Abstract
Aims/Introduction
Type 2 diabetes mellitus has been a leading cause of chronic kidney disease (CKD), with a heterogeneous distribution worldwide. Optimal healthcare planning requires an understanding of how the burden of CKD as a result of type 2 diabetes mellitus has changed over time and geographic location, as well as the potential roles of sociodemographic, clinical and behavioral factors in these changes.
Materials and Methods
We used the Global Burden of Disease data from 1990 to 2017 at the global, regional and national levels to investigate changes in the incidence, death and disability‐adjusted life years of CKD as a result of type 2 diabetes mellitus, incorporating both epidemiological research and risk factor monitoring.
Results
The incident cases of CKD as a result of type 2 diabetes mellitus worldwide in 2017 had increased by 74% compared with 1990; total disability‐adjusted life years had increased by 113%, mainly attributable to population expansion and demographic transition. The Sociodemographic Index was significantly and negatively correlated with overall CKD as a result of type 2 diabetes mellitus burden. However, in 82 countries and territories, the burden was not alleviated in parallel with socioeconomic development.
Conclusions
CKD as a result of type 2 diabetes mellitus has been the main contributor to the increasing burden of CKD over the past several decades. We suggest a more pragmatic approach focusing on early diagnosis, primary care and adequate follow up to reduce mortality and the long‐term burden in low‐to‐middle Sociodemographic Index regions. Interventions should address high systolic blood pressure, as well as overweight and obesity problems, especially in high‐income regions.
Keywords: Chronic kidney disease, Global Burden of Disease, Type 2 diabetes mellitus
On a global scale, a modest decrease in the age‐standardized incidence rate of chronic kidney disease as a result of type 2 diabetes mellitus has been observed. Low‐ to middle‐income countries are suffering from a disproportionally higher burden of mortality rather than of morbidity compared with relatively more affluent counties. From 1990 to 2017, 82 of 195 countries failed to alleviate the burden of chronic kidney disease in patients with type 2 diabetes mellitus in parallel with sociodemographic development. Meanwhile, there are potential opportunities for countries across the entire development spectrum. High body mass index is accounting for an increasingly greater proportion of the age‐standardized disability‐adjusted life years rate globally.
Introduction
Chronic kidney disease (CKD) is an important public health problem because of its increasing burden, as shown by general population‐based studies 1 , 2 , 3 . CKD as a result of type 2 diabetes mellitus appears to be the main contributor to the increase in CKD burden over the past several decades 3 , 4 , 5 , and it was the only cause of CKD to show a significant increase in the age‐standardized disability‐adjusted life year (DALY) rate, which changed by 9.5% (95% uncertainty interval [UI] 4.3–13.7) from 1990 to 2017 6 . This can be partially explained by an increase in the prevalence of diabetes associated with population aging, urbanization, industrialization, changes in nutrition, epidemics of obesity and low levels of physical activity 7 . However, the global importance of CKD as a result of type 2 diabetes mellitus has not yet been widely acknowledged.
A detailed quantitative analysis of the global, regional, and national burden and disparities in the burden of CKD as a result of type 2 diabetes mellitus over the past 30 years has not been carried out. The current study used the Global Burden of Disease (GBD) data from 1990 to 2017 to describe the incidence, mortality and DALYs of CKD as a result of type 2 diabetes mellitus, their disparities according to sociodemographic regions and countries, and the attributable risk factors. We aimed to provide policymakers with the evidence required to allocate resources appropriately to achieve optimal health outcomes.
Methods
Study data
The GBD study carried out in 2017 aimed to determine the incidence, prevalence, mortality, and DALYs of 359 diseases and injuries, and 84 risk factors by age and sex in 195 countries and territories from 1990 to 2017. The methodological details of GBD 2017 have been reported elsewhere 8 , 9 , 10 . In the present study, we extracted data from the GBD Collaborative Network, and data on the incidence, death rate, DALYs and attributable risk factors for CKD as a result of type 2 diabetes mellitus at the global, regional and national levels were analyzed. CKD is defined as a permanent loss of renal function, as shown by the estimated glomerular filtration rate <60 mL/min/1.73 m2 or urinary albumin : creatinine ratio >30 mg/g, or both. CKD as a result of type 2 diabetes is a subtype of CKD that could be attributable to diabetes mellitus type 2 etiologically. 8 DALYs is calculated by summing up the years of life lost and years lived with disability 9 . The estimated burden attributable to a certain risk factor represents DALYs that could have been avoided if the risk exposure was reduced to the theoretical minimum exposure level 10 . The downloaded tool is available in the Global Health Data Exchange and contains core summary results for GBD 2017 (http://ghdx.healthdata.org/gbd‐results‐tool).
This study categorized 195 countries into five regions in terms of the Sociodemographic Index (SDI), which quantifies the fertility rate, income per capita and educational attainment into a range from 0 to 1. Age‐standardized rates are also available to universalize the population size and age structure, referring to rates per 100,000 population based on the updated standard population age structure from the GBD 2017 population estimates for all national locations 11 .
Statistical analysis
The estimated annual percentage change (EAPC) is a widely used measure for evaluating the time trends of various rates over a specified interval. A regression line was fitted to the natural logarithm of the rates; that is, y = α + βx + ε, where y is the natural logarithm (rate), and x is the calendar year. The EAPC was calculated as 100 × (exp[β] − 1), and its 95% UI was obtained using a linear regression model 12 .
We applied a decomposition analysis to determine the additive contribution of explanatory factors that drove the change in DALYs between 1990 and 2017. The overall difference in DALYs was decomposed according to the age structure, population growth and age‐specific DALY rates (epidemiological changes).
To gain an intuitive understanding of the inequity in the burden of CKD as a result of type 2 diabetes mellitus worldwide, we defined the global burden among 50‐year‐olds as the reference and estimated the equivalent age in 195 countries with the same burden. To evaluate the lowest potential age‐standardized DALY rate based on a given SDI, we carried out a frontier analysis using data for the 195 countries from 1990 to 2017. A non‐parametric data envelope analysis was applied to produce a non‐linear frontier 13 .
