Abstract
Background
Women with breast cancer are more likely to develop cognitive impairment (CI), insomnia, fatigue, and mood disturbance than individuals with other cancers. The main objectives of this study were to establish the prevalence of CI and examine the relationships between CI, insomnia, fatigue, and mood over the first year of breast cancer treatment.
Methods
Participants were recruited after diagnosis and completed validated measures of insomnia, objective and perceived CI, fatigue, and mood disturbance at four time points during the first year of treatment. A random intercepts cross‐lagged panel model assessed relationships among symptoms over time.
Results
The sample included 98 women. Prevalence of objective CI ranged from 3.1% to 8.2% throughout the year, whereas 36.7% demonstrated a clinically meaningful decline in perceived CI from baseline to 4 months, which remained relatively stable. Greater perceived CI was associated with more fatigue (β = −0.78, z = 17.48, p < .01) and symptoms of insomnia (β = −0.58, z = 5.24, p < .01). Short‐term fluctuations in perceived CI (p < .05), but not fatigue or insomnia, predicted future perceived CI. Fatigue (p < .001) was a significant predictor of future reported symptoms of fatigue and insomnia.
Conclusion
Subjective CI is more prevalent than objective impairments. Fatigue, insomnia, and perceived CI remain stable and are associated during the first year of treatment. Changes in insomnia and fatigue may have little effect on future perceived cognition. Women with breast cancer likely require targeted intervention for these side effects.
Keywords: breast cancer, cognitive impairment, perceived cognitive impairment, insomnia, fatigue, mood disturbance
This study recruited 98 women with non metastatic breast cancer shortly after diagnosis and assessed their objective and perceived cognitive function alongside their levels of insomnia, fatigue, and mood disturbance at 4 time points: shortly after diagnosis and 4 months, 8 months, and 12 months later. Subjective impairment was more prevalent than objective impairment and both remained stable across the year. Perceived impairment in cognition was associated with fatigue and insomnia symptoms, suggesting the need for interventions that can address co‐occurring symptoms.
1. BACKGROUND
Cognitive impairment (CI) in women with breast cancer typically presents as difficulties with memory, learning, attention, executive function, and/or processing speed. 1 , 2 Estimates of the prevalence of CI vary widely as a result of inconsistency in definitions and measurement of CI; however, research suggests that roughly one‐third of breast cancer patients experience CI prior to treatment, which increases to 75 percent during active treatment, and remains at 35 percent in the months and years after treatment completion. 3
CI seldom occurs in isolation and is typically observed alongside other distressing symptoms, including insomnia, fatigue, and psychological distress. 4 Women with breast cancer are more likely to develop insomnia, 5 fatigue, 6 and mood disturbance 7 than individuals with other cancers and their noncancer peers. The available research supports an association between CI, insomnia, fatigue, and mood symptoms, although the direction of the relationships has not been elucidated. Cross‐sectional data have illustrated that breast cancer patients with mild, moderate, and severe insomnia symptoms report greater perceived CI than patients without insomnia symptoms. 8 Studies have also demonstrated a relationship between subjective CI, and fatigue, 9 anxiety, 10 , 11 and depression. 11 This co‐occurrence suggests these symptoms may result from shared physiological and/or behavioral mechanisms that may together contribute to CI above and beyond the contribution of a single side effect. It is also possible that the presence of one symptom exacerbates others, which further contributes to greater overall symptom burden and worse CI. Prospective studies are needed to explore the relative contribution of insomnia, fatigue, and mood disturbance to CI during the first year of active treatment.
2. OBJECTIVE
The first objective of the current study was to characterize the prevalence of objective CI and change in perceived CI among women with breast cancer during the first year of treatment. The second objective was to examine the relationships between perceived CI, insomnia, fatigue, and mood over time. We hypothesized that symptoms of insomnia, fatigue, and mood disturbance at earlier time points would predict higher levels of perceived CI at later time points.
3. METHODS
3.1. Participants
One‐hundred women with breast cancer were recruited from a regional cancer clinic after receiving their breast cancer diagnosis (i.e., after receiving surgery but prior to beginning adjuvant treatment). Eligibility criteria were: (a) female sex; (b) English‐speaking; (c) over 18‐years of age; (d) a diagnosis of stage I‐III breast cancer; and (e) scheduled to receive adjuvant hormone therapy (i.e., tamoxifen or an aromatase inhibitor), chemotherapy, radiation, or trastuzumab if indicated). Exclusion criteria included: (a) previous treatment for cancer or currently undergoing treatment; (b) presence of a sleep disorder other than insomnia that was not currently managed, such as sleep apnea; (c) presence of a psychological disorder that was not stable and/or would impair the individual's ability to participate in the study, such as schizophrenia; and (d) a score lower than 24 on the Mini Mental State Examination (MMSE; i.e., a score suggestive of severe cognitive impairment).
3.2. Procedure
The oncologists screened clinical charts to identify potentially eligible women. Assessments occurred shortly after their clinic visit and after informed consent was obtained. A medical, psychological and sleep disorder screener and the MMSE 12 were administered during the first assessment to evaluate inclusion and exclusion criteria. Participants were assessed four times over the course of one year: prior to treatment (i.e., T1), and 4‐ (i.e., T2), 8‐ (i.e., T3), and 12‐months after commencing treatment (i.e., T4).
