Abstract
Background and Aim:
Irritable bowel syndrome (IBS) is a complex and heterogeneous disorder. Sensory, motor and barrier dysfunctions are the key physiological endophenotypes of IBS. Our aim is to review studies evaluating barrier dysfunction in adults and children with IBS, as well as to link those changes with IBS symptomatology and quality of life.
Methods:
A comprehensive and systematic review of multiple databases was performed up to March 2020 to identify studies comparing intestinal permeability in IBS patients with healthy controls. Both in vivo and in vitro studies were considered.
Results:
We identified 66 studies, of which 27 used intestinal probes to quantify barrier function. The prevalence of barrier dysfunction differed between PI-IBS (17–50%), IBS-D (37–62%) and IBS-C (4–25%). At a group level, permeability was increased compared with healthy controls in IBS-D (9/13 studies) and PI-IBS (4/4 studies), but only a minority of IBS-C (2/7 studies) and not in the only IBS-M study. All four studies in children with IBS demonstrated loss of barrier function. A heterogeneous set of tight junction genes were found to be altered in small and large intestines of adults with IBS, but these have not been evaluated in children. Positive associations were identified between barrier dysfunction and bowel disturbances (6/9 studies), abdominal pain (9/13 studies), overall symptom severity (1/6 studies), depression and anxiety (1/1 study) and quality of life (1/4 studies). Fecal slurry or supernatants of IBS patients were found to induce barrier disruption in animal models (5/6 studies).
Conclusions:
Barrier dysfunction is present in a significant proportion of adult and all pediatric IBS studies, especially in the IBS-D and PI-IBS subtype. The majority of studies indicated a positive association between loss of barrier function and symptoms such as abdominal pain and changes in the bowel function.
Keywords: functional gastrointestinal disorders, immune cells, microbiome, occludin, zonula occludens
Introduction
Irritable bowel syndrome (IBS) is a chronic bowel disorder characterized by recurrent abdominal pain related to defecation and changes in bowel habits.1 Clinically, IBS patients are characterized by their predominant aberrant bowel pattern as diarrhea-predominant (IBS-D), constipation-predominant (IBS-C) or mixed (IBS-M).1 Increasing evidence points toward the presence of pathophysiological disturbances in subsets of IBS.1,2 These include alterations in visceral sensitivity, gastrointestinal (GI) motility, intestinal permeability, the microbiome and the immune function.1–3 Furthermore, several risk factors for the development of IBS have been identified, among which infectious gastroenteritis appears to the most predominant.4,5 However, the development of new therapeutics is hampered by heterogeneous presentation and difficulties in phenotypic characterization.3
With a surface area of up to 40 m2, the digestive tract presents a large interface from which to interact with the external environment while serving many critical homeostatic functions.6 The intestinal barrier protects the internal environment from a continuous exposure to pathogens and antigens, while at the same time being responsible for the uptake of nutrients and water.7 To fulfill these conflicting functions, the gut has evolved into a complex system of multiple defensive layers, consisting of physical, biochemical and immune components.8,9 First, intrinsic secretions of the GI tract as well as products of commensal microbes prevent the colonization of pathogens.8,10–13 Second, the adherent mucus layer, a network consisting of mucin polymers produced by the goblet cells, coats the intestinal epithelium, providing a barrier between the host and the microbiome, while also entrapping pathogens.14 Third and perhaps most importantly, the epithelial barrier itself, consisting of a single layer of epithelial cells interconnected by tight junctions, adherens junctions and desmosomes, provides the strongest physical defense against submucosal access of noxious luminal substances.15,16 Fourth, the immune cells in the mucosa and in the lamina propria (e.g. dendritic cells, mast cells or macrophages) mount protective responses through the production of immunoglobulins, cytokines and many other critical immunomodulators.17 In addition to physical and chemical components of the barrier, the propulsive motility of the gut also plays an important role in defending the internal environment.18
The intestinal epithelia also have important absorptive and secretory roles, necessitating the ability of ions, molecules and solutes to cross the intestinal epithelium. This can be accomplished via the transcellular or paracellular pathways.19,20 Three distinct paracellular pathways have been proposed.21 First is the pore pathway, a high-capacity size- and charge-selective pathway regulated by the members of the claudin (CLDN) family.21,22 Second is the leak pathway, a non-selective low-capacity pathway predominantly regulated by zonula occludens-1 (ZO-1), occludin and myosin light chain kinase (MLCK).23,24 Finally, the unrestricted pathway opens due to loss of tight junction complexes typically as a result of cell death, apoptosis or mucosal damage. This route can allow passage of large macromolecules and even microbes across the epithelium.25 Barrier dysfunction has been linked to visceral hypersensitivity and pain in IBS, presumably due to exposure of submucosal neuronal and immune apparatus to the luminal microbes, antigens and other mediators. A recent animal and a human study demonstrated that inhibition or restoration of barrier dysfunction can correct visceral hypersensitivity26 and pain27 in IBS, respectively. However, direct evidence for an impaired barrier to causally result in visceral hypersensitivity is lacking. Moreover, the significance of small bowel versus colonic barrier dysfunction is poorly explored in IBS. It is possible that postprandial symptoms may be mediated by an impaired barrier in the proximal small bowel, whereas symptoms of lower abdominal pain and urgency are driven by colonic involvement.28 Lastly, different measurements of barrier structure and function are often interpreted without appropriate context. Whereas in vivo studies such as those using saccharide administration reflect the end result of integrated host physiology including barrier function, studies with biopsies in Ussing chambers devoid of the neuro-vascular input and studies with luminal mediators or structural studies using electron microscopy provide significantly different pieces of information.
Previous narrative reviews on barrier dysfunction in IBS29,30 provide few details on population characteristics, comorbidities such as psychological distress, and methodological details (in vivo, ex vivo and in vitro). Furthermore, associations of barrier dysfunction with IBS symptomatology and evidence for barrier dysfunction in children with IBS have not been summarized. We therefore performed a systematic review of studies investigating disturbances in intestinal permeability (in vivo) in IBS patients, evaluating the presence of ex vivo and in vitro barrier dysfunction in both children and adults and potential associations of barrier changes with IBS symptomatology and quality of life (QoL) measures.
Methods
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed while conducting and writing this systematic review.31
Selection criteria
We included peer-reviewed studies reporting on IBS defined by Rome criteria (I, II, III or IV) or by physician diagnosis. Studies that did not describe how the IBS diagnosis was determined were excluded. Studies across all age groups assessing in vivo, ex vivo and/or in vitro measurements of barrier function were included. Only studies comparing IBS patients with either a healthy control group or those using a predefined cut-off value of normality were included. Studies focusing on animal models were excluded unless human samples were used to modulate barrier function. Narrative reviews, guidelines, editorials, conference summaries, conference abstracts, case reports, study protocols and non-English studies were also excluded.
Data sources and search strategy
After an initial search by the authors, an experienced librarian (LCH) performed an extensive search to retrieve additional articles (last search conducted on 18 March 2020). The databases included MEDLINE and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews and Scopus. Combinations of subject headings and keywords were used to search for the primary concepts. Selected terms include: “irritable bowel,” “irritable colon,” “permeability,” “tight junction,” “adherens junction,” “desmosomes,” “claudin,” “occludin,” and “zona occludens.” For the full search strategy, please refer to the Supplementary Materials. Identified records were imported into Endnote X9 software and combined to remove duplicates. Based upon title and abstract, one investigator (NH) excluded studies that did not focus on the research questions of interest. Subsequently, two investigators (NH and AE) independently reviewed the remaining full-text articles in more detail to assess whether they contained relevant information and met the inclusion criteria. Any disagreements in study selection were resolved by discussion and consensus with the senior investigators (BDW and MG). Finally, reference lists of all included studies were hand-searched to identify additional studies.
Data collection
One investigator (NH) extracted data using a standardized form in Microsoft Excel. The first author, the year of publication, the number of patients in the IBS and control groups and the diagnostic criteria used to identify IBS patients was abstracted. In addition, we extracted clinical characteristics of the studied populations including the IBS subtypes according to predominant stool pattern, age, gender, body mass index (BMI), psychological distress, symptom severity and QoL. For in vivo permeability studies, the details on methodology were abstracted (probes used, sample collection time, dietary restrictions, etc.). For in vitro permeability studies, the site of collected specimen and the experimental technique(s) were summarized. For interventional studies, only baseline parameters were extracted.
Assessment of quality and risk of bias
The risk of bias and the overall quality of all selected studies were assessed using the AXIS Tool, consisting of 20 items.32 Two authors (NH and HC) independently reviewed all included studies. The inter-rater agreement between the two reviewers was 92%. Disagreements were resolved by one of the senior investigators (BDW), who scored all discrepant items.
Results
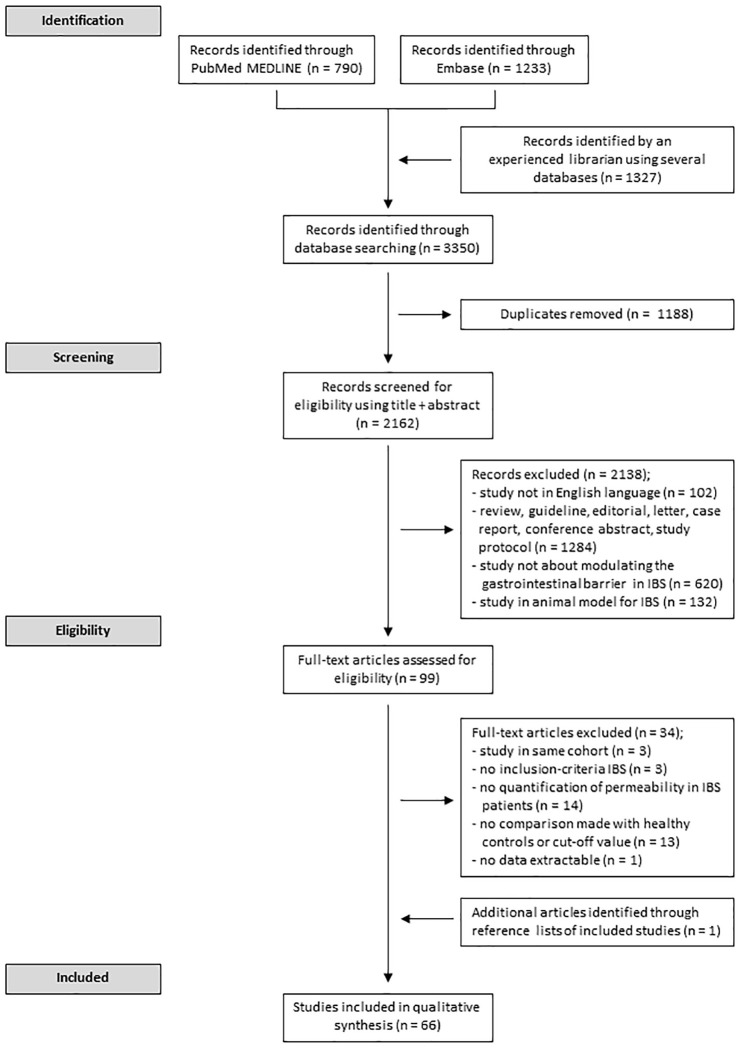
The search strategy resulted in the identification of 3350 unique records. After screening abstract and full text, a total of 66 unique articles met the inclusion criteria. Of these, one study was identified after screening reference lists. A flow chart summarizing the study screening selection is shown in Figure 1. Results of the Quality Assessment are shown in Supplementary Table 1. The median quality score for the included studies was 15 (range 10–19; <14 in nine studies, 14–16 in 48 studies and >16 in 10 studies). Owing to the large heterogeneity in the protocols followed to quantify intestinal permeability, a meta-analysis was not deemed feasible. Thus, the included studies are reviewed in a systematic way.
Figure 1.
Study schematic. Flowchart describing process for screening and selection of studies included in the systematic review.
In vivo measurements of intestinal permeability in adults
Participant characteristics
Twenty-seven studies evaluated intestinal barrier function in adult IBS patients after the administration of permeability probes.33–59 Ten studies were conducted in the United States, five in Italy, four in the United Kingdom, three in The Netherlands, two in China, one in Canada, one in Hungary and one in South Korea. IBS was diagnosed according to the Rome criteria in 25 studies (two Rome I, six Rome II and 17 Rome III). In two studies, IBS diagnosis was based on a clinician evaluation. Of the 25 studies employing the Rome criteria, 11 included >1 subtype, nine only IBS-D, two only IBS-C and three did not report a subtype. Four studies specified including patients with post-infection IBS (PI-IBS). The proportion of women ranged between 34–100% (<60% females in seven studies; 60–80% in 10 studies; >80% in 10 studies). Twelve studies reported the BMI of their populations, which ranged between 22 and 34 kg m–2 (normal (<25 kg m–2) in six studies; overweight (25–30 kg m–2) in three studies; and obese (>30.0 kg m–2) in three studies.
Methodological differences
Twenty-five studies quantified permeability by measuring the renal excretion of orally ingested and gastrointestinally absorbed probes and two quantified the probe in serum. Characteristics of the included studies are reported in Table 1, and additional demographic characteristics (country/region, gender, age, BMI, anxiety and depression scores, symptom and QoL scores) are in Supplementary Table 2. A number of probes, including mono- and disaccharides, 51Chromium ethylene diamine tetra acetic acid (51Cr-EDTA) and polyethylene glycol (PEG) polymers were used. An ideal probe molecule should not be degraded or metabolized in the human body or urine and cause no toxic effects. Furthermore, the molecule should not be present naturally (e.g. ingested via food) and fully and rapidly excreted via the urine. Finally, its measurement should be sensitive and accurate.41,60,61 A large proportion of studies (22) combine the administration of a monosaccharide, such as mannitol, with the administration of a disaccharide, such as lactulose. With a diameter of 8 Å, mannitol is a smaller molecule than lactulose (13 Å). Mannitol can traverse the pore pathway as well as the larger leak and unrestricted pathways. Due to its larger size, lactulose can only move across the intestinal barrier via the leak pathway or through the unrestricted pathway.62 Therefore, an increase in the lactulose-to-mannitol ratio (LMR) reflects a disruption of the epithelial barrier, normalized against the total paracellular transport. This becomes critical when comparing conditions such as celiac disease where there is a loss of absorptive surface area (and hence decreased mannitol excretion) in addition to increased leak through paracellular pathways (and, hence, increased lactulose excretion). Since sucrose is absorbed rapidly, it is thought to be a marker of gastric or gastroduodenal permeability.50 Three studies investigated its absorption in IBS patients.47,50,57 In contrast with the other sugars, the artificial disaccharide sucralose is not broken down by colonic bacteria, making it a more suitable marker for colonic permeability. Four studies reported the use of sucralose to reflect colonic barrier function.38,47,50,53 Other less commonly used saccharides were raffinose, L-arabinose, and L-rhamnose.
