Abstract
Understanding the mechanisms by which natural anti‐freeze proteins protect cells and tissues from cold could help to improve the availability of donor organs for transplantation.

The first successful organ transplant in humans was performed in 1954 by Joseph Murray, who used a patient’s twin as a kidney donor. Murrays’ breakthrough paved the way for organ transplantation and the number of transplanted organs has grown ever since. For example, in 2017, a total of 139.024 solid organs—mostly kidney, liver, heart, lung, pancreas, and small bowel—were transplanted (Fig 1A). But this number only reflects 10% of the worldwide need; many patients still die of end‐stage organ failure while on a waiting list. The limited number of donor organs contributes only partially to this shortage. Many donor organs are not transplanted eventually owing to inefficient preservation techniques that shorten their extracorporeal lifetime. In fact, the percentage of donor organs that are unused is estimated to range from around 25% for kidneys and livers up to 70–80% for hearts and lungs (Giwa et al, 2017); Fig 1B).
Figure 1. Organ transplantation and preservability status.
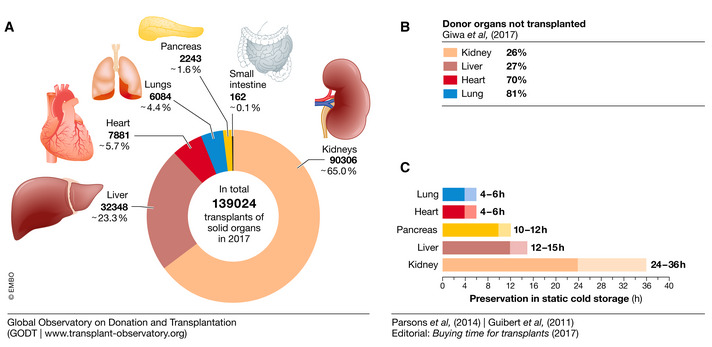
Statistics show a positive correlation between the duration of ex vivo preservation and the number of organ transplants. Number of solid organs transplanted in 2017 (A). Percentage of organs failed to be transplanted (B). Duration of solid organ ex vivo preservation in static cold storage (C). Sources: Data from the Global Observatory on Donation and Transplantation and (Parsons et al, 2014), (Guibert et al, 2011) and (Editorial: Buying time for transplants (2017))
Many donor organs are not transplanted eventually owing to inefficient preservation techniques that shorten their extracorporeal lifetime.
To address the shortage of donor organs and decrease the number of organs that go to waste, biobanks could efficiently store viable tissues and organs until transplantation. Yet, the current standard for ex vivo preservation of donor organs is static cold storage (4–8°C) which, depending on the organ, ensures viable conservation for only some hours; hearts are typically viable for a maximum of only 4 h (Fig 1C). In addition, this approach leads to hypothermic damage and to ischemia/reperfusion injury.
Hence, there is an urgent need for strategies that prolong the viable preservation of donor organs. Two main strategies have emerged for cryopreservation and subzero storage, both of which cool tissues below the freezing point. While subzero storage just below 0°C may suffice for short‐term preservation, cryopreservation at −80°C or even lower temperatures is required for long‐term storage in biobanks. A proof‐of‐principle study already demonstrated that subzero preservation extended the preservation of rat hearts up to 24 h after collection (Amir et al, 2004); cryopreservation of whole hearts is currently not possible. The main reason is that lowering the temperature below the freezing point of water leads to ice formation, which causes cell damage and destroys tissues. One of the main challenges in biomedical research for organ transplantation is therefore finding non‐toxic and biocompatible antifreeze compounds that enable subzero storage and cryopreservation without causing tissue damage. An additional benefit is a larger time window to perform evaluation in terms of organ size and human leukocyte antigens matching and preparing the recipient patient to increase the chance of a successful transplantation.
