Abstract
Mycotoxin exposure is common in the poultry industry. Deoxynivalenol (DON) is usually detected at levels below the maximum threshold (5000 ppb), but depending on diet and age, broiler performance can be affected. We evaluated the effects of 900 ppb and 2300 ppb DON on the performance, intestinal morphometry, and lesion scores of broiler chickens. One-day-old male Ross broilers (n = 736) were divided into 4 treatments with 8 replicates each, and a pen containing 23 birds was the experimental unit. The animals were fed diets naturally contaminated with two levels of DON: 900 (Low DON—LD) or 2300 (Moderate DON—MD) ppb, with or without activated charcoal, over 28 days. After this, all birds were fed a marginally DON-contaminated diet without charcoal. During the first 28 days, body weight gain (BWG) and feed conversion ratio (FCR) were significantly impaired when broilers were fed a MD diet without activated charcoal. Even after feeding a marginally contaminated diet from D28–35, birds previously fed the MD diet presented a significantly lower performance. The villus height:crypt depth (VH:CD) ratio was significantly higher in the ileum from 14-day-old broilers fed the MD when compared with the LD diet. At D28, the MD diet caused decreased villus height (VH) and increased crypt depth (CD), affecting VH:CD ratio in both intestinal segments, with higher levels in the jejunum from 28-day-old broilers fed a non-supplemented LD diet. Broiler production was negatively affected by DON, even at moderate levels (2300 ppb).
Keywords: deoxynivalenol, Ross broilers, intestine, performance, carryover
1. Introduction
Among the factors that negatively affect animal production, mycotoxins play an important role because these natural contaminants are ubiquitous contaminants in feeds and feedstuffs [1]. Besides this, cereal byproducts that are rejected for human consumption during processing for mycotoxin removal, as well as raw materials such as dried distillers’ grains with solubles (DDGS), can enter the animal feed chain, increasing the exposure risk [2]. In most cases, contamination levels are low enough to ensure compliance with feed safety recommendations. However, mycotoxin contamination might still exert adverse effects on animals, and, at an economic level, the major mycotoxins risks are linked to suboptimal production and not to disease.
There is evidence that even at levels below authorities’ feed safety recommendations, deoxynivalenol (DON) may decrease resistance to infectious disease in broilers [3]. A previous study showed that broilers fed a diet containing 3000–4000 ppb DON, i.e., at levels below the European maximum guidance (5000 ppb in the complete diet) [4], are predisposed to develop necrotic enteritis. Also, broilers fed 1500 ppb DON combined with 20,000 ppb fumonisins are more susceptible to coccidiosis [5]. This poses a concern for the broiler industry, and there is an ongoing discussion about the reduction of antibiotics and the potential impact on the use of anticoccidials, particularly those from the class of ionophores. This class of anticoccidial fits the classical definition of an antibiotic because they have some antibacterial activity. This means that the importance of mycotoxins in the poultry industry may increase in a situation where ionophore anticoccidials are banned from feed. Besides this, the subclinical and indirect effects of mycotoxins are often underestimated because no typical mycotoxicosis symptoms are observed. In general, mycotoxins may be involved in numerous subclinical symptoms, and will potentiate the negative effect of diseases or simply lead to impaired performance.
To mimic on-farm conditions, it is important to expose broilers to feed naturally contaminated with mycotoxins as opposed to experimental contamination with synthetic mycotoxins. Furthermore, realistic contamination levels have to be considered when applying naturally contaminated diets. For instance, it is not common to find feedstuffs highly contaminated with fumonisins. Therefore, the probability of producing a diet with a final concentration between 15,000 ppb and 20,000 ppb (20,000 ppb is the European threshold level in complete feed) [4] is extremely low. The maximum acceptable level of DON in cereals and cereal products used for feed production is 8000 ppb, while for maize byproduct feed materials, it is 12,000 ppb (EC 576/2006) [4]. Considering that northwestern European broiler diets contain approximately 30% wheat, it will be difficult to reach 4000 ppb in the final diet. Based on this, we can expect only low to moderate levels of DON in broiler diets, e.g., 1000 ppb to 3000 ppb.
The type of feedstuff added to the diet also interferes with intestinal health and broiler performance. Wheat and rye evoke increased intestinal viscosity in broilers due to high levels of soluble non-starch polysaccharides (NSP), and this results in impaired nutrient digestibility and predisposes to infections [6,7]. This effect is usually prevented by the inclusion of enzymes that break down soluble NSP. For this trial, we decided to keep the inclusion level of wheat close to practice, whereas a small amount of rye was included to induce a mild intestinal challenge.
For this study, we exposed broilers during the starter and grower phase to two different levels of DON (900 ppb or 2300 ppb). In the finisher period, they received a diet with a negligible level of DON (57.3 ppb) to evaluate carryover effects. As a positive control, activated charcoal was tested at each DON level. Furthermore, feed was not supplemented with coccidiostats or NSP enzymes, with the aim to evaluate animal performance and intestinal integrity.
2. Results
2.1. Broiler Performance
The average body weight of the birds at the start of the trial was 42.7 g (42.4–43.0 g) for all treatments. The lowest body weight (BW) at D14 and D28 was observed in broilers fed the moderate DON (MD), regardless of the dietary supplementation with activated charcoal. Dietary supplementation with activated charcoal improved BW only at D14. Although birds were fed a diet with negligible levels of DON and no activated charcoal at D35, the birds previously fed with MD diets presented a significantly lower BW (Table 1).
Table 1.
Mean (± SD) effects of deoxynivalenol (DON) and activated charcoal on body weight (BW; g) of the birds at D14, D28, and D35.
| Treatment | DON | D14 | D28 | D35 | |||
|---|---|---|---|---|---|---|---|
| No Additive | MD | 530 ± 9 | 1696 ± 69 | 2362 ± 88 | |||
| Activated Charcoal | MD | 545 ± 20 | 1762 ± 117 | 2382 ± 89 | |||
| No Additive | LD | 551 ± 23 | 1830 ± 52 | 2464 ± 71 | |||
| Activated Charcoal | LD | 563 ± 11 | 1822 ± 78 | 2438 ± 95 | |||
| MD | 538 ± 17 | a | 1729 ± 99 | a | 2372 ± 86 | a | |
| LD | 557 ± 19 | b | 1826 ± 64 | b | 2451 ± 82 | b | |
| No Additive | 541 ± 20 | a | 1763 ± 91 | 2413 ± 93 | |||
| Activated Charcoal | 554 ± 18 | b | 1792 ± 101 | 2410 ± 94 | |||
| Effect | p-value | LSD | p-value | LSD | p-value | LSD | |
| DON | 0.004 | 12.5 | 0.001 | 54.8 | 0.02 | 64.6 | |
| Additive | 0.042 | 12.5 | 0.28 | 54.8 | 0.94 | 64.6 | |
| DON × Additive | 0.83 | 17.7 | 0.18 | 77.4 | 0.48 | 91.3 | |
MD: Moderate DON level (2300 ppb in the diet); LD: Low DON level (900 ppb in the diet), LSD: Least significant difference. (a–c) Values followed by a different letter within a column differ significantly (p ≤ 0.05).
During the starter (D0–14; Table 2) and grower (D14–28; Table 3) periods, there were no interactions between DON level and activated charcoal. The lowest body weight gain (BWG) and highest feed conversion ratio (FCR) at D14 and D28 was observed when birds were fed the MD diet, regardless of the dietary supplementation with activated charcoal (Table 2 and Table 3). Activated charcoal improved BWG at D14 only but did not improve FCR (Table 2). None of the diets affected feed intake (FI) in the starter (D0–14) and grower (D14–28) periods (Table 2 and Table 3).
Table 2.
Mean (± SD) effects of DON and activated charcoal in the starter phase (D0–14) on body weight gain (BWG; g), feed intake (FI; g), and feed conversion ratio (FCR; g/g) of broilers.
| BWG | FI | FCR | |||||
|---|---|---|---|---|---|---|---|
| Treatment | DON | (g) | (g) | (g/g) | |||
| No Additive | MD | 488 ± 9 | 578 ± 11 | 1.186 ± 0.016 | |||
| Activated Charcoal | MD | 502 ± 20 | 586 ± 22 | 1.168 ± 0.011 | |||
| No Additive | LD | 508 ± 23 | 588 ± 21 | 1.158 ± 0.020 | |||
| Activated Charcoal | LD | 520 ± 11 | 597 ± 18 | 1.148 ± 0.035 | |||
| MD | 495 ± 17 | a | 582 ± 17 | 1.177 ± 0.016 | b | ||
| LD | 514 ± 18 | b | 593 ± 20 | 1.153 ± 0.028 | a | ||
| No Additive | 498 ± 20 | a | 583 ± 17 | 1.172 ± 0.023 | |||
| Activated Charcoal | 511 ± 18 | b | 592 ± 20 | 1.158 ± 0.027 | |||
| Effect | p-value | LSD | p-value | LSD | p-value | LSD | |
| DON | <0.01 | 12.5 | 0.17 | 14.9 | <0.01 | 0.0163 | |
| Additive | 0.03 | 12.5 | 0.27 | 14.9 | 0.10 | 0.0163 | |
| DON × Additive | 0.72 | 17.7 | 0.97 | 21.0 | 0.62 | 0.0231 | |
MD: Moderate DON level (2300 ppb in the diet); LD: Low DON level (900 ppb in the diet). (a,b) Values followed by a different letter within a column differ significantly (p ≤ 0.05).
