Abstract
Mice are the most common animal model to investigate human disease and explore physiology. Mice are practical, cost efficient, and easily used for genetic manipulations. Although variability in cardiac structure and function among mouse strains is well noted, the effect of mouse strain on vascular stiffness indices is not known. Here, we compared mouse strain-dependent differences in key vascular stiffness indices among frequently used inbred mouse strains—C57Bl/6J, 129S, and Bl6/129S. In young healthy animals, baseline blood pressure and heart rate were identical in all strains, and independent of gender. However, both active in vivo and passive ex vivo vascular stiffness indices exhibited distinct differences. Specifically, both male and female 129S animals demonstrated the highest tensile stiffness, were least responsive to acetylcholine-induced vasorelaxation, and showed the lowest pulse wave velocity (PWV), an index of in vivo stiffness. C57Bl/6J mice demonstrated the highest PWV, lowest tensile stiffness, and the highest response to acetylcholine-induced vasorelaxation. Interestingly, within each strain, female mice had more compliant aortas. C57Bl/6J mice had thinner vessel walls with fewer layers, whereas 129S mice had the thickest walls with the most layers. Values in the Bl6/129S mixed background mice fell between C57Bl/6J and 129S mice. In conclusion, we show that underlying vascular properties of different inbred wild-type mouse strains are distinct, despite superficial similarities in blood pressure. For each genetic modification, care should be taken to identify proper controls, and conclusions might need to be verified in more than one strain to minimize the risk of false positive studies.
Keywords: Mouse model, Strains, Vasculature
Introduction
Mice are the animal model most commonly used to simulate human disease and explore physiology. They are practical and cost efficient and can readily undergo genetic modification to cater to any research need. A plethora of different inbred mouse strains are commercially available. Inbred mice have the advantage of genetic homogeneity; however, different strains can exhibit significant genetic divergence [1]. Basic cardiovascular indices such as blood pressure can also vary greatly among different mouse strains [2]. However, this fundamental fact is often overlooked, and researchers might assume that these strains are identical in key physiologic components, such as blood pressure, vascular stiffness, and vasoreactivity, as well as the underlying mechanisms.
In cardiovascular studies, the C57Bl/6J strain (black 6) is widely used, as are the 129S and mixed Bl6/129S strains. Prior research has shown that cardiac function can vary greatly between strains, with C57Bl/6J maintaining better cardiac function than other inbred strains after an ischemic insult [3]. Furthermore, echocardiographic, exercise response, and telemetric variables can vary between strains, with C57Bl/6J mice demonstrating relative eccentric cardiac hypertrophy and increased exercise tolerance—comparable to an “athlete’s heart” [4]. The data on vascular properties, however, are not as robust [5, 6]. Ryan et al. [5] have previously reported that endothelial function varies in certain inbred mouse strains, despite the mice being normotensive, with C57Bl/6J exhibiting a very robust response to endothelium-dependent vasorelaxation.
Vascular stiffness is gaining acceptance as a predictor of cardiovascular mortality and as an indicator of major cardiovascular events, such as perioperative myocardial infarctions, heart failure, stroke, and death [7, 8]. Vascular stiffening results from complex alterations to both composition of and interactions between the constituent cellular and structural elements of the vessel [7, 9]. However, despite recent advances, the specific molecular mechanisms underlying these changes are still poorly understood. Clinically, increases in the indices of vascular stiffness (such as pulse wave velocity [PWV]) correlate independently with poor outcomes [8]. Understanding the biochemical mechanisms of vascular stiffness is an essential first step to developing targeted therapies [10–12]. Investigators often choose a specific mouse strain for this research because it happens to be the background strain for a certain mutation that is being studied, or, almost by default, they choose the black 6 strain when a normotensive mouse is needed [13]. Given the prominent role of mice in research on vascular stiffness, our aim in this study was to determine if frequently used strains exhibit differences in vascular properties, specifically vascular stiffness.
Methods
Animals
Three different mouse strains were used in this study: C57Bl/6J, 129S, and the mixed background Bl6/129S. Both male and female mice were used (n = 10 animals per group and gender, 8–12 weeks of age). Animals were maintained in the Johns Hopkins University School of Medicine animal care facility on a 12:12–h light–dark cycle and were fed and watered ad libitum. All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University.
Wire myography
Vasoreactivity of isolated aortic rings was studied as previously described [12–15]. Vasocontractile response was determined with a phenylephrine dose response (10−9–10−5 mol/L) and normalized to a potassium chloride response (60 mM). Next, the aortas were preconstricted with phenylephrine (10−6 mol/L; Sigma-Aldrich), endothelium-dependent responses were determined with acetylcholine (10−9–10−5 mol/L), and endothelium-independent vasorelaxation was investigated by using sodium nitroprusside (10−9–10−5 mol/L; Sigma-Aldrich).
