Key Points
Question
After induction therapy and 2 blocks of consolidation chemotherapy, does 1 cycle of blinatumomab compared with a third course of consolidation chemotherapy before allogeneic hematopoietic stem cell transplant improve event-free survival in high-risk first-relapse B-cell acute lymphoblastic leukemia (B-ALL) in children?
Findings
In this randomized clinical trial that included 108 children with high-risk first-relapse B-ALL, treatment with blinatumomab compared with chemotherapy for consolidation treatment resulted in a statistically significant hazard ratio for event-free survival of 0.33 after a median of 22.4 months of follow-up.
Meaning
Blinatumomab compared with chemotherapy for consolidation treatment improved event-free survival in children with relapsed B-ALL.
Abstract
Importance
Blinatumomab is a CD3/CD19-directed bispecific T-cell engager molecule with efficacy in children with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL).
Objective
To evaluate event-free survival in children with high-risk first-relapse B-ALL after a third consolidation course with blinatumomab vs consolidation chemotherapy before allogeneic hematopoietic stem cell transplant.
Design, Setting, and Participants
In this randomized phase 3 clinical trial, patients were enrolled November 2015 to July 2019 (data cutoff, July 17, 2019). Investigators at 47 centers in 13 countries enrolled children older than 28 days and younger than 18 years with high-risk first-relapse B-ALL in morphologic complete remission (M1 marrow, <5% blasts) or with M2 marrow (blasts ≥5% and <25%) at randomization.
Intervention
Patients were randomized to receive 1 cycle of blinatumomab (n = 54; 15 μg/m2/d for 4 weeks, continuous intravenous infusion) or chemotherapy (n = 54) for the third consolidation.
Main Outcomes and Measures
The primary end point was event-free survival (events: relapse, death, second malignancy, or failure to achieve complete remission). The key secondary efficacy end point was overall survival. Other secondary end points included minimal residual disease remission and incidence of adverse events.
Results
A total of 108 patients were randomized (median age, 5.0 years [interquartile range {IQR}, 4.0-10.5]; 51.9% girls; 97.2% M1 marrow) and all patients were included in the analysis. Enrollment was terminated early for benefit of blinatumomab in accordance with a prespecified stopping rule. After a median of 22.4 months of follow-up (IQR, 8.1-34.2), the incidence of events in the blinatumomab vs consolidation chemotherapy groups was 31% vs 57% (log-rank P < .001; hazard ratio [HR], 0.33 [95% CI, 0.18-0.61]). Deaths occurred in 8 patients (14.8%) in the blinatumomab group and 16 (29.6%) in the consolidation chemotherapy group. The overall survival HR was 0.43 (95% CI, 0.18-1.01). Minimal residual disease remission was observed in more patients in the blinatumomab vs consolidation chemotherapy group (90% [44/49] vs 54% [26/48]; difference, 35.6% [95% CI, 15.6%-52.5%]). No fatal adverse events were reported. In the blinatumomab vs consolidation chemotherapy group, the incidence of serious adverse events was 24.1% vs 43.1%, respectively, and the incidence of adverse events greater than or equal to grade 3 was 57.4% vs 82.4%. Adverse events leading to treatment discontinuation were reported in 2 patients in the blinatumomab group.
Conclusions and Relevance
Among children with high-risk first-relapse B-ALL, treatment with 1 cycle of blinatumomab compared with standard intensive multidrug chemotherapy before allogeneic hematopoietic stem cell transplant resulted in an improved event-free survival at a median of 22.4 months of follow-up.
Trial Registration
ClinicalTrials.gov Identifier: NCT02393859
This randomized trial compares the effects of blinatumomab, an antibody construct that links CD3+ T cells to CD19+ leukemia cells, vs consolidation chemotherapy as a third consolidation block before allogeneic hematopoietic stem cell transplant (HST) on event-free survival in children with high-risk first-relapse B-cell acute lymphoblastic leukemia (B-ALL).
Introduction
Approximately 15% of children with B-cell acute lymphoblastic leukemia (B-ALL) relapse after frontline chemotherapy.1 The prognosis of these children depends mainly on the site of relapse and time from diagnosis to relapse, although some recurrent chromosomal abnormalities can affect outcomes.2,3,4 According to the site and timing of relapse, children are categorized as having standard- or high-risk first-relapse B-ALL.5 Children with high-risk first-relapse B-ALL are candidates to receive allogeneic hematopoietic stem cell transplant when a second cytomorphologic complete remission is achieved; allogeneic hematopoietic stem cell transplant is a very effective approach for preventing further recurrence in these patients.6
Blinatumomab is a CD3/CD19-directed bispecific T-cell engager molecule that engages T cells to lyse CD19-expressing B cells.7 Blinatumomab has demonstrated antileukemic activity in a phase 1/2 study conducted in children with relapsed or refractory B-ALL8 and induced high rates of complete minimal residual disease response in adults and children with molecularly resistant B-ALL.9,10
This study was a multicenter, randomized, phase 3 clinical trial designed to compare blinatumomab with intensive multidrug consolidation chemotherapy as the third consolidation block before allogeneic hematopoietic stem cell transplant for children with high-risk first relapse of B-ALL.
Methods
Trial Design, Oversight, and Participants
The study protocol and statistical analysis plan are provided in Supplement 1 and Supplement 2. The trial protocol was approved by the ethics committee or institutional review board at each participating center. Parents or a legally acceptable representative provided written informed consent.
In this multicenter, open-label, randomized, phase 3 clinical trial, investigators at 47 centers in 13 countries (eTable 1 in Supplement 3) enrolled children older than 28 days and younger than 18 years with Philadelphia chromosome–negative, high-risk, first-relapse, B-ALL, defined according to the International BFM Study Group and IntReALL Consortium risk classification5 (eTable 2 in Supplement 3). Children were required to have M1 marrow (<5% morphologic blasts) or M2 marrow (≥5% but <25% morphologic blasts) at randomization. The aim of the trial was to investigate the efficacy of blinatumomab to reduce residual leukemia burden before allogeneic hematopoietic stem cell transplant and, by that, to thereby improve outcome after allogeneic hematopoietic stem cell transplant. Only patients completing induction and the first 2 cycles of standard consolidation therapy were included in the trial. Patients refractory to induction or relapsing during the first 2 blocks of consolidation chemotherapy were excluded. Patient legal representatives consented, and randomization was performed immediately before the study therapy and after the second consolidation course. Complete inclusion and exclusion criteria are provided (eAppendix in Supplement 3).
Randomization
Eligible patients were randomly assigned in a 1:1 ratio via an interactive voice-response system with a computer-generated random number to receive a third consolidation course with either blinatumomab or consolidation chemotherapy. Randomization was stratified by 2 age groups (1-9 years and other [<1 year and >9 years], the latter 2 “age groups” having a worse prognosis than the former) and 3 bone marrow/minimal residual disease categories (M1 marrow with minimal residual disease level ≥10-3, M1 marrow with minimal residual disease level <10-3, and M2 marrow); minimal residual disease and cytomorphologic bone marrow status were determined at the end of induction and end of the second consolidation course, respectively. A diagnostic lumbar puncture was performed before randomization and children with evidence of central nervous system involvement at randomization were considered per-protocol ineligible.
Treatment
Eligible patients received induction therapy and 2 blocks of consolidation therapy (eTables 3 and 4 in Supplement 3), chosen among the IntReALL HR 2010, ALL-REZ BFM 2002, ALL R3, COOPRALL, and AIEOP ALL REC 2003 protocols at the investigators’ discretion.11,12,13,14,15 Patients were then randomized to receive a third consolidation course with either blinatumomab (15 μg/m2/d for 4 weeks by continuous intravenous infusion) or consolidation chemotherapy according to the IntReALL HR 2010 protocol (eTables 3 and 4 in Supplement 3). The consolidation chemotherapy block of the IntReALL HR 2010 protocol is routinely used in the AIEOP-BFM protocols16 during the consolidation phase of high-risk patients. Patients in the blinatumomab group received dexamethasone (5 mg/m2) before treatment on day 1 to prevent first-dose adverse events. Dose modification details are provided (eAppendix in Supplement 3). Patients who achieved a second complete remission (M1 marrow) after completion of either blinatumomab or consolidation chemotherapy treatment could undergo allogeneic hematopoietic stem cell transplant.