All assessments were completed in person or remotely via telehealth for those located in rural areas. Self‐report measures were mailed to those completing telehealth assessments along with a postage paid return envelope.
3.3. Measures
All clinical variables were abstracted from medical charts, and a demographics questionnaire was used to characterize the sample.
3.3.1. Subjective cognitive measures
Perceived cognitive impairment was assessed using the Perceived Cognitive Impairment (PCI) subscale of the Functional Assessment of Cancer Therapy—Cognitive Function (FACT‐Cog), version 3. 13 Higher scores on the FACT‐cog reflect fewer perceived cognitive problems and better quality of life. A decline of 5.6 points on the PCI subscale represents clinically meaningful perceived declines in cognitive functioning. 14
3.3.2. Objective cognitive measures
The use of the following objective cognitive measures was based on the recommendations put forth by the International Cognition and Cancer Task Force (ICCTF). 15 Considering the rural and remote population distribution of Newfoundland and Labrador, only measures that have been validated for administration via telehealth were used. 16 , 17 , 18 The Hopkins Verbal Learning Test‐Revised (HVLT‐R) is a measure of verbal learning and memory, including immediate recall, delayed recall, and delayed recognition. 19 The Controlled Oral Word Association Test (COWAT) measures verbal fluency and cognitive and motor speed that involves areas of executive functioning such as cognitive flexibility, strategy utilization, suppression of interference, and response inhibition. 20 Letter‐Number Sequencing (LNS) measures working memory, attention, and cognitive control.
3.3.3. Symptom measures
The Insomnia Severity Index (ISI) 21 is a seven‐item measure of insomnia severity and higher scores are indicative of more severe insomnia symptoms. The Hospital Anxiety and Depression Scale (HADS) is a 14‐item self‐report measure with subscales that measure anxiety and depression symptoms in the past week. 22 Higher scores indicate worse mood symptoms. The Multidimensional Fatigue Inventory‐Short Form (MFSI‐SF) is a 30‐item self‐report measure used to assess the various manifestations of fatigue. 23 Higher scores are reflective of greater levels of fatigue.
3.4. Statistical analysis
3.4.1. Missing data analysis
One participant discontinued participation halfway through the first assessment and was removed due to the large proportion of missing data. One participant was identified as a multivariate outlier (Mahalanobis distance exceeding the χ2(9) critical of 37.70), 24 and their data were removed. Little's test for missing completely at random (MCAR) indicated that data were missing completely at random, χ 2 = 2588.223, p > .999. Missing data were singly imputed using estimation‐maximization in SPSS 26.
3.4.2. Prevalence of decline in perceived CI
Difference scores in perceived CI relative to baseline were calculated over time and prevalence of decline in perceived CI was quantified as the proportion of women who experienced a change greater than the recognized cutoff of 5.6 points on the PCI subscale. Lower scores on the FACT‐cog PCI subscale are indicative of worse perceived CI.
3.4.3. Changes in perceived CI, insomnia, fatigue, and mood across time
A series of four (Time: T1, T2, T3, T4) repeated measures analysis of variances (ANOVAs) were conducted with cognition, insomnia, fatigue, and mood as dependent variables to evaluate change across time. Greenhouse‐Geisser corrections were used in cases where sphericity was violated. Significant time effects were followed up with pairwise comparisons using the Bonferroni correction to adjust for inflation in family‐wise error associated with performing multiple statistical tests.
3.4.4. Prevalence of objective CI
Objective CI was defined in concordance with the International Cancer and Cognition Task Force (ICCTF) recommendations of ≥2 standard deviations (SD) below published normative means on at least one objective cognitive test or ≥1.5 SD below published normative means on two or more objective tests. 15 Frequencies were tabulated to characterize the prevalence of objective CI at each time point.
3.4.5. Relationship between cognition, insomnia, fatigue, and mood across time
Structural equation modeling was used to examine how perceived CI related to insomnia and fatigue over time. Our original intention was to evaluate symptoms of insomnia, fatigue, and mood; however, mood was excluded from the analysis because of concerns of multicollinearity with fatigue. The inclusion of mood into the model did not appreciably alter results. Cross‐lagged panel models (CLPM) estimate the covariation of multiple variables across time points—while accounting for all other variables in the model—to infer causal influences. We used a Random Intercepts Cross‐Lagged Panel Model (RI‐CLPM) to assess causal pathways between perceived CI, insomnia, and fatigue. The RI‐CLPM breaks down observed variables into two latent components, including trait‐like, time‐invariant, or “between‐person” factors that are controlled for and state‐like, time‐varying, or “within person” factors that are used to estimate autoregressive and cross‐lagged effects for hypothesis testing. We chose the random intercepts variant of the CLPM because our interest was in evaluating the within‐person causal relations between variables after taking between‐person factors that are stable over time into account. For further details on the RI‐CLPM, see Hamaker et al., 2015 25 and Lim et al., 2016. 26
Syntax for the RI‐CLPM was generated using the R‐package riclpmr, 27 which was implemented using lavaan 28 in the R programing language. 29 To simplify, we evaluated a model in which the lagged effects (i.e., cross paths and stability paths) and correlated error terms at 4‐, 8‐, and 12‐months were constrained to be equal over time; constraining our terms in this manner implicitly assumes that the nature and magnitude of the relationship between a given variable at T‐1 and another variable at time T is always the same from one time window to the next. Supporting this decision, the more parsimonious constrained model was favored over a model where these parameters were free to vary as evaluated using either the Akaike Information Criterion (ΔAIC = 9.6 in favor of the simpler model) or the Bayesian Information Criterion (ΔBIC = 87.1 in favor of the simpler model). Model fit was evaluated using the Tucker‐Lewis incremental fit index (TLI 30 ), and root mean square error of approximation (RMSEA 31 ) where values ≥0.90 and ≤0.08 were considered good fit, respectively. 32
4. RESULTS
4.1. Demographic & clinical characteristics
Three hundred and forty‐three women with breast cancer were approached between January of 2017 and February of 2019; refer to Figure 1 for a study flow diagram. Data from 98 participants were analyzed with an attrition rate of 15%. On average, participants were 60 years of age (range 29–83) and had 13.6 years of education (range 7–25). Refer to Table 1.