Immunohistochemistry
Mouse thoracic aortic rings were cut into 2 mm lengths, formalin fixed, paraffin embedded, sectioned at 6 μm, and mounted onto slides. Hematoxylin and eosin, Masson trichrome, and Movat pentachrome stains were applied in the Department of Pathology Reference Histology laboratory with standard methods. Images were digitally captured with a Laxco microscope at 10×, 20×, and 40× magnification.
PWV
PWV was measured noninvasively by using a high-frequency, high-resolution Doppler spectrum analyzer [11, 16–18]. Mice were anesthetized with 1.5% isoflurane and placed supine on a heated (37 °C) plate. A 10 MHz probe was used to record the aortic pulse waves at thorax and abdomen separately at a distance of 4 cm. The electrocardiograph was recorded simultaneously and the time taken by the wave to travel from thoracic aorta to abdominal aorta was measured by using the R wave of the electrocardiograph as a fixed point. Subsequently, PWV was calculated as the separation distance divided by the pulse transit time between the two points.
Blood pressure measurements
After slow acclimatization over 10 days, awake animals were gently restrained in a translucent plastic cone with the tail exposed. Blood pressure was determined with a tail-cuff measurement system (Kent Scientific).
Tensile testing
We analyzed the elastic properties of two to three aortic rings from each animal by tensile testing as previously described [12–14]. Briefly, the aorta was harvested and cut into 2 mm rings. We obtained transverse and longitudinal images of each sample to calculate vessel dimensions (lumen diameter, wall thickness, and length). Samples were mounted on an electromechanical puller (DMT). After calibration, the pins were moved apart with an electromotor and displacement and force were recorded continuously. Engineering stress (S) was calculated by normalizing force (F) to the initial stress-free area of the specimen (S = F/2t × L; where t = thickness and L = length of the sample). Engineering strain (λ) was calculated as the ratio of displacement to the initial stress-free diameter. Stress–strain relationships were represented by the equation S = α exp (βλ), where α and β are constants. α and β were determined by nonlinear regression for each sample and were used to generate stress–strain curves by treating the x-axis as a continuous variable.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Student’s t test was used to compare two means. Sample size (n) is indicated for each reported value. For statistical evaluation, two means were compared by the nonparametric Wilcoxon rank-sum test and groups were compared with the Kruskal–Wallis nonparametric test with Dunn’s comparison. For multiple comparisons, two-way analysis of variance with Bonferroni post hoc analysis was used. Means were considered to be statistically different at P < 0.05.
Results
Baseline cardiovascular properties
Blood pressure was identical in all three strains (P > 0.05 for all comparisons; Fig. 1) with no differences noted by gender or strain in systolic blood pressure (C57Bl/6J: 105.3 ± 2.5 mmHg, Bl6/129S: 100.0 ± 1.9 mmHg, 129S: 105.8 ± 3.4mmHg; Fig. 1a), diastolic blood pressure (C57Bl/6J: 79.2 ± 2.8 mmHg, Bl6/129S: 73.0 ± 2.1 mmHg, 129S: 80.7 ± 3. 7 mmHg; Fig. 1b), or mean arterial pressure (C57Bl/6J: 87.5 ± 2.7 mmHg, Bl6/129S: 82.4 ± 1.9 mmHg, 129S: 88.7 ± 3.6 mmHg; Fig. 1c). Heart rate (in beats per minute [bpm]), which was measured simultaneously, was also identical in all three groups (C57Bl/6J: 329.5 ± 11.5 bpm, Bl6/129S: 339.3 ± 15.0 bpm, 129S: 333.3 ± 18.7 bpm; Fig. 1d).
Fig. 1.