Table 3.
Mean (± SD) effects of DON and activated charcoal in the grower phase (D14–28) on body weight gain (BWG; g), feed intake (FI; g), and feed conversion ratio (FCR; g/g) of broilers.
| BWG | FI | FCR | |||||
|---|---|---|---|---|---|---|---|
| Treatment | DON | (g) | (g) | (g/g) | |||
| No Additive | MD | 1166 ± 75 | 1814 ± 54 | 1.561 ± 0.080 | |||
| Activated Charcoal | MD | 1217 ± 102 | 1849 ± 95 | 1.523 ± 0.078 | |||
| No additive | LD | 1279 ± 38 | 1888 ± 56 | 1.476 ± 0.021 | |||
| Activated Charcoal | LD | 1259 ± 71 | 1861 ± 70 | 1.480 ± 0.039 | |||
| MD | 1191 ± 91 | a | 1831 ± 77 | 1.542 ± 0.079 | b | ||
| LD | 1269 ± 56 | b | 1875 ± 63 | 1.478 ± 0.030 | a | ||
| No Additive | 1222 ± 82 | 1851 ± 66 | 1.519 ± 0.072 | ||||
| Activated Charcoal | 1238 ± 88 | 1855 ± 81 | 1.501 ± 0.064 | ||||
| Effect | p-value | LSD | p-value | LSD | p-value | LSD | |
| DON | <0.01 | 50.4 | 0.10 | 51.2 | <0.01 | 0.0401 | |
| Additive | 0.52 | 50.4 | 0.88 | 51.2 | 0.39 | 0.0401 | |
| DON × Additive | 0.16 | 71.3 | 0.23 | 72.4 | 0.31 | 0.0567 | |
MD: Moderate DON level (2300 ppb in the diet); LD: Low DON level (900 ppb in the diet). (a,b) Values followed by a different letter within a column differ significantly (p ≤ 0.05).
During the finisher phase (D28–35), the broilers were fed a marginally contaminated diet (57.3 ppb DON) without activated charcoal. No dietary effects were observed in the performance parameters in this period (Table 4).
Table 4.
Mean (± SD) effects of DON and activated charcoal in the finisher phase (D28–35) on body weight gain (BWG; g), feed intake (FI; g), and feed conversion ratio (FCR; g/g) of broilers.
| BWG | FI | FCR | |||||
|---|---|---|---|---|---|---|---|
| Treatment | DON | (g) | (g) | (g/g) | |||
| No Additive | MD | 666 ± 105 | 1162 ± 81 | 1.767 ± 0.168 | |||
| Activated Charcoal | MD | 620 ± 57 | 1148 ± 40 | 1.860 ± 0.124 | |||
| No Additive | LD | 634 ± 42 | 1173 ± 62 | 1.854 ± 0.085 | |||
| Activated Charcoal | LD | 616 ± 56 | 1141 ± 61 | 1.858 ± 0.078 | |||
| MD | 643 ± 85 | 1157 ± 62 | 1.814 ± 0.151 | ||||
| LD | 625 ± 49 | 1149 ± 61 | 1.856 ± 0.079 | ||||
| No Additive | 650 ± 79 | 1168 ± 70 | 1.811 ± 0.136 | ||||
| Activated Charcoal | 618 ± 55 | 1144 ± 50 | 1.859 ± 0.100 | ||||
| Effect | p-value | LSD | p-value | LSD | p-value | LSD | |
| DON | 0.49 | 52.7 | 0.92 | 45.5 | 0.36 | 0.0919 | |
| Additive | 0.23 | 52.7 | 0.31 | 45.5 | 0.29 | 0.0919 | |
| DON × Additive | 0.58 | 74.5 | 0.71 | 64.3 | 0.33 | 0.1299 | |
MD: Moderate DON level (2300 ppb in the diet); LD: Low DON level (900 ppb in the diet). MD and LD diets were given in the start (D0–14) and grower (D14–28) periods only. During the finisher period, all birds were fed a diet with negligible (57.3 ppb) DON levels.
Considering the complete feeding period (D0–35; Table 5), no interactions between DON and activated charcoal were observed, but broiler chickens fed the MD diet during the starter and grower phase presented a significantly lower BWG and a higher FCR, even if they were fed a marginally contaminated diet in the finisher period.
Table 5.
Mean (± SD) effects of DON and activated charcoal in the complete feeding period (D0–35) on body weight gain (BWG; g), feed intake (FI; g), and feed conversion ratio (FCR; g/g) of broilers.
| BWG | FI | FCR | |||||
|---|---|---|---|---|---|---|---|
| Treatment | DON | (g) | (g) | (g/g) | |||
| No Additive | MD | 2319 ± 88 | 3555 ± 77 | 1.534 ± 0.041 | |||
| Activated Charcoal | MD | 2339 ± 89 | 3583 ± 110 | 1.532 ± 0.038 | |||
| No Additive | LD | 2421 ± 71 | 3649 ± 110 | 1.508 ± 0.026 | |||
| Activated Charcoal | LD | 2396 ± 95 | 3600 ± 97 | 1.503 ± 0.028 | |||
| MD | 2329 ± 86 | a | 3569 ± 93 | 1.533 ± 0.038 | b | ||
| LD | 2408 ± 82 | b | 3624 ± 104 | 1.505 ± 0.026 | a | ||
| No Additive | 2370 ± 93 | 3612 ± 104 | 1.521 ± 0.035 | ||||
| Activated Charcoal | 2368 ± 94 | 3591 ± 101 | 1.518 ± 0.036 | ||||
| Effect | p-value | LSD | p-value | LSD | p-value | LSD | |
| DON | 0.02 | 64.5 | 0.13 | 73.2 | 0.04 | 0.0249 | |
| Additive | 0.94 | 64.5 | 0.76 | 73.2 | 0.81 | 0.0249 | |
| DON × Additive | 0.48 | 91.3 | 0.29 | 103.5 | 0.91 | 0.0352 | |
MD: Moderate DON level (2300 ppb in the diet); LD: Low DON level (900 ppb in the diet). (a,b) Values followed by a different letter within a column differ significantly (p ≤ 0.05).
2.2. Intestinal Analysis
2.2.1. Jejunum and Ileum Morphometry
The effects of DON and activated charcoal on jejunum and ileum morphometry were evaluated by comparing villus height (VH) (µm), crypt depth (CD) (µm), villus height:crypt depth (VH:CD) ratio, and villus area (µm2) among the treatments at D14 (Table 6), D28 (Table 7), and D35 (Table 8). The VH:CD ratio was significantly higher in the ileum from 14-day-old broilers fed the MD when compared with the LD diet (Table 6). The VH and VH:CD ratio in the jejunum from 28-day-old broilers showed a significant interaction, indicating that broilers fed a non-supplemented MD diet had the shortest villi and lowest VH:CD ratio when compared with the non-supplemented LD diet. These differences were statistically significant. When LD diets were supplemented with activated charcoal, a significant decrease in VH was observed (Table 7).
Table 6.