FIGURE 1.
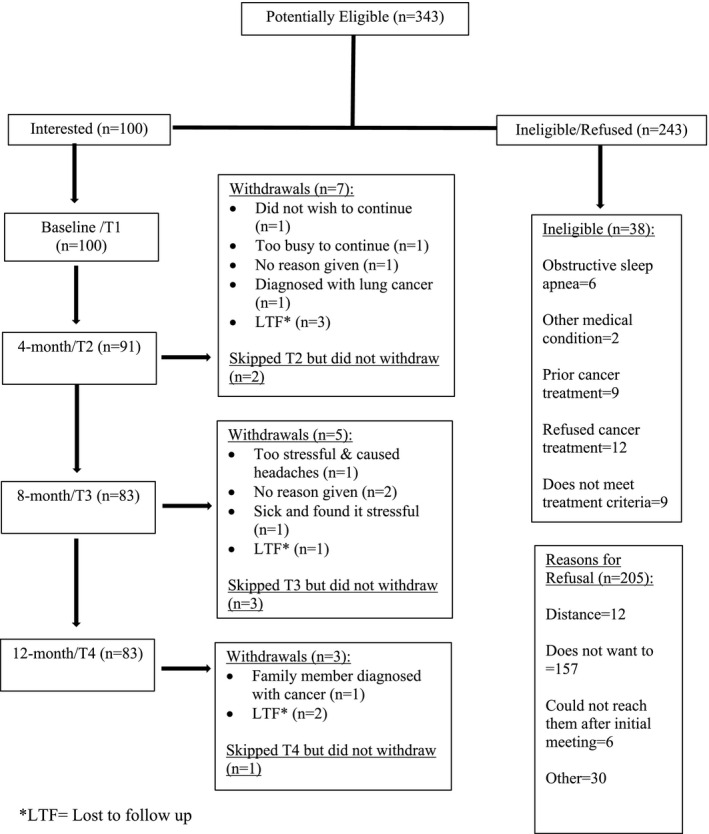
Study flow diagram demonstrating the number of eligible participants and the number of participants who withdrew at each time point. One‐hundred participants completed a baseline assessment. A total of 15 participants dropped out of the study
TABLE 1.
Demographic and clinical characteristics
|
(N = 98) N (%) |
||
|---|---|---|
| Age at enrollment (mean ± SD) | 60.12 ± 11.05 (range = 29–83) | |
| BMI (mean ± SD) | 29.70 ± 7.26 (range = 17.02–51.70) | |
| Marital Status | Married/Committed relationship | 70 (71.4%) |
| Divorced | 7 (7.1%) | |
| Single | 8 (8.2%) | |
| Widowed | 12 (12.2%) | |
| Other | 1 (1.0%) | |
| Number of children | None | 8 (8.2%) |
| One or two | 50 (51.1%) | |
| Three or more | 40 (40.8%) | |
| Race | White/Caucasian | 94 (95.9%) |
| Other | 4 (4.1%) | |
| Education | Some high school (<11 years) | 16 (16.3%) |
| High school (11 years) | 18 (18.4%) | |
| College (12–14) | 28 (28.6%) | |
| Postsecondary (≥15) | 36 (36.7%) | |
| Currently employed | Yes | 36 (36.7%) |
| No | 62 (63.3%) | |
| Premenopausal | Yes | 25 (25.5%) |
| No | 71 (72.4%) | |
| Unsure | 2 (2.0%) | |
| Surgery | Lumpectomy | 40 (40.8%) |
| Simple mastectomy | 48 (49.0%) | |
| Modified radical mastectomy | 10 (10.2%) | |
| Sentinel node biopsy | 74 (75.5%) | |
| Axillary lymph node dissection | 15 (15.3%) | |
| Adjuvant therapy | Chemotherapy | 22 (22.4%) |
| Radiation | 52 (53.1%) | |
| Trastuzumab | 3 (3.6%) | |
| Hormone therapy | Tamoxifen | 19 (19.4%) |
| Aromatase inhibitor | 79 (80.6%) | |
| T Stage | T1 | 67 (68.4%) |
| T2 | 24 (24.5%) | |
| T3 | 6 (6.1%) | |
| T4 | 1 (1.0%) | |
| Estrogen receptor positive | Yes | 98 (100.0%) |
| No | 0 (0.0%) | |
| Progesterone receptor positive | Yes | 91 (92.9%) |
| No | 7 (7.1%) | |
| HER2 positive | Yes | 5 (5.1%) |
| No | 93 (94.9%) |
Caucasians made up 95.9% of the sample. On average, participants were 60.1 years old (range 29–83) and had 13.6 years of education (range 7–25).