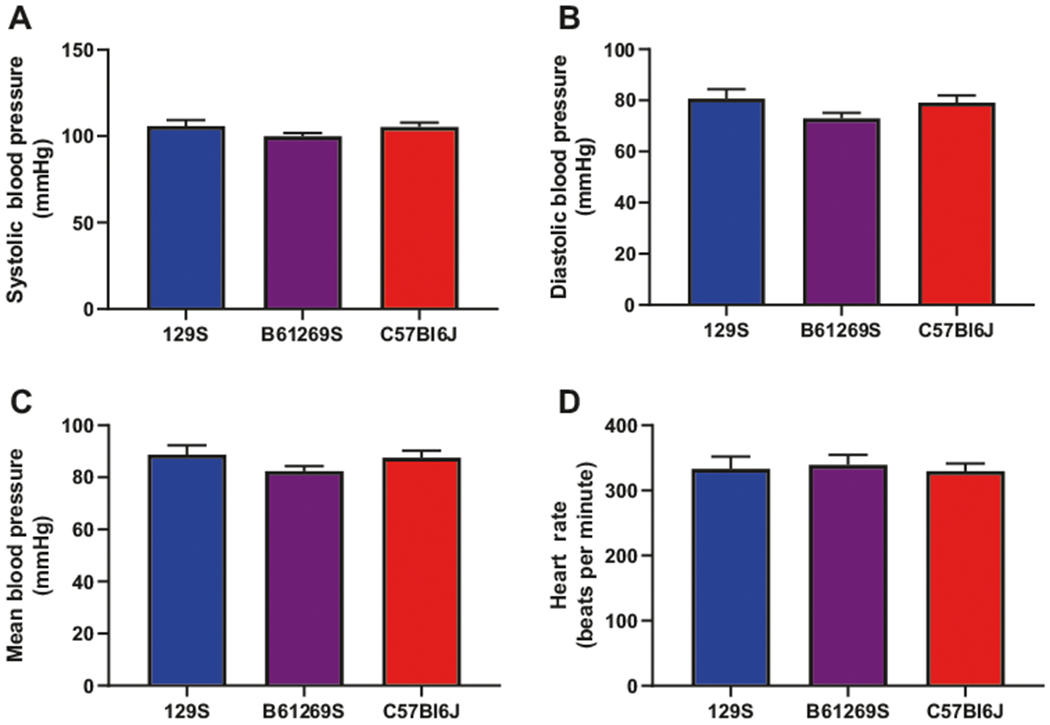
Blood pressure and heart rate. Blood pressure measured noninvasively via tail-cuff was identical in all three mouse strains. Neither systolic (a), nor diastolic (b), nor mean blood pressure (c) differed among the groups. d Heart rate also showed no significant difference among the groups (n = 10 mice per group)
Ex vivo passive vascular stiffness
The mechanical modulus of the aorta from the three mouse strains was determined by tensile testing with an electromechanical puller. The three mouse strains exhibited significant differences in ex vivo tensile properties, with 129S mice demonstrating the highest passive vascular stiffness (P < 0.001 129S vs. B6/129S and 129S vs. C57Bl/6J; Fig. 2a), though B6/129S mice were not significantly different from C57Bl/6J mice. The maximum stress value at a strain of 3 was 42,265 mN/mm2 in 129S mice, 11,265 mN/mm2 in B6/129S mice, and 8698 mN/mm2 in C57Bl/6J mice. Aortic rings from female mice were markedly more compliant when compared with male mice of the same strain (Fig. 2b; P < 0.01 for male vs. female).
Fig. 2.
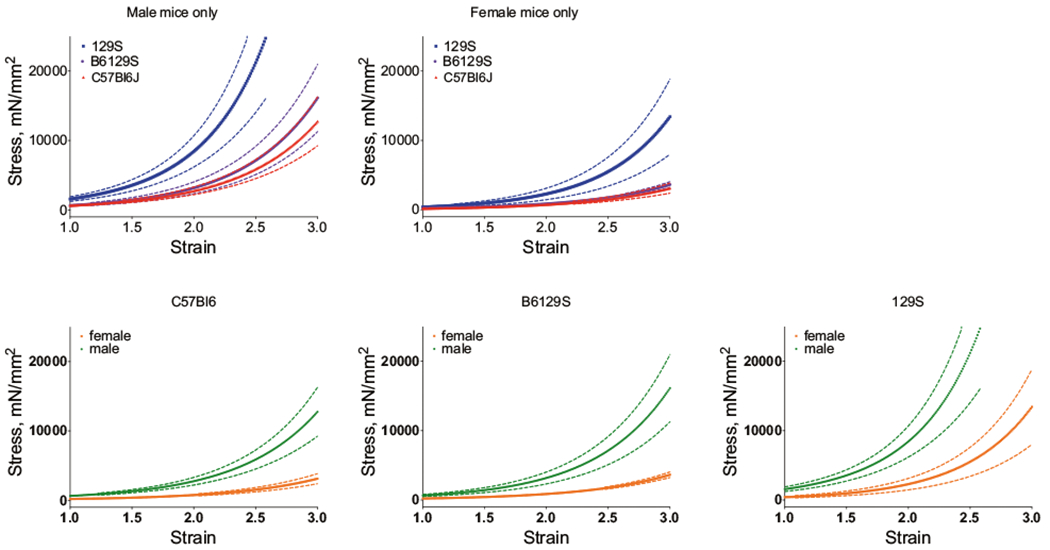
Ex vivo tensile testing. The stress–strain relationship of the aortas from the three mouse strains showed significant differences in ex vivo tensile properties. a The highest passive vascular stiffness was observed in 129S mice, with aortas from B6/129S and C57Bl/6J mice being significantly less stiff (n = 10, p < 0.0001 for 129S vs. B6/129S and 129S vs. C57Bl/6J). This was true for male mice (ai) and female mice (aii); bi–iii Passive stiffness was lower in female mice vs. male mice in all three strains (n = 5, male vs. female p <0.0001 for all strains)
Endothelial function and vasoreactivity