Natural antifreeze proteins
The solution may be found in nature since a wide variety of organisms face similar challenges. Fish living under the polar caps are constantly exposed to freezing water; plants and insects survive extreme cold during the winter. These organisms need to control and prevent the formation of ice since rapidly expanding ice in their vasculature, and other tissues would be lethal. In contrast to warm‐blooded animals, plants and insects cannot regulate their body temperature. Instead, they rely on other coping strategies to survive at extremely low temperatures. In addition to colligative, that is, concentration‐dependent, antifreeze molecules such as polyols and sugars, these organisms also produce a wide variety of ice‐binding proteins (IBPs), that control the nucleation and growth of ice crystals (Fig 2A).
Figure 2. Functional and structural diversity of AFPs.
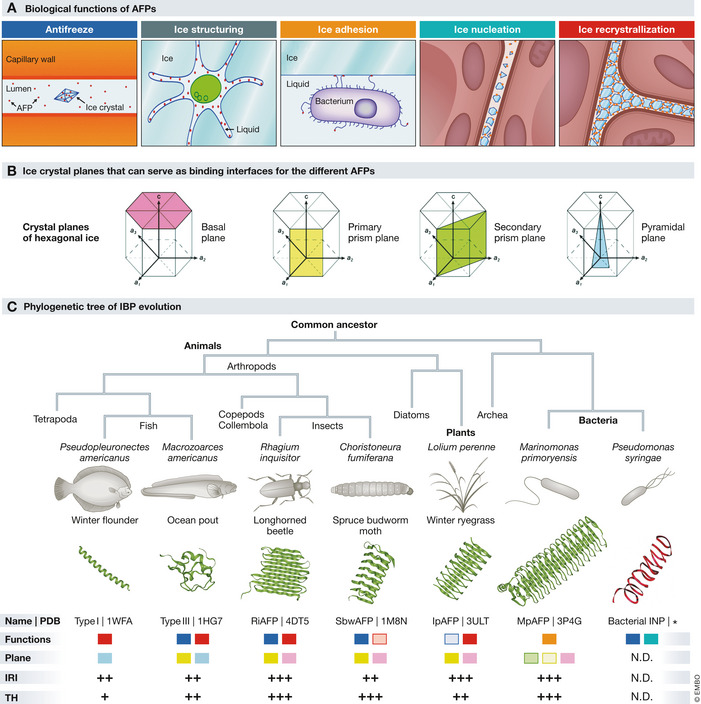
Five biological functions of AFPs (A). Four ice‐crystal planes that can serve as binding interfaces for the different AFPs (B). Phylogenetic tree of IBP evolution. IBP structures found in several organisms are shown to highlight the structural diversity. For each of these IBPs, the specific functions and plane selectivity (color coding as in panels A and B), IRI and TH activity are indicated. + low activity, ++ moderate activity, +++ high activity. *predicted structure, from Graether and Jia (2001). N.D., not determined (C). Sources: (Bar Dolev et al, 2016; Voets, 2017).
A subclass of IBPs possesses potent antifreeze activity and are therefore called antifreeze proteins (AFPs). AFPs can either generate a thermal hysteresis (TH) gap and/or control ice‐recrystallization inhibition (IRI). TH activity prohibits the growth of ice and lowers the freezing point temperature: nucleated ice crystals remain small until the temperature falls below the TH gap, only then crystals start growing again owing to secondary nucleation events. In contrast, IRI activity prevents the maturation of small crystals into larger ones but does not reduce the freezing temperature (Fig 2A; Box 1). Because IBPs have evolved by convergent evolution, a large variety of these proteins with different structures and differential TH and IRI activity exist (Fig 2B and C). AFPs with the highest TH activity that reduce the freezing temperature by up to 13 °C are found in insects while the highest IRI activity is observed in antifreeze glycoproteins (AFGPs) from arctic fish (Bar Dolev et al, 2016; Voets, 2017). Their biological nature and non‐colligative activity make AFPs a non‐toxic alternative that can be used at much lower concentrations compared to the currently used synthetic antifreeze compounds. Their TH activity may be exploited to expand static cold storage to subzero temperatures, while IRI activity and ice shaping may enable cryopreservation.