Table 1.
Studies comparing in vivo permeability in adult IBS patients with healthy controls or without controls.
| Reference | Study population | Permeability probe | Dietary restrictions | Timing of urine or blood collection | Cut-off value of normality | Results | |
|---|---|---|---|---|---|---|---|
| Comparison with healthy volunteers | Proportion of ↑ permeability | ||||||
| Studies with a control population | |||||||
| Studies collecting urine over 0–8 h, representing proximal GI permeability | |||||||
| Russo et al.55 | IBS-D (n = 28) HV (n = 19) |
10 g lactulose 5 g mannitol 100 mL of H2O |
Overnight fast: YES | 0–5 h | NA |
Lactulose: IBS-D 0.3% versus HV 0.4% (p = NS) Mannitol: IBS-D 11.0% versus HV 12.2% (p = NS) LMR: IBS-D 0.02 versus HV 0.03 (p = NS) |
NA |
| Lobley et al.33 | IBS (n = 62) HV (n = 40) |
2 g L-arabinose 20 g lactose 8 g raffinose 250 mL of H2O |
Overnight fast: YES Water ad libitum after 2 h, food not allowed |
0–5 h | Ra/Ara >0.06 |
L-arabinose: IBS 15.1% versus HV 17.5% (p < 0.01) Raffinose: IBS 0.2% versus HV 0.3% (p = NS) Ra/Ara: IBS versus HV (p = NS) |
IBS 2% versus HV 0% |
| Mattioli et al.44 | IBS-C (n = 32) HV (n = 23) |
5 g lactulose 2 g D-mannitol 100 mL of H2O |
Overnight fast: YES Intake of 500 mL of H2O after 30 min, fasting for the first 2 hof the collection period |
0–5 h | LMR >0.052 |
Lactulose: IBS-C 0.6% versus HV 0.5% (p = NS) Mannitol: IBS-C 17.1% versus HV 19.8% (p = NS) LMR: IBS-C 0.04 versus HV 0.03 (p < 0.05) |
IBS-C 25% versus HV 9–13% |
| Del Valle-Pinero et al.47 | IBS (n = 20) HV (n = 39) |
10 g sucrose 5 g lactulose 1 g mannitol 0.1 g sucralose 100 mL of H2O |
Overnight fast: YES Water ad libitum, food not allowed |
0–5 h | NA |
Sucrose: IBS 0.03% versus HV 0.04% (p = 0.118) LMR: IBS 0.01 versus HV 0.01 (p = 0.45) Sucralose: IBS 5.4% versus HV 9.1% (p = 0.011) |
NA |
| Linsalata et al.57 | IBS-D (n = 39) HV (n = 20) |
40 g sucrose 10 g lactulose 5 g mannitol 100 mL of H2O |
Overnight fast: YES | 0–5 h | LMR ⩾0.035 | Sucrose, lactulose, mannitol, LMR: IBS-D versus HV (p = NS) | IBS-D 46% versus HV 0% |
| Russo et al.58 | IBS-D (n = 34) HV (n = 17) |
40 g sucrose 10 g lactulose 5 g mannitol 100 mL of H2O |
Overnight fast: YES | 0–5 h | LMR ⩾0.035 |
Lactulose: IBS-D 0.4% versus HV 0.2% (p = 0.1212) Mannitol: IBS-D 11.0% versus HV 13.0% (p = 0.0386) LMR:IBS-D 0.04 versus HV 0.02 (p = 0.0091) |
IBS-D 44% |
| Spiller et al.35 | PI-IBS (n = 10) HV (n = 10) |
5 g lactulose 2 g mannitol 100 mL of H2O |
Overnight fast: YES Test meal of 100 mL of Fortisip (200 kcal) before intake probes |
0–6 h | LMR >0.03 | LMR: PI-IBS 0.06 versus HV 0.009 (p = 0.005) | PI-IBS 50% versus HV 0% |
| Kerckhoffs et al.41 | IBS-A (n = 3) IBS-C (n = 3) IBS-D (n = 8) HV (n = 15) |
40 g sucrose 5 g lactulose 2 g mannitol 100 mL of H2O |
Overnight fast: YES Water ad libitum, food not allowed |
0–6 h | LMR >0.03 | LMR: IBS 0.01 versus HV 0.01 (p = NS) | IBS 21% versus HV 0% |
| Marshall et al.36 | IBS (n = 132), mostly PI-IBS HV (n = 86) |
100 g sucrose 5 g lactulose 2 g mannitol 500 mL of H2O 1.5 g of flavored drink crystals |
Overnight fast: NO | Overnight | LMR ⩾0.025 | LMR: ↑ in IBS versus HV (p = 0.007) | IBS 16% versus HV 8% |
| Park et al.39 | IBS-A (n = 3) IBS-C (n = 8) IBS-D (n = 27) HV (n = 12) |
PEG 400 PEG 3350 |
Overnight fast: ND | 0–8 h | NA |
PEGR: IBS 0.8 versus HV 0.4 (p < 0.05) PEGR: IBS-A 0.8 versus IBS-C 0.7 versus IBS-D 0.9 (p = NS) |
NA |
| Valentin et al.56 | IBS-D (n = 15) HV (n = 12) |
1 g lactulose 0.1 g 13C mannitol |
Overnight fast: YES Water ad libitum, standardized breakfast (egg, toast, water) after 2 h, standardized lunch (chicken, potato and water) after 6 h |
0–2 h 2–8 h |
NA |
13C mannitol: IBS-D 0.2 versus HV (p = NS) LMR: IBS-D versus HV (p = NS) 2–8 h 13 C mannitol: IBS-D 0.2 versus HV (p = NS) LMR: IBS-D versus HV (p = NS) |
NA |
| Studies collecting urine over 0–24 h, representing distal or whole GI tract permeability | |||||||
| Dunlop et al.37 | IBS-C (n = 15) PI-IBS-D (n = 15) HV (n = 15) |
1.8 MBq of 100 μL of 51Cr-EDTA 100 mL of H2O 200 mL of Fortisip (300 kcal) |
Overnight fast: YES Drinking allowed after 3 h, food after 5 h |
0–3 h 3–5 h 5–24 h |
NA | 0–3 h
PI-IBS-D 0.2% versus IBS-C 0.1% versus HV 0.1% (p = 0.02 overall, p = 0.037 for PI-IBS-D versus HV, p = 0.004 for PI-IBS versus IBS-C, p = NS for IBS-C versus HV) 3–5 h PI-IBS-D 0.2% versus IBS-C 0.1% versus HV 0.3% (p = 0.08) 5–24 h PI-IBS-D 0.8% versus IBS-C 0.9% versus HV 1.0% (p = 0.2) |
NA |
| Dunlop et al.37 | PI-IBS-D (n = 15) nonPI-IBS-D (n = 15) HV (n = 12) |
1.8 MBq of 100 μL of 51Cr-EDTA 100 mL of H2O 200 mL of Fortisip (300 kcal) |
Overnight fast: ND | 0–6 h 6–24 h |
NA | 0–6 h
Non-PI-IBS-D 0.8% versus PI-IBS-D 0.4% versus HV 0.3% (p = 0.001 overall, p = 0.028 for nonPI-IBS-D versus HV, p = 0.001 for PI-IBS-D versus HV, p = 0.004 for non-PI-IBS-D versus PI-IBS-D) 6–24 h Non-PI-IBS-D 1.2% versus PI-IBS-D 1.0% versus HV 0.8% (p = 0.1 overall, p = 0.04 for non-PI-IBS-D versus HV, p = 0.5 for PI-IBS-D versus HV) |
NA |
| Zeng et al.38 | IBS-D (n = 29) HV (n = 12) |
10 g lactulose 5 g mannitol 5 g sucralose 100 mL of H2O |
Overnight fast: YES Water and food allowed after 2 h |
0–5 h 5–24 h |
LMR >0.025
Sucralose >42.1 mg |
0–5 h
LMR: IBS-D 0.04 versus HV 0.02 (p = 0.002) 0–24 h Sucralose: IBS-D 44.3 mg versus 31.4 mg (p = 0.028) |
LMR: IBS-D 62% Sucralose: IBS-D 52% |
| Zhou et al.40 | IBS-D (n = 54) HV (n = 22) |
5 g lactulose 2 g mannitol 100 mL of H2O |
Overnight fast: YES | 0–24 h | LMR ⩾0.07 | NA | IBS-D 39% versus HV 0% |
| Kerckhoffs et al.41 | IBS-A (n = 3) IBS-C (n = 3) IBS-D (n = 8) HV (n = 15) |
5 g PEG 400 1.5 g PEG 1500 5 g PEG 4000 10 g PEG 10000 100 mL of H2O containing 0.1% sorbate |
Overnight fast: YES Water ad libitum, food allowed after 6 h |
0–2 h 2–4 h 4–6 h 6–8 h 8–10 h 10–12 h 12–14 h 14–16 h 16–24 h |
NA |
PEG 400: IBS 26.0% versus HV 27.9% (p = NS) PEG 1500: IBS 1.0% versus HV 1.3% (p = NS) PEG 4000: IBS 0.0% versus HV 0.02% (p = NS) |
NA |
| Zhou et al.42 | IBS-D (n = 19) HV (n = 10) |
5 g lactulose 2 g mannitol 100 mL of H2O |
Overnight fast: ND | 0–5 h 6–24 h |
LMR ⩾0.07 | NA | 0–5 h
IBS-D 42% versus HV 0% 6–24 h IBS-D 42% versus HV 0% |
| Gecse et al.43 | IBS-C (n = 12) IBS-D (n = 18) HV (n = 10) |
1.8 MBq of 100 μL of 51Cr-EDTA 100 mL of H2O 200 mL of Fortisip (300 kcal) |
Overnight fast: YES Drinking allowed after 3 h, food after 5 h |
0–3 h 3–5 h 5–24 h |
NA | 0–3 h
IBS-C 0.3% versus IBS-D 0.6% versus HV 0.6% (p < 0.05 for IBS-C versus HV, p = NS for IBS-D versus HV) 3–5 h IBS-C 0.4% versus IBS-D 0.6% versus HV 0.4% (p = NS) 5–24 h IBS-C 0.7% versus IBS-D 2.7% versus HV 1.0% (p = NS for IBS-C versus HV, p < 0.05 for IBS-D versus HV) 0–24 h IBS-C 1.3% versus IBS-D 3.9% versus HV 2.0% (p = NS for IBS-C versus HV, p < 0.05 for IBS-D versus HV) |
NA |
| Rao et al.45 | IBS-D (n = 12) HV (n = 12) |
1 g lactulose 0.2 g mannitol 250 mL of H2O containing Tc-99m DTPA |
Overnight fast: YES Water ad libitum, standardized breakfast (egg, toast, water) after 2 h, standardized lunch (chicken, potato and water) after 6 h, all food allowed after 8 h |
0–0.5 h 0.5–1 h 1–1.5 h 1.5–2 h 2–4 h 4–6 h 6–8 h 8–24 h |
NA | 0–2 h
Mannitol: ↑ IBS-D versus HV (p = 0.056) Lactulose, LMR: IBS-D versus HV (p = NS) 2–8 h Mannitol: ↑ IBS-D versus HV (p = 0.0489) Lactulose, LMR: IBS-D versus HV (p = NS) 8–24 h Lactulose: ↑ IBS-D versus HV (p = 0.097) Mannitol, LMR: IBS-D versus HV (p = NS) |
NA |
| Rao et al.45 | IBS-D (n = 12) HV (n = 12) |
1 g lactulose 0.2 g mannitol Methacrylate-coated capsule in 250 mL of H2O |
Overnight fast: YES Water ad libitum, standardized breakfast (egg, toast, water) after 2 h, standardized lunch (chicken, potato and water) after 6 h, all food allowed after 8 h |
0–0.5 h 0.5–1 h 1–1.5 h 1.5–2 h 2–4 h 4–6 h 6–8 h 8–24 h |
NA | 0–2 h
Lactulose, mannitol, LMR: IBS-D versus HV (p = NS) 2–8 h Lactulose, mannitol, LMR: IBS-D versus HV (p = NS) 8–24 h Lactulose, mannitol, LMR: IBS-D versus HV (p = NS) |
NA |
| Vazquez-Roque et al.63 | IBS-D (n = 45) HV (n = 12) |
1 g lactulose 0.2 g mannitol |
Overnight fast: YES Water ad libitum, standardized breakfast (egg, toast, water) after 2 h, standardized lunch (chicken, potato and water) after 6 h, all food allowed after 8 h |
0–0.5 h 0.5–1 h 1–1.5 h 1.5–2 h 2–4 h 4–6 h 6–8 h 8–24 h |
NA | 0–2 h
Lactulose: ↑ IBS-D versus HV (p < 0.001) Mannitol: ↑ IBS-D versus HV (p < 0.001) 8–24 h: LMR: ↑ IBS-D versus HV (p = 0.106) Lactulose, mannitol: IBS-D versus HV (p = NS) |
NA |
| Swan et al.48 | IBS-C (n = 18) IBS-D (n = 37) C. jejuni 6m post-enteritis HV (n = 26) |
1.8 MBq of 51Cr-EDTA 50 mL of H2O 200 mL of Fortisip (150 kcal) |
Overnight fast: YES Drinking allowed after 3 h, food after 5 h |
0–3 h 3–6 h 6–24 h |
NA | 0–3 h
PI-IBS versus IBS-C versus IBS-D versus HV (p = NS) 3–6 h PI-IBS 0.7% versus IBS-C 0.6% versus IBS-D 0.6% versus HV 0.5% (p = 0.025 for PI-IBS versus HV, p = 0.9 for IBS-C versus HV, p = 0.9 for IBS-D versus HV) 6–24 h PI-IBS versus IBS-C versus IBS-D versus HV (p = NS) |
NA |
| Camilleri et al.59 | IBS-C (n = 30) IBS-D (n = 64) HV (n = 30) |
1 g lactulose 0.2 g mannitol 240 mL of H2O |
Overnight fast: ND | 0–2 h 2–4 h 4–6 h 6–8 h 8–24 h |
NA | 0–2 h
Mannitol: IBS-C 264.8 mg versus IBS-D 444.3 mg versus HV 355.2 mg (p = 0.039) 8–24 h Mannitol: IBS-C 65.8 mg versus IBS-D 45.5 mg versus HV 43.6 mg (p = 0.708) |
NA |
| Mujagic et al.50 | IBS-C (n = 21) IBS-D (n = 34) IBS-M (n = 30) IBS-U (n = 6) HV (n = 94) |
1 g sucrose 1 g lactulose 0.5 g L-rhamnose 1 g sucralose 1 g erythritol 150 mL of H2O |
Overnight fast: YES Water ad libitum, other drinks and food allowed after 5 h |
0–5 h 5–24 h |
NA | 0–5 h
Sucrose: IBS-C 7.4 μmol versus IBS-D 4.2 μmol versus IBS-M 6.6 versus HV 2.4 μmol (p = 0.880) LRR: IBS-C 0.02 versus IBS-D 0.02 versus IBS-M 0.02 versus HV 0.01 (p = 0.022 for IBS-D versus HV, p = NS for other groups) 5–24 h SER: IBS-C 0.009 versus IBS-D 0.008 versus IBS-M 0.008 versus HV 0.010 (p = NS) 0–24 h SER: IBS-C 0.008 versus IBS-D 0.009 versus IBS-M 0.010 versus HV 0.009 (p = NS) |
NA |
| Li et al.53 | IBS-D (n = 40) HV (n = 10) |
5 g lactulose 2 g mannitol 2 g sucralose 100 mL of H2O |
Overnight fast: YES | 0–5 h 5–24 h |
LMR > 0.025 | 0–5 h
LMR: IBS-D 0.02 versus HV 0.02 (p = 0.010) 0–24 h Sucralose: 23.3 mg versus HV 21.7 mg (p = 0.574) |
IBS-D 48% versus HV 10–20% |
| Peters et al.54 | IBS-C (n = 19) HV (n = 18) |
1 g lactulose 0.1 g 12C mannitol 0.1 g 13C mannitol 250 mL of H2O |
Overnight fast: ND | 0–2 h 2–8 h 8–24 h |
NA | 0–2 h
Lactulose: IBS-C 1.2 mg versus HV 1.0 mg (p = 0.53) 13 C mannitol: IBS-C 12.1 mg versus HV 13.2 mg (p = 0.39) LMR: IBS-C 0.01 versus HV 0.007 (p = 0.25) 8–24 h Lactulose: IBS-C 0.9 mg versus HV 0.5 mg (p = 0.75) 13 C mannitol: IBS-C 3.1 mg versus HV 3.9 mg (p = 0.08) LMR: IBS-C 0.02 versus HV 0.01 (p = 0.87) |
NA |
| Studies collecting blood samples | |||||||
| Keszthelyi et al.49 | IBS-C (n = 5) IBS-D (n = 7) IBS-M (n = 3) HV (n = 15) |
1 g lactulose 0.5 g L-rhamnose |
Overnight fast: YES | 1 hour | NA |
LRR: IBS 12 × 10–3
versus HV 6.3 × 10–3 (p = 0.06) No differences between IBS subtypes |
NA |
| Paganelli et al.34 | IBS (n = 14) HV (n = 10) |
Fresh cow milk (10 mL/kg) | Overnight fast: YES | 2 h 4 h |
B-lactoglobulin ⩾0.3 ng/mL | NA | IBS 21% |
| Studies without a control population | |||||||
| Zhou et al.51 | IBS-C (n = 74) IBS-D (n = 109) HV (n = 36) |
5 g lactulose 2 g mannitol 100 mL of H2O |
Overnight fast: YES | 0–24 h | LMR ⩾0.07 | NA | IBS-C 4% versus IBS-D 37% |
| Jarrett et al.52 | IBS-C (n = 11) IBS-D (n = 27) IBS-M (n = 38) IBS-U (n = 4) |
6.375 g lactulose 1.275 g mannitol 127.5 mL of H2O |
Overnight fast: NO, but administration after a fasting period of 4 h, after the evening meal. Administration of probe solution directly followed by drinking 240 mL of H2O |
0–24 h | LMR >0.015 | LMR: IBS-C 0.01 versus IBS-D 0.01 versus IBS-M 0.01 versus IBS-U 0.02 (p = 0.111) | IBS 28% |
51Cr-EDTA, chromium-51-ethylenediamine tetraacetic acid; C, Campylobacter; HV, healthy volunteers; IBS, irritable bowel syndrome; IBS-A, irritable bowel syndrome with alternating stool pattern; IBS-C, irritable bowel syndrome with constipation; IBS-D, irritable bowel syndrome with diarrhea; IBS-M, irritable bowel syndrome with mixed stool pattern; IBS-U, unsubtypted irritable bowel syndrome; La/Ara, lactose-to-L-arabinose ratio; LMR, lactulose to mannitol ratio; LRR, lactulose to L-rhamnose ratio; NA, not applicable; ND, not described; PEG, polyethylene glycol; PEGR, polyethylene glycol 400 to polyethylene glycol 3350 ratio; PI-IBS, post-infection irritable bowel syndrome; Ra/Ara, raffinose to L-arabinose ratio; SER, sucralose-to-erythritol ratio.