Mean (± SD) effects of DON and activated charcoal on morphometric parameters and ileum at D14.
| Jejunum | Ileum | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Villus Height | Crypt Depth | VH:CD | Villus Area | Villus Height | Crypt Depth | VH:CD | Villus Area | ||||||||||
| Treatment | DON | (µm) | (µm) | ratio | (mm2) | (µm) | (µm) | ratio | (mm2) | ||||||||
| No Additive | MD | 829 ± 110 | 160 ± 31 | 5.3 ± 0.6 | 81 ± 15 | 663 ± 44 | 158 ± 16 | 4.3 ± 0.5 | 64 ± 5.8 | ||||||||
| Activated Charcoal | MD | 830 ± 99 | 165 ± 31 | 5.2 ± 0.8 | 90 ± 17 | 671 ± 103 | 158 ± 47 | 4.6 ± 1.4 | 74 ± 29 | ||||||||
| No Additive | LD | 783 ± 72 | 180 ± 41 | 4.6 ± 1.4 | 82 ± 11 | 591 ± 69 | 195 ± 75 | 3.4 ± 1.0 | 63 ± 14 | ||||||||
| Activated Charcoal | LD | 841 ± 70 | 184 ± 33 | 4.7 ± 1.0 | 92 ± 21 | 670 ± 113 | 173 ± 21 | 4.0 ± 0.8 | 78 ± 20 | ||||||||
| MD | 829 ± 101 | 162 ± 30 | 5.2 ± 0.7 | 86 ± 16 | 667 ± 77 | 158 ± 34 | 4.4 ± 1.0 | b | 69 ± 21 | ||||||||
| LD | 812 ± 75 | 182 ± 36 | 4.7 ± 1.2 | 87 ± 17 | 630 ± 99 | 184 ± 54 | 3.7 ± 0.9 | a | 71 ± 18 | ||||||||
| No Additive | 806 ± 93 | 170 ± 37 | 5.0 ± 1.1 | 82 ± 13 | 627 ± 67 | 176 ± 56 | 3.8 ± 0.9 | 64 ± 11 | |||||||||
| Activated Charcoal | 835 ± 83 | 175 ± 32 | 5.0 ± 0.9 | 91 ± 18 | 671 ± 105 | 165 ± 36 | 4.3 ± 1.1 | 76 ± 24 | |||||||||
| Effect | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | |
| DON | 0.59 | 66.1 | 0.13 | 25.6 | 0.14 | 0.75 | 0.83 | 13.2 | 0.25 | 63.8 | 0.07 | 28.0 | 0.03 | 0.66 | 0.85 | 13.7 | |
| Additive | 0.37 | 66.1 | 0.71 | 25.6 | 0.97 | 0.75 | 0.16 | 13.2 | 0.17 | 63.8 | 0.43 | 28.0 | 0.18 | 0.66 | 0.08 | 13.7 | |
| DON × Additive | 0.37 | 93.5 | 0.96 | 36.1 | 0.82 | 1.06 | 0.98 | 18.7 | 0.26 | 90.3 | 0.42 | 39.5 | 0.67 | 0.93 | 0.72 | 19.4 | |
MD: Moderate DON level (2300 ppb in the diet); LD: Low DON level (900 ppb in the diet). (a,b) Values followed by a different letter within a column differ significantly (p ≤ 0.05).
Table 7.
Mean (± SD) effects of DON and activated charcoal on morphometric parameters of jejunum and ileum at D28.
| Jejunum | Ileum | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Villus Height | Crypt Depth | VH:CD | Villus Area | Villus Height | Crypt Depth | VH:CD | Villus Area | ||||||||||
| Treatment | DON | (µm) | (µm) | ratio | (mm2) | (µm) | (µm) | ratio | (mm2) | ||||||||
| No Additive | MD | 922 ± 113 | a | 232 ± 34 | 4.1 ± 0.9 | a | 182 ± 128 | 832 ± 192 | 235 ± 73 | b | 3.9 ± 1.4 | a | 119 ± 31 | ||||
| Additive | MD | 1002 ± 120 | ab | 214 ± 34 | 4.8 ± 0.9 | ab | 180 ± 168 | 876 ± 126 | 199 ± 41 | ab | 4.7 ± 1.4 | ab | 98 ± 19 | ||||
| No Additive | LD | 1073 ± 138 | b | 186 ± 23 | 5.9 ± 1.1 | b | 120 ± 16 | 951 ± 182 | 177 ± 26 | a | 5.5 ± 1.3 | b | 151 ± 113 | ||||
| Additive Charcoal | LD | 962 ± 153 | a | 212 ± 63 | 4.8 ± 1.2 | ab | 146 ± 63 | 888 ± 210 | 214 ± 41 | ab | 4.3 ± 1.2 | ab | 192 ± 171 | ||||
| MD | 962 ± 120 | 223 ± 34 | 4.5 ± 1.0 | 181 ± 144 | 854 ± 158 | 217 ± 60 | 4.3 ± 1,4 | 109 ± 27 | |||||||||
| LD | 1017 ± 152 | 199 ± 47 | 5.4 ± 1.2 | 133 ± 46 | 920 ± 193 | 196 ± 38 | 4.9 ± 1,4 | 171 ± 141 | |||||||||
| No Additive | 998 ± 144 | 209 ± 36 | 5.0 ± 1.3 | 151 ± 94 | 892 ± 191 | 206 ± 61 | 4.7 ± 1.5 | 135 ± 81 | |||||||||
| Activated Charcoal | 982 ± 134 | 213 ± 49 | 4.8 ± 1.1 | 163 ± 123 | 882 ± 167 | 206 ± 40 | 4.5 ± 1.3 | 145 ± 127 | |||||||||
| Effect | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | |
| DON | 0.16 | 76.8 | 0.12 | 30.1 | 0.03 | 0.78 | 0.23 | 80.2 | 0.34 | 137.6 | 0.20 | 47.7 | 0.18 | 0.92 | 0.12 | 78.9 | |
| Additive | 0.68 | 76.8 | 0.81 | 30.1 | 0.65 | 0.78 | 0.77 | 80.2 | 0.89 | 137.6 | 1.00 | 35.8 | 0.60 | 0.92 | 0.80 | 78.9 | |
| DON × Additive | 0.02 | 108.6 | 0.15 | 42.6 | 0.03 | 1.10 | 0.72 | 113.5 | 0.43 | 194.6 | 0.04 | 37.0 | 0.041 | 1.305 | 0.43 | 111.6 | |
MD: Moderate DON level (2300 ppb in the diet); LD: Low DON level (900 ppb in the diet). (a,b) Values followed by a different letter within a column differ significantly (p ≤ 0.05).
Table 8.
Mean (± SD) effects of DON and activated charcoal on morphometric parameters of jejunum and ileum at D35.
| Jejunum | Ileum | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Villus Height | Crypt Depth | VH:CD | Villus Area | Villus Height | Crypt Depth | VH:CD | Villus Area | ||||||||||
| Treatment | DON | (µm) | (µm) | ratio | (mm2) | (µm) | (µm) | ratio | (mm2) | ||||||||
| No Additive | MD | 1170 ± 169 | 256 ± 64 | 4.9 ± 1.3 | 212 ± 143 | 905 ± 124 | 205 ± 45 | 4.7 ± 1.6 | 170 ± 124 | ||||||||
| Additive | MD | 1166 ± 222 | 250 ± 61 | 4.9 ± 1.3 | 289 ± 156 | 887 ± 230 | 203 ± 33 | 4.5 ± 1.2 | 114 ± 39 | ||||||||
| No Additive | LD | 1120 ± 206 | 240 ± 55 | 4.9 ± 1.4 | 279 ± 126 | 835 ± 141 | 207 ± 65 | 4.3 ± 1.1 | 98 ± 24 | ||||||||
| Additive Charcoal | LD | 1175 ± 129 | 266 ± 43 | 4.6 ± 0.9 | 257 ± 155 | 895 ± 106 | 202 ± 36 | 4.6 ± 0.8 | 142 ± 80 | ||||||||
| MD | 1168 ± 190 | 253 ± 60 | 4.9 ± 1.2 | 250 ± 150 | 896 ± 182 | 204 ± 38 | 4.6 ± 1.4 | 142 ± 90 | |||||||||
| LD | 1148 ± 166 | 253 ± 49 | 4.7 ± 1.1 | 268 ± 138 | 865 ± 125 | 205 ± 50 | 4.4 ± 0.9 | 120 ± 62 | |||||||||
| No Additive | 1145 ± 182 | 248 ± 58 | 4.9 ± 1.3 | 245 ± 137 | 870 ± 133 | 206 ± 55 | 4.5 ± 1.3 | 134 ± 91 | |||||||||
| Activated Charcoal | 1171 ± 175 | 258 ± 51 | 4.7 ± 1.1 | 273 ± 151 | 891 ± 173 | 203 ± 33 | 4.5 ± 1.0 | 128 ± 63 | |||||||||
| Effect | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | |
| DON | 0.78 | 145.4 | 1.00 | 43.8 | 0.72 | 0.98 | 0.74 | 105.6 | 0.60 | 120.7 | 0.99 | 36.2 | 0.72 | 0.87 | 0.39 | 51.5 | |
| Additive | 0.72 | 145.4 | 0.65 | 43.8 | 0.81 | 0.98 | 0.60 | 105.6 | 0.72 | 120.7 | 0.86 | 36.2 | 0.99 | 0.87 | 0.83 | 51.5 | |
| DON × Additive | 0.68 | 205.6 | 0.47 | 62.0 | 0.71 | 1.39 | 0.35 | 149.4 | 0.51 | 170.8 | 0.93 | 51.4 | 0.51 | 1.24 | 0.06 | 72.8 | |
MD: Moderate DON level (2300 ppb in the diet); LD: Low DON level (900 ppb in the diet). MD and LD diets were given in the start (D0–14) and grower (D14–28) periods only. During the finisher period, all birds were fed a diet with negligible (57.3 ppb) DON levels.