Gender did not affect vasoconstrictive responses measured in this study. The vasoconstrictive response of aortic rings to the thromboxane A2 analog U46619 was similar in all of the mouse strains (Fig. 3a). The maximum vasoconstrictive response was: 265.8 ± 32.8% in C57Bl/6J mice, 218.6 ± 5.7% in B6/129S mice, and 224.5 ± 10.8% in 129S mice (P > 0.05). Vasoconstriction elicited by the alpha-adrenergic agonist phenylephrine (PE) was similar in the 129S and B6/129S mice (Fig. 3b). However, the PE-induced vasoconstriction response was markedly higher in aortic rings from C57Bl/6J mice 68.2 ± 9.3%) than in those from 129S and B6/129S mice (62.0 ± 8.3% and 45.9 ± 6.7%). The maximum vasodilatory response to acetylcholine was significantly different in between strains, 81.8 ± 6.5% in C57Bl/6J mice, 46.0 ± 5.5% in B6/129S mice, and 22.2 ± 3.7% in 129S mice (P < 0.0001). Consistent with prior work [5], maximum contractility was lowest in C57Bl/6J mice (397.1 ± 50.9% in C57Bl/6J mice, 544.4 ± 62.0% in B6/129S mice, and 665.2 ± 83.6% in 129S mice; Fig. 3c).
Fig. 3.
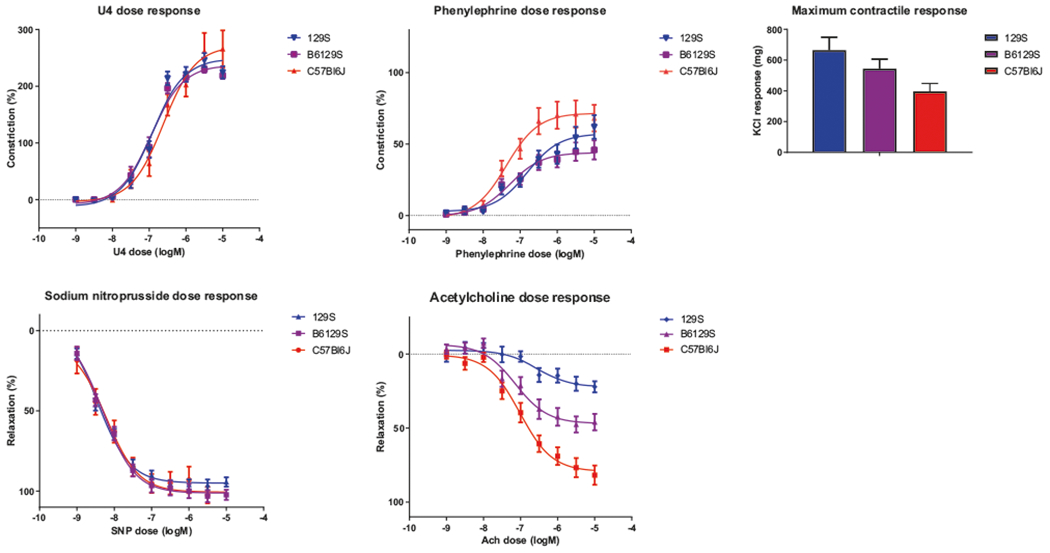
Vasoreactivity of aortic rings. a Wire myography showed no difference in U46619-induced vasoconstriction among the three mouse strains. However, in comparison to 129S and B6/129S mice, C57Bl/6J mice had the greatest alpha-1-dependent vasoconstrictive response to phenylephrine (b) and the lowest maximum contractile response to potassium chloride (KCl) (c). Similarly there was no difference in the nitric endothelium-independent response to sodium nitroprusside (SNP) (d), but C57Bl/6J did show a much more robust nitric oxide-dependent response to acetylcholine (Ach) (e). (n = 10 per group, P < 0.001 for 129S vs. C57Bl/6J, 129S vs. B6/129S, and C57Bl/6J vs. B6/129S)
We next examined vasorelaxation of PE-preconstricted vessels. The endothelium-independent vasodilatory response to sodium nitroprusside was identical in all three strains (both the maximum response and the EC50; Fig. 3d). The maximum vasodilatory response was 110.3 ± 4.4% in C57Bl/6J mice, 102.3 ± 3.0% in B6/129S mice, and 94.3 ± 2.8% in 129S mice (P > 0.05). Interestingly the three groups exhibited distinct differences in the endothelium-dependent response to acetylcholine (both the maximum response and the EC50; Fig. 3e).