Urine collection times varied between 2 h and 24 h post administration of the probes (15/25 collected for up to 24 h). In early studies, a urine collection period of 2 or 3 h was considered to represent gastroduodenal permeability, 5–6 h as a marker for the small intestinal permeability and ⩾8 h for colonic permeability. However, Rao et al.45 observed that in healthy volunteers, 62% of the ingested liquid solution has already reached the colon at 2 h. In addition, alterations in intestinal transit like that seen in IBS-D and IBS-C will also affect the interpretation of the involved region in the GI tract. We believe that probe recovery after the first 2 h likely represents distal small bowel and colonic permeability, certainly in IBS-D patients. The migration and absorption of the probes throughout the GI lumen could be influenced by the intake of food or drinks.64 In the majority of studies, probe solution was ingested after an overnight fast. In two studies, however, urine was collected overnight after drinking the probe solution in the evening. Participants were not allowed to consume solid foods after the ingestion of the probe solution in 13 studies, whereas in 14 studies, no dietary restrictions were reported. Standardized meals were provided in only three studies. The regulations for the intake of water were also quite variable among the studies (restricted for 2–3 h, ad libitum or no limitations). In three studies, the intake of probes accompanied a caloric drink.
Permeability measurements
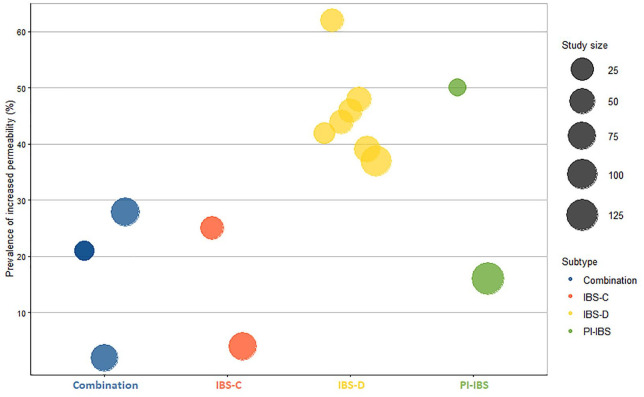
Fourteen studies used a “normal” cut-off value to determine the percentage of IBS patients with an increased intestinal permeability.33–36,38,40–42,44,51–53,57,58 These were either based on earlier experiments or newly recruited healthy controls. Cut-off values for the LMR ranged between 0.015 and 0.07.40,42,51,52 The prevalence of increased permeability in IBS was highly variable, with 2–62% of the IBS subjects showing increased intestinal permeability versus 0–15% in controls.33–36,38,40–42,44,51–53,57,58 An overview is shown in Figure 2. When assessing cumulative differences compared with controls, 14 studies using varying urine collection time points concluded increased intestinal permeability in IBS patients,35–39,43–45,48,50,53,58,59,63 while no differences were detected in eight studies.33,41,49,52,54–57 Remarkably, one study found a decreased colonic permeability in IBS patients.47
Figure 2.
Proportion of patients with increased in vivo permeability in the different IBS subtypes. IBS-D represented by the highest number of studies, which show a much higher proportion of patients (39–62%) with increased permeability. Larger studies tend to have a lower proportion of patients with increased permeability compared with smaller studies.*
*Only studies that reported a proportion of IBS patients with increased permeability were included in this figure. Combination, ⩾1 subtype.
IBS-C, constipation-predominant irritable bowel syndrome; IBS-D, diarrhea-predominant irritable bowel syndrome; PI-IBS, post-infection irritable bowel syndrome.
Assessing by IBS subtype, nine studies reported an increased permeability in IBS-D compared with healthy controls,37–39,43,45,50,53,58,63 whereas four studies did not.48,56,57,55 The prevalence of increased permeability ranged between 37% and 62%.38,40,42,51,53,57,58 In IBS-C, two studies39,44 found increased permeability compared with healthy controls, whereas five studies showed no group differences.37,48–50,54 Moreover, one study reported decreased gastroduodenal permeability, compared with controls.43 The prevalence of increased permeability ranged between 4% and 25% compared with ~9% in controls.44,51 Intestinal permeability was normal in one study investigating IBS-M patients.
Four studies focused on in vivo permeability in PI-IBS populations, all of which demonstrated increased permeability compared with controls.35/–37,48 A small study (n = 10) found that 50% of PI-IBS patients had increased permeability,35 while this dropped to 16% in a larger study from the Walkerton outbreak cohort (n = 132).36 The two other studies did not estimate the prevalence, but did show that cumulative small intestinal permeability was increased.37,48
Confocal laser endomicroscopy
Two studies used confocal laser endomicroscopy to visualize intercellular junctions in vivo in IBS patients.65,66 The epithelial gap density was increased in the ileum of both IBS-C and IBS-D patients.65 However, in the other study, no changes were seen in the rectosigmoid of IBS-D patients.66 More studies are needed to evaluate the usefulness of the technique in evaluating permeability in the context of IBS.
In summary, 9/13 IBS-D studies and anywhere from one-third to two-thirds of the patients demonstrate increased intestinal permeability. On the contrary, IBS-C patients likely do not have increased intestinal permeability as studies are either negative or the proportion of patients with increased intestinal permeability is not much different from the controls.
Permeability studies in pediatric populations
Four studies have been performed in pediatric IBS populations, all using saccharide probes (Table 2).67–70 Two of these studies also included children with functional abdominal pain (FAP), a functional GI disorder with abdominal pain but no bowel disturbances.67,68 As many as 59% of children with IBS or FAP had increased intestinal permeability (based on LMR cut-off <0.034).68 Shulman et al.67 found increased colonic permeability, but did not detect changes in gastric and small intestinal permeability in the IBS/FAP population compared with healthy controls. A subsequent study found that small intestinal and whole-gut permeability was changed in children with IBS but not FAP.70 Sex was found to have an effect on intestinal permeability in children with IBS.70 Girls but not boys had an increased sucrose recovery, a marker of gastric barrier function. Conversely, boys but not girls had an increased 0–3 h LMR, suggesting increased small intestinal permeability. Lastly, colonic permeability was also only increased in boys.70
Table 2.
Studies comparing in vivo permeability in pediatric IBS patients with healthy controls.
| Reference | Study population | Permeability probe | Dietary restrictions | Timing of urine collection | Results | |
|---|---|---|---|---|---|---|
| Comparison with healthy volunteers | Proportion of ↑ permeability | |||||
| Studies with a control population | ||||||
| Shulman et al.67 | IBS or FAP (n = 93) HV (n = 52) Age IBS: 8.2 ± 1.4 Age HV: 8.5 ± 1.3 |
12.75 g sucrose 6.375 g lactulose 1.275 g mannitol 1.275 g sucralose 127.5 mL of H2O |
Overnight fast: YES 240 mL of H2O directly after the probe ingestion, followed by 3 h of fasting |
0–3 h |
Sucrose: IBS/FAP 0.02% versus HV 0.02% (p = NS) Lactulose: IBS/FAP 0.10% versus HV 0.09% (p = NS) Mannitol: IBS/FAP 7.6% versus HV 7.6% (p = NS) Sucralose: IBS/FAP 0.4% versus HV 0.4% (p = NS) SCLR: IBS/FAP 0.6 versus HV 0.4 (p = 0.001) LMR: IBS/FAP 0.06 versus HV 0.07 (p = NS) SALR: IBS/FAP 1.0 versus HV 0.8 (p = 0.05) |
NA |
| Francavilla et al.68 | IBS or FAP (n = 54) HV (n = 55) Age IBS: 6.4 ± 2.0 Age HV: range 5–12 |
5 g lactulose 2 g mannitol 150 mL of H2O |
Overnight fast: YES Water allowed after 30 min, food not allowed |
0–5 h | LMR: IBS/FAP 0.04 versus HV 0.03 (p < 0.01) | IBS/FAP 59% |
| Gervasoni et al.69 | IBS-C (n = 2) IBS-D (n = 8) IBS-U (n = 5) HV (n = 10) Age IBS: range 5–16 Age HV: range 5–16 |
5 g lactulose 1 g mannitol 120 mL of H2O |
Overnight fast: YES | 0–6 h |
Lactulose: IBS versus HV (p = NS) Mannitol: IBS versus HV (p = NS) LMR: IBS 0.10 versus HV 0.01 (p < 0.05) LMR: IBS-D 1.2 versus IBS-C + IBS-U 0.5 (p < 0.05) |
NA |
| Shulman et al.70 | IBS (n = 95) FAP (n = 25) HV (n = 60) Age IBS: 9.4 ± 1.5 Age FAP: 9.2 ± 1.7 Age HV: 9.7 ± 1.6 |
10 g sucrose 5 g lactulose 1 g mannitol 1 g sucralose (in a capsule, due to taste) 127.5 mL of H2O |
Overnight fast: YES 240 mL of H2O directly after the probe ingestion |
0–3 h 3–24 h |
0–3 h
Sucrose: IBS 0.06% versus FAP 0.08% versus HV 0.04% (p = 0.49 overall, p = 0.646 for IBS versus FAP, p = 0.335 for IBS versus HV, p = 0.328 for FAP versus HV) Lactulose: IBS 0.2% versus FAP 0.2% versus HV 0.1% (p = 0.59) Mannitol: IBS 7.8% versus FAP 10.1% versus HV 9.3% (p = 0.05) LMR: IBS 0.12 versus FAP 0.08 versus HV 0.07 (p = 0.624 for IBS versus FAP, p = 0.023 for IBS versus HV, p = 0.178 for FAP versus HV) 0–24 h Lactulose: IBS 0.6% versus FAP 0.6% versus HV 0.5% (p = 0.52) Mannitol: IBS 22.1% versus FAP 24.6% versus HV 25.0% (p = 0.12) LMR: IBS 0.2 versus FAP 0.1 versus HV 0.1 (p = 0.538 for IBS versus FAP, p = 0.050 for IBS versus HV, p = 0.315 for FAP versus HV) Sucralose: IBS 1.8% versus FAP 1.4% versus HV 1.8% (p = 0.23 overall, p = 0.279 for IBS versus FAP, p = 0.248 for IBS versus HV, p = 0.045 for FAP versus HV) |
NA |
BW, bodyweight; FAP, functional abdominal pain; HV, healthy volunteers; IBS, irritable bowel syndrome; IBS-C, irritable bowel syndrome with constipation; IBS-D, irritable bowel syndrome with diarrhea; IBS-U, unsubtyped irritable bowel syndrome; LMR, lactulose-to-mannitol ratio; SALR, sucralose to lactulose ratio; SCLR, sucrose-to-lactulose ratio.