Outcomes
The primary end point was event-free survival, for which events were relapse, death, second malignancy, or failure to achieve complete remission. Overall survival (time from randomization to death) was a key secondary efficacy end point. Secondary end points included cumulative incidence of relapse, minimal residual disease remission at end of treatment, survival status at 100 days after allogeneic hematopoietic stem cell transplant, and incidence of adverse events. An exploratory end point was CD19 status at relapse. Additional secondary end points (incidence of antiblinatumomab antibody formation, pharmacokinetic sampling for blinatumomab concentrations for population pharmacokinetic analysis, and blinatumomab steady-state concentrations) are not reported in this article.
Assessments
Complete remission was defined as M1 marrow (representative bone marrow aspirate or biopsy with <5% blasts with satisfactory cellularity and regenerating hematopoiesis), peripheral blood without blasts, and absence of extramedullary leukemic involvement. Cytomorphologic bone marrow assessments were conducted at University Hospital Schleswig–Holstein, Kiel.
Minimal residual disease was assessed by real-time quantitative polymerase chain reaction (PCR) of clonal T-cell receptor or immunoglobulin gene rearrangements and multicolor flow cytometry.17,18 PCR is considered the criterion standard for quantifying minimal residual disease and has been validated by the study groups managing pediatric and adult patients with ALL.19,20 Assessment of minimal residual disease by PCR and flow cytometry was conducted in parallel, unless material was limited, in which case PCR, the most sensitive and robust investigator-independent method, was used. PCR assessments were conducted at Charité Campus Virchow-Klinikum Pädiatrie m.S. Onkologie und Hämatologie, Berlin, Germany, and multicolor flow cytometry at Charles University, Prague, Czech Republic and Westmead Hospital, Sydney, Australia; assay sensitivity was greater than or equal to 10-4. Minimal residual disease remission was defined as minimal residual disease less than 10-4.
Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. Serious adverse events included events that were fatal; were life threatening; required hospitalization or prolonged hospitalization; resulted in disability or incapacity, congenital anomaly, or birth defect; or were other medically important events. Adverse events were continuously collected on or after the first dose of protocol-specified therapy and up to and including 30 days after the end of protocol-specified therapy if the patient did not proceed to allogeneic hematopoietic stem cell transplant or more than 90 days after allogeneic hematopoietic stem cell transplant.
Sample Size and Power Calculation
An enrollment target of approximately 202 patients and the observation of 94 events was estimated to provide approximately 84% power and a noncured hazard ratio of 0.63 in the blinatumomab vs consolidation chemotherapy group at a 2-sided α level of .05. The calculation was based on a control true cure rate of 40% (Arend von Stackelberg, MD, Charité-Universitätsmedizin Berlin, email on October 27, 2013), a control true median event-free survival of 7 months among noncured patients, a true treatment cure rate of 56.2%, and a true treatment median event-free survival of 11.1 months among noncured patients (a noncured hazard ratio of 0.63). The 4.1-month improvement in median event-free survival was considered clinically meaningful in this high-risk first-relapse pediatric B-ALL population because of their poor outcome with standard of care and anticipated benefit of less toxicity based on the data of trial MT103-202 and expanded access cases.5,21,22,23
Statistical Analysis
The primary end point was event-free survival, calculated from randomization to the date of relapse or M2 marrow after achievement of complete remission, failure to achieve complete remission at the end of treatment, second malignancy, or death owing to any cause, whichever occurred first. Patients who did not achieve complete remission after treatment or who died before the end-of-treatment disease assessment were assigned an event-free survival duration of 1 day. Patients who prematurely ended study before observing an event-free survival event were censored at their last evaluable disease assessment date; only 6 of the 108 patients discontinued study prematurely before having an event-free survival event. Patients still alive and event-free were censored on their last disease assessment date.
Two interim analyses were planned to assess benefit when approximately 50% and approximately 75% of the total number of event-free survival events were observed. Stopping for benefit was based on the O’Brien-Fleming24 member of the family of Lan-DeMets25 α spending functions, which is more conservative during early interim analyses. The critical P value corresponding to this spending function was .003 for the 50% interim analysis.
Patients were analyzed according to their randomized treatment group, regardless of the treatment they received after they were randomized (ie, intention-to-treat analysis). Analysis of efficacy included all patients who underwent randomization; analysis of safety included all patients given either blinatumomab or consolidation chemotherapy. Time-to-event end points were summarized with the Kaplan-Meier method, and treatment groups were compared with 2-sided stratified log-rank tests. A Cox regression model also tested for a treatment-by-subgroup interaction (an interaction term with a P < .10 may be suggestive of an inconsistent treatment effect). Subgroups included age (1-9 years vs other [<1 year and >9 years]), bone marrow (M1 vs M2 marrow), minimal residual disease categories (<10-3 vs ≥10-3), sex (boys vs girls), time to relapse (<18 months vs ≥18 months and ≤30 months), and extramedullary disease at relapse (yes vs no). Treatment effects were described with a hazard ratio with 95% CI, estimated with a stratified Cox regression model. Percentages of patients with minimal residual disease remission were summarized with an exact binomial 95% CI. Patient incidences of all treatment-emergent adverse events were summarized. The cumulative incidence of relapse was analyzed with an extension of the Cox regression model, whereby deaths that occurred before relapse and were considered unrelated to an otherwise undocumented relapse were treated as a competing risk.26 Descriptive statistics identified the extent of missing data. A post hoc analysis of event-free survival accounting for the effect of study center was performed with a semiparametric gamma frailty model treating study center as a random effect. A sensitivity analysis was performed to estimate the treatment effect of overall survival conditioning on the time at which patients in the consolidation chemotherapy group received blinatumomab.27 The statistical software used for the analyses was SAS version 9.4. Because of the potential for type I error owing to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
Results
Trial Population and Treatment
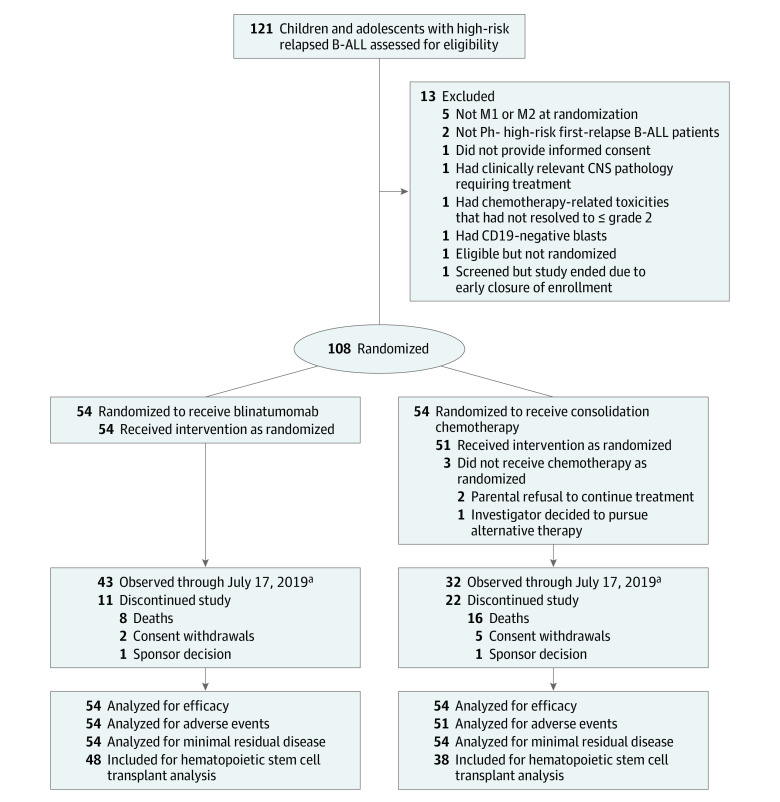
Patients were enrolled between November 2015 and July 2019. After approximately 50% of the total event-free survival events had occurred (49 of the total 94 events; data cutoff date, July 17, 2019), the independent data monitoring committee recommended early termination of enrollment because the prespecified criterion to declare benefit in favor of blinatumomab was met (the observed P < .001 was less than the threshold of .004). This analysis included 108 patients who had undergone randomization (54, blinatumomab; 54, consolidation chemotherapy); 54 of 54 patients (100.0%) received 1 cycle of blinatumomab and 51 of 54 patients (94.4%) received consolidation chemotherapy (Figure 1). Six patients randomized to the consolidation chemotherapy group received blinatumomab before allogeneic hematopoietic stem cell transplant; 5 of 6 patients received blinatumomab after a relapse event (3, M3; 2, M2 marrow) after consolidation chemotherapy and 1 of 6 patients received blinatumomab because of refractory minimal residual disease–positive M1 marrow after consolidation chemotherapy. Two patients in the blinatumomab group and none in the consolidation chemotherapy group were continuing trial treatment at the analysis. The baseline demographics and disease characteristics of the 2 treatment groups were comparable, except there was a higher percentage of female patients in the consolidation chemotherapy group than in the blinatumomab group (59.3% vs 44.4%) (Table 1). No patient younger than 1 year was included in the trial. Induction therapy and the first 2 courses of consolidation were balanced between the 2 groups.