Abbreviations: BMI, Body Mass Index; HER2, human epidermal growth factor receptor 2.
4.2. Prevalence of objective CI and changes in perceived CI
Prior to commencing chemotherapy, radiation, and/or hormonal therapies, 6.1% of participants met the ICCTF criteria for objective CI at baseline; 8.2% met criteria at 4 months; 7.1% at 8 months; and 3.1% at 12 months. In contrast, 36.7% of participants demonstrated a clinically meaningful decline in perceived CI from baseline to 4 months and this remained relatively constant throughout the study period. See Table 2. Exploratory comparisons were performed to determine whether those who reported a clinically meaningful decline in perceived CI differed from those who did not report a decline on demographic, clinical, or symptom measures. Individuals with perceived CI at 4 months reported more hours of physical activity at baseline (4.49 ± 4.72) than those without perceived CI (2.76 ± 2.77), t(96) = 2.29, p = .02, Cohen's d = 0.48. Those with greater perceived CI also reported greater insomnia (11.74 vs. 8.64; p = .02, Cohen's d = 0.50), greater fatigue (22.71 vs. 7.47; p = .001, Cohen's d = 0.78), and greater depressive symptoms (5.58 vs. 2.98; p = .002, Cohen's d = 0.73) than those without perceived CI. No other differences were observed between groups on demographic, clinical, or symptom characteristics.
TABLE 2.
Prevalence of objective CI and changes in perceived CI across time
|
(N = 98) N (%) |
||
|---|---|---|
| Pretreatment assessment (T1) | Objective cognitive impairment | 6 (6.1%) |
| 4‐month assessment (T2) | Objective cognitive impairment | 8 (8.2%) |
| Significant decline in perceived cognition from T1 to T2 | 36 (36.7%) | |
| 8‐month assessment (T3) | Objective cognitive impairment | 7 (7.1%) |
| Significant decline in perceived cognition from T1 to T3 | 35 (35.7%) | |
| 12‐month assessment (T4) | Objective cognitive impairment | 3 (3.1%) |
| Significant decline in perceived cognition from T1 to T4 | 35 (35.7%) |
Six participants (6.1%) met criteria for objective cognitive impairment prior to commencing treatment, 8.2% at T2 7.1% at T3, and 3.1% at T4. A significant decline in perceived cognitive functioning was observed in 36.7% of participants from T1 to T2, 35.7% from T2 to T3, and 35.7% from T3 to T4.
Abbreviation: CI, cognitive impairment.
4.3. Changes in perceived CI, insomnia, fatigue, and mood
There was a statistically significant difference in perceived CI across the 12 months [F(2.629, 255.049) = 8.60, p < .001]. Perceived CI scores at baseline were significantly better than perceived CI scores at 4‐, 8‐, and 12‐months. There was a statistically significant difference in insomnia severity between time points [F(3, 291) = 11.13, p < .001]. Insomnia increased at 4‐months and remained stable across the remaining assessments. Fatigue scores changed significantly over time [F(2.337, 226.658) = 7.47, p < .001], with increases from baseline observed at both 4‐ and 8‐months. Fatigue scores decreased again at 12‐months but was not significantly different from any other time point. There was a statistically significant difference in depression scores over time [F(3, 291) = 4.14, p = .007]. Depression scores at baseline were significantly lower than scores at 4‐months. The remaining pairwise comparisons between T2, T3, and T4 were not statistically significant. There was not a statistically significant difference in anxiety symptoms over time [F(3, 291) = 0.338, p = .798]. See Table 3.
TABLE 3.
One‐way ANOVAS demonstrating changes in PCI, insomnia, fatigue, and mood across time
| T1 | T2 | T3 | T4 | F | p | ||
|---|---|---|---|---|---|---|---|
| PCI c | Mean | 56.45 | 52.96 b | 52.68 b | 52.62 b | 8.60 | .000 |
| SD | 10.09 | 10.65 | 10.73 | 10.79 | |||
| Insomnia | Mean | 6.81 | 9.91 b | 9.24 b | 8.48 a | 11.13 | .000 |
| SD | 6.03 | 6.10 | 6.04 | 6.08 | |||
| Fatigue | Mean | 5.84 | 12.77 b | 13.10 b | 10.03 | 7.47 | .000 |
| SD | 17.95 | 19.85 | 18.56 | 17.31 | |||
| Depression | Mean | 3.04 | 3.97* | 3.64 | 3.34 | 4.14 | .007 |
| SD | 3.12 | 3.61 | 2.99 | 2.85 | |||
| Anxiety | Mean | 5.85 | 5.88 | 5.98 | 5.66 | .338 | .798 |
| SD | 4.07 | 3.70 | 3.60 | 3.51 |
A significant difference in scores was found in perceived cognitive impairment (p = .000) from T1 to T2, T1 to T3, and T1 to T4; a significant difference in scores was found in insomnia (p = .000) from T1 to T2, T1 to T3, and T1 to T4; a significant difference was found in fatigue scores (p = .000) from T1 to T2 and T1 to T3; a significant difference was found in depression scores (p = .007) from T1 to T2; no significant differences were found in anxiety scores across time.