Their biological nature and non‐colligative activity make AFPs a non‐toxic alternative that can be used at much lower concentrations compared to the currently used synthetic antifreeze compounds.
Although the potential of nature’s antifreeze toolbox for organ preservation has been widely appreciated, our understanding of how these remarkable proteins work is still far from complete. It is however clear that there is no “winner” molecule for a one‐size‐fits‐all method but rather a variety of different preservation methods for different biological samples fine‐tuned to their sensitivity and intended application after storage. Several strategies have been considered for preserving biological samples that range from conservation at physiological temperatures (35–37°C) down to cryopreservation at −196°C, the temperature of liquid nitrogen. While whole organs are currently preserved at hypothermic conditions (4–8°C), cryopreservation is the method of choice for long‐term storage of single cells.
Cold damages cells
In most vertebrates, the metabolic rate is halved for each 10°C reduction in body temperature. This fundamental principle underlies the preservation of cells, tissues, and organs at hypothermic conditions as the reduction of metabolism, energy expenditure, and oxygen demand increases tolerance to ischemia, the temporary halt of blood flow (Fig 3). However, while short‐term exposure to hypothermia is usually reversible, longer exposure and colder preservation temperatures can cause permanent and irreversible damage. As the temperature drops, cell membranes become leaky and allow an inward flux of ions, disrupting osmolality and often leading to water uptake, protein denaturation, and activation of cell death pathways. Furthermore, hypothermia has other intracellular effects, such as increased cellular acidosis, production of reactive oxygen species, depletion of ATP reserves, and cytoskeleton disruption (Rubinsky, 2003). To counteract these effects, preservation solutions were developed with the aim of maintaining the ionic and osmotic balance, supplying preserved samples with energy alternatives and preventing the formation of free radicals. To minimize ischemic injury further, ex vivo perfusion of donor organs has proven useful. Despite some advances, however, hypothermic preservation is inherently limited to hours mainly owing to the incomplete metabolic stop. As such, longer preservation requires a complete block of metabolism, which means colder temperatures (Fig 3).
Figure 3. Reduced temperature increases preservability by suppressing the metabolic rate.
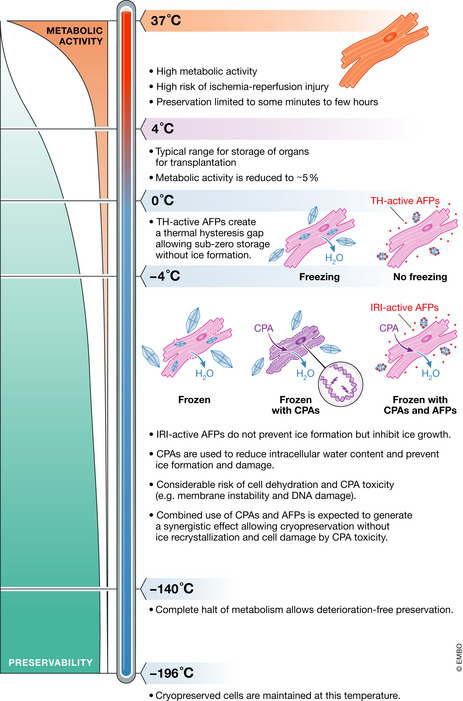
Organ preservation typically involves cooling to prevent the depletion of energy reserves. Donor hearts are preserved in static cold storage at 4°C where there is no risk of freezing but preservation times are limited. Subzero storage allows further repression of the metabolic activity to increase preservation duration. A complete metabolic stop and indefinite storage periods require cryopreservation at cryogenic temperatures (−196°C). However, at these temperatures, tissues are subjected to harmful ice crystals and dehydration. AFPs with TH activity can be used to reduce freezing temperatures in subzero storage, whereas IRI‐active AFPs can reduce cell damage during freezing. Combined use of cell‐penetrating CPAs and AFPs may allow reducing the dependence on high CPA concentrations, while maintaining intra‐ and extracellular protection.Sources: (Best, 2015; Giwa et al, 2017; Kim et al, 2017; Rubinski, 2003).