Figure 1. Flow of Patients Through the Trial of Blinatumomab vs Chemotherapy in Children With B-Cell Acute Lymphoblastic Leukemia (B-ALL).
Randomized patients were stratified based on age and bone marrow/minimal residual disease. M1 marrow is indicated as <5% morphologic blasts; M2 marrow, 5%-<25% morphologic blasts. Ph− indicates Philadelphia chromosome negative, ie, patients with Philadelphia chromosome were excluded.
aData cutoff date occurred when 50% of total enrollment experienced defined study survival events. The final analysis is planned for January 2023.
Table 1. Demographic and Clinical Characteristics of Children at Randomization in the Trial of Blinatumomab vs Chemotherapy in B-ALL.
| No. (%) | ||
|---|---|---|
| Blinatumomab (n = 54) | Consolidation chemotherapy (n = 54) | |
| Age, y | ||
| Median (range) | 6 (1-17) | 5 (1-17) |
| Distributiona | ||
| 1-9 | 39 (72.2) | 38 (70.4) |
| 10-18 | 15 (27.8) | 16 (29.6) |
| Sex | ||
| Boys | 30 (55.6) | 22 (40.7) |
| Girls | 24 (44.4) | 32 (59.3) |
| Raceb | ||
| White | 50 (92.6) | 43 (79.6) |
| Other | 3 (5.6) | 5 (9.3) |
| Asian | 1 (1.9) | 3 (5.6) |
| Black or African American | 0 | 3 (5.6) |
| Ethnicityc | ||
| Not Hispanic or Latino | 53 (98.1) | 51 (94.4) |
| Hispanic or Latino | 1 (1.9) | 3 (5.6) |
| B-cell precursor subtyped | ||
| Common ALL | 31 (57.4) | 29 (53.7) |
| Pre–B-ALL | 20 (37.0) | 19 (35.2) |
| Pro–B-ALL | 3 (5.6) | 6 (11.1) |
| Genetic abnormalities at diagnosis of first high-risk relapse | ||
| Favorable prognosis | 8 (14.8) | 10 (18.5) |
| Hyperdiploidy | 6 (11.1) | 6 (11.1) |
| t(12;21)(p13;q22)/TEL-AML1 | 2 (3.7) | 4 (7.4) |
| Unfavorable prognosise | 7 (13.0) | 9 (16.7) |
| t(v;11q23)/KMT2A rearranged | 2 (3.7) | 6 (11.1) |
| t(1;19)(q23;p13.3)/E2A-PBX1 | 2 (3.7) | 2 (3.7) |
| Hypodiploidy | 2 (3.7) | 0 |
| Prognosis undefined | 5 (9.3) | 6 (11.1) |
| History of extramedullary relapse at diagnosis of first high-risk relapse | 10 (18.5) | 14 (25.9) |
| Central nervous system | 8 (14.8) | 11 (20.4) |
| Testis | 1 (1.9) | 1 (1.9) |
| Other | 1 (1.9) | 2 (3.7) |
| M1 (bone marrow aspirate or biopsy with <5% blasts) assessment per central laboratory, No./total evaluable (%)f | 54/54 (100.0) | 51/53 (96.2) |
| Minimal residual disease at screening for randomization ≥10-4 blastsg | 29 (53.7) | 28 (51.9) |
| Time from first diagnosis to relapse, mean (SD), mo | 21.9 (8.0) | 22.8 (12.3) |
| Time from first diagnosis to relapse, mo | ||
| ≥18 and ≤30 | 32 (59.3) | 28 (51.9) |
| <18 | 19 (35.2) | 22 (40.7) |
| >30 | 3 (5.6) | 4 (7.4) |
| Performance score, No./total evaluable (%)h | ||
| 100 | 25/52 (48.1) | 18/51 (35.3) |
| 90 | 19/52 (36.5) | 18/51 (35.3) |
| 80 | 7/52 (13.5) | 13/51 (25.5) |
| 70 | 1/52 (1.9) | 1/51 (2.0) |
| 60 | 0/52 | 1/51 (2.0) |
Abbreviations: B-ALL, B-cell acute lymphoblastic leukemia; PCR, polymerase chain reaction.
Although children younger than 1 year and older than 28 days were allowed to enroll in the trial, there were no patients younger than 1 year who were enrolled. Because the trial enrolled first-relapse patients, it would take time for an infant to receive a diagnosis, receive treatment, and relapse within the first year of age.
Race was reported on the electronic case report form in accordance with guardian report. Options included American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or other Pacific Islander, White, or other.
Ethnicity was reported on the electronic case report form in accordance with guardian report. Options included Hispanic or Latino and not Hispanic or Latino.
B-precursor subclassification is based on the immunophenotype of the lymphoblasts. For pro–B-ALL, lymphoblasts express CD19, CD34, CD22, TdT, and cytoplasmic CD79a, but do not express CD10. For common ALL, lymphoblasts also express CD10. For pre–B-ALL, lymphoblasts express CD22, CD34, CD19, TdT, cytoplasmic CD79a, CD10, and the cytoplasmic μ heavy chain.
One patient in the blinatumomab group with IAMP21 and 1 in the consolidation chemotherapy group with t(17;19)(q22;p13)/TCF3-HLF also carried a genetic abnormality predicting an unfavorable prognosis.
Evaluated after 2 blocks of high-risk consolidation and before study treatment.
All patients had minimal residual disease of bone marrow specimens evaluated by PCR and/or flow cytometry to detect ALL cells. If a patient had minimal residual disease evaluated by both PCR and flow cytometry, the value obtained by PCR was included in the analysis. Minimal residual disease is defined by the presence of at least 0.01% (ie, ≥10-4) ALL cells in a bone marrow specimen and predicts the likelihood of relapse.
Lansky performance score28 is recorded for patients younger than 16 years and Karnofsky performance score29 is recorded for those aged 16 years or older. The Lansky scale assesses performance in children with cancer via a parental assessment of usual play activity during the prior week, ranging from “unresponsive” (0%) and “no play; does not get out of bed” (10%) to “minor restrictions in physically strenuous activity” (90%) and “fully active, normal” (100%). The Karnofsky Performance Scale uses patient and caregiver evaluation to rate functional status from 0 to 100 from death (0%) to full independent ability to complete activities of daily living (100%).