Two studies correlating intestinal permeability with symptoms found no significant associations with abdominal pain symptoms or stool characteristics.67,68 Of note, McOmber et al.71 showed that both siblings and parents of children with IBS had increased small bowel permeability, suggesting a possible role of genetic and/or environmental factors. At close to 60%, increased intestinal permeability may be more strongly associated in children with IBS than in adults. Additionally, it is clear that sex has an effect on barrier dysfunction in IBS in children, with males showing more prominent changes.
Ex vivo and in vitro measurement of gastrointestinal barrier function in adults
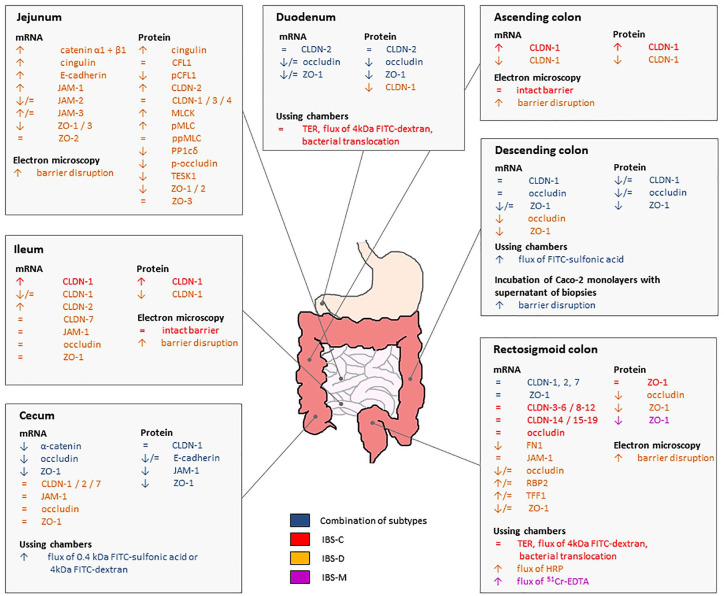
Thirty studies have assessed mucosal barrier properties using biopsy samples (12 IBS-D,38,63,66,72–80 two IBS-C,54,81 one IBS-M82 and 15 >1 IBS subtypes49,51,83–95). An overview of these studies is given in Table 3. Furthermore, the results are summarized in Figure 3.
Table 3.
Studies assessing in vitro or ex vivo gastrointestinal barrier function in adult IBS patients.
| Reference | Study population | Findings |
|---|---|---|
| Duodenum | ||
| Keszthelyi et al.49 | IBS-C (n = 5) IBS-D (n = 7) IBS-M(n = 3) HV (n = 15) |
- PCR: ↓ occludin, ZO-1 in IBS versus HV - Immunohistochemistry: ↓ occludin, ZO-1 in IBS versus HV |
| Zhou et al.51 | IBS-C (n = 74) IBS-D (n = 109) HV (n = 36) |
- PCR: ↑ mi-RNA-29a, mi-RNA-29b, mi-RNA-29c in IBS-D with ↑ permeability but not with = permeability or in IBS-C versus HV - Northern blot: ↑ mi-RNA-29a, mi-RNA-29b in IBS-D with ↑ permeability but not with = permeability versus HV - Immunoblot: ↓ CLDN-1, NKRF in IBS-D with ↑ permeability but not with = permeability versus HV |
| Peters et al.54 | IBS-C (n = 19) HV (n = 18) |
- Ussing chambers: = TER, flux of 4kDa FITC-dextran and translocation of E. coli in IBS-C versus HV |
| Fritscher-Ravens et al.95 | IBS-C (n = 14) IBS-D(n = 52) IBS-M (n = 42) HV (n = 14) |
- PCR: = CLDN-2, occludin, ZO-1 in IBS versus HV - Immunohistochemistry: ↓ occludin, = CLDN-2 in IBS versus HV |
| Jejunum | ||
| Martínez et al.72 | IBS-D (n = 25) HV (n = 23) |
- RNA microarray + IPA: tight junction signaling pathways are associated with IBS-D versus HV - PCR: ↓ ZO-1, ZO-3, = ZO-2 in IBS-D versus HV - Immunofluorescence: ↓ ZO-1, ZO-2, = ZO-3 in IBS-D versus HV |
| Martínez et al.73 | IBS-D (n = 45) HV (n = 30) |
- Immunofluorescence: ↑ MLCK, pMLC, ↓ PP1cδ, = ppMLC in IBS-D versus HV - Immunofluorescence: ↑ occludin staining in cytoplasm, ↓ occludin staining at tight junction complexes in IBS-D versus HV - Western blot: ↑ CLDN-2, ↓ p-occludin, = CLDN-1, CLDN-3, CLDN-4, occludin in IBS-D versus HV - EM: ↑ apical intercellular distance, proportion of dilated junctions, percentage of junctions with perijunctional cytoskeleton condensation |
| Martínez et al.74 | IBS-D (n = 43) HV (n = 26) |
- mRNA sequencing (exploration cohort): ↑ E-cadherin, catenin α1 + β1, cingulin, JAM-1, JAM-3, ↓ JAM-2 in IBS-D versus HV - nCounter RNA sequencing (validation cohort): ↑ E-cadherin, catenin α1 + β1, cingulin, JAM-1, = JAM-2, JAM-3 - mRNA sequencing + IPA: ↑ tight junction signaling, caveolar-mediated endocytosis signaling, actin cytoskeleton signaling, epithelial adherens junction signaling - PCR: ↓ has-miRNA-125b-5p, has-miRNA-16-5p in IBS-D versus HV - Western blot: ↑ cingulin, CLDN-2 in IBS-D versus HV |
| Rodiño-Janeiro et al.75 | IBS-D (n = 15) HV (n = 16) |
- Proteomics: ↓ pCFL1, TESK1, = CFL1 in IBS-D versus HV - Proteomics + IPA: alterations in actin cytoskeleton function, clathrin-mediated endocytosis signaling, actin cytoskeleton signaling, caveolar-mediated endocytosis signaling and integrin signaling |
| Ileum | ||
| Turcotte et al.65 | IBS-C (n = 4) IBS-D (n = 12) HV (n = 18) |
- CLE: ↑ epithelial gap density in IBS versus HV |
| Cheng et al.83 | IBS-C (n = 30) IBS-D (n = 33) HV (n = 30) |
- PCR: ↑ CLDN-1 in IBS-C versus HV, ↓ CLDN-1 in IBS-D versus HV - Western blot: ↑ CLDN-1 in IBS-C versus HV, ↓ CLDN-1 in IBS-D versus HV - Immunohistochemistry: ↑ CLDN-1 in IBS-C versus HV, ↓ CLDN-1 in IBS-D versus HV - EM: ↑ mucus secretion, mucus bubble fusion in goblet cells, tracer extravasation in IBS-C and IBS-D versus HV, ↑ width of gaps between tight junctions in 70% of patients in IBS-D versus HV, = intercellular tight junction structures in IBS-C versus HV |
| Ishimoto et al.76 | IBS-D (n = 17) HV (n = 20) |
- PCR: ↑ CLDN-2, = CLDN-1, CLDN-7, JAM-1, occludin, ZO-1 in IBS-D versus HV |
| Cecum | ||
| Vivinus-Nébot et al.84 | IBS-C (n = 10) IBS-D (n = 13) IBS-M (n = 11) HV (n = 15) |
- Ussing chambers: ↑ flux of 4kDa FITC-dextran in IBS versus HV |
| Wilcz-Villega et al.85 | IBS-A (n = 12) IBS-D(n = 22) HV (n = 12) |
- Immunofluorescence: ↓ JAM-1 in IBS versus HV |
| Vivinus-Nébot et al.86 | IBS-C (n = 15) IBS-D (n = 18) IBS-M (n = 18) HV (n = 27) |
- Ussing chambers: ↑ flux of 0.4 kDa FITC-sulfonic acid in IBS versus HV - PCR: ↓ α-catenin, occludin, ZO-1 in IBS versus HV |
| Wilcz-Villega et al.87 | IBS-A (n = 12) IBS-D (n = 24) HV (n = 12) |
- Immunofluorescence: ↓ E-cadherin, ZO-1, = CLDN-1 in IBS versus HV - Immunohistochemistry: = E-cadherin in IBS versus HV |
| Ishimoto et al.76 | IBS-D (n = 17) HV (n = 20) |
- PCR: = CLDN-1, CLDN-2, CLDN-7, JAM-1, occludin, ZO-1 in IBS-D versus HV |
| Ascending colon | ||
| Cheng et al.83 | IBS-C (n = 30) IBS-D (n = 33) HV (n = 30) |
- PCR: ↑ CLDN-1 in IBS-C versus HV, ↓ CLDN-1 in IBS-D versus HV - Western blot: ↑ CLDN-1 in IBS-C versus HV, ↓ CLDN-1 in IBS-D versus HV - Immunohistochemistry: ↑ CLDN-1 in IBS-C versus HV, ↓ CLDN-1 in IBS-D versus HV - EM: ↑ mucus secretion, mucus bubble fusion in goblet cells, tracer extravasation in IBS-C and IBS-D versus HV, ↑ width of gaps between tight junctions in 80% of patients in IBS-D versus HV, = intercellular tight junction structures in IBS-C versus HV |
| Descending colon | ||
| Piche et al.88 | IBS-A (n = 5) IBS-C (n = 3) IBS-D (n = 4) HV (n = 5) |
- Ussing chambers: ↑ flux of FITC-sulfonic acid in IBS versus HV - PCR: ↓ ZO-1, = occludin in IBS versus HV - Caco-2 cell monolayers incubated with biopsy supernatant: ↑ flux of 4kDa FITC-dextran, ↓ TER in IBS versus HV - Caco-2 cell monolayers incubated with biopsy supernatant + PCR: ↓ ZO-1, = occludin in IBS versus HV |
| Coëffier et al.89 | IBS-A (n = 4) IBS-C (n = 8) IBS-D (n = 13) HV (n = 18) |
- PCR: = occludin in IBS versus HV - Western blot: ↓ occludin in IBS versus HV |
| Bertiaux-Vandaele et al.90 | IBS-A (n = 15) IBS-C (n = 14) IBS-D (n = 19) HV (n = 33) |
- PCR: = CLDN-1, occludin, ZO-1 in IBS versus HV - Western blot: ↓ CLDN-1, occludin, ZO-1 in IBS-D versus HV, ↓ ZO-1, = CLDN-1, occludin in IBS-A and IBS-C versus HV |
| Vazquez-Roque et al.63 | IBS-D (n = 25) HV (n = 16) |
- PCR: ↓ occludin, ZO-1, = CLDN-1 in IBS-D versus HV |
| Barbaro et al.94 | IBS-C (n = 8) IBS-D (n = 9) IBS-M (n = 11) HV (n = 7) |
- Caco-2 cell monolayers incubated with biopsy supernatant: ↑ flux of FITC-sulfonic acid in IBS versus HV |
| Rectosigmoid colon | ||
| Zeng et al.38 | IBS-D (n = 30) HV (n = 12) |
- PCR: ↓ occludin, ZO-1 in IBS-D versus HV - EM: Staining of junctional complexes among colonic enterocytes was faint and discontinuous in 33% of IBS-D, compared with HV |
| Lee et al.77 | IBS-D (n = 20) HV (n = 30) |
- Ussing chambers: ↑ flux of HRP in IBS-D versus HV |
| Lee et al.78 | IBS-D (n = 16) HV (n = 7) |
- Ussing chambers: ↑ flux of HRP in IBS-D versus HV |
| Camilleri et al.80 | IBS-D (n = 9) HV (n = 9) |
- RNA sequencing: ↑ RBP2, TFF1, ↓ FN1, WDR72, = CLDN-1, MMP1, MUC20, occludin, ZO-1 in IBS-D versus HV - PCR: ↓ FN1, = CLDN-1 occludin, RBP2, TFF1, ZO-1 in IBS-D versus HV |
| Camilleri et al.93 | IBS-C (n = 10) IBS-D (n = 47) HV (n = 17) |
- RNA sequencing: ↓ CLDN-1, FN1, = ZO-1, OCLN, RBP2, TFF1 in IBS-D versus HV, ↓ OCLN, = ZO-1, CLDN-1, RBP2, FN1, TFF1 in IBS-C versus HV |
| Zhen et al.79 | IBS-D (n = 42) HV (n = 20) |
- Western blot: ↓ occludin in IBS-D versus HV - Immunohistochemistry: staining of occludin was faint and discontinuous in IBS-D versus HV |
| Ishimoto et al.76 | IBS-D (n = 17) HV (n = 20) |
- PCR: = CLDN-1, CLDN-2, CLDN-7, JAM-1, occludin, ZO-1 in IBS-D versus HV |
| Peters et al.54 | IBS-C (n = 19) HV (n = 18) |
- Ussing chambers: = TER, flux of 4kDa FITC-dextran and translocation of E. coli in IBS-C versus HV - PCR: = CLDN-1, CLDN-2, CLDN-3, CLDN-4, CLDN-5, CLDN-6, CLDN-7, CLDN-8, CLDN-9, CLDN-10, CLDN-11, CLDN-12, CLDN-14, CLDN-15, CLDN-16, CLDN-17, CLDN-18, CLDN-19, occludin, ZO-1, ZO-2, ZO-3 in IBS-C versus HV |
| Videlock et al.92 | IBS-C (n = 10) IBS-D (n = 10) HV (n = 10) |
- Microarray profiling analysis: 1270 DETs for IBS-C versus HV (↑MUC-20, ↓ MYLK2, WDR72), no DETs meeting FDR <0.05 in IBS versus HV or IBS-D versus HV (= FN1, OCLN, TFF1, TJP1) - WGCNA: ↓ cell junction module in IBS-D but not IBS-C versus HV |
| Lee et al.96 | IBS-C (n = 33) IBS-D (n = 21) IBS-M (n = 5) HV (n = 36) |
- PCR: ↓ ZO-1 in females but not males, = CLDN-1, occludin in IBS-D versus HV (no differences in IBS-C and IBS-M) - Western blot: ↓ ZO-1 in IBS-D and IBS-M but not IBS-C versus HV |
| Zhao et al.66 | IBS-D (n = 10) HV (n = 10) |
- CLE: No differences in epithelial architecture, no fluorescein leakage into the lumen in IBS-D versus HV - EM: ↑ apical intercellular distance, percentage of dilated intercellular junctions, dilatation and destruction of adherens junctions and desmosomes in IBS-D versus HV |
| Katinios et al.82 | IBS-M (n = 15) HV (n = 15) |
- Ussing chambers: ↓ TER, ↑ flux of 51Cr-EDTA in IBS-M versus HV |
| Colon – unspecified location | ||
| Annaházi et al.81 | IBS-C (n = 14) HV (n = 33) |
- Western blot: ↓ occludin in IBS-C versus HV |
| Zhou et al.51 | IBS-C (n = 74) IBS-D (n = 109) HV (n = 36) |
- PCR: ↑ mi-RNA-29a, mi-RNA-29b with ↑ permeability but not with = permeability or in IBS-C versus HV, = mi-RNA-29c in IBS-D versus HV - Northern blot: ↑ mi-RNA-29a, mi-RNA-29b in IBS-D with ↑ permeability but not with = permeability versus HV - Western blot: ↓ CLDN-1, NKRF in IBS-D with ↑ permeability but not with = permeability versus HV - Immunoblot: ↓ CLDN-1, NKRF in IBS-D with ↑ permeability but not with = permeability versus HV |
| Tulic et al.97 | IBS (n = 8) HV (n = 6) |
- Ussing chambers: ↑ flux of FITC-sulfonic acid in IBS versus HV |
| Bednarska et al.91 | IBS-C (n = 8) IBS-D (n = 8) IBS-M (n = 21) HV (n = 15) |
- Ussing chambers: ↑ flux of 51Cr-EDTA and translocation of bacteria, ↓ TER after 0-30-60 but not 90 min in IBS versus HV - Immunofluorescence: = occludin, ZO-1 in IBS versus HV |
51Cr-EDTA chromium-51-ethylenediamine tetraacetic acid; CFL1, cofilin 1; CLDN, claudin; CLE, confocal laser endomicroscopy; DET, differentially expressed transcript; E, Escherichia; EM, electron microscopy; FDR, false discovery rate; FITC, fluorescein isothiocyanate; FN1, fibronectin-1; HRP, horseradish peroxidase; HV, healthy volunteers; IBS, irritable bowel syndrome; IBS-A, irritable bowel syndrome with alternating stool pattern; IBS-C, irritable bowel syndrome with constipation; IBS-D, irritable bowel syndrome with diarrhea; IBS-M, irritable bowel syndrome with mixed stool pattern; IPA, ingenuity pathways analysis; JAM, junctional adhesion molecule; MLCK, myosin light chain kinase; MMP1, matrix metalloprotease-1; mRNA, messenger ribonucleic acid; MUC20, mucin 20; MYLK2, myosin light chain kinase 2; NKRF, NF-kappa-β repressing factor; OCLN, occludin isof. B precursor; pCFL1, phosphorylated cofilin 1; PCR, polymerase chain reaction; pMLC, phosphorylated myosin light chain; p-occludin, phosphorylated occludin; PP1cδ, protein phosphatase 1 catalytic subunit delta; ppMLC, di-phosphorylated myosin light chain; RBP2, retinoblastoma binding protein 2; RNA, ribonucleic acid; TER, transepithelial electrical resistance; TESK1, testis-associated actin remodeling kinase 1; TFF1, trefoil factor 1; TJP1, tight junction protein ZO-1 isof. A; WDR72, WD repeat domain 72; WGCNA, weighted gene coexpression network analysis; ZO, zona occludens.
