Abstract
Background and Objectives
Atrial high-rate episodes (AHREs) can be continuously detected by cardiovascular implantable electronic devices (CIEDs); however, the predictors of clinically relevant AHREs are unclear.
Methods
This prospective multicenter study monitored 816 patients (median age 73 years, 40.4% male) without atrial fibrillation (AF) from September 2017 to July 2020. AHREs was defined as a programmed atrial detection rate >220 beats/min. The reference values of 6 minutes and 6 hours were set to analyze clinical implication of AHREs based on previously published data that the 6 minutes excluded most episodes of oversensing.
Results
During a median follow-up of 18 months (interquartile interval 9–26 months), AHREs with the longest durations of >15 seconds, >6 minutes, and >6 hours and clinically documented AF by electrocardiography were noted in 246 (30.1%), 112 (13.7%), 49 (6.0%), and 24 (2.9%) patients, respectively. Among patients developing AHREs >6 minutes, 102 (91.1%) of 112 patients were identified at the 6-month visit. Patients with AHREs >6 minutes had higher proportions of sick sinus syndrome, subjects with atrial premature beat >1% on Holter monitoring, and larger left atrium (LA) size than patients with AHREs ≤6 minutes. Multivariable logistic regression analysis showed that LA diameter >41 mm (odds ratio [OR], 2.08; 95% confidence interval [95% CI], 1.25–3.45), and sick sinus syndrome (OR, 3.22; 95% CI, 1.91–5.43) were associated with AHREs >6 minutes.
Conclusions
In patients with LA diameter >41 mm, and sick sinus syndrome before CIEDs implantation is associated with risk of developing AHREs >6 minutes.
Trial Registration
ClinicalTrials.gov Identifier: NCT03303872
Keywords: Arrhythmia; Pacemaker, artificial; Sick sinus syndrome; Left atrium
INTRODUCTION
Cardiac implantable electronic devices (CIEDs) can be programmed to monitor and identify atrial high-rate episodes (AHREs), also described as “subclinical atrial fibrillation (SCAF),” which is defined as asymptomatic atrial fibrillation (AF), atrial tachycardia (AT), and atrial flutter (AFL) of short duration. Individuals may benefit from using CIEDs to detect AHREs as increasing evidence indicates that AHREs are associated with stroke risk. However, the expected stroke risk in patients with AHREs was much lower than that in clinically documented AF patients with similar risk profile.1),2),3),4),5) Most studies used an episode duration longer than 5–6 min to define AHREs,3),6),7),8),9),10) although a reported false-positive rate of 17.3%, making physician review more essential compared with AHREs lasting >6 hours, a false-positive rate of 3.3%.11) Current guidelines on non-valvular AF recommend anticoagulation therapy in patients with CHA2DS2-VASc scores ≥2 and consideration of anticoagulation therapy in patients with CHA2DS2-VASc scores of 1.12),13) Recently, the European Heart Rhythm Association suggested consideration of anticoagulation therapy for patients with AHREs >5.5 hours and with two additional stroke risk factors.14) However, the optimal primary stroke prevention indication and strategy remain uncertain.
Previous studies have reported various predictors of AHREs in patients with CIEDs. In the ASSERT study,1) lower resting heart rate and sinus node disease were associated with the prediction of AHREs. Other studies reported that a history of heart failure predicted AHREs,9) while others reported that a history of recurrent transient ischemic attack (TIA) and heart failure (HF),15) increased age, increased left atrium (LA) size, and lower systolic blood pressure predicted AHREs.16) A meta-analysis including 10 studies found that baseline characteristics; diabetes mellitus (DM); old age; thromboembolism events; congestive heart failure, hypertension (HTN), age ≥75 years, DM, stroke, CHADS2 score; and coronary artery disease (CAD) were not associated with the prediction of AHREs.17) Therefore, AHREs predictors have not been well established and the optimal treatment for preventing stroke primarily in AHREs is uncertain. Thus, the aim of the present study was to prospectively investigate clinical predictors for AHREs and evaluate the clinical development of AF and cardiovascular events according to the presence of AHREs.
METHODS
Study design and methods
The evaluation of AF occurrence in sick sinus syndrome and atrioventricular node disease patients after pacemaker implantation (AF-Pacemaker Study) was a prospective, multicenter, observational study performed in patients with AF aged >18 years attending any of the 11 tertiary hospital centers comprising all geographical regions of Korea. The study enrollment period started in September 2017 and ended in July 2020.
The AF-pacemaker study aims to investigate the occurrence and management (including ablation therapy) of device-detected AF/AT episodes in patients with post-pacemaker implantation through a prospective, non-randomized, non-blinded, observational, multicenter design. The study is conducted in compliance with the ethical rules of the Declaration of Helsinki (2013) as a statement of ethical principles for medical research involving human subjects by The World Medical Association and approved by the Institutional Review Board of Yonsei University Health System (1-2017-0008). This study is registered at ClinicalTrials.gov (NCT03303872). The Ethics Committees of all 11 tertiary hospital centers (Severance Hospital, Seoul National University Bundang Hospital, Seoul National University Hospital, Donga University Medical Center, Keimyung University Hospital, Ewha Womans University Medical Center, Daegu Catholic University Medical Center, Korea University Medical Center, Eulji University Hospital, CHA Bundang Medical Center, and Kangneung Asan Medical Center) approved this study, and all patients provided informed consent for their inclusion.
Study population
The study population included patients 1) eligible for permanent pacemaker implantation based on the 2016 revised Korean indication guideline on cardiac pacemaker implantation, 2) with an estimated atrial pacing percentage >40% under sinus rhythm (Lower rate ≥60 bpm, close hysteresis, and rest rate), 3) estimated ventricular pacing percentage >40% under sinus rhythm (lower rate ≥40 bpm, dual chamber pacing, close hysteresis, and rest rate). Data collection was usually conducted by personnel with no clinical activity assigned to the project. Data were entered into a common electronic database to limit inconsistencies and errors and provided online help for key variables. Each center could see its data and those of the other participating centers.