Efficacy
Primary Outcome: Event-Free Survival
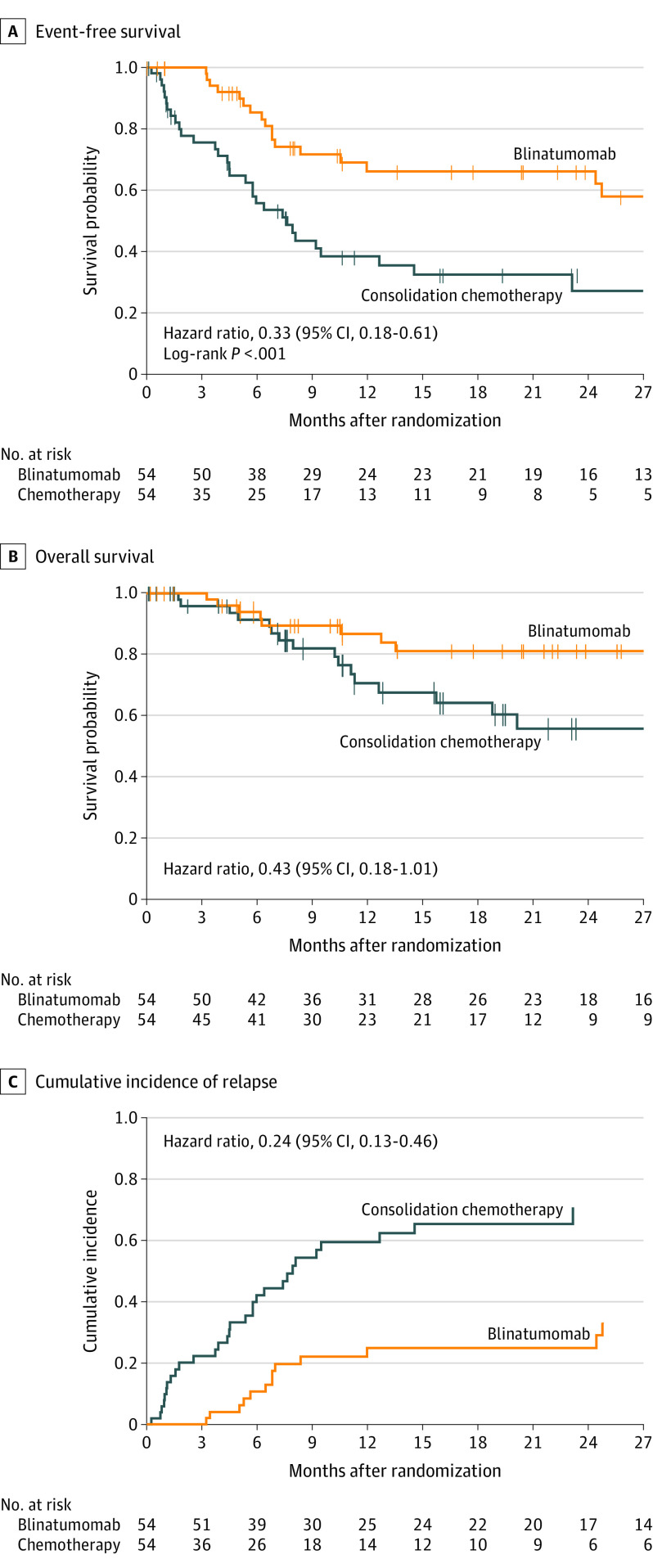
The median follow-up time for event-free survival was 22.4 months (interquartile range, 8.1-34.2). Sites of relapse are provided (Table 2). Events were reported for 17 of 54 patients (31.5%) in the blinatumomab group and 31 of 54 (57.4%) in the consolidation chemotherapy group (Table 2, Figure 2A). Event-free survival was significantly prolonged after blinatumomab compared with consolidation chemotherapy (P < .001 by the stratified log-rank test). The event-free survival hazard ratio from a stratified Cox proportional hazard model was 0.33 (95% CI, 0.18-0.61) in favor of blinatumomab. The 24-month Kaplan-Meier estimate of event-free survival rate was 66.2% (95% CI, 50.1%-78.2%) in the blinatumomab group and 27.1% (95% CI, 13.2%-43.0%) in the consolidation chemotherapy group (Figure 2A).
Table 2. Event-Free Survival, Overall Survival, Minimal Residual Disease Remission, and Allogeneic Hematopoietic Stem Cell Transplant Outcomes.
| No. (%) | Absolute difference, % (95% CI) |
||
|---|---|---|---|
| Blinatumomab (n = 54) | Consolidation chemotherapy (n = 54) | ||
| Primary end point | |||
| Event-free survival | 37 (69) | 23 (43) | |
| Events | 17 (31) | 31 (57) | |
| Total relapses | 13 (24) | 29 (54) | |
| Isolated bone marrow | 6 (11) | 12 (22) | |
| M2 marrow after achievement of complete remission | 4 (7) | 12 (22) | |
| Combined bone marrow | 2 (4) | 0 | |
| Central nervous system extramedullary | 1 (2) | 2 (4) | |
| Extramedullary at other sitesa | 0 | 3 (6) | |
| Death from any cause other than relapse | 4 (7)b | 2 (4)c | |
| Failure to achieve complete remission after treatment with investigational product | 0 | 0 | |
| Second malignancy | 0 | 0 | |
| Secondary end points | |||
| Overall survival | |||
| Death from any cause | 8 (15) | 16 (30) | |
| Minimal residual disease remission by minimal residual disease status at baseline (minimal residual disease evaluable set)d,e,f | Blinatumomab remission, No./total evaluable (%) | Consolidation chemotherapy remission, No./total evaluable (%) | |
| Minimal residual disease remission | 17/20 (85) | 20/23 (87) | −2.0 (−31.2 to 28.0) |
| No minimal residual disease remission | 27/29 (93) | 6/25 (24) | 69.1 (45.4 to 85.5) |
| Total | 44/49 (90) | 26/48 (54) | 35.6 (15.6 to 52.5) |
| Blinatumomab (n = 48) | Consolidation chemotherapy (n = 38) | ||
| Subset of patients who underwent allogeneic hematopoietic stem cell transplant in second complete remission | |||
| Time to transplant, median (range), mo | 1.9 (1 to 3) | 1.7 (1 to 3) | |
| Stem cell source | |||
| Peripheral blood | 20 (42) | 9 (24) | |
| Bone marrow | 24 (50) | 24 (63) | |
| Cord blood | 4 (8) | 5 (13) | |
| Donor type | |||
| Matched sibling | 12 (25) | 10 (26) | |
| Haploidentical relatedg | 13 (27) | 10 (26) | |
| Matched unrelated | 17 (35) | 12 (32) | |
| Mismatched unrelated | 6 (13) | 6 (16) | |
| Receipt of conditioning total body irradiation | 27 (56) | 18 (47) | |
| Receipt of conditioning chemotherapy | 21 (44) | 20 (53) | |
| Died after receiving a hematopoietic stem cell transplant | |||
| Transplant-related deathh | 4 (8) | 4 (11) | |
| Due to relapse/disease progression | 3 (6) | 8 (21) | |
Abbreviation: PCR, polymerase chain reaction.
Testicular extramedullary relapse was not observed in either group.
All events occurred after allogeneic hematopoietic stem cell transplant: hemophagocytic lymphohistiocytosis (in the context of acute graft rejection), respiratory failure due to pneumonia, hepatic failure (developing after graft-vs-host disease), and infection (in the context of graft-vs-host disease).
Causes of death included acute respiratory failure (occurring after allogeneic hematopoietic stem cell transplant) and fungal sinusitis.