In vivo vascular stiffness
In vivo stiffness is the sum total of the contributions from the vascular matrix and vascular tone/vascular smooth muscle cell stiffness. PWV, a reliable and well-accepted index of in vivo vascular stiffness, was measured noninvasively by Doppler. PWV was highest in C57Bl/6J mice (stiffer vasculature) and lowest in 129S mice (more compliant vasculature, P = 0.02). The results for B6/129S mice fell between these two extremes (4.10 ± 0.19 m/s in C57Bl/6J mice, 3.73 ± 0.23 m/s in B6/129S mice, and 3.27 ± 0.13 m/s in 129S mice; Fig. 4). No correlation to gender was noted.
Fig. 4.
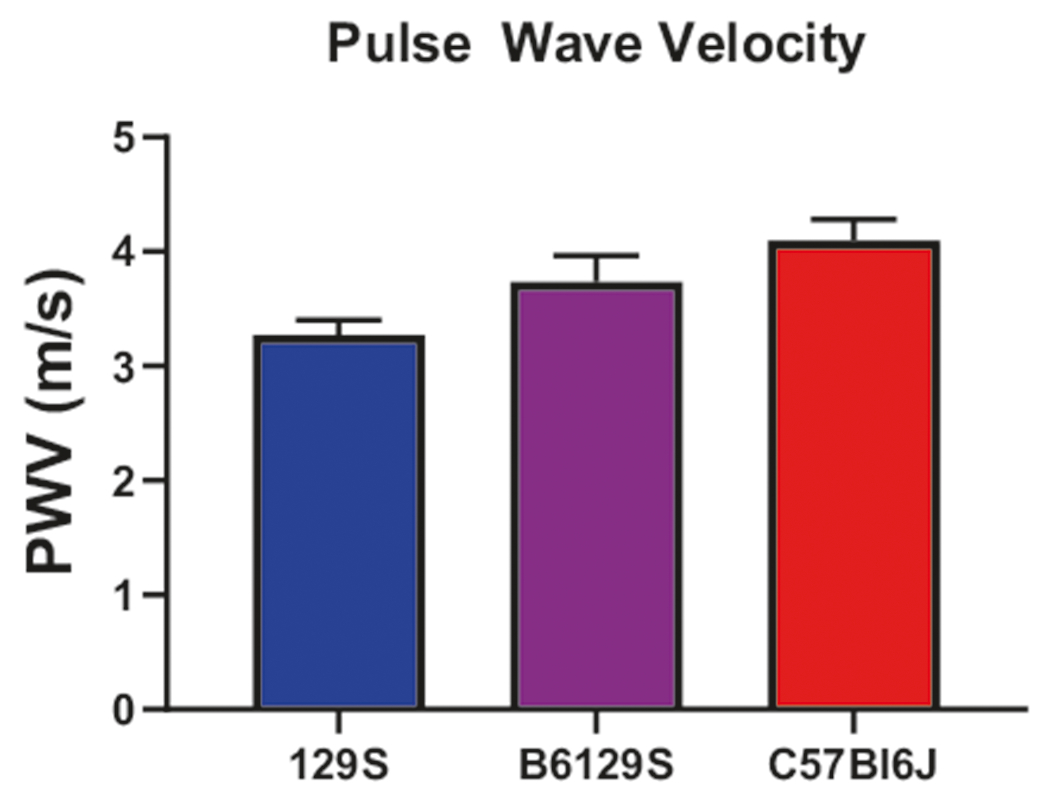
Pulse wave velocity (PWV). In vivo vascular stiffness was highest in C57Bl/6J mice and lowest in 129S mice (n = 10 mice per group)
Vascular composition and structure
Aortic cross sections were examined by immunohistochemical analysis. Hematoxylin and eosin staining were used to determine lumen diameter and wall thickness, and MOVAT and Masson’s Trichrome were used to examine collagen and elastin content (Fig. 5). We observed significant differences in vessel wall thickness between the three strains, with 129S animals having the thickest vessel walls and C57Bl/6J having the thinnest (C57Bl/6J: 45.3 ± 1.58 μm, B6/129S: 55.6 ± 2.3 μm, 129S: 67.0 ± 0.56 μm; C57Bl/6J vs. B6/129S: P = 0.001, C57Bl/6J vs. 129S: P < 0.0001, B6/129S vs. 129S: P = 0.0006; Fig. 6a). Interestingly the mean number of distinct lamellar units (elastic lamella and adjacent smooth muscle cells) [19] was also different, consistent with the results of vessel wall thickness (C57Bl/6J: 4.43 ± 0.20 μm, B6/129S: 4.83 ± 0.17 μm, 129S: 5.33 ± 0.21 μm; C57Bl/6J vs. B6/129S: P = 0.48, C57Bl/6J vs. 129S: P = 0.014, B6/129S vs. 129S: P = 0.26; Fig. 6b). No differences were noted in the collagen/elastin content or the elastic lamella by strain or gender.