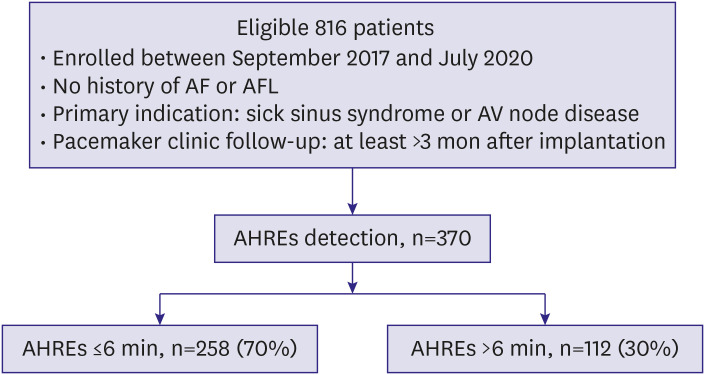
A total of 816 consecutive patients were enrolled at 11 tertiary centers and implanted with a St. Jude Medical dual-chamber rate-adaptive pacemaker (Assurity PM2240) with stored electrogram capabilities for sick sinus syndrome (sinus bradycardia, sinus pause ≥3 seconds, tachy-brady syndrome, sinus node dysfunction, and chronotropic incompetence) or atrioventricular block (high degree/complete atrioventricular block). Patients were eligible for inclusion in the study if they were at least 19 years of age and were available for periodic observation after pacemaker implantation. We excluded patients with severe liver dysfunction (aspartate transaminase/alanine transferase ≥3 times the normal upper limit), severe renal dysfunction (serum creatinine ≥3.5 mg/dL or creatinine clearance ≤30 mL/min) including dialysis, pregnant, lactating, or with malignant cancer, dilated cardiomyopathy, hypertrophic cardiomyopathy, severe valvular heart disease, or life expectancy ≤12 months from enrollment. Patients were also excluded if they had AF at baseline before pacemaker (Figure 1).
Figure 1.
Flow diagram showing study participants.
AF = atrial fibrillation; AFL = atrial flutter; AHRE = atrial high rate episode; AV = atrioventricular.
Baseline assessment and patient follow-up
We collected data on each patient's baseline demographic characteristics, clinical information, 12-lead electrocardiogram (ECG), laboratory examination, medications, Holter monitoring, treadmill test, and transthoracic echocardiography before pacemaker implantation. The baseline AF rhythm for patient exclusion was reviewed from documented AF on surface ECG/Holter/treadmill test. Device interrogation information was obtained from a clinic visit at 6, 12, and 18 months after pacemaker implantation. There was allowed up to 3 months before and after each clinic visit interval. The AHREs were interrogated to compare ECG traces and pacemaker AHREs data at the end of the ambulatory monitoring period. The clinicians were blinded to the results of the atrial diagnostic data. A Clinical Events Committee classified causes of death and adjudicated all suspected clinical events with blinding to indications of pacemaker implantation. An ECG Core Laboratory reviewed the ECGs and confirmed AF diagnoses.
Pacemaker programming and AHREs detection
The pacemakers incorporated bipolar atrial and ventricular leads (Tendril MRI LPA1200M, Isoflex Optim 1944/1948, Tendril ST Optim 1888TC) in all patients. The atrial and ventricular leads were placed in the right atrial appendage and right ventricular apex, respectively. An AHREs detection rate of 220 beats/min was programmed and storage of up to four atrial electrograms of 12-second duration on automatic detection of AHREs was activated. At every visit, the longest durations of all AHREs were ascertained. The longest AHREs durations were classified >15 vs. ≤15 seconds, >6 vs. ≤6 minutes, and >6 vs. ≤6 hours. Clinical events including cerebrovascular accidents (CVAs), major/minor bleeding, myocardial infarction (MI), and all-cause deaths were recorded.
Statistical analysis
Continuous variables were presented as mean±standard deviations for normally distributed values or medians and interquartile interval (IQI) for non-normally distributed values and categorical variables as numbers and percentages in each group. The baseline characteristics of the two groups were compared using Student's t- or Mann–Whitney U-tests for continuous variables and Pearson's χ2 or Fisher's exact tests for categorical variables. We used a logistic regression model for multivariate analysis to assess predictors of AHREs after adjusting for other clinical events. Based on age, sex, and body mass index (BMI), we built a multivariate logistic model by adding variables that were p values <0.10 into the univariate models. Logistic regression analysis was also used to confirm the statistical significance between AHREs predictors and longest duration. Associations were presented as odds ratios (ORs) with 95% confidence intervals (CIs). All analyses were performed using SAS version 9.4 (SAS Institute) and R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria). Two-tailed p values <0.05 indicated statistical significance.
RESULTS
Between September 2017 and July 2020, this study enrolled 816 patients with a median follow-up of 18 months. The median patient age was 73 (65, 79) years and 60% were women. Atrioventricular block (58.1%) was the most common indication for CIEDs implantation, followed by sick sinus syndrome (41.9%). The median CHA2DS2-VASc score was 3 (2, 4); 677 (83.0%) of patients had scores ≥2 and 66.9% had hypertension. The median LA diameter was 40 mm (36, 45), the left ventricular ejection fraction (LVEF) was 65% (60, 70) by transthoracic echocardiography, and the PR interval was 184 ms (164, 204) by electrocardiography. Fifty-five (7.1%) showed atrial premature contraction (APC) at >1% per 24 hours. Overall, 358 (43.9%) patients were taking renin-angiotensin-aldosterone system inhibitors (Table 1).