Figure 3.
Overview of in vitro and ex vivo barrier function changes in the different parts of the gastrointestinal tract of IBS patients. Most studies are available from the rectosigmoid colon, jejunum and the cecum and studied an IBS-D population. There is a significant heterogeneity in the target proteins assessed and the methodology used for determination of changes in vitro and ex vivo.*
*Results of studies that did not specify the exact colonic region where biopsies were taken were not included in this figure, but are discussed in the main text.
Functional studies
Nine studies assessed barrier function of biopsy samples in Ussing chambers (one IBS-C,54 two IBS-D,77,78 one IBS-M,82 four multiple subtypes84,86,88,91 and one without subtyping97). In the single IBS-C study, transepithelial electrical resistance measurements and the flux of probes across the mucosal biopsy were similar to controls in both the duodenum and the rectosigmoid colon.54 The eight other studies all reported increased permeability in IBS patients compared with controls in biopsies taken in different locations along the GI tract.77,78,82,84,86,88,91,97 In biopsies of the cecum84,86 and the descending colon,88 paracellular flux of FITC-labeled probes (0.4–4 kDa) was higher in IBS patients compared with healthy controls. As opposed to the study in IBS-C, the intestinal barrier was disrupted in the rectosigmoid colon of patients with IBS-D77,78 and IBS-M.82 Interestingly, one study noted increased translocation of E. coli and S. typhimurium across the epithelium, indicating that paracellular as well as transcellular transport might be affected in IBS.91 Taken together, these studies suggest a functional disruption of the intestinal barrier in IBS patients, especially for the IBS-D and IBS-M subtypes. Although studies with mixed populations do not report on differences between IBS-C and the other subtypes, the negative studies in IBS-C likely indicate that permeability disturbances might be less relevant in this subtype.
Molecular and structural studies
Duodenum
Three studies (all multiple subtypes) evaluated duodenal epithelial barrier.49,51,95 Occludin and ZO-1 protein expression were decreased but CLDN-2 expression was unchanged in study populations consisting of multiple IBS subtypes.49,95 Furthermore, CLDN-1 expression was decreased in IBS-D patients, compared with healthy controls.51 Hence, duodenal barrier function seems perturbed in IBS, although potential differences between subtypes require further exploration.
Jejunum
Four studies (all IBS-D) examined the jejunal mucosal barrier, all of which provided evidence for a disrupted epithelial barrier.72–75 Transcriptomic studies described changes in tight junction and adherens junction signaling pathways,72,98 while protein assessment revealed alterations in actin–cytoskeleton function and signaling.75 An up-regulation of E-cadherin, catenin α1 and β1 and cingulin was seen.74 The up-regulation of CLDN-2 but not CLDN-1, CLDN-3 and CLDN-4 was noted in two other studies.73,74 Furthermore, contraction of the peri-junctional actinmyosin ring was seen in one study.73 Several molecular targets within the jejunum that are important for maintaining proper barrier function appear to be affected in IBS patients.
Terminal ileum
Two studies investigated changes in the terminal ileum.76,83 Electron microscopy showed increased mucus secretion and larger intercellular gaps in IBS-D.83 Furthermore, CLDN-2 was up-regulated, while the expression of CLDN-7 and other regulatory tight junction molecules such as occludin, junctional adhesion molecule (JAM)-1 and ZO-1 was similar to controls.76 One study found a decreased expression of CLDN-1 in IBS-D,83 whereas the other study did not.76 In IBS-C, CLDN-1 was up-regulated and electronic microscopy showed intact tight junction complexes.83 Collectively, tight junction complexes in the terminal ileum are predominantly altered in IBS-D but unchanged in IBS-C.
Cecum
Four studies (one IBS-D, three multiple subtypes) determined changes in the cecum. Three of these found a disrupted barrier,85–87 whereas one did not.76 The expression of JAM-1 and E-cadherin was decreased in IBS-A and IBS-D, compared with healthy controls.85,87 However, a study by Ishimoto and colleagues did not find any changes in the expression of tight junction molecules in IBS-D.76 Paracellular permeability in the cecum is disrupted in IBS, but the associated molecular changes require further exploration.
Ascending colon
Only one study focused on the epithelial barrier in the ascending colon,83 and found widened intercellular gaps in 80% of IBS-D patients and a decreased expression of CLDN-1 compared with controls. There was no evidence for disrupted tight junctions in IBS-C. However, mucus secretion in IBS-C was impaired, suggesting the mucus barrier could be dysfunctional.83
Descending colon
Five studies (one IBS-D, four mixed) investigated the descending colon, all of which found evidence for a disrupted barrier.63,88–90,94 Exposure of Caco-2 monolayers to biopsy supernatant of IBS patients increased flux of probes.88,94 Furthermore, two studies found a decreased protein expression of occludin in colonic biopsies of IBS patients,89,90 whereas differences in mRNA expression were conflicting across studies (for details, refer to Table 3).63,89,90 Taken together, these studies clearly indicate the presence of increased intestinal permeability in the descending colon, although it remains unclear if differences exist between IBS subtypes.
Rectosigmoid colon
Being most easily accessible, intestinal barrier function in the rectosigmoid colon is most extensively described. Eight studies included an IBS-D population,38,66,76,79,80,92,93,96 four IBS-C54,92,93,96 and one IBS-M.96 Seven of the ten studies found barrier dysfunction in IBS-D patients.38,66,77–80,96 One found increased apical intercellular distances, as well as destruction of adherens junctions and desmosomes.66 Evidence about the specific molecular changes involved is conflicting, but suggests a role for both occludin and ZO-1 (Table 3).38,76,79,80,92,93,96 Five out of six studies examining the expression of several tight junction molecules found at least one significant change compared with healthy controls.38,79,80,93,96 A study by Lee et al.96 demonstrated that the differences in barrier function of IBS patients could be gender related, since they observed alterations in the expression of ZO-1 in females but not in males. Two studies in IBS-C did not find any alterations in the expression of tight junction proteins.54,96 However, two other studies revealed alterations in the tight junction genes in IBS-C patients (Table 3).92,93 Katinios and colleagues demonstrated an increased paracellular flux in sigmoid biopsies in IBS-M compared with controls.82 Although mRNA levels of tight junction molecules were similar to those in healthy controls, ZO-1 protein expression was decreased in a small study in IBS-M patients (n = 5).96
The wealth of studies specifically examining the rectosigmoid colon has outlined that barrier function is mainly impaired in IBS-D and IBS-M, but not in IBS-C. This assertion, however, needs context as there is limited literature for IBS-C and IBS-M studies.
Colon (unidentified site)
Three studies (one IBS-C and two multiple subtypes) investigated colonic permeability without presenting the sampling site in the colon,51,81,91 two of which found changes in the expression of the tight junction molecules occludin and CLDN-1.51,81 However, Bednarska and colleagues did not observe differences in the expression of occludin and ZO-1, even though they noted alterations in barrier function Ussing chamber experiments.91
Broadly, the rectosigmoid and descending colon are the most studied parts of the bowel and although preponderance of IBS-D studies demonstrates evidence of barrier dysfunction, the tight junction proteins involved and location along the GI tract are highly variable. Longitudinal sampling of the GI tract in the same individual may be able to provide a more complete understanding of barrier dysfunction in IBS. An example of such an attempt was duodenal and colonic assessment in IBS-C in our study, which showed unchanged barrier at both sites.54 Furthermore, it should be noted that a change in the tight junction gene or protein expression or the ultrastructure does not necessarily imply an impaired barrier function.
Studies involving fecal samples
An overview of studies using IBS fecal samples to study the effects on permeability using a combination of in vitro and in vivo approaches is presented in Table 4.81,99–104
Table 4.
Studies assessing effects of fecal slurries or fecal supernatant from adult IBS patients on barrier function in immortalized cell monolayers, organoids, intestinal tissue from rodents or germ-free mice.
| Reference | Study population | Model | Findings |
|---|---|---|---|
| In vitro: cell cultures | |||
| Annaházi et al.81 | IBS-C, HV | In vitro assay | - Recombinant occludin degradation assay: ↑ occludin degradation by FSN from IBS-C versus HV |
| Annaházi et al.81 | IBS-C, HV | T84 cell monolayer | - In vitro permeability: ↑ flux of 4kDa FITC-dextran in monolayers exposed to FSN from IBS-C versus HV |
| Edogawa et al.102 | IBS with high FPA, IBS with low FPA | Caco-2 cell monolayer | - In vitro permeability: ↓ TER, ↑ flux of 4kDa Texas Red dextran in monolayers exposed to FSN from IBS with high FPA versus IBS with low FPA - Western blot + immunofluorescence: ↓ occludin, ↑ pMLC/MLC and co-localization with phalloidin, internalization of CLDN-2 in monolayers exposed to FSN from IBS with high FPA versus IBS with low FPA |
| Han et al.103 | IBS-D, HV | Human colonoids | - In vitro permeability: ↓ retention of injected 4kDa FITC-dextran in colonoids exposed to FSN from IBS-D versus HV |
| In vivo: colonic infusion of FSN in mice | |||
| Gecse et al.99 | IBS-A, IBS-C, IBS-D, HV | C57BL/6J mice | - Ussing chambers: ↑ flux of 4kDa FITC-dextran in IBS-D versus HV but not IBS-C or IBS-A versus HV - Western blot: ↑ pMLC in IBS-D versus HV - Immunohistochemistry: pronounced and diffuse labeling of pMLC in epithelial cells and ZO-1 staining in the intracellular compartment, suggesting intensive internalization in IBS-D versus HV |
| Annaházi et al.81 | IBS-C, HV | C57BL/6J mice | - In vivo probes: ↑ uptake of 4kDa FITC-dextran in the blood after 4 h but not 1 h in mice exposed to FSN from IBS-C versus HV - Western blot: ↓ occludin expression in colon from mice exposed to FSN from IBS-C versus HV |
| Nébot-Vivinus et al.104 | IBS, HV | C57BL/6 mice | - In vivo probes: ↑ excretion of 51Cr-EDTA via urine in mice exposed to FSN from IBS versus HV |
| In vivo: humanized rodent models | |||
| Crouzet et al.100 | IBS-C, HV | Humanized germ-free Fisher 344 albino rats | - In vivo probes: no difference in excretion of 51Cr-EDTA via urine in rats humanized with stool from IBS-C versus HV |
| De Palma et al.101 | IBS-D, HV | Humanized germ-free Swiss mice | - Ussing chambers: ↑ flux of 51Cr-EDTA in colon tissue but = flux in jejunal tissue of mice humanized with stool from IBS-D versus HV |
| Edogawa et al.102 | IBS with high FPA, IBS with low FPA, HV | Humanized germ-free Swiss Webster mice | - In vivo probes: ↑ uptake of creatinine in the blood in mice humanized with stool from IBS with high FPA versus HV, no differences in uptake of 4 kDa FITC-dextran and 70 kDa rhodamine-dextran |
51Cr-EDTA chromium-51-ethylenediamine tetraacetic acid; FITC-dextran, fluorescein isothiocyanate dextran; FPA, fecal proteolytic activity; FSN, fecal supernatant; HV, healthy volunteers; IBS, irritable bowel syndrome; IBS-A, irritable bowel syndrome with alternating stool pattern; IBS-C, irritable bowel syndrome with constipation; IBS-D, irritable bowel syndrome with diarrhea; pMLC, phosphorylated myosin light chain; ZO-1, zona occludens-1.