… while short‐term exposure to hypothermia is usually reversible, longer exposure and colder preservation temperatures can cause permanent and irreversible damage.
At cryogenic temperatures, −80°C or lower, biological samples can theoretically be preserved for decades without any deterioration (Rubinsky, 2003). It is the freezing and thawing however that causes most of the cellular damage. When cells, tissues, or organs are cryopreserved, they first undergo a transient yet harmless hypothermia. As the temperature falls below the freezing point, water turns into solid ice crystals which contain essentially only water molecules. The solute concentration of the remaining liquid thus increases markedly, which generates an osmotic imbalance across the cell membrane as ice predominantly forms in the extracellular space. This forces water to exit the cells to equalize the concentration of solutes and, as a consequence, cells dehydrate, which is a major source of cell damage during cryopreservation (Rubinsky, 2003; Fig 3).
As the cytoplasm loses water, intracellular proteins may denature or undergo structural modifications that render them dysfunctional. The remaining intracellular water will eventually nucleate and form ice crystals that puncture (sub)cellular membranes and/or generate mechanical strain (Karlsson & Toner, 1996). Furthermore, extracellular ice can disrupt the extracellular matrix (ECM) organization and/or proteins regulating cell‐cell or cell‐ECM interactions, which are essential for functions at tissue and organ level (Schenke‐Layland et al, 2006). Clearly, a delicate balance must be struck between dehydration and ice formation, which can be achieved to some extent with a fast cooling rate. While slow cooling is associated with extreme dehydration and damage, fast cooling still increases the likelihood of ice being formed within the cell.
To prevent dehydration, cryoprotective agents (CPAs) were introduced. Cell‐permeable CPAs increase the intracellular concentration of solutes and lower the osmotic differential between the cytosol and the extracellular environment. However, by maintaining a larger cytoplasmic volume, cryoprotectants may have the unwanted effect of increasing the chance of intracellular ice formation. This can be avoided by resorting to a very fast cooling rate—usually by rapid immersion into liquid nitrogen—during which liquids become highly viscous and never enter a frozen state. This technique is called vitrification and has been very useful for the cryopreservation of human oocytes and embryos. The drawback is the high toxicity of cryoprotectants, particularly when used at the required high concentrations. Furthermore, vitrification is currently limited to small samples as larger specimens, notably organs, would require much faster and homogenous cooling and rewarming rates.
Cells may also undergo further damage during rewarming. As the temperature increases, particularly when the rewarming rate is slow, ice recrystallization into bigger and sharp crystals can occur and damage cell membranes. Moreover, the extracellular space becomes rapidly hypotonic, as a consequence of ice thawing, and the osmotic pressure promotes water movement into the cell, which can swell and eventually burst. To prevent this type of damage, thawing is typically done quickly as well.
Antifreeze proteins for organ preservation
After decades of research, we have now identified the challenges for and bottlenecks of the preservation of biological samples, but progress remains limited. Strategies to improve hypothermic preservation should aim at further reducing metabolic activity, which can be achieved by subzero storage. In turn, better cryopreservation strategies should focus on inhibiting ice recrystallization during freezing and thawing and in parallel abrogate the use of toxic CPAs to replace them with non‐toxic alternatives.
TH and IRI‐active AFPs are now being explored to achieve freeze avoidance or tolerance in subzero preservation and cryopreservation strategies, respectively. However, despite the potential of AFPs, research is still ongoing to investigate whether and how these can be used to improve subzero storage and cryopreservation of cells, tissues, and organs.