Table 1. Baseline characteristics of the overall population (n=816).
| Variables | Values | |
|---|---|---|
| Age (years) | 73 (65, 79) | |
| Male, No. (%) | 329 (40.4) | |
| Body mass index (kg/m2) | 24.1 (22.1, 26.2) | |
| Systolic blood pressure (mmHg) | 135 (121, 147) | |
| Diastolic blood pressure (mmHg) | 71 (64, 80) | |
| Heart rate (per min) | 60 (50, 72) | |
| Smoking (n=670) | ||
| Never | 561 (83.7) | |
| Former/current | 109 (16.3) | |
| Alcohol (n=670) | ||
| Never | 557 (83.1) | |
| Former/current | 113 (16.9) | |
| CHA2DS2-VAS score* | 3 (2, 4) | |
| CHA2DS2-VAS score, group | ||
| 0 | 24 (2.9) | |
| 1 | 115 (14.1) | |
| ≥2 | 677 (83.0) | |
| CHF | 31 (3.8) | |
| Hypertension | 546 (66.9) | |
| Age ≥75 years | 364 (44.6) | |
| Diabetes | 228 (27.9) | |
| Prior stroke/TIA | 85 (10.4) | |
| Vascular disease | 80 (9.8) | |
| CKD | 64 (7.8) | |
| Dyslipidemia | 249 (30.5) | |
| Laboratory findings | ||
| Estimated GFR (mL/min/1.73 m2) | 77.6 (62.0, 91.6) | |
| Free T4 (ng/dL) | 1.1 (1.0, 1.3) | |
| TSH (µIU/mL) | 1.9 (1.1, 3.2) | |
| Echocardiography | ||
| LA diameter (mm) | 40 (36, 45) | |
| LVEF (%) | 65 (60, 70) | |
| E/Em | 11.6 (8.9 14.9) | |
| Electrocardiogram | ||
| PR interval (ms) | 184 (164, 204) | |
| QRS duration (ms) | 108 (90, 144) | |
| QTc interval (ms) | 455 (422, 488) | |
| Medications | ||
| ARB/ACEi | 358 (43.9) | |
| Beta blocker | 122 (15.0) | |
| Calcium channel blocker | 254 (31.1) | |
| Statin | 349 (42.8) | |
| Diuretics | 181 (22.2) | |
| Holter recording | ||
| APC >1% | 58 (7.1) | |
| VPC >1% | 54 (6.6) | |
| Pacemaker indication | ||
| Sick sinus syndrome | 342 (41.9) | |
| AV block | 474 (58.1) | |
The data are presented as number (%), mean±standard deviation, or median (interquartile interval). Non-parametric continuous variables, as assessed using the Kolmogorov–Smirnov method, were analyzed using the Mann–Whitney U test.
ACEi = angiotensin-converting-enzyme inhibitor; AF = atrial fibrillation; APC = atrial premature complex; ARB = angiotensin receptor blocker; AV = atrioventricular; BUN = blood urea nitrogen; CHF = congestive heart failure; CKD = chronic kidney disease; E/Em = the ratio of the early diastolic mitral inflow velocity (E) to the early diastolic mitral annular velocity (Em); GFR = glomerular filtration rate; LA = left atrium; LVEF = left ventricular ejection fraction; QTc = corrected QT; TIA = transient ischemic attack; TSH = thyroid-stimulating hormone; VPC = ventricular premature complex.
*The CHA2DS2-VAS score is a measure of the risk of stroke in patients with atrial fibrillation, with scoring ranging from 0 to 9 and higher scores indicating greater risk. Congestive heart failure, hypertension, age 75 years or older (doubled), diabetes, stroke (doubled), vascular disease, age 65 to 74 years, sex category (female).
AHRE duration criteria
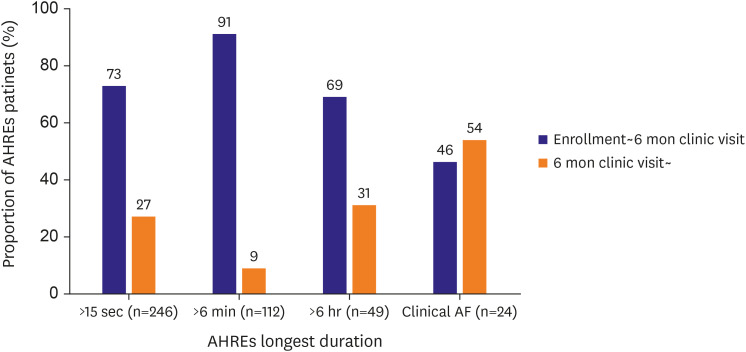
Analysis of the longest AHREs durations showed large variability (Supplementary Table 1). Using the AHREs cut-off, AHREs >6 minutes were identified in 102 (12.5%) patients between enrollment and the 6-month follow-up and 112 (13.7%) patients during the entire study period. Longest AHREs lasting >6 hours were found in 34 (4.2%) patients between enrollment and the 6-month follow-up, and 49 (6.0%) patients during the entire study period. Overall, 24 (2.9%) of the 816 patients experienced newly developed AF, as documented by 12-lead ECG during the follow-up period. In patients with confirmed AHRE, the most AHRE was observed within 6 months after pacemaker implantation. In contrary, more than half of the patients diagnosed with newly detected AF were identified more than 6 months after implantation (Figure 2).
Figure 2.
Distributions of cardiac implantable electronic device-detected AHREs, and clinical AF during study period.
AF = atrial fibrillation; AHRE = atrial high rate episode.
Patient characteristics: longest AHREs duration >6 minutes versus ≤6 minutes
Comparison of the baseline characteristics at the time of CIEDs implantation between the groups with AHREs ≤ or >6 minutes is shown in Table 2. These patients did not differ significantly with respect to age, sex, BMI, estimated glomerular filtration rate, thyroid function, LVEF, E/Em, PR interval, history of smoking, alcohol consumption, proportion of congestive heart failure, HTN, DM, prior stroke/TIA, vascular disease, CHA2DS2-VAS score, chronic kidney disease, and dyslipidemia. Medication use including renin-angiotensin-aldosterone system inhibitors, calcium channel blockers, statins, and diuretics also did not differ significantly between patients with longest AHRE durations >6 and ≤6 minutes. Patients with the longest AHREs durations >6 minutes had larger LA diameter, a higher proportion of APC >1% on Holter monitoring, beta-blockade use, and sick sinus syndrome among CIEDs implantation indications compared to patients with longest AHREs duration ≤6 minutes. Comparison based on a longest AHREs duration cut-off of 6 hours showed relatively differences in LA diameter and proportion of prior stroke/TIA, APC >1% on Holter monitoring, beta-blockade use (Table 3).
