicate that a poorly structured and disorganized matrix of the ligament is less capable of restraining external loading, thus resulting in a greater amount of laxity. T2* relaxation times could be indicative of intrinsic ACL properties, and T2* relaxation time has been used as a measure of ligamentous composition.7,41 Hence, it was our initial expectation that longer T2* relaxation times may be related to greater AKL. However, current data did not fully support this expectation.
The findings were consistent with this directional relationship, with longer T2* relaxation time being weakly associated with greater AKL. Although the explained variance in AKL was small and only trended toward significance (R 2 = 0.04; P = .062), this is the first study, to the best of our knowledge, to report the relationship of intrinsic ligamentous characteristics assessed using T2* relaxation times with AKL in healthy in vivo ACLs. A study of T2* relaxation times in 15 cadaveric limbs reported a similar level of nonsignificant predictivity of median T2* relaxation times to yield loads (R 2 = 0.05; P = .44).6 However, the authors suggested that the lack of significant findings was because of natural age-restricted distributions of T2* and ligament volume in their sample of limbs with a median age of 54 years.6 Given the relatively low associations, further work is needed to understand the clinical relevance of this measure in understanding the resiliency of the ACL to an applied load.
While MRI offers noninvasive measurements of ligament size and structural quality, it is costly and inaccessible to many clinicians. The findings support the use of AKL as a cost-effective clinically accessible measure of ligament structure and function that can be examined in the physician’s office as well as sports medicine clinics to inform the standard of care for ACL tears as well as injury risk screening. Understanding the intrinsic ACL properties that contribute to AKL may also serve to advance prevention efforts that focus on increasing ligament strength. To date, ACL injury prevention programs have primarily focused on neuromuscular training to protect the ACL from external loading.35,40,44 However, animal studies have suggested that ligaments are dynamic tissues that respond to exercise training and can increase in size, strength, and stiffness if the training protocol is sufficient to load the bone-ligament-bone complex and stimulate changes in collagen structure.10,12,27,46,47,55 While bone and tendon adaptations to resistance training are well established in humans,16,28 ligament adaptations have rarely been studied. Interventions designed to increase ACL intrinsic properties to better withstand external loads and resist deformation during sport may be a promising direction for future research.
Limitations
Because this study was based on obtaining a wide range of AKL measurements in male and female participants, a limitation was the wide included range of physical activity levels as evidenced by the large SD in Marx activity rating scores (Table 1), which may have confounded the current findings and hurt external validity in populations at a high risk of noncontact ACL injuries. Previous evidence that physical activity may affect AKL in the noninjured knee has been reported.33 However, collecting a wider range of activity levels could help us to better understand the gross relationship of ACL morphometry and structural composition to AKL. The recruited male and female participants were both from active populations. While the study was limited to healthy limbs, it included 10 female participants who reported an ACL injury in the contralateral limb to the testing limb. This was done to potentially include a greater distribution of ligament sizes, quality, and functionality in the sample population. It is possible that the inclusion of only female participants may have biased the outcome. Furthermore, the ACL was the only structure that was analyzed as being responsible for AKL. There was no analysis of peripheral instabilities, major meniscal lesions, bone sizes, slope deformities, or posterior cruciate ligament insufficiencies. Additionally, it is important to note that the implications of the current study are limited by the unknown relationship between knee laxity and failure loads of healthy, native ACL tissue. However, the study provided a possible direction on noninvasive measures of ligament quality.
Conclusion
The current main findings were that after adjusting for sex and weight, a smaller ACL volume was the primary predictor of greater AKL, with a longer T2* relaxation time trending toward a significant contribution to greater AKL. The results help to provide an initial step toward our understanding of contributions to in vivo ligament function. Future studies should be done to continue to address factors associated with increased AKL to help focus future intervention efforts in enhancing ligament strength and reducing the prospective risk factor of high laxity.
Acknowledgment
This work was supported by the Gateway MRI Center at the University of North Carolina at Greensboro.