Fig. 5.
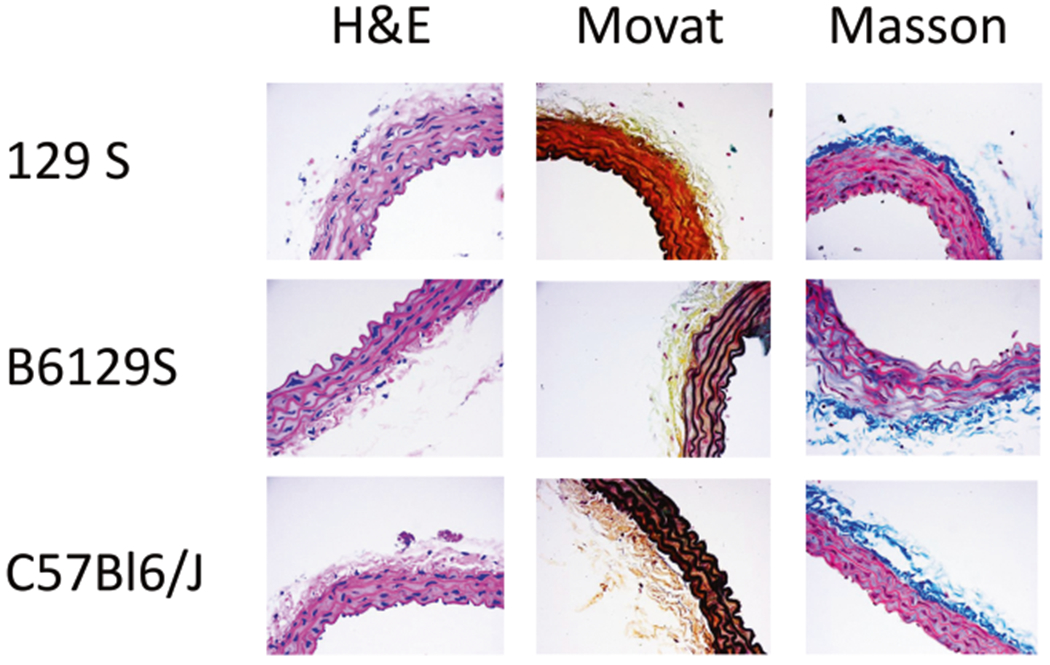
Immunohistochemistry. Representative images of hematoxylin and eosin-stained aortic sections from each of the three mouse strains show differences in wall thickness and vessel layers. No differences were noted in the collagen/elastin content (Movat staining) or the elastic lamella (Masson’s Trichrome staining)
Fig. 6.