The methodologies are described in detail in Data S1. All statistical analyses and graphics were carried out using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Burden of CKD as a result of type 2 diabetes mellitus
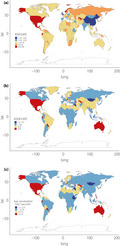
The incident cases of CKD as a result of type 2 diabetes mellitus worldwide increased by 74% (95% UI 37–92), from 1,354,548 in 1990 to 2,352,496 in 2017. The age‐standardized incidence rate (ASIR) decreased by an average of 0.40% (95% UI −0.47 to −0.33) per year during the study period (from 31.92 per 100,000 in 1990 to 29.15 per 100,000 in 2017). The largest decrease in ASIR occurred in China (EAPC −1.79, 95% UI −2.13 to −1.44), whereas the largest increase was observed in the USA (EAPC 0.64, 95% UI 0.40–0.87). CKD as a result of type 2 diabetes mellitus led to 348,959 deaths (95% UI 306,839–395,903) globally in 2017, representing a 145% increase compared with 1990. The age‐standardized death rate (ASDR) increased by an average of 0.80% (95% UI = 0.74–0.86) globally. CKD as a result of type 2 diabetes mellitus was responsible for 8,122,240 DALYs (95% UI 7,117,016–9,245,108) in 2017, representing a large 2.13‐fold increase from the 3,819,981 DALYs in 1990. The age‐standardized DALY rate increased steadily (EAPC 0.43, 95% UI 0.39–0.47) between 1990 and 2017, from 92.12 per 100,000 in 1990 to 100.85 per 100,000 in 2017. The increase was largest in El Salvador, at an average of 4.34% (95% UI 3.80–4.88) per year. Notably, the USA was among the countries and territories (henceforth referred to as “countries”) showing a significant increase in the age‐standardized DALY rate (EAPC 3.05, 95% UI 2.73–3.37; Table 1, Figures 1,S1).
Table 1.
Chronic kidney disease as a result of type 2 diabetes mellitus numbers and age‐standardized rates globally and by Sociodemographic Index quintile
| Region metric | All ages (95% UI) | Age‐standardized rate/100,000 (95% UI) | ||||||
|---|---|---|---|---|---|---|---|---|
| 2017 n Male + female | 2017 n Male | 2017 n Female | 1990–2017 change | 2017 Male + female | 2017 Male | 2017 Female | 1990–2017 change | |
| Global | ||||||||
| Incidence | 2,352,496 (2,063,870–2,680,907) | 1,096,618 (956,874–1,260,600) | 1,255,878 (1,102,679–1,428,199) | 0.74 (0.37–0.92) | 29.15 (25.62–33.24) | 28.68 (25.05–32.76) | 29.62 (26.05–33.68) | −0.09 (–0.22 to 0) |
| Deaths | 348,959 (306,839–395,903) | 177,783 (152,861–203,857) | 171,176 (151,399–192,499) | 1.45 (1.3–1.57) | 4.50 (3.95–5.10) | 5.12 (4.42–5.87) | 3.98 (3.52–4.48) | 0.19 (0.12–0.24) |
| DALYs | 8,122,240 (7,117,016–9,245,108) | 4,277,520 (3,696,214–4,896,674) | 3,844,720 (3,377,393–4,343,161) | 1.13 (1.02–1.22) | 100.85 (88.43–114.79) | 112.71 (97.73–128.79) | 90.21 (79.18–101.98) | 0.09 (0.04–0.14) |
| Low SDI | ||||||||
| Incidence | 260,507 (227,908–297,705) | 124,342 (108,199–144,036) | 136,165 (119,523–155,229) | 0.81 (0.21–1.14) | 34.71 (30.42–39.75) | 33.63 (29.34–38.81) | 35.77 (31.48–40.56) | −0.07 (−0.24 to 0.04) |
| Deaths | 29,265 (24,797–35,733) | 16,398 (13,556–21,970) | 12,867 (10,822–15,174) | 0.92 (0.75–1.11) | 4.89 (4.16–6.02) | 5.41 (4.46–7.28) | 4.37 (3.66–5.11) | −0.1 (−0.17 to −0.02) |
| DALYs | 786,251 (670,435–930,703) | 433,698 (365,303–557,594) | 352,553 (302,284–406,577) | 0.81 (0.67–0.98) | 111.39 (95.40–132.03) | 123.14 (103.11–157.52) | 99.78 (85.80–115.21) | −0.13 (−0.19 to –0.05) |
| Low‐middle SDI | ||||||||
| Incidence | 423,104 (371,682–486,695) | 200,437 (175,435–232,547) | 222,666 (195,918–255,105) | 0.83 (0.28–1.11) | 34.01 (29.81–38.78) | 33.39 (29.18 −38.23) | 34.63 (30.43–39.40) | −0.05 (−0.22 to 0.04) |