During cryopreservation, temperatures are lowered far beyond the freezing point of water and beyond the TH gap of AFPs, which increases the risk that harmful large and sharp ice crystals form. Here, IRI and the ice‐shaping activity of AFPs can help to reduce crystal size and control the shape of crystals to reduce mechanical damage during freezing and thawing. Various studies have shown that addition of AFPs during cryopreservation improves the viability of a variety of isolated mammalian cells including sperm, oocytes, red blood cells, hepatocytes, and cardiomyocytes without compromising their differentiation capacity or functionality (Kim et al, 2017). In contrast, cryopreservation of tissues and organs remains more challenging and has not been successfully achieved. Furthermore, although some studies have reported positive effects of AFPs on viability on a cellular and tissue level, others have achieved contrasting results and report little to no effect of AFPs upon freezing (Kim et al, 2017).
One major drawback of AFPs is their inability to penetrate the cell membrane and protect the intracellular compartment. Therefore, future efforts should focus on developing cell‐permeable AFP analogues or complementary CPAs. Additionally, reducing the ice‐volume fraction is still required to ensure the availability of diffusive nutrients to preserve cellular material and to suppress freeze‐induced dehydration. Although this will always require conventional colligative CPAs, their concentration can potentially be lowered as higher ice‐volume fractions are tolerated by the addition of IRI‐active AFPs. Finally, it should be considered that even if AFPs successfully alleviate freeze‐induced damage, tissue complexity and function can still be compromised. As such, AFPs alone are likely insufficient and additional cryopreservation methods will be needed to avert all damage while retaining functionality. In short, more work is necessary to determine whether AFPs are viable agents for long‐term storage of tissues or if other (complementary) avenues should be explored.
While the formation of harmful ice crystals is unavoidable in cryopreservation, TH‐active AFPs can potentially be effective to avoid freezing during subzero storage. Addition of these AFPs would allow a further reduction in storage temperature, which lowers the metabolic rate and prolongs organ preservation by hours (Fig 3).
Promising results by Amir and colleagues showed that rat hearts stored for 18 and 24 h in the presence of 15 mg/ml type III AFPs at −1.3°C displayed significantly better morphology and contractility after reperfusion compared to hearts stored at 4°C. Evaluation by histological staining and electron microscopy showed reduced necrosis along with better preservation of cardiomyocyte ultrastructure, mitochondrial integrity and undisrupted z‐lines (Amir et al, 2004). An older study by Rubinsky and colleagues obtained similar results in liver tissue. Interestingly, they performed storage experiments at −3°C with AFGP concentrations (1 mg/ml) that were not enough to generate a TH gap large enough to avoid freezing altogether. However, AFGPs inhibited ice formation and recrystallization sufficiently to result in good preservation of liver ultrastructure and improved bile production after cooling (Rubinsky et al, 1994).
Exploring Nature’s diversity
While these and other studies demonstrate the potential of IRI and TH‐active AFPs for cryopreservation of single cells and subzero storage of more complex tissues, studies remain few and AFPs have not transitioned from the research stage into clinic. Most studies use fish type I or type III AFP or insect AFGPs (Kim et al, 2017); these are easily produced, and their activity has been well characterized, but more potent AFPs exist. For example, insect and plant AFPs have both higher IRI and TH activity, which enables subzero storage temperatures as low as −10°C and with lower concentrations compared to fish AFPs (Bar Dolev et al, 2016). Additionally, these proteins bind prism as well as basal planes of ice crystals resulting in more blunt‐shaped crystals that are less prone to cause mechanical damage (Kim et al, 2017; Fig 2). In order to overcome such problems, future efforts can be focused to identify and engineer new AFP variants. For example, a mutation in threonine 18 of type III AFP results in the development of blunt rather than sharp bipyramidal crystals while maintaining IRI activity (DeLuca et al, 1996).