Footnotes
Final revision submitted July 19, 2020; accepted August 4, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: This work was supported by the Gateway MRI Center at the University of North Carolina at Greensboro. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the University of North Carolina at Greensboro (study No. 15-0393).
References
- 1. Amiel D, Ishizue KK, Harwood FL, Kitabayashi L, Akeson WH. Injury of the anterior cruciate ligament: the role of collagenase in ligament degeneration. J Orthop Res. 1989;7(4):486–493. [DOI] [PubMed] [Google Scholar]
- 2. Anderson AF, Dome DC, Gautam S, Awh MH, Rennirt GW. Correlation of anthropometric measurements, strength, anterior cruciate ligament size, and intercondylar notch characteristics to sex differences in anterior cruciate ligament tear rates. Am J Sports Med. 2001;29(1):58–66. [DOI] [PubMed] [Google Scholar]
- 3. Beveridge JE, Machan JT, Walsh EG, Kiapour AM. Magnetic resonance measurements of tissue quantity and quality using T(2)* relaxometry predict temporal changes in the biomechanical properties of the healing ACL. J Orthop Res. 2018;36(6):1701–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beynnon BD, Johnson RJ, Toyama H, et al. The relationship between anterior-posterior knee laxity and the structural properties of the patellar tendon graft: a study in canines. Am J Sports Med. 1994;22(6):812–820. [DOI] [PubMed] [Google Scholar]
- 5. Biercevicz AM, Akelman MR, Fleming BC, Walsh EG, Murray MM. Improving the clinical efficiency of T(*) mapping of ligament integrity. J Biomech. 2014;47(10):2522–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biercevicz AM, Akelman MR, Rubin LE, et al. The uncertainty of predicting intact anterior cruciate ligament degeneration in terms of structural properties using T(2)(*) relaxometry in a human cadaveric model. J Biomech. 2015;48(6):1188–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Biercevicz AM, Murray MM, Walsh EG, et al. T2* MR relaxometry and ligament volume are associated with the structural properties of the healing ACL. J Orthop Res. 2014;32(4):492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boden BP, Dean GS, Feagin JA, Jr, Garrett WE, Jr. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–578. [DOI] [PubMed] [Google Scholar]
- 9. Boden BP, Sheehan FT, Torg JS, Hewett TE. Noncontact anterior cruciate ligament injuries: mechanisms and risk factors. J Am Acad Orthop Surg. 2010;18(9):520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burroughs P, Dahners LE. The effect of enforced exercise on the healing of ligament injuries. Am J Sports Med. 1990;18(4):376–378. [DOI] [PubMed] [Google Scholar]
- 11. Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee: a biomechanical study. J Bone Joint Surg Am. 1980;62(2):259–270. [PubMed] [Google Scholar]
- 12. Cabaud HE, Chatty A, Gildengorin V, Feltman RJ. Exercise effects on the strength of the rat anterior cruciate ligament. Am J Sports Med. 1980;8(2):79–86. [DOI] [PubMed] [Google Scholar]
- 13. Chaudhari AM, Zelman EA, Flanigan DC, Kaeding CC, Nagaraja HN. Anterior cruciate ligament-injured subjects have smaller anterior cruciate ligaments than matched controls: a magnetic resonance imaging study. Am J Sports Med. 2009;37(7):1282–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chu CR, Williams AA. Quantitative MRI UTE-T2* and T2* show progressive and continued graft maturation over 2 years in human patients after anterior cruciate ligament reconstruction. Orthop J Sports Med. 2019;7(8):2325967119863056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Culav EM, Clark CH, Merrilees MJ. Connective tissues: matrix composition and its relevance to physical therapy. Phys Ther. 1999;79(3):308–319. [PubMed] [Google Scholar]