Table 2. Baseline characteristics for longest AHRE duration ≤6 min (n=258) versus >6 min (n=112).
| Variables | AHRE ≤6 min (n=258) | AHRE >6 min (n=112) | p value | |
|---|---|---|---|---|
| Age (years) | 73 (65–78) | 74 (67–80) | 0.358 | |
| Male | 102 (39.5) | 48 (42.9) | 0.629 | |
| Body mass index (kg/m2) | 24.0 (22.3–26.0) | 23.9 (22.1–26.1) | 0.532 | |
| Systolic blood pressure (mmHg) | 136 (124–150) | 133 (120–145) | 0.064 | |
| Diastolic blood pressure (mmHg) | 71 (65–80) | 70 (63–80) | 0.328 | |
| Heart rate (/min) | 60 (50–74) | 60 (50–72) | 0.595 | |
| Smoking | 0.539 | |||
| Never | 209 (81.0) | 92 (82.1) | ||
| Former/current | 49 (19.0) | 20 (17.8) | ||
| Alcohol | 0.558 | |||
| Never | 202 (78.3) | 90 (80.3) | 1.000 | |
| Former/current | 56 (21.7) | 22 (19.7) | ||
| CHA2DS2-VAS score* | 3 (2, 4) | 3 (2, 4) | 0.611 | |
| CHA2DS2-VAS score, group | 0.924 | |||
| 0 | 8 (3.1) | 4 (3.6) | ||
| 1 | 38 (14.7) | 15 (13.4) | ||
| ≥2 | 212 (82.2) | 93 (83.0) | ||
| CHF | 8 (3.1) | 3 (2.7) | 1.000 | |
| Hypertension | 177 (68.6) | 72 (64.3) | 0.488 | |
| Age ≥75 years | 116 (45.0) | 54 (48.2) | 0.643 | |
| Diabetes | 69 (26.7) | 25 (22.3) | 0.443 | |
| Prior stroke/TIA | 26 (10.1) | 17 (15.2) | 0.219 | |
| Vascular disease | 20 (7.8) | 13 (11.6) | 0.319 | |
| CKD | 22 (8.5) | 10 (8.9) | 0.925 | |
| Dyslipidemia | 76 (29.5) | 27 (24.1) | 0.572 | |
| Laboratory findings | ||||
| Estimated GFR (mL/min/1.73 m2) | 81.3 (65.1–95.0) | 79.5 (62.0–89.0) | 0.200 | |
| Free T4 (ng/dL) | 1.2 (1.0–1.3) | 1.1 (1.0–1.4) | 0.602 | |
| TSH (µIU/mL) | 1.8 (1.0–3.2) | 2.0 (1.0–3.3) | 0.611 | |
| Echocardiography | ||||
| LA diameter (mm) | 40 (35–45) | 42 (37–45) | 0.034 | |
| LVEF (%) | 67 (60–71) | 65 (60–70) | 0.380 | |
| E/Em | 11.0 (8.4–14.5) | 11.4 (8.3–14.8) | 0.601 | |
| Electrocardiogram | ||||
| PR interval (ms) | 184 (167–207) | 184 (163–207) | 0.469 | |
| QRS duration (ms) | 112 (90–144) | 98 (88–134) | 0.150 | |
| QTc interval (ms) | 455 (425–488) | 451 (421–477) | 0.304 | |
| Medications | ||||
| ARB/ACEi | 116 (45.0) | 42 (37.5) | 0.223 | |
| Beta blocker | 27 (10.5) | 28 (25.0) | 0.001 | |
| Calcium channel blocker | 81 (31.4) | 31 (27.7) | 0.554 | |
| Statin | 96 (37.2) | 43 (38.4) | 0.921 | |
| Diuretics | 54 (20.9) | 25 (22.3) | 0.871 | |
| Holter recording | ||||
| APC >1% | 13 (5.0) | 16 (14.3) | 0.005 | |
| VPC >1% | 14 (5.4) | 11 (9.8) | 0.186 | |
| Pacemaker indication | <0.001 | |||
| Sick sinus syndrome | 80 (31.0) | 71 (63.4) | ||
| AV block | 178 (69.0) | 41 (36.6) | ||
The data are presented as number (%) or median (interquartile interval). Non-parametric continuous variables, as assessed using the Kolmogorov–Smirnov method, were analyzed using the Mann-Whitney U test.
ACEi = angiotensin-converting-enzyme inhibitor; AHREs = atrial high rate episodes; APC = atrial premature complex; ARB = angiotensin receptor blocker; AV = atrioventricular; BUN = blood urea nitrogen; CHF = congestive heart failure; CKD = chronic kidney disease; E/Em = the ratio of the early diastolic mitral inflow velocity (E) to the early diastolic mitral annular velocity (Em); GFR = glomerular filtration rate; LA = left atrium; LVEF = left ventricular ejection fraction; QTc = corrected QT; TIA = transient ischemic attack; TSH = thyroid-stimulating hormone; VPC = ventricular premature complex.
*The CHA2DS2-VAS score is a measure of the risk of stroke in patients with atrial fibrillation, with scoring ranging from 0 to 9 and higher scores indicating greater risk. Congestive heart failure, hypertension, age 75 years or older (doubled), diabetes, stroke (doubled), vascular disease, age 65 to 74 years, sex category (female).
Table 3. Baseline characteristics for longest AHRE duration ≤6 hours (n=321) versus >6 hours (n=49).