Regarding the ileum, the crypt depth was highest when broilers chickens were fed a non-supplemented MD diet and lowest when the birds were fed the non-supplemented LD diet. This resulted also in the significantly lowest and highest VH:CD ratio when the birds were fed the MD and LD diets, respectively. No dietary effect was observed on the morphometry of the jejunum and ileum from 35-day-old broilers (Table 8).
2.2.2. Goblet Cell Counting
The effects of DON and activated charcoal on jejunum and ileum mucus production were evaluated by counting the number of goblet cells per villus and by determining the density of these goblet cells according to the villus area (µm2) among the treatments at D14 (Table 9), D28 (Table 10), and D35 (Table 11). Dietary effects were observed only at D14, where a significant increase in goblet cell density was observed in the ileum from broilers fed the MD diet, regardless of the supplementation with activated charcoal (Table 9).
Table 9.
Mean (± SD) effects of DON and activated charcoal on the number and density of goblet cells in jejunum and ileum of broilers at D14.
| Treatment | DON | Jejunum | Ileum | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N° Goblet Cells | Goblet Cells/μm2 | N° Goblet Cells | Goblet Cells/μm2 | ||||||
| No Additive | MD | 111 ± 21 | 1.4 ± 0.2 | 104 ± 18 | 1.6 ± 0.2 | ||||
| Activated Charcoal | MD | 126 ± 29 | 1.4 ± 0.2 | 119 ± 63 | 1.6 ± 0.2 | ||||
| No Additive | LD | 99 ± 20 | 1.2 ± 0.3 | 84 ± 24 | 1.3 ± 0.3 | ||||
| Activated Charcoal | LD | 112 ± 29 | 1.2 ± 0.3 | 101 ± 41 | 1.3 ± 0.4 | ||||
| MD | 119 ± 26 | 1.4 ± 0.2 | 111 ± 45 | 1.6 ± 0.2 | b | ||||
| LD | 106 ± 25 | 1.2 ± 0.3 | 93 ± 34 | 1.3 ± 0.3 | a | ||||
| No Additive | 105 ± 21 | 1.3 ± 0.3 | 94 ± 23 | 1.5 ± 0.3 | |||||
| Activated Charcoal | 119 ± 29 | 1.3 ± 0.3 | 110 ± 52 | 1.4 ± 0.3 | |||||
| Effect | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | |
| DON | 0.14 | 17.0 | 0.06 | 0.17 | 0.20 | 29.7 | 0.02 | 0.21 | |
| Additive | 0.10 | 17.0 | 0.88 | 0.17 | 0.28 | 29.7 | 0.67 | 0.21 | |
| DON × Additive | 0.90 | 24.0 | 0.92 | 0.24 | 0.91 | 42.0 | 0.86 | 0.29 | |
MD: Moderate DON level (2300 ppb in the diet); LD: Low DON level (900 ppb in the diet). (a,b) Values followed by a different letter within a column differ significantly (p ≤ 0.05).
Table 10.
Mean (± SD) effects of DON and activated charcoal on the number and density of goblet cells in jejunum and ileum of broilers at D28.
| Treatment | DON | Jejunum | Ileum | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N° Goblet Cells | Goblet Cells/μm2 | N° Goblet Cells | Goblet Cells/μm2 | ||||||
| No Additive | MD | 143 ± 56 | 1.0 ± 0.6 | 160 ± 88 | 1.4 ± 0.7 | ||||
| Activated Charcoal | MD | 151 ± 65 | 1.2 ± 0.7 | 134 ± 28 | 1.4 ± 0.4 | ||||
| No Additive | LD | 147 ± 41 | 1.3 ± 0.5 | 161 ± 49 | 1.4 ± 0.7 | ||||
| Activated Charcoal | LD | 164 ± 56 | 1.2 ± 0.5 | 172 ± 95 | 1.5 ± 1.1 | ||||
| MD | 147 ± 59 | 1.1 ± 0.6 | 147 ± 65 | 1.4 ± 0.6 | |||||
| LD | 155 ± 48 | 1.2 ± 0.5 | 167 ± 73 | 1.4 ± 0.9 | |||||
| No Additive | 145 ± 47 | 1.1 ± 0.5 | 160 ± 69 | 1.4 ± 0.7 | |||||
| Activated Charcoal | 157 ± 59 | 1.2 ± 0.6 | 153 ± 71 | 1.4 ± 0.8 | |||||
| Effect | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | |
| DON | 0.64 | 36.5 | 0.52 | 0.40 | 0.41 | 47.2 | 0.95 | 0.50 | |
| Additive | 0.49 | 36.5 | 0.67 | 0.40 | 0.76 | 47.2 | 0.87 | 0.50 | |
| DON × Additive | 0.80 | 51.7 | 0.52 | 0.57 | 0.42 | 66.7 | 0.94 | 0.70 | |
MD: Moderate DON level (2300 ppb in the diet); LD: Low DON level (900 ppb in the diet).
Table 11.
Mean (± SD) effects of DON and activated charcoal on the number and density of goblet cells in jejunum and ileum of broilers at D35.
| Treatment | DON | Jejunum | Ileum | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N° Goblet Cells | Goblet Cells/μm2 | N° Goblet Cells | Goblet Cells/μm2 | ||||||
| No Additive | MD | 81.3 ± 36.5 | 0.5 ± 0.2 | 73.0 ± 26.5 | 0.6 ± 0.4 | ||||
| Activated Charcoal | MD | 65.0 ± 29.1 | 0.3 ± 0.2 | 72.2 ± 33.4 | 0.6 ± 0.1 | ||||
| No Additive | LD | 76.2 ± 46.7 | 0.4 ± 0.4 | 73.6 ± 21.8 | 0.8 ± 0.3 | ||||
| Activated Charcoal | LD | 84.8 ± 28.8 | 0.5 ± 0.3 | 65.5 ± 34.8 | 0.6 ± 0.3 | ||||
| MD | 73.1 ± 33.0 | 0.4 ± 0.2 | 72.6 ± 30 | 0.6 ± 0.3 | |||||
| LD | 80.5 ± 37.0 | 0.4 ± 0.3 | 69.5 ± 28 | 0.7 ± 0.3 | |||||
| No Additive | 78.7 ± 40.1 | 0.4 ± 0.3 | 73.3 ± 23 | 0.7 ± 0.3 | |||||
| Activated Charcoal | 74.9 ± 29.8 | 0.4 ± 0.3 | 68.8 ± 33 | 0.6 ± 0.2 | |||||
| Effect | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | |
| DON | 0.59 | 27.9 | 0.89 | 0.21 | 0.73 | 17.8 | 0.38 | 0.16 | |
| Additive | 0.78 | 27.9 | 0.63 | 0.21 | 0.61 | 17.8 | 0.22 | 0.16 | |
| DON × Additive | 0.37 | 39.5 | 0.20 | 0.30 | 0.68 | 25.2 | 0.10 | 0.22 | |
MD: Moderate DON level (2300 ppb in the diet); LD: Low DON level (900 ppb in the diet). MD and LD diets were given in the start (D0–14) and grower (D14–28) periods only. During the finisher period, all birds were fed a diet with negligible (57.3 ppb) DON levels.
2.2.3. Intestinal Lesion Scores
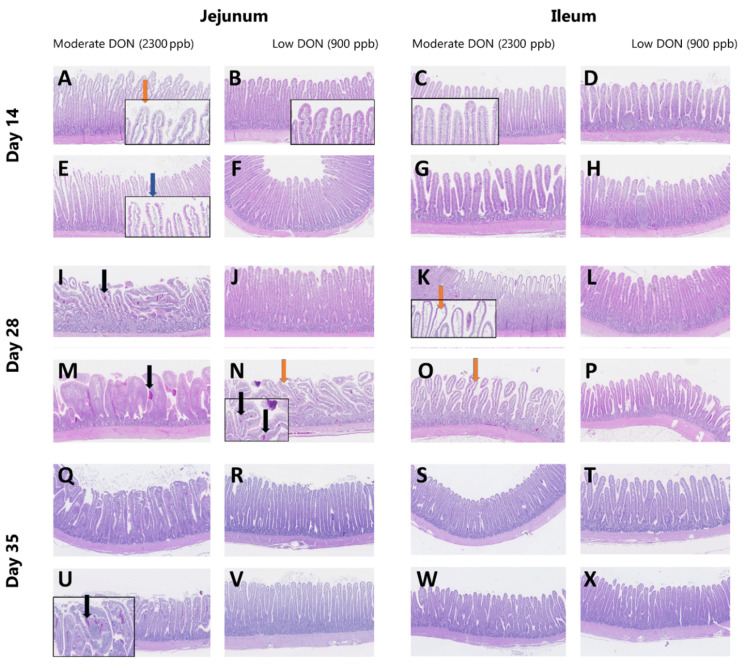
Lesion scores were given to the jejunum and ileum on a scale from 0 to 6 based on the Chiu/Park scoring method, where the higher the score, the higher the degree of damage. At D14, lesion scores were significantly increased when birds were fed a MD diet supplemented with activated charcoal, whereas at D28, the highest lesion scores were observed in broiler chickens receiving a non-supplemented MD died. No dietary effects were observed at D35 (Table 12). Figure 1 illustrates examples of jejunum and ileum sections from broilers fed MD and LD diets supplemented or not with activated charcoal.