| Deaths | 70,030 (60,290–81,419) | 38,150 (31,024–44,730) | 31,879 (37,046–27,281) | 1.25 (1.06–1.43) | 6.61(5.69–6.67) | 7.55 (6.21–8.86) | 5.76 (4.92–6.67) | 0.06 (−0.02 to 0.15) |
| DALYs | 1,800,887 (1,542,337–2,067,625) | 980,298 (813,454–1,135,050) | 820,589 (706,592–945,278) | 1.08 (0.93–1.23) | 150.13 (129.31–171.79) | 168.93 (140.10–196.06) | 132.61 (114.43–156.56) | 0.02 (−0.06 to 0.09) |
| Middle SDI | ||||||||
| Incidence | 583,963 (512,850–667,380) | 278,046 (243,459–317,437) | 305,917 (267,582–350,036) | 1.03 (0.46–1.3) | 25.87 (22.68–29.58) | 25.57 (22.35–29.23) | 26.15 (22.92–29.83) | –0.06 (−0.23 to 0.03) |
| Deaths | 124,801 (107,996–141,733) | 65,126 (54,432–74,652) | 59,675 (52,475–67,285) | 1.93 (1.7–2.1) | 6.14 (5.31–6.96) | 6.92 (5.72–7.91) | 5.47 (4.82–6.16) | 0.16 (0.07–0.22) |
| DALYs | 2,933,391 (2,551,167–3,329,395) | 1,568,835 (1,321,386–1,786,662) | 1,364,556 (1,194,741–1,545,625) | 1.53 (1.38–1.67) | 131.66 (114.67–149.32) | 149.92 (123.50–167.92) | 117.66 (103.20–133.13) | 0.11 (0.04–0.16) |
| High‐middle SDI | ||||||||
| Incidence | 465,632 (405,314–534,640) | 204,678 (177,599–235,904) | 260,954 (228,354–297,081) | 0.61 (0.38–0.75) | 25.63 (22.46–29.28) | 24.18 (21.12–27.74) | 26.95 (23.65–30.61) | −0.14 (−0.23 to −0.07) |
| Deaths | 47,620 (41,585–54,568) | 23,265 (19,918–27,284) | 24,355 (21,240–27,660) | 1.16 (0.99–1.28) | 2.81 (2.45–3.21) | 3.22 (2.78–3.76) | 2.52 (2.20–2.86) | 0.06 (−0.02 to 0.12) |
| DALYs | 1,146,288 (995,292–1,315,358) | 572,617 (490,814–661,176) | 573,671 (499,115–654,542) | 0.82 (0.72–0.91) | 63.75 (55.44–72.88) | 69.75 (60.33–80.44) | 58.86 (51.11–67.08) | −0.04 (−0.09 to 0) |
| High SDI | ||||||||
| Incidence | 613,315 (533,337–706,082) | 286,258 (246,723–331,251) | 327,057 (286,210–375,040) | 0.54 (0.42–0.66) | 28.34 (24.59–32.47) | 28.86 (25.02–33.20) | 27.90 (24.36–31.85) | −0.07 (−0.14 to 0) |
| Deaths | 76,017 (654,40–86,671) | 34,223 (29,383–39,648) | 41,794 (35,939–47,453) | 1.48 (1.31–1.65) | 2.97 (2.59–3.37) | 3.35 (2.89–3.87) | 2.68 (2.34–3.03) | 0.28 (0.22–0.33) |
| DALYS | 1,426,713 (1,253,433–1,613,573) | 707,054 (616,874–805,570) | 719,659 (633,554–806,863) | 0.98 (0.9–1.07) | 64.77 (56.96–73.38) | 71.88 (63.01–81.67) | 58.65 (51.42–66.43) | 0.17 (0.12–0.21) |
DALYs, disability‐adjusted life years; SDI, Sociodemographic Index.
Figure 1.
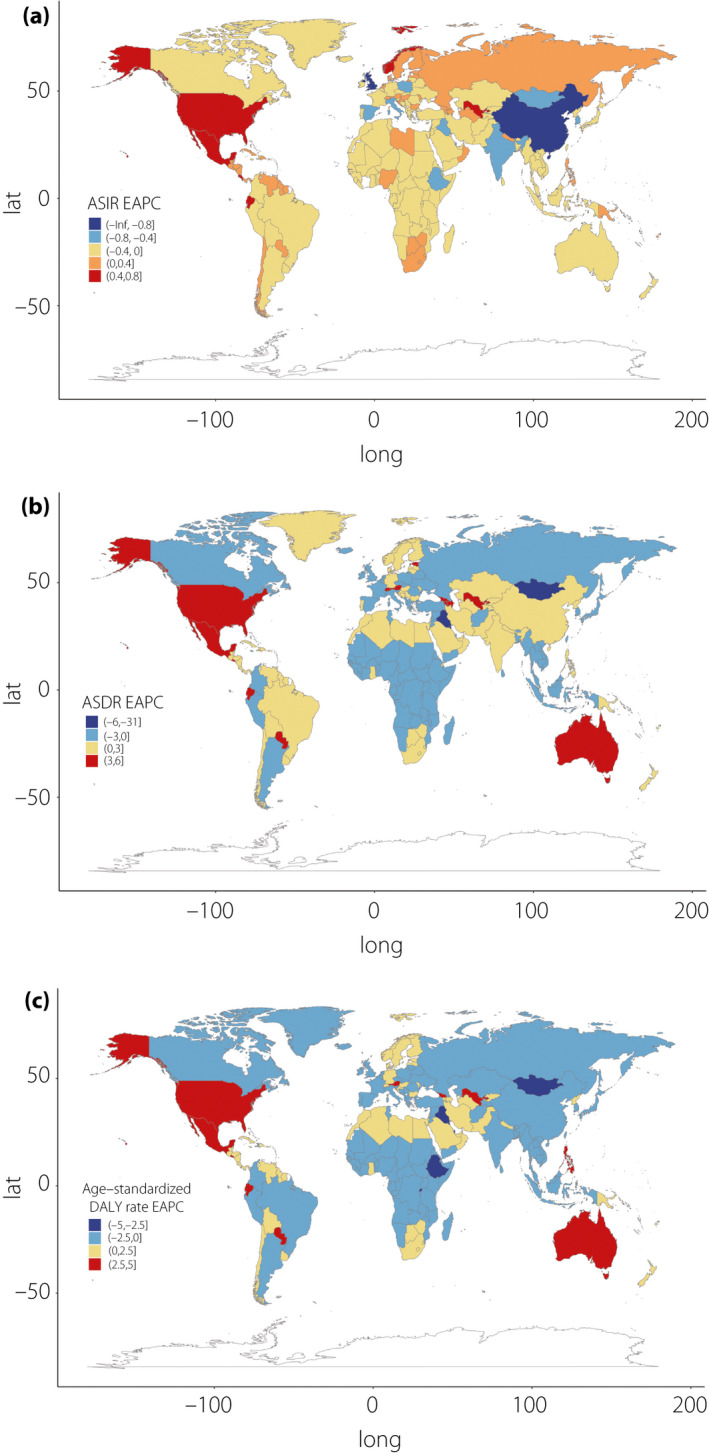
Estimated annual percentage change (EAPC) in (a) age‐standardized incidence, (b) death and (c) disability‐adjusted life year (DALY) rates between 1990 and 2017.
On observation from SDI regions, the incident cases of CKD as a result of type 2 diabetes mellitus increased in all quintiles, with the rise being largest (at 2.03‐fold) in middle SDI countries. The ASIR was highest in the low and low‐middle SDI quintiles during the study period, although it generally decreased across all five SDI regions. The total deaths and DALYs for CKD as a result of type 2 diabetes mellitus increased in all regions, by the most in the middle SDI quintile (2.93‐ and 2.53‐fold, respectively). Despite the decreasing trends in ASIR, the ASDR increased in all except the low SDI quintile, in which it decreased by approximately 10% (95% UI −17 to −2). The age‐standardized DALY rate increased in the low‐middle, middle and high SDI quintiles, whereas it decreased in the low and high‐middle SDI quintiles. The secular trends in ASDR and age‐standardized DALY rate were comparable, as DALYs were mainly attributable to years of life lost in all SDI regions.