| Variables | AHREs ≤6 hours (n=321) | AHREs >6 hours (n=49) | p value | |
|---|---|---|---|---|
| Age (years) | 73 (66–79) | 75 (67–79) | 0.621 | |
| Male | 127 (39.6) | 23 (46.9) | 0.410 | |
| Body mass index (kg/m2) | 24.0 (22.2–25.9) | 24.4 (22.5–26.5) | 0.598 | |
| Systolic blood pressure (mmHg) | 136 (123–149) | 130 (117–142) | 0.170 | |
| Diastolic blood pressure (mmHg) | 72 (65–80) | 70 (63–77) | 0.190 | |
| Heart rate (/min) | 62 (50–75) | 62 (51–74) | 0.933 | |
| Smoking | 0.881 | |||
| Never | 264 (82.2) | 41 (83.6) | ||
| Former/current | 57 (17.8) | 8 (16.4) | ||
| Alcohol | 0.560 | |||
| Never | 266 (82.9) | 40 (81.6) | ||
| Former/current | 55 (17.1) | 9 (18.4) | ||
| CHA2DS2-VAS score* | 3 (2–4) | 3 (2–5) | 0.155 | |
| CHA2DS2-VAS score, group | 0.573 | |||
| 0 | 11 (3.4) | 1 (2.0) | ||
| 1 | 48 (15.0) | 5 (10.2) | ||
| ≥2 | 262 (81.6) | 43 (87.8) | ||
| CHF | 8 (2.5) | 3 (6.1) | 0.346 | |
| Hypertension | 215 (67.0) | 34 (69.4) | 0.864 | |
| Age, ≥75 years | 144 (44.9) | 26 (53.1) | 0.358 | |
| Diabetes | 85 (26.5) | 9 (18.4) | 0.299 | |
| Prior stroke/TIA | 31 (9.7) | 12 (24.5) | 0.005 | |
| Vascular disease | 26 (8.1) | 7 (14.3) | 0.252 | |
| CKD | 27 (8.4) | 5 (10.2) | 0.189 | |
| Dyslipidemia | 92 (28.7) | 11 (22.4) | 0.509 | |
| Laboratory findings | ||||
| Estimated GFR (mL/min/1.73 m2) | 80.7 (64.4–94.0) | 81.0 (60.8–91.0) | 0.634 | |
| Free T4 (ng/dL) | 1.1 (1.0–1.3) | 1.1 (1.0–1.4) | 0.840 | |
| TSH (µIU/mL) | 1.7 (1.0–3.1) | 2.2 (1.2–4.6) | 0.209 | |
| Echocardiography | ||||
| LA diameter (mm) | 40 (35–45) | 42 (39–45) | 0.149 | |
| LVEF (%) | 66 (60–70) | 65 (60–70) | 0.838 | |
| E/Em | 11.0 (8.1–14.3) | 11.8 (8.9–16.2) | 0.366 | |
| Electrocardiogram | ||||
| PR interval (ms) | 185 (166–208) | 180 (164–204) | 0.286 | |
| QRS duration (ms) | 111 (90–143) | 94 (86–122) | 0.102 | |
| QTc interval (ms) | 455 (424–488) | 451 (425–476) | 0.292 | |
| Medications | ||||
| ARB/ACEi | 135 (42.1) | 23 (46.9) | 0.625 | |
| Beta blocker | 47 (14.6) | 8 (16.3) | 0.926 | |
| Calcium channel blocker | 97 (30.2) | 15 (30.6) | 1.000 | |
| Statin | 118 (36.8) | 21 (42.9) | 0.508 | |
| Diuretics | 66 (20.6) | 13 (26.5) | 0.446 | |
| Holter recording | ||||
| APC >1% | 25 (7.8) | 4 (8.2) | 1.000 | |
| VPC >1% | 21 (6.5) | 4 (8.2) | 0.908 | |
| Pacemaker indication | 0.001 | |||
| Sick sinus syndrome | 120 (37.4) | 31 (63.3) | ||
| AV block | 201 (62.6) | 18 (36.7) | ||
The data are presented as number (%) or median (interquartile interval). Non-parametric continuous variables, assessed using the Kolmogorov–Smirnov method, were analyzed using Mann-Whitney U tests.
ACEi = angiotensin-converting-enzyme inhibitor; AHREs = atrial high rate episodes; APC = atrial premature complex; ARB = angiotensin receptor blocker; AV = atrioventricular; BUN = blood urea nitrogen; CHF = congestive heart failure; CKD = chronic kidney disease; E/Em = the ratio of the early diastolic mitral inflow velocity (E) to the early diastolic mitral annular velocity (Em); GFR = glomerular filtration rate; LA = left atrium; LVEF = left ventricular ejection fraction; QTc = corrected QT; TIA = transient ischemic attack; TSH = thyroid-stimulating hormone; VPC = ventricular premature complex.
*The CHA2DS2-VAS score is a measure of the risk of stroke in patients with atrial fibrillation, with scoring ranging from 0 to 9 and higher scores indicating a greater risk. Congestive heart failure, hypertension, age 75 years or older (doubled), diabetes, stroke (doubled), vascular disease, age 65 to 74 years, sex category (female).
Predictors of longest AHREs duration >6 minutes
During the follow-up period, 112 of the 816 (13.7%) patients had AHREs with a longest duration >6 min. Univariate logistic regression analysis showed that LA diameter >41 mm (OR, 2.05; 95% CI, 1.29–3.28; p=0.003), APC >1% on Holter monitoring before CIEDs implantation (OR, 3.14; 95% CI, 1.46–6.78; p=0.004), and sick sinus syndrome for CIEDs implantation (OR, 3.85; 95% CI, 2.42–6.14; p<0.001) predicted a higher probability of longest AHREs duration >6 minutes. The sensitivity analysis of atrial pacing ratio rather than the diagnosis of sick sinus syndrome demonstrated consistent findings (Supplementary Table 2).
Multivariable logistic regression analysis demonstrated that LA diameter >41 mm (OR, 2.08; 95% CI, 1.25–3.45; p=0.005), and prior sick sinus syndrome (OR, 3.22; 95% CI, 1.91–5.43; p<0.001) were significantly associated with the probability of AHREs with a longest duration >6 minutes (Table 4). Comparison of AHREs with a longest duration cut-off of 6 hours showed that prior stroke/TIA (OR, 1.62; 95% CI, 1.08–2.43; p=0.021), LA diameter >41 mm (OR, 1.96; 95% CI, 1.00–3.85; p=0.050), and sick sinus syndrome for CIEDs implantation (OR, 2.52; 95% CI, 1.27-4.99; p=0.008) were independently associated with longest AHREs duration >6 hours (Table 5).