In vitro: cell cultures
T84 monolayers incubated with fecal supernatant (FSN) of IBS-C patients had an increased flux of 4kDa FITC-dextran and a loss of occludin as compared with supernatants from healthy controls.81 Edogawa et al.102 demonstrated that FSN from IBS patients (predominantly PI-IBS) with a high fecal proteolytic activity increased paracellular permeability in Caco-2 cell monolayers, compared with FSN of patients with a low fecal proteolytic activity. Additionally, high proteolytic activity-exposed monolayers had a lower expression of occludin and internalization of claudin-2, indicating likely involvement of both leak and pore pathways. Lastly, colonoids exposed to FSN of IBS-D patients were more permeable to 4kDa FITC-dextran than those exposed to FSN of healthy controls.103
In vivo: colonic infusion of FSN in mice
Infusion of FSN of IBS patients in the colon of mice resulted in an increased urinary excretion of 51Cr-EDTA.104 Another study demonstrated that mouse colonic epithelium exposed to FSN of IBS-D patients was more permeable to FITC-dextran in Ussing chambers, whereas epithelium exposed to FSN of IBS-C or IBS-M patients was not.99 In contrast, FSN of IBS-C patients increased colonic flux to FITC-dextran in a study by Annaházi and colleagues.81 At a molecular level, decreased colonic expression of occludin was also seen.81 Furthermore, ZO-1 immunostaining showed intracellular internalization in response to IBS supernatants, which has been associated with weakening of the barrier.99 Finally, colonic infusion of FSN resulted in an increase in the expression of phosphorylated myosin light chain, which has also been linked to a loss of barrier integrity.99
In vivo: humanized rodent models
Three studies have investigated the role of the microbiome in regulating intestinal permeability by gavaging human fecal slurry in germ-free rodents.100–102 Three to six weeks later, barrier function was quantified. Urine excretion of orally administered 51Cr-EDTA was unchanged in rats humanized with the fecal slurry from IBS-C patients. Although the gastrointestinal barrier was intact, these rats did display signs of visceral hypersensitivity.100 In contrast, De Palma et al.101 detected a disrupted in vitro colonic but not jejunal barrier following gavage with fecal slurry of IBS-D patients. In mice that were humanized with stool from IBS patients with a high proteolytic activity, creatinine but not 4kDa FITC-dextran or 70 kDa rhodamine-dextran crossed the barrier at a higher rate in vivo.102 Proteolytic activity seems to be one of the important factors driving barrier dysfunction, since mice humanized with stool from an IBS patient with a low fecal proteolytic activity had an intact barrier similar to healthy volunteers.102
Collectively, in vitro cell culture and in vivo models using rodents have shed light on the potential mechanisms underlying barrier disruption in IBS patients. Again, greater evidence is available for IBS-D mediators to affect barrier compared with IBS-C and suggests an effect of proteases in mediation of barrier dysfunction by the luminal contents.
Barrier dysfunction and IBS symptomatology
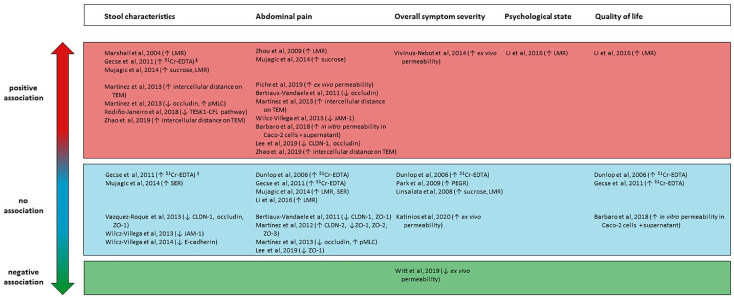
Association with abdominal pain
One study found a modest but significant relationship between an increased in vivo intestinal permeability and severity of abdominal pain (Figure 4).50 Zhou et al.40 found that an increased LMR was associated with somatic hypersensitivity in response to thermal stimulation as well as visceral hypersensitivity to rectal distension. However, three other studies did not find correlations between abdominal pain and in vivo permeability.37,43,53 In a small study visualizing the colonic mucosa ultrastructurally, intercellular gaps correlated with the frequency of abdominal pain66 and similar findings were noted in jejunal73 and colonic mucosa.88 Lastly, in vitro barrier changes caused by colonic biopsy supernatants associated with both the severity and the frequency of abdominal pain.94 The colonic expression of the tight junction molecules CLDN-1, ZO-1 and occludin was correlated with abdominal pain, although only the occludin expression remained significant in a multivariate analysis.90 In a recent study, colonic mRNA expression of occludin and CLDN-1 showed a threefold and tenfold decrease in the patients experiencing more pain.96 A lower cecal expression of JAM-1 was shown to be associated with more severe abdominal pain in IBS-M but not IBS-D patients.85 However, CLDN-1 and ZO-1 expression in the cecum were not correlated with abdominal pain.87 Furthermore, changes in zonula occludens 1–3, CLDN-2, occludin or pMLC expression in the jejunum could not be linked to either the intensity or the frequency of abdominal pain.72,73
Figure 4.
Overview of studies reporting associations between barrier function and stool characteristics, abdominal pain, overall symptom severity, psychological functioning and quality of life in IBS patients. A positive association (red color) indicates study concluded barrier dysfunction to be positively correlated with a more severe symptomatology in IBS patients versus no correlation (blue color) versus a negative correlation (green color).§
§Gecse and colleagues found an association between an increased intestinal permeability and stool frequency, but no association between stool consistency and increased intestinal permeability.
51Cr-EDTA, 51Cr-EDTA, chromium-51-ethylenediamine tetraacetic acid; CFL, cofilin; CLDN, claudin; JAM-1, junctional adhesion molecule 1; LMR, lactulose-to-mannitol ratio; PEGR, polyethylene glycol 400 to polyethylene glycol 3350 ratio; pMLC, phosphorylated myosin light chain; SER, sucralose-to-erythritol ratio; TEM, transmission electron microscopy; TESK1, testis-associated actin remodeling kinase 1; ZO, zona occludens.
Studies based on in vivo permeability have provided mixed results for the association with abdominal pain. However, ultrastructural as well as gene expression studies provide a more consistent association of barrier dysfunction with abdominal pain. Additional work needs to be done to better understand how changes in tight junction proteins mediate visceral pain in IBS.
Associations with stool characteristics
A positive correlation was found between the severity of diarrhea and both gastroduodenal and small intestinal permeability.50 Stool frequency, but not stool consistency, was found to associate with whole-gut permeability in PI-IBS and colonic permeability in IBS-D patients.36,43 Ultrastructural disruption of tight junctions in jejunum and colorectum of IBS-D patients correlated with both greater stool frequency and looser consistency.66,73 Furthermore, jejunal expression of occludin and pMLC correlated with the severity of diarrhea in IBS-D.73 In female IBS-D patients, there was a downregulation of the TESK1/CFL pathway in the jejunum, which is involved in regulating cytoskeleton dynamics associated with bowel movements.75 No significant correlations between stool characteristics and cecal expression of E-cadherin or JAM-1 could be detected85,87 or with duodenal or colonic expression of ZO-1, claudin-1 and occludin in another study.46 Although results were mixed, a greater number of studies associate small bowel changes with stool frequency.
Associations with overall severity scores
Vivinus-Nébot et al.86 found a moderate positive correlation between IBS symptom severity and cecal paracellular permeability of IBS-C, IBS-D and IBS-M patients. However, an opposite result was found by Witt et al.,105 who found a negative correlation between IBS severity scores and colonic paracellular permeability. Four other studies did not detect significant correlations between the overall symptom severity and barrier disruption, regardless of the IBS subtype.37,39,57,82 IBS symptom severity is a composite measure of pain and bowel dissatisfaction which is probably driven by multiple factors, and changes in intestinal permeability may only partially drive the overall symptom severity.
Association with psychological functioning
IBS-D patients with an increased permeability scored higher on the Hospital Anxiety and Depression Scale for both anxiety and depression subscales.53 In another study, the effect of anxiety and depression symptoms on barrier function was found to be small and not statistically significant.50 Taken together, these studies suggest a potential for cross-talk between gut barrier function and psychological stress in IBS but also a lack of conclusive evidence for the same hypothesis.
Association with quality of life
One study associated an increased intestinal permeability with a decrease in QoL,53 whereas three did not.37,43,94 Li and colleagues observed a lower QoL in the subgroup of IBS-D patients with increased small intestinal permeability.53 However, no significant correlations between intestinal permeability in IBS-C or PI-IBS patients and items from the IBS QoL questionnaire were found in a small study.37 This was subsequently shown in other IBS subtypes as well.43,94 Thus, there is overall weak evidence for permeability to explain overall QoL. This is not unexpected considering the complex and heterogeneous nature of the disease and the fact that only a variable subset of IBS patients have increased permeability, which likely results in weaker correlations for composite measures like symptom severity and QoL.
Discussion
This review provides several useful insights into barrier dysfunction in IBS. First, this is the largest systematic review and the first to be performed using PRISMA guidelines (includes 67 studies). Second, the criteria used to diagnose and subtype IBS were critically assessed in relation to the changes in the barrier function. Third, we comprehensively assessed methodologies (in vivo, ex vivo and in vitro) used to measure barrier function while also stratifying the studies according to the location along the GI tract. Fourth, barrier changes were associated with IBS symptomatology (abdominal pain, stool characteristics, symptom severity), psychological comorbidities and QoL. Finally, we included pediatric studies which, although limited in number, provide a reflection of barrier dysfunction in that population.
Increased intestinal permeability was present in 37–62% of IBS-D and 16–50% of PI-IBS patients. More IBS-C studies showed unchanged permeability compared with controls, and the ones showing increased permeability had smaller proportions of patients with increased permeability (4–25%). Unfortunately, the prevalence of barrier disruption in IBS-M remains unclear. Another important finding is that changes in the expression of tight junction genes or the ultrastructure were not specific to any particular region of the intestinal tract. However, only a limited number of studies examined different regions in the bowel.51,54,76,83 In three studies, findings were consistent across the different regions,51,76,83 whereas one study detected an increased expression of CLDN-2 in the ileum, but not in the cecum or the rectosigmoid colon.76 The mucosal and luminal milieu, microbiome106,107 and motility108,109 exhibit biological differences both spatially as well as between individuals. This makes exploration of different regions of the gut within the same volunteers essential to comprehensively understand changes in mucosal barrier function.
Demographic factors can influence barrier function as well. A recent study found that elderly IBS patients have greater disruption of small intestinal barrier compared with their younger counterparts.110 Another study, however, found no effect of adjusting for age on permeability changes in IBS-D.50 Furthermore, the expression of several tight junction (occludin, ZO-1 and CLDN-1) and adherens junction (JAM-1 and E-cadherin) molecules was not correlated with age in IBS patients.49,85,87,90 There is an established female predominance in IBS. Some studies in healthy volunteers found a lower intestinal permeability in females than males,111–113 although this was not confirmed by others.114–116 In IBS patients, sex differences in barrier function were noted.36,50,70,96 Sucrose excretion in IBS males was higher than females, indicating increased gastroduodenal permeability in males.50 Furthermore, in the Walkerton cohort, IBS males had higher permeability.36 In contrast with these findings, ZO-1 expression was decreased in female IBS-D patients, whereas this was unchanged in males.96 Other in vitro studies did not find any sex differences in tight junction gene expression in the duodenum, cecum and descending colon.49,85,87,90 Thus, the interaction between sex and permeability is still incompletely understood. Lastly, obesity has been associated with an impaired barrier function.117–120 Two studies in IBS patients found a positive association between permeability and BMI.50,90 BMI was a strong confounder of sucrose excretion in one study.50 Furthermore, occludin expression was lower in obese patients (BMI >30 kg m–2) compared with non-obese patients.90 Compared with controls, IBS patients had a significantly higher BMI in two studies, which raises the question whether increased permeability in these particular studies is due to the effects of BMI.45,59 Hence, we believe BMI should always be taken into consideration when designing and analyzing permeability studies.
We aimed to comprehensively document differences in the experimental protocols used to assess in vivo permeability. We observed large differences in the cut-off values (LMR >0.015–0.07). However, cut-off values were mainly derived from prospectively enrolled healthy volunteers or historic controls from the same geographic region. When assessing future studies based on cut-off values, it is most ideal if those were obtained from a demographically and geographically similar population and ideally by the same investigators using identical protocols. The intake of food or drinks affects the passage of probe solutions throughout the GI lumen.64 An overnight fasting period was reported in the majority of studies discussing dietary restrictions. Because 14/25 in vivo studies did not document restrictions imposed on their patients, it cannot be excluded that these affected the outcome. The urine collection time varied strongly across studies. Interestingly, studies using shorter collection periods also showed significant differences between IBS patients and controls, suggesting involvement of the proximal gut in the pathophysiology of IBS, which has been relatively underexplored with most studies focusing on the distal colon. We observed a positive correlation between barrier dysfunction and the diarrhea severity. Changes in claudin proteins can impair ionic fluxes which can perturb net absorption of water across the colonic epithelium. How these changes in tight junction proteins may lead to physiological changes contributing to diarrhea remains to be understood.21,121 Psychological disturbances such as anxiety and depression are highly prevalent in IBS patients,122,123 and can modulate intestinal barrier dysfunction, potentially via hypothalamic–pituitary adrenal axis-induced mast cell activation.124,125 Acute stress in healthy volunteers has been shown to increase intestinal permeability as well.124 Chronic anxiety and depression can by itself impair barrier function.126 Although there is some suggestion that IBS patients with anxiety and depression have greater impairment of barrier functions, understanding the precise role of psychological factors will require cohorts of IBS patients with and without these psychological factors.53,127 One such cohort is our PI-IBS cohort that is fairly low in anxiety and depression scores but still demonstrates a high prevalence of impaired barrier function.102 A recent randomized clinical trial by Zhou and colleagues demonstrated that glutamine supplementation in PI-IBS (IBS-D) patients with increased intestinal permeability resulted in improvement of stool frequency, consistency, abdominal pain, overall IBS symptom severity scores, and a reduction of intestinal permeability.27 This suggests intestinal permeability can be specifically targeted resulting in an improvement of barrier function and clinical symptoms.
Both biopsy and fecal supernatants from IBS patients impair barrier in vitro, suggesting a role of peripheral mediators. The exact mediators and their mechanism of action have yet to be fully unraveled, but food antigens,128 proteases,81,99,102 bile acids129 and short-chain fatty acids130 are among the top targets. The recent development of humanized animal models as well as organoids provides unique platforms to study the effects of specific patient-derived mediators on host environment that more closely resemble the complexity of humans. Although evidence in children with IBS is limited, it points to permeability as a relevant pathophysiological mechanism in approximately 60% of the patients.68 Research in children is mainly hampered by the lack of structural investigations, and mainly focuses on the non-invasive in vivo permeability assays. McOmber and colleagues found an increased permeability in healthy siblings and parents of children with IBS, indicating a familial predisposition toward the development of barrier disruption.71 Several genetic variations in IBS patients have been described, some of which, like CDH-1, have been associated with increased permeability.131–134
We recognize our review has limitations. Heterogeneity in experimental protocols used to assess in vivo permeability in regard to the type and amount of permeability probes used, dietary restrictions for patients and the timing of urine collections make it hard to reach concrete conclusions. Therefore, we summarized the differences in the protocols used and accounted for them when interpreting the results. Similarly, tight junction gene expression studies are hard to interpret, due to differences in genes, examined sites, and methodology. Clinical characteristics such as age, BMI and gender can confound permeability assays and, ideally, should be accounted for in the analysis and interpretation of individual studies. Unfortunately, most studies reviewed did not use multivariable statistics so the differences observed might be influenced, either positively or negatively, by any of these variables. The clinical diversity of IBS populations resulted in a low number of studies per IBS subtype, making it difficult to formally assess the presence of publication bias. Finally, most studies did not provide IBS severity and psychiatric comorbidities, which limited comprehensive assessment of their associations with intestinal permeability.
