Abstract
EFSA assessed the role of seropositive wild boar in African swine fever (ASF) persistence. Surveillance data from Estonia and Latvia investigated with a generalised equation method demonstrated a significantly slower decline in seroprevalence in adult animals compared with subadults. The seroprevalence in adults, taking more than 24 months to approach zero after the last detection of ASFV circulation, would be a poor indicator to demonstrate the absence of virus circulation. A narrative literature review updated the knowledge on the mortality rate, the duration of protective immunity and maternal antibodies and transmission parameters. In addition, parameters potentially leading to prolonged virus circulation (persistence) in wild boar populations were reviewed. A stochastic explicit model was used to evaluate the dynamics of virus prevalence, seroprevalence and the number of carcasses attributed to ASF. Secondly, the impact of four scenarios on the duration of ASF virus (ASFV) persistence was evaluated with the model, namely a: (1) prolonged, lifelong infectious period, (2) reduction in the case‐fatality rate and prolonged transient infectiousness; (3) change in duration of protective immunity and (4) change in the duration of protection from maternal antibodies. Only the lifelong infectious period scenario had an important prolonging effect on the persistence of ASF. Finally, the model tested the performance of different proposed surveillance strategies to provide evidence of the absence of virus circulation (Exit Strategy). A two‐phase approach (Screening Phase, Confirmation Phase) was suggested for the Exit Strategy. The accuracy of the Exit Strategy increases with increasing numbers of carcasses collected and tested. The inclusion of active surveillance based on hunting has limited impact on the performance of the Exit Strategy compared with lengthening of the monitoring period. This performance improvement should be reasonably balanced against an unnecessary prolonged ‘time free’ with only a marginal gain in performance. Recommendations are provided for minimum monitoring periods leading to minimal failure rates of the Exit Strategy. The proposed Exit Strategy would fail with the presence of lifelong infectious wild boar. That said, it should be emphasised that the existence of such animals is speculative, based on current knowledge.
Keywords: African swine fever, domestic pig, epidemiology, freedom of infection, management, risk factor, seasonality, surveillance, wild boar
Summary
Term of Reference 1 (ToR 1) of the mandate of the European Commission requested EFSA to (1) clarify the risk factors possibly contributing to African swine fever (ASF) persistence in affected areas over a number of years in wild boar populations and (2) assess the role of seropositive wild boar in the context of ASF infection, and in particular in areas without evidence of recent virus circulation.
The first subquestion of ToR 1 (ToR 1.1) was related to the role of seropositive wild boar in ASF persistence, and specifically how ASF seroprevalence in the adult and subadult wild boar population evolves after the last detection of a polymerase chain reaction (PCR)‐positive sample. To address this question, surveillance data from Estonia, Latvia and Sardinia submitted to EFSA's data collection framework were investigated with a generalised equation method. The objective was to study the evolution of the seroprevalence in adult (≥ 1 year old) and subadult (< 1 year old) wild boar after the last detection of a PCR‐positive sample in a given local administrative unit in Latvia and Estonia. The model demonstrated a more rapid decrease towards zero seroprevalence among subadult animals compared with adult animals subsequent to the last detection of a PCR‐positive sample, both in Estonia and Latvia. The decline in seroprevalence in adult animals compared with subadults was much slower, taking more than 24 months to approach zero. For this reason, seroprevalence in adults is a poor indicator to demonstrate the absence of virus circulation. In Sardinia, a decline in virus and seroprevalence has been observed from 2015. In the Anglona‐Gallura subregion of Sardinia, ASF appears to have faded out as no PCR‐positive animals have been detected since 2015. Nonetheless, seropositive adult animals were detected as recently as January 2020.
Although not explicitly mentioned in ToR 1, during initial discussions with the European Commission, it was queried whether current surveillance activities would be able to reliably detect the presence of clusters of virus when virus prevalence is very low. This became the second subquestion of ToR 1 (ToR 1.2). The disease freedom methodology which considers different risk for the sampled subgroups (hunted and found dead animals) was used to estimate the combined confidence in disease freedom. It was assumed that the risk of finding ASF in found dead animals is 60 times higher than in hunted animals. Based on Estonian data, the current sampling intensities were found to be insufficient to detect infection in many Estonian Local Administrative Unit 1 (LAU 1) regions, based on the assumption of 1% disease prevalence and homogenous geographical distribution of infected animals. Instead, it was concluded that intensive sampling would be required to demonstrate the absence of virus circulation based on mainly active surveillance (hunted animals). The collection of the number of samples required to achieve at least 95% confidence in freedom from infection would probably be unfeasible under field conditions.
In this Opinion, a spatial‐explicit stochastic model has been used to test different surveillance strategies, based on available surveillance tools and achievable sampling efforts. A previously documented spatially explicit stochastic model (Grimm et al., 2006, 2010; Grimm, 2020) was chosen (e.g. modelling infectious diseases in wild boar at http://www.ecoepi.eu/ASFWB) for this purpose. In addition to standard testing of wild boar hunted or found dead, the potential inclusion of serological testing of young wild boar as an indicator of freedom from infection was also considered.
The third subquestion of ToR 1 requested an update of aspects of ASF epidemiology that are still subject to considerable scientific uncertainty (ToR 1.3), including the implications of these uncertainties for any conclusions drawn. This information is directly relevant to the stochastic models. In this context, a narrative literature review was conducted. Several relevant epidemiological attributes were identified including the mortality rate due to ASF, the duration of protective immunity and duration of maternal antibodies and transmission parameters.
The true mortality caused by ASF at the population level is difficult to estimate due to the occurrence of non‐ASF‐related mortality, such as hunting. Recent estimates from Poland and Latvia attributed around 80% of the mortality in the wild boar population to ASF. The case‐fatality rate due to ASF experimental infections of wild boar with ASFV genotype II strains is likely above 95%.
The duration of protective immunity in animals recovering from ASF has not been well studied and is considered a knowledge gap. Recent studies have demonstrated a lack of protection even 4 months post‐immunisation with attenuated ASFV strains. There was no clear correlation between protection and antibody levels. However, protection from clinical disease may still last for several months in animals recovering from the disease. Re‐infection of these animals, however, cannot be excluded.
The duration of maternal antibodies in piglets of sows surviving ASF is not known. According to the literature, the longest time that maternal antibodies against ASFV have been found in piglets is 7 weeks. However, true long‐term studies are missing. Maternal antibodies against other pig diseases such as Classical Swine Fever virus and porcine parvovirus have been shown to last up to 2–4 months, and up to 6 months for Aujeszky's disease virus. In all cases, some individuals will show antibodies for prolonged periods.
The transmission parameter estimates from experimental studies are dependent on the experimental setting and conditions. The use of differing humane endpoints (moment when euthanising the animals in animal experiments) is particularly relevant. The estimates from field studies are influenced by various factors that affect contact rates between animals, e.g. farm management. The point estimates for transmission parameters obtained in experimental conditions fall within a relatively narrow range (R0: 5.0–6.1). The parameters calculated based on field data are more variable, being lowest for ASFV genotype I in Sardinia (R0 ranging from 1.2 to 2.7) and highest for genotype II outbreaks in Russia (R0 ranging from 4.4 to 17.3). There are no experimental data on transmission of ASFV from infected carcasses to susceptible wild boar. The studies estimating R0 for wild boar are based on field data and incorporate the effect of all transmission routes. The transmission parameter estimates from field data are influenced by local conditions (e.g. population density and management of wild boar) and disease intervention measures, which all have an effect on contact rates between the animals and animal groups.
The fourth subquestion of ToR 1 (ToR 1.4) was related to parameters that could potentially lead to prolonged virus circulation (persistence) in wild boar populations in an affected area. This subquestion was addressed through a narrative literature review.
Firstly, possible hypotheses for persistence of ASFV in the environment were reviewed. African swine fever virus is known to be highly stable under a wide range of environmental conditions. Several modelling studies reported in the scientific literature demonstrated that more than half of all transmission events in wild boar populations are attributed to contact between live wild boar and infectious carcasses. The behaviour of wild boar towards dead conspecifics is likely to be one of avoidance, but with occasional contact of infectious material around dead animals. For this reason, carcass removal is considered an important control measure for ASF. Also, possible persistence of ASFV through biological and mechanical vectors was reviewed. Scavenger mammal and bird species represent a minor risk factor for spreading ASF in wild boar populations but may contribute to reducing local virus persistence by removing infected carcasses. Based on current knowledge, Ornithodoros spp., belonging to the Argasidae family of soft ticks, is the only tick genus that can be considered a competent vector that is able to replicate and transmit ASFV. Ticks of the O. erraticus complex are present in parts of the European, trans‐Caucasus countries and Russian Federation territories and may be important in maintaining the local foci of the ASFV within traditional pig management systems. However, they do not play an active role in the geographic spread of ASFV. Furthermore, European wild boars rest above the ground rather than in protected burrows, thereby reducing the opportunity for Ornithodoros spp. infestation. Ticks of the O. erraticus complex have not been reported from central or northern Europe.
ASFV DNA has been detected in some biting arthropods in outbreak farms in Lithuania and Romania. However, their potential role in the mechanical transmission in ASFV needs to be clarified. Specifically, conclusive evidence of their role in ASFV transmission will require consideration of virus isolation studies on arthropods caught on outbreak pig farms and laboratory experimental transmission studies; and to link this evidence with studies on arthropod foraging strategies and habitat use.
Secondly, factors relating to wild boar that could possibly contribute to ASFV persistence were reviewed. The last decade of ASF in Europe has demonstrated that ASFV can persist in wild boar populations without re‐infection from domestic pigs. Viral persistence in wild boar populations is influenced by both host and environmental factors. Direct transmission between live wild boar is primarily to other individuals within the same social group. Furthermore, habitat quality is important, and the presence of large, well‐connected forests favours unrestricted wild boar movement and contact. At higher boar densities, there is increased potential for direct transmission because of increased within‐group contacts, and indirect transmission through contact of wild boar with infected carcasses and contaminated environments. As wild boar density falls, viral persistence is likely to be facilitated by viral survival in infectious carcasses. There is no evidence of a population density threshold for spontaneous ASF fade‐out. The potential role of surviving infectious animals in long‐term transmission is still controversial. Although virus can be isolated from survivors for roughly 60–70 days following initial infection, there is no evidence of a major role for these long‐term infectious animals in maintaining virus circulation from either field experience or long‐term studies.
Thirdly, the review focused on possible factors that are intrinsic characteristics of the virus that could contribute to virus persistence in wild boar populations. The ASFV strains in the current European epidemic belong to the p72 genotype II. These strains are usually highly virulent, inducing an acute form of ASF with a case‐fatality rate approaching 95%, regardless of age, dose or route of administration. There have been several examples of naturally occurring, attenuated genotype II strains during the current epidemic, in Estonia and Latvia, but they appear to have disappeared from the wild boar populations, possibly due to their reduced ability to generate infectious carcasses. Circulation of genotype I in Europe is limited to Sardinia, following introduction in 1978. The genotype I strains circulating in Sardinia have always been associated with high virulence. In recent years, however, the virus was isolated from apparently healthy pigs. The presence of less virulent ASFV strains in Sardinia has never been confirmed, although the field observations are highly suggestive.
Finally, human‐induced factors that could lead to virus persistence were reviewed. Although the spread of ASF in wild boar populations can continue without re‐infection from domestic pigs, there are some examples of spillover from domestic pigs to wild boar, such as introduction and spread into a country through indirect contacts with infected meat or products in Europe. The risk related to infected meat and products from domestic pigs and wild boar is often associated with illegal movements of such products or with small free‐ranging backyard farms where animals are illegally fed with untreated food leftovers or catering waste. Human activity remains an important contributor to both ASF persistence and expansion in wild boar populations, including hunting activities with poor biosecurity. In the current epidemic, there have been multiple examples of long‐distance translocations of infection, which could plausibly only be related to human activity.
Term of Reference 2 (ToR 2) of the mandate requested EFSA to define pathway(s) to ASF freedom in relevant areas, in accordance with the Strategic approach to the management of African Swine Fever for the EU and recommend criteria for defining an area as free from ASF in wild boar. In this task, EFSA was asked to take account of the results of wild boar testing (in particular, antibody detection and virus identification).
As a first step, a spatial‐explicit stochastic model was used to simulate the spread of ASF in Estonia, based on surveillance data submitted to EFSA's data collection framework, generating the temporal dynamics of virus prevalence, seroprevalence and the number of carcasses attributed to ASF infection throughout the epidemic in the wild boar population at the scale of the local administrative unit 1 (LAU 1). The area covered by LAU 1 units varied between less than 1,000 and 5,000 km2. Throughout the ASF epidemic, a low virus prevalence was observed with a median of about 2% at the peak of epidemic (1–4% as central 50% interval), and the virus prevalence was very low during the 6 months prior to virus extinction in an LAU 1 region in Estonia (median virus prevalence below 0.5% with 0.1–2% as central 50% interval). The median seroprevalence in subadults declined to 0% within 1 year (9–18 months as central 50% interval) after local extinction of ASFV in an LAU 1 region in Estonia, whereas the same decline in adults took more than 3 years. The median number of wild boar dying because of ASF was around 150 carcasses per LAU 1 at the peak of epidemic (100–300 central 50% interval across runs and LAU 1 units) and 1 year before local extinction about 40 carcasses (10–150 central 50% interval across runs and LAU 1 units).
As a second step, the model was used to test the impact of those attributes contributing to ASF epidemiology that could potentially contribute to prolonged virus circulation (persistence) in wild boar populations in an affected area. Specifically, four scenarios were evaluated, including: (1) the potential existence of wild boar with prolonged infectious period (carriers) (scenario 1); (2) a reduction in the case‐fatality rate and a lengthened period of transient infectiousness among surviving animals (scenario 2); (3) a change in the duration of protective immunity among animals surviving ASFV infection (scenario 3); and a change in the duration of protection from maternal antibodies on the duration of virus circulation (scenario 4).
In scenario 1, there was a more marked difference in the serological profile of subadult compared with adult animals with an increasing proportion of carriers involved. The seroprevalence in subadults was lower than in adults and the decline in seroprevalence much slower in the years prior to regional extinction, as the proportion of carriers increases. Furthermore, carcass numbers attributable to ASF were lower and the decline in carcass numbers much slower in the years prior to regional extinction, as the proportion of carriers increases. In scenario 2, variation in case‐fatality alone did not substantially impact the duration of virus circulation, given transient infectiousness of about 1 week among surviving animals. There was an impact on duration of virus circulation when the duration of transient infectiousness among surviving animals was increased to 4 weeks, however, final fade‐out was only marginally affected. For scenarios 3 and 4, the impact on duration of virus circulation was minimal.
As a third step, the model was used to test the performance of different proposed surveillance strategies that could be implemented to provide evidence of the absence of virus circulation (Exit Strategy). To make sure the Exit Strategy would be feasible to implement in the field, different combinations of duration and intensity of existing surveillance tools (active surveillance based on hunting and passive surveillance based on wild boar found dead) were tested in several iterations of the stochastic model. For the active surveillance, only testing on the subadult wild boar was included in these iterations, as it was already shown that inclusion of serology of adult wild boar would be poor indicator to demonstrate the absence of virus circulation, since it would take up to 3 years before seropositive wild boar would disappear from the population after the virus was eliminated.
After these first iterations of the stochastic model, which are reported in the External Scientific Report (Lange et al., 2021), it became evident that as a general principle, a two‐phase approach (Screening Phase, Confirmation Phase) would be advisable for the Exit Strategy, based on knowledge of virological and serological prevalence profiles. Further model simulations evaluated different Exit Strategy options, which varied by surveillance options and intensity and the length of the monitoring period during each phase. Each option was assessed in terms of performance (failure rate, being the per cent of simulations for which it was falsely concluded that virus is absent) and ‘time free’ (the time lag between point of viral extinction and time when an exit decision is possible).
It was demonstrated that the accuracy of the Exit Strategy approach to demonstrate freedom of ASFV circulation in a wild boar population increased with an increasing number of carcasses being routinely collected and tested. However, the Exit Strategy will only be feasible if the duration and intensity of the passive surveillance can be sustained under field conditions. To increase the feasibility of the Exit Strategy approach, a longer monitoring phase with routine surveillance effort (the Screening Phase) and a shorter monitoring phase with the maximum surveillance possible under field conditions (the Confirmation Phase) is proposed. Lengthening of the monitoring periods leads to an improvement in Exit Strategy performance; however, this performance improvement should be reasonably balanced against an unnecessary prolonged ‘time free’ with only a marginal gain in performance of the Exit Strategy.
In general, the inclusion of active surveillance in the Exit Strategy has very limited impact on the performance compared with a lengthening the overall monitoring period. A declining seroprevalence in subadults can add information about the fade‐out of the epidemic and trigger the decision to initiate the Exit Strategy, however including this surveillance activity during the Exit Strategy only marginally improves its performance.
Furthermore, it was demonstrated by the model that the scenario based on a decreased case‐fatality rate, with surviving animals having a longer (but still transient) period of infectivity, would not influence the outcomes of the Exit Strategy approach. In contrast, the proposed Exit Strategy would fail with the presence of lifelong infectious carrier animals. That said, it should be emphasised that the existence of such carriers is speculative, based on current knowledge.
Assuming a higher natural mortality that is not caused by ASF or hunting in the model, reduced the probability of finding infected carcasses in an affected area and therefore reduced the performance of passive surveillance. This is due to the dilution effect for detecting infected carcasses by the increased proportions of carcasses of wild boar that died due to reasons other than ASF. Therefore, a more cautious approach may be advisable in those regions where the natural mortality rates are uncertain or known to be higher than the assumed 10% natural mortality that is not caused by ASF or hunting.
Based on the model outcomes, several practical examples of an Exit Strategy, both for large affected areas and for smaller areas after a focal introduction of ASF were provided.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
ASF is an infectious lethal disease affecting domestic pigs and wild boar. It can be transmitted via direct animal contact or via dissemination of contaminated food or equipment. This disease has serious economic implications for pig meat and related sectors, including indirect costs related to trade restrictions. The persistence of the disease in wild boar and the limited number of control measures available represents a challenge for the whole EU agricultural sector, in particular the pig farming industry. There is no vaccine or cure despite active ongoing research.
From the beginning of 2014 until now, genotype II of ASF has been notified in Belgium, Bulgaria, the Czech Republic, Estonia, Greece, Latvia, Lithuania, Poland, Romania and Slovakia causing very serious concerns. The disease has also been reported in Belarus, Moldova, Serbia, Russia and Ukraine, which creates a constant risk for all the Member States that share a border with these third countries. In Italy (Sardinia only) genotype I of ASFV has been present since 1978 in domestic pigs and wild boar. The remainder of Italy remains free of the disease. The entire island is considered as part IV in terms of regionalisation, the only area in the EU subject to this type of restriction at present.
Member States and the Commission are continuously updating the ‘Strategic approach to the management of African Swine Fever for the EU’ and the related legislation. There is knowledge, legislation, scientific, technical and financial tools in the EU to effectively tackle ASF.
Active surveillance in wild boar consists of hunting and testing healthy wild boar. It mainly aims to measure the number of infected animals over the susceptible population to assess changes in the prevalence of infection. According to EFSA's ‘epidemiological analyses of African swine fever in the EU’ published on 30 January 2020 (hereafter ‘the latest EFSA report’), active surveillance can provide indications on the effectiveness of the ASF control measures on the prevalence of infected animals and pave the way to the design of an ‘Exit Strategy’.
The latest and previous EFSA reports give a general priority to passive surveillance over active surveillance. Enhanced passive surveillance systems should be first in place to ensure timely detection of ASF. However, the latest EFSA report reported also that ‘in areas where ASF has been present in the wild boar population for more than 1 year, active surveillance is the suited approach to monitor the effect of interventions on the prevalence of infected animals and building evidence to regain ASF‐free status’.
To explore the options for an ‘Exit Strategy’, the Commission intends to mandate EFSA to evaluate all the necessary elements and specific measurement of prevalence (through active and passive surveillance).
1.1.2. Terms of Reference (ToR)
In accordance with Article 29 of Regulation (EC) No 178/2002, EFSA is requested to provide a Scientific Opinion to address the following questions:
Specific to Estonia and Latvia, EFSA should clarify: i) the risk factors possibly contributing to ASF persistence in affected areas over a number of years in wild boar populations; and ii) the role of seropositive wild boar in the context of ASF infection, and in particular in areas with no current evidence of virus circulation.
EFSA should define pathway(s) to ASF freedom in relevant areas, in accordance with the Strategic approach to the management of African Swine Fever for the EU and recommend criteria for defining an area as free from ASF in wild boar. In this task, EFSA should take into account the results of wild boar testing (in particular, antibody detection and virus identification) and the results in relation to the identification of wild boar carcasses (with differing time since death).
1.2. Interpretation of the Terms of Reference
ToR 1: Specific to Estonia and Latvia, EFSA should clarify: (i) the risk factors possibly contributing to ASF persistence in affected areas over a number of years in wild boar populations; and (ii) the role of seropositive wild boar in the context of ASF infection, and in particular in areas with no current evidence of virus circulation.
During discussions at the kick‐off meeting, the expectations from the European Commission were further specified. EFSA should consider a situation where there is no evidence of virus circulation (i.e. no PCR‐positive test results and only seropositive animals found in an area) in the context of the current approaches of passive and active surveillance, and with this situation in mind, ToR 1 was split into four subquestions:
Subquestion 1: What is the role of the seropositive wild boar in ASF persistence, after a long period without PCR‐positive results? This was further narrowed down into: How does ASF seroprevalence in the adult and subadult wild boar subpopulation evolve after the last detection of a PCR‐positive sample?
Subquestion 2: How reliable are the surveillance results from Latvia and Estonia for the purpose of demonstrating the absence of virus circulation? Although not specifically mentioned in the ToR 1, it was suggested by the requester of this mandate during the first working group meeting that Sardinia is included in the list of countries to be considered in this task.
Subquestion 3: Are there updates on aspects of ASF epidemiology that are still subject to considerable scientific uncertainty?
Subquestion 4: Which factors could potentially lead to prolonged virus circulation (persistence)?
Figure 1 displays these four subquestions of ToR 1, the data sources and methods used to address them and the sections in this Opinion where this is reported.
Figure 1.
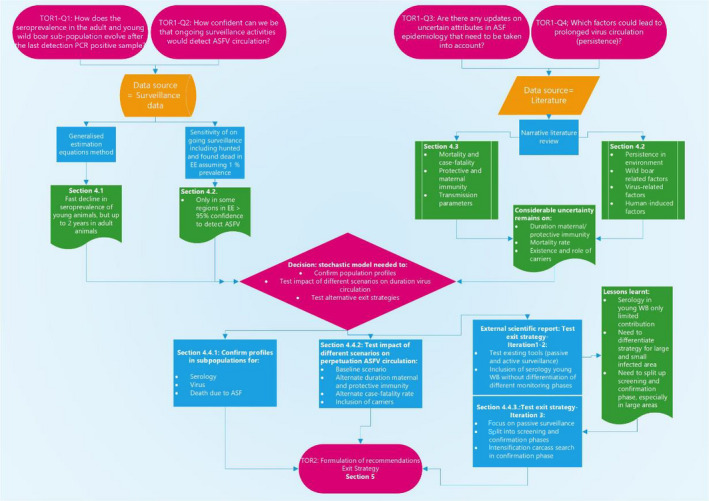
Flow chart displaying terms of references, subquestions, data sources and assessment sections
To address subquestion 1 and subquestion 2, surveillance data from Estonia, Latvia and Sardinia were investigated. Firstly, the wild boar surveillance data from Estonia, Latvia and Sardinia were analysed using a general estimation equation methodology, to study temporal trends in the proportions of seropositive samples in the year immediately prior to and following the last PCR‐positive observation in the country, to get a better understanding of this phase of the epidemic. For instance, a gradual decline in the number of seropositive samples in the year after the last PCR‐positive sample could indicate the fading out of infection in the population, whereas the observation of an oscillating seroprevalence curve would indicate ongoing virus circulation.