Minimal residual disease remission is defined as <10-4 blast cells. Minimal residual disease remission was analyzed at end of treatment (cycle 1, day 29) of investigational product. Patients who were part of the minimal residual disease evaluable set and were missing postbaseline disease assessments were considered not to have achieved a response. Patients assessed included those in the minimal residual disease evaluable set who had minimal residual disease status at baseline as defined earlier and minimal residual disease response at end of treatment (cycle 1, day 29) of investigational product for the respective assessment methods.
The minimal residual disease evaluable set included patients for whom evaluable baseline minimal residual disease marker could be found with either of the minimal residual disease assessment methods: PCR or flow cytometry.
For minimal residual disease status at baseline, if both a PCR and flow cytometry value was available, then the minimal residual disease PCR value was taken because PCR is more sensitive.
Includes mismatched sibling, haploidentical mother, and haploidentical father.
Causes of transplant-related death in the blinatumomab group included hepatic failure (n = 1), respiratory failure (n = 1), hemophagocytic lymphohistiocytosis (n = 1), and graft-vs-host disease with fungal infection (n = 1). Causes in the consolidation chemotherapy group were fungal sinusitis (n = 1), myocardial infarction (n = 1), multiorgan failure (n = 1), and acute respiratory failure (n = 1).
Figure 2. Efficacy End Points of Blinatumomab vs Chemotherapy.
Vertical bars indicate censoring. A, Event free survival from randomization to relapse, all-cause death, second malignancy, or failure to achieve complete remission at treatment end. Patients who did not achieve remission or died before assessment were assigned 1 day of event-free survival. Patients alive and event free were censored on their last assessment date. Median (IQR) observation: 22.4 (8.1-34.2) months. B, Overall survival from randomization to death from any cause. Median (IQR) observation: 19.5 (7.8-31.6) months. C, Cumulative incidence of relapse, with death due to other causes as a competing factor, truncated at last relapse event (23.1 months chemotherapy and 24.8 months blinatumomab). Median (IQR) observation: 22.4 (8.1-34.2) months.
A post hoc analysis accounting for the effect of study center resulted in a hazard ratio of 0.37 (95% CI, 0.20-0.66), a value similar to the primary analysis hazard ratio stated earlier.
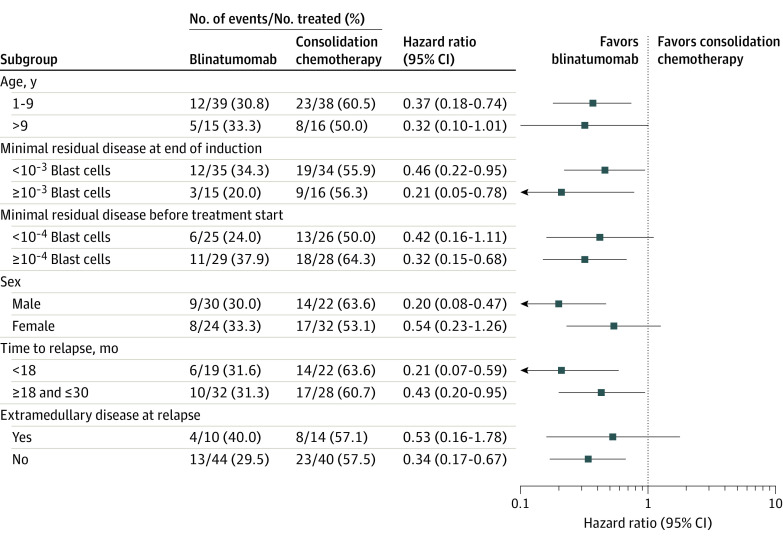
The hazard ratio remained in favor of blinatumomab in all specified subgroups (Figure 3). Among patients with very early relapse (<18 months from initial diagnosis to relapse), events were reported for 6 of 19 patients (31.6%) in the blinatumomab group and 14 of 22 (63.6%) in the consolidation chemotherapy group (hazard ratio = 0.21; 95% CI, 0.07-0.59) (Figure 3; eTable 5 in Supplement 3). Fewer events were observed in blinatumomab-treated patients than in those given the consolidation chemotherapy block independent of the minimal residual disease level measured at the end of induction or before treatment start (Figure 3).
Figure 3. Event-Free Survival by Study Subgroups.
A Cox regression model tested for treatment × subgroup interaction.
Key Secondary Outcome
Overall Survival
The median follow-up time for overall survival was 19.5 months (range, 0.1-44.1 months). There were 24 deaths, 8 (14.8%) in the blinatumomab group and 16 (29.6%) in the consolidation chemotherapy group. The overall survival hazard ratio from a stratified Cox proportional hazard model was 0.43 (95% CI, 0.18-1.01) (Figure 2B; eTable 6 in Supplement 3). A sensitivity analysis that estimated the treatment effect conditioning on the time at which 13 patients in the consolidation chemotherapy group received blinatumomab resulted in a hazard ratio for overall survival of 0.35 (95% CI, 0.12-1.01) (eTable 7 in Supplement 3).
Among patients with very early relapse, there were 9 deaths, 2 of 19 patients (10.5%) in the blinatumomab group and 7 of 22 (31.8%) in the consolidation chemotherapy group (hazard ratio, 0.23; 95% CI, 0.05-1.13) (eTable 8 in Supplement 3).
Other Secondary Outcomes
Minimal Residual Disease
The proportion of patients who had minimal residual disease remission, as defined by having fewer than 10-4 blast cells on a bone marrow aspirate within 29 days of treatment initiation, was assessed by PCR and flow cytometry. Minimal residual disease remission by PCR was observed in 90% of patients (44/49) in the blinatumomab group and in 54% (26/48) in the consolidation chemotherapy group (absolute percentage difference, 35.6% [95% CI, 15.6%-52.5%]) (Table 2). In the subgroup of patients who were in minimal residual disease remission at baseline, most remained there in both groups: 85% in the blinatumomab group and 87% in the consolidation chemotherapy group. In the subgroup of patients who had detectable minimal residual disease at baseline (>10-4), 93% of patients (27/29) treated with blinatumomab achieved minimal residual disease remission after treatment compared with 24% (6/25) treated with consolidation chemotherapy (absolute percentage difference, 69.1% [95% CI, 45.4%-85.5%]). Minimal residual disease remission by flow cytometry was comparable to that observed with PCR and was observed in more patients in the blinatumomab vs consolidation chemotherapy group (90.6% [48/53] vs 60.4% [32/53]).
Allogeneic Hematopoietic Stem Cell Transplant
Because patients participating in this clinical trial were high risk, the intent was for all patients achieving a second complete remission to undergo an allogeneic hematopoietic stem cell transplant while in continuous complete remission. Forty-eight patients (88.9%) in the blinatumomab group and 38 (70.4%) in the consolidation chemotherapy group underwent allogeneic hematopoietic stem cell transplant while in second continuous complete remission (Table 2). Of these patients, 56.3% in the blinatumomab group and 47.4% in the consolidation chemotherapy group received total body irradiation as part of the conditioning regimen; 8.3% of patients (4/48) in the blinatumomab group and 10.5% (4/38) in the consolidation chemotherapy group experienced transplant-related fatalities. Three of 48 patients (6.3%) in the blinatumomab group and 8 of 38 (21.1%) in the consolidation chemotherapy group died after receiving allogeneic hematopoietic stem cell transplant owing to relapse. A Kaplan-Meier estimate of mortality at 100 days after allogeneic hematopoietic stem cell transplant was 5.6% (95% CI, 1.4% to 20.5%) for the consolidation chemotherapy group and 4.2% (95% CI, 1.1% to 15.6%) for the blinatumomab group. The Kaplan-Meier median time to death was not reached for either treatment group.