Conclusion
Barrier dysfunction is present in a significant proportion of patients with IBS, especially in the IBS-D and PI-IBS subtypes. Future studies should attempt to use standardized experimental protocols to increase reproducibility. Furthermore, potential confounders like age, BMI, sex, psychological factors and diet should be adjusted for before drawing conclusions. The mechanisms underlying barrier dysfunction in IBS need to be studied but several studies have pointed to potential drivers. These include mast cell activation,78,91 microbiome changes,102 diet46 and mediators such as vasoactive intestinal polypeptide,91 serotonin,49 serine proteases49 and cysteine proteases.81 Further research is necessary to identify specific therapeutic targets for addressing increased permeability and assays to determine patient subsets that are most likely to benefit from those targets, underscoring the need for personalized treatment of IBS.
Supplemental Material
Supplemental material, sj-pdf-1-tag-10.1177_1756284821993586 for Intestinal barrier dysfunction in irritable bowel syndrome: a systematic review by Nikita Hanning, Adam L. Edwinson, Hannah Ceuleers, Stephanie A. Peters, Joris G. De Man, Leslie C. Hassett, Benedicte Y. De Winter and Madhusudan Grover in Therapeutic Advances in Gastroenterology
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NH is supported by a grant from the Flemish Institute for Research (FWO-SBO grant nr. S001017) and a grant from the University of Antwerp (TOP grant nr. 35018). MG is supported by NIDDK K23 103911 and R03 120745
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Benedicte Y. De Winter  https://orcid.org/0000-0003-0327-6304
https://orcid.org/0000-0003-0327-6304
Madhusudan Grover  https://orcid.org/0000-0001-5092-0831
https://orcid.org/0000-0001-5092-0831
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Nikita Hanning, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota, USA; Laboratory of Experimental Medicine and Pediatrics (LEMP) and Infla-Med, research consortium of excellence, University of Antwerp, Antwerp, Belgium.
Adam L. Edwinson, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota, USA
Hannah Ceuleers, Laboratory of Experimental Medicine and Pediatrics (LEMP) and Infla-Med, research consortium of excellence, University of Antwerp, Antwerp, Belgium.
Stephanie A. Peters, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, Minnesota, USA
Joris G. De Man, Laboratory of Experimental Medicine and Pediatrics (LEMP) and Infla-Med, research consortium of excellence, University of Antwerp, Antwerp, Belgium
Leslie C. Hassett, Mayo Clinic Libraries, Mayo Clinic, Rochester, Minnesota, USA
Benedicte Y. De Winter, Division of Gastroenterology, Laboratory of Experimental Medicine and Pediatrics, Universiteitsplein 1, Antwerp, 2610, Belgium Department of Gastroenterology and Hepatology, Antwerp University Hospital (UZA), Antwerp, Belgium.
Madhusudan Grover, Department of Medicine and Physiology, Enteric NeuroScience Program, 200 First St SW, Rochester, MN 55905, USA.
References
- 1. Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med 2017; 376: 2566–2578. [DOI] [PubMed] [Google Scholar]
- 2. Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol 2016; 1: 133–146. [DOI] [PubMed] [Google Scholar]
- 3. Simrén M, Tack J. New treatments and therapeutic targets for IBS and other functional bowel disorders. Nat Rev Gastroenterol Hepatol 2018; 15: 589–605. [DOI] [PubMed] [Google Scholar]
- 4. Sibelli A, Chalder T, Everitt H, et al. A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol Med 2016; 46: 3065–3080. [DOI] [PubMed] [Google Scholar]
- 5. Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology 2017; 152: 1042–1054.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helander HF, Fändriks L. Surface area of the digestive tract - revisited. Scand J Gastroenterol 2014; 49: 681–689. [DOI] [PubMed] [Google Scholar]
- 7. Maynard CL, Elson CO, Hatton RD, et al. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012; 489: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johansson ME, Phillipson M, Petersson J, et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 2008; 105: 15064–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ehmann D, Wendler J, Koeninger L, et al. Paneth cell α-defensins HD-5 and HD-6 display differential degradation into active antimicrobial fragments. Proc Natl Acad Sci U S A 2019; 116: 3746–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corr SC, Li Y, Riedel CU, et al. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci U S A 2007; 104: 7617–7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kommineni S, Bretl DJ, Lam V, et al. Bacteriocin production augments niche competition by enterococci in the mammalian gastrointestinal tract. Nature 2015; 526: 719–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamada N, Kim YG, Sham HP, et al. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science 2012; 336: 1325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johansson ME, Hansson GC. Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol 2016; 16: 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pelaseyed T, Hansson GC. Membrane mucins of the intestine at a glance. J Cell Sci 2020; 133: jcs240929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Otani T, Nguyen TP, Tokuda S, et al. Claudins and JAM-A coordinately regulate tight junction formation and epithelial polarity. J Cell Biol 2019; 218: 3372–3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schlegel N, Meir M, Heupel W-M, et al. Desmoglein 2-mediated adhesion is required for intestinal epithelial barrier integrity. Am J Physiol Gastrointest Liver Physiol 2010; 298: G774–G783. [DOI] [PubMed] [Google Scholar]
- 17. Allaire JM, Morampudi V, Crowley SM, et al. Frontline defenders: goblet cell mediators dictate host-microbe interactions in the intestinal tract during health and disease. Am J Physiol Gastrointest Liver Physiol 2018; 314: G360–G377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benguettat O, Jneid R, Soltys J, et al. The DH31/CGRP enteroendocrine peptide triggers intestinal contractions favoring the elimination of opportunistic bacteria. PLoS Pathog 2018; 14: e1007279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watson CJ, Rowland M, Warhurst G. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am J Physiol Cell Physiol 2001; 281: C388–C397. [DOI] [PubMed] [Google Scholar]
- 20. Krug SM, Amasheh S, Richter JF, et al. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell 2009; 20: 3713–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsai PY, Zhang B, He WQ, et al. IL-22 upregulates epithelial claudin-2 to drive diarrhea and enteric pathogen clearance. Cell Host Microbe 2017; 21: 671–681.e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weber CR, Raleigh DR, Su L, et al. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem 2010; 285: 12037–12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marchiando AM, Shen L, Graham WV, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol 2010; 189: 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turner JR, Buschmann MM, Romero-Calvo I, et al. The role of molecular remodeling in differential regulation of tight junction permeability. Semin Cell Dev Biol 2014; 36: 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol 2017; 14: 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Long Y, Du L, Kim JJ, et al. MLCK-mediated intestinal permeability promotes immune activation and visceral hypersensitivity in PI-IBS mice. Neurogastroenterol Motil 2018; 30: e13348. [DOI] [PubMed] [Google Scholar]
- 27. Zhou Q, Verne ML, Fields JZ, et al. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut 2019; 68: 996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vanheel H, Vicario M, Vanuytsel T, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut 2014; 63: 262–271. [DOI] [PubMed] [Google Scholar]
- 29. Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2012; 303: G775–G785. [DOI] [PubMed] [Google Scholar]
- 30. Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability - a new target for disease prevention and therapy. BMC Gastroenterology 2014; 14: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Downes MJ, Brennan ML, Williams HC, et al. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016; 6: e011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lobley RW, Burrows PC, Warwick R, et al. Simultaneous assessment of intestinal permeability and lactose tolerance with orally administered raffinose, lactose and L-arabinose. Clin Sci (Lond) 1990; 79: 175–183. [DOI] [PubMed] [Google Scholar]
- 34. Paganelli R, Fagiolo U, Cancian M, et al. Intestinal permeability in irritable bowel syndrome. Effect of diet and sodium cromoglycate administration. Ann Allergy 1990; 64: 377–380. [PubMed] [Google Scholar]
- 35. Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 2000; 47: 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marshall JK, Thabane M, Garg AX, et al. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther 2004; 20: 1317–1322. [DOI] [PubMed] [Google Scholar]
- 37. Dunlop SP, Hebden J, Campbell E, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol 2006; 101: 1288–1294. [DOI] [PubMed] [Google Scholar]
- 38. Zeng J, Li YQ, Zuo XL, et al. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2008; 28: 994–1002. [DOI] [PubMed] [Google Scholar]
- 39. Park JH, Park DI, Kim HJ, et al. The relationship between small-intestinal bacterial overgrowth and intestinal permeability in patients with irritable bowel syndrome. Gut Liver 2009; 3: 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 2009; 146: 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kerckhoffs AP, Akkermans LM, de Smet MB, et al. Intestinal permeability in irritable bowel syndrome patients: effects of NSAIDs. Dig Dis Sci 2010; 55: 716–723. [DOI] [PubMed] [Google Scholar]
- 42. Zhou Q, Souba WW, Croce CM, et al. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 2010; 59: 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gecse K, Roka R, Sera T, et al. Leaky gut in patients with diarrhea-predominant irritable bowel syndrome and inactive ulcerative colitis. Digestion 2011; 85: 40–46. [DOI] [PubMed] [Google Scholar]
- 44. Mattioli F, Fucile C, Marini V, et al. Assessment of intestinal permeability using sugar probes: influence of urinary volume. Clin Lab 2011; 57: 909–918. [PubMed] [Google Scholar]
- 45. Rao AS, Camilleri M, Eckert DJ, et al. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol Gastrointest Liver Physiol 2011; 301: G919–G928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 2013; 144: 903–911.e903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Del Valle-Pinero AY, Van Deventer HE, Fourie NH, et al. Gastrointestinal permeability in patients with irritable bowel syndrome assessed using a four probe permeability solution. Clin Chim Acta 2013; 418: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Swan C, Duroudier NP, Campbell E, et al. Identifying and testing candidate genetic polymorphisms in the irritable bowel syndrome (IBS): association with TNFSF15 and TNFα. Gut 2013; 62: 985–994. [DOI] [PubMed] [Google Scholar]
- 49. Keszthelyi D, Troost FJ, Jonkers DM, et al. Serotonergic reinforcement of intestinal barrier function is impaired in irritable bowel syndrome. Aliment Pharmacol Ther 2014; 40: 392–402. [DOI] [PubMed] [Google Scholar]
- 50. Mujagic Z, Ludidi S, Keszthelyi D, et al. Small intestinal permeability is increased in diarrhoea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders. Aliment Pharmacol Ther 2014; 40: 288–297. [DOI] [PubMed] [Google Scholar]
- 51. Zhou Q, Costinean S, Croce CM, et al. MicroRNA 29 targets nuclear factor-κB-repressing factor and Claudin 1 to increase intestinal permeability. Gastroenterology 2015; 148: 158–169.e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jarrett ME, Cain KC, Barney PG, et al. Balance of autonomic nervous system predicts who benefits from a self-management intervention program for irritable bowel syndrome. J Neurogastroenterol Motil 2016; 22: 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li L, Xiong L, Yao J, et al. Increased small intestinal permeability and RNA expression profiles of mucosa from terminal ileum in patients with diarrhoea-predominant irritable bowel syndrome. Dig Liver Dis 2016; 48: 880–887. [DOI] [PubMed] [Google Scholar]
- 54. Peters SA, Edogawa S, Sundt WJ, et al. Constipation-predominant irritable bowel syndrome females have normal colonic barrier and secretory function. Am J Gastroenterol 2017; 112: 913–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Russo F, Chimienti G, Linsalata M, et al. The obestatin/ghrelin ratio and ghrelin genetics in adult celiac patients before and after a gluten-free diet, in irritable bowel syndrome patients and healthy individuals. Eur J Gastroenterol Hepatol 2017; 29: 160–168. [DOI] [PubMed] [Google Scholar]
- 56. Valentin N, Camilleri M, Carlson P, et al. Potential mechanisms of effects of serum-derived bovine immunoglobulin/protein isolate therapy in patients with diarrhea-predominant irritable bowel syndrome. Physiol Rep 2017; 5: e13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Linsalata M, Riezzo G, D’Attoma B, et al. Noninvasive biomarkers of gut barrier function identify two subtypes of patients suffering from diarrhoea predominant-IBS: a case-control study. BMC Gastroenterol 2018; 18: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Russo F, Chimienti G, Riezzo G, et al. Adipose tissue-derived biomarkers of intestinal barrier functions for the characterization of diarrhoea-predominant IBS. Dis Markers 2018; 2018: 1827937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Camilleri M, Shin A, Busciglio I, et al. Validating biomarkers of treatable mechanisms in irritable bowel syndrome. Neurogastroenterol Motil 2014; 26: 1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chadwick VS, Phillips SF, Hofmann AF. Measurements of intestinal permeability using low molecular weight polyethylene glycols (PEG 400). I. Chemical analysis and biological properties of PEG 400. Gastroenterology 1977; 73: 241–246. [PubMed] [Google Scholar]
- 61. Grover M, Camilleri M, Hines J, et al. (13) C mannitol as a novel biomarker for measurement of intestinal permeability. Neurogastroenterol Motil 2016; 28: 1114–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ordiz MI, Davitt C, Stephenson K, et al. EB 2017 article: interpretation of the lactulose:mannitol test in rural Malawian children at risk for perturbations in intestinal permeability. Exp Biol Med (Maywood) 2018; 243: 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vazquez-Roque MI, Camilleri M, Smyrk T, et al. Association of HLA-DQ gene with bowel transit, barrier function, and inflammation in irritable bowel syndrome with diarrhea. Am J Physiol Gastrointest Liver Physiol 2012; 303: G1262–G1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jones RB, Dockray GJ, Thompson DG. The effects of fasting duration on gastric emptying in man, an exploration of the role of the endocannabinoid system and inter-individual responsiveness. Neurogastroenterol Motil 2012; 24: 928–e461. [DOI] [PubMed] [Google Scholar]
- 65. Turcotte JF, Kao D, Mah SJ, et al. Breaks in the wall: increased gaps in the intestinal epithelium of irritable bowel syndrome patients identified by confocal laser endomicroscopy (with videos). Gastrointest Endosc 2013; 77: 624–630. [DOI] [PubMed] [Google Scholar]