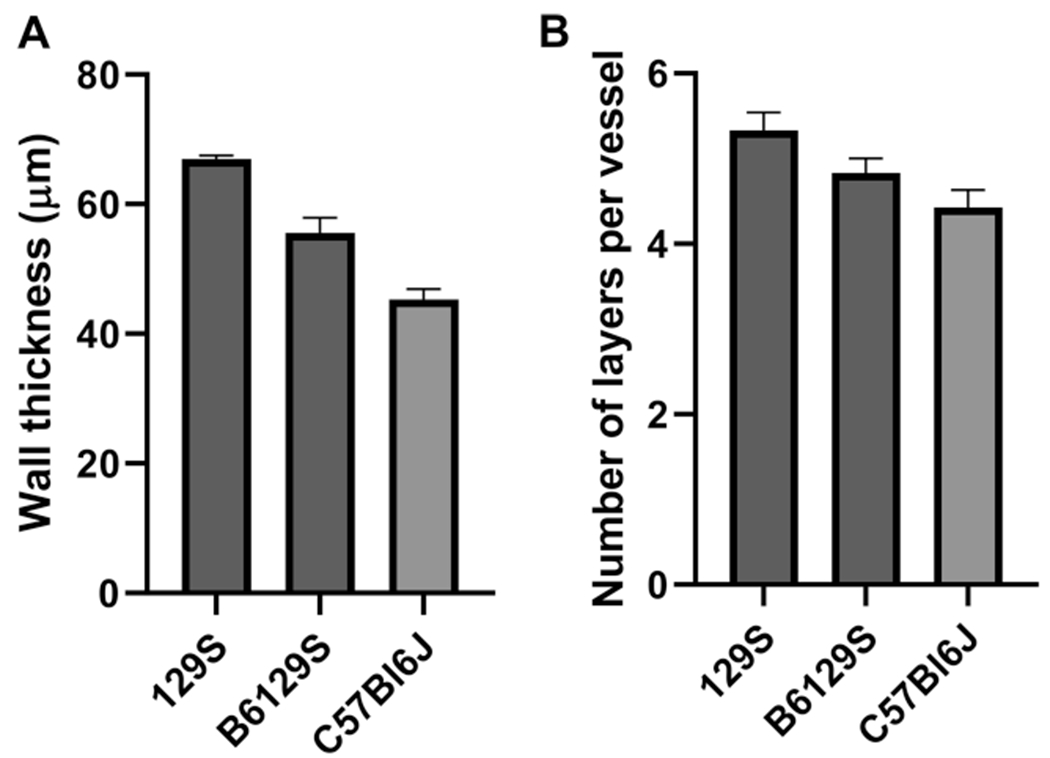
Bar graphs of wall thickness and number of vessel layers. a The mouse strains exhibited significant differences in vessel wall thickness, with 129S mice having the thickest vessel walls and C57Bl/6J mice having the thinnest. b Mean number of distinct lamellar units (layers) was also different (n = 5 mice per group for each stain)
Discussion
In this study we evaluated vascular phenotype differences among inbred mouse strains of different backgrounds that are frequently used in cardiovascular studies. Our results show that the major elastic vessel, the aorta, has distinct properties in different strains and within male and female animals. Interestingly these differences do not translate into overt differences in blood pressure or heart rate and are likely underappreciated when investigators are selecting mouse models.
Increased stiffening of the central vasculature is a hallmark of aging and hypertension. It is also highly predictive of major adverse cardiovascular events, such as perioperative myocardial infarction, heart failure, and death [8, 20]. Furthermore, it has been shown previously that vascular stiffness is closely related to age-associated hypertension, which precedes the development of clinical hypertension [11, 12]. This vascular stiffening results from complex alterations to both composition of and interactions between the constituent cellular and structural elements of the vessel, including extracellular matrix remodeling [13, 14, 20, 21]. It is also known that different mouse strains demonstrate a certain degree of genetic variation and differences in blood pressures. Given these potential differences, we elected to study frequently used mouse strains that have identical normotensive blood pressures at baseline [1, 2]. A limitation of our study is that we performed tail-cuff blood pressure measurements. Despite being a measure in awake animals, this approach provides a daily spot measurement and not continuous monitoring, as would have been possible with a telemetry system. To overcome this limitation of the tail-cuff method, repeated measurements were obtained in each mouse over 7–10 days prior to harvest of aorta for ex vivo analyses.
We found distinct differences in active and passive vascular stiffness among the three strains tested, as well as between male and female animals. C57Bl/6J mice had the most compliant aortas and exhibited greater PE-induced vasoconstriction and acetylcholine-induced vasorelaxation responses when compared with the other strains. Moreover, C57Bl/6J mice had thinner vessel walls with fewer lamellar units, whereas 129S mice had the thickest walls with the most lamellar units. However, contrary to the passive stiffness, in vivo stiffness (PWV), blood pressure, and heart rate were similar in all of the groups. Overall, the low ex vivo/passive vascular compliance of the thin vessels is offset by high contractility and higher active tone, resulting in similar in vivo vascular stiffness. These results suggest that the response of C57Bl/6J mice to hypertensive stimuli would be distinct from that of the other two strains tested in this study. This finding has important implications for choosing a mouse strain for cardiovascular studies, as the underlying properties of different mouse strains are distinct, despite superficial similarities in blood pressure. When using homozygous knockout mouse models, the selection of a control wild-type group should be carefully considered to most closely match the knockout mouse. In general, our data suggest that C57Bl6 mice, yield the highest dynamic range for vasoreactivity and stiffness studies. Conversely, 129S mice are a poor choice for vasoreactivity and stiffness studies. The ideal study design would use a het/het breeding strategy that would yield wild-type and homozygous littermate mice. In cases where mouse models have been bred to homogeneity, the use of the same background strain as control wild types is critical. Finally, studies might need to be verified in more than one background strain and in both genders to maximize the robust interpretation of preclinical data.