Relapse
Relapse (from randomization to relapse/date of censoring) occurred in 13 of 54 patients (24.1%) in the blinatumomab group and 29 of 54 (53.7%) in the consolidation chemotherapy group. The stratified hazard ratio for the cumulative incidence of relapse from a Cox proportional hazard model was 0.24 (95% CI, 0.13-0.46) (Figure 2C), and the cumulative incidence rates of relapse at 24 months were 24.9% (95% CI, 13.2%-38.5%) in the blinatumomab group and 70.8% (95% CI, 50.7%-83.9%) in the consolidation chemotherapy group. One patient in each treatment group experienced CD19-negative relapse.
Adverse Events
Adverse events were collected from start of therapy until 90 days after allogeneic hematopoietic stem cell transplant or 30 days for patients not receiving such transplant. Adverse events were reported in all patients (54/54) in the blinatumomab group and 96.1% (49/51) in the consolidation chemotherapy group (Table 3); adverse events occurring in at least 5% of patients in the blinatumomab group are shown in eTable 9 in Supplement 3. No fatal adverse events were reported. In the blinatumomab group, adverse events with a patient incidence of greater than or equal to 25% were pyrexia (81.5%, 44/54), nausea (40.7%, 22/54; 1 grade ≥3), headache (35.2%, 19/54), stomatitis (35.2%, 19/54; during conditioning therapy for allogeneic hematopoietic stem cell transplant), and vomiting (29.6%, 16/54; grade ≤2). In the consolidation chemotherapy group, adverse events with a patient incidence of 25% or more were stomatitis (57.4%, 31/51), anemia (45.1%, 23/51), thrombocytopenia (39.2%, 20/51), neutropenia (35.3%, 18/51), and febrile neutropenia (25.5%, 13/51).
Table 3. Number of Patients Experiencing Adverse Eventsa.
| No. of patients (%) | ||
|---|---|---|
| Blinatumomab (n = 54) | Consolidation chemotherapy (n = 51) | |
| Any adverse event (including serious adverse events) | 54 (100.0) | 49 (96.1) |
| Any serious adverse eventb | 13 (24.1) | 22 (43.1) |
| Adverse event leading to discontinuation of trial treatment | 2 (3.7) | 0 |
| Grade ≥3 adverse event | 31 (57.4) | 42 (82.4) |
| Grade ≥3 adverse events in ≥3% of patients in either group | 31 (57.4) | 42 (82.4) |
| Thrombocytopeniac | 10 (18.5) | 18 (35.3) |
| Stomatitisd | 10 (18.5) | 16 (31.4) |
| Neutropeniae | 9 (16.7) | 16 (31.4) |
| Anemia | 8 (14.8) | 21 (41.2) |
| Leukopeniaf | 4 (7.4) | 4 (7.8) |
| Pyrexia | 3 (5.6) | 0 |
| Elevated liver enzyme levelsg | 3 (5.6) | 9 (17.6) |
| Aplasia | 2 (3.7) | 4 (7.8) |
| Febrile neutropenia | 2 (3.7) | 13 (25.5) |
| Hypotension | 2 (3.7) | 1 (2.0) |
| Hypokalemia | 1 (1.9) | 2 (3.9) |
| Epistaxis | 0 | 3 (5.9) |
| Cytopeniah | 0 | 2 (3.9) |
| Hepatotoxicity not otherwise specified | 0 | 2 (3.9) |
Adverse events were coded with MedDRA version 22.1 and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03. Patients who experienced the same event multiple times were counted once and their worst grade is reported.
Serious adverse events included events that were fatal; were life threatening; required hospitalization or prolonged hospitalization; resulted in disability or incapacity, congenital anomaly, or birth defect; or were other medically significant events as determined by the reporter. There were no fatal adverse events in either group. Seriousness criteria are defined by regulations. Grading is a measurement of severity against a predefined scale. Although there exists some overlap between seriousness and grading (eg, grade 5 and fatal events), they are not entirely synonymous.
Adverse events for MedDRA-preferred terms “thrombocytopenia” and “platelet count decreased.”
Adverse events for MedDRA-preferred terms “stomatitis” and “mucosal inflammation”; occurred after completion of blinatumomab treatment and considered to be unrelated to blinatumomab treatment.
Adverse events for MedDRA-preferred terms “neutropenia” and “neutrophil count decreased.”
Adverse events for MedDRA-preferred terms “leukopenia” and “white blood cell count decreased.”
Alanine aminotransferase increased, alanine aminotransferase, aspartate aminotransferase increased, aspartate aminotransferase, γ-glutamyltransferase increased, or hypertransaminasemia.
One patient had pancytopenia, and 1 had cytopenia in 2 cell lines.
The patient incidence of adverse events of grade 3 or higher was 57.4% (31/54) in the blinatumomab group and 82.4% (42/51) in the consolidation chemotherapy group (Table 3). Adverse events of grade 3 or higher with a patient incidence of 10% or more included thrombocytopenia and stomatitis (each 18.5%, 10/54), neutropenia (16.7%, 9/54), and anemia (14.8%, 8/54) in the blinatumomab group, and anemia (41.2%, 21/51), thrombocytopenia (35.3%, 18/51), neutropenia and stomatitis (each 31.4%, 16/51), febrile neutropenia (25.5%, 13/51), and elevated liver enzyme levels (17.6%, 9/51) in the consolidation chemotherapy group. Adverse events leading to treatment discontinuation were reported in 2 patients (3.7%; 1 grade 3 nervous system disorder, 1 grade 4 seizure) in the blinatumomab group and none in the consolidation chemotherapy group.
The incidence of serious adverse events was 24.1% in the blinatumomab group and 43.1% in the consolidation chemotherapy group (Table 3 and eTable 10 in Supplement 3). The most frequently reported serious adverse events were neurologic symptoms and seizure (each 3.7%, 2/54) in the blinatumomab group and febrile neutropenia (17.6%, 9/51) in the consolidation chemotherapy group.
The incidence of grade 3 or higher neutropenia and febrile neutropenia in the blinatumomab group was 16.7% and 3.7%, respectively. The incidence of grade 3 or higher neutropenia and febrile neutropenia in the consolidation chemotherapy group was 31.4% and 25.5%, respectively. Ten patients (18.5%) allocated to receive blinatumomab experienced infections of grade 3 or higher; in 7 patients, infections occurred after receipt of allogeneic hematopoietic stem cell transplant preparative regimens; and 9.8% of patients (5/51) given consolidation chemotherapy developed grade 3 or higher infections.
The incidence rates of neurologic events were 48.1% in the blinatumomab group and 29.4% in the consolidation chemotherapy group; 3 patients in the blinatumomab group and 1 in the consolidation chemotherapy group had neurologic events that were grade 3 or 4 (eTable 11 in Supplement 3). Two patients in the blinatumomab group and 1 in the consolidation chemotherapy group experienced cytokine release syndrome at less than grade 3.
Discussion
In this randomized clinical trial in children with high-risk first-relapse B-ALL, 1 cycle of blinatumomab as the third consolidation block before allogeneic hematopoietic stem cell transplant was associated with improved event-free survival and lower risk of leukemia recurrence compared with consolidation chemotherapy.
The lower risk of disease recurrence—resulting in better event-free survival—in patients administered blinatumomab is consistent with data showing that minimal residual disease remission before allogeneic hematopoietic stem cell transplant improves posttransplant outcomes in childhood ALL.13,30,31 Blinatumomab treatment therefore may represent a valuable consolidation treatment that appears to be more effective than conventional chemotherapy before transplant for this patient population.
Several trials have demonstrated that improved survival and response to blinatumomab are associated with low leukemia burden.8,9,10 In this study, the outcomes of patients in minimal residual disease remission or with low persistent leukemia burden were improved after 1 cycle of blinatumomab (as late consolidation treatment) followed by allogeneic hematopoietic stem cell transplant. This finding provides a rationale for evaluating the role of blinatumomab in children with chemotherapy-sensitive leukemia, including newly diagnosed or low-risk first-relapse B-ALL. Such studies (eg, NCT03914625, NCT03117751, NCT03643276) are underway worldwide and will define the role of blinatumomab in these clinical contexts.