- 66. Zhao DY, Qi QQ, Long X, et al. Ultrastructure of intestinal mucosa in diarrhea-predominant irritable bowel syndrome. Physiol Int 2019; 106: 225–235. [DOI] [PubMed] [Google Scholar]
- 67. Shulman RJ, Eakin MN, Czyzewski DI, et al. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J Pediatr 2008; 153: 646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Francavilla R, Miniello V, Magistà AM, et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics 2010; 126: e1445–e1452. [DOI] [PubMed] [Google Scholar]
- 69. Gervasoni J, Schiattarella A, Giorgio V, et al. Validation of an LC-MS/MS method for urinary lactulose and mannitol quantification: results in patients with irritable bowel syndrome. Dis Markers 2016; 2016: 5340386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shulman RJ, Devaraj S, Heitkemper M. Gut permeability is affected by sex and increased in children with irritable bowel syndrome but not in functional abdominal pain. Neurogastroenterol Motil 2020; 32: e13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McOmber M, Rafati D, Cain K, et al. Increased gut permeability in first-degree relatives of children with irritable bowel syndrome or functional abdominal pain. Clin Gastroenterol Hepatol 2020; 18: 375–384.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Martínez C, Vicario M, Ramos L, et al. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol 2012; 107: 736–746. [DOI] [PubMed] [Google Scholar]
- 73. Martínez C, Lobo B, Pigrau M, et al. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut 2013; 62: 1160–1168. [DOI] [PubMed] [Google Scholar]
- 74. Martínez C, Rodiño-Janeiro BK, Lobo B, et al. miR-16 and miR-125b are involved in barrier function dysregulation through the modulation of claudin-2 and cingulin expression in the jejunum in IBS with diarrhoea. Gut 2017; 66: 1537–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rodiño-Janeiro BK, Martínez C, Fortea M, et al. Decreased TESK1-mediated cofilin 1 phosphorylation in the jejunum of IBS-D patients may explain increased female predisposition to epithelial dysfunction. Sci Rep 2018; 8: 2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ishimoto H, Oshima T, Sei H, et al. Claudin-2 expression is upregulated in the ileum of diarrhea predominant irritable bowel syndrome patients. J Clin Biochem Nutr 2017; 60: 146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lee H, Park JH, Park DI, et al. Mucosal mast cell count is associated with intestinal permeability in patients with diarrhea predominant irritable bowel syndrome. J Neurogastroenterol Motil 2013; 19: 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhen Y, Chu C, Zhou S, et al. Imbalance of tumor necrosis factor-α, interleukin-8 and interleukin-10 production evokes barrier dysfunction, severe abdominal symptoms and psychological disorders in patients with irritable bowel syndrome-associated diarrhea. Mol Med Rep 2015; 12: 5239–5245. [DOI] [PubMed] [Google Scholar]
- 79. Camilleri M, Carlson P, Acosta A, et al. RNA sequencing shows transcriptomic changes in rectosigmoid mucosa in patients with irritable bowel syndrome-diarrhea: a pilot case-control study. Am J Physiol Gastrointest Liver Physiol 2014; 306: G1089–G1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Annaházi A, Ferrier L, Bézirard V, et al. Luminal cysteine-proteases degrade colonic tight junction structure and are responsible for abdominal pain in constipation-predominant IBS. Am J Gastroenterol 2013; 108: 1322–1331. [DOI] [PubMed] [Google Scholar]
- 81. Katinios G, Casado-Bedmar M, Walter SA, et al. Increased colonic epithelial permeability and mucosal eosinophilia in ulcerative colitis in remission compared with irritable bowel syndrome and health. Inflamm Bowel Dis 2020; 26: 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cheng P, Yao J, Wang C, et al. Molecular and cellular mechanisms of tight junction dysfunction in the irritable bowel syndrome. Mol Med Report 2015; 12: 3257–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vivinus-Nébot M, Dainese R, Anty R, et al. Combination of allergic factors can worsen diarrheic irritable bowel syndrome: role of barrier defects and mast cells. Am J Gastroenterol 2012; 107: 75–81. [DOI] [PubMed] [Google Scholar]
- 84. Wilcz-Villega EM, McClean S, O’Sullivan MA. Mast cell tryptase reduces junctional adhesion molecule-a (JAM-A) expression in intestinal epithelial cells: implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. Am J Gastroenterol 2013; 108: 1140–1151. [DOI] [PubMed] [Google Scholar]
- 85. Vivinus-Nébot M, Frin-Mathy G, Bzioueche H, et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut 2014; 63: 744–752. [DOI] [PubMed] [Google Scholar]
- 86. Wilcz-Villega E, McClean S, O’Sullivan M. Reduced E-cadherin expression is associated with abdominal pain and symptom duration in a study of alternating and diarrhea predominant IBS. Neurogastroenterol Motil 2014; 26: 316–325. [DOI] [PubMed] [Google Scholar]
- 87. Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 2009; 58: 196–201. [DOI] [PubMed] [Google Scholar]
- 88. Coëffier M, Gloro R, Boukhettala N, et al. Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. Am J Gastroenterol 2010; 105: 1181–1188. [DOI] [PubMed] [Google Scholar]
- 89. Bertiaux-Vandaele N, Youmba SB, Belmonte L, et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol 2011; 106: 2165–2173. [DOI] [PubMed] [Google Scholar]
- 90. Bednarska O, Walter SA, Casado-Bedmar M, et al. Vasoactive intestinal polypeptide and mast cells regulate increased passage of colonic bacteria in patients with irritable bowel syndrome. Gastroenterology 2017; 153: 948–960.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Videlock EJ, Mahurkar-Joshi S, Hoffman JM, et al. Sigmoid colon mucosal gene expression supports alterations of neuronal signaling in irritable bowel syndrome with constipation. Am J Physiol Gastrointest Liver Physiol 2018; 315: G140–G157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Camilleri M, Carlson P, Acosta A, et al. Colonic mucosal gene expression and genotype in irritable bowel syndrome patients with normal or elevated fecal bile acid excretion. Am J Physiol Gastrointest Liver Physiol 2015; 309: G10–G20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Barbaro MR, Fuschi D, Cremon C, et al. Escherichia coli Nissle 1917 restores epithelial permeability alterations induced by irritable bowel syndrome mediators. Neurogastroenterol Motil. Epub ahead of print 28 June 2018. DOI: 10.1111/nmo.13388. [DOI] [PubMed] [Google Scholar]
- 94. Fritscher-Ravens A, Pflaum T, Mösinger M, et al. Many patients with irritable bowel syndrome have atypical food allergies not associated with immunoglobulin E. Gastroenterology 2019; 157: 109–118.e5. [DOI] [PubMed] [Google Scholar]
- 95. Tulic MK, Vivinus-Nebot M, Rekima A, et al. Presence of commensal house dust mite allergen in human gastrointestinal tract: a potential contributor to intestinal barrier dysfunction. Gut 2016; 65: 757–766. [DOI] [PubMed] [Google Scholar]
- 96. Wohlfarth C, Schmitteckert S, Härtle JD, et al. miR-16 and miR-103 impact 5-HT4 receptor signalling and correlate with symptom profile in irritable bowel syndrome. Sci Rep 2017; 7: 14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lee JY, Kim N, Park JH, et al. Expression of neurotrophic factors, tight junction proteins, and cytokines according to irritable bowel syndrome subtype and sex. J Neurogastroenterol Motil 2020; 26: 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lee JW, Park JH, Park DI, et al. Subjects with diarrhea-predominant IBS have increased rectal permeability responsive to tryptase. Dig Dis Sci 2010; 55: 2922–2928. [DOI] [PubMed] [Google Scholar]
- 99. Gecse K, Róka R, Ferrier L, et al. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut 2008; 57: 591–599. [DOI] [PubMed] [Google Scholar]
- 100. Crouzet L, Gaultier E, Del’Homme C, et al. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol Motil 2013; 25: e272–e282. [DOI] [PubMed] [Google Scholar]
- 101. De Palma G, Lynch MDJ, Lu J, et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med 2017; 9: eaaf6397. [DOI] [PubMed] [Google Scholar]
- 102. Edogawa S, Edwinson AL, Peters SA, et al. Serine proteases as luminal mediators of intestinal barrier dysfunction and symptom severity in IBS. Gut. Epub ahead of print 28 March 2019. DOI: 10.1136/gutjnl-2018-317416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Han X, Lee A, Huang S, et al. Lactobacillus rhamnosus GG prevents epithelial barrier dysfunction induced by interferon-gamma and fecal supernatants from irritable bowel syndrome patients in human intestinal enteroids and colonoids. Gut Microbes 2019; 10: 59–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nébot-Vivinus M, Harkat C, Bzioueche H, et al. Multispecies probiotic protects gut barrier function in experimental models. World J Gastroenterol 2014; 20: 6832–6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Witt ST, Bednarska O, Keita ÅV, et al. Interactions between gut permeability and brain structure and function in health and irritable bowel syndrome. Neuroimage Clin 2019; 21: 101602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Duan R, Zhu S, Wang B, et al. Alterations of gut microbiota in patients with irritable bowel syndrome based on 16S rRNA-targeted sequencing: a systematic review. Clin Transl Gastroenterol 2019; 10: e00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sundin J, Aziz I, Nordlander S, et al. Evidence of altered mucosa-associated and fecal microbiota composition in patients with irritable bowel syndrome. Sci Rep 2020; 10: 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Camilleri M, McKinzie S, Busciglio I, et al. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2008; 6: 772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Deiteren A, Camilleri M, Burton D, et al. Effect of meal ingestion on ileocolonic and colonic transit in health and irritable bowel syndrome. Dig Dis Sci 2010; 55: 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wilms E, Troost FJ, Elizalde M, et al. Intestinal barrier function is maintained with aging - a comprehensive study in healthy subjects and irritable bowel syndrome patients. Sci Rep 2020; 10: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Suenaert P, Bulteel V, Den Hond E, et al. The effects of smoking and indomethacin on small intestinal permeability. Aliment Pharmacol Ther 2000; 14: 819–822. [DOI] [PubMed] [Google Scholar]
- 112. Edogawa S, Peters SA, Jenkins GD, et al. Sex differences in NSAID-induced perturbation of human intestinal barrier function and microbiota. FASEB J 2018; 32: fj201800560R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Suenaert P, Bulteel V, Den Hond E, et al. In vivo influence of nicotine on human basal and NSAID-induced gut barrier function. Scand J Gastroenterol 2003; 38: 399–408. [DOI] [PubMed] [Google Scholar]
- 114. Prytz H, Benoni C, Tagesson C. Does smoking tighten the gut? Scand J Gastroenterol 1989; 24: 1084–1088. [DOI] [PubMed] [Google Scholar]
- 115. Blomquist L, Bark T, Hedenborg G, et al. Evaluation of the lactulose/mannitol and 51Cr-ethylenediaminetetraacetic acid/14C-mannitol methods for intestinal permeability. Scand J Gastroenterol 1997; 32: 805–812. [DOI] [PubMed] [Google Scholar]
- 116. Shaikh M, Rajan K, Forsyth CB, et al. Simultaneous gas-chromatographic urinary measurement of sugar probes to assess intestinal permeability: use of time course analysis to optimize its use to assess regional gut permeability. Clin Chim Acta 2015; 442: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wilbrink J, Bernards N, Mujagic Z, et al. Intestinal barrier function in morbid obesity: results of a prospective study on the effect of sleeve gastrectomy. Int J Obes (Lond) 2020; 44: 368–376. [DOI] [PubMed] [Google Scholar]
- 118. Damms-Machado A, Louis S, Schnitzer A, et al. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am J Clin Nutr 2017; 105: 127–135. [DOI] [PubMed] [Google Scholar]
- 119. Genser L, Aguanno D, Soula HA, et al. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J Pathol 2018; 246: 217–230. [DOI] [PubMed] [Google Scholar]
- 120. Rainone V, Schneider L, Saulle I, et al. Upregulation of inflammasome activity and increased gut permeability are associated with obesity in children and adolescents. Int J Obes (Lond) 2016; 40: 1026–1033. [DOI] [PubMed] [Google Scholar]
- 121. Tang Y, Clayburgh DR, Mittal N, et al. Epithelial NF-kappaB enhances transmucosal fluid movement by altering tight junction protein composition after T cell activation. Am J Pathol 2010; 176: 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology 2002; 122: 1140–1156. [DOI] [PubMed] [Google Scholar]
- 123. Fond G, Loundou A, Hamdani N, et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 2014; 264: 651–660. [DOI] [PubMed] [Google Scholar]
- 124. Vanuytsel T, van Wanrooy S, Vanheel H, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 2014; 63: 1293–1299. [DOI] [PubMed] [Google Scholar]
- 125. Alonso C, Guilarte M, Vicario M, et al. Acute experimental stress evokes a differential gender-determined increase in human intestinal macromolecular permeability. Neurogastroenterol Motil 2012; 24: 740–746, e348–e349. [DOI] [PubMed] [Google Scholar]
- 126. Stevens BR, Goel R, Seungbum K, et al. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018; 67: 1555–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Shulman RJ, Jarrett ME, Cain KC, et al. Associations among gut permeability, inflammatory markers, and symptoms in patients with irritable bowel syndrome. J Gastroenterol 2014; 49: 1467–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Aguilera-Lizarraga J, Mondelaers S, Florens M, et al. 245 evidence for a new mechanism underlying persistent visceral hypersensitivity and increased permeability in a model of post-infectious IBS. Gastroenterology 2015; 148: S-55. [Google Scholar]
- 129. Camilleri M, Busciglio I, Acosta A, et al. Effect of increased bile acid synthesis or fecal excretion in irritable bowel syndrome-diarrhea. Am J Gastroenterol 2014; 109: 1621–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sun Q, Jia Q, Song L, et al. Alterations in fecal short-chain fatty acids in patients with irritable bowel syndrome: a systematic review and meta-analysis. Medicine (Baltimore) 2019; 98: e14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zucchelli M, Camilleri M, Andreasson AN, et al. Association of TNFSF15 polymorphism with irritable bowel syndrome. Gut 2011; 60: 1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Camilleri M, Carlson P, McKinzie S, et al. Genetic susceptibility to inflammation and colonic transit in lower functional gastrointestinal disorders: preliminary analysis. Neurogastroenterol Motil 2011; 23: 935–e398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wouters MM, Lambrechts D, Knapp M, et al. Genetic variants in CDC42 and NXPH1 as susceptibility factors for constipation and diarrhoea predominant irritable bowel syndrome. Gut 2014; 63: 1103–1111. [DOI] [PubMed] [Google Scholar]
- 134. Villani AC, Lemire M, Thabane M, et al. Genetic risk factors for post-infectious irritable bowel syndrome following a waterborne outbreak of gastroenteritis. Gastroenterology 2010; 138: 1502–1513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tag-10.1177_1756284821993586 for Intestinal barrier dysfunction in irritable bowel syndrome: a systematic review by Nikita Hanning, Adam L. Edwinson, Hannah Ceuleers, Stephanie A. Peters, Joris G. De Man, Leslie C. Hassett, Benedicte Y. De Winter and Madhusudan Grover in Therapeutic Advances in Gastroenterology