Table 4. Logistic regression analysis of factors associated with longest AHRE >6 min.
| Variables | Univariate analysis | Multivariable analysis* | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | ||
| Age | 1.01 (0.99–1.03) | 0.476 | 1.00 (0.98–1.03) | 0.774 | |
| Male | 1.15 (0.73–1.80) | 0.550 | 1.36 (0.81–2.26) | 0.244 | |
| Body mass index | 0.98 (0.91–1.05) | 0.508 | 0.95 (0.88–1.03) | 0.236 | |
| Hypertension | 0.82 (0.52–1.32) | 0.416 | |||
| Diabetes | 0.79 (0.47–1.33) | 0.370 | |||
| Prior stroke/TIA | 1.60 (0.83–3.08) | 0.162 | |||
| Vascular disease | 1.56 (0.75–3.26) | 0.235 | |||
| CKD | 1.05 (0.48–2.30) | 0.900 | |||
| Dyslipidemia | 0.76 (0.46–1.27) | 0.294 | |||
| LA diameter | 1.03 (0.99–1.07) | 0.105 | |||
| ≤41 mm (reference) | |||||
| >41 mm | 2.05 (1.29–3.28) | 0.003 | 2.08 (1.25–3.45) | 0.005 | |
| LVEF | 0.99 (0.96–1.02) | 0.342 | |||
| PR interval | 1.00 (0.99–1.00) | 0.205 | |||
| APC at Holter monitoring | |||||
| APC <1% (reference) | |||||
| APC >1% | 3.14 (1.46–6.78) | 0.004 | 1.54 (0.67–3.54) | 0.314 | |
| Pacemaker indication | |||||
| AV block (reference) | |||||
| Sick sinus syndrome | 3.85 (2.42–6.14) | <0.001 | 3.22 (1.91–5.43) | <0.001 | |
AHREs = atrial high rate episodes; APC = atrial premature contraction; AV = atrioventricular; CI = confidence interval; CKD = chronic kidney disease; ECG = electrocardiogram; LA = left atrium; LVEF = left ventricular ejection fraction; TIA = transient ischemic attack.
*Adjusted covariates include age, sex, body mass index, LA diameter >41 mm, APC >1% on Holter monitoring, pacemaker indication.
Table 5. Logistic regression of factors associated with AHREs (>6 hours).
| Variables | Univariate analysis | Multivariable analysis* | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | ||
| Age | 1.01 (0.98–1.04) | 0.631 | 1.00 (0.96–1.03) | 0.757 | |
| Male | 1.35 (0.74–2.47) | 0.329 | 1.82 (0.93–3.54) | 0.080 | |
| Body mass index | 1.02 (0.93–1.12) | 0.688 | 1.00 (0.90–1.12) | 0.950 | |
| Hypertension | 1.12 (0.58–2.14) | 0.738 | |||
| Diabetes | 0.63 (0.29–1.34) | 0.228 | |||
| Prior stroke/TIA | 3.03 (1.43–6.42) | 0.004 | 1.62 (1.08–2.43) | 0.021 | |
| Vascular disease | 1.89 (0.77–4.63) | 0.163 | |||
| CKD | 1.24 (0.45–3.38) | 0.678 | |||
| Dyslipidemia | 0.71 (0.35–1.45) | 0.349 | |||
| LA diameter | 1.02 (0.97–1.07) | 0.375 | |||
| ≤41 mm (reference) | |||||
| >41 mm | 2.16 (1.14–4.10) | 0.018 | 1.96 (1.00–3.85) | 0.050 | |
| LVEF | 0.99 (0.95–1.03) | 0.597 | |||
| PR interval | 0.99 (0.98–1.00) | 0.120 | |||
| APC in Holter monitoring implantation | |||||
| APC <1% (reference) | |||||
| APC >1% | 1.05 (0.35–3.17) | 0.928 | |||
| Pacemaker indication | |||||
| AV block (reference) | |||||
| Sick sinus syndrome | 2.89 (1.55–5.38) | 0.001 | 2.52 (1.27–4.99) | 0.008 | |
AHREs = atrial high rate episodes; APC = atrial premature contraction; AV = atrioventricular; CI = confidence interval; CKD = chronic kidney disease; ECG = electrocardiogram; LA = left atrium; LVEF = left ventricular ejection fraction; TIA = transient ischemic attack.
*Adjusted covariates include age, sex, body mass index, prior stroke/TIA, LA diameter >41 mm, pacemaker indication.
Associations between AHREs and clinical outcomes
Total of four CVA events were observed during the study period. The incidence rates (IRs, percent per person-year) of CVA, major/minor bleeding, MI, and all-cause death were 2.30%, 2.30%, 0%, and 0%, respectively, in patients with AHREs >6 minutes. For patients with AHREs ≤6 minutes, these IRs were 0.31%, 0.95%, 0.31%, and 0.31%, respectively. For the AHREs cut-off 6 hours, similar trends were observed (Supplementary Tables 3 and 4).
DISCUSSION
This study observed a substantial incidence in AHREs among patients 19 years of age or older with a history of sick sinus syndrome or atrioventricular node disease who had undergone pacemaker implantation and were free of clinical AF. AHREs with a longest duration >6 minutes were detected in 12.5% of patients within 6 months after implantation and were detected at least once during a median follow-up period of 18 months in 13.7% of patients. Episodes of subclinical atrial tachyarrhythmias were almost five times as common as episodes of clinical AF. During the course of the study, AHREs detection was confirmed in all clinical AF patients. Clinical AF developed in 21.4% (24/112) of patients with longest AHREs duration >6 minutes and 48.9% (24/49) of patients with longest AHREs duration >6 hours, suggesting that there can be a gap between subclinical events and clinical detection. Larger LA size (>41 mm) and sick sinus syndrome for CIEDs implantation were significant predictors of longest AHREs duration >6 minutes. By means of longest AHREs duration >6 hours, prior history of stroke or TIA, larger LA size (>41 mm), and sick sinus syndrome were significant predictors. However, we did not demonstrate a meaningful relationship between AHREs and clinical events including CVA, bleeding, MI, and all-cause death.