In addition, seroprevalence in young and adult animals was studied separately, as higher seroprevalence in the adult age class would indicate fade out of infection in the population. It is known that surviving wild boar will remain seropositive for the remaining time of their life, and that a significantly lower seroprevalence in young wild boar would be suggestive of no new recent infection. These exploratory analyses of the field surveillance data were carried both for data pooled at both a country and regional level, to see if there was any regional difference in the progression of the epidemic.
To address the second subquestion of ToR 1, the sensitivity of ongoing surveillance including hunted and found dead was calculated for the surveillance data from Estonia, assuming 1% prevalence.
To address the third subquestion of ToR 1, a literature review was be conducted by working group experts to provide an update on all the relevant epidemiological attributes of ASFV, which are still associated with a high uncertainty and could have an impact on the possible outcomes of surveillance activities to demonstrate freedom of virus circulation. This could be the mortality rate in affected wild boar populations, the proportion of infected wild boar surviving an infection, the presence of infectious carriers, the duration of protective immunity and maternal antibodies and direct and indirect transmission rates. The outcomes of the review will inform a spatial‐explicit stochastic model, which will be the third component to address in this ToR.
To address the fourth subquestion of ToR 1 also a narrative literature review was performed to study if there are any scientific updates on potential risk factors that can lead to persistence of ASFV in wild boar populations, including parameters related to wild boar density or population structure, the virulence of the ASFV strain, the duration of virus survival in the environment or the presence and/or role of soft ticks. This review also included knowledge of any possible virus shedding by seropositive animals that have survived the infection, and the role of seropositive animals in the epidemiology.
ToR 2: EFSA should define pathway(s) to ASF freedom in relevant areas, in accordance with the Strategic approach to the management of African Swine Fever for the EU and recommend criteria for defining an area as free from ASF in wild boar. In this task, EFSA should take into account the results of wild boar testing (in particular, antibody detection and virus identification) and the results in relation to the identification of wild boar carcasses (with differing time since death).
Also, for this ToR, the Expectation from the European Commission was further clarified. Firstly, it was agreed that proving freedom from ASFV infection should be interpreted as to provide evidence to substantiate the absence of ASFV circulation. It was expected that EFSA would provide scientifically reliable, but also practically implementable, tools to enable MSs to provide evidence to substantiate the absence of ASFV circulation in wild boar populations and to support the revision of regionalisation towards totally or partially lifting restrictions.
EFSA was required to evaluate if ongoing surveillance activities are sufficient to provide evidence to substantiate the absence of virus circulation and support revision of regionalisation, and to suggest how these surveillance activities could possibly be improved, taking into account the outcomes of ToR 1.
Key points that had to be taken into account when developing a surveillance strategy to substantiate the absence of disease:
-
1
Size of spatial unit, defined (smaller) geographic areas.
During the final phase of the epidemic, ASF can persist in small localised clusters, with a prevalence pattern that is not geographically homogeneous. Consequently, an administrative approach focusing on large geographic regions (e.g. relying on sample size calculations based on a prevalence threshold across a country) will not be sufficient for virus detection, unless impractically high samples sizes are used. It is possible to calculate sample size requirements for smaller geographic (sub)regions.
-
2
Output‐based criteria achievable in the field.
The Exit Strategy should integrate output‐based criteria, based on surveillance activities that rely on sampling intensities that are achievable in the field through a sustainable hunting intensity (active surveillance) and detection of carcasses of dead animals (passive surveillance).
-
3
Sampling approach, guided by population management.
Seropositive animals will remain positive throughout life. Therefore, the frequency and timing of sampling for seroprevalence are less important. For virus detection, in contrast, the frequency of sampling is very important. Hence, it is expected that surveillance strategies to detect viral presence will be different to those that seek to clarify seroprevalence.
It was decided that, based on the results from the literature reviews and the analysis from the surveillance data, a stochastic model would be needed to address ToR 2, to:
confirm the dynamic profiles in the subpopulation for the serology, virology and number of carcasses generated due to the disease in the population;
test the impact of the different scenarios for each uncertain attribute in the ASF epidemiology (carriers, immunity, reduced case‐fatality rate) on the perpetuation of ASFV in wild boar populations;
test different Exit Strategy opinions, to justify the formulation of the most optimal Exit Strategy criteria.
2. Data
2.1. African swine fever surveillance data from Estonia, Latvia and Sardinia
Data from the wild boar surveillance (PCR and serological test results) were submitted to EFSA's data collection framework (DCF) and were used to estimate the apparent seroprevalence in the regions in the countries. The data used for the analysis included the LAU 1 region, the sampling date, sample matrix, age class, sex and the PCR and serological result. Data were available from surveillance activities carried out since the first incursion in 2014 throughout the year in Estonia and Latvia. In Sardinia, surveillance data from 2014 were submitted to the DCF which were collected mostly during hunting activities in the winter period (from November to January).
3. Methodologies
3.1. Exploration of surveillance data
3.1.1. Estonia and Latvia
The data submitted to EFSA's DCF for the different LAU 1 regions in Latvia and Estonia were explored and the data pertaining the serological results of the samples tested from 1 year before the last detection of a PCR‐positive sample in an LAU 1 region until present were analysed.
For Latvia, all ELISA‐positive samples were tested by either one of the confirmatory immunoblotting (IB) test or immunoperoxidase test (IPT) and the test results of these confirmatory tests were used as the results of the serology test.
For Estonia, in some occasions, the ELISA‐positive results of the confirmatory tests were not reported, and then, the ELISA test results were used as the outcome of the serology tests. This may have caused overestimation of the seroprevalences for Estonia as 5% of ELISA‐positive test results appear to be false positive in comparison with confirmatory test in Estonian data. However, this was considered acceptable for the purpose of this analysis taking into account that the ELISA test is somewhat of lower sensitivity compared with the IPT test (Gallardo et al., 2015). A map showing the seroprevalence (proportions of seropositive samples) was provided. The different regions were pooled to study the temporal evolution considering time 0 as the last date at which a PCR‐positive result was reported in each region. A histogram of the frequency of samples tested by ELISA for all LAU 1 regions from the last PCR‐positive report was presented, differentiating between two age classes (young, < 1 year and adult, > 1 year).
The general estimation equation (GEE) model (considering Binomial family) (Agresti, 2019) was used to estimate the seroprevalence dynamics for both age groups. The analysis considered the year before the last PCR positive and the 2 years after, including restricted cubic spline (Durrleman and Simon, 1989) time effect. The analyses presented in this report are based on the exchangeable working correlation structure given that seroprevalence estimated based on different working correlation structures were similar and it is known that estimations based on the exchangeable correlation structure have an appropriate marginal interpretation in the case of informative cluster sizes (Williamson et al., 2003). Analyses were performed in R (version 3.5.3), using the geepack package to fit the models (Yan, 2002; Yan and Fine, 2004; Højsgaard et al., 2006) and the MuMIn package for model selection (Barton, 2018).
As the focus was on the population temporal behaviour of animals in the two age classes, the model included an effect associated with the two age classes as well as a restricted cubic spline temporal effect and their interaction.
The model fitted for both periods (before and after) included the interaction term between the time effect and the AGE group of the animal, the model considering the interaction and the ones without considering the AGE group effect were fitted using GEE and the respective quasi‐Akaike information criterion (QIC) was computed. The model with the smallest QIC (Pan, 2001) was chosen to represent a better fitting model.
3.1.2. Sardinia
The data submitted to EFSA's DCF for the different LAU 1 regions in Sardinia were explored, and analysis was conducted on serological results from samples that were tested more than 1 year after the last detection of a PCR‐positive sample in an LAU 1 region in Anglona‐Gallura in 2015).
As all the samples tested for ASFV antibodies by ELISA were confirmed by confirmatory test (IB or IPT) since the beginning of the introduction of ASF in Sardinia, the IB/IPT test results were used for the analysis. Virological and serological ASF trends in Sardinia were evaluated and a map showing the seroprevalence (proportions of seropositive samples) was provided. The Anglona‐Gallura subregion was chosen as reference, since this is the wild boar management unit with the longest period without any PCR‐positive test result (in this management unit, the most recent positive PCR test result was reported in 2015). Considering time 0 as the last date at which a PCR‐positive result was reported in Anglona‐Gallura, a histogram of the frequency of samples tested by IB/IPT from the last PCR‐positive report was presented, differentiating between two age classes (young, < 1 year and adult, ≥ 1 year). It should be noted that most of the Sardinian surveillance data were generated during active surveillance, i.e. during hunting activities. The passive surveillance samples are mainly from animals that died during road accidents, as no enhanced carcass searching is ongoing, and this resulted in several months (i.e. nine) of few data between hunting seasons. Therefore, only trends in the prevalence over a longer period (several years) could be investigated and performing the GEE analysis since the last PCR‐positive sample would require more frequent sampling efforts distributed all over the year and was therefore not performed.
3.2. Sensitivity of surveillance activities in Estonia
Information submitted to the DCF by Estonia was used to estimate the confidence of the surveillance system in place to detect ASF at an assumed 1% design prevalence, considering sampling effort per year and LAU 1 in the two groups, hunted and found dead wild boar. For each LAU 1, the date of the last PCR positive was identified, and the number of samples taken in 52‐week intervals starting from that date was counted. The area of each LAU 1 was calculated and the total wild boar population in each LAU 1 was estimated as the area in square km multiplied by 0.3 (considering estimated post‐farrowing population size based on hunting bag). A test sensitivity of 100% was assumed to estimate the overall confidence.
The disease freedom methodology considering different risk for the subgroups (see section 2.3 in EFSA, 2012) was used to estimate the combined confidence in disease freedom. It was assumed that the probability of finding ASF in found dead is 60 times higher than in hunted animals and the proportion of found dead that are expected to be found in the area is 1% of the total population of wild boar (WB) in the area and the rest are included in the other subgroup.
3.3. Narrative literature review
3.3.1. Literature review on potential risk factors possibly contributing to ASF persistence in affected areas in wild boar populations
Type of literature review: A narrative literature review was carried out to identify possible parameters that could have a prolonging effect on the duration of virus circulation in wild boar in an affected area.
Review question: What are the possible epidemiological, environmental, management and demographic parameters that contribute to prolongation of the time of circulation of ASFV in wild boar?
Search keywords: virus circulation, persistence, duration, model, ASFV detection, wild boar.
Relevance criteria: Does the paper study a possible impact on the duration of the ASFV circulation in wild boar?
The expected outcomes of the review were to:
identify the parameters that are hypothesised in literature to prolong the circulation ASFV in wild boar in an area;
describe the underlying mechanism;
describe and appraise the evidence based on the presence of experimental/field evidence provided in the papers.
3.3.2. Literature review on epidemiological attributes of African swine fever virus genotypes I and II that have still a high uncertainty
Type of literature review: A narrative literature review was carried out with the objective to update the parameters of the spatially explicit, stochastic, individual‐based demographic model and to identify scenarios for possible mechanism prolonging circulation of ASFV in wild boar populations.
Which attributes will be reviewed: The identification of the parameters and mechanistic scenarios was based on the fulfilment of at least one of the following criteria:
Parameters for which a gap in knowledge was identified in the model documentation (refer to the ODD protocol annexed to Lange et al., 2018) or which were considered uncertain due to limited knowledge or difficulties in generating field evidence (see also Section 3.3).
-
Scenarios representing plausible mechanisms leading to prolonged circulation of the infection in an affected wild boar population):
-
–
case‐fatality rate
-
–
presence and duration of ‘surviving infectious animals with long‐term transmission
-
–
duration of protective immunity and maternal antibodies
-
–
direct transmission rate and indirect transmission rate.
-
–
The case‐fatality rate in pig or wild boar due to experimental inoculation with ASFV genotype II field strains from Europe was estimated through an extensive literature review (ELR). The literature review protocol published by Dórea et al. (2017) has been followed.
Time period to be covered: The literature review was restricted to identify only more recent studies than already summarised in Table 2 of the model documentation i.e. the Overview, Design and Details (ODD) protocol (Lange et al., 2018).
Table 2.
Quasi‐Akaike information criterion of models before and after the last PCR‐positive detection in area for two age classes
| Before last PCR positive | After last PCR positive | |
|---|---|---|
| Age included | 2,770.63 | 1,424.80 |
| Age not included | 2,824.77 | 1,507.08 |
3.4. Spatially explicit stochastic model
A stochastic spatially explicit individual‐based model was developed to understand the impact of different epidemiological scenarios on the course of an ASF outbreak in wild boar populations. The main model outcomes reported for analysis addressed the duration of circulating infection in a geographical area, the population size, the proportion of virologically and serologically positive animals and the number of carcasses from animals that had succumbed to ASF infection, each over 20 years after ASFV introduction.
3.4.1. The model objectives
-
To compare the duration of circulating infection in simulated wild boar populations in Estonia based on alternative model scenarios that represent mechanisms potentially associated with prolonged virus circulation:
-
–
carrier animals
-
–
reduced case‐fatality rate and prolonged period of transient infectiousness among surviving animals
-
–
loss of protective immunity among surviving animals
-
–
duration of protection from maternal immunity.
-
–
To explore the temporal evolution of three parameters (virus prevalence, antibody prevalence particularly in subadults, the number of carcasses from animals that succumbed to ASF infection) during the period before and following local virus extinction in simulated wild boar populations in Estonia.
To test decision criteria, robust if possible, according to different assumptions regarding persistence mechanisms and epidemiological scenarios, that could be used to underpin stages of an Exit Strategy for ASF control. If the evaluation of general criteria is not possible, partial/specialised criteria according to distinct epidemiological scenarios should be addressed.
3.4.2. The model context
Model documentation and validity
The model is spatially explicit, mechanistic and individual based. Spatially explicit approaches are advised whenever the effectivity of control measures is of quantitative interest and explicit resource needs are considered. The model links individual animal behaviour to the strategic outcome of measures applied over thousands of square kilometres. This upscaling of expert knowledge about detailed processes to the level at which management evaluation is performed, is the main advantage over implicit modelling techniques. The observer level (landscape or population) is emergent of the detailed processes and their mechanistic interaction, i.e. infection status is testable per individual, as census or on a spatio‐temporal sample basis.
The model is fully documented following a recognised protocol to describe complex models (Grimm et al., 2006, 2010; Grimm, 2020). The ODD protocol is proposed to allow communication and reliable reconstruction of complex research models. The ODD protocol of the model is open access (e.g. Modelling infectious diseases in wild boar at http://www.ecoepi.eu/ASFWB). The documentation was proven sufficient to reconstruct the wild boar model by independent international academia (Halasa et al., 2019).
Here, we add information from the model documentation, which is important to be shared for models dedicated for decision‐making (POE; Grimm et al., 2020), i.e. overview of a model's purpose, its principle organisation and the evidence of being fit for purpose.
Purpose: The model aims at assessment of ASF spread in European wild boar populations and the evaluation of population structure and temporal profiles informing a possible surveillance strategy applied in areas affected by ASF towards the possible end of virus circulation.
Organisation: Entities and scales. The ASF wild boar model is a compilation of a spatially explicit, stochastic, individual‐based demographic model for wild boars (Sus scrofa) in a structured landscape of habitat patches (grid of core home ranges). Superimposed is a transmission and disease course model for the ASFV. The model comprises three entities: spatial habitat units, connecting edges between these units and wild boar individuals. All processes take place on a raster map of spatial habitat units. Each cell represents a functional classification of a landscape denoting habitat quality. The square cells of the model landscape represent 9 km2 (3 × 3 km), encompassing a wild boar group's core home range. At run time, habitat quality is interpreted as breeding capacity, i.e. the number of female boars that are allowed to have offspring (explicit density regulation). Habitat quality allows implementing an external data set of spatial wild boar density distribution. Habitat cells are connected by edges to the neighbouring eight cells. Connecting edges represent space between core habitat areas that is shared among neighbouring herds. Each habitat cell and each connecting edge may contain carcasses of locally succumbed wild boar to infection. The third model entities are the individual wild boars. State variables of host individuals are the position, the sex and the age in weeks, resulting in age classes: piglet (< 8 months ± 6 weeks), subadult (< 2 years ± 6 weeks) and adult. Age class transition event is stochastic in an interval range. Each host individual has a location, which denotes its home range cell on the raster grid as well as its family group. Furthermore, the individual host animal comprises an epidemiological status (susceptible, lethally infected, non‐lethally infected, immune after recovery or protected by transient maternal antibodies). Subadult wild boar may disperse during the dispersal period dependent on their demographic status (disperser or non‐disperser).
Processes and scheduling: The model proceeds in weekly time steps. Processes of each time step are performed as applicable: virus release, transmission events, dispersal of subadults, reproduction, ageing, mortality, hunting (for surveillance and depopulation) and control measures. Submodels are executed in this order. In the first week of each year, mortality probabilities are assigned stochastically to the age classes representing annual fluctuations in boar living conditions, and boars are assigned to breed or not, according to the carrying capacity of their home range cell. Transmission of ASF infection is operated by direct contacts within groups of socialising wild boar hosts and with carcasses deposited in the habitat landscape.
Evidence: The model software was already extensively verified (recoded and technically tested). Internal validation was performed by international experts of wild boar and disease ecology via several projects (incl. previous EFSA expert groups and panels). External validation was achieved using wild boar distribution data, dynamic patterns of multiple infections (CSF, ASF, FMD) and spatio‐temporal notification data. Model predictions were post hoc validated by the matching of model predictions with observational data, i.e. host habitat model (Jordt et al., 2016) spatial and temporal spread of FMD infection in wildlife ruminants (Dhollander et al., 2016); contact probability to carcasses (Lange and Thulke, 2017 vs. Probst et al., 2017); probably fade‐out of ASF from limited wild boar populations (EFSA, 2014, Figure 2, vs. Schulz et al., 2019).
Figure 2.
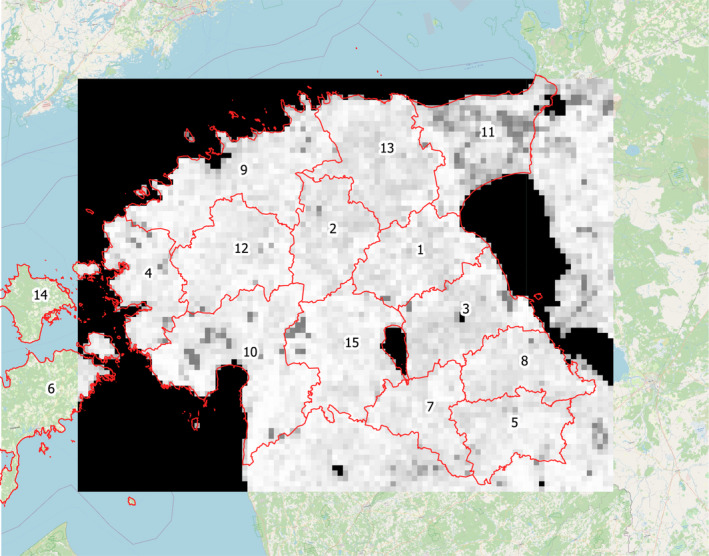
- The lighter a grid cell is coloured, the more wild boar would be sustained within the location due to assumed habitat quality, e.g. feed resources. Administrative subregions are delineated in red and numbered for further reference. The total area covered is about 45,000 km2 with each subregion having an area of about 2–5,000 km2
Specific model amendments
The model includes scenarios for known and recently discussed aspects of ASF epidemiology and control. The model represents the explicit spatial clustering of infection and plausible alternative mechanisms for virus persistence. The model reflects non‐homogenous methods of surveillance (passive, active).
The model simulations were performed on real habitat geography for wild boar in Estonia. Local abundance of wild boar varies according to habitat geography, and the total population is calibrated with the reported average density of wild boar in the simulated area before ASF (e.g. Estonia). Subsequent local densities emerge from the simulated spread of ASF.
The simulation respects administrative subregions according to LAU 1 level (see Figure 2). Wild boar habitat geography was derived from vegetation coverage (Jedrzejewska et al., 1994) and calibrated to the overall population density estimate of wild boar in Estonia prior to ASF incursion. The lighter a grid cell is coloured, the more wild boar would be sustained within the location due to assumed habitat quality e.g. feed resources. Administrative subregions are delineated in red and numbered for further reference. The total area covered is about 45,000 km2 with each subregion having an area of about 2–5,000 km2) and all model output is stratified either by LAU 1 units or the total landscape. The areas covered by LAU 1 units vary between less than 1,000 towards 5,000 km2.
The model represents detailed information relevant for the potential diagnostic outcome if a live animal is shot and tested for antigen or antibody (Table 1).
Table 1.
Time since infection and interpretation of diagnostic results in shot wild boar (after Blome et al., 2020)
| Time since infection | Diagnostic outcome | Infection status in model |
|---|---|---|
| Week 1 (3–10 dpi) | Virus positive, PCR+ and seronegative | Infectious |
| Weeks 2–8 (10–60 dpi) | Virus positive, PCR+ and seropositive | Not infectious and immunea |
| Weeks 9–14 (60–100 dpi) | Virus negative, PCR+ and seropositive | Not infectious and immunea |
| From week 15 (> 100 dpi) | Virus negative, PCR− and seropositive | Not infectious and immunea |
In the scenario ‘prolonged infectivity of survivors’, the status is infectious in weeks 2–4 followed by recovery.
3.4.3. Model outputs
Output recording the population profile
In order to inform the development of Exit Strategy criteria, the population dynamics after introduction of the ASF infection into the simulation landscape were recorded. Different profiles of the population are derived for the total landscape and 13 subunits (LAU 1) by:
diagnostic status/outcome in accordance with Table 1 using week post‐infection, individual disease course (lethal or non‐lethal) and age of the animal;
age cohort;
mortality reasons;
carcass presence considering seasonally varying decomposition.
Fade‐out events are recorded in time per simulation on the:
regional scale including the whole simulation landscape as such
unit scale referring e.g. to LAU 1 resolution (see Figure 2).
Fade‐out is defined as:
no infectious object/infectious live animal remained in the area (i.e. all infectious animals died and all carcasses from individuals that succumbed to the infection are decomposed);
during the following 3 years, no new ASF infections occurred in the evaluated area (no reintroduction events).
This definition assures true fade‐out in the focused area, as well as sufficient time a posteriori to elicit the diagnostic and population profiles to inform the Exit Strategy criteria.
The output of diagnostic and population profiles from study areas facilitates rigorous comparison of different epidemiological scenarios, while respecting the behaviour of the epidemic in time and space (the spatio‐temporal characteristics of the outbreak, including the shape of the epidemic curve and speed of propagation, related to the DCF data from Estonia).
Thus, for potential epidemiological scenarios – representing knowledge gaps or mechanisms possibly impacting the overall duration of infection circulation within a given area – the impact on the patterns of surveillance results (number/prevalence of PCR‐positive and seropositive animals, by age) could be revealed by comparing the reference model output with the respective output data from the model simulations considering the scenario.
Output recording the performance of proposed Exit Strategy protocols
The overall performance of proposed Exit Strategies was tested on repeated simulations of stochastic ASF spread in the simulation area. The sampling strategy proposed for an Exit Strategy was applied from the beginning of the outbreak simulations and reference criteria (e.g. no virus detections) continuously evaluated. If the simulated surveillance efforts fulfilled the proposed Exit Strategy criteria, meaning the tested area would be declared as free from ASF in wild boar, the decision proposed by the surveillance sample (sample‐based knowledge) was compared with the true status in the model population (perfect knowledge). The performance of the investigated Exit Strategy, i.e. the system sensitivity and specificity, was evaluated from all simulation runs. For all decisions, the time between true virus fade‐out and exit decision was measured. False‐negative decisions (decisions that would lead to a declaration of freedom of ASF, while in reality this is not the case) were analysed regarding the achieved sampling targets.
3.4.4. Epidemiological scenarios
The epidemiological scenarios are based on the results of the literature review in step 2:
case fatality
infectious period of animals surviving the infection
presence/duration of ‘infectious carrier’ status
lifelong vs transient immunity in animals that survive ASFV infection
duration of protection by maternal antibodies.
4. Assessment
4.1. Exploration of surveillance data from Estonia, Latvia and Sardinia to study patterns in sero‐ and virus prevalence
4.1.1. Estonia
A rapid decrease in the numbers of detected cases as well as the prevalence of ASF PCR‐ and/or antibody‐positive animals and a depletion of the wild boar population in infected areas has been observed in wild boar surveillance data in Estonia since 2018 following the peak of the epidemic in 2016–2017 (Schulz et al., 2019). Until a recent outbreak in August 2020, the previous clusters of PCR‐positive wild boar in Estonia were detected in late 2018 to early 2019 on the west coast of the country and on the eastern border with Russia (EFSA, 2020). The outbreaks near the eastern border in Estonia were probably epidemiologically linked to the ASF situation over the border in Russia, where ASFV circulation was registered from September to November 2018 (Schulz et al., 2020). The last outbreak in domestic pigs was registered in September 2017 and Estonia has self‐declared freedom from ASF in domestic pigs according to OIE rules in November 2019 (OIE, 2020). Since February 2019 until August 2020, only cases of seropositive wild boar were sporadically detected in Estonia. During 2020 until August all detected seropositive wild boars were in the ‘older than one year age’ class. In July, two PCR‐negative and seropositive piglets were hunted on island Saaremaa, both reported to be younger than 6 months (M. Kristian, pers. commun.) indicating that these animals may have had maternal antibodies. No virus has been detected consecutively in wild boars hunted or found dead in this area or Saaremaa island in general.
In late August 2020, a new cluster of PCR‐positive wild boar was detected in one hunting ground located in Raplamaa county in the western part of the country. Until 14 December 2020, 13 (11 found dead, 2 hunted) PCR‐positive wild boars were detected in this hunting ground, all located in an area with a radius of approximately 3 km. In October 2020, three PCR‐negative and seropositive wild boars younger than 1 year old were hunted in the same area, likely to be the surviving piglets of the infected group (M. Kristian, pers. commun.).
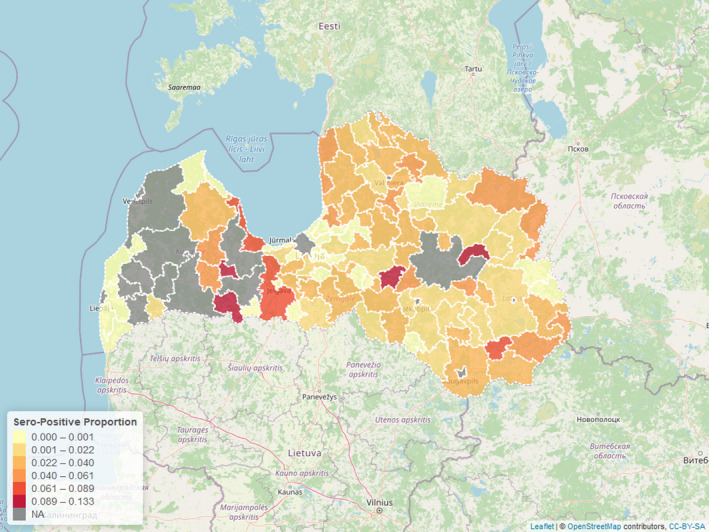
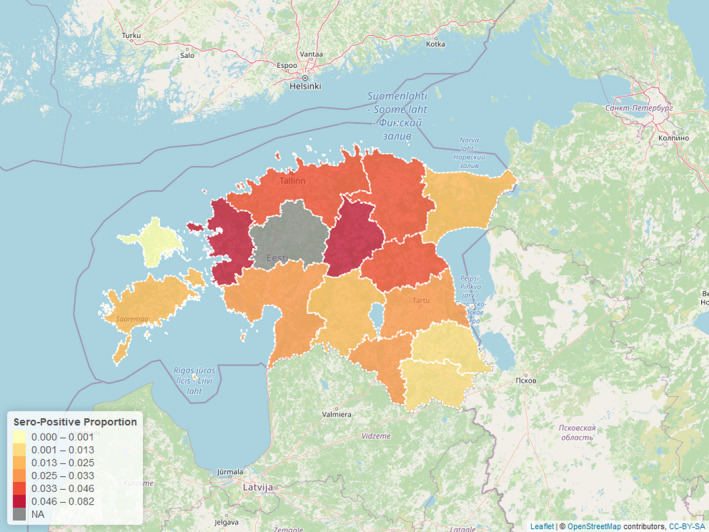
Figure 3 displays the seroprevalence in each LAU 1 in Estonia for the period from the last PCR‐positive sample until 31 August 2020, with darker colours representing a higher seroprevalence (proportions of samples tested with ELISA that were positive).
Figure 3.
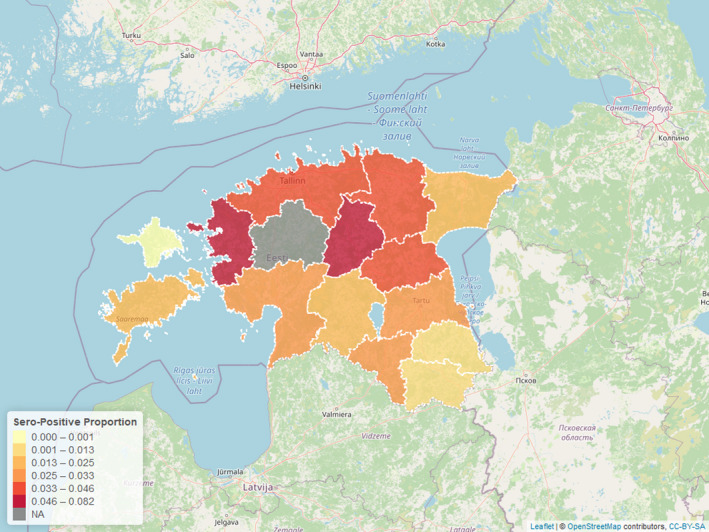
- NA: ASF PCR‐positive wild boar present.
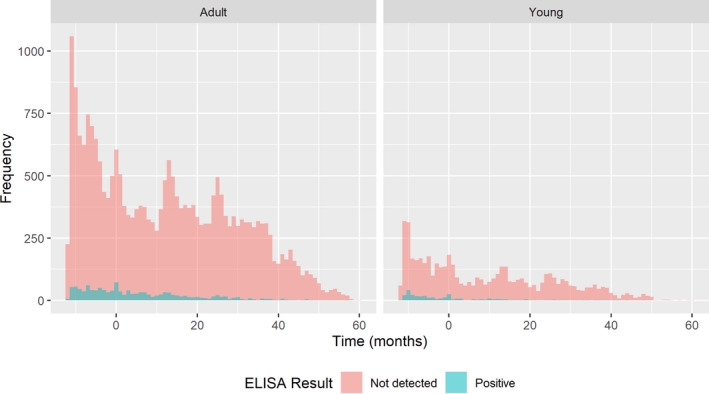
The regions were pooled to study the temporal evolution of infection, with time 0 being the last date at which a PCR‐positive result was reported per LAU 1 region. As shown in Figure 4, seropositive animals in each region are uncommon, particularly more than 24 months after the last PCR‐positive animal had been identified.
Figure 4.

- Month 0 in the x‐axis represents the last PCR‐positive results (end 2018 to beginning 2019). Data include 12 months before time 0 and 48 months after.
A GEE model was used to obtain population estimates for the seroprevalence after accounting for potential correlation between results coming from the same age class and LAU 1 region. It was fitted from the 12‐month period prior to the last PCR‐positive result, and afterwards in order to compare the temporal trend before and after virus circulation in a region. The moment of detecting the last PCR‐positive sample in a particular LAU 1 region was considered as time point = 0. To evaluate the fitness of the models, the QICs for both models were calculated and displayed in Table 2.
A difference between QICs of the fitted models of more than 10 is considered relevant (Pan, 2001) and the differences between the QICs of the model with and without age was larger than 50. This implies that there were differences in the prevalence time between the two age groups.
Figure 5 presents the seroprevalence for different age classes, highlighting differences in the temporal trend before and after the last PCR‐positive sample in Estonia.
Figure 5.
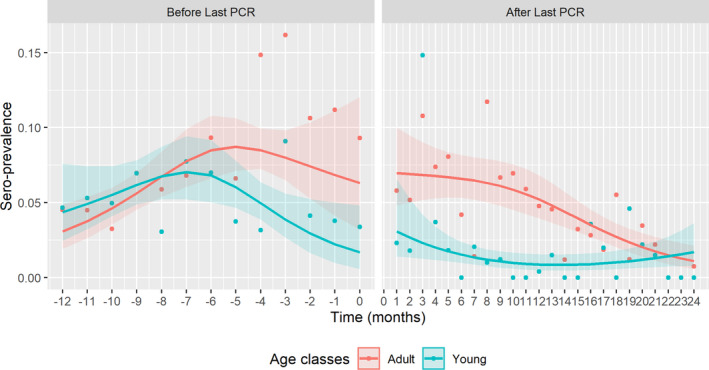
- Time point 0 = moment of detecting last PCR‐positive sample in particular LAU 1 region.
4.1.2. Latvia
The first cases of ASF in Latvia were detected in June 2014 near the border to Belarus, and ASFV spread locally in the wild boar population. Thirty‐two outbreaks in domestic pigs and 217 cases in wild boar had been notified by the end of 2014 (Oļševskis et al., 2016). In November 2020, around 90% of the territory of Latvia was affected by ASF.
More than 5,000 ASF cases have been confirmed in wild boar out of almost 75,000 animals tested since the disease introduction in Latvia. As a consequence, ASF spread within wild boar population in Latvia and 67 outbreaks in pig holdings have been confirmed since 2014 in ASF‐infected areas and more than 45,000 pigs have been culled and destroyed.
In a recent study, ASF surveillance data collected during the first five hunting seasons since the introduction of ASF in Latvia was analysed with the aim to investigate the course of the ASF epidemic in wild boar in Latvia through the dynamics of ASFV prevalence and seroprevalence in wild boar considering also the age class. The results of this study revealed an increase in serologically positive and PCR‐negative wild boar samples from active surveillance over time. When comparing the results by age class, the highest ASFV prevalence was observed in the wild boar younger than 1 year, whereas the seroprevalence was higher in the older animals. These findings demonstrate that only a small proportion of affected animals survive an infection, but accumulation of their numbers over time led to the increase in seroprevalence (Oļševskis et al., 2020).
As a result of ASF spread in the wild boar population, as well as implementation of disease control measures targeted to its reduction, the wild boar population in Latvia has decreased by more than 70% since 2013 when the estimated number of wild boar was 74,107 (Oļševskis et al., 2020).
In 2020, 297 ASF cases had been confirmed in wild boar in Latvia by the 6 November. Out of the 297 ASF cases, the presence of ASF virus genome was confirmed only in 106 wild boar (77 found dead and 29 hunted). In the majority of ASF cases (n = 191), only the presence of antibodies was confirmed. Most of ASF virus‐positive wild boar (n = 103) originated from the western part of Latvia, where the epidemic wave is currently present. Only three ASFV‐positive cases (one roadkill and two animals found dead) were found in the eastern part of Latvia, where the disease was introduced in 2014. The last ASFV detection in the eastern part of Latvia was in July 2020.
The location of seropositive cases (n = 191) in wild boar was distributed almost equally in the territory of Latvia with 56% of cases in the western part and 44% in the eastern part.
In 2020, three ASF outbreaks were confirmed in pig holdings located in the western part of Latvia, in areas where most of ASF virus‐positive cases in wild boar are detected.
Similar to the result from Estonia, seropositive animals in each region are rare, particularly more than 24 months after the last PCR‐positive animal had been identified (Time point 0 = moment of detecting last PCR‐positive sample in particular LAU 1 region) (Figure 6). To evaluate the fitness of the models, the QICs for both models were calculated and displayed in Table 3.
Figure 6.
- NA: No ELISA tests results were submitted to EFSA after the last PCR positive in the specific LAU region.
Table 3.
Quasi‐Akaike information criterion of models before and after the last PCR‐positive results detection in area for two age classes
| Before last PCR positive | After last PCR positive | |
|---|---|---|
| Age included | 4,048.80 | 3,458.89 |
| Age not included | 4,075.5 | 3,503.66 |
A difference between QICs of the fitted models of more than 10 is considered relevant (Pan, 2001) and the differences between the QICs of the model with and without age was larger than 25. This implies that there were differences in the prevalence time between the two age groups.
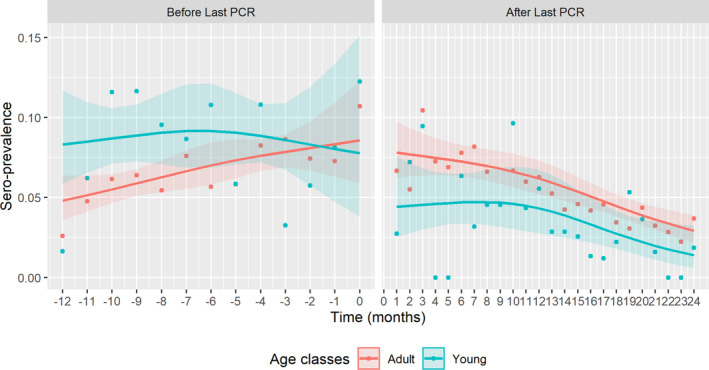
Figure 7 presents the seroprevalence for different age classes, highlighting differences in the temporal trend in Latvia for the period before compared with the time period after the last PCR positive.
Figure 7.
- Time point 0 = moment of detecting last PCR‐positive sample in particular LAU 1 region.
4.1.3. Sardinia
ASFV was first introduced in Sardinia by contaminated meat in 1978 (Contini et al., 1982) and still persists. The disease was first detected in domestic pigs and subsequently spread all over the susceptible Sardinian populations (i.e. domestic pigs, wild boar, free‐ranging pigs) (Wilkinson, 1984; Giammarioli et al., 2011). The particular epidemiological cycle of ASF in Sardinian and the presence of several specific risk factors (i.e. socio‐cultural traditions) allowed a perfect endemic condition and the persistence of the disease until now (Mur et al., 2014; Cappai et al., 2018).
The main role of illegal free‐ranging pigs in disease persistence and the secondary role of wild boar in the maintenance of the disease have been recently demonstrated (Laddomada et al., 2019; Franzoni et al., 2020; Loi et al., 2020). Free‐ranging pigs have the historical role of making the link between domestic pigs and wild boar in the ASF Sardinian epidemiological cycle, as recently reconfirmed (Cappai et al., 2018; Bosch et al., 2020). Moreover, given the very high prevalence detected in these animals and the recently reported the absence of any clinical signs, their possible role as ASFV carriers has been hypothesised, contributing to virus transmission and environmental contamination (Franzoni et al., 2020).
In Sardinia, less than 10% of the total tested samples is provided by passive surveillance and most of these samples are from wild boar killed in road traffic accidents. Most of the wild boar samples, however, are taken during active surveillance and the sampling period is limited to the hunting season, which lasts from November to January (Cappai et al., 2020). Because the data in Sardinia are submitted mostly during the hunting season that lasts from November until the end of January, during the rest of the year very few wild boars have been sampled and tested for ASF and the data were not considered sufficient to perform GEE analysis to study the trend of seroprevalence in young and adult animals separately.
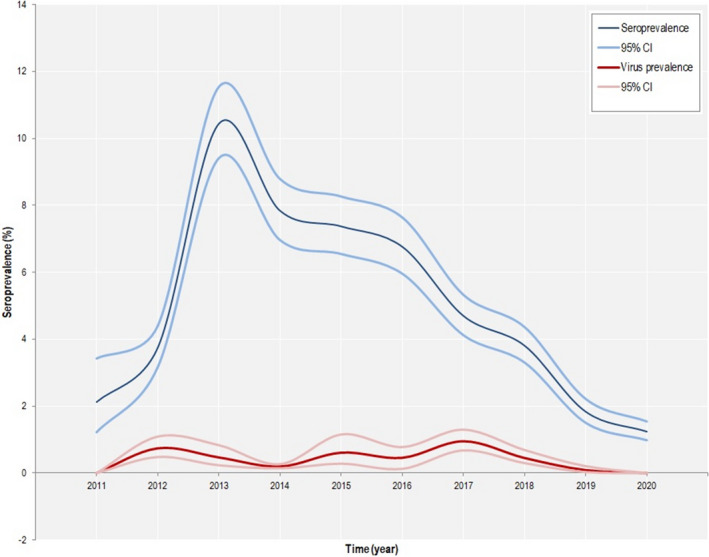
After several years of constant persistence of the disease on the island, a decreasing trend in both virus and seroprevalence has been observed from 2015 in wild boar population (Figure 8), and the last outbreaks in domestic pig dates back to September 2018.
Figure 8.
Results of the GEE model, displaying the seroprevalence in different age classes in the months before and after the last PCR‐positive sample in Latvia
In wild boar, the geographical distribution of disease has mainly been limited to the middle of Sardinia (Figure 9).
Figure 9.
Virus prevalence and seroprevalence ASF trend in Sardinia in wild boar
The last ASF PCR positive in wild boar in Sardinia dates back to April 2019 (two animals found dead), but seropositive wild boar are still found, although limited to four areas (Figure 10). Otherwise, different disease trends have been observed in the different wild boar management units and some of these that were infected for years have been recently defined as free from the disease (i.e. Anglona‐Gallura) (Loi et al., 2020).
Figure 10.
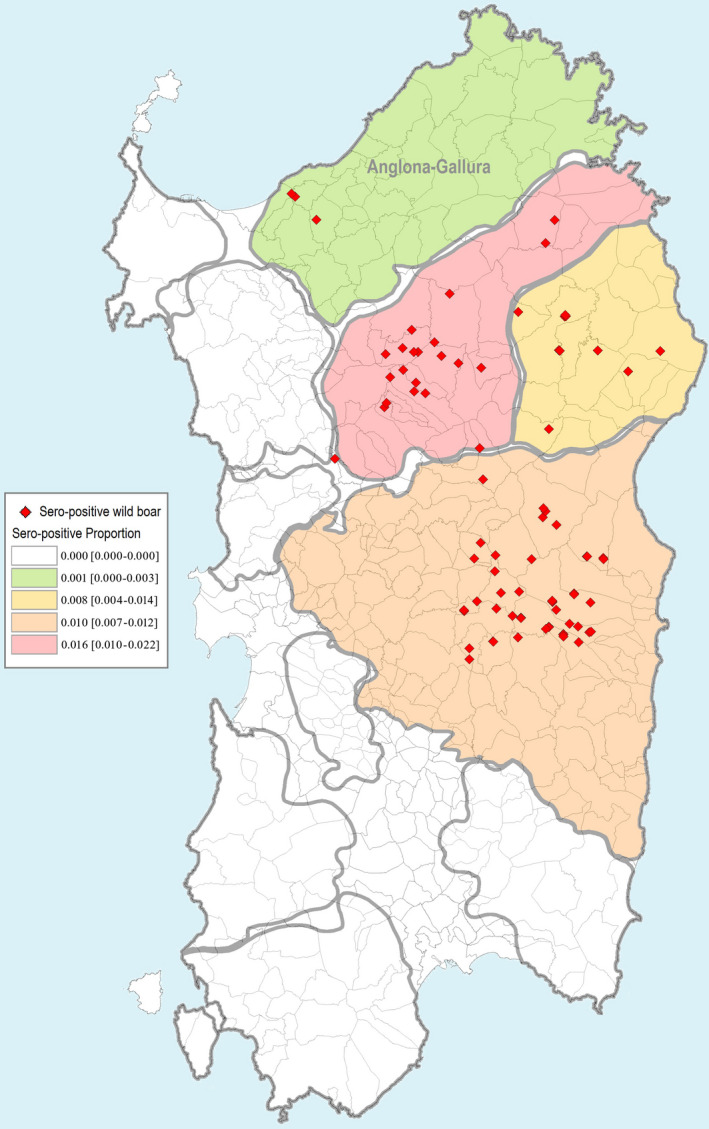
Proportion of seropositive samples in Sardinia (95% confidence intervals), submitted in period May 2019 to January 2020
From the last PCR‐positive detection until October 2020, all the 509 samples taken during the passive surveillance and tested for ASFV were negative.
Figure 10 displays the frequency of samples that were serologically tested by ELISA and confirmed by IB/IPT in Anglona‐Gallura. The figure clearly demonstrates the very low numbers of seropositive samples both in young and adult animals. After several years of disease persistence in Anglona‐Gallura (Feliziani et al., 2010), the ASFV circulation spontaneously faded out and only few seropositive adult animals are still detected (Figure 10).
In Sardinia, a decline in virus and seroprevalence has been observed from 2015. In Anglona‐Gallura, ASF appears to have faded out as only a few seropositive adult animals are still detected.
Figure 11.
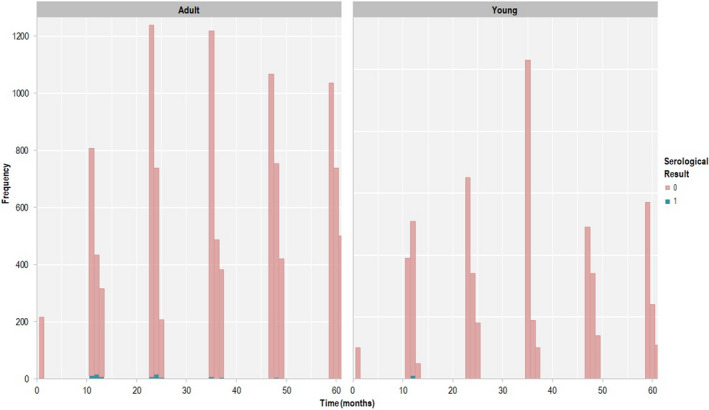
- 0 = negative, 1 = positive.
The decrease in the ASF seroprevalence in Anglona‐Gallura is strongly related to the current high compliance with the last ASF Eradication Plan rules (i.e. no illegal free‐ranging pigs, high‐level of biosecurity and regular veterinary checks in domestic pig farms, adequate number of samples from active surveillance during hunting season (further details can be found in Loi et al., 2019)).
4.1.4. Common observations on the role of seropositive animals
As a general conclusion, the seroprevalence has decreased during the period since the last detection of PCR‐positive wild boar in a region, in the three countries. Furthermore, the decrease is more rapid among animals of less than 1 year old, particularly in Estonia. The fast decline in seroprevalence in the younger animals indicates that there is an increasing proportion of naïve young animals over time, resulting from a reduced or the absence of virus circulation.
In Latvia and Estonia, conversely, 12 months before the last PCR‐positive sample was found in a region, in general the seroprevalence was higher in the young compared with the older age class, consistent with strong association between the presence of virus (PCR)‐positive animals and increased seroprevalence in young animals. The reason for this age‐related difference in antibody response given the presence of PCR‐positive animals is uncertain, but it could be related to less frequent aggregation of adult animals as population numbers are low. In contrast, family groups (sow and piglets) are more likely to remain intact. In addition, it was speculated that the possible higher survival rate of young wild boar could play a role in the observed higher seroprevalence (Nurmoja et al., 2017b; Sehl et al., 2020), or the more frequent exposure of younger animals to infected carcasses (Probst et al., 2017).
4.2. Sensitivity of ongoing surveillance activities in Estonia
The confidence in disease freedom achieved in each LAU 1 region is presented in Figures 12 and 13 for respective 52‐week intervals, and it can be seen that for some of the periods and LAU 1 regions, the estimated confidence was above 95% (3 in the first 52 weeks period and 5 in the second period of 52 weeks), while other ones were estimated to be below the desired 95% confidence level.
Figure 12.
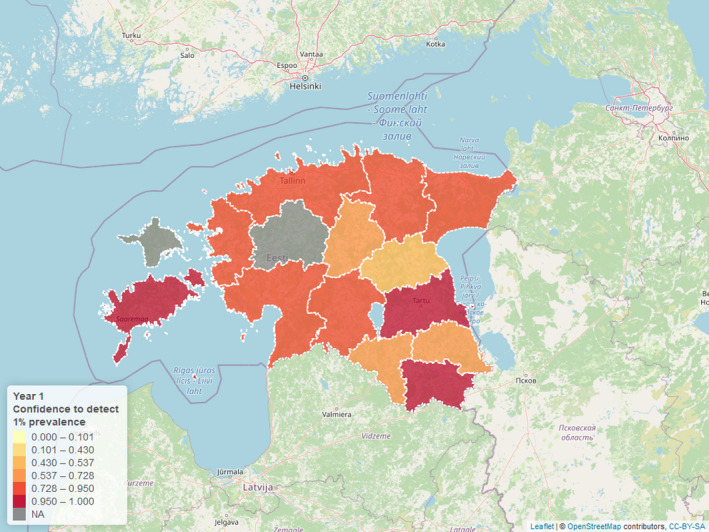
Estimated confidence achieved to detect ASF in LAU 1 regions assuming 1% prevalence for the first 52‐week surveillance period after the last PCR‐positive findings
Figure 13.
Estimated confidence achieved to detect ASF in LAU 1 regions assuming 1% prevalence for the second 52‐week surveillance period after the last PCR‐positive findings
In conclusion, the current sampling intensities are insufficient to detect infection assuming 1% prevalence in many Estonian Local Administrative Unit 1 (LAU 1) regions, based on the assumption of homogenous geographical distribution of infected animals (Figures 12 and 13). Considering the assumptions used in this analysis (population proportion and relative risks in each subgroup), this can be seen as a somewhat over optimistic estimation of the confidence achieved in the LAU 1 with the sampling intensities reported.
Although these ongoing surveillance activities were not designed for demonstrating the absence of virus circulation, they could trigger the final surveillance steps needed to prove the absence of virus circulation. Furthermore, considering the comparatively higher efficiency of passive (in comparison to active) surveillance to detect the virus, these final steps of proving freedom from infection should focus as much as possible on passive surveillance.
4.3. Possible hypotheses for persistence of African swine fever virus in wild boar populations
4.3.1. Viral persistence in the environment
Survival in the environment and carcasses
African swine fever virus is highly stable under a wide range of environmental conditions (Blome et al., 2020). Consequently, the virus can remain viable in the environment for longer than the length of the infectious period in the live host making the indirect transmission through contact with infected carcass more likely to be compared with direct contact with live infectious animals. The spatial spread of ASF, as observed during the current epidemic, could only be replicated using spatial models if account was taken of environmental transmission through infected carcasses. Compared with direct transmission alone, indirect transmission through infected carcasses was found to prolong the duration of viral persistence by two orders of magnitude (Lange et al., 2018). Using a different spatially explicit model to estimate the proportion of transmission events that should be attributed to contact between a live host and contaminated carcass, Pepin et al. (2020) proposed that 53–66% of transmission events in wild boar populations were carcass based. Similar results were obtained across four simulated landscapes with different levels of wild boar density [a landscape of high (2 boar/km2) and low (0.5 boar/km2) density patches, homogenous landscapes with densities of 1, 1.5 and 2 boar/km2].
Wild boars are recognised as efficient scavengers of any carcass other than wild boar (Selva et al., 2005). The behaviour of wild boar towards dead conspecifics is more controversial, based on both observational and modelling perspectives, and may be influenced by climatic and other environmental factors. Probst et al. (2017) monitored 32 wild boar carcasses on nine study sites in northeast Germany using photo‐trapping under field conditions. Based on the pictures taken by the game cameras, wild boar approached the carcasses, but true scavenging was not observed. Wild boar were interested in the soil surrounding the carcasses, both in summer and winter, with the contacts mainly consisting of sniffing and poking on the carcass. There was no evidence for intra‐species scavenging, although piglets were observed several times chewing bare bones once sceletonisation of the carcasses was complete. A similar study with seven wild boar carcasses was carried out in the Czech Republic (Cukor et al., 2020). Under these conditions, direct contact and also true cannibalism was observed. Cannibalism occurred rather late, i.e. on average after 70 days. Using a mechanistic procedure to fit observational data to a spatio‐temporally explicit model framework, Lange and Thulke (2017) concluded that the behaviour of wild boar to dead conspecifics is likely to be one of avoidance, except for occasional contact with infectious material around dead animals. Similar conclusions (i.e. avoidance of dead conspecifics) were drawn from observational studies in Białowieża Forest in Poland (Selva et al., 2005). In broad terms, it was concluded that contact, as observed with dead conspecifics, could lead to transmission. For this reason, carcass removal is considered a crucial control measure.
The time of decay of wild boar carcasses lying on the ground in nature is mainly dependent on the age (the body mass) of the animal, surrounding climatic conditions (season of the year and exposure to direct sunlight and water) and abundance of scavenging insects, birds and other animals (Probst et al., 2017, 2020). In an experiment with wild boar carcasses in cages (without access of scavenging birds and animals), advanced decomposition and skeletonisation were observed 3–44 and 11–433 days post‐mortem, respectively. However, one carcass did not reach skeletonisation even after 2 years. Carcasses in the sun decomposed faster than the sheltered ones, while standing water around the carcass may slow down the decay (Probst et al., 2020). In a field study where scavenging birds and animals were feeding on wild boar carcasses the time until complete skeletonisation was estimated to take between 4 days (young female in summer) and 3 months (adult male boar sunken in a wallow in winter) (Probst et al., 2017). In conclusion, there is large variability in the time of decay of carcasses in nature and factors described above have to be considered when estimating the time of death of found dead animals.
Taking into account stability data for meat and related products, it can be assumed that ASFV is stable in carcasses while they are frozen (reviewed by Fila and Woźniakowski, 2020). Additional environmental components and factors were studied by Mazur‐Panasiuk and Woźniakowski (2020). The laboratory‐scale study comprised artificially contaminated water, wet soil and wet leaf litter. Tests for viral genome and infectious virus were carried out over 14 days at different ambient temperatures. In addition, virus survival was tested in different organs and in spleen samples that were kept at different ambient temperatures on soil, leaf litter, straw, hay and grain, and in water. Again, genome detection was possible over the whole study period across all matrices and environmental conditions. Infectious virus was isolated from artificially contaminated water over the whole study period. ASFV was isolated from contaminated soil and leaf litter immediately after the virus had been added to this matrix, but not subsequently. There was complete loss of virus infectivity, independent of temperature conditions, after a short, 3‐day period of incubation. With regard to inactivation during putrefaction of spleen tissue on different matrices, room temperature led to rapid decay with no influence of the underlying matrix (half‐life 0.44 days). At 4°C, virus was viable over at least 56 days in water, straw and hay. Samples on soil and grain were inactivated after 28 days, and leaf litter caused even faster loss of infectivity (below the detection limit between days 7 and 14). Based on the estimated half‐life values, the authors concluded that the investigated tissues are predicted to remain infectious for 353–713 days at −20°C, 35–136 days at +4°C and from 9 to 17 days at +23°C.
Recently, a supplementary study was conducted to assess ASFV survival in buried wild boar carcasses (Zani et al., 2020). Burial was practiced in some affected countries, e.g. in Lithuania, when removal was not possible. For the purpose of the study, carcasses of ASFV‐infected wild boar buried in Lithuania at different time points and 20 locations have been excavated and retested for the presence of infectious ASFV by in vitro assays and for viral genome by qPCR. Moreover, pooled soil samples were investigated. While viral genome was detected in all buried carcasses and soil samples, virus isolation was not successful from any of the samples tested in this framework.
Carlson et al. (2020) also targeted soil contamination in a recent study. To this end, different soil matrices were spiked with ASFV‐positive blood and tested for viral genome and infectious virus after incubation at different ambient temperatures. As expected, viral genome was detected over the whole study period of 4 weeks in all soil matrices. Soil pH, structure and ambient temperature played a significant role in the stability of infectious ASFV. Infectious ASFV was demonstrated in specimens originating from sterile sand for at least 3 weeks, and from ordinary beach sand for up to 2 weeks. In yard soil, infectious ASFV was demonstrated for 1 week, and in soil from a swampy area for 3 days. Virus was not recovered from two acidic forest soils. The study also comprised a small mitigation component with citric acid or calcium hydroxide. Both compounds led to complete inactivation in the experimental set‐up. Treatment of carcass collection points with disinfectants could therefore be considered for additional risk reduction.
It has to be mentioned that very old, bioassay‐based studies by Kovalenko et al. (1964) reported much longer infectivity times in soil and other matrices (reviewed by Chenais et al., 2019; Fila and Woźniakowski, 2020). However, these studies dealt with large quantities of rather difficult materials inoculated into pigs. The undoubted correlation of the observed signs with an ASFV infection may not have been clearly proven.
The stability of ASFV on crops was recently assessed by Fischer et al. (2020). Briefly, the effect of drying and heat treatment on inactivation of ASFV was tested on six different types of field crops, namely wheat, barley, rye, triticale, corn and peas. Contamination was performed with infectious blood. ASFV genome was detected in all samples by PCR, including samples that had been dried for 2 h and incubated for 1 h at 75°C. Conversely, after 2 h drying, no infectious virus could be detected using virus isolation in porcine macrophages in combination with the detection of ASFV by the haemadsorption test (HAT).
Biological and mechanical vectors
The above‐mentioned study to assess the behaviour of wild boar towards dead conspecifics (Probst et al., 2017) also created the opportunity to study their scavenging activities (Probst et al., 2019). Using digital cameras, 22 vertebrates were detected at the study sites that included two mammal species and three bird species scavenging. The most frequently detected species was the raccoon dog (Nyctereutes procyonoides) (44% of all visits). Raccoon dogs, red foxes (Vulpes vulpes) and buzzards (Buteo buteo) scavenged in the warm and the cold season, while ravens (Corvus corax) and white‐tailed eagles (Haliaeetus albicilla) scavenged only in the cold season. In summer, insects removed most of the carcass biomass. Removal of materials from the carcass sites was a rare event. The authors concluded that scavengers represented a minor risk factor for spreading ASF but may contribute to reducing local virus persistence by removing infected carcasses.
Several studies have investigated a possible role of arthropods in ASF transmission, prompted by the observed summer peak in ASF cases. Studies from Australia (Muzari et al., 2010) and France (Baldacchino et al., 2014) have demonstrated that tabanids are opportunistic feeders on a range of ungulates and wild fauna, including feral pigs and wild boar, respectively. Mellor et al. (1987) presented the first evidence of possible transmission of ASFV by S. calcitrans (Diptera; Muscidae) in transmission laboratory trials. However, as yet, there is no field evidence of a role of biting insects in the mechanical or biological transmission of ASFV, and this has been identified as a knowledge gap (EFSA, 2019). In a so far unpublished study from Estonia, different blood sucking arthropods were trapped in areas with ASFV in wild boar and tested for viral genome. Neither biting midges or mosquitoes nor hard ticks or tabanids were found positive by PCR (Forth and Zani, pers. commun.).
A small laboratory‐scale study targeted blowfly larvae as possible reservoirs and mechanical vectors of ASFV (Forth et al., 2018). In brief, larvae of two commonly found blowfly species, Lucilia sericata and Calliphora vicina, were experimentally bred on ASFV‐infected spleen tissue. After different time intervals, developing larvae and pupae were tested for infectious virus and viral DNA. By qPCR, contamination of the blowfly larvae and pupae with ASFV‐DNA could be demonstrated even after several washing steps, proving the uptake of virus during feeding in the larval stage. However, infectious virus could never be isolated. The results even suggest that the larvae had inactivating properties (probably effects of the salivary secretions).
In an earlier review, EFSA AHW Panel (2010b) concluded that Ornithodoros, belonging to the Argasidae family of soft ticks was the only tick genus able to transmit ASFV. The Ornithodoros erraticus complex, present in parts of the European, trans‐Caucasus countries and Russian Federation territories, may be important in maintaining the local foci of the ASFV within traditional pig management systems, surviving in old shelters/sties with crevices, in particular for genotype I that circulated in Europe in the 1980s. However, these ticks do not play an active role in the geographical spread of the virus and, in addition, O. erraticus and O. verrucosus failed to transmit genotype II in laboratory trials (Pereira de Oliveira et al., 2019). As yet, ticks of the O. erraticus complex have not been reported from central or northern Europe (Vial, 2009; Boinas et al., 2014), and there is insufficient evidence of soft tick–wild boar contact in Germany based on methods to detect antibodies in wild boar against salivary antigens of Ornithodoros spp. (Pietschmann et al., 2016). Furthermore, in contrast with warthogs in Africa, European wild boars rest above the ground and not inside protected burrows, thereby reducing the opportunity for Ornithodoros spp. infestation (EFSA AHW Panel, 2010a). Another potential pathway of transmission related to vectors is the direct ingestion by animals (i.e. pigs) of artificially ASFV (Georgia 2007/1 strain)‐infected O. erraticus ticks, but it is unknown if this pathway would be relevant for wild boar (Pereira de Oliveira et al., 2020).
Subsequently, the vector competence for ASFV of Ixodes ricinus or Dermacentor reticulatus, two commonly found hard tick species in Europe, has been assessed using in vitro feeding experiments (de Carvalho Ferreira et al., 2014). There was no evidence of ASFV replication and these species are unlikely to be relevant biological vectors of ASFV. In these experiments, viral DNA could be detected for up to 8 weeks after feeding in some cases, although it was not possible to determine the precise moment when infectious ASFV (i.e. viable virus) may have been cleared from these hard ticks. Prior to this point of clearance, Frant et al. (2017) have speculated that these ticks may act as mechanical vectors. In field studies conducted to date (Frant et al., 2017; Blome et al., 2020), ASFV has not been detected in hard ticks in central Europe and the Baltic States.
4.3.2. Factors relating to wild boar
Ecological and demographic factors
Since 2014 and up to 31 August 2020, 27,158 cases in wild boar and 4,500 outbreaks in domestic pigs were reported to the Animal Disease Notification System in the current epidemic of ASFV genotype 2 in the EU. Several authors have concluded that ASF can persist in wild boar populations without re‐infection from domestic pigs (Oļševskis et al., 2016; Podgórski and Śmietanka, 2018; Dixon et al., 2020). This is in contrast with the earlier outbreak in Spain, where inadequate biosecurity in outdoor pig production facilities and the presence of soft ticks (Ornithodoros erraticus) each contributed to viral persistence (Mur et al., 2012). Based on a recent modelling study, viral persistence may also be constrained in discrete and limited populations of wild boar, e.g. following ASF introduction into the wild boar population in the Forest of Dean in the UK (Croft et al., 2020). The interplay between host and environmental factors is likely to contribute to viral persistence in wild boar populations. The risk posed by infected carcasses is substantial, and indirect transmission through contact with infected carcasses is considered an important contributor to long‐term ASF persistence, as highlighted previously. To this point, it should be noted that no experimental data are available documenting transmission of ASFV from infected carcasses to susceptible wild boar. Wild boar scavenge carcasses, certainly in Spain (Carrasco‐Garcia et al., 2018) but less so in Germany (Probst et al., 2017), and ASFV can remain stable and infectious for an extended period leading to a prolonged period of environmental contamination (Probst et al., 2017). Furthermore, viral persistence in the environment is favoured in eastern and central Europe by the cold and moist climatic conditions in winter (Chenais et al., 2019; Mazur‐Panasiuk et al., 2019).
In contrast, and despite the abundance and considerable mobility of wild boar, movement probably plays only a limited role in ASF dynamics at a broader scale. In the large part, this is due to the lethality of ASF infection, with a case‐fatality rate of 90–100% within 10–20 days post‐infection (Blome et al., 2012). In addition, there is substantial contact within a single social group, but limited contact between different social groups, given a social structure centred on matrilineal social groups with a few subadult and adult females and their offspring (Podgórski et al., 2014). As a consequence, direct transmission between live wild boar is primarily to other individuals within the same social group.
Habitat quality is associated with ASF incidence and persistence. The presence of large, well‐connected forests is of particular importance in central and eastern Europe, favouring unrestricted wild boar movement and high contact rates between susceptible and infected individuals (Podgórski et al., 2019) and facilitating contact within wild boar meta‐populations (EFSA AHW Panel, 2015; Bosch et al., 2016). The ASF‐infected area of Belgium was found to expand more quickly inside, compared with outside, forested areas (Dellicour et al., 2020). Suitable wild boar habitat can vary seasonally, particularly in areas where crops are being cultivated (Thurfjell et al., 2009).
With respect to host factors, ASF transmission is assumed to be density dependent, i.e. increasing contact rates are associated with increased population density. In agreement, several authors have noted a positive association between wild boar density and ASF occurrence, including EFSA (2017) and Nurmoja et al. (2017a). The latter authors found a positive association between wild boar numbers and ASF occurrence in wild boar in Estonia. Similarly, Podgórski et al. (2019) observed a positive effect of wild boar abundance on the probability of detecting an ASF case. They highlight the contribution of two different, non‐mutually exclusive, processes underlying this density‐dependent pattern of ASF incidence. At higher boar densities, leading to larger wild boar family groups, there is increased potential for direct transmission between wild boar as a consequence of increased within‐group contacts, as well as increased opportunities for indirect transmission through contact of wild boar with infected carcasses and contaminated environments. As wild boar density falls, leading to reduced opportunities for contact and direct transmission, viral persistence is likely to be facilitated by viral survival in infectious carcasses (Podgórski et al., 2019).
The spread of ASF is slow (a median of 8–17 km/year in the Baltic countries in 2017–2018; EFSA, 2018; a median of 3–12 km/year in affected Member States in 2018–2019; EFSA, 2020) and spatially limited, even at high densities, with a rapid decay in ASF case probability with distance from previous infection (Podgórski et al., 2019). ASF spread has been observed in areas of very low (i.e. 0.1 animal/km2) wild boar density, and there is no evidence of a population threshold for spontaneous ASF fade‐out (EFSA AHW Panel, 2018). Croft et al. (2020) suggest that reduction in outbreak severity is influenced by wild boar distribution rather than density or overall population size. Bosch et al. (2016) came to similar conclusions, but in a different context, suggesting that wild boar presence was a more important indicator than wild boar density for the risk of ASF introduction.
Long‐term infectious animals
Long‐term surviving infectious animals, often referred to as carriers or persistently infectious animals, have been discussed as an important factor for viral maintenance, especially in an endemic situation. The role of such infectious animals in long‐term transmission is still controversially discussed (Ståhl et al., 2019). Some of the controversy around ‘persistence’ of ASFV is probably rather a matter of definition. Without doubt, virus and especially viral genome can be detected in surviving animals for a relatively long time following infection (Petrov et al., 2018). In the absence of truly neutralising antibodies, virus can be isolated from survivors for roughly 60–70 days following initial infection. Viral genome can be detected for an even longer period (~ 100 days). The latter showed no transmission to sentinel animals, and no virus in survivors beyond 100 days (Nurmoja et al., 2017b; Petrov et al., 2018). However, there is no evidence of a major role of potential surviving infectious animals from field experience and long‐term studies (Ståhl et al., 2019). Recently Ståhl et al. (2019) published a systematic review investigating the exact question of whether there is experimental or field‐based evidence for surviving infectious animals with long‐term transmission. Assessing the experimental studies included in the review, it becomes clear that most of the recent trials showed quite comparable results in terms of excretion pattern. However, the interpretation of results and definition of persistence or carrier state was different. As an example, the conclusion drawn by Petrov et al. (2018) of no evidence for a carrier state is in contrast with the findings of de Carvalho Ferreira et al. (2012), with the same genotype I virus and who speak of persistently infected animals. However, it has to be kept in mind that the study designs and lengths were different. This is also true for the recently published follow‐up study by Eblé et al. (2019). Contact transmission occurred after the acute phase of infection but still a rather short period after initial infection, in a phase that one could still call a recovery phase. In general, long‐term detectability of both virus and genome was quite similar in the studies mentioned above.
Fila and Woźniakowski (2020) mentioned that very small doses of the virus may cause asymptomatic spread and virus shedding. The source of this information or related data remains unclear. In the model‐based study by O'Neill et al. (2020) it has been shown, that long‐term‐infectious animals, given they exist, support time horizon of infection circulation. However, it has been demonstrated previously that there is no need for long‐term infectious animals in order to replicate the observed spatio‐temporal spread of ASF in wild boar using different spatially explicit simulation models (e.g. Figure 14, Pepin et al., 2020; Lange, 2015).
Figure 14.
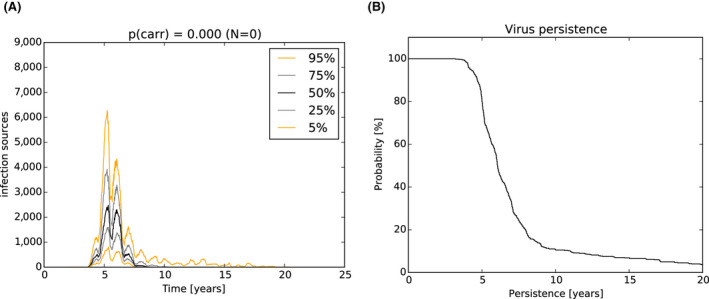
- (A) Temporal dynamics and variability in the number of infectious objects (live animals and carcasses attributable to ASF infection) in simulated wild boar populations of Estonia between ASF introduction and fade‐out. The simulation was conducted for 100 runs over the full territory of Estonia (except islands) and the variation between simulated runs is presented as percentiles. The timing of fade‐out was variable between years 10 and 25 and 5% of runs did not fade out by year 20. (B) Duration of circulation of ASFV infection in simulated wild boar populations of Estonia. Commencing at the moment of introduction of infection into the simulation area (t = 0, x‐axis) the graph shows the probability of ASF persistence, calculated as the percentage of 600 simulation runs (y‐axis) with infectious objects still present.
4.3.3. Characteristics of the virus
The ASFV strains in the current European epidemic, apart from Sardinia where genotype I is circulating, belong to the p72 genotype II, indicating a common origin in the Caucasus (Guinat et al., 2016a). These strains are highly virulent, inducing per‐acute to acute disease with up to 100% case fatality within 7–10 days (Blome et al., 2020), regardless of age, dose or route of administration (Gallardo et al., 2018).
Although ASFV is a very stable DNA virus with a low mutation rate (Dixon et al., 2020), there have been several examples of naturally occurring, attenuated virus variants during the current epidemic. In north‐eastern Estonia in 2014, an ASFV variant with reduced virulence was identified (Zani et al., 2018), further to field evidence of differing outbreak characteristics in this region compared with the rest of Estonia (Nurmoja et al., 2017b). This attenuated phenotype was associated with a deletion of 14,560 base pairs at the 5′ end, and genome reorganisation by duplication (Zani et al., 2018). This strain has not been detected subsequently in Estonia or elsewhere, consistent with the authors’ speculation that attenuated phenotypes with lower mortality rates in swine, in the absence of a reservoir vector, will probably vanish due to the animals clearing the virus before it is transmitted via bloody excretions or the dead animal's carcass (Zani et al., 2018). This is consistent with the results of an individual‐based, spatially explicit ASF model, which suggested that attenuated ASFV types were unlikely to establish in the field when competing with non‐attenuated types (Meier, 2017). A further mutation was observed in south‐eastern Estonia, the GII‐CVR2 variant, but only over a short time window (July 2015 to March 2016) in four municipalities within the county of Tartu (Gallardo et al., 2018; Vilem et al., 2020). The disappearance of this strain could possibly have been connected to its reduced virulence. In Latvia in 2017, an attenuated and non‐haemadsorbing genotype II ASFV, ASFV Lv/17/WB/Rie1, was isolated from a hunted wild boar in Latvia. This animal was both virus and antibody positive. During subsequent experimental challenge with this strain, pigs developed nonspecific clinical signs and, in some cases, remained asymptomatic, showing intermittent and weak viraemia and a high antibody response (Gallardo et al., 2019). This attenuated strain, which was subsequently isolated in 2019 (but not in 2018) from the same region of Latvia, is currently further considered as a potential vaccine candidate (Barasona et al., 2019).
Up to recent years in Sardinia, the genotype I ASFV strains have always been isolated from symptomatic domestic pigs (i.e. with per‐acute or acute clinical signs) or from hunted/dead wild boar with unknown health status (Torresi et al., 2020). However, during the last 3 years (2017–2020), the virus has been isolated from illegal free‐ranging pigs without clinical signs of ASF. The presence of less virulent ASFV strains has never been confirmed, although the field observations are highly suggestive of their presence in Sardinia. Studies on this issue are ongoing. It is speculated that there has been gradual disease evolution within the island leading to an attenuation of virulence, along with other factors (i.e. animal resistance and tolerance) (Franzoni et al., 2020).
4.3.4. Human‐induced factors
Spillover from domestic cycles, including illegal movement of infected meat and products
The spread of ASF in wild boar populations can continue without re‐infection from domestic pigs (Dixon et al., 2020). Nonetheless, there have been some examples of spillover from domestic pigs to wild boar, both in the Russian Federation and the EU. In the Russian Federation, involvement of domestic pigs in the epidemic occurred due to direct contact between infected wild boars and free‐ranging pigs. In the earlier stages of the epidemic in the Russian Federation, infection was primarily in domestic pigs, with wild boar acting as sentinels for the presence of disease as a result of spillover infection from domestic pigs (FAO, 2013). Similarly, infection of wild boars in the Tver region in 2012, an area far removed from the earlier affected southern regions, is likely to have occurred from infected domestic pigs (Gogin et al., 2013). In the EU, ASF in both domestic pig and wild boar populations was a common feature at the start of the current epidemic (Pejsak et al., 2014; Oļševskis et al., 2016), and the known risk of viral transmission from wild boar to domestic pigs (Boklund et al., 2020), related to levels of farm biosecurity (Zani et al., 2019; Gavier‐Widén et al., 2020), is likely to be reciprocated through well‐described transmission routes (Guinat et al., 2016a). In the earlier Spanish outbreak, factors known to complicate ASF eradication included low levels of biosecurity in outdoor domestic pig production systems, the absence of an adequate identification and traceability systems for herds and pig movements and continuous contact between infected and susceptible domestic pigs, wild boar and soft ticks (Mur et al., 2012). In Sardinia, ASF persistence is strongly associated with socio‐economic factors, culture and traditional practices on the island (Cappai et al., 2018; Loi et al., 2019; Laddomada et al., 2019). In the study by Cappai et al. (2018), deprivation (cultural and material deprivation, lack of resources and overcrowding) and low educational level were each significantly associated with ASF risk.
There are several examples of ASF introduction and spread into a country through indirect contacts with infected meat or products in Europe (Chenais et al., 2019). ASFV was introduced into Sardinia in 1978 through contaminated pig products, and has persisted subsequently (Contini et al., 1982). The same route was hypothesised for the introduction of ASFV in Georgia in 2007 (Beltrán‐Alcrudo et al., 2018), and for cases of long‐distance ‘disease jumping’ in the Russian Federation (Gogin et al., 2013; Oganesyan et al., 2013; Kolbasov et al., 2018). The risk related to infected meat and pork products is often associated with illegal movements (i.e. tourism) or small free‐ranging backyard farms where animals are fed with untreated food leftovers or catering waste (Guinat et al., 2016a). The annual probability of ASFV infection of domestic pigs in EU countries by legal/illegal movement of pig meat products was quantitative assessed to be very low (6 × 10−4) and limited to only a few countries. In these countries, this risk was linked to the amount of infected meat from infected pigs entering the country, the high average number of pigs on each backyard farm and/or the absolute number of backyard farms (Taylor et al., 2020). Even if waste pork products are properly disposed of, imported meat is more likely to end up at a landfill site, which can be easily accessed by wild boar, rather than being fed as swill to backyard pigs.
Wild boar management
Human activity is an important contributor to both ASF persistence and expansion. Hunting management (sustained winter feeding, avoidance of females with piglets, hunting bags below natural recruitment rate) can alter wild boar ecology and ASF epidemiology (Guberti et al., 2018; Chenais et al., 2019). Poor biosecurity during hunting, particularly the mismanagement of carcass offal, can facilitate local transmission within wild boar habitats, and between domestic pigs and wild boar (Dixon et al., 2020). Conversely, hunting groups can play a key role, in collaboration with authorities, to improve detection of wild boar carcasses. Using data from Sardinia, Cappai et al. (2018) found that collaboration with hunting organisations was significantly associated with improved ASF detection. Similarly, Loi et al. (2019) observed that the compliance of hunting organisations with hunting season management rules was associated with a reduction in a calculated ASF risk index, being a summary measure of the risk level in each of Sardinia's municipalities.
There are also multiple examples of long‐distance translocation of infection (Beltran‐Alcrudo et al., 2019; Dellicour et al., 2020), which can only plausibly be related to human activity. Furthermore, in several network analyses conducted by EFSA (2017, 2018, 2019), there were multiple examples of cases in wild boar across affected Member States that could not plausibly be explained by boar‐to‐boar transmission. These are assumed to be human‐related translocations. Finally, the analysis of the spatio‐temporal patterns of ASFV notifications in the Baltic member state and Poland revealed the impact of human‐mediated translocation on the expansion of the epidemic. Without inclusion of human‐mediated translocations, an adequate reconstruction of the continental ASFV spread was impossible (Lange et al., 2018; Figure 6).
4.4. Update on epidemiological attributes of African swine fever virus genotypes I and II that have still a high uncertainty
4.4.1. Mortality rate
Field data
The true impact of ASF at the population level is poorly understood. In almost all infected countries, hunting has been carried out in infected wild boar populations, making it difficult to disentangle the relative impact of mortality caused by ASF compared with deaths directly attributable to hunting.
Using wild boar camera trap data from Poland, Morelle et al. (2020) estimated that ASF virus and hunting activities decreased the infected wild boar population by 94.8%, including 78.3% attributed to ASF‐related mortality and 21.7% due to hunting‐related mortality. Additionally, in Poland, in a protected area where hunting is forbidden, 83.8% (SD 25.5%) of the wild boar died because of ASF.
The available data from Belgium (September–July 2020) have shown a collapse in the wild boar population in the infected area, which encompasses about 600 km2 (Licoppe et al., 2020). Prior to the ASF incursion, the hunting bag was estimated to be 3.6 wild boar/km2 but had dropped to 0.17 wild boar/km2 at the end of the epidemic, a decrease of more than 20‐fold (Licoppe, pers. comm.). During this period, 2,158 animals were collected, including 801 animals that died because of ASF (37.1%), 356 that died of unknown causes other than ASF (16.5%), 587 wild boar that were hunted (27.2%), 29 trapped (6%) and 197 shot at night (9.1%). About 5% were killed for other reasons.
In several Latvian hunting grounds affected by ASF, mortality caused by the virus in the first year was estimated to be 79.4% (SD 15.6%), while hunting‐related mortality in the same hunting grounds was 7.0% (SD 0.72%) (M. Seržants, Food and Veterinary Service of Latvia, pers. commun.).
In the Zlin area of the Czech Republic, within the 57.2 km2 of the high‐risk area where ASFV has been detected, 241 animals were found dead of which 190 (79%) were ASFV positive, while 247 (50.6% of all dead animals recorded) had been shot. As a result of both ASF and wild boar management, the local wild boar population decreased from 8.4 (June 2017) to 0.17 (31 January 2018) wild boar/km2 (Tomas Jarosil, SVA Czech Republic, pers. commun.; Marcon et al., 2019).
Experimental infections: case‐fatality
ASF kills wild boar with an estimated case‐fatality rate of about 95% in experimental infections (Dixon et al., 2020). Since the introduction and spread of ASFV genotype II in Europe in 2007, several experimental infections of pigs and wild boar with genotype II field strains from Europe have been performed to study the clinical course of the infection (Table 4). The number of experimental animals that died due to the infection, either naturally or on welfare grounds, could provide an indication of the case‐fatality rate.
Table 4.
Case‐fatality rate observed during experimental infections
| Experimental animal group | Reference | ASFV genotype II strain | Target | Sample size | Route | Doses | Duration (dpi) | Died due to disease | Euthanised due to disease | Case‐fatality rate |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bernard et al. (2016) | Ambaton 02 | DP | 18 | ID | 104 HAD50 | 8 | 0 | 18 | 100 |
| 2 | Bernard et al. (2016) | Ambaton 02 | DP | 12 | ID | 102 HAD50 | 8 | 0 | 12 | 100 |
| 3 | Pietschmann et al. (2015) | Armenia 08 | WB | 6 | ON | 10 HAD50 | 36 | 6 | 0 | 100 |
| 4 | Pietschmann et al. (2015) | Armenia 08 | DP | 6 | ON | 100 HAD50 | 36 | 6 | 0 | 100 |
| 5 | Pietschmann et al. (2015) | Armenia 08 | DP | 6 | ON | 10 HAD50 | 36 | 6 | 0 | 100 |
| 6 | Pietschmann et al. (2015) | Armenia 08 | WB | 6 | ON | 100 HAD50 | 36 | 6 | 0 | 100 |
| 7 | Frederic et al. (2015) | Georgia 2007/1 | DP | 3 | IM | 103 TCID50 | 6 | 3 | 0 | 100 |
| 8 | Vlasova et al. (2015) | Kashino 04/13 | DP | 4 | IH | 50 HAD50 | 21 | 4 | 0 | 100 |
| 9 | Vlasova et al. (2015) | Kashino 04/13 | DP | 2 | DC | 503 HAD50 | 15 | 2 | 0 | 100 |
| 10 | Vlasova et al. (2015) | Boguchary 06/13 | DP | 4 | IH | 50 HAD50 | 11 | 4 | 0 | 100 |
| 11 | Vlasova et al. (2015) | Boguchary 06/13 | DP | 2 | IM | 503 HAD50 | 9 | 2 | 0 | 100 |
| 12 | Vlasova et al. (2015) | Boguchary 06/13 | DP | 2 | DC | 503 HAD50 | 9 | 2 | 0 | 100 |
| 13 | Vlasova et al. (2015) | Karamzino 06/13 | DP | 4 | IH | 50 HAD50 | 21 | 4 | 0 | 100 |
| 14 | Vlasova et al. (2015) | Karamzino 06/13 | DP | 2 | IM | 503 HAD50 | 11 | 2 | 0 | 100 |
| 15 | Vlasova et al. (2015) | Karamzino 06/13 | DP | 2 | DC | 503 HAD50 | 15 | 2 | 0 | 100 |
| 16 | Vlasova et al. (2015) | K 08/13 | DP | 4 | IM | 503 HAD50 | 11 | 4 | 0 | 100 |
| 17 | Vlasova et al. (2015) | Vyazma 08/13 | DP | 4 | IH | 50 HAD50 | 15 | 4 | 0 | 100 |
| 18 | Vlasova et al. (2015) | Vyazma 08/13 | DP | 2 | IM | 503 HAD50 | 8 | 2 | 0 | 100 |
| 19 | Vlasova et al. (2015) | Stavropol 01/08 | DP | 4 | IH | 50 HAD50/503 HAD50 | 9 | 4 | 0 | 100 |
| 20 | Guinat et al. (2014) | Georgia 2007/1 | DP | 16 | DC | 14 | 0 | 16 | 100 | |
| 21 | Guinat et al. (2014) | Georgia 2007/1 | DP | 16 | DC | 18 | 0 | 16 | 100 | |
| 22 | Guinat et al. (2014) | Georgia 2007/1 | DP | 16 | IM | 102 HAD50 | 12 | 1 | 15 | 100 |
| 23 | Karalyan et al. (2012) | Field strain from Armenia and Georgia | DP | 9 | IM | 7 | 9 | 100 | ||
| 24 | Gabriel et al. (2011) | Armenia/2008 | WB | 1 | IM | 103 HAD50 | 25 | 1 | 0 | 100 |
| 25 | Gabriel et al. (2011) | Armenia/2008 | WB | 3 | DC | NA | 25 | 3 | 0 | 100 |
| 26 | Gabriel et al. (2011) | Armenia/2008 | DP | 3 | DC | NA | 20 | 3 | 0 | 100 |
| 27 | Gabriel et al. (2011) | Armenia/2008 | WB | 6 | O | 25 | 6 | 0 | 100 | |
| 28 | Olesen et al., (2018) | POL/2015/Podlaskie/Lindholm | DP | 4 | DC | 7 | 0 | |||
| 29 | Olesen et al. (2018) | POL/2015/Podlaskie/Lindholm | DP | 4 | DC | NA | 21 | 0 | ||
| 30 | Olesen et al. (2018) | POL/2015/Podlaskie/Lindholm | DP | 4 | DC | NA | 21 | 0 | ||
| 31 | Olesen et al. (2018) | POL/2015/Podlaskie/Lindholm | DP | 4 | DC | 106 MTC | 21 | 0 | ||
| 32 | Olesen et al. (2017) | POL14/WB7397#13 | DP | 4 | DC | NA | 14 | 0 | 4 | 100 |
| 33 | Olesen et al. (2017) | POL14/WB7397#13 | DP | 6 | DC | NA | 18 | 2 | 33.3 | |
| 34 | Olesen et al. (2017) | POL14/WB7397#13 | DP | 4 | IN | 104.5 TCID50 | 11 | 0 | 4 | 100 |
| 35 | Olesen et al. (2017) | POL14/WB7397#13 | DP | 4 | DC | NA | 12 | 0 | 4 | 100 |
| 36 | Olesen et al. (2017) | POL14/WB7397#13 | DP | 4 | DC | NA | 12 | 0 | 4 | 100 |
| 37 | Olesen et al. (2017) | POL14/WB7397#13 | DP | 4 | DC | NA | 14 | 1 | 2 | 75 |
| 38 | Olesen et al. (2017) | POL14/WB7397#13 | DP | 4 | IN | 104.5 TCID50 | 11 | 1 | 3 | 100 |
| 39 | Olesen et al. (2017) | POL14/WB7397#13 | DP | 4 | DC | NA | 16 | 2 | 50 | |
| 40 | Olesen et al. (2017) | POL14/WB7397#13 | DP | 4 | DC | NA | 14 | 0 | 4 | 100 |
| 41 | Karalyan et al. (2017) | NR | DP | 8 | IM | 104 HAD50 | 7 | 0 | 8 | 100 |
| 42 | Gallardo et al. (2017) | LT14/1490 | DP | 8 | IM | No pdf | 19 | 8 | 0 | 100 |
| 43 | Gallardo et al. (2017) | LT14/1490 | DP | 10 | DC | NA | 61 | 9 | 0 | 90 |
| 44 | Popescu et al. (2017) | Georgia 2007/1 | DP | 10 | IM | 104 HAD50 | 4 | 0 | 10 | 100 |
| 45 | Donnell et al. (2016) | Georgia 2007 | DP | 5 | IM | 103 HAD50 | 8 | 0 | 5 | 100 |
| 46 | Donnell et al. (2016) | Georgia 2007 | DP | 5 | IM | 103 HAD50 | 0 | 5 | 100 | |
| 47 | Donnell et al. (2016) | Georgia 2007 | DP | 5 | IM | 103 HAD50 | 9 | 0 | 5 | 100 |
| 48 | Donnell et al. (2017) | Georgia | DP | 5 | IM | 104 HAD50 | 7 | 0 | 5 | 100 |
| 49 | Donnell et al. (2017) | Georgia | DP | 5 | IM | 104 HAD50 | 7 | 0 | 5 | 100 |
| 50 | Burmakina et al. (2016) | Congo K‐49 | DP | 3 | IM | 103 HAU | 6 | 3 | 0 | 100 |
| 51 | Burmakina et al. (2016) | Congo K ‐49 | DP | 3 | IM | 103 HAU | 7 | 3 | 0 | 100 |
| 52 | Burmakina et al. (2016) | Congo K ‐49 | DP | 3 | IM | 103 HAU | 8 | 3 | 0 | 100 |
| 55 | Sanford et al. (2016) | Georgia | DP | 2 | IM | 104 TCID50 | 8 | 0 | 2 | 100 |
| 56 | Sanford et al. (2016) | Georgia | DP | 5 | IM | 104 TCID50 | 8 | 0 | 5 | 100 |
| 57 | Gallardo et al. (2018) | Es15/WB‐Tartu14 ASFV | DP | 2 | IM | 10 HAD50 | 78 | 2 | 0 | 100 |
| 58 | Gallardo et al. (2018) | Es15/WB‐Valga‐6 ASFV | DP | 2 | IM | 10 HAD50 | 78 | 1 | 1 | 100 |
| 59 | Gallardo et al. (2018) | Es15/WB‐Tartu14 ASFV | DP | 4 | DC | NA | 78 | 2 | 0 | 50 |
| 60 | Gallardo et al. (2018) | Es15/WB‐Valga‐6 ASFV | DP | 4 | DC | NA | 78 | 2 | 0 | 50 |
| 61 | Zani et al. (2018) | ASFV from N‐E Estonia | mini‐DP | 12 | ON | 105 HAU | 36 | 1 | 2 | 25 |
| 62 | Zani et al. (2018) | ASFV from N‐E Estonia | DP | 5 | ON | 105 HAU | 36 | 0 | 0 | 0 |
| 63 | Zani et al. (2018) | ASFV from N‐E Estonia | WB | 5 | ON | 106.5 HAU | 17 | 2 | 3 | 100 |
| 64 | Zhao et al. (2019) | DP/HLJ/18 | DP | 1 | IM | NR | 14 | 1 | 0 | 100 |
| 65 | Zhao et al. (2019) | DP/HLJ/18 | DP | 2 | IM | NR | 14 | 2 | 0 | 100 |
| 66 | Zhao et al. (2019) | DP/HLJ/18 | DP | 3 | IM | NR | 14 | 3 | 0 | 100 |
| 67 | Zhao et al. (2019) | DP/HLJ/18 | DP | 3 | IM | NR | 14 | 3 | 0 | 100 |
| 68 | Zhao et al. (2019) | DP/HLJ/18 | DP | 2 | DC | NR | 14 | 2 | 0 | 100 |
| 69 | Gallardo et al. (2019) | Lv17/WB/Rie1 (non‐HAD) | DP | 2 | IM | 10 TCID50 | 126 | 0 | 1 | 50 |
| 70 | Gallardo et al. (2019) | Lv17/WB/Rie1 (non‐HAD) | DP | 4 | DC | NA | 126 | 0 | 3 | 75 |
| 71 | Gallardo et al. (2019) | Lv17/WB/Zieme3 (HAD) | DP | 1 | IM | 10 TCID50 | 126 | 1 | 100 | |
| 72 | Gallardo et al. (2019) | Lv17/WB/Zieme3 (HAD) | DP | 1 | DC | NA | 126 | 0 | 1 | 100 |
| 73 | Cadenas‐Fernandez et al. (2020) | ASFV Arm07 isolate | WB | 2 | IM | 10 HAD50 | 48 | 0 | 2 | 100 |
| 74 | Walczak et al. (2020) | Pol18_28298_O111 | DP | 8 | IN | 1000 HAU | 32 | 7 | 0 | 87.5 |
| 75 | Walczak et al. (2020) | Pol18_28298_O111 | DP | 6 | IN | 500 HAU | 24 | 4 | 2 | 100 |
| 76 | Walczak et al. (2020) | Pol18_28298_O111 | DP | 8 | IN | 5 HAU | 21 | 6 | 2 | 100 |
| 77 | Borca et al. (2020) | Georgia 2007/1 | DP | 5 | IM | 102 HAD50 | 0 | 5 | 100 | |
| 78 | Lokhandwala et al. (2019) | ASFV‐Georgia 2007/1 | DP | 5 | IN | 104 TCID50 | 5 | 1 | 2 | 60 |
| 79 | Lokhandwala et al. (2019) | ASFV‐Georgia 2007/1 | DP | 5 | IN | 104 TCID50 | 5 | 2 | 1 | 60 |
DC: direct contact with infected animals; DP: domestic pig; dpi: days post‐inoculations; HAD: haemadsorption doses; HAU: haemadsorbing units; ID: intradermal; IH: inhalation; IM: intramuscular; IN: intranasal; MTC: Maximum tolerable concentration; NA: not applicable; NR: not reported; O: oral; ON: oronasal; TCID: tissue culture infectious dose; V: vector bite; WB: wild boar.
An extensive review of literature retrieved 27 papers about experimental infections with ASFV genotype II, and showed that in 62 of 79 different experimental animal groups (groups of either wild boar or domestic pigs which were inoculated in the same experiment with the same doses of the same ASFV strain with the same infection route), all animals in the group died due to the disease or had to be euthanised because of welfare reasons. In contrast, Gallardo et al. (2018, 2019) and Zani et al. (2018) reported a case‐fatality between 0 and 75% during the course of the experiment using attenuated field strains from Estonia and Latvia. Olesen et al. (2017, 2018) also reported a relatively low number of animals infected with a Polish field strain that died during a 3‐week experiment (i.e. none or only one‐third of the pigs either died or were euthanised on welfare grounds during the experiment).
Cross‐study comparisons must be done with care. Bias in estimates of the case‐fatality rates cannot be avoided due to the differences in study duration, the use of different humane endpoints in experimental protocols in different laboratories or relatively small sample size in this type of experiment. For instance, in the experiment of Lokhandwala et al. (2019), only 60% of the animals died due to the disease, or were euthanised for welfare reasons, due to the clinical signs induced by the disease, but the experiment only lasted for 5 days and only five animals were included.
4.4.2. Duration of protective immunity and maternal antibodies
Protective immunity
The duration of protective immunity in animals surviving from ASF has not been well studied and is considered a knowledge gap (Blome et al., 2020). In experimental challenges with attenuated and virulent strains of ASFV, it has been demonstrated that animals recovering from the infection are protected against a subsequent challenge with homologous virus (Mebus and Dardiri, 1980; King et al., 2011). However, not all of these animals are protected from becoming re‐infected. In a recent study with virulent strains of ASF genotype II virus, three out of four recovered pigs that became re‐infected 78 days post first challenge infection experienced a short transient viraemia after the second challenge exposure. One of the animals showed mild clinical signs of ASF. No transmission occurred when these challenged survivor animals were housed together with susceptible sentinel pigs (Gallardo et al., 2018).
In many vaccine trials only, short‐term protection (few weeks after the first immunisation) has been demonstrated. Sánchez‐Cordón et al. (2020) demonstrated that immunised animals were not protected against homologous virulent challenge at day 130 after primary infection with attenuated Benin 97/1 strains. However, Stone et al. (1968) have shown partial protection of immunised pigs after 117 days and Sereda et al. (2020) have stated that the protection lasted at least 4 months post‐immunisation with attenuated ASFV strains, suggesting that the protection from clinical disease may last at least several months in animals recovering from the disease. Re‐infection of these animals, however, cannot be excluded.
Maternal antibodies
Intrauterine infection of fetuses with ASFV has seldom been registered, although abortions are a frequent outcome of the disease in pregnant sows. Schlafer and Mebus (1987) observed fetal infection in one challenge experiment. The infected fetuses were born dead. In a recent challenge experiment, in which pregnant minipigs were infected with ASF genotype II virus, the fetuses remained uninfected (Zani et al., 2018). The fetal infection, if occurring, seems to be fatal for fetuses. Therefore, the prenatal infection of live born piglets is unlikely.
The duration of maternal antibodies in piglets of sows surviving ASF is not known. In the only published challenge experiment of its kind, piglets with maternal antibodies of 7 weeks of age were inoculated with virulent virus that resulted in elevated rectal temperatures and viraemia for most of the challenged piglets (Schlafer et al., 1984a). This is the longest time period for ASFV maternal antibodies in piglets reported in the literature.
Duration of maternal antibodies of other viral infections of pigs and wild boar gives an indication of the possible time range for maternal antibodies against viruses that may persist in piglets. The classical swine fever virus (RNA virus) and porcine parvovirus (DNA virus) maternal antibodies have been shown to last in piglets for 2–4 months (Kaden and Lange, 2004; Fenati et al., 2009), whereas Aujeszky's disease virus (DNA virus) maternal antibodies lasted up to 6 months (Müller et al., 2005).
4.4.3. Transmission parameters
The transmission parameters of ASFV genotype II have been estimated both from data obtained from animal experiments and from using the field data. The summary of the results of direct transmission parameters investigated in these studies is presented Table 5.
Table 5.
Direct transmission parameter estimates for ASFV genotype II and Sardinian strain of genotype I
| Subspecies | Genotype of the virus | Study type | Parameter values | Reference |
|---|---|---|---|---|
| Pigs and wild boar | Georgia II | Animal experiment Wild boar only group Wild boar/domestic pigs mixed group | R0 within pen: 5.0 (95% CI: 1.4–10.7) 6.1 (95% CI: 0.6–14.5) | Pietschmann et al. (2015) |
| Pigs | Georgia II | Animal experiment | Pig‐ to‐pig R0: 5.0 (95% CI 2.4–9.1) | Guinat et al. (2016b) |
| Pigs | Georgia II | Animal experiment | β within pen: 1.05 (95% CI 0.62–1.72) | Nielsen et al. (2017) |
| Pigs | Georgia II | Observational (Field data) | R0 within farm: from 8 to 11 | Gulenkin et al. (2011) |
| Pigs | Georgia II | Observational (Field data) | R0 within herd: from 4.4 to 17.3 | Guinat et al. (2018) |
| Pigs | Sardinia I | Observational (Field data) | R0 within herd: min: 1.20 (95% CI: 0.22–2.26) max: 2.67 (95% CI: 0.75–4.59) | Franzoni et al. (2020) |
R0 – basic reproduction number.
β – number of infectious contacts per infectious animal per time unit.
The transmission parameter estimates from experimental studies are dependent on both the experimental setting and conditions, including the number of animals used in the experiment and the size of the pen in which the animals are kept. Nevertheless, the experimental conditions probably mimic the conditions that pigs are held in commercial pig farms as well as the contact patterns between animals in wild boar social groups in nature.
There are no experimental data on transmission of ASFV from infected carcasses to susceptible wild boar. The studies estimating the basic reproduction number (R0) for genotype II ASF virus in wild boar are based on field data and incorporate the effect of all transmission routes. The following R0 estimates have been obtained from different affected countries:
Russia 1.58 (95% CI: 1.13–3.77) (Iglesias et al., 2016)
Czechia 1.95 (Marcon et al., 2019)
Belgium 1.65 (Marcon et al., 2019).
The R0 estimates for ASFV genotype I in Sardinia calculated per hunting management unit have been somewhat lower but in a similar range: minimum 1.12 (95% CI: 1.10–1.15) and maximum 1.17 (95% CI: 1.01–1.33) (Loi et al., 2020).
The transmission parameter estimates from the field data are influenced by local conditions (e.g. population density and management of wild boar), which have an effect on contact rates between the animals and animal groups. Also, the applied intervention measures have an effect on the estimates (removal of carcasses, fencing, hunting). The R0 estimates for wild boar are similar to between pig herd R0 calculated from Russian data (between 2 and 3; Iglesias et al., 2016), indicating that this represents the most likely R0 between social groups of wild boars (Marcon et al., 2019).
The indirect transmission parameters have been estimated for domestic pigs in experimental conditions by Guinat et al. (2016b). Four pens were located in two rooms (two pens in each) allowing indirect contact between inoculated and susceptible pigs in adjacent pens, most likely to be through airborne transmission and small amounts of urine and faeces passing under the fence. Nose‐to‐nose contacts were prevented. The R0 between pen in this experiment was estimated to be 2.7 with 95% CI 0.7–5.2 (Guinat et al., 2016b). Based on data from this experiment the estimated β between pen [the number of infectious contacts per infectious animal in one time unit (day)] was 0.46 with 95% CI 0.17–1.00 (Nielsen et al., 2017).
The infection probability per carcass has been determined using spatial–temporal data of ASF notifications in wild boar in Animal Disease Notification System (ADNS) and a spatial‐explicit ASF simulation model (Lange and Thulke, 2017). In order to simulate the most similar spatio‐temporal pattern of ASF notifications (epidemiological model) to observed one (ADNS database) a low probability of transmission by carcasses (βcarc ~ 0.15) was required, while the possibility of contact with carcasses by live hosts had to be maximal (Paccess 0.9–0.99).
4.5. Spatial‐explicit stochastic model outputs
4.5.1. Characteristics of simulated outbreaks in Estonia
The following chapter considers the virological and serological profiles of wild boar populations in regions of Estonia following simulated ASF spread in a spatial‐explicit stochastic model. These data are used to inform the plausibility of sets of criteria relevant to a proposed pathway to demonstrate the absence of ASFV circulation in the wild boar population. We subsequently refer to this as the Exit Strategy.
This section considers the duration of virus circulation, the number of virologically and serologically positive animals and the number of carcasses attributed to ASF infection throughout the epidemic in a regional wild boar population.
Time horizon of virus circulation
Figure 14A presents the epidemic curve over time, in the simulated landscape and highlights the variability in this curve, as reflected in different stochastic repetitions (100). Time to fade‐out over the simulation landscape is represented using a standard survival curve, based on an extended number of simulation runs (600; Figure 14B). There is a period of epidemic spatial spread (up to about year 5), subsequent fade‐out following saturation of the entire simulated landscape (between years 5 and 10), and stochastic fade‐out of the remaining 10% of simulation runs (beyond year 10).
Temporal profile of virus‐positive animals
Figure 15 presents the simulated dynamics of virus prevalence in Estonia during the 3 years after introduction of ASF into LAU 1 units (A) and during the final stage prior to fade‐out in LAU 1 units (B). The typical epidemiological curve with rapid incline and slower decline is visible in (a), which is the standard way of presenting these epidemic data. Following a realignment of data to t = 0 (b), this being the time point of fade‐out, there is a steep decline in virus prevalence in the last year prior to fade‐out, which is probably due to collapse in the susceptible population that occurred once the whole LAU 1 unit had been infected.
Figure 15.
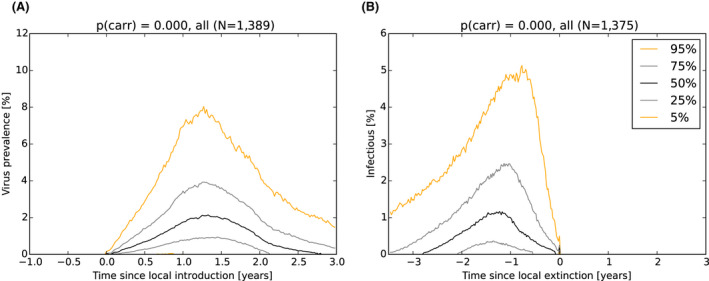
- Variation over time between simulated runs and different LAU 1 units is presented using percentiles. Data are aligned by the week of first ASF incursion (A, left), and by the date (t = 0) in each LAU 1 unit when the last infectious animal had succumbed to ASF (B, right). Weekly prevalence was calculated given the present population number (i.e. number of animals alive). The data series comprise 100 runs and 13 LAU 1 units. The simulation was conducted over the full territory of Estonia (except islands).
Temporal profiles of serologically positive animals
Figure 16 shows the corresponding dynamics of serologically positive animals. The data are presented for the adult cohort (A, B) and the subadult cohort (C, D). Following local virus extinction, adult seropositive animals are detectable for an extended period, whereas seropositive subadults can only be detected for a limited time. For these two reasons, the serology of adults is not useful for the development of the Exit Strategy, while the disappearance of seropositive subadults from a surveillance sample may be suggestive of local extinction and a plausible exit point. These outputs are comparable with the seroprevalence analysis done in Section 4.1.1 for Estonia.
Figure 16.
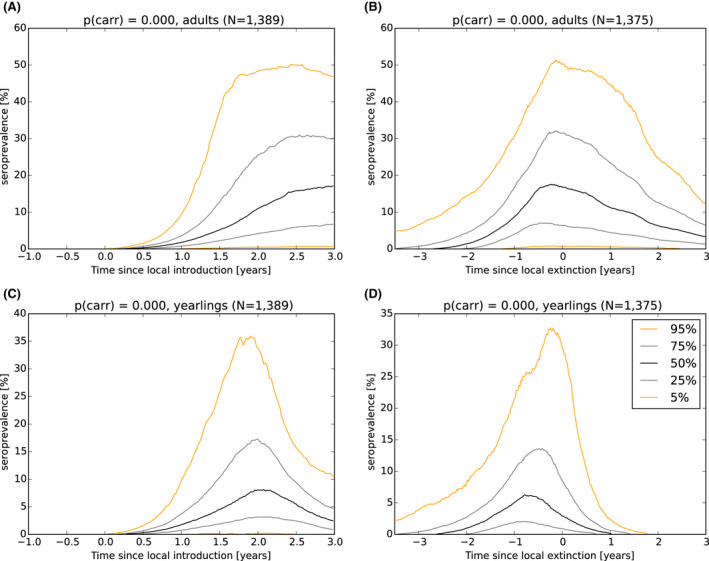
- Variation over time between simulated runs and different LAU 1 units is presented using percentiles. Data are aligned either by time of initial ASF incursion (A+C) or time of local extinction (B+D), per LAU 1 unit (t = 0). Weekly prevalence was calculated based on population numbers at that point in time. The data series (N = 1,223) comprise 100 runs and 13 LAU 1 units. The simulation was conducted over the full territory of Estonia (except islands).
Temporal profiles of fatality associated to ASFV infection
Figure 17 represents the temporal dynamics of the number of carcasses attributable to ASF infection in affected LAU 1 units. The standard view, with the data aligned by date of ASFV introduction (A), highlights the seasonal fluctuation in the number of carcasses present in the environment, which reflects the seasonal change of carcass decomposition due to, for example, temperature fluctuations (Section 4.3.1). In (B), with the data aligned to the date when the last infectious animal had succumbed to ASF in each LAU 1 unit, there is a substantial number of carcasses of animals that died due to ASF during the period shortly prior to viral fade‐out. In comparison with the number of virus‐positive animals (see Lange et al., 2021), the ratio between the number of virus‐positive pigs that are alive and the number of carcasses attributable to ASF infection of animals that died of ASF is always greater that 1:5, which suggests the greater usefulness of intensive carcass collection to inform a potential exit decision.
Figure 17.
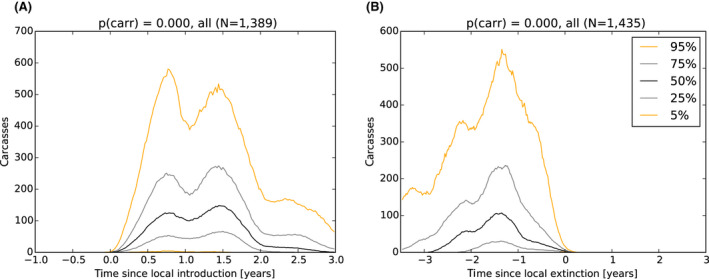
Temporal dynamics of the number of carcasses attributable to ASF infection that were present in the environment in simulated wild boar populations in Estonia at the LAU 1 unit Variation over time between simulated runs and different LAU 1 units is presented using percentiles. The data represent the actual number of carcasses present after accounting for time to decomposition, and not the weekly incidence. Data are aligned by the week of the first ASF incursion (A), and date when the last infectious animal had succumbed (B), per LAU 1 unit (t = 0). The data series comprise 100 runs and 13 LAU 1 units. The simulation was conducted over the full territory of Estonia (except islands).
4.5.2. Scenarios representing mechanisms that potentially could prolong circulation of ASFV infection within an area
This section addresses potential mechanisms (‘scenarios’) that could impact the time of circulation of ASFV infection in wild boar populations. These mechanisms, which are each linked with current scientific uncertainties or gaps in knowledge, may interfere with criteria associated with proposed Exit Strategies.
For each scenario, the impact on the duration of circulation of infections was analysed and compared with the standard model as outlined in Section 4.5.1. For two scenarios, there is evidence of a substantial change in the duration of virus circulation [carriers (see section on Scenario 1: Prolonged infectious period (that is, carrier animals)) and prolonged infectious period with low lethality (see section on Scenario 2: Reduced case‐fatality rate and a lengthened period of transient infectiousness among surviving animals)]. For the carrier scenario, the diagnostic profiles (i.e. virus positive, seropositive) and carcass volume are presented in the presence and the absence of carrier animals. For all other scenarios (those without evidence of substantial change in the duration of virus circulation), see Lange et al. (2021) for detailed profiles.
Scenario 1: Prolonged infectious period (i.e. carrier animals)
The scenario shown in Figure 18 introduces the concept of carrier status into the model, while acknowledging that there is uncertainty around this concept (as elaborated in Section 4.3.2). The model outcomes demonstrate that the presence of carriers can change the duration of virus circulation, dependent on the proportion of animals that are carriers (Figure 18). With this scenario, the model intentionally did not alter the proportion of animals that survive the infection and become immune (i.e. a transient course of infection). The proportion of lifelong infectious animals was added to the 0.05 infected animals that, on average, survive infection in the standard model (black line).
Figure 18.

- Fade‐out graphs reflect the effect of differing proportions of infected animals developing into a (lifelong) carrier status (worst case version). Following initial introduction of infection in the simulation area (t = 0, x‐axis), the graph presents the percentage of simulation runs that still contained either infectious animals or carcasses attributable to ASF infection (y‐axis) at a given point of time.
For comparison, the temporal dynamics of virus (PCR+) prevalence, without and with carrier animals, is presented in Figure 19.
Figure 19.
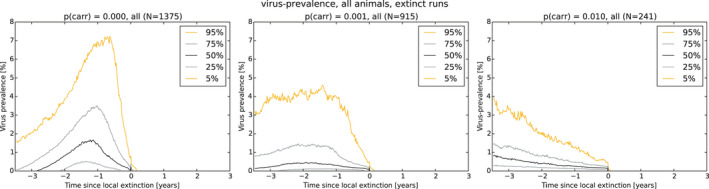
- Variation over time between simulated runs and different LAU 1 units is presented using percentiles. Data are aligned by the week that the last infectious animal succumbed to ASF, per LAU 1 unit (t = 0). Left: standard model, no animals develop lifelong infectivity; Middle: 1 out of 1,000 infected animals develops lifelong infectivity; and Right: 1 out of 100 infected animals develops lifelong infectivity. Weekly prevalence was calculated given the present population number (i.e. number of animals alive). Of the original data series (comprising 100 runs and 13 LAU 1 units), only those simulation runs in which fade‐out occurred are presented here. The simulation was conducted over the full territory of Estonia (except the islands). Additional plots are presented in Lange et al. (2021), including the numbers of virus positives.
The decline of virus prevalence was substantially slower in the presence of carriers in comparison with the standard model.
For comparison, the temporal dynamics of seroprevalence in subadults, without and with carrier animals, is presented in Figure 20.
Figure 20.
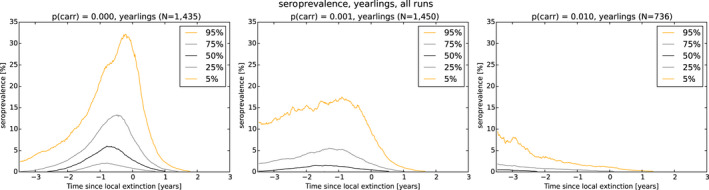
- Variation over time between simulated runs and different LAU 1 units is presented using percentiles. Data are aligned by the date (t = 0) in each LAU 1 unit when the last infectious animal had succumbed to ASF. Left: standard model, no animals develop lifelong infectivity; Middle: 1 out of 1,000 infected animals develops lifelong infectivity; and Right: 1 out of 100 infected animals develops lifelong infectivity. The weekly prevalence was calculated given the current population number (i.e. the number of animals alive) in the respective age class. Of the original data series (comprising 100 runs and 13 LAU 1 units), only those simulation runs in which fade‐out occurred are presented here. The simulation was conducted over the full territory of Estonia (except the islands).
Figure 20 shows the three seroprevalence profiles with an increasing proportion of infected animals with carrier status (left to right: zero, one in a thousand and one in a hundred infected animals). In comparison with the virus prevalence in adult animals (Figure 16B), there is a more marked difference in the seroprofile of subadult animals with an increasing proportion of carriers involved. In comparison with adults, the seroprevalence in subadults is lower and the decline in seroprevalence much slower in the years prior to regional extinction, as the proportion of carriers increases.
For comparison, the temporal dynamics of carcasses of animals succumbed to infection, without and with carrier animals, is presented in Figure 21.
Figure 21.
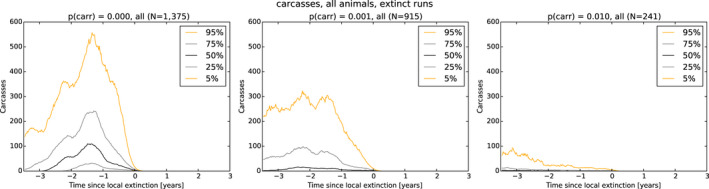
- Variation over time between simulated runs and different LAU 1 units is presented using percentiles. The data represent the actual number of carcasses present after accounting for time to decomposition, and not the weekly incidence. Data are aligned by the week of the last infectious animal present per LAU 1 unit (t = 0). Left: standard model, no animals develop lifelong infectivity; Middle: 1 out of 1,000 infected animals develops lifelong infectivity; and Right: 1 out of 100 infected animals develops lifelong infectivity. Of the original data series (comprising 100 runs and 13 LAU 1 units), only those simulation runs in which fade‐out occurred are presented here. The simulation was conducted over the full territory of Estonia (except islands).
Figure 21 presents the temporal carcass dynamics with an increasing proportion of infected animals with carrier status (left to right zero, one in a thousand and one in a hundred infected animals). Similar to the seroprevalence profiles in subadults (Figure 20), carcass numbers attributable to ASF are lower and the decline in carcass numbers much slower in the years prior to regional extinction, as the proportion of carriers increases.
Scenario 2: Reduced case‐fatality rate and a lengthened period of transient infectiousness among surviving animals
The second scenario considers the impact on virus persistence of a reduced case‐fatality rate (5% surviving, 10%, 20%) coupled with a prolonged period of transient infectivity (1, 4 weeks) among animals that survive ASFV infection. As highlighted in Figure 22A, variation in case‐fatality alone did not substantially impact the duration of virus circulation, given transient infectivity of about 1 week among surviving animals. The related diagnostic and population profiles do not differ from those in Section 4.5.1 (see Lange et al., 2021). There was an impact on duration of virus circulation when the duration of transient infectivity among surviving animals was increased to 4 weeks (Figure 22B), however, final fade‐out was only marginally affected. The related diagnostic and population profiles do not differ from those in Section 4.5.1 (see Lange et al., 2021). Indeed, viral and serological prevalence both exhibit an even stronger dynamics towards viral extinction compared with the standard scenario, and therefore, the relevant exit criteria are not influenced.
Figure 22.
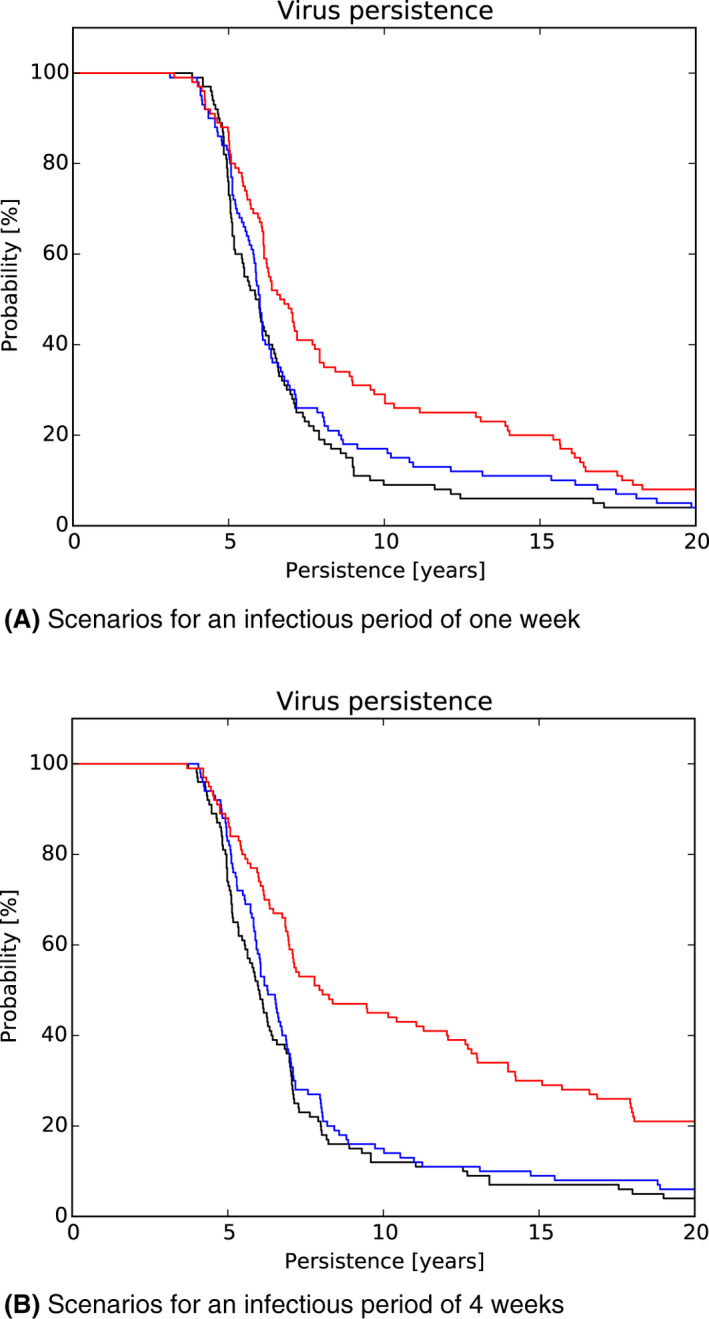
- The simulations compare the standard model (black line, transient infections 5%) with scenarios assuming greater case survival (blue line 10%; red line 20%). Additionally, the transiently infected animals had an infectious period of either 1 week (A) or 4 weeks (B). Fade‐out graphs show the proportion of simulation runs that still contained either infectious animals or carcasses attributable to ASF infection (y‐axis) if the infection was introduced in the simulation area at t = 0 (x‐axis).
Scenarios 3 and 4: Loss of protective immunity and duration of protection from maternal antibodies
The third and fourth scenarios consider, respectively, the impact of the duration of protective immunity among animals surviving ASFV infection and the duration of protection from maternal antibodies on the duration of virus circulation. For each scenario (Figure 23A: loss of protective immunity among surviving animals: no loss (standard model) vs. loss after 52 weeks; Figure 23B: duration of protection from maternal antibodies: 12 (standard model) 7, 0 weeks), the impact on duration of virus circulation was minimal. Furthermore, the related prevalence and population profiles do not differ from those in Section 4.5.1 (see Lange et al., 2021).
Figure 23.
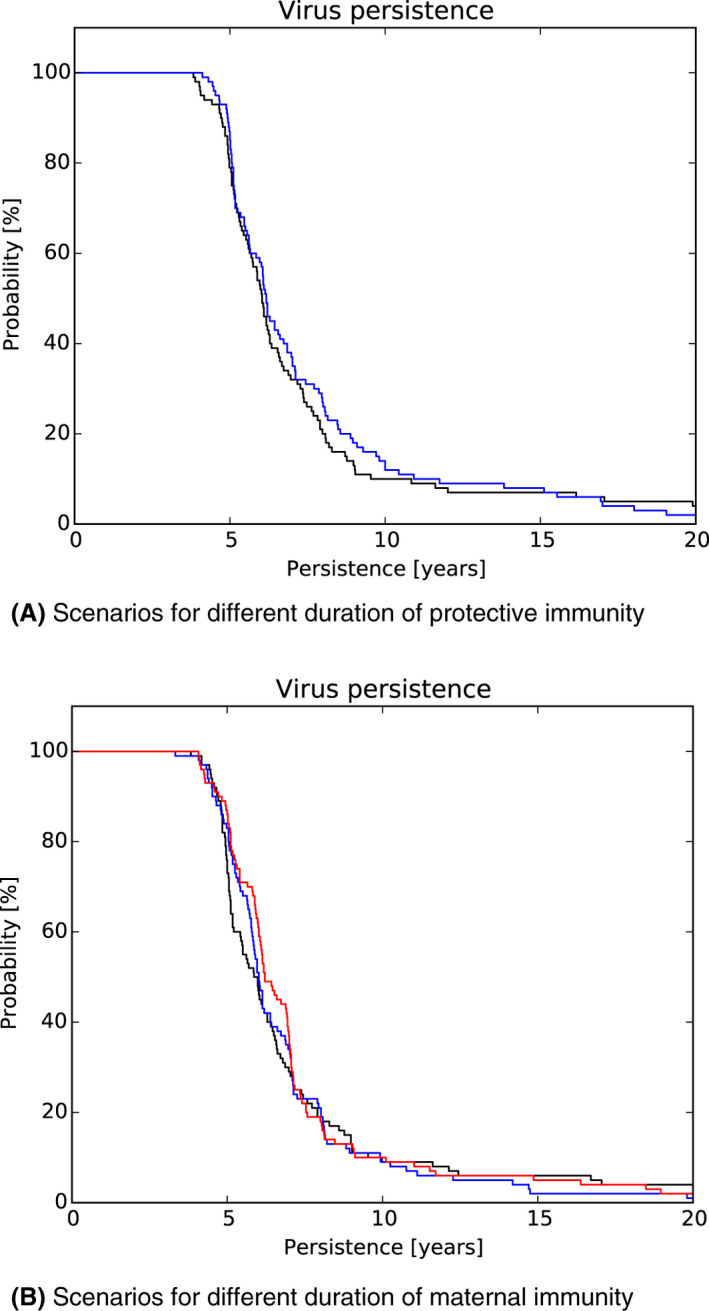
- Fade‐out graphs show the proportion of simulation runs that still contained either infectious animals or carcasses attributable to ASF infection (y‐axis) if the infection was introduced in the simulation area at t = 0 (x‐axis).
4.5.3. Evaluating proposed Exit Strategy criteria
General principles
The data analysis and the model simulations provide an understanding of virus and seroprevalence (obtained through active surveillance) and carcass abundance (passive surveillance) within the simulated wild boar population (see Sections 4.1 and 4.5.1). With this information, it is possible to identify, consider and subsequently test a range of decision criteria, which are based on aspects of passive and active surveillance. The outcome will be presented in Chapter 5. The following paragraphs are intended to introduce the general approach of the proposed Exit Strategy, and to highlight uncertainties to be considered in association with the final strategy recommendation.
The proposed approach, in general, will consider two phases. First, a Screening Phase with a focus on virus detection, using routine surveillance. This phase should continue for a defined period, as considered in detail later. The approach only switches to a Confirmation Phase if no virus is found during the Screening Phase. During the Confirmation Phase, the aim is to maximise the surveillance effort without finding evidence of virus circulation. There is a need to parameterise the proposed Exit Strategy, to determine the appropriate duration and minimum surveillance effort in both the Screening and Confirmation Phases.
The motivation of the two‐phase approach is based on the logic that an exit scenario will be conducted during a period when there are very few infected animals (with these animals being difficult to detect) and very few virus‐positive carcasses. This is confirmed in the virological and serological prevalence profiles, as reported previously (Figures 15 and 16). In 50% of simulation runs, less than 0.5% of animals are virus positive shortly before viral extinction. Furthermore, in wildlife populations, such as wild boar, representative sampling is difficult to achieve in a practical sense, and unbiased estimates of population characteristics, such as density, virus prevalence and seroprevalence, cannot be obtained. Given this, the evidence to demonstrate the absence of ASFV circulation has to be accumulated over time (Thulke et al., 2000). Nonetheless, the time horizon and surveillance effort proposed with an Exit Strategy must be sustainable under field conditions. For this reason, a longer phase with routine surveillance effort (the Screening Phase) and a shorter (minimal) phase with increased surveillance effort (the maximum possible under field conditions) (the Confirmation Phase) is proposed. It is logical that virological information is used as the primary criterion in the Exit Strategy, with serological information from subadults considered as a possible secondary criterion if no virus is detected. The secondary criterion is linked to the finding that in 50% of runs and after about 1 year following viral extinction, subadult animals with positive serological status are no longer present (see Figure 16).
The model was used to evaluate the two‐phase Exit Strategy by considering the failure rate given differing monitoring periods during each phase. The failure rate is defined as the percentage of false exit decisions that were obtained by proposing freedom from ASF while (undetected) infectious objects (live animals and carcasses attributable to ASF infection) were still present in the simulation area. The outcome of this process is presented using a contour plot (Figure 24), which illustrates the failure rate for combinations of differing monitoring periods during the Screening (x‐axis) and Confirmation (y‐axis) Phases. The failure rate was calculated across multiple simulations (100 per combination).
Figure 24.
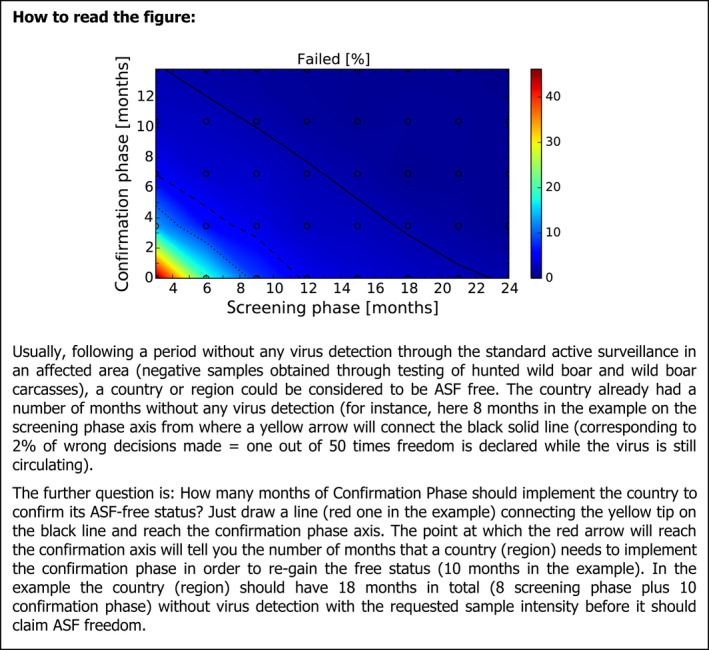
- The failure rate is presented given differing monitoring periods during the Screening and Confirmation Phases. The surveillance effort was 1 carcass per 1,000 km2 per year during the Screening Phase (x‐axis) and 2 carcasses (doubled intensity) during the Confirmation Phase (y‐axis). The colours represent the percentage of trials in which the Exit Strategy would have failed, i.e. obtaining a false‐negative result by proposing freedom from ASF while (undetected) infectious objects were still present in the simulation area. Lines show isoclines for failure rate of 2% (solid), 5% (dashed) and 10% (dotted). The random spread scenario ignores the human translocations that were observed in Estonia during 2015–2018 (EFSA, 2018).
Box 1: Example of how to interpret a heat diagram
Assessing the importance of the passive surveillance component
The efficiency of passive surveillance for ASF detection has previously been reported (EFSA AHW Panel, 2010b; EFSA, 2018; Lange, 2015; Gervasi et al., 2020). Importantly, the accuracy of the Exit Strategy in supporting a decision on viral extinction is increased with an increasing number of carcasses that are routinely collected and tested. This is illustrated in Figure 25 where the percentage of false exit decisions (the probability of false‐negative results) decreases (15.57%, 5.41%, 3.91% and 2.44%) concurrent with an increase in the percentage of all carcasses that are collected during the Confirmation Phase in the wild boar area (resulting in 0, 1, 2 and 6 carcasses per year and 1,000 km2, respectively). The box plots also demonstrate the increasing variability in the number of carcasses collected in the different model runs in each sampled area.
Figure 25.
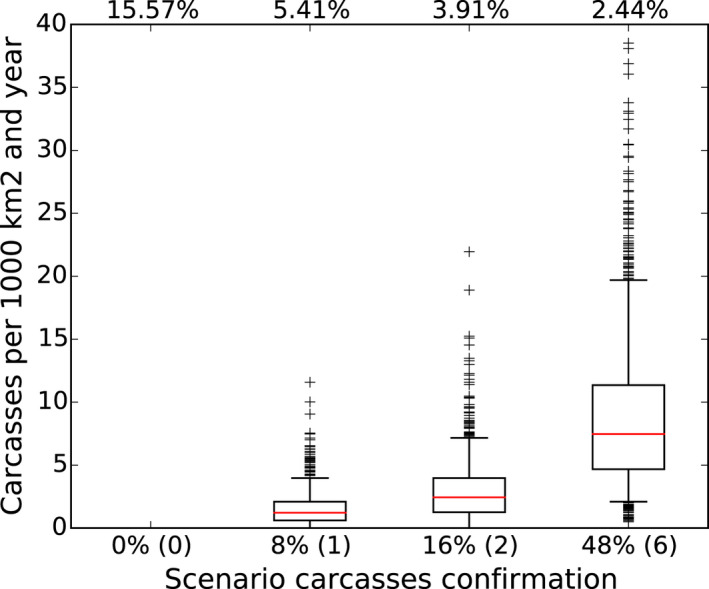
- The scenario replicates 1 carcass collected per year and 1,000 km2 in the Screening Phase, but varies the intensity of carcass detection during the Confirmation Phase (bottom legend), namely 0, 1, 2 or 6 carcasses per 1,000 km2 per year (corresponding to 0%, 8% 16% and 48% carcass detection probability). The box plots present the variation in the number of carcasses that were actually collected in LAU 1 units and across model runs. The data are shown only for one specific combination of monitoring periods: Screening Phase of 6 months, Confirmation Phase of 7 months (a total of 13 months). At the top of the figure, the resulting strategy performance is shown in terms of the probability of a false decision.
Assessing the importance of active surveillance component
As highlighted previously in Section 4.5.1, the response in the seroprevalence profile among subadult wild boar in ASF affected populations occurs within about 1 year following viral extinction. In contrast, a similar response among adult animals is slower and does not provide information that could be used as an Exit Strategy criterion.
The impact of different components of active surveillance on the performance of the Exit Strategy can be demonstrated through a comparison of model simulations that do or do not include active surveillance within the two‐phase approach. Figure 26 summarises this information, highlighting several conclusions that can be drawn following a systematic investigation of different Exit Strategies. For details of the stepwise development process, see Lange et al. (2021).
Figure 26.
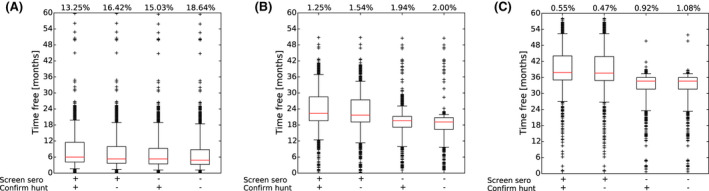
- Box plots reflect either the inclusion or omission of active sampling of subadults for serology during the Screening Phase (sero+, done; sero−, omitted) and of any active surveillance during the Confirmatory Phase (hunt+, all tested for viral genome and antibody; hunt−, no shot animals are tested). The box plots present a summary of the length of time that a LAU 1 unit was free of ASFV infection before the Exit Strategy came to a decision for that LAU 1 unit. The data are shown only for specific combinations of monitoring periods during the Screening or Confirmation Phase: (A) 3 + 4 (a total of 7) months; (B) 12 + 11 (23) months; (C) 24 + 14 (38) months. At the top of the figures, the resulting strategy performance is shown in terms of the probability of a false decision.
First, consider the performance of each Exit Strategy option, which is represented at the top of each figure (13.25%, 16.42%, etc.). This is the probability of a false decision, i.e. concluding from surveillance outcome that the virus is absent when in fact it is not. Comparison between (A) (left), (B) (centre) and (C) (right) demonstrates how a lengthening of the monitoring period leads to an improvement in Exit Strategy performance, reflecting the importance of the accumulation over time of evidence for freedom. Prolonging the monitoring periods (from 7 to 38 months in Figure 26) provides increasing confidence about the safety of the resulting exit decisions, from below 85% in (A) up to more than 99% in (C). However, this performance improvement needs to be balanced against an increase in the ‘time free’, i.e. the time lag between point of viral extinction and time when an exit decision is possible. This trade‐off may require an economical evaluation of surveillance efforts compared with commercial costs of restrictions, which is beyond the scope of this mandate.
Second, the four performance values on the top of each individual diagram highlight the limited impact of active surveillance within the broader Exit Strategy. In each of these three diagrams, the value at the far left reflects the maximum application of active surveillance (in addition to passive surveillance, hunted animals are tested for viral genome (hunt B+) and subadult animals are tested for serology (sero A+) in both the Screening and Confirmation Phases). In contrast, the value at the far right of each diagram reflects the Exit Strategy performance in which subadult serology is omitted in both phases (sero A−) and virological investigations of hunted animals is limited to the Screening Phase (hunt B−), i.e. active surveillance was not performed at all in the Confirmation Phase. Furthermore, the inclusion of active surveillance in the Exit Strategy had a very limited impact on performance compared with a lengthening of the monitoring period. To illustrate this, consider Figure 26A, in which the choice of the monitoring periods (Screening plus Confirmation Phases) is extremely short (7 months in total). With this scenario, the difference between the performance value at the far left (13.25%; Figure 26A) and the far right (18.64%; Figure 26A), i.e. the effect of active surveillance, is minimal compared with the improvement that is achieved when the monitoring period is prolonged, either to 23 months, resulting in a failure rate between 1.25% and 2.0% (Figure 26B), or 38 months, with a failure rate between 0.55% and 1.08% (Figure 26C). In conclusion, the contribution of active surveillance to an Exit Strategy appears less important than either passive surveillance (carcasses collected) and the lengths of the monitoring periods during the Screening and Confirmation Phases.
Based on earlier investigations of subadult serological profiles following viral extinction (Figure 16), it appeared that serological surveillance of sub‐adults may add information about the adequacy of an exit decision. However, further investigation has shown that this information is of marginal benefit. In hindsight, this conclusion is logical given the greater efficiency of passive, compared with active, surveillance for case finding, as highlighted previously (Gervasi et al., 2020). Specifically, information from subadult serology will be redundant in the presence of robust passive surveillance. Furthermore, if virus circulation were still present, signals will be picked up more rapidly through passive surveillance from carcasses than from active (i.e. serological) surveillance of subadults.
Active (serological) surveillance of subadults is not only without value but comes at an extra cost for the Exit Strategy. If it were to be included within the protocol, the detection of seropositive subadults would argue for a return to the start of the exit procedure. This will prolong the period between viral extinction and the exit decision, as demonstrated by Figure 26(B) and (C) (scenarios with sero A+ compared with those with sero A−), even though viral extinction had already occurred.
Assessing the importance of the carrier scenarios
Two possible mechanisms prolonging viral circulation in a regional wild boar population were identified as potentially important, including carrier animals (i.e. animals with lifelong infectivity, section: Scenario 1: Prolonged infectious period (i.e. carrier animals)) and transiently infected survivors (with 20%, rather than 5%, of animals surviving the infection with an infectious period of greater than 4 weeks, rather than 1; see section on Scenario 2: Reduced case‐fatality rate and a lengthened period of transient infectiousness among surviving animals). Both concepts are motivated by knowledge of other wild boar infections, specifically classical swine fever, long‐term infectious animals [chronic infections or persistently infected (PI) piglets, Kramer‐Schadt et al., 2007] facilitate continuous circulation of that infection in wildlife populations (Kramer‐Schadt et al., 2009). The epidemiology of ASF and CSF is not compatible, in part due to the large difference in case fatality between the two diseases. Nonetheless, the impact of a potential increase in duration of the infectious period on the estimated duration of viral circulation of ASF in wildlife needs to be considered (Figures 18 and 22B).
As outlined in section on Scenario 2: Reduced case‐fatality rate and a lengthened period of transient infectiousness among surviving animals, the virological and serological profiles do not differ from the standard scenario if there is an increase in survival, with surviving animals having a longer (but still transient) period of infectivity (Figure 22 ). Similarly, this scenario does not influence the outcomes of the exit approach proposed here.
In contrast, virus circulation is substantially influenced by the scenario of lifelong carrier animals because these animals dominate the epidemiological situation. A slower decline in virus prevalence is observed, and seropositive and virus‐positive animals may be present, albeit in very small numbers, over long periods. Similarly, there will be a low number of carcasses from infected animals over an extended period. In this scenario, exit is a ‘trial and error game’, with carrier animals contributing for years to ASF spread in an affected region. As a consequence, a proposed exit approach will repeatedly result in a restarting of the Screening Phase due to ongoing infection circulation and the detection of low number of infected carcasses. As reflected in Figure 27, the respective result is disappointing, both in terms of performance and time free (Figure 27). In conclusion, an Exit Strategy is problematic in the presence of carrier animals. That said, it should be emphasised that the existence of carriers is speculative, based on current knowledge.
Figure 27.
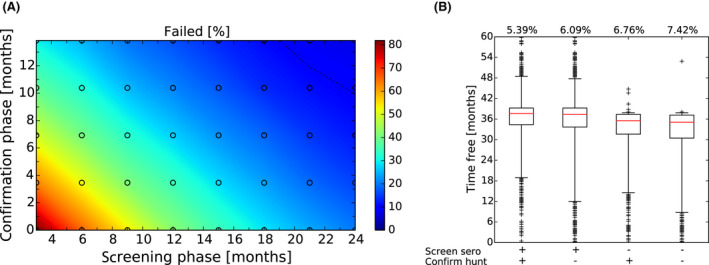
- (A) Model outcome of different parameterisations of an Exit Strategy in terms of failure rate assuming 1 lifelong carrier animal for every 100 ASF‐infected wild boar. The surveillance effort was 1 carcass per 1,000 km2 per year during the Screening Phase (x‐axis) and 2 carcasses (doubled intensity) in the Confirmation Phase (y‐axis). The colours represent the percentage of trials in which the Exit Strategy would have failed, i.e. obtaining a false‐negative result by proposing freedom from ASF while (undetected) infectious objects were still present in the simulation area. Lines show isoclines for failure rate of 2% (solid), 5% (dashed) and 10% (dotted). The random spread scenario takes account of the human translocations that were observed in Estonia during 2015–2018 (EFSA, 2018). (B) Comparison of different Exit Strategy options given differing levels of active surveillance during the Screening and Confirmation Phases. Equivalent to Figure 23C for the maximum considered monitoring periods of 24 + 14 months, but assuming 1 in 100 surviving animals being lifelong in an infectious carrier status.
Assessing the impact of natural mortality in a wild boar population
Assumptions with respect to the ‘estimated relative mortality not attributed to hunting’ in a wild boar population has implications for passive surveillance (see Lange et al., 2021 for details), specifically the effectiveness of passive surveillance for ASF case detection. In brief, an increased contribution of natural mortality will lead to increased carcass numbers, which in turn will decrease ASFV detection probability per carcass if the passive surveillance requirements remain unchanged (e.g. testing a single carcass per 1,000 km2 per year). In other words, there is a dilution of the effectiveness of passive surveillance due to increased numbers of non‐infected carcasses.
The model parameterisation of relative mortality not attributed to hunting was in accordance with the scientific literature regarding the portion of animals annually removed from the population (35–60%; Focardi et al., 1996; Gaillard et al., 1987). To split the data into mortality attributable to hunting and natural deaths, the following information was retrieved (J. Vicente pers. comm.), highlighting some variation in estimated relative mortalities:
If there were uncertainty about natural mortality rates in a region, a more conservative exit criteria would be advisable that can be derived from model outputs using the upper bound of natural mortality (i.e. 80% mortality due to hunting and 20% due to natural mortality), as presented in Figure 28.
90% due to hunting and 10% due to natural mortality (Focardi et al., 1996; V. Guberti pers. comm.),
80% due to hunting and 20% due to natural mortality (Toïgo et al., 2010),
values in‐between the two others (84% due to hunting/16% natural mortality) (Keuling et al., 2013). In comparison with Figures 24 and 28 highlights the extent to which the sensitivity of passive surveillance efforts is decreased with increasing numbers of negative carcasses in the landscape.
Figure 28.
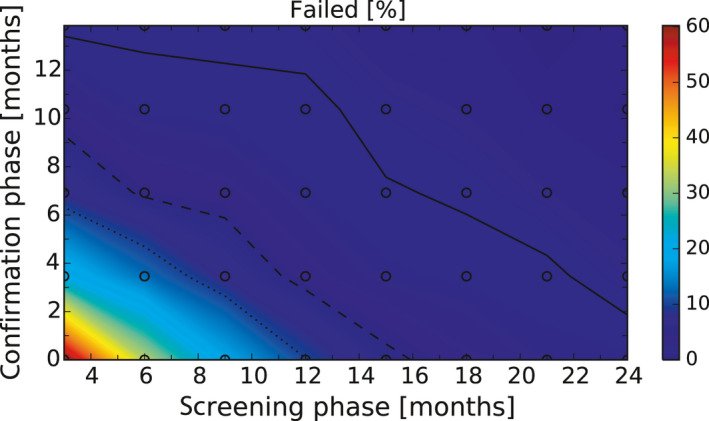
- The surveillance effort was 1 carcass per 1,000 km2 per year during the Screening Phase (x‐axis) and 2 carcasses (doubled intensity) during the Confirmation Phase (y‐axis). The colours represent the percentage of trials in which the Exit Strategy would have failed, i.e. obtaining a false‐negative result by proposing freedom from ASF while (undetected) infectious objects were still present in the simulation area. Lines show isoclines for failure rate of 2% (solid), 5% (dashed) and 10% (dotted). The random spread scenario takes account of the human translocations that were observed in Estonia during 2015–2018 (EFSA, 2018).
5. Recommendations for the Exit Strategy approach
The previous chapter have outlined population‐level events that occur prior to and following local virus extinction. Based on this information, a comprehensive range of two‐phase combinations were applied to identify the Exit Strategy that was best suited to various situations. Modelling and sensitivity analysis were used, with the aims:
to minimise the time delay between extinction and final decision;
to minimise the failure rate (the probability of a false decision on disease freedom).
Table 6 lists the minimum requirements during the Screening and Confirmation Phases of an Exit Strategy to demonstrate freedom of ASFV circulation under two different epidemiological scenarios and in line with EU ASF control strategy, including:
freedom following an eradication scenario (i.e. local containment of the epidemic in a small area);
freedom following a control scenario (i.e. countrywide spread of the epidemic) (European Commission, 2015).
Table 6.
Minimum requirements during Exit Strategy proposed for two different epidemiological scenarios
| Exit Strategy I | Exit Strategy II | |||
|---|---|---|---|---|
| Target | Freedom following eradication scenario (see EU strategya) | Freedom following control scenario (see EU strategya) | ||
| Local containment of epidemic in small area, e.g. the past epidemics in the affected area in Czechia and Belgium | Countrywide spread of epidemic, large area, e.g. Estonia and Latvia | |||
| Screening Phase (SP): all samples negative | ||||
| Passive surveillance | Number of carcasses = 2% of hunting bag prior to ASF introduction (2% HB) | Test at least 1 carcass per 1,000 km2 per year (SP 1)b (baseline intensity) | ||
| Active surveillance | No specific requirements | Test all hunting bag for virus | ||
| Confirmation Phase (CP): all samples negative | ||||
| Passive surveillance | Number of carcasses = 2% of hunting bag prior to ASF introduction (2% HB) | Test at least 1, 2 or 6c carcasses per 1,000 km2 per year (CP1, CP2 and CP3, respectively)b (increased intensity) | ||
| Active surveillance | No specific requirements | Test all hunting bag for virus | ||
| Minimum monitoring periods | Combination of duration Screening Phase (phase A) with the adequate period for Confirmation Phase (phase B) can be seen in Figure 29 | Combination of duration monitoring period Screening Phase with the adequate period for Confirmation Phase can be seen in Figures 30–32. Example: To achieve a failure rate of maximum 2% (solid line) after 12 months applying Exit Strategy II's Screening Phase (including 1 carcass per 1,000 km2 per year) one may need to monitor further 11 months in the Confirmation Phase with 1 carcass per year and 1,000 km2 (Figure 30), 7 months with 2 carcasses (Figure 31), 3 months when collecting 6 carcasses (Figure 32) | ||
Annex III of the Strategic approach to the management of African Swine Fever for the EU (European Commission, 2015).
Carcass collection efforts assumed to be distributed in time and space.
The greater the intensity of carcass collection was chosen the shorter the monitoring period of the Confirmation Phase has to last.
Any PCR‐positive sample obtained during any sampling effort (including sampling conducted in addition to the requirements listed in Table 6) will require a reset of the start date of the procedure, resulting in a requirement to return to the start of the Screening Phase.
These requirements were based on the assumption that 10% of non‐ASF‐related mortality is due to natural mortality and 90% to hunting. As highlighted above, if there were uncertainty about natural mortality rates in a region, more conservative exit criteria would be advisable that can be derived from model outputs using the upper bound of natural mortality (i.e. 80% mortality due to hunting and 20% due to natural mortality). If serology of subadults is undertaken (including any sampling conducted in addition to the requirements listed in Table 6), the detection of seropositive subadults would also require a return to the date of the start of the Screening Phase.
Depending on the epidemiological situation, if PCR‐positive skeletonised carcasses are detected, it is recommended that virus isolation is performed to verify the viability of the virus (Fischer et al., 2020; Zani et al., 2020). This is because PCR is able to detect the virus genome even if the virus is no longer viable/infectious. It is rarely possible to accurately determine the date of death of animals on the basis of skeletal remains.
Animals killed in car accidents should be considered as hunted animals in the Exit Strategy.
The Exit Strategy recommendations were formulated per 1,000 km2 but should be applied to the specific region size. For example, for a region of 2,500 km2, the required sample sizes provided in Table 4 have to be multiplied by 2.5, rounded upwards. It is expected that the samples are distributed as evenly as possible in time and space in order to provide a good representation of the wild boar population of interest.
Figure 29.
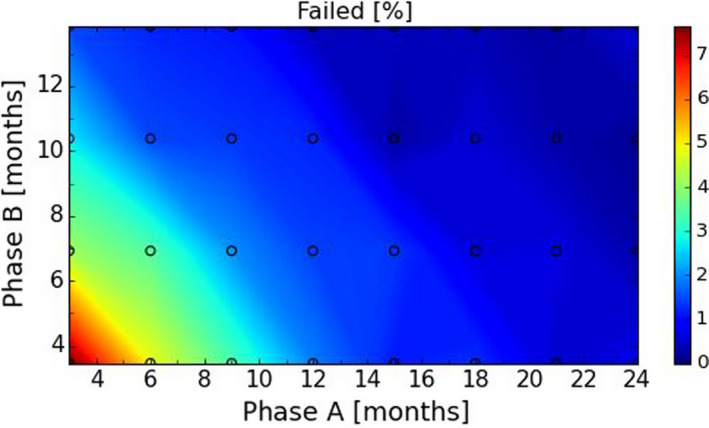
SP 2% HB + CP 2% HB as carcass; no active surveillance
Figure 30.
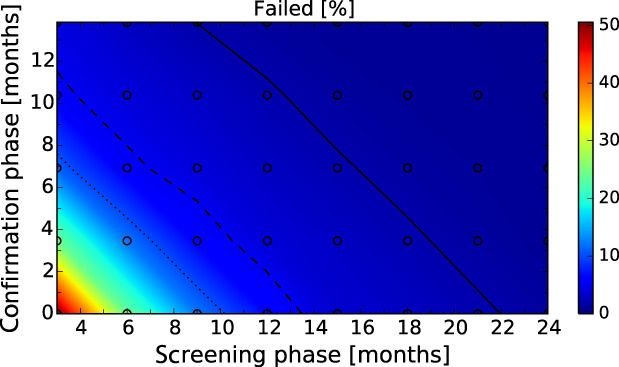
SP 1 + CP 1 carcass; no serology
Figure 31.
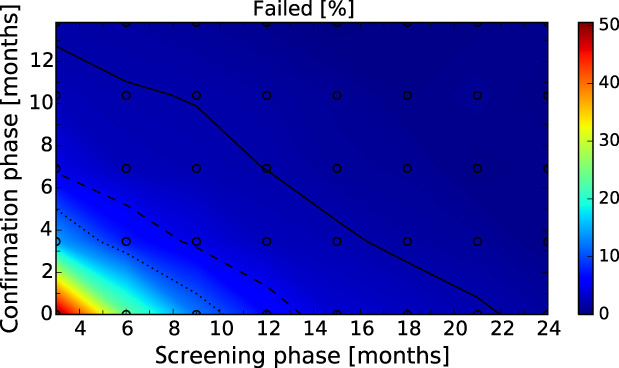
SP 1 + CP 2 carcasses; no serology
Figure 32.
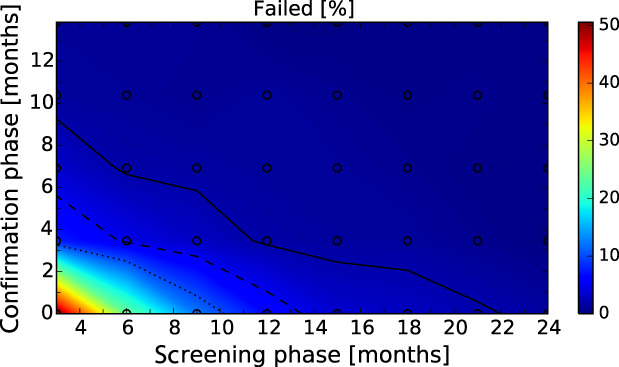
SP 1 + CP 6 carcasses; no serology
6. Conclusions
6.1. Exploration of surveillance data from Estonia, Latvia and Sardinia
Estonia and Latvia
Since the last detection of PCR‐positive wild boar in a given LAU 1 region in Latvia and Estonia, the seroprevalence in young wild boar (< 1 year old) decreased more rapidly and was lower than in older wild boar (≥ 1 year old). This could indicate the fade‐out of virus circulation.
The decline in seroprevalence in adult animals took more than 24 months to approach zero. For this reason, seroprevalence in adults is a poor indicator to demonstrate the absence of virus circulation.
The decreased population due to management and disease makes it more challenging to find carcasses for passive surveillance.
Sardinia
In Sardinia, a decline in virus and seroprevalence has been observed from 2015. In Anglona‐Gallura, ASF appears to fade out as, since December 2015, no virus‐positive and, since January 2020, no seropositive wild boar have been detected.
6.2. Sensitivity of ongoing surveillance activities in Estonia
The current sample intensity (mainly based on active surveillance) is insufficient to detect virus that could persist in small pockets or low prevalence.
The ongoing surveillance activities were not designed for demonstrating the absence of ASF virus circulation, although they could trigger the final surveillance steps needed to prove the absence of ASF virus circulation.
6.3. Possible hypotheses for persistence of African swine fever virus in wild boar populations, based on literature review
6.3.1. Viral persistence in the environment
Survival in the environment and carcasses
African swine fever virus is highly stable under a wide range of environmental conditions. Based on modelling studies, more than half of the transmission events in wild boar populations are attributed to contact between live wild boar and infectious carcass.
Wild boar are efficient scavengers of any carcass other than wild boar. The behaviour of wild boar towards dead conspecifics is likely to be one of avoidance, with occasional contact of infectious material around dead animals. Carcass removal is considered an important control measure.
In the environment, the stability of ASFV in tissues from infected wild boar varies in different matrices and at different temperatures. At −20°C, the virus remained viable in spleen, kidney and lungs from infected pigs for prolonged periods of time. At 4°C, ASFV could only be isolated immediately after artificial contamination of water, wet soil and wet leaf litter. In contrast, virus was viable over at least 56 days in water, straw and hay. At 23°C, putrefaction of spleen led to rapid decay of ASFV survival (half‐life 0.44 days) regardless of the underlying matrix.
Persistence through biological and mechanical vectors
Scavenger mammal and bird species are expected to represent a minor risk factor for spreading ASF in wild boar populations but may contribute to reducing local virus persistence by removing infected carcasses.
There is no field evidence of a role for biting arthropods (midges, mosquitoes, hard ticks, tabanids etc.) in the mechanical or biological transmission of ASFV. There was no evidence of ASFV replication in Ixodes ricinus or Dermacentor reticulatus, two commonly found hard tick species in Europe, and ASFV has not been detected in hard ticks in central Europe and the Baltic States.
Based on current knowledge, Ornithodoros spp., belonging to the Argasidae family of soft ticks, is the only tick genus able to transmit ASFV. Ticks of the O. erraticus complex are present in parts of the European, trans‐Caucasus countries and Russian Federation territories and may be important in maintaining the local foci of the ASFV within traditional pig management systems. However, they do not play an active role in the geographic spread of ASFV. Furthermore, European wild boars rest above the ground and not inside protected burrows, thereby reducing the opportunity for Ornithodoros spp. infestation. Ticks of the O. erraticus complex have not been reported from central or northern Europe.
6.3.2. Factors relating to wild boar
Ecological and demographic factors
ASF can persist in wild boar populations without re‐infection from domestic pigs.
Viral persistence in wild boar populations is influenced by both host and environmental factors. Direct transmission between live wild boar is primarily due to other individuals within the same social group. Furthermore, habitat quality is important, and the presence of large, well‐connected forests favours unrestricted wild boar movement and contact.
At higher boar densities, there is increased potential for direct transmission as a consequence of increased within‐group contacts, and indirect transmission through contact of wild boar with infected carcasses and contaminated environments. Viral persistence is likely to be facilitated by viral survival in infectious carcasses.
There is no evidence of a population density threshold for spontaneous ASF fade‐out.
Long‐term infectious animals
Although the term ‘carriers’ is commonly used, there is no common definition in the context of ASF, and therefore, this term is not used in the conclusions.
The potential role of surviving infectious animals in long‐term transmission is still controversial.
Although virus can be isolated and transmission from survivors can occur for roughly 60–70 days following initial infection, there is no evidence from either field experience or long‐term studies of a major role of these long‐term infectious animals in maintaining the virus circulation.
6.3.3. Characteristics of the virus
The ASFV strains in the current European epidemic belong to the p72 genotype II. These strains are highly virulent, inducing an acute form of ASF with a case‐fatality rate approaching 95%, regardless of age, dose or route of administration.
There have been several examples of naturally occurring, attenuated genotype II strains during the current epidemic in Estonia and Latvia, but they seem to have disappeared from the wild boar populations, possibly due to their reduced virulence.
Circulation of genotype I in Europe is limited to Sardinia, following introduction in 1978. These genotype I strains have always been associated with high virulence. In recent years, however, the virus was isolated from apparently healthy pigs. The presence of less virulent ASFV strains has never been confirmed, although the field observations are highly suggestive.
6.3.4. Human‐induced factors
Although the spread of ASF in wild boar populations can continue without re‐infection from domestic pigs, there are some examples of spill over from domestic pigs to wild boar.
The risk related to infected meat and products from domestic pigs and wild boar is often associated with illegal movements of such products or with small free‐ranging backyard farms where animals are illegally fed with untreated food leftovers or catering waste.
Human activity is an important contributor to both ASF persistence and expansion in wild boar populations, including hunting activities with poor biosecurity. There are also multiple examples of long‐distance translocation of infection, which can only plausibly be related to human activity.
6.4. Update on epidemiological attributes of African swine fever virus genotypes I and II that have still a high uncertainty
6.4.1. Mortality rate
The true mortality caused by ASF at the population level is difficult to estimate due to the occurrence of non‐ASF-related mortality, for instance caused by hunting. Recent estimates from Poland and Latvia attributed around 80% of the mortality in the affected wild boar populations to ASF.
The case‐fatality rate due to ASF experimental infections with ASFV genotype II of wild boar is likely above 95%.
6.4.2. Duration of protective immunity and maternal antibodies
Protective immunity
The duration of protective immunity in animals surviving from ASF has not been well studied and is considered a knowledge gap.
Recent studies demonstrated protection of at least 4 months post‐immunisation with attenuated ASFV strains, indicating that the protection from clinical disease may last at least several months in animals recovering from the disease. Re‐infection of these animals, however, cannot be excluded.
Maternal antibodies
The duration of maternal antibodies in piglets of sows surviving ASF is not known. The longest time maternal antibodies against ASFV have been found in piglets according to the literature is 7 weeks.
Maternal antibodies against other pig diseases such as Classical Swine Fever virus and porcine parvovirus have been shown to last up to 2–4 months, and up to 6 months for Aujeszky's Disease virus.
6.4.3. Transmission parameters
The transmission parameter estimates from experimental studies are dependent on the experimental setting and conditions. The estimates from field studies are influenced by various farm management and other factors having effect on contact rates between animals.
The parameters calculated based on field data are more variable, being lowest for ASFV genotype I in Sardinia (ranging from 1.2 to 2.7) and highest for genotype II outbreaks in Russia (ranging from 4.4 to 17.3).
There are no experimental data on transmission of ASFV from infected carcasses to susceptible wild boar. The studies estimating R0 for wild boar are based on field data and incorporate the effect of all transmission routes.
The transmission parameter estimates from the field data are influenced by local conditions (e.g. population density and management of wild boar) and disease intervention measures which all have an effect on contact rates between the animals and animal groups.
The point estimates for transmission parameters obtained in experimental conditions fall within a relatively narrow range (R0: 5.0–6.1).
The R0 estimates for wild boar are similar to between pig herd R0, indicating that they represent most likely to be the R0 between social groups of wild boars.
Modelling the spread of ASF in wild boar a low infection probability per carcass had to be assumed (βcarc ~ 0.15) to be able to simulate the ASF epidemic in wild boar coherent to observed spatial–temporal notification data in ADNS.
6.5. Spatial‐explicit stochastic model outputs
6.5.1. Characteristics of simulated outbreaks in Estonia
In simulated wild boar populations in Estonia, the probability of virus persistence following introduction falls to ~ 50% 7 years after virus introduction, reducing to approximately 10% after 10 years.
Throughout the simulated ASF epidemic, a low prevalence of ASF virus positive animals is observed with a median of about 2% at the peak of epidemic (1–4% for the 25th and 75th percentile), and prevalence is very low 6 months prior to virus extinction in an LAU 1 region in Estonia (median ASF virus prevalence below 0.5%; 25th and 75th percentile of 0.1–2%).
The median seroprevalence in subadults declined to 0% within 1 year (9 and 18 months for the 25th and 75th percentile) after local extinction of ASFV in an LAU 1 region in Estonia. In adults, this decline took more than 3 years. This outcome is in line with the analysis of the surveillance results of Estonia (see conclusions in Section 6.1).
The median number of wild boar deaths attributable to ASF is around 150 carcasses per LAU 1 at the peak of epidemic (100–300 central 50% interval across runs and LAU 1 units) and about 40 carcasses (10–150 central 50% interval across runs and LAU 1 units) a year prior to local extinction.
6.5.2. Scenarios representing mechanisms potentially prolonging circulation of infection within an area
The presence of life‐long infectious animals into the model, whilst acknowledging that there is uncertainty around their existence, prolonged circulation of ASF infection in wild boar populations and habitat resulting in an approximately 90% probability of virus persistence for longer than 20 years following viral introduction. The decline of virus and seroprevalence prior to local extinction was substantially slowed in the presence of such animals.
A reduction in case‐fatality rate and a lengthened period of transient infectiousness among surviving animals did not increase virus persistence.
Loss of protective immunity and reduced duration of protection by maternal antibodies does not increase virus persistence.
6.5.3. Evaluating proposed Exit Strategy criteria
As a general principle, a two‐phase approach (Screening Phase, Confirmation Phase) is proposed for the Exit Strategy, based on knowledge of virological and serological prevalence profiles. The motivation for this approach is several fold. An exit scenario will be conducted during a period when there are very few infected animals (with these animals being difficult to detect) and very few virus‐positive carcasses. Furthermore, the time horizon and surveillance effort proposed with an Exit Strategy must be sustainable under field conditions.
Model simulations have been used to evaluate different Exit Strategy options, which vary by surveillance options and intensity, and the length of the monitoring period during each phase. Each option was assessed in terms of performance (failure rate, being the per cent of simulations for which it was falsely concluded that virus is absent) and ‘time free’ (the time lag between point of viral extinction and time when an exit decision is possible).
The accuracy of the Exit Strategy approach to demonstrate freedom of ASFV circulation in a wild boar population is increased with an increasing number of carcasses being routinely collected and tested. However, the Exit Strategy will only be feasible if the duration and intensity of the passive surveillance can be sustained under field conditions. This is most likely to be achieved with a longer monitoring phase during routine surveillance effort (the Screening Phase) and a shorter monitoring phase of increased surveillance effort (the Confirmation Phase).
Lengthening of the monitoring periods leads to an improvement in Exit Strategy performance; however, this performance improvement should be reasonably balanced against an unnecessary prolonged ‘time free’ with only a marginal gain in performance of the Exit Strategy.
Increased intensity of passive surveillance is associated with a substantial increase in Exit Strategy performance.
In general, the inclusion of active surveillance in the Exit Strategy has very limited impact on the performance compared with a lengthening the overall monitoring period.
A declining seroprevalence in subadults can add information about the fade‐out of the epidemic and trigger the decision to initiate the Exit Strategy, however, including this surveillance activity during the Exit Strategy only marginally improves its performance. This is because information from subadult serology will be redundant in the presence of robust passive surveillance.
An Exit Strategy is problematic in the presence of lifelong infectious carrier animals. That said, it should be emphasised that the existence of such carriers is speculative, based on current knowledge.
Higher natural mortality that is not caused by ASF or hunting reduces the probability of finding infected carcasses in an affected area, and therefore reduces the performance of passive surveillance. If there were uncertainty about natural mortality rates in a region, a conservative exit criterion would be advisable that can be derived from model outputs using the upper bound of natural mortality (i.e. 80% mortality due to hunting and 20% due to natural mortality).
6.5.4. Additional conclusions
Depending on the epidemiological situation, if PCR‐positive, skeletonised carcass remains are detected, it is recommended that virus isolation is performed to verify the viability of the virus. This is because PCR is able to detect the virus genome even if the virus is no longer viable/infectious.
It is rarely possible to accurately determine the date of death of animals on the basis of skeletal remains.
Animals killed in car accidents should be considered as hunted animals in the Exit Strategy.
The Exit Strategy recommendations were formulated per 1,000 km2 and therefore need to be scaled with the size of the specific region of application. It is expected that the samples are distributed as evenly as possible in time and space in order to provide a good representation of the wild boar population of interest.
7. Recommendations for further research
Several knowledge gaps still exist pertaining to the epidemiology of ASF, for instance relating to the:
persistence of maternal antibodies against ASFV and the duration of the immunity in survivors;
long‐term transmission of ASFV by wild boar surviving infection (e.g. possible carriers, virus shedders);
duration of the infectiveness of the environment contaminated with ASFV, role of the environment as a source of the infection for wild boar and domestic pigs;
role of vectors, mainly arthropods, in mechanic or biologic transmission of ASF in the EU.
reduction of ASFV virulence due to long‐term exposure (i.e. Sardinia) and circulation of less virulent strains
Abbreviations
- ADNS
Animal Disease Notification System
- ASF
African swine fever
- ASFV
ASF virus
- CP
Confirmation Phase
- DCF
Data collection framework
- ELR
Extensive literature review
- HAT
Haemadsorption test
- ODD
Overview, Design and Details
- PI
Persistently infected
- PSA
Peste Suina Africana
- QIC
Quasi‐Akaike Information Criterion
- RiBESS
Risk based estimate of system sensitivity
- SP
Screening Phase
- ToR
Terms of Reference
- WB
Wild boar
Supporting information
Flow chart displaying terms of references, subquestions, data sources and assessment sections
Seroprevalence (seropositive proportion) in Estonia, from the last PCR‐positive sample in each LAU 1 region until 31 August 2020
Seroprevalence (seropositive proportion) in Latvia from the last PCR‐positive sample in each LAU 1 region until 31 August 2020
Estimated confidence achieved to detect ASF in LAU 1 regions assuming 1% prevalence for the first 52‐week surveillance period after the last PCR‐positive findings
Estimated confidence achieved to detect ASF in LAU 1 regions assuming 1% prevalence for the second 52‐week surveillance period after the last PCR‐positive findings
Suggested citation: European Food Safety Authority (EFSA) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Depner K, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortazar Schmidt C, Herskin M, Michel V, Miranda Chueca MÁ, Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Stahl K, Velarde A, Winckler C, Abrahantes JC, Dhollander S, Ivanciu C, Papanikolaou A, Van der Stede Y, Blome S, Guberti V, Loi F, More S, Olsevskis E, Thulke HH and Viltrop A, 2021. ASF Exit Strategy: Providing cumulative evidence of the absence of African swine fever virus circulation in wild boar populations using standard surveillance measures. EFSA Journal 2021:19(3):6419, 72 pp. 10.2903/j.efsa.2021.6419
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00424
Panel members: Julio Alvarez, Dominique Joseph Bicout, Paolo Calistri, Klaus Depner, Julian Ashley Drewe, Bruno Garin‐Bastuji, Jose Luis Gonzales Rojas, Christian Gortázar Schmidt, Mette Herskin, Virginie Michel, Miguel Ángel Miranda Chueca, Søren Saxmose Nielsen, Paolo Paquali, Helen Clare Roberts, Liisa Helena Sihvonen, Hans Spoolder, Karl Stahl, Antonio Velarde, Arvo Viltrop and Christoph Winckler.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: The AHAW Panel wishes to thank the following for the support provided to this scientific output: the trainee Elisabeth Dorbek‐Kolin and the ad‐interim staff Cristina Rapagnà (AHAW team, ALPHA unit, EFSA).
Adopted: 21 January 2021
References
- Agresti A, 2019. An Introduction to Categorical Data Analysis, 3rd Edition. John Wiley and Sons, New York. [Google Scholar]
- Baldacchino F, Desquesnes M, Mihok S, Foil LD, Duvallet G and Jittapalapong S, 2014. Tabanids: neglected subjects of research, but important vectors of disease agents!. Infect Genet Evol., 28, 596–615. 10.1016/j.meegid.2014.03.029 Epub 2014 Apr 13. PMID: 24727644. [DOI] [PubMed] [Google Scholar]
- Barasona JA, Gallardo C, Cadenas‐Fernández E, Jurado C, Rivera B, Rodríguez‐Bertos A, Arias M and Sánchez‐Vizcaíno JM, 2019. First oral vaccination of Eurasian wild boar against African swine fever virus genotype II. Frontiers in Veterinary Science, 6, 137. 10.3389/fvets.2019.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton K, 2018. MuMIn: Multi‐Model Inference, R Package Version. 1.42.1. Available online: https://CRAN.R-project.org/package=MuMIn [Google Scholar]
- Beltran‐Alcrudo D, Falco JR, Raizman E and Dietze K, 2019. Transboundary spread of pig diseases: the role of international trade and travel. BMC Veterinary Research, 15, 4. 10.1186/s12917-019-1800-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán‐Alcrudo D, Kukielka EA, de Groot N, Dietze K, Sokhadze M and Martínez‐López B, 2018. Descriptive and multivariate analysis of the pig sector in Georgia and its implications for disease transmission. PLoS ONE, 13, e0202800. 10.1371/journal.pone.0202800. PMID: 30142224; PMCID: PMC6108502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard J, Hutet E, Paboeuf F, Randriamparany T, Holzmuller P, Lancelot R, Rodrigues V, Vial L and Le Potier M‐F, 2016. Effect of O. porcinus tick salivary gland extract on the african swine fever virus infection in domestic pig. PLOS ONE, 11, e0147869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blome S, Gabriel C, Dietze K, Breithaupt A and Beer M, 2012. High virulence of African swine fever virus Caucasus isolate in European wild boars of all ages. Emerging Infectious Diseases, 18, 708. 10.3201/eid1804.111813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blome S, Franzke K and Beer M, 2020. African swine fever – a review of current knowledge. Virus Research, 287, 198099. 10.1016/j.virusres.2020.198099 [DOI] [PubMed] [Google Scholar]
- Boinas F, Ribeiro R, Madeira S, Palma M, de Carvalho IL, Nncio S and Wilson AJ, 2014. The medical and veterinary role of Ornithodoros erraticus complex ticks (Acari: Ixodida) on the Iberian peninsula. Journal of Vector Ecology, 39, 238–248. 10.1111/jvec.12098 [DOI] [PubMed] [Google Scholar]
- Boklund A, Dhollander S, Vasile TC, Abrahantes JC, Bøtner A, Gogin A, Villeta LCG, Gortázar C, More SJ, Papanikolaou A, Roberts H, Stegeman A, Ståhl K, Thulke HH, Viltrop A, der Stede YV and Mortensen S, 2020. Risk factors for African swine fever incursion in Romanian domestic farms during 2019. Scientific Reports, 10, 10215. 10.1038/s41598-020-66381-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borca MV, Ramirez‐Medina E, Silva E, Vuono E, Rai A, Pruitt S, Holinka LG, Velazquez‐Salinas L, Zhu J and Gladue DP, 2020. Development of a Highly Effective African Swine Fever Virus Vaccine by Deletion of the I177L Gene Results in Sterile Immunity against the Current Epidemic Eurasia