Blinatumomab also appeared to be more effective than chemotherapy as the third consolidation block before allogeneic hematopoietic stem cell transplant in patients experiencing very early relapse (relapse within 18 months from diagnosis), a challenging subset of patients with a dismal prognosis.5,32 Moreover, previously published studies have shown that the efficacy of blinatumomab, like that of other immunotherapies but unlike chemotherapy, does not seem to be affected by the presence of high-risk genetic abnormalities.10,33,34
The toxicity profile of blinatumomab was consistent with that expected in patients with limited leukemia burden. The incidence of hematologic toxicities, including febrile neutropenia, was lower in the blinatumomab group than in the consolidation chemotherapy group; thus, blinatumomab may offer a safety benefit over intensive multidrug consolidation chemotherapy. This observation is important because standard, intensive-consolidation, multidrug chemotherapy after induction chemotherapy is commonly associated with the occurrence of toxicities that may be fatal or reduce the likelihood of proceeding to allogeneic hematopoietic stem cell transplant.35 Neurologic toxicity and cytokine release syndrome have been reported to be peculiar toxicities associated with the use of blinatumomab.36 Only 3 patients in the blinatumomab group and 1 in the consolidation chemotherapy group had grade 3 or 4 neurologic events, and there were no reported events of cytokine release syndrome of grade 3 or higher in the blinatumomab group, likely because of the low leukemic burden of patients. The greater incidence of grade 3 or higher infections in the blinatumomab group could be explained by the adverse event reporting period ending 30 days after the last dose of investigational product. This period ended later for patients randomized to receive blinatumomab owing to the duration of administration, often overlapping with subsequent anticancer therapy. Specifically, 7 of 10 blinatumomab group infections and 0 of 5 consolidation chemotherapy group infections occurred after receipt of allogeneic hematopoietic stem cell transplant preparative regimens. Consistent with this observation, previous studies in children and adults treated with blinatumomab have not shown any increased risk of infection after blinatumomab.37,38,39
The Children's Oncology Group conducted a randomized trial in which children, adolescents, and young adults with first-relapse B-ALL after induction therapy received either 2 cycles of intensive multidrug chemotherapy or 2 cycles of blinatumomab as consolidation treatment before allogeneic hematopoietic stem cell transplant.39 As in this study, treatment with blinatumomab resulted in less severe toxicities, higher rates of minimal residual disease remission, greater likelihood of proceeding to allogeneic hematopoietic stem cell transplant, and improved overall survival; minimal residual disease clearance was observed after the first cycle of blinatumomab.
Preliminary data suggest that CD19 chimeric antigen receptor T-cell therapy may be effective in children with multiple relapsed or refractory childhood B-ALL or with disease relapsing after allogeneic hematopoietic stem cell transplant, including persistent minimal residual disease40; however, grade 3 or 4 adverse events, including neurotoxicity and cytokine release syndrome, were reported in approximately one-third of patients.41 To date, to our knowledge, chimeric antigen receptor T-cell therapy has not been evaluated in children with first-relapse B-ALL. Because patients with ALL often need immediate treatment, a potential advantage of blinatumomab is that it is an already manufactured, immediately available drug, whereas chimeric antigen receptor T-cell therapy requires patient-specific engineering and therefore may not be as readily available. Lack of head-to-head comparisons between immunotherapies precludes any definitive conclusions on their relative roles and merits.
Limitations
This study has several limitations. First, because only 1 course of consolidation therapy with blinatumomab was applied, it is unknown whether additional cycles with or without replacement of either some or all consolidation chemotherapy blocks could further improve patient outcomes. Second, the number of patients who experienced an isolated extramedullary involvement at first high-risk relapse was too low to allow inference of definitive conclusions on the efficacy of blinatumomab in comparison with consolidation chemotherapy in this patient subset. Third, studies are needed to evaluate the role of blinatumomab in pediatric patients experiencing standard-risk first relapse of B-ALL.
Conclusions
Among children with high-risk first-relapse B-ALL, treatment with 1 cycle of blinatumomab compared with standard intensive multidrug chemotherapy before allogeneic hematopoietic stem cell transplant resulted in an improved event-free survival at a median of 22.4 months of follow-up.
Trial Protocol
Statistical Analysis Plan
Principal Investigators
eAppendix
eTable 1. Enrollment by Region and Country
eTable 2. Risk Stratification by Time From Diagnosis to Relapse and Site of Relapse According to IntReALL Risk Classification
eTable 3. Components of High-Risk Consolidation Therapy
eTable 4. Age-Adapted Doses of Intrathecal Chemotherapy
eTable 5. Subgroup Analysis of Event-Free Survival
eTable 6. Overall Survival
eTable 7. Estimated Latent Treatment Effect on Overall Survival Without Subsequent Blinatumomab Drop-in by Consolidation Chemotherapy Group
eTable 8. Subgroup Analysis of Overall Survival
eTable 9. Adverse Events Reported for >5% of Patients in the Blinatumomab Group
eTable 10. Serious Adverse Events
eTable 11. Neurologic Events and Cytokine Release Syndrome
Data Sharing Statement
References
- 1.Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541-1552. doi: 10.1056/NEJMra1400972 [DOI] [PubMed] [Google Scholar]
- 2.Irving JA, Enshaei A, Parker CA, et al. Integration of genetic and clinical risk factors improves prognostication in relapsed childhood B-cell precursor acute lymphoblastic leukemia. Blood. 2016;128(7):911-922. doi: 10.1182/blood-2016-03-704973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krentz S, Hof J, Mendioroz A, et al. Prognostic value of genetic alterations in children with first bone marrow relapse of childhood B-cell precursor acute lymphoblastic leukemia. Leukemia. 2013;27(2):295-304. doi: 10.1038/leu.2012.155 [DOI] [PubMed] [Google Scholar]
- 4.Malempati S, Gaynon PS, Sather H, La MK, Stork LC; Children’s Oncology Group . Outcome after relapse among children with standard-risk acute lymphoblastic leukemia: Children’s Oncology Group study CCG-1952. J Clin Oncol. 2007;25(36):5800-5807. doi: 10.1200/JCO.2007.10.7508 [DOI] [PubMed] [Google Scholar]
- 5.Locatelli F, Schrappe M, Bernardo ME, Rutella S. How I treat relapsed childhood acute lymphoblastic leukemia. Blood. 2012;120(14):2807-2816. doi: 10.1182/blood-2012-02-265884 [DOI] [PubMed] [Google Scholar]
- 6.Peters C, Schrappe M, von Stackelberg A, et al. Stem-cell transplantation in children with acute lymphoblastic leukemia: a prospective international multicenter trial comparing sibling donors with matched unrelated donors: the ALL-SCT-BFM-2003 trial. J Clin Oncol. 2015;33(11):1265-1274. doi: 10.1200/JCO.2014.58.9747 [DOI] [PubMed] [Google Scholar]
- 7.Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell–engaging antibody. Science. 2008;321(5891):974-977. doi: 10.1126/science.1158545 [DOI] [PubMed] [Google Scholar]
- 8.von Stackelberg A, Locatelli F, Zugmaier G, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34(36):4381-4389. doi: 10.1200/JCO.2016.67.3301 [DOI] [PubMed] [Google Scholar]
- 9.Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522-1531. doi: 10.1182/blood-2017-08-798322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locatelli F, Zugmaier G, Mergen N, et al. Blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia: results of the RIALTO trial, an expanded access study. Blood Cancer J. 2020;10(7):77. doi: 10.1038/s41408-020-00342-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckert C, Henze G, Seeger K, et al. Use of allogeneic hematopoietic stem-cell transplantation based on minimal residual disease response improves outcomes for children with relapsed acute lymphoblastic leukemia in the intermediate-risk group. J Clin Oncol. 2013;31(21):2736-2742. doi: 10.1200/JCO.2012.48.5680 [DOI] [PubMed] [Google Scholar]
- 12.Parker C, Waters R, Leighton C, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010;376(9757):2009-2017. doi: 10.1016/S0140-6736(10)62002-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paganin M, Zecca M, Fabbri G, et al. Minimal residual disease is an important predictive factor of outcome in children with relapsed “high-risk” acute lymphoblastic leukemia. Leukemia. 2008;22(12):2193-2200. doi: 10.1038/leu.2008.227 [DOI] [PubMed] [Google Scholar]
- 14.Domenech C, Mercier M, Plouvier E, et al. First isolated extramedullary relapse in children with B-cell precursor acute lymphoblastic leukaemia: results of the Cooprall-97 study. Eur J Cancer. 2008;44(16):2461-2469. doi: 10.1016/j.ejca.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 15.Charité–University Hospital of Berlin . International study for treatment of standard risk childhood relapsed ALL. Accessed February 1, 2021. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2012-000793-30
- 16.Möricke A, Zimmermann M, Valsecchi MG, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood. 2016;127(17):2101-2112. doi: 10.1182/blood-2015-09-670729 [DOI] [PubMed] [Google Scholar]
- 17.van der Velden VH, Cazzaniga G, Schrauder A, et al. ; European Study Group on MRD Detection in ALL (ESG-MRD-ALL) . Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21(4):604-611. doi: 10.1038/sj.leu.2404586 [DOI] [PubMed] [Google Scholar]
- 18.Mejstríková E, Hrusak O, Borowitz MJ, et al. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. 2017;7(12):659. doi: 10.1038/s41408-017-0023-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brüggemann M, Gökbuget N, Kneba M. Acute lymphoblastic leukemia: monitoring minimal residual disease as a therapeutic principle. Semin Oncol. 2012;39(1):47-57. doi: 10.1053/j.seminoncol.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 20.Brüggemann M, Schrauder A, Raff T, et al. ; European Working Group for Adult Acute Lymphoblastic Leukemia (EWALL); International Berlin-Frankfurt-Münster Study Group (I-BFM-SG) . Standardized MRD quantification in European ALL trials: proceedings of the Second International Symposium on MRD assessment in Kiel, Germany, 18-20 September 2008. Leukemia. 2010;24(3):521-535. doi: 10.1038/leu.2009.268 [DOI] [PubMed] [Google Scholar]
- 21.Tallen G, Ratei R, Mann G, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2010;28(14):2339-2347. doi: 10.1200/JCO.2009.25.1983 [DOI] [PubMed] [Google Scholar]
- 22.Handgretinger R, Zugmaier G, Henze G, Kreyenberg H, Lang P, von Stackelberg A. Complete remission after blinatumomab-induced donor T-cell activation in three pediatric patients with post-transplant relapsed acute lymphoblastic leukemia. Leukemia. 2011;25(1):181-184. doi: 10.1038/leu.2010.239 [DOI] [PubMed] [Google Scholar]
- 23.Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell–engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493-2498. doi: 10.1200/JCO.2010.32.7270 [DOI] [PubMed] [Google Scholar]
- 24.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549-556. doi: 10.2307/2530245 [DOI] [PubMed] [Google Scholar]
- 25.Gordon Lan KK, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659-663. doi: 10.1093/biomet/70.3.659 [DOI] [Google Scholar]
- 26.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 27.Branson M, Whitehead J. Estimating a treatment effect in survival studies in which patients switch treatment. Stat Med. 2002;21(17):2449-2463. doi: 10.1002/sim.1219 [DOI] [PubMed] [Google Scholar]
- 28.Lansky SB, List MA, Lansky LL, Ritter-Sterr C, Miller DR. The measurement of performance in childhood cancer patients. Cancer. 1987;60(7):1651-1656. doi: [DOI] [PubMed] [Google Scholar]
- 29.Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2(3):187-193. doi: 10.1200/JCO.1984.2.3.187 [DOI] [PubMed] [Google Scholar]
- 30.Bader P, Kreyenberg H, Henze GH, et al. ; ALL-REZ BFM Study Group . Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27(3):377-384. doi: 10.1200/JCO.2008.17.6065 [DOI] [PubMed] [Google Scholar]
- 31.Ruggeri A, Michel G, Dalle JH, et al. Impact of pretransplant minimal residual disease after cord blood transplantation for childhood acute lymphoblastic leukemia in remission: an Eurocord, PDWP-EBMT analysis. Leukemia. 2012;26(12):2455-2461. doi: 10.1038/leu.2012.123 [DOI] [PubMed] [Google Scholar]
- 32.Cooper SL, Brown PA. Treatment of pediatric acute lymphoblastic leukemia. Pediatr Clin North Am. 2015;62(1):61-73. doi: 10.1016/j.pcl.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mouttet B, Vinti L, Ancliff P, et al. Durable remissions in TCF3-HLF positive acute lymphoblastic leukemia with blinatumomab and stem cell transplantation. Haematologica. 2019;104(6):e244-e247. doi: 10.3324/haematol.2018.210104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forero-Castro M, Montaño A, Robledo C, et al. Integrated genomic analysis of chromosomal alterations and mutations in B-cell acute lymphoblastic leukemia reveals distinct genetic profiles at relapse. Diagnostics. 2020;10(7):455. doi: 10.3390/diagnostics10070455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmiegelow K, Attarbaschi A, Barzilai S, et al. ; Ponte di Legno Toxicity Working Group . Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol. 2016;17(6):e231-e239. doi: 10.1016/S1470-2045(16)30035-3 [DOI] [PubMed] [Google Scholar]
- 36.Stein AS, Schiller G, Benjamin R, et al. Neurologic adverse events in patients with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab: management and mitigating factors. Ann Hematol. 2019;98(1):159-167. doi: 10.1007/s00277-018-3497-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. doi: 10.1056/NEJMoa1609783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinelli G, Boissel N, Chevallier P, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study. J Clin Oncol. 2017;35(16):1795-1802. doi: 10.1200/JCO.2016.69.3531 [DOI] [PubMed] [Google Scholar]
- 39.Brown PA, Ji L, Xu X, et al. A randomized phase 3 trial of blinatumomab vs chemotherapy as post-reinduction therapy in high and intermediate risk (HR/IR) first relapse of B-acute lymphoblastic leukemia (B-ALL) in children and adolescents/young adults (AYAs) demonstrates superior efficacy and tolerability of blinatumomab: a report from Children's Oncology Group Study AALL1331. Blood. 2019;134(suppl 2):LBA-1. doi: 10.1182/blood-2019-132435 [DOI] [Google Scholar]
- 40.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507-1517. doi: 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curran KJ, Margossian SP, Kernan NA, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood. 2019;134(26):2361-2368. doi: 10.1182/blood.2019001641 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
Principal Investigators
eAppendix
eTable 1. Enrollment by Region and Country
eTable 2. Risk Stratification by Time From Diagnosis to Relapse and Site of Relapse According to IntReALL Risk Classification
eTable 3. Components of High-Risk Consolidation Therapy
eTable 4. Age-Adapted Doses of Intrathecal Chemotherapy
eTable 5. Subgroup Analysis of Event-Free Survival
eTable 6. Overall Survival
eTable 7. Estimated Latent Treatment Effect on Overall Survival Without Subsequent Blinatumomab Drop-in by Consolidation Chemotherapy Group
eTable 8. Subgroup Analysis of Overall Survival
eTable 9. Adverse Events Reported for >5% of Patients in the Blinatumomab Group
eTable 10. Serious Adverse Events
eTable 11. Neurologic Events and Cytokine Release Syndrome
Data Sharing Statement