- 16. Farr JN, Laddu DR, Going SB. Exercise, hormones and skeletal adaptations during childhood and adolescence. Pediatr Exerc Sci. 2014;26(4):384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fleming BC, Vajapeyam S, Connolly SA, Magarian EM, Murray MM. The use of magnetic resonance imaging to predict ACL graft structural properties. J Biomech. 2011;44(16):2843–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622–627. [DOI] [PubMed] [Google Scholar]
- 19. Grood ES, Walz-Hasselfeld KA, Holden JP, et al. The correlation between anterior-posterior translation and cross-sectional area of anterior cruciate ligament reconstructions. J Orthop Res. 1992;10(6):878–885. [DOI] [PubMed] [Google Scholar]
- 20. Hashemi J, Breighner R, Chandrashekar N, et al. Hip extension, knee flexion paradox: a new mechanism for non-contact ACL injury. J Biomech. 2011;44(4):577–585. [DOI] [PubMed] [Google Scholar]
- 21. Hashemi J, Chandrashekar N, Mansouri H, Slauterbeck JR, Hardy DM. The human anterior cruciate ligament: sex differences in ultrastructure and correlation with biomechanical properties. J Orthop Res. 2008;26(7):945–950. [DOI] [PubMed] [Google Scholar]
- 22. Hewett TE, Paterno MV, Myer GD. Strategies for enhancing proprioception and neuromuscular control of the knee. Clin Orthop Relat Res. 2002;402:76–94. [DOI] [PubMed] [Google Scholar]
- 23. Hinton RY, Rivera VR, Pautz MJ, Sponseller PD. Ligamentous laxity of the knee during childhood and adolescence. J Pediatr Orthop. 2008;28(2):184–187. [DOI] [PubMed] [Google Scholar]
- 24. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311–319. [PMC free article] [PubMed] [Google Scholar]
- 25. Jamison ST, Flanigan DC, Nagaraja HN, Chaudhari AM. Side-to-side differences in anterior cruciate ligament volume in healthy control subjects. J Biomech. 2010;43(3):576–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lai YS, Chen WC, Huang CH, et al. The effect of graft strength on knee laxity and graft in-situ forces after posterior cruciate ligament reconstruction. PLoS One. 2015;10(5):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laros GS, Tipton CM, Cooper RR. Influence of physical activity on ligament insertions in the knees of dogs. J Bone Joint Surg Am. 1971;53(2):275–286. [PubMed] [Google Scholar]
- 28. Legerlotz K, Marzilger R, Bohm S, Arampatzis A. Physiological adaptations following resistance training in youth athletes: a narrative review. Pediatr Exerc Sci. 2016;28(4):501–520. [DOI] [PubMed] [Google Scholar]
- 29. Liu SH, Yang RS, al-Shaikh R, Lane JM. Collagen in tendon, ligament, and bone healing: a current review. Clin Orthop Relat Res. 1995;318:265–278. [PubMed] [Google Scholar]
- 30. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363–2370. [DOI] [PubMed] [Google Scholar]
- 31. Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213–218. [DOI] [PubMed] [Google Scholar]
- 32. Matzat SJ, van Tiel J, Gold GE, Oei EH. Quantitative MRI techniques of cartilage composition. Quant Imaging Med Surg. 2013;3(3):162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Medrano D, Jr, Smith D. A comparison of knee joint laxity among male and female collegiate soccer players and non-athletes. Sports Biomech. 2003;2(2):203–212. [DOI] [PubMed] [Google Scholar]
- 34. Myer GD, Ford KR, Paterno MV, Nick TG, Hewett TE. The effects of generalized joint laxity on risk of anterior cruciate ligament injury in young female athletes. Am J Sports Med. 2008;36(6):1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Myer GD, Sugimoto D, Thomas S, Hewett TE. The influence of age on the effectiveness of neuromuscular training to reduce anterior cruciate ligament injury in female athletes: a meta-analysis. Am J Sports Med. 2013;41(1):203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakamura N, Hart DA, Boorman RS, et al. Decorin antisense gene therapy improves functional healing of early rabbit ligament scar with enhanced collagen fibrillogenesis in vivo. J Orthop Res. 2000;18(4):517–523. [DOI] [PubMed] [Google Scholar]