As shown in Figure 2, the age‐standardized DALY rate showed a unimodal distribution among different age groups for both sexes in 2017; the rate distribution pattern was similar in all SDI quintiles with peak values in people aged 85–89 years. The DALY rate in men was generally higher than that in women, except for the people aged >85 years in a low SDI region. Notably, at the global level, there were an estimated 1,255,878 incident cases of CKD as a result of type 2 diabetes mellitus in women compared with 1,096,618 in men, whereas the ASIR in women slightly exceeded that in men in 2017 (Table 1, Figures 2,S2).
Figure 2.
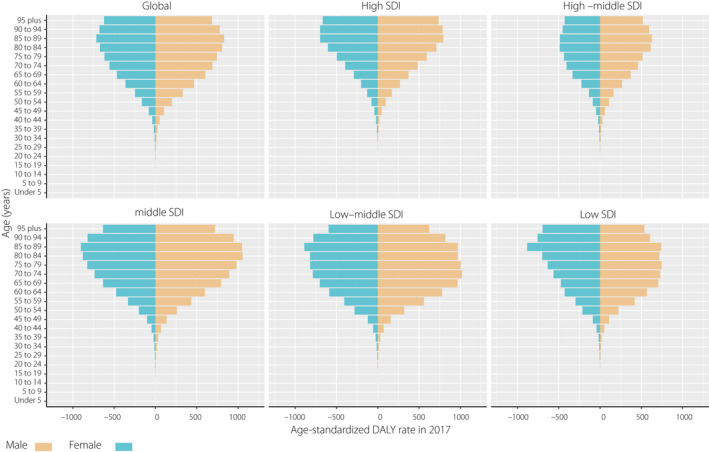
Age‐standardized disability‐adjusted life year (DALY) rate by sex and age in 2017. SDI, Sociodemographic Index.
Decomposition analysis of the increase in DALYs
Decomposition analysis showed that population growth contributed 45.94% to the increased burden of DALYs between 1990 and 2017, followed by aging (42.78%) and epidemiological changes (11.28%) worldwide. The contribution of aging to the overall DALYs was most pronounced in the high‐middle SDI quintile (70.89%), followed by the middle (56.79%), high (51.29%), low‐middle (32.99%) and low (22.98%) SDI quintiles. Most of the increase in DALYs was driven by population growth in the low (103.60%) and low‐middle (66.51%) SDI quintiles. The epidemiological changes showed various trends over the 28‐year analysis period, with a negative contribution in low and high‐middle SDI quintiles (−26.58 and −8.01%, respectively), and a notable positive contribution of 24.51% in the high SDI quintile (Table 2).
Table 2.
Changes in disability‐adjusted life years by population‐level determinants from 1990 to 2017 globally and by Sociodemographic Index quintile
| Location | Overall difference † | Change due to population‐level determinants (contribution to the total change) | ||
|---|---|---|---|---|
| Aging ‡ | Population growth § | Epidemiological changes ¶ | ||
| Global | 4,302,258.98 | 1,840,535.972 (42.78%) | 1,976,597.73 (45.94%) | 485,125.307 (11.28%) |
| High SDI | 707,882.99 | 363,048.8603 (51.29%) | 171,331.9108 (24.20%) | 173,502.2049 (24.51%) |
| High‐middle SDI | 516,766.77 | 366,329.3238 (70.89%) | 191,852.9424 (37.13%) | −41,415.49135 (−8.01%) |
| Middle SDI | 1,776,209.54 | 1,008,685.954 (56.79%) | 573,257.0779 (32.27%) | 194,266.4759 (10.94%) |
| Low‐middle SDI | 935,285.86 | 308,557.5868 (32.99%) | 622,102.0237 (66.51%) | 4,626.221747 (0.49%) |
| Low SDI | 352,956.06 | 81,121.49878 (22.98%) | 365,666.0095 (103.60%) | −93,831.44438 (−26.58%) |
Change in disability‐adjusted life years (DALYs) number between 2017 and 1990.
Change in DALYs number as a result of change in the age structure.
Change in DALYs number as a result of change in population number.
Change in DALYs number as a result of epidemiological changes. Epidemiological changes refer to the DALY number change when age structure and population hold constant. SDI, Sociodemographic Index.
SDI and burden of CKD as a result of type 2 diabetes mellitus
As shown in Figure 3, significant negative associations were found between SDI and ASIR (ρ = −0.52, P < 0.001), ASDR (ρ = −0.27, P < 0.001) and the age‐standardized DALY rate (ρ = −0.30, P < 0.001) in 2017. The disease‐related burden will be expected to show a downward temporal trend with an improving socioeconomic status.
Figure 3.