Abbreviations: PCI, perceived cognitive impairment; SD, standard deviation.
Statistically different from T1 with p < .05.
Statistically different from mean T1 score with p < .001.
Lower scores on the PCI subscale of the FACT‐cog are indicative of worse perceived cognitive impairment.
4.4. Relationships between change in perceived cognition, insomnia, and fatigue over time
The RI‐CLPM evaluating covariation between change in perceived cognition, insomnia, and fatigue over time indicated good model fit, TLI = 0.97, RMSEA = 0.058; for simplification purposes, an example of the RI‐CLPM is illustrated in Figure 2 using two variables. The means, residual variance, and random effect variance estimates for cognition, fatigue, and insomnia and full model figure are included in Table S1 and Figure S1. At the trait‐like level, there was a strong negative association between perceived CI and fatigue (β = −0.78, z = 17.48, p < .01) and symptoms of insomnia (β = −0.58, z = 5.24, p < .01), indicating that women who reported high perceived CI reported high fatigue and symptoms of insomnia. There was also a strong positive trait‐level association between fatigue and symptoms of insomnia (β = 0.74, z = 9.70, p < .01), indicating that women who reported high fatigue reported high symptoms of insomnia.
FIGURE 2.
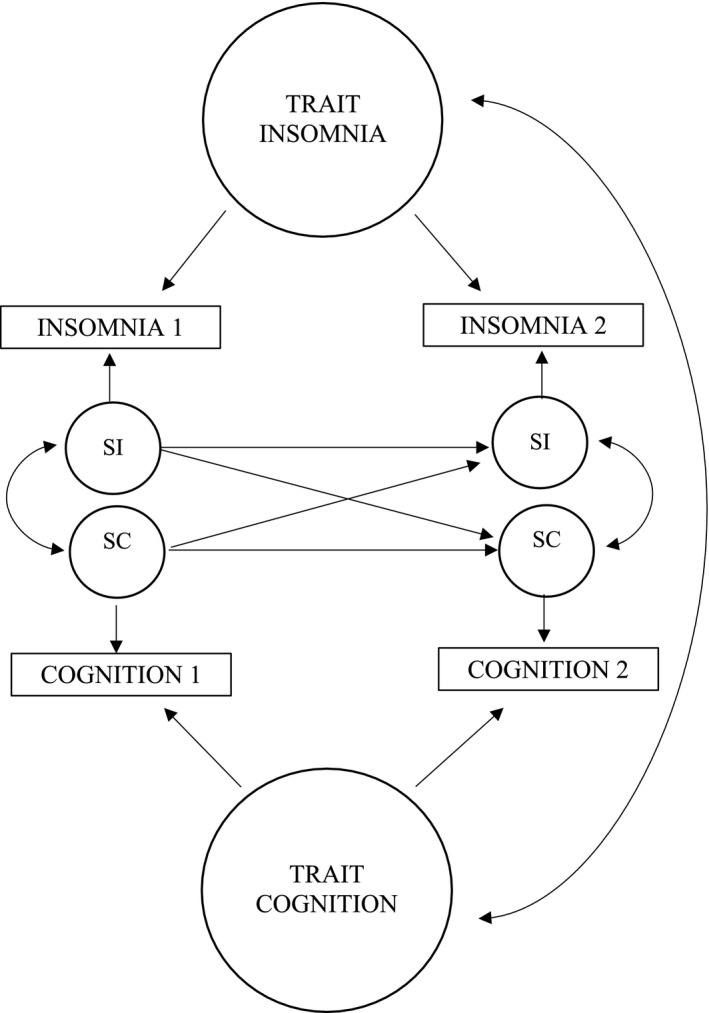
Example of the RI‐CLPM depicting the relationship between two variables—cognition and insomnia—over time. SC, state cognition; SF, state fatigue; SI, state insomnia
After accounting for the above trait‐level associations, perceived CI at a preceding timepoint predicted perceived CI at the following measurement (p < .05). Neither symptoms of insomnia, nor fatigue predicted future perceived CI. A similar association was found for fatigue, wherein fatigue experienced at one timepoint predicted future reported fatigue (p < .01). Neither symptoms of insomnia, nor perceived CI from one time point predicted future reported fatigue. Symptoms of insomnia at one timepoint did not predict symptoms of insomnia at the following measurement. Fatigue (p < .001), but not perceived cognitive impairment, was a significant predictor of future reported symptoms of insomnia.