- 37. Nordin M, Frankel VH. Basic Biomechanics of the Musculoskeletal System. Lea & Febiger; 1989. [Google Scholar]
- 38. Noyes FR, Grood ES, Butler DL, Malek M. Clinical laxity tests and functional stability of the knee: biomechanical concepts. Clin Orthop Relat Res. 1980;146:84–89. [PubMed] [Google Scholar]
- 39. Quapp KM, Weiss JA. Material characterization of human medial collateral ligament. J Biomech Eng. 1998;120(6):757–763. [DOI] [PubMed] [Google Scholar]
- 40. Renstrom P, Ljungqvist A, Arendt E, et al. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42(6):394–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schmitz RJ, Wang HM, Kraft RA, et al. Regional differences in anterior cruciate ligament imaging biomarkers: T2 and T2* values. Muscles Ligaments Tendons J. 2018;8(2):238–245. [Google Scholar]
- 42. Shultz SJ, Schmitz RJ, Kong Y, et al. Cyclic variations in multiplanar knee laxity influence landing biomechanics. Med Sci Sports Exerc. 2012;44(5):900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shultz SJ, Shimokochi Y, Nguyen AD, et al. Measurement of varus-valgus and internal-external rotational knee laxities in vivo, part II: relationship with anterior-posterior and general joint laxity in males and females. J Orthop Res. 2007;25(8):989–996. [DOI] [PubMed] [Google Scholar]
- 44. Sugimoto D, Myer GD, Bush HM, et al. Compliance with neuromuscular training and anterior cruciate ligament injury risk reduction in female athletes: a meta-analysis. J Athl Train. 2012;47(6):714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taylor JB, Wang HM, Schmitz RJ, et al. Multiplanar knee laxity and perceived function during activities of daily living and sport. J Athl Train. 2015;50(11):1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tipton CM, James SL, Mergner W, Tcheng TK. Influence of exercise on strength of medial collateral knee ligaments of dogs. Am J Physiol. 1970;218(3):894–902. [DOI] [PubMed] [Google Scholar]
- 47. Tipton CM, Vailas AC, Matthes RD. Experimental studies on the influences of physical activity on ligaments, tendons and joints: a brief review. Acta Med Scand Suppl. 1986;711:157–168. [DOI] [PubMed] [Google Scholar]
- 48. Uhorchak JM, Scoville CR, Williams GN, et al. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003;31(6):831–842. [DOI] [PubMed] [Google Scholar]
- 49. Wang HM, Shultz SJ, Ross SE, et al. Sex comparisons of in vivo anterior cruciate ligament morphometry. J Athl Train. 2019;54(5):513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang HM, Shultz SJ, Ross SE, et al. ACL size and notch width between ACLR and healthy individuals: a pilot study. Sports Health. 2020;12(1):61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang HM, Shultz SJ, Schmitz RJ. Association of anterior cruciate ligament width with anterior knee laxity. J Athl Train. 2016;51(6):460–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Westermann RW, Wolf BR, Elkins JM. Effect of ACL reconstruction graft size on simulated Lachman testing: a finite element analysis. Iowa Orthop J. 2013;33:70–77. [PMC free article] [PubMed] [Google Scholar]
- 53. Woo SL-Y, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex: the effects of specimen age and orientation. Am J Sports Med. 1991;19(3):217–225. [DOI] [PubMed] [Google Scholar]
- 54. Woodford-Rogers B, Cyphert L, Denegar CR. Risk factors for anterior cruciate ligament injury in high school and college athletes. J Athl Train. 1994;29(4):343–346. [PMC free article] [PubMed] [Google Scholar]
- 55. Zuckerman J, Stull GA. Effects of exercise on knee ligament separation force in rats. J Appl Physiol. 1969;26(6):716–719. [DOI] [PubMed] [Google Scholar]