Each organ or tissue has specific structures and functions, and preserving their complexity and function will therefore require different strategies. Targeted engineering of tailor‐made AFP‐based preservation strategies requires a much better understanding of the antifreeze mechanisms of AFPs at the molecular level. Furthermore, decreasing cellular temperatures beyond the freezing point requires optimization of intracellular protection, which could be achieved by generation of AFP analogues that permeate the cell membrane; delivery of AFPs directly to the cytoplasm; and use of AFPs in combination with cell‐permeable CPAs. It is anticipated that AFPs and CPAs will produce a synergistic effect and, therefore, require lower concentrations of both to achieve improved cryopreservation.
Targeted engineering for tailor‐made AFP‐based preservation strategies requires a much better understanding of the antifreeze mechanisms of AFPs at the molecular level.
In addition to optimized freezing strategies, we would also need better measures to determine whether preserved tissues retained their endogenous physiological properties. Cell viability assays together with microscopic techniques are often used to quantify the rate of cell death and ultrastructural perturbations. Additionally, functional assays help to understand the state of a tissue or organ, such as contraction for the heart or bile production for the liver (Rubinsky et al, 1994; Amir et al, 2004). While these assays are crucial, they only paint a partial picture. Modern transcriptomic and proteomic as well as epigenetic characterization techniques would help to characterize the state of cells and tissues during cooling strategies. This will allow for early detection of changes in the genetic and proteomic profile of the cells at the stage of protocol development.
Modern transcriptomic and proteomic as well as epigenetic characterization techniques would help to characterize the state of cells and tissues during cooling strategies.
Mechanistic understanding of AFPs
Even though previous studies have explored various AFPs for cryopreservation and subzero storage, consensus has not been reached on the optimal levels of IRI/TH activity for the respective storage techniques. Interestingly, although IRI and TH activity of most of the identified AFPs has been mapped, the mechanistic effects responsible for the respective functions remain largely unknown. As a consequence, further improvement of cryopreservation or subzero storage involving AFPs is stalled. Understanding the underlying principles of ice‐binding and activity‐affecting mutations, like the T18N, would allow rational design of AFPs with desired traits. For example, de novo ice‐binders could be generated from stable protein backbones that can be controlled for their size and affinity and that are highly active, plane selective, less toxic and controlled for shaping ice into blunt rather than sharp crystals.
To further understand the mechanisms by which AFPs work, the adsorption‐inhibition hypothesis was formulated and is generally adopted to explain TH activity by AFPs. This model proposes that AFPs are “pinned” tightly to the ice‐water interface which prevents ice‐growth at this location. As a consequence, ice is only able to grow between AFPs resulting in locally highly curved ice lattices that are energetically unfavorable for further growth, reducing freezing temperature (Bar Dolev et al, 2016; Fig 2). One central assumption in this model is that AFPs are tightly spaced and irreversibly bound to ice. However, although ice‐binding activity is obviously pivotal, important properties, such as spacing and binding kinetics of the structurally diverse AFPs, remain elusive and debated. Furthermore, several AFPs possess high IRI activity but have low TH activity. While the adsorption‐inhibition hypothesis is mainly developed for TH function, it does not predict IRI activity. Moreover, the model does not explain how TH and IRI activity can be decoupled even though they rely on the same fundamental principles. One likely explanation for the differential activity of AFPs is that differences in affinities, interprotein distances, and behavior at the ice‐crystal lattice may underlie their function as either strong TH or IRI‐active molecules.
It has been challenging to elucidate these dependencies and understand structure‐function relationships, because both single AFPs and their collective behavior need to be observed to correlate their biophysical properties and activity. So far, AFP activity and selectivity are commonly studied by measurements of ice‐crystal growth, shape, and freezing temperatures; visualization of fluorescently labeled AFPs at ice‐crystal planes and/or molecular dynamic (MD) simulations. While the experimental approaches have provided important insights about the collective behavior of AFPs, they do not offer the resolution to interrogate the single‐molecule behavior of AFPs. In contrast to the experimental work, theoretical MD simulations provide details at molecular length scales and have contributed to our understanding of the interactions and arrangements of AFPs on the crystal lattice. However, the gap in resolution between experimental and computational approaches has led to apparently contrasting results, which are yet to be reconciled.