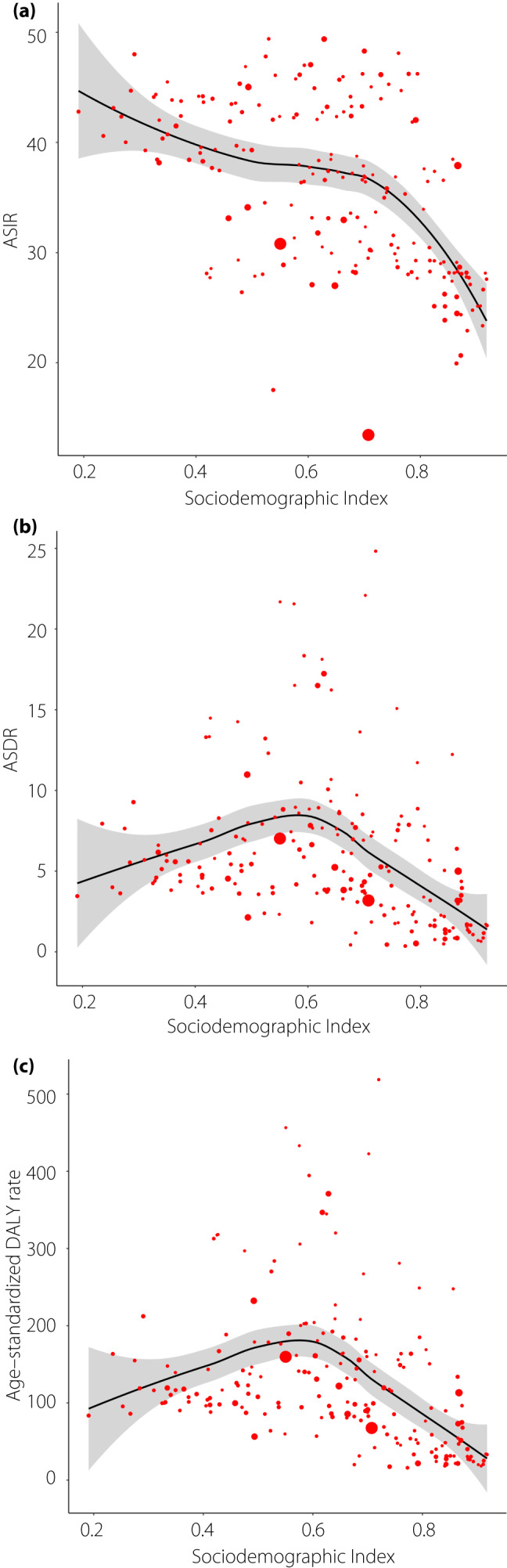
The association between (a) age‐standardized incidence, (b) death, (c) disability‐adjusted life year (DALY) rate and Sociodemographic Index. (a) ρ = −0.52, P < 0.001; (b) ρ = −0.27, P < 0.001; (c) ρ = −0.30, P < 0.001; Each circle represents a country; circle size corresponds to population number. The P indices and ρ values were derived from Pearson’s correlation analysis.
The global average DALY rate for CKD as a result of type 2 diabetes mellitus among 50‐year‐olds in 2017 was 137.2 per 100,000 persons. Taking this as a reference for comparisons with other countries, the estimated equivalent ages varied widely, from being oldest (at 81.5 years) in Belarus to youngest (at 35.7 years) in the Solomon Islands. As shown in Figure 4, countries with a higher SDI generally experience a lower burden of CKD as a result of type 2 diabetes mellitus: the estimated equivalent ages were 56.1, 55.9, 47.8, 46.9 and 48.6 years in high, high‐middle, middle, low‐middle and low SDI countries, respectively. The results for all 195 countries are provided in Table S2.
Figure 4.
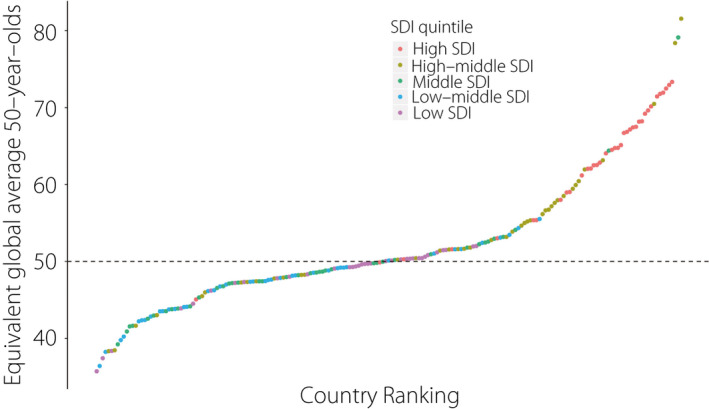
Comparing the equivalent ages to global average 50‐year‐olds across countries in 2017. SDI, Sociodemographic Index.
Years of life lost accounted for 82.13% of the age‐standardized DALY rate associated with CKD as a result of type 2 diabetes mellitus in 2017 globally. The proportion was lowest in the high SDI quintile (68.64%), followed by the high‐middle (75.86%), low (84.06%), middle (86.92%) and low‐middle (87.15%) SDI quintiles (Figure S3).
Frontier analysis based on age‐standardized DALY rates
Although there was generally a negative correlation between SDI and the age‐standardized DALY rate, we noted substantial heterogeneity for countries with similar SDIs. Overall, the lowest observed DALY rate showed a downward trend as SDI increased, and the frontier remained steady when SDI exceeded 0.75. We set countries with small effective distance changes (between −10 and 10) from 1990 to 2017 as the moderate group, referring to countries along with comparable decreasing DALY rate as the SDI increased. Additionally, we defined the overperforming and underperforming groups to classify the 195 countries into separate groups based on their relative performance. A total of 63 countries were categorized into the moderate group (e.g., China, Brazil, Russia and Germany), 50 into the overperforming group (e.g., Japan, Mongolia, South Korea and Turkey) and 82 countries into the underperforming group (e.g., the USA, Australia, India and Mexico). Notably, the SDI for the USA increased from 0.78 to 0.87 between 1990 and 2017, meanwhile the corresponding DALY rate increased from 54.91 to 113.17 (Table S3).
Furthermore, the top five countries with the lowest effective differences from the frontier were Belarus, Ukraine, Iceland, Lithuania and Moldova in 2017 (range 0.24–3.25). The top five countries with the largest effective differences were Mauritius, Marshall Islands, Federated States of Micronesia, American Samoa and El Salvador (range 376.49–502.58; Figure 5).
Figure 5.