5. DISCUSSION
5.1. Prevalence of objective cognitive impairment over time
This study is one of the first to investigate objective and perceived CI before and during the first year of treatment for breast cancer. Prevalence of objective CI remained relatively consistent, with estimates ranging from 3.1% to 8.2%. Prior to receiving any treatment, 6.1% of our sample presented with objective CI. Other research has found that women who exhibit pretreatment objective CI do so on only the most challenging attention and memory tests. 33 While the prevalence of objective CI observed in this study during and after breast cancer treatment is lower than reported in previous studies, it appears to be consistent with prevalence rates observed in healthy, cancer‐free populations. 34 The discrepancy between the prevalence rates observed in the present study and estimates found in other studies may be attributable to different sample characteristics and/or the lower sample sizes reported in some of the previous research, which would affect the generalizability of the results to the larger population of women with breast cancer. This discrepancy may also be attributable to differences in the definition and measurement of CI. 35 Specifically, we assessed for objective CI using three objective measures. Thus, it is possible that the lower number of tests included in this study resulted in a more conservative estimate of CI. More research that follows recommended guidelines for assessment of CI is needed to increase confidence in the findings and make more accurate comparisons across studies.
5.2. Changes in perceived cognitive impairment, insomnia, fatigue, and mood over time
In addition to a worsening of insomnia, fatigue, and depression, just over one third of the sample reported clinically meaningful declines in their perception of their cognitive functioning that persisted throughout the study duration. When compared to prevalence of objective CI, these results suggest that it is the perception of cognitive functioning that is of greater concern among women undergoing breast cancer treatment. A number of studies on cognition and cancer have reported weak or absent associations between objective and subjective cognition. 36 , 37 As such, perceived CI may be a more accurate reflection of the impact of even subtle changes in cognition on functioning and quality of life. 37 , 38 Studies with larger samples are required to better understand associations between perceived and objective CI, insomnia, fatigue, and mood disturbance over time.
5.3. Relationships between change in cognition, insomnia, and fatigue over time
Results from the structural equation modeling demonstrated that trait‐like symptoms of fatigue, insomnia, and perceived CI endured over the 12‐month study duration. These side effects were also strongly associated, suggesting that women who experience problems in one domain were more likely to report problems in others. After accounting for trait‐like associations, neither insomnia nor fatigue were predictive of later CI, which refuted our initial hypothesis and suggests that recent fluctuations in insomnia and fatigue may have little effect on perceived cognition months later. Short‐term fluctuations in fatigue did, however, predict subsequent fatigue and insomnia. These results, in part, mirror results from previous research indicating that the presence of fatigue earlier in treatment is one of the strongest predictors of posttreatment fatigue. 39 , 40 , 41 Women with breast cancer also have worse performance on measures of processing speed when they report relatively high levels of fatigue. 42
Prompt intervention may change the trajectory of insomnia, fatigue, and perceived CI over time given that women are experiencing these concerns early on in treatment. Physical activity (both aerobic and resistance/strength training) is considered a category 1 recommendation (i.e., highest level of evidence) for fatigue by the National Comprehensive Cancer Network. 43 A meta‐analysis of randomized controlled trials demonstrated significant reductions in fatigue levels following exercise interventions. 44 Physical activity has also proven beneficial for CI, which may be at least partially explained by improvements in symptoms of depression that result from increased behavioral activation. 45 Further, there is evidence that even low intensity exercises can benefit cognition. 46 Physicians and their patients would thus benefit from discussions around the incorporation of physical activity into the treatment plan at diagnosis and before beginning treatment.
In the present study, state‐like insomnia did not predict itself over time, suggesting that the trait‐like component represents a more meaningful target for intervention due to its overriding importance over time. Thus, the best interventions for insomnia will be those with enduring long‐term effects (e.g., Cognitive Behaviour Therapy for Insomnia; CBT‐I). CBT‐I has demonstrated immediate and long‐lasting effects for reduction of insomnia severity in cancer patients, 47 and has been shown to reduce fatigue over time. 48 As such, CBT‐I might be an important first‐line treatment option for breast cancer patients with a primary insomnia complaint and secondary fatigue symptoms.
6. STRENGTHS AND LIMITATIONS
The present study's unique use of objective measures of cognitive function recommended by the ICCTF in combination with an empirically validated subjective cognitive measure increases the confidence in the prevalence estimates observed. This study is also the first to use structural equation modeling to investigate the relationships between perceived CI, insomnia, fatigue and mood throughout the first year of treatment. This statistical method allowed us to explore directionality of associations between symptoms; however, we cannot fully infer causality because of the potential influence of other unmeasured variables. Lastly, the present model is calibrated only to detect fairly slow associations (e.g., the impact that having fatigue a few months prior would have on cognitive abilities today). If more rapid relations existed, we would be unable to detect them using the chosen analysis.
7. CONCLUSION & IMPLICATIONS
Women report symptoms of CI, insomnia, and fatigue at cancer diagnosis, and these concerns are associated and remain stable across the first year of treatment. Naturally occurring fluctuations in fatigue and insomnia at any given point in time do not predict future CI symptomatology, suggesting that early identification and targeted interventions are required to bring about meaningful improvements in perceived CI. To address this issue, it would be beneficial to invest in the development of multicomponent interventions that can be effectively tailored to address diverse distressing concerns among breast cancer patients in a manner that is cost‐effective. Future research should focus on the early identification and delivery of interventions for cognitive, psychological, and behavioral concerns in women with breast cancer in order to improve overall recovery and well‐being.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Nicole Rodriguez: Conceptualization, formal analysis, funding acquisition, investigation, project administration, writing—original draft, and writing—review and editing. Jonathan Fawcett: Conceptualization, data curation, formal analysis, methodology, software, supervision, and writing—review and editing. Joshua Rash: Conceptualization, formal analysis, supervision, and writing—review and editing. Renee Lester: Resources, supervision, and writing—review and editing. Erin Powell: Resources, supervision, and writing—review and editing. Connor MacMillan: Data Curation, resources, supervision, and writing—review and editing. Sheila Garland: Conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, writing—original draft, and writing– review and editing.