Acknowledgments
Funding This work was supported by two Stimulating and Advancing ACCM Research (StAAR) grants from the Department of Anesthesiology and Critical Care Medicine at Johns Hopkins University (to LS and JS), a JHU Discovery Award and MedImmune research award (to LS), and a K08 grant (K08HL145132) from the National Heart Lung and Blood Institute (to JS).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Doevendans PA, Daemen MJ, de Muinck ED, Smits JF. Cardiovascular phenotyping in mice. Cardiovasc Res. 1998;39:34–49. [DOI] [PubMed] [Google Scholar]
- 2.Schlager G, Weibust RS. Genetic control of blood pressure in mice. Genetics. 1967;55:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnabei MS, Palpant NJ, Metzger JM. Influence of genetic background on ex vivo and in vivo cardiac function in several commonly used inbred mouse strains. Physiol Genom. 2010; 42A:103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoit BD, Kiatchoosakun S, Restivo J, Kirkpatrick D, Olszens K, Shao H, et al. Naturally occurring variation in cardiovascular traits among inbred mouse strains. Genomics. 2002;79:679–85. [DOI] [PubMed] [Google Scholar]
- 5.Ryan MJ, Didion SP, Davis DR, Faraci FM, Sigmund CD. Endothelial dysfunction and blood pressure variability in selected inbred mouse strains. Arterioscler Thromb Vasc Biol. 2002;22:42–8. [DOI] [PubMed] [Google Scholar]
- 6.Fujii M, Hara H, Meng W, Vonsattel JP, Huang Z, Moskowitz MA. Strain-related differences in susceptibility to transient forebrain ischemia in SV-129 and C57black/6 mice. Stroke. 1997;28:1805–10. [DOI] [PubMed] [Google Scholar]
- 7.Di Lazzaro V, Pilato F, Dileone M, Tonali PA, Ziemann U. Dissociated effects of diazepam and lorazepam on short-latency afferent inhibition. J Physiol. 2005;569:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27. [DOI] [PubMed] [Google Scholar]
- 9.Steppan J, Barodka V, Berkowitz DE, Nyhan D. Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol Res Pract. 2011;2011:263585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh YJ, Pau VC, Steppan J, Sikka G, Bead VR, Nyhan D, et al. Role of tissue transglutaminase in age-associated ventricular stiffness. Amino Acids. 2017;49:695–704. [DOI] [PubMed] [Google Scholar]
- 11.Steppan J, Wang H, Bergman Y, Rauer MJ, Tan S, Jandu S, et al. Lysyl oxidase-like 2 depletion is protective in age-associated vascular stiffening. Am J Physiol Heart Circ Physiol. 2019;317:H49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steppan J, Sikka G, Jandu S, Barodka V, Halushka MK, Flavahan NA, et al. Exercise, vascular stiffness, and tissue transglutaminase. J Am Heart Assoc. 2014;3:e000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung SM, Jandu S, Steppan J, Belkin A, An SS, Pak A, et al. Increased tissue transglutaminase activity contributes to central vascular stiffness in eNOS knockout mice. Am J Physiol Heart Circ Physiol. 2013;305:H803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steppan J, Bergman Y, Viegas K, Armstrong D, Tan S, Wang H, et al. Tissue transglutaminase modulates vascular stiffness and function through crosslinking-dependent and crosslinking-independent functions. J Am Heart Assoc. 2017;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barreto Ortiz S, Hori D, Nomura Y, Yun X, Jiang H, Yong H, et al. Opsin 3 and 4 mediate light-induced pulmonary vasorelaxation that is potentiated by G protein-coupled receptor kinase 2 inhibition. Am J Physiol Lung Cell Mol Physiol. 2018;314:L93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hori D, Dunkerly-Eyring B, Nomura Y, Biswas D, Steppan J, Henao-Mejia J, et al. miR-181b regulates vascular stiffness age dependently in part by regulating TGF-beta signaling. PLoS ONE. 2017;12:e0174108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, et al. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genom. 2016;48:826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steppan J, Tran HT, Bead VR, Oh YJ, Sikka G, Bivalacqua TJ, et al. Arginase inhibition reverses endothelial dysfunction, pulmonary hypertension, and vascular stiffness in transgenic sickle cell mice. Anesth Analg. 2016;123:652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–43. [DOI] [PubMed] [Google Scholar]
- 21.Steppan J, Tran H, Benjo AM, Pellakuru L, Barodka V, Ryoo S, et al. Alagebrium in combination with exercise ameliorates age-associated ventricular and vascular stiffness. Exp Gerontol. 2012; 47:565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