To finally elucidate the molecular mechanisms of antifreeze activity and bridge the present knowledge gap, new technologies are needed which enable direct measurements of the essential parameters that underlie AFP activity, such as “pinning” and interprotein spacing. Observation of AFPs at the single‐molecule level will allow correlation between the molecular behavior and the respective activity and provide direct evidence and input for the refinement of MD simulations to understand and predict AFP behavior. Over the past decades, a subset of fluorescence based super‐resolution microscopy (SRM) techniques have been developed that are diffraction unlimited and can potentially resolve the dynamics of individual AFPs at the ice‐water interface with high spatiotemporal resolution. Data from these techniques can help to refine MD simulations and lead to a complete data‐based model to predict both TH and IRI activity in single or multiple AFP subtypes. Such understanding of the structure‐function relationships of AFPs could be adopted in the engineering of new AFP variants, similar to T18N, or bio‐inspired antifreeze compounds with controlled activity as well as other desired properties such as non‐harmful crystal shapes. These insights will be highly valuable to get AFPs into clinical use.
… understanding of the structure‐function relationships of AFPs could be adopted in the engineering of new AFP variants […] or bio‐inspired antifreeze compounds with controlled activity …
Given the elevated mortality rate associated with end‐stage organ failure, improved cellular, tissue, and organ preservation strategies are highly sought after and will have great socioeconomic impact. AFPs could provide a solution to reduce temperatures and minimize damage during subzero storage and cryopreservation. However, although addition of AFPs has led to some promising experimental results, implementation in clinical settings is yet to be achieved. Major steps are therefore needed to understand the beneficial effects during the preservation of biological materials and elucidate key fundamental principles of antifreeze activity to engineer non‐toxic AFPs and AFP‐inspired synthetic compounds with optimal properties for preservation. Finally, new avenues of AFP activity can be explored to further increase the antifreeze toolbox. It is imperative to continue investigating these biological antifreezes and alternatives to increase the pool of transplantable organs.
Box 1: Ice‐binding by IBPs modulates nucleation, growth and shaping of ice crystals.
Thermal hysteresis (TH) activity is associated with freeze avoidance and allows preservation at temperatures below 0°C. Non‐colligative freezing point depression and melting inhibition by IBPs creates a so‐called thermal hysteresis gap between the freezing and melting points of embryonic ice crystals in which further growth ice crystals is stopped. Nascent crystals thus remain tiny within this small temperature gap, so that blood circulation is maintained.
Ice‐recrystallization inhibition (IRI) activity prevents disproportionation of ice crystals of different shapes and dimensions. Without IBPs, ice crystals fuse, ripen, and reshape to reduce the total surface‐to‐volume ratio and thereby minimize the free energy of the system. In the presence of IRI‐active IBPs, ice crystals remain numerous, yet sufficiently small so that the vasculature is not blocked. This property is therefore associated with freeze tolerance and holds potential for cryopreservation of biological samples.
Ice‐shaping activity results from the differential affinity of IBPs toward the four crystallographic planes of the ice‐crystal lattice. The plane(s) decorated by antifreeze proteins is (partially) prohibited from growing further, which enables IBPs to sculpt the growing ice crystal. “Blunt” shapes associated with basal plane affinity are considered favorable for preservation as these are less prone to cause mechanical damage. By contrast, pyramidal plane affinity causes sharp needle‐like bipyramidal shapes that can puncture membranes.
Ice‐nucleating activity is attributed to ice‐binding sites on IBPs that arrange water molecules to resemble an ice‐like lattice. These ordered water molecules serve as a nucleation point to induce freezing at temperatures above the homogeneous nucleation temperature of water.
Conflict of interest
LL reports consultancy fees to UMCU from Abbott, Medtronic, Vifor, Novartis, and research materials from Roche and Sopachem (all outside of the current work).