Table 12.
Mean (± SD) effects of DON and activated charcoal on jejunum and ileum Chiu/Park intestinal lesion scores *.
| Jejunum | Ileum | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | DON | D14 | D28 | D35 | D14 | D28 | D35 | ||||||
| No Additive | MD | 0.3 ± 0.2 | a | 3.1 ± 1.3 | b | 1.5 ± 0.7 | 0.1 ± 0.1 | 1.3 ± 0.8 | 0.7 ± 0.7 | ||||
| Additive | MD | 1.1 ± 0.8 | b | 2.2 ± 1.4 | ab | 1.2 ± 1.3 | 0.3 ± 0.3 | 1.2 ± 1.2 | 0.9 ± 0.9 | ||||
| No Additive | LD | 1.0 ± 1.2 | ab | 1.3 ± 1.6 | a | 1.0 ± 0.7 | 0.2 ± 0.2 | 0.6 ± 0.6 | 0.6 ± 0.6 | ||||
| Additive Charcoal | LD | 0.2 ± 0.4 | a | 2.3 ± 1.2 | ab | 0.3 ± 0.6 | 0.0 ± 0.0 | 1.5 ± 1.1 | 0.4 ± 0.3 | ||||
| MD | 0.7 ± 0.7 | 2.7 ± 1.4 | 1.3 ± 1.0 | 0.2 ± 0.2 | 1.2 ± 0.9 | 0.8 ± 0.8 | |||||||
| LD | 0.6 ± 0.6 | 1.8 ± 1.5 | 0.6 ± 0.7 | 0.1 ± 0.1 | 1.0 ± 1.0 | 0.5 ± 0.5 | |||||||
| No Additive | 0.7 ± 0.7 | 2.2 ± 1.7 | 1.2 ± 0.7 | 0.1 ± 0.1 | 0.9 ± 0.9 | 0.7 ± 0.7 | |||||||
| Activated Charcoal | 0.7 ± 0.7 | 2.2 ± 1.3 | 0.7 ± 0.7 | 0.1 ± 0.1 | 1.4 ± 1.1 | 0.7 ± 0.7 | |||||||
| Effect | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | p-value | LSD | |
| DON | 0.75 | 0.56 | 0.07 | 0.92 | 0.06 | 0.69 | 0.30 | 0.19 | 0.53 | 0.61 | 0.34 | 0.60 | |
| Additive | 0.97 | 0.56 | 0.94 | 0.92 | 0.16 | 0.69 | 0.80 | 0.19 | 0.13 | 0.61 | 0.96 | 0.60 | |
| DON × Additive | <0.01 | 0.80 | 0.04 | 1.31 | 0.62 | 0.98 | 0.08 | 0.28 | 0.10 | 0.86 | 0.57 | 0.85 | |
MD: Moderate DON level (2300 ppb in the diet); LD: Low DON level (900 ppb in the diet). MD and LD diets were given in the start (D0–14) and grower (D14–28) periods only. During the finisher period, all birds were fed a diet with negligible (57.3 ppb) DON levels. * The higher the score, the more villus damage. (a,b) Values followed by a different letter within a column differ significantly (p ≤ 0.05).
Figure 1.
Illustrative images of periodic acid–Schiff (PAS)-hematoxylin-stained sections of jejunum and ileum from broilers fed the experimental diets. Orange arrows indicate denuded lamina propria, blue arrow shows damage in villus tip, and black arrows indicate blood in the villi. Scale bars = 100 μm.
2.2.4. Intestinal Viscosity
The results concerning viscosity in the duodenum on D14, D28, and D35 are shown in Table 13. Intestinal viscosity was significantly increased in 14-day-old broilers fed the MD diet, regardless of the dietary supplementation with activated charcoal. No other changes were observed in the other feeding phases.
Table 13.
Mean (± SD) effects of DON and activated charcoal on intestinal viscosity (cP) at D14, D28, and D35.
| Treatment | DON | D14 (cP) | D28 (cP) | D35 (cP) | |||
|---|---|---|---|---|---|---|---|
| No Additive | MD | 16.6 ± 3.1 | 6.4 ± 3.8 | 2.2 ± 0.3 | |||
| Activated Charcoal | MD | 14.6 ± 2.9 | 7.9 ± 2.6 | 2.1 ± 0.2 | |||
| No Additive | LD | 9.1 ± 2.2 | 9.7 ± 3.7 | 2.2 ± 0.2 | |||
| Activated Charcoal | LD | 10.5 ± 1.2 | 9.6 ± 6.0 | 2.3 ± 0.2 | |||
| MD | 15.6 ± 3.2 | b | 7.1 ± 3.2 | 2.1 ± 0.3 | |||
| LD | 9.8 ± 1.9 | a | 9.6 ± 4.8 | 2.2 ± 0.2 | |||
| No Additive | 12.9 ± 4.7 | 8.0 ± 4.0 | 2.2 ± 0.3 | ||||
| Activated Charcoal | 12.6 ± 2.9 | 8.7 ± 4.5 | 2.2 ± 0.2 | ||||
| Effect | p-value | LSD | p-value | LSD | p-value | LSD | |
| DON | <0.001 | 1.83 | 0.15 | 3.39 | 0.32 | 0.19 | |
| Additive | 0.75 | 1.83 | 0.66 | 3.39 | 0.95 | 0.19 | |
| DON × Additive | 0.06 | 2.59 | 0.62 | 4.80 | 0.32 | 0.27 | |
MD: Moderate DON level (2300 ppb in the diet); LD: Low DON level (900 ppb in the diet). MD and LD diets were given in the start (D0–14) and grower (D14–28) periods only. During the finisher period, all birds were fed a diet with negligible (57.3 ppb) DON levels. (a,b) Values followed by a different letter within a column differ significantly (p ≤ 0.05).
3. Discussion
In the present study, we demonstrate that even at moderate levels (2300 ppb), DON can impair the performance of broiler chickens. Also, this negative effect is not mitigated when the chickens are subsequently fed a diet with negligible levels (57.3 ppb) of DON for 7 days. Anticipating the potentially increasing impact of mycotoxins when ionophore anticoccidials are banned from feed, we decided not to supplement the experimental diets with anticoccidials. In a previous study, broiler chickens fed diets containing 1600 ppb DON and simultaneously challenged with Eimeria spp. [5] had an impaired performance, and one of the factors involved was the ability of DON to modulate the host immune response to coccidial infections [8]. In the present study, instead of challenging the birds with Eimeria spp. oocysts, we excluded anticoccidials from the diet formulation. Coccidiosis not only leads to clinical signs but can also result in poor performance [9]. Another factor that can challenge gut function is an increase in digesta viscosity, because it can decrease nutrient digestibility [7] and increases the retention time of the diet in the intestine, inducing competition with gut microbiota for digestible nutrients. Subsequently, this increases the risks of infection [10]. Therefore, in the present study, we used a wheat-based diet without NSP enzymes together with rye at an inclusion level of 5% in the starter (D0–14) and grower (D14–28) diets, thus increasing viscosity.
Although the diets were contaminated with a variety of mycotoxins, the negative impact observed in the present study was basically caused by DON. Besides DON, the other mycotoxins present in the diets were deoxynivalenol-3-glucoside (DON-3-G), enniatins B and B1 (ENNB+B1), zearalenone (ZEN), ochratoxin A (OTA), alternariol (AOH), and alternariol methyl ether (AME). The levels of ZEN, OTA, AOH, and AME were negligible. When DON is conjugated with glucose, resulting in the modified form of DON-3-G, it becomes unable to bind to the ribosome peptidyl transferase center, the main target of intestinal toxicity [11]. In pigs but not in broiler chickens, DON-3-G can be hydrolyzed to DON [12]. Therefore, the observed dietary levels between 10.7 ppb and 1670 ppb in the present study should not be a reason for concern. Enniatin B has a low intestinal toxicity in broiler chickens [13]. Furthermore, it was remarkable that ENNB+B1 levels were higher in MD than in LD diets.