Supporting information
Fig S1
Table S1
Funding information
Ms. Rodriguez received funding from the Canadian Centre for Applied Research in Cancer Control (ARCC). ARCC receives core funding from the Canadian Cancer Society (Grant #2015‐ 703549). Dr. Garland is supported by a New Investigator Award from the Beatrice Hunter Cancer Research Institute (BHCRI). The research was also supported by seed funding from Memorial University of Newfoundland.
DATA AVAILABILITY STATEMENT
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. This research has been reviewed by the Interdisciplinary Committee on Ethics in Human Research and found to be in compliance with Memorial University's Ethics policy.
REFERENCES
- 1. Joly F, Giffard B, Rigal O, et al. Impact of cancer and its treatments on cognitive function: advances in research from the Paris international cognition and cancer task force symposium and update since 2012. J Pain Symptom Manage. 2015;50(6):830‐841. [DOI] [PubMed] [Google Scholar]
- 2. Tao JJ, Visvanathan K, Wolff AC. Long term side effects of adjuvant chemotherapy in patients with early breast cancer. Breast. 2015;24(Suppl 2):149‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer‐related cognitive impairment. Int Rev Psychiatry. 2014;26(1):102‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sanford SD, Beaumont JL, Butt Z, Sweet JJ, Cella D, Wagner LI. Prospective longitudinal evaluation of a symptom cluster in breast cancer. J Pain Symptom Manage. 2014;47(4):721‐730. [DOI] [PubMed] [Google Scholar]
- 5. Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19(3):895‐908. [DOI] [PubMed] [Google Scholar]
- 6. Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528‐538. [DOI] [PubMed] [Google Scholar]
- 7. Maass SW, Roorda C, Berendsen AJ, Verhaak PF, de Bock GH. The prevalence of long‐term symptoms of depression and anxiety after breast cancer treatment: a systematic review. Maturitas. 2015;82(1):100‐108. [DOI] [PubMed] [Google Scholar]
- 8. Liou KT, Ahles TA, Garland SN, et al. The relationship between insomnia and cognitive impairment in breast cancer survivors. JNCI Cancer Spectr. 2019;3(3):pkz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gullett JM, Cohen RA, Yang GS, et al. Relationship of fatigue with cognitive performance in women with early‐stage breast cancer over 2 years. Psychooncology. 2019;28(5):997‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramalho M, Fontes F, Ruano L, Pereira S, Lunet N. Cognitive impairment in the first year after breast cancer diagnosis: a prospective cohort study. Breast. 2017;32:173‐178. [DOI] [PubMed] [Google Scholar]
- 11. Janelsins MC, Heckler CE, Peppone LJ, et al. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age‐matched controls: an analysis from a nationwide, multicenter. Prospective Longitudinal Study J Clin Oncol. 2017;35(5):506‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 13. FACITQuestionnaires . FACIT Questionnaires. FACT‐Cog Scoring and Interpretation Materials. https://www.facit.org/facitorg/questionnaires. Published 2020. Accessed April, 2020.
- 14. Bell MA‐O, Dhillon HM, Bray VJ, Vardy JL. Important differences and meaningful changes for the Functional Assessment of Cancer Therapy‐Cognitive Function (FACT‐Cog). LID ‐ 48. J Patient Rep Outcomes. 2018;12(2):018‐0071. [Google Scholar]
- 15. Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703‐708. [DOI] [PubMed] [Google Scholar]
- 16. Scurrey S, Garland SN, Thoms J, Laing K. Evaluating the experience of rural individuals with prostate and breast cancer participating in research via telehealth. Rural Remote Health. 2019;19(3):52‐69. [DOI] [PubMed] [Google Scholar]
- 17. Munro Cullum C, Hynan LS, Grosch M, Parikh M, Weiner MF. Teleneuropsychology: evidence for video teleconference‐based neuropsychological assessment. J Int Neuropsychol Soc. 2014;20(10):1028‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turner TH, Horner MD, Vankirk KK, Myrick H, Tuerk PW. A pilot trial of neuropsychological evaluations conducted via telemedicine in the Veterans Health Administration. Telemed J E Health. 2012;18(9):662‐667. [DOI] [PubMed] [Google Scholar]
- 19. Brandt J. The Hopkins Verbal Learning Test: development of a new verbal memory test with six equivalent forms. Clin Neuropsychol. 1991;5(2):125–142. [Google Scholar]
- 20. Benton AL, Hamsher K, Rey GL, Sivan AB. Multilingual Aphasia examination, 3rd edn. Iowa City, IA: AJA Associates; 1994. [Google Scholar]
- 21. Morin CM. Insomnia: psychological assessment and management. New York, NY: Guilford Press; 1993. [Google Scholar]
- 22. Mitchell AJ, Meader N, Symonds P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: a meta‐analysis. J Affect Disord. 2010;126(3):335‐348. [DOI] [PubMed] [Google Scholar]
- 23. Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory‐short form. J Pain Symptom Manage. 2004;27(1):14‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tabachnick B, Fidell L. Using multivariate statistics. Boston: Pearson; 2012. [Google Scholar]
- 25. Hamaker EL, Kuiper RM, Grasman RP. A critique of the cross‐lagged panel model. Psychol Methods. 2015;20(1):102‐116. [DOI] [PubMed] [Google Scholar]
- 26. Lim MH, Rodebaugh TL, Zyphur MJ, Gleeson JF. Loneliness over time: the crucial role of social anxiety. J Abnorm Psychol. 2016;125(5):620‐630. [DOI] [PubMed] [Google Scholar]
- 27. Flournoy J. riclpmr: Generate lavaan syntax for RI‐CLPM. R package version 0.1.0.9000; 2020.
- 28. Rosseel Y. lavaan: An R Package for Structural Equation Modeling. J Stat Softw. 2012;48:1‐36. [Google Scholar]
- 29. R Core Team . R: A language and environment for statistical imputing. Version 3.6.2. Vienna, Austria: R Foundation for Statistical Imputing; 2019. [Google Scholar]
- 30. Tucker LR, Lewis C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika. 1973;38:1‐10. [Google Scholar]
- 31. Steiger JHL. J. C. Statistically‐based tests for the number of factors. Paper presented at the Psychometric Society. Iowa City, IA: Psychometrika; 1980. [Google Scholar]
- 32. Hooper D, Coughlan J, Mullen M. Structural equation modelling: guidelines for determining model fit. Electr J Business Res Methods. 2008;6:53‐60. [Google Scholar]
- 33. Kaiser J, Dietrich J, Amiri M, et al. Cognitive performance and psychological distress in breast cancer patients at disease onset. Front Psychol. 2019;10:2584. 10.3389/fpsyg.2019.02584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sachdev PS, Lipnicki DM, Kochan NA, et al. The prevalence of mild cognitive impairment in diverse geographical and ethnocultural regions: the COSMIC collaboration. PLoS One. 2015;10(11):e0142388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schilder CM, Seynaeve C, Linn SC, et al. The impact of different definitions and reference groups on the prevalence of cognitive impairment: a study in postmenopausal breast cancer patients before the start of adjuvant systemic therapy. Psychooncology. 2010;19(4):415‐422. [DOI] [PubMed] [Google Scholar]
- 36. Ahles TA, Hurria A. New challenges in psycho‐oncology research IV: cognition and cancer: conceptual and methodological issues and future directions. Psychooncology. 2018;27(1):3‐9. [DOI] [PubMed] [Google Scholar]
- 37. Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev. 2012;38(7):926‐934. [DOI] [PubMed] [Google Scholar]
- 38. Myers JS. Cancer‐ and chemotherapy‐related cognitive changes: the patient experience. SeminOncolNurs. 2013;29(4):300‐307. [DOI] [PubMed] [Google Scholar]
- 39. Bower JE. Cancer‐related fatigue–mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gutenkunst SL, Vardy JL, Dhillon HM, Bell ML. Correlates of cognitive impairment in adult cancer survivors who have received chemotherapy and report cognitive problems. Supportive Care Cancer. 2020; 10.1007/s00520-020-05616-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim HJ, Jung SO, Kim H, Abraham I. Systematic review of longitudinal studies on chemotherapy‐associated subjective cognitive impairment in cancer patients. Psychooncology. 2020;29(4):617‐631. 10.1002/pon.5339. [DOI] [PubMed] [Google Scholar]
- 42. Small BJ, Jim HSL, Eisel SL, Jacobsen PB, Scott SB. Cognitive performance of breast cancer survivors in daily life: role of fatigue and depressed mood. Psychooncology. 2019;28(11):2174‐2180. 10.1002/pon.5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. National Comprehensive Cancer Network . NCNN guidelines for supportive care. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf. Published 2020. Accessed May, 2020.
- 44. Juvet LK, Thune I, Elvsaas I, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow‐up: a meta‐analysis. Breast. 2017;33:166‐177. [DOI] [PubMed] [Google Scholar]
- 45. Bedillion MF, Ansell EB, Thomas GA. Cancer treatment effects on cognition and depression: the moderating role of physical activity. Breast. 2019;44:73‐80. 10.1016/j.breast.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 46. Gokal K, Munir F, Ahmed S, Kancherla K, Wallis D. Does walking protect against decline in cognitive functioning among breast cancer patients undergoing chemotherapy? Results from a small randomised controlled trial. PLoS One. 2018;13(11):e0206874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ma Y, Hall DL, Ngo LH, Liu Q, Bain PA, Yeh GY. Efficacy of cognitive behavioral therapy for insomnia in breast cancer: a meta‐analysis. Sleep Med Rev. 2020;55:101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zachariae R, Amidi A, Damholdt MF, et al. Internet‐delivered cognitive‐behavioral therapy for insomnia in breast cancer survivors: a randomized controlled trial. J Natl Cancer Inst. 2018;110(8):880‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Table S1
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. This research has been reviewed by the Interdisciplinary Committee on Ethics in Human Research and found to be in compliance with Memorial University's Ethics policy.