Acknowledgements
We apologize that we were unable to cite and discuss all relevant papers due to space limitations. This work was financially supported by the European Union (ERC‐2014‐StG Contract No. 635928), the Dutch Science Foundation (NWO ECHO Grant No. 712.016.002), the Dutch Ministry of Education, Culture and Science (Gravity Program 024.001.035), the Netherlands Heart Foundation (Dekker Senior Clinical Scientist 2019 Grant No. 2019T056) and the alliance between Eindhoven University of Technology, Utrecht University and the University Medical Center Utrecht.
EMBO reports (2021) 22: e52162
Contributor Information
Linda W van Laake, Email: L.W.vanLaake@umcutrecht.nl.
Ilja K Voets, Email: i.voets@tue.nl.
References
- Amir G, Rubinsky B, Horowitz L, Miller L, Leor J, Kassif Y, Mishaly D, Smolinsky AK, Lavee J (2004) Prolonged 24‐hour subzero preservation of heterotopically transplanted rat hearts using antifreeze proteins derived from arctic fish. Ann Thorac Surg 77: 1648–1655 [DOI] [PubMed] [Google Scholar]
- Bar Dolev M, Braslavsky I, Davies PL (2016) Ice‐binding proteins and their function. Annu Rev Biochem 85: 515–542 [DOI] [PubMed] [Google Scholar]
- Best BP (2015) Cryoprotectant Toxicity: Facts, Issues, and Questions. Rejuvenation research 18: 422–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca CI, Chao H, Sönnichsen FD, Sykes BD, Davies PL (1996) Effect of type III antifreeze protein dilution and mutation on the growth inhibition of ice. Biophys J 71: 2346–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giwa S, Lewis JK, Alvarez L, Langer R, Roth AE, Church GM, Markmann JF, Sachs DH, Chandraker A, Wertheim JA et al (2017) The promise of organ and tissue preservation to transform medicine. Nat Biotechnol 35: 530–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graether SP, Jia Z (2001) Modeling Pseudomonas syringae ice‐nucleation protein as a beta‐helical protein. Biophysical journal 80: 1169–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guibert EE, Petrenko AY, Balaban CL, Somov AY, Rodriguez JV, Fuller BJ (2011) Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie 38: 125–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson JOM, Toner M (1996) Long‐term storage of tissues by cryopreservation: critical issues. Biomaterials 17: 243–256 [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee JH, Hur YB, Lee CW, Park SH, Koo BW (2017) Marine antifreeze proteins: structure, function, and application to cryopreservation as a potential cryoprotectant. Marine Drugs 15(2): 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall A (2017) Buying time for transplants. Nat Biotechnol 35: 801 [DOI] [PubMed] [Google Scholar]
- Parsons RF, Guarrera JV (2014) Preservation solutions for static cold storage of abdominal allografts: which is best? Current opinion in organ transplantation 19: 100–107 [DOI] [PubMed] [Google Scholar]
- Rubinsky B, Arav A, Hong JS, Lee CY (1994) Freezing of mammalian livers with glycerol and antifreeze proteins. Biochem Biophys Res Commun 200: 732–741 [DOI] [PubMed] [Google Scholar]
- Rubinsky B (2003) Principles of low temperature cell preservation. Heart Fail Rev 8: 277–284 [DOI] [PubMed] [Google Scholar]
- Schenke‐Layland K, Madershahian N, Riemann I, Starcher B, Halbhuber KJ, Konig K, Stock UA (2006) Impact of cryopreservation on extracellular matrix structures of heart valve leaflets. Ann Thorac Surg 81: 918–926 [DOI] [PubMed] [Google Scholar]
- Voets IK (2017) From ice‐binding proteins to bio‐inspired antifreeze materials. Soft Matter 13: 4808–4823 [DOI] [PMC free article] [PubMed] [Google Scholar]