Increasing the DON level from 900 ppb to 2300 ppb in the starter and grower diets resulted in a decreased BWG. This negative effect on BWG was partly counteracted by activated charcoal only in the MD diet. The same pattern was observed with FCR, which was negatively affected in broilers fed the MD diet without activated charcoal. One must bear in mind that the goal of this study was not to promote activated charcoal as a DON adsorbent, since it may also absorb nutrients [14], but to use it as an extra control [15]. Instead, it was shown that even when supplementing a diet with a compound that can bind DON, performance losses are not completely avoided. When the diet was replaced by a marginally contaminated feed in the finisher phase (D28–35), no differences in performance were observed in this period. However, the final body weight of the birds fed the MD diet during the starter and grower period was impaired, regardless of the supplementation with activated charcoal. Moreover, considering the complete production period (D0–35), BWG and FCR were impaired in broilers fed the MD diet compared with those fed the LD diet, whereas FI was not affected by the experimental diets. Based on a previous study, DON has a low oral availability (~19%) and a high plasma clearance (~0.12 L/min kg) in broilers [16]. Therefore, the impaired performance was not a result of mycotoxin accumulation or anorexia caused by DON. Previously, Lucke et al. [17] did not observe any difference in broiler performance after 28 days of dietary exposure to 5000 ppb DON, and only observed differences after 35 days when BWG was significantly decreased. Besides the dietary differences, in this later study, broiler chickens were fed diets artificially contaminated with DON. Importantly, to achieve homogeneity, this mycotoxin was mixed with inulin at a rate of 0.03% to 0.20% depending on the desired DON dietary level. Inulin is a fructo-oligosaccharide (FOS) able to increase the production of cecal butyric acid, which provides energy for the growth of the intestinal epithelium [18]. In another study, broiler performance was even improved, and intestinal viscosity decreased when the diet was contaminated with 1500 ppb DON [19]. The former authors suggested that physicochemical alterations in the wheat, caused by mycotoxins, resulted in better nutrient digestibility and in a lower viscosity in the jejunum and ileum. However, we did not observe a similar decrease in viscosity, which was measured in the duodenum. Instead, we observed an increase when birds were fed the MD diet during the starter phase. The prolonged exposure time to DON did not affect duodenal viscosity at D28 (8–9 cP), although it was still considerably higher than that observed in birds fed a marginally contaminated diet in the finisher period (2.2 cP). Also, the finisher diet contained NSP enzymes, which can explain the reduced viscosity in this phase. In a similar study, Dänicke et al. [19] fed a control diet resulting in high ileal viscosity (above 25 mPa-s, i.e., 25 cP), which was considerably higher than that observed in our study. Viscosity can be increased by including rye and excluding NSP enzymes [20] as we did in the present study. An increase in viscosity will negatively influence nutrient digestibility and absorption, decreasing BWG and increasing FCR. Therefore, it is not surprising that this diet, combined with moderate DON contamination, impaired broiler performance. Nonetheless, differences in DON levels and dietary composition do not allow a comparison between the study of Dänicke et al. [19] and the current study. Also, the wheat batch we used was naturally contaminated with a mixture of mycotoxins and not submitted to induced infection with a specific Fusarium strain, and nutritional composition may vary among batches. Recently, we showed that broiler chickens fed a corn-based diet (low in NSP) contaminated with ~3500 ppb DON and ~50 ppb of its derivatives 3+15 Ac-DON presented an impaired FCR, indicating that intestinal viscosity is not crucial to observe the negative impact of DON on performance [21]. Likewise, a longitudinal study also showed that mixtures of mycotoxins below EU recommendation levels impair the performance of broiler chickens [22].
Intestinal morphometric changes in the first 14 days of exposure were negligible and limited to an increase in the VH:CD ratio in the ileum of broilers fed the MD diet, regardless of the presence of activated charcoal, indicating that cell proliferation decreased in broilers fed the MD diet without resulting in immediate villus shortening. This was expected, since DON decreases cell proliferation and the crypt is responsible for cell renewing and maintenance of villus length. After 28 days, however, broilers fed the MD diet presented a significantly lower villus height and VH:CD ratio than those fed the LD diet. This leads us to infer that birds fed the MD diet were probably trying to maintain villus height by a compensatory increase in the proliferation in the crypt, which required extra energy. Besides this, shortened villi and deeper crypts will result in suboptimal nutrient absorption and impaired animal performance [23,24]. This fits with the observed decreased BWG and increased FCR at D28. Besides, the ileum presented a higher VH:CD ratio due to an increase in crypt depth, showing that in this intestinal section, cell proliferation was increased to keep villus height similar to the expected (LD diet) levels. Such a compensatory mechanism costs energy and will result in suboptimal performance. At D35, no differences were observed, because the finisher diet had negligible levels of DON and intestinal cell turnover takes around 48 h to 96 h [25]. The number of goblet cells was not affected by the treatments. In a study with pigs, it was shown that DON causes oedema in the lamina propria and contact loss between lamina propria and enterocytes [26]. However, in the present study, the tested DON levels were not able to cause such effects. Based on the morphometric analysis and lesion scores in the jejunum and ileum, it can be confirmed that the jejunum was the intestinal section more sensitive to DON than the ileum, as reported before for broilers submitted to DON exposure [27] or other sources of stress [28].
In conclusion, broiler chickens fed a diet containing moderate levels of DON (2300 ppb) will perform inefficiently. Further, the influence of mycotoxins on poultry performance should be assessed not only on its toxicity per se, but also considering animal age, dietary composition, and the presence of other types of additives, such as NSP enzymes and anticoccidials.
4. Materials and Methods
4.1. Animal Ethics Statement
The experiment was conducted according to the guidelines of the Animal and Human Welfare Codes/Laboratory practice codes in the Netherlands. The protocol was approved by the Ethics Review Committee: Body of Animal Welfare at SFR (AVD246002016450), approval date: 28 February 2019.
4.2. Broilers and Housing
One-day-old male Ross 308 broilers purchased from a local commercial hatchery were used in this study, with 4 dietary treatments of 184 chicks each (divided among 8 replicate pens with 23 chicks each). The birds were housed in 32 floor pens with wood shavings as bedding material in the broiler facilities of Schothorst Feed Research, Lelystad, The Netherlands. Each pen (2.2 m2) had 1 feeder and 3 drinking nipples. Birds were kept until 35 days of age. The ambient temperature was gradually decreased from 34.5 °C at the arrival of the birds to 19.4 °C at 35 days of age. Room temperature and relative humidity were recorded daily. Light was provided continuously for the first 24 h to give birds the opportunity to readily find feed and water. After that, the light schedule was 22L (light): 2D (dark) for 1 day and then 8L: 4D: 10L: 2D for the remaining experimental period, complying with European Union (EU) legislation of a minimum of 6 h of darkness from the second day onward, of which at least 4 h was uninterrupted darkness. Birds were vaccinated against Newcastle Disease at D10 and against Infectious Bursal Disease at D20 of the trial. The health status of the flock was monitored by a poultry veterinarian. Throughout the experimental period, all animals were monitored daily for abnormalities, such as abnormal behavior, clinical signs of illness, and mortality.
4.3. Diets and Experimental Design
The experiment comprised 4 dietary treatments in a factorial design with DON (2 levels; moderate—MD and low—LD) in diets with or without activated charcoal (2 g/kg diet; Norit, Carbomix, KELA Pharma, Sint-Niklaas, Belgium), applied as a DON adsorbent [15]. Diets were prepared with wheat batches naturally contaminated with different DON levels (1650 ppb and 6880 ppb), together with other mycotoxins. The recommended maximum level of DON in poultry diet is 5000 ppb (EU Commission Directive 2003/100/EC). Therefore, in the present study, the obtained 900 ppb and 2300 ppb DON in the final diet were considered as low (LD) and moderate (MD) DON levels, respectively. Treatments were randomly allocated per block to pens, where each treatment was repeated 8 times. The pen was the experimental unit, and each pen contained 23 broilers. Dietary treatments are summarized in Table 14, together with their mycotoxin composition. Although the supplemented diets were made from the same basal diet of each MD or LD level, the levels of DON were measured in all diets to calculate the mean contamination level. The mean levels of DON in the LD diets during the starter and grower phases were 881 ± 4 ppb and 876 ± 92 ppb, respectively. The mean levels of DON in the MD diets during the starter and grower phases were 2130 ± 99 ppb and 2290 ± 99 ppb, respectively. The finisher diet was prepared with marginally contaminated feedstuffs, reaching a DON level of 57 ppb. All diets were analyzed by an independent and accredited laboratory (Primoris, Belgium). A multi-mycotoxin test was applied, showing that DON was the main contaminant and that other mycotoxins were found at negligible levels. The nutrient composition of the diets is given in the Supplementary Table S1.
Table 14.