Most studies of patients with CIEDs have shown a high incidence of CIEDs-detected AHREs.1),3),4),6),8),9),11),18),19) A growing body of clinical data supports the hypothesis that AHREs are associated with an elevated risk of developing further clinical AF and stroke; however, the stroke risk is relatively lower than that in patients with clinical AF due to the short and rare nature of AHREs.14) Data from ASSERT trial1) showed that AHREs lasting more than 6 min in the first 3 months after CIED implantation were associated with a 5.6-fold increased hazard of clinical AF during a mean 2.5-year follow-up. Similar results were reported in the MOST trial,3) in which AHREs lasting at least 5 minutes were associated with a 5.9-fold increased hazard of clinical AF during median 2.3-year follow-up. Clinical atrial tachyarrhythmias on surface ECG occurred in 25.7% of patients in the MOST trial, 15.8% of patients in the ASSERT trial, and 28.9% of patients in the ASSERT II trial, which investigated patients ≥65 years of age.1),3),20) In subsets of ASSERT patients, older age, higher BMI, and longer AHRE episodes were associated with AF progression.21) In our study, 21.4% and 48.9% of patients with AHREs >6 minutes and >6 hours were confirmed to have clinically documented AF during follow-up. As the number of patients was small, we could not proceed with further analysis. Although AHREs are more likely to allow detection of ECG-documented AF, about 80% of patients with AHREs >6 minutes never develop ECG-documented AF in the subsequent years, probably due to the infrequent and short nature of AHREs in most patients and missing link that we have not proven yet.
We detected four CVA events during the median 18-month follow-up. Our findings did not demonstrate an increased risk of CVA in patients with CIEDs developing AHREs. However, a meta-analysis of seven studies22) showed that AHREs were associated with a 2.4-fold increased risk of stroke. Although the definitions of AHREs duration varied significantly between studies, subsequent stratified analyses showed that most events occurred in patients with >24-hours AHREs.23) Therefore, the predominant evidence suggests a dose-response relationship between longest AHREs duration and stroke risk. Long-term follow-up is necessary to determine the relationship between AHREs and stroke risk in this registry.
The increased risk associated with developing AHREs may be similar to that for AF, reinforcing the concept that AHREs and AF are likely to represent a clinical continuum in the spectrum of atrial tachyarrhythmias. Some predictors for AHREs have been identified in patients with CIEDs. In the ASSERT trial,1) sinus node dysfunction and decreased resting heart rate were associated with the prediction of AHREs. Gonzales et al.9) reported that previous HF predicted AHREs, while Cheung et al.24) found that sinus node disease and a higher proportion of ventricular pacing were associated with AHREs. A meta-analysis of 28 dual-chamber device studies showed that the clinical variables related to AF including increasing age, HTN, DM, CAD, embolic events, and CHADS2 were not associated with the prediction of AHREs. In our study, the independent predictors of AHREs with longest duration >6 minutes were larger LA size (>41 mm), and sick sinus syndrome before CIED implantation. Between patients who developed AHREs and those who did not, an LA size of 41 mm was a useful threshold to identify patients who would develop AHREs. Although total APC >1% on Holter monitoring showed predictive value for AHREs development in univariate analysis, this variable was not associated in the multivariate analysis. Prior stroke/TIA, LA diameter >41 mm, and sick sinus syndrome were the predictors for longest AHREs duration >6 hours. These differences may be explained in part by differences in the definitions of AHREs duration. Therefore, our data indicate new predictors including prior stroke/TIA and LA diameter >41 mm before CIEDs implantation.
This study has several limitations. First, although this was a prospective and multicenter study, the study design was non-randomized and observational. The study duration was relatively short compared to that in previous studies, the enrolled population was small, and additional potential confounding factors may have significantly affected the results. Second, the study population was limited to patients with pacemakers with generally normal LV systolic function. Therefore, the results cannot be directly applied to implantable cardioverter-defibrillator or cardiac resynchronization therapy populations, whose prevalence of CIEDs-detected AF may be higher. Third, although AHREs lasting >6 hours can improve the positive predictive value,11) current guidelines12) consider AHREs lasting >5 minutes as clinically relevant, and the majority of studies3),6),7),8),9),10)used an episode duration of AHRE longer than 5–6 minutes. Therefore, it may be reasonable to analyze the results based on AHREs lasting >6 minutes. Finally, very few clinical events were observed during the follow-up period, including four cases of stroke. Patients are being enrolled and the follow-up period is relatively short; therefore, analysis of long-term data is required.
AHREs occurred frequently in patients with pacemakers who had a history of prior stroke/TIA, LA diameter >41 mm, and sick sinus syndrome before CIEDs implantation but no prior diagnosis of clinical AF. However, there was a lack of data regarding whether AHREs increased the stroke risk. More long-term follow-up data are required to determine the clinical impact of AHREs.
Footnotes
Funding: This study was supported by research grants from the Korean Healthcare Technology R&D project funded by the Ministry of Health & Welfare (HI15C1200, HC19C0130), and from the Abbott Medical Korea Ltd.
Dr. Boyoung Joung has served as a speaker for Bayer, BMS/Pfizer, Medtronic, and Daiichi-Sankyo and received research funds from Medtronic and Abbott.
Conflict of Interest: Dr. Choi received research grants from Bayer, BMS/Pfizer, Biosense Webster, Chong Kun Dang, Daiichi-Sankyo, Samjinpharm, Sanofi-Aventis, Seers Technology, Skylabs, and Yuhan. The other authors have no financial conflicts of interest.
- Conceptualization: Kim M, Kim TH, Yu HT, Choi EK, Park HS, Park J, Lee YS, Kang KW, Shim J, Sung JH, Oh IY, Joung B.
- Data curation: Kim M, Yu HT, Choi EK, Park HS, Park J, Lee YS, Kang KW, Shim J, Sung JH, Oh IY, Joung B.
- Formal analysis: Kim M, Kim TH, Joung B.
- Funding acquisition: Joung B.
- Investigation: Kim M, Kim TH, Yu HT, Choi EK, Park HS, Lee YS, Kang KW, Shim J, Sung JH, Oh IY, Joung B.
- Methodology: Kim M, Kim TH, Yu HT, Oh IY, Joung B.
- Project administration: Kim TH, Joung B.
- Resources: Kim TH, Yu HT, Choi EK, Oh IY, Joung B.
- Software: Kim M, Joung B.
- Supervision: Kim TH, Yu HT, Choi EK, Park HS, Park J, Oh IY, Joung B.
- Validation: Kim M, Kim TH, Yu HT, Joung B.
- Visualization: Joung B.
- Writing - original draft: Kim M, Kim TH.
- Writing - review & editing: Kim M, Kim TH, Yu HT, Oh IY, Joung B.
SUPPLEMENTARY MATERIALS
Distributions according to longest AHRE duration
Sensitivity analysis for sick sinus syndrome: logistic regression analysis of factors associated with longest AHRE >6 minutes
Clinical outcomes occurring after CIED implantation according to AHRE duration of ≤6 minutes vs. >6 minutes
Clinical outcomes occurring after CIED implantation according to AHRE duration of ≤6 hours vs. >6 hours
References
- 1.Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 2.Brambatti M, Connolly SJ, Gold MR, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129:2094–2099. doi: 10.1161/CIRCULATIONAHA.113.007825. [DOI] [PubMed] [Google Scholar]
- 3.Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST) Circulation. 2003;107:1614–1619. doi: 10.1161/01.CIR.0000057981.70380.45. [DOI] [PubMed] [Google Scholar]
- 4.Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–480. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 5.Kim D, Yang PS, Jang E, et al. 10-year nationwide trends of the incidence, prevalence, and adverse outcomes of non-valvular atrial fibrillation nationwide health insurance data covering the entire Korean population. Am Heart J. 2018;202:20–26. doi: 10.1016/j.ahj.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Tse HF, Lau CP. Prevalence and clinical implications of atrial fibrillation episodes detected by pacemaker in patients with sick sinus syndrome. Heart. 2005;91:362–364. doi: 10.1136/hrt.2003.027219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capucci A, Santini M, Padeletti L, et al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005;46:1913–1920. doi: 10.1016/j.jacc.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 8.Mittal S, Stein K, Gilliam FR, 3rd, Kraus SM, Meyer TE, Christman SA. Frequency, duration, and predictors of newly-diagnosed atrial fibrillation following dual-chamber pacemaker implantation in patients without a previous history of atrial fibrillation. Am J Cardiol. 2008;102:450–453. doi: 10.1016/j.amjcard.2008.03.080. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez M, Keating RJ, Markowitz SM, et al. Newly detected atrial high rate episodes predict long-term mortality outcomes in patients with permanent pacemakers. Heart Rhythm. 2014;11:2214–2221. doi: 10.1016/j.hrthm.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler PD, Glotzer TV, Daoud EG, et al. Detection of previously undiagnosed atrial fibrillation in patients with stroke risk factors and usefulness of continuous monitoring in primary stroke prevention. Am J Cardiol. 2012;110:1309–1314. doi: 10.1016/j.amjcard.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman ES, Israel CW, Nair GM, et al. Positive predictive value of device-detected atrial high-rate episodes at different rates and durations: an analysis from ASSERT. Heart Rhythm. 2012;9:1241–1246. doi: 10.1016/j.hrthm.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 13.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 14.Gorenek B. Device-detected subclinical atrial tachyarrhythmias: definition, implications and management-an European Heart Rhythm Association (EHRA) consensus document, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLEACE) (vol 19, pg 1556, 2017) Europace. 2018;20:658. doi: 10.1093/europace/eux163. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen KB, Madsen C, Sandgaard NC, Diederichsen AC, Bak S, Brandes A. Subclinical atrial fibrillation in patients with recent transient ischemic attack. J Cardiovasc Electrophysiol. 2018;29:707–714. doi: 10.1111/jce.13470. [DOI] [PubMed] [Google Scholar]
- 16.Lopes RD, Alings M, Connolly SJ, et al. Rationale and design of the apixaban for the reduction of thrombo-embolism in patients with device-detected sub-clinical atrial fibrillation (ARTESiA) trial. Am Heart J. 2017;189:137–145. doi: 10.1016/j.ahj.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Belkin MN, Soria CE, Waldo AL, et al. Incidence and clinical significance of new-onset device-detected atrial tachyarrhythmia: a meta-analysis. Circ Arrhythm Electrophysiol. 2018;11:e005393. doi: 10.1161/CIRCEP.117.005393. [DOI] [PubMed] [Google Scholar]
- 18.Gillis AM, Morck M. Atrial fibrillation after DDDR pacemaker implantation. J Cardiovasc Electrophysiol. 2002;13:542–547. doi: 10.1046/j.1540-8167.2002.00542.x. [DOI] [PubMed] [Google Scholar]
- 19.Healey JS, Martin JL, Duncan A, et al. Pacemaker-detected atrial fibrillation in patients with pacemakers: prevalence, predictors, and current use of oral anticoagulation. Can J Cardiol. 2013;29:224–228. doi: 10.1016/j.cjca.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Healey JS, Alings M, Ha A, et al. Subclinical atrial fibrillation in older patients. Circulation. 2017;136:1276–1283. doi: 10.1161/CIRCULATIONAHA.117.028845. [DOI] [PubMed] [Google Scholar]
- 21.Wong JA, Conen D, Van Gelder IC, et al. Progression of device-detected subclinical atrial fibrillation and the risk of heart failure. J Am Coll Cardiol. 2018;71:2603–2611. doi: 10.1016/j.jacc.2018.03.519. [DOI] [PubMed] [Google Scholar]
- 22.Mahajan R, Perera T, Elliott AD, et al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J. 2018;39:1407–1415. doi: 10.1093/eurheartj/ehx731. [DOI] [PubMed] [Google Scholar]
- 23.Van Gelder IC, Healey JS, Crijns HJ, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339–1344. doi: 10.1093/eurheartj/ehx042. [DOI] [PubMed] [Google Scholar]
- 24.Cheung JW, Keating RJ, Stein KM, et al. Newly detected atrial fibrillation following dual chamber pacemaker implantation. J Cardiovasc Electrophysiol. 2006;17:1323–1328. doi: 10.1111/j.1540-8167.2006.00648.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distributions according to longest AHRE duration
Sensitivity analysis for sick sinus syndrome: logistic regression analysis of factors associated with longest AHRE >6 minutes
Clinical outcomes occurring after CIED implantation according to AHRE duration of ≤6 minutes vs. >6 minutes
Clinical outcomes occurring after CIED implantation according to AHRE duration of ≤6 hours vs. >6 hours