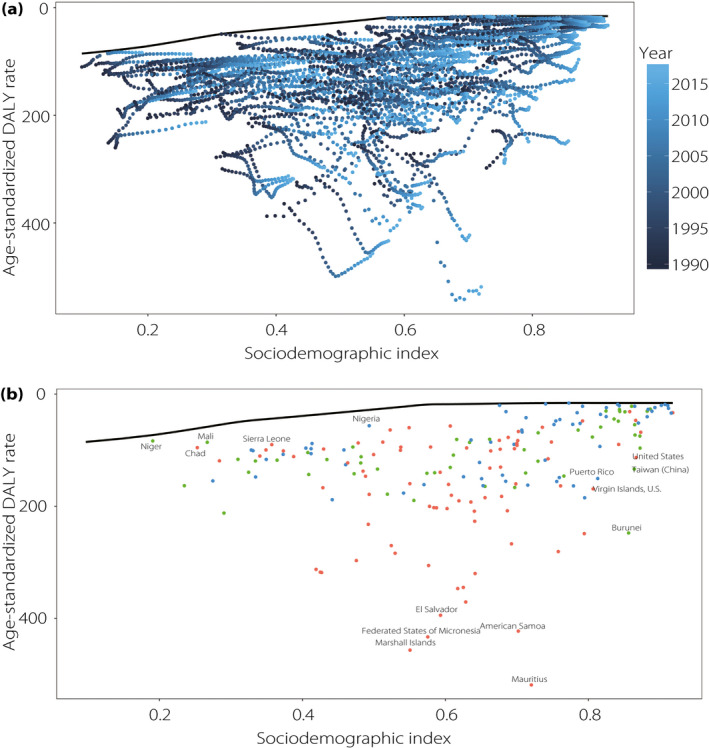
(a) Frontier analysis based on Sociodemographic Index (SDI) and age‐standardized disability‐adjusted life year (DALY) rate from 1990 to 2017. Color scale represents the years from 1990 shown in dark blue to 2017 shown in light blue. The frontier is shown in black color. (b) Frontier analysis based on SDI and age‐standardized chronic kidney disease as a result of type 2 diabetes mellitus DALY rate in 2017. The frontier is shown in black color; countries and territories are represented as dots. The top five countries with the largest effective difference, examples of countries with low effective difference and low SDI (<0.5), and examples of countries (territories) with relatively high effective difference and high SDI (>0.8) are labeled in black. Blue dots indicate countries in the moderate group with compatible declining DALY rate as SDI increased. Green dots indicate countries in the overperforming group. Red dots indicate countries in the underperforming group.
Burden attributable to risk factors
CKD as a result of type 2 diabetes mellitus is always accompanied by impaired kidney function and a high fasting plasma glucose level. We focused on DALYs as a result of high systolic blood pressure, high body mass index (BMI), diet high in sodium and lead exposure in this study. As of 2017, 45.72% of the global age‐standardized DALY rate was attributable to hypertension, with a steady trend from 1990 globally and across all except the high SDI region. High BMI contributed 34.55% of the total burden, with the proportion increasing rapidly during the study period across all SDI quintiles. The increase was largest for the high SDI region, from a 33.64% contribution in 1990 to one of 43.75% in 2017, resulting in high BMI being a leading risk factor since 2015 in high SDI countries. Diet high in sodium and lead exposure were responsible for 12.35 and 4.69% of the global burden in 2017, respectively, and this pattern was stable and comparable among all SDI quintiles over time (Figure 6).
Figure 6.
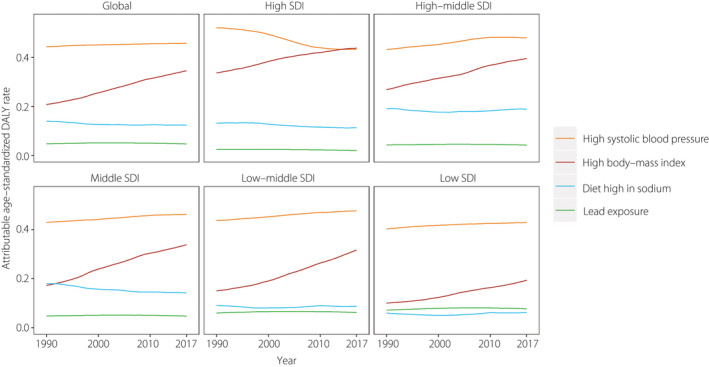
Contributions of risk factors to age‐standardized disability‐adjusted life year (DALY) rate globally and in Sociodemographic Index (SDI) quintiles.
Discussion
Diabetic kidney disease (DKD) develops in approximately 40% of patients who have diabetes and is the leading cause of CKD worldwide 14 . The decrease in ASIR most likely reflects progress made in the management of hyperglycemia and the complications of type 2 diabetes mellitus despite the prevalence of diabetes globally having been increasing since 1980 7 , 15 . This encouraging trend is further supported by data from the Steno Diabetes Center, which reported contemporary patients with type 2 diabetes mellitus are less likely to develop renal impairment than were patients from 1983 to 2003 16 , possibly as a result of overall increased use of glucose‐lowering medications and renin–angiotensin–aldosterone system inhibitors.
China has experienced the largest decrease in ASIR at a national level. Similarly, in a cohort study using data of 8,811 community‐based patients with diabetes in China, the percentage of isolated estimated glomerular filtration rate <60 mL/min/1.73 m2 and isolated proteinuria (either alone or in combination) was comparatively lower compared with results from the UK Prospective Diabetes Study involving 4,006 patients with diabetes 17 , 18 . By contrast, the USA saw a significant increase in the AISR of CKD as a result of type 2 diabetes mellitus, along with high EAPC from 1990 to 2017. The overall prevalence of DKD did not change significantly among adults in the USA with diabetes from 1988 to 2014, and the prevalence of DKD in the USA increased in proportion to the prevalence of diabetes 19 , 20 . In addition, in the USA, the prevalence of type 2 diabetes mellitus among children and adolescents has increased by 30.5% between 2001 and 2009 21 , and its incidence has increased 4.8% annually between 2002 and 2012 22 .
The burden of CKD as a result of type 2 diabetes mellitus quantified as the deaths and DALYs increased steadily over the study period at the global level. Notably, the mortality risk is known to be enormous, nearly 20% per year in patients with elevated plasma creatinine or RRT long before end‐stage kidney disease 18 . As both the incidence and prevalence of type 2 diabetes mellitus increase substantially with age, and elderly diabetes patients are more likely to have other comorbidities, the DALY rate of population aged >65 years was significantly higher 23 . Despite the overall heavier burden in men in 2017, the exceeding cases and incidence rates for women suggest a potential upcoming rapid rise in the prevalence of CKD as a result of type 2 diabetes mellitus in women. Several explanatory factors have been put forward for this, including overestimating disease presence in women by glomerular filtration rate‐estimating equations, declining protective hormone levels with age for women and relevant social factors, such as women being more likely to present for screening or symptom diagnosis in comparison with men 24 .
Aging was the more prominent driver in the middle, high‐middle and high SDI nations with higher life expectancies, and population growth was the main force in the low‐middle and low SDI nations known for high fertility and lower life expectancy. In accordance with the decreasing trend of the age‐standardized DALY rate in low and high‐middle SDI regions, epidemiological changes contributed negatively in those areas, which represents a favorable finding and shows that some progress has been made in the prevention and treatment of CKD as a result of type 2 diabetes mellitus 25 .
Higher SDI countries have greater financial resources to establish a better healthcare system, and thereby implement more effective and timely treatments 26 , 27 . Furthermore, our equivalent age analysis showed a staggering difference between countries of 46 years in the highest and lowest equivalent ages compared with the global average 50‐year‐olds, and the deviations present a call to action for healthcare providers to narrow the gap in health burden.
The years of life lost accounted for 84.06 and 87.15% of DALYs in the low and low‐middle SDI quintiles, respectively, significantly exceeding those in the high (68.64%) and high‐middle (75.86%) SDI quintiles. This finding showed that the deterioration in health statuses in these regions was primarily driven by premature deaths rather than disability. There are several possible explanations for this: (i) lack of health awareness and low likelihood of detecting disease in the early stage; (ii) failure of the primary healthcare system to respond promptly and effectively; and (iii) less available access to high‐cost interventions (e.g., RRT and kidney transplantation) 28 , 29 , 30 . Given the restricted sociodemographic resources, we suggest that policies and practices in underdeveloped countries should focus on facilitating cost‐effective screening, early diagnoses and low‐cost treatments, such as peritoneal dialysis, to minimize the risk of CKD progression in diabetes patients, which will have a substantial cost impact for health systems 31 , 32 . As excess mortality in type 2 diabetes mellitus patients is largely confined to those with CKD 33 , the value of identifying CKD in a timely manner and consequently optimizing care in a proper manner is potentially great.
Some countries have made considerable progress alongside a rapid socioeconomic development from 1990 to 2017. Meanwhile, 82 countries showed disappointing performance (e.g., the USA and Australia). In 2017, we found countries with DALY rates that were distant from the frontier, suggesting that there are considerable opportunities to narrow the gap, as well as exemplars that showed leading performance at all levels of the development spectrum, which should encourage countries with similar SDIs to optimize their available resources to achieve better health outcomes. Low socioeconomic development is not an impossible barrier to overcome, and the health progress enabled by sociodemographic prosperity might be overwhelmed by other forces. This heterogeneity could be partially explained by the shift of prevalence patterns of attributable risk factors (i.e., hyperglycemia, hypertension, obesity) 34 , meanwhile therein lies the opportunity to break out of the narrative that managing kidney disease burden just requires addressing diabetes and hypertension, as data are emerging on the relationship of smoking and ambient pollution with diabetic nephropathy burden in different parts of the world 35 , 36 , 37 .
High BMI has made an increasing contribution to the DALY rate across all SDI quintiles from 1990 to 2017. This finding is consistent with a previous report that the prevalence of obesity in adults is highest in high‐SDI countries and is continuing to increase rapidly 38 , which highlights the need for a continued focus on BMI surveillance. Furthermore, reducing dietary sodium intake represents another therapeutic opportunity to improve nephropathy in diabetes patients 39 .
The present study was subject to several limitations. First, we relied on estimates generated by the GBD study group, and although GBD methodologies and results are considered reliable and robust, they are necessarily limited by the quality of the available data 8 , 9 . Second, CKD etiology proportion models informed by data from end‐stage kidney disease registries and the Geisinger Health System in Pennsylvania might also have affected the precision of some of the estimated levels and trends of disease burden 8 . Third, certain established risk factors for CKD as a result of type 2 diabetes mellitus have not been elaborated in the database, and therefore are not estimated separately in the present study.
The global burden of CKD as a result of type 2 diabetes mellitus quantified as ASDR and age‐standardized DALY rate has substantially increased from 1990 to 2017. Our decomposition analysis identified population expansion and demographic transition as the main factors driving the increasing DALYs worldwide, and there was a significant variation in demographic and epidemiological changes across SDI regions. Despite a negative correlation between socioeconomic status and the corresponding burden, we ascertained that certain countries across the development spectrum showed performance that exceeded expectations, whereas others suffered an increasing DALY rate despite sociodemographic prosperity. Given restricted resources, we suggest that there should be more of a focus on initiating causal pathways and interventions at early stages in order to alleviate the death burden in low‐to‐middle SDI countries. Overweight and obesity are increasing public health problems across all SDI levels as far as CKD as a result of type 2 diabetes mellitus is concerned. There is an urgent need to implement more tailored prevention programs (e.g., diet modification or physical activity programs) and targeted campaigns against overweight and obesity, especially in high‐income countries.
Disclosure
The authors declare no conflict of interest.
Supporting information
Data S1 | Supplementary methods.
Table S1 | Chronic kidney disease as a result of type 2 diabetes mellitus numbers and age‐standardized rates at the country and regional level.
Table S2 | Equivalent age in 195 countries and territories compared with global 50‐year‐olds in 2017.
Table S3 | Frontier disability‐adjusted life years and effective difference by country or territory.
Figure S1 | (a) Age‐standardized incidence, (b) death and (c) disability‐adjusted life years (DALY) rates in 2017.
Figure S2 | (a) Age‐standardized incidence, (b) death and (c) disability‐adjusted life years (DALY) rates globally and in Sociodemographic Index (SDI) quintiles between 1990 and 2017.
Figure S3 | The ratio of age‐standardized years of life lost rate and disability‐adjusted life years (DALY) rate globally and in Sociodemographic Index (SDI) quintile.
Acknowledgments
We appreciate the work by the Global Burden of Disease study 2017 collaborators.
J Diabetes Investig 2021; 12: 346–356
References
- 1. Zhang L, Long J, Jiang W, et al. Trends in chronic kidney disease in China. N Engl J Med 2016; 375: 905–906. [DOI] [PubMed] [Google Scholar]
- 2. Fraser SDS, Roderick PJ. Kidney disease in the Global Burden of Disease Study 2017. Nat Rev Nephrol 2019; 15: 193–194. [DOI] [PubMed] [Google Scholar]
- 3. Xie Y, Bowe B, Mokdad AH, et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 2018; 94: 567–581. [DOI] [PubMed] [Google Scholar]
- 4. Jager KJ, Fraser SDS. The ascending rank of chronic kidney disease in the global burden of disease study. Nephrol Dial Transplant 2017; 32(suppl_2):ii121–ii128. [DOI] [PubMed] [Google Scholar]
- 5. Wang F, Yang C, Long J, et al. Executive summary for the 2015 Annual Data Report of the China Kidney Disease Network (CK‐NET). Kidney Int 2019; 95: 501–505. [DOI] [PubMed] [Google Scholar]
- 6. Global, regional, and national burden of chronic kidney disease, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395: 709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Collaboration NCDRF . Worldwide trends in diabetes since 1980: a pooled analysis of 751 population‐based studies with 4.4 million participants. Lancet 2016; 387: 1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. GBD 2017 Disease and Injury Incidence and Prevalence Collaborator . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. GBD 2017 Causes of Death Collaborators . Global, regional and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018; 392: 1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. GBD 2017 DALYs and HALE Collaborators . Global, regional, and national disability‐adjusted life‐years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1859–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. GBD 2017 Population and Fertility Collaborators . Population and fertility by age and sex for 195 countries and territories, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1995–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao S, Yang WS, Bray F, et al. Declining rates of hepatocellular carcinoma in urban Shanghai: incidence trends in 1976–2005. Eur J Epidemiol. 2012; 27: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. GBD 2015 Healthcare Access and Quality Collaborators . Healthcare Access and Quality Index based on mortality from causes amenable to personal health care in 195 countries and territories, 1990–2015: a novel analysis from the Global Burden of Disease Study 2015. Lancet 2017; 390: 231–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 2017; 12: 2032–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ruospo M, Saglimbene VM, Palmer SC, et al. Glucose targets for preventing diabetic kidney disease and its progression. Cochrane Database Syst Rev 2017; 6: CD010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andresdottir G, Jensen ML, Carstensen B, et al. Improved survival and renal prognosis of patients with type 2 diabetes and nephropathy with improved control of risk factors. Diabetes Care 2014; 37: 1660–1667. [DOI] [PubMed] [Google Scholar]
- 17. Gao B, Wu S, Wang J, et al. Clinical features and long‐term outcomes of diabetic kidney disease ‐ A prospective cohort study from China. J Diabetes Complicat 2019; 33: 39–45. [DOI] [PubMed] [Google Scholar]
- 18. Adler AI, Stevens RJ, Manley SE, et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003; 63: 225–232. [DOI] [PubMed] [Google Scholar]
- 19. de Boer IH, Rue TC, Hall YN, et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011; 305: 2532–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Afkarian M, Zelnick LR, Hall YN, et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA 2016; 316: 602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dabelea D, Mayer‐Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014; 311: 1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mayer‐Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med 2017; 376: 1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boyle JP, Thompson TJ, Gregg EW, et al. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010; 8: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thomas B. The global burden of diabetic kidney disease: time trends and gender gaps. Curr Diab Rep 2019; 19: 18. [DOI] [PubMed] [Google Scholar]
- 25. Harris DC, Dupuis S, Couser WG, et al. Training nephrologists from developing countries: does it have a positive impact? Kidney Int Suppl 2011; 2012: 275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cervantes L, Tuot D, Raghavan R, et al. Association of Emergency‐Only vs Standard Hemodialysis With Mortality and Health Care Use Among Undocumented Immigrants With End‐stage Renal Disease. JAMA Intern Med. 2018; 178: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Osman MA, Alrukhaimi M, Ashuntantang GE, et al. Global nephrology workforce: gaps and opportunities toward a sustainable kidney care system. Kidney Int Suppl 2011; 2018: 52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bello AK, Johnson DW, Feehally J, et al. Global Kidney Health Atlas (GKHA): design and methods. Kidney Int Suppl 2011; 2017: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Htay H, Alrukhaimi M, Ashuntantang GE, et al. Global access of patients with kidney disease to health technologies and medications: findings from the Global Kidney Health Atlas project. Kidney Int Suppl 2011; 2018: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crews DC, Bello AK, Saadi G. Burden, access, and disparities in kidney disease. Kidney Int 2019; 95: 242–248. [DOI] [PubMed] [Google Scholar]
- 31. Karopadi AN, Mason G, Rettore E, et al. Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant 2013; 28: 2553–2569. [DOI] [PubMed] [Google Scholar]
- 32. Muralidharan A, White S. The need for kidney transplantation in low‐ and middle‐income countries in 2012: an epidemiological perspective. Transplantation 2015; 99: 476–481. [DOI] [PubMed] [Google Scholar]
- 33. Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013; 24: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Collaborators GBDRF . Global regional and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392: 1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liao D, Ma L, Liu J, et al. Cigarette smoking as a risk factor for diabetic nephropathy: a systematic review and meta‐analysis of prospective cohort studies. PLoS One 2019; 14: e0210213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jhee JH, Joo YS, Kee YK, et al. Secondhand smoke and CKD. Clin J Am Soc Nephrol 2019; 14: 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chin WS, Chang YK, Huang LF, et al. Effects of long‐term exposure to CO and PM2.5 on microalbuminuria in type 2 diabetes. Int J Hyg Environ Health 2018; 221: 602–608. [DOI] [PubMed] [Google Scholar]
- 38. Collaborators GBDO, Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017; 377: 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lambers Heerspink HJ, Navis G, Ritz E. Salt intake in kidney disease–a missed therapeutic opportunity? Nephrol Dial Transplant 2012; 27: 3435–3442. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 | Supplementary methods.
Table S1 | Chronic kidney disease as a result of type 2 diabetes mellitus numbers and age‐standardized rates at the country and regional level.
Table S2 | Equivalent age in 195 countries and territories compared with global 50‐year‐olds in 2017.
Table S3 | Frontier disability‐adjusted life years and effective difference by country or territory.
Figure S1 | (a) Age‐standardized incidence, (b) death and (c) disability‐adjusted life years (DALY) rates in 2017.
Figure S2 | (a) Age‐standardized incidence, (b) death and (c) disability‐adjusted life years (DALY) rates globally and in Sociodemographic Index (SDI) quintiles between 1990 and 2017.
Figure S3 | The ratio of age‐standardized years of life lost rate and disability‐adjusted life years (DALY) rate globally and in Sociodemographic Index (SDI) quintile.