Evaluated and mean levels of DON and other mycotoxins in the starter (D0–14), grower (D1–28), and finisher (D28–35) diets.
| Experimental Diets | ||||
|---|---|---|---|---|
| Mycotoxins Levels (ppb) |
Moderate DON (MD) | MD + Activated Charcoal |
Low DON (LD) | LD + Activated Charcoal |
| Starter Diet (D0–14) | ||||
| DON | 2060 | 2200 | 878 | 884 |
| DON-3-Glucoside | 132 | 132 | 99 | 454 |
| Enniatin B | 28.2 | 32.3 | 90.4 | 61.8 |
| Enniatin B1 | 13.1 | 8.5 | 16.0 | 17.3 |
| Alternariol | 10.7 | - | - | 3.3 |
| Alternariol ME | - | - | - | 2.4 |
| Grower Diet (D14-28) | ||||
| DON | 2360 | 2220 | 941 | 811 |
| DON-3-Glucoside | 1670 | 1480 | 851 | 632 |
| Zearalenone | - | - | 18.2 | 16.6 |
| Ochratoxin | - | - | - | 3.3 |
| Enniatin B | 36.7 | 41.7 | 58.5 | 60.5 |
| Enniatin B1 | 8.7 | 10.4 | 15.8 | 16.6 |
| Alternariol | 4.2 | - | 3.8 | 2.1 |
| Alternariol ME | 2.1 | 2.2 | 2.7 | - |
| Finisher Diet (D14–28) | ||||
| DON | 57.3 | 57.3 | 57.3 | 57.3 |
| Enniatin B | 8.4 | 8.4 | 8.4 | 8.4 |
| Beauvericin | 6.1 | 6.1 | 6.1 | 6.1 |
4.4. Performance and Litter Score
Broilers were weighed per pen on D0, D14, D28, and D35, and feed consumption and mortality were recorded throughout the experimental period. Body weight gain (BWG), feed intake (FI), and feed conversion ratio (FCR) were determined in the cumulative phases from D0–14, D14–28, D28–35, and D0–35. Litter quality was visually scored at D14, D28, and D35 on a scale of 1–10, with 1 indicating low quality (wet) and 10 indicating high quality (dry and friable) [29].
4.5. Intestinal Analysis
4.5.1. Jejunum and Ileum Morphometry and Goblet Cell Counting
Samples of jejunum and ileum (we randomly selected 1 bird/pen on D14, D28, and D35) were collected and fixed in buffered formalin for histological analysis. In brief, histological slides (periodic acid–Schiff (PAS) counterstaining with hematoxylin staining) from the jejunum and ileum from each bird were scanned by the NanoZoomer-XR (Hamamatsu Photonics KK, Hamamatsu, Japan). The scanned slides were viewed through the viewer software (NDP.view2; Hamamatsu, Japan) and analyzed using the analysis software (NDP.analyze; Hamamatsu, Japan). Villus height (VH), crypt depth (CD), and villus area (µm2) from each individual bird were measured (5 villi per intestinal segment). The measurements of VH and CD were used to calculate the VH:CD ratio. The number of goblet cells and goblet cell density per villus were also quantified in scans of Alcian Bleu sections, serial to the PAS-hematoxylin sections. Only intact villi were measured.
4.5.2. Jejunum and Ileum Lesion Scores
To evaluate the degree of mucosal damage, the Chiu/Park scale was applied [30]. In brief, the mucosa was classified from normal if presenting an intact structure with no visible damage (degree 0) to severely damaged (degree 6), as previously described [28]:
Degree 0: Intact without visible damage.
Degree 1: Damage in subepithelial space at villus tips.
Degree 2: Extension of subepithelial space with moderate lifting.
Degree 3: Massive lifting down the sides of villi with some denuded villi.
Degree 4: Denuded villi with dilated capillaries.
Degree 5: Disintegration of lamina propria.
Degree 6: Crypt injury.
To statistically compare the degrees among the treatments, a composite score per treatment was determined by averaging the score from each bird. For this, the percentage of villi with a specific degree was multiplied by its respective degree. This calculation was performed for each degree per treatment, and the sum obtained was considered the composite score [31].
4.5.3. Intestinal Viscosity
Digesta samples from the duodenum were collected at D14 (pooled sample of 3 birds per pen), D28 (1 bird per pen), and D35 (1 bird per pen). The samples were submitted to viscosity determination according to the AOAC protocol. In brief, each sample was centrifuged for 10 min at 3500× g and at 4 °C. The supernatant was filtrated with a serum filter tube and placed on ice. Viscosity of the supernatant was measured at 20 °C with a digital Brookfield DV-II LVCP viscometer (Brookfield Engineering, Middleboro, MA, USA) according to the “plate and cone” method.
4.6. Statistical Analysis
Observations were marked as outliers to be excluded from the dataset prior to statistical analyses if the residual (fitted—observed value) was more than 2.5× standard error of the parameter. If at least 1 of the response parameters FI, BWG, or FCR was an outlier, then all 3 records were dropped for that particular observation in that measurement period. Body weight was analyzed separately from the other production parameters. Data regarding intestinal morphometry and goblet cells counting were used without removing outliers. The experimental data were analyzed with ANOVA (GenStat Version 19.0, 2018, Hemel Hempstead, UK). Each pen was an experimental unit. Given the factorial design, the statistical model used to analyze the data was:
| Y = μ + blocki + DONj + Additivek + DON∗Additivejk + eijk |
In which:
Y = Response parameter
μ = General mean
Blockj = Effect of block (i = 1…8)
DONj = Effect of DON (j = 1, 2)
Additivek = Effect of Additive (k = 1, 2)
DON*Additivejk = Effect of the interactions between DON and Additive
Errorijk = Error term
Treatment means were compared by least significant difference (LSD). Values with p ≤ 0.05 were considered statistically significant.
Abbreviations
| AME | Alternariol Methyl Ether |
| ANOVA | Analysis of variance |
| AOAC | Association of Official Analytical Chemists |
| AOH | Alternariol |
| BWG | Body Weight Gain |
| CD | Crypt depth |
| cP | Centipoise |
| DDGS | Distiller’s dried grains with solubles |
| DON | Deoxynivalenol |
| DON-3-G | Deoxynivalenol-3-Glucoside |
| ENNB+B1 | Enniatins B and B1 |
| EU | European Union |
| FCR | Feed conversion ratio |
| FI | Feed intake |
| FOS | Fructo-oligosaccharides |
| LD | Low deoxynivalenol |
| LSD | Least significant difference |
| MD | Moderate deoxynivalenol |
| NSP | Non-starch polysaccharides |
| OTA | Ochratoxin A |
| PAS | Periodic Acid–Schiff |
| VH | Villus height |
| VH:CD ratio | Villus height:Crypt depth ratio |
| ZEN | Zearalenone |
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6651/13/2/170/s1, Table S1. Composition of the experimental diets.
Author Contributions
Conceptualization: R.R.S. and E.v.E.; Funding acquisition: R.R.S. and E.v.E.; Investigation and Project administration: R.R.S.; Validation: R.R.S. and E.v.E.; Writing original draft: R.R.S. and E.v.E.; Writing review & editing: R.R.S. and E.v.E. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by ABZ Diervoeding B.V., De Hoop Mengvoeders B.V., Hankkija Oy, and Vitelia Voeders B.V. The authors would like to thank Andre de Ruijter, Eija Valkonen, Erja Koivunen, Gerard Raedts, Jacco Vessies, and Tom Lemmens for supporting the project.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of SFR (AVD246002016450), approval date: 28 February 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
This study evaluates the effect of low (900 ppb) and moderate (2300 ppb) levels of deoxynivalenol (DON) on the performance and intestinal integrity of broilers fed a diet with or without activated charcoal as a toxin binder. Impaired performance was observed in broilers fed a diet containing 2300 ppb DON for 28 days. Although the replacement of the contaminated diet by a diet marginally contaminated with DON mitigated the negative effect of DON after 1 week, the losses related to the complete production period were not recovered. Furthermore, 2300 ppb DON negatively affected intestinal morphology.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pinotti L., Ottoboni M., Giromini C., Dell’Orto V., Cheli F. Mycotoxin contamination in the EU feed supply chain: A focus on cereal byproducts. Toxins. 2016;8:45. doi: 10.3390/toxins8020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magnoli A.P., Poloni V.L., Cavaglieri L. Impact of mycotoxin contamination in the animal feed industry. Curr. Opin. Food Sci. 2019;29:99–108. doi: 10.1016/j.cofs.2019.08.009. [DOI] [Google Scholar]
- 3.Antonissen G., Van Immerseel F., Pasmans F., Ducatelle R., Haesebrouck F., Timbermont L., Verlinden M., Janssens G.P.J., Eeckhaut V., Eeckhout M., et al. The mycotoxin deoxynivalenol predisposes for the development of Clostridium perfringens-induced necrotic enteritis in broiler chickens. PLoS ONE. 2014;9:e108775. doi: 10.1371/journal.pone.0108775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Commission Recommendation 2016/1319/EC of 29 July 2016 amending Commission Recommendation 2006/576/EC on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union. 2016;L208:58–60. [Google Scholar]
- 5.Grenier B., Dohnal I., Shanmugasundaram R., Eicher S.D., Selvaraj R.K., Schatzmayr G., Applegate T.J. Susceptibility of broiler chickens to coccidiosis when fed subclinical doses of deoxynivalenol and fumonisins—special emphasis on the immunological response and the mycotoxin interaction. Toxins. 2016;8:231. doi: 10.3390/toxins8080231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tellez G., Latorre J.D., Kuttappan V.A., Hargis B.M., Hernandez-Velasco X. Rye affects bacterial translocation, intestinal viscosity, microbiota composition and bone mineralization in turkey poults. PLoS ONE. 2015;10:e0122390. doi: 10.1371/journal.pone.0122390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayres V.E., Broomhead J.N., Li X., Raab R.M., Moritz J.S. Viscosity and growth response of broilers fed high fiber diets supplemented with a corn-produced recombinant carbohydrase. J. Appl. Poultry Res. 2019;28:826–836. doi: 10.3382/japr/pfz039. [DOI] [Google Scholar]
- 8.Girgis G.N., Barta J.R., Girish C.K., Karrow N.A., Boermans H.J., Smith T.K. Mycotoxins and an organic mycotoxin adsobent on immune cell dynamics in the jejunum of chickens infected with Eimeria maxima. Vet. Immunol. Immunopathol. 2010;138:218–223. doi: 10.1016/j.vetimm.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Noack S., Chapman H.D., Selzer P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019;118:2009–2026. doi: 10.1007/s00436-019-06343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raza A., Bashir A., Tabassum R. An update on carbohydrases: Growth performance and intestinal health of poultry. Heliyon. 2019;5:e01437. doi: 10.1016/j.heliyon.2019.e01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierron A., Mimoun S., Murate L.S., Loiseau N., Lippi Y., Bracarense A.P.F.L., Liaubet L., Schatzmayr G., Berthiller F., Moll W.D., et al. Intestinal toxicity of the masked mycotoxin deoxynivalenol-3-β-glucoside. Arch. Toxicol. 2016;90:2037–2046. doi: 10.1007/s00204-015-1592-8. [DOI] [PubMed] [Google Scholar]
- 12.Broekaert N., Devreese M., van Bergen T., Schauvliege S., De Boevre M., De Saeger S., Vanhaecke L., Berthiller F., Michlmayr H., Malachova A., et al. In vivo contribution of deoxynivalenol-3-β-D-glucoside to deoxynivalenol exposure in broiler chickens and pigs: Oral bioavailability, hydrolysis and toxicokinetics. Arch. Toxicol. 2017;91:699–712. doi: 10.1007/s00204-016-1710-2. [DOI] [PubMed] [Google Scholar]
- 13.Fraeyman S., Croubels S., Devreese M., Ducatelle R., Rychlik M., Antonissen G. Chronic dietary intake of enniatin B in broiler chickens has low impact on intestinal morphometry and hepatic histology, and shows limited transfer to liver tissue. Toxins. 2018;10:45. doi: 10.3390/toxins10010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avantaggiato G., Havenaar R., Visconti A. Evaluation of the intestinal absorption of deoxynivalenol and nivalenol by an in vitro gastrointestinal model, and the binding efficacy of activated charcoal and other adsorbent materials. Food Chem. Toxicol. 2004;42:817–824. doi: 10.1016/j.fct.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Devreese M., Antonissen G., De Backer P., Croubels S. Efficacy of active carbon towards the absorption of deoxynivalenol in pigs. Toxins. 2014;6:2998–3004. doi: 10.3390/toxins6102998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osselaere A., Devreese M., Goossens J., Vandenbroucke V., De Baere S., De Backer P., Croubels S. Toxicokinetic study and absolute oral bioavailability of deoxynivalenol, T-2 toxin and zearalenone in broiler chickens. Food Chem. Toxicol. 2013;51:350–355. doi: 10.1016/j.fct.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Lucke A., Doupovec B., Paulsen P., Bohm Q.Z. Effects of low to moderate levels of deoxynivalenol on feed and water intake, weight gain, and slaughtering traits of broiler chickens A. Mycotoxin Res. 2017;33:261–271. doi: 10.1007/s12550-017-0284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng P.Y., Kim W.K. Review: Roles of prebiotics in intestinal ecosystem of broilers. Front. Vet. Sci. 2018;5:245. doi: 10.3389/fvets.2018.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dänicke S., Valenta H., Matthes S. On the interactions between Fusarium toxin-contaminated wheat and nonstarch polysaccharide hydrolyzing enzymes in diets of broilers on performance, intestinal viscosity, and carryover of deoxynivalenol. Poult. Sci. 2007;86:291–298. doi: 10.1093/ps/86.2.291. [DOI] [PubMed] [Google Scholar]
- 20.Smulikowska S., Mieczkowska A., Nguyen C.V., Babelewska M. The influence of digesta viscosity on the development of the stomach, on in vitro small intestinal motility and on digestion of nutrients in broiler chicken. J. Anim. Feed Sci. 2002;11:683–694. doi: 10.22358/jafs/67925/2002. [DOI] [Google Scholar]
- 21.Santos R.R., Molist F. Effect of different dietary levels of corn naturally contaminated with DON and its derivatives 3+15 Ac-DON and DON-3-glucoside on the performance of broilers. Heliyon. 2020;6:e05257. doi: 10.1016/j.heliyon.2020.e05257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolawole O., Graham A., Donaldson C., Owens B., Abia W.A., Meneely J., Alcorn M.J., Connolly L., Elliott C.T. Low doses of mycotoxin mixtures below EU regulatory limits can negatively affect the performance of broiler chickens: A longitudinal study. Toxins. 2020;12:e433. doi: 10.3390/toxins12070433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z.R., Hu C.H., Xia M.S., Zhan X.A., Wang M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- 24.Awad W.A., Ghareeb K., Abdel-Raheem S., Böhm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broilers chickens. Poult. Sci. 2009;88:49–56. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- 25.Potten C.S. Stem cells in the gastrointestinal epithelium: Numbers, characteristics and death. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1998;353:821–830. doi: 10.1098/rstb.1998.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheat S., Gerez J.R., Cognie J., Alassane-Kpembi I., Bracarense A.P.F.L., Raymond-Letron I., Oswald I.P., Kolf-Dlauw M. Nivalenol has a greater impact than deoxynivalenol on pig jejunum mucosa in vitro on explants and in vivo on intestinal loops. Toxins. 2015;7:1945–1961. doi: 10.3390/toxins7061945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osselaere A., Santos R.R., Hautekiet V., De Backer P., Chiers K., Ducatelle R., Croubels S. Deoxynivalenol impairs hepatic and intestinal gene expression of selected oxidative stress, tight junction and inflammation proteins in broiler chickens, but addition of an adsorbing agent shifts the effects to the distal parts of the small intestine. PLoS ONE. 2013;8:e69014. doi: 10.1371/journal.pone.0069014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos R.R., Awati A., Roubos-van den Hil P., Tersteeg-Zijderveld M.H.G., Koolmees P.A., Fink-Gremmels J. Quantitative histo-morphometric analysis of heat stress related damage in the small intestines of broiler chickens. Avian Pathol. 2015;44:19–22. doi: 10.1080/03079457.2014.988122. [DOI] [PubMed] [Google Scholar]
- 29.Dersjant-Li Y., Van de Belt K., Van der Klis J.D., Kettunen H., Awati A. Effect of multi-enzymes in combination with a direct-fed microbial on performance and welfare parameters in broilers under commercial production settings. J. Appl. Poult. Res. 2015;24:80–90. doi: 10.3382/japr/pfv003. [DOI] [Google Scholar]
- 30.Quaedackers J.S., Beuk R.J., Bennet L., Charlton A., Oude Egbrink M.G., Gunn A.J., Heineman E. An evaluation of methods for grading histologic injury following ischemia/reperfusion of the small bowel. Transplant Proc. 2000;32:1307–1310. doi: 10.1016/S0041-1345(00)01238-0. [DOI] [PubMed] [Google Scholar]
- 31.Santos R.R., Awati A., Roubos-van den Hil P., van Kempen T.A.T.G., Tersteeg-Zijderveld M.H.G., Koolmees P.A., Smits C., Fink-Gremmels J. Effects of a feed additive blend on broilers challenged with heat stress. Avian Pathol. 2019;48:582–601. doi: 10.1080/03079457.2019.1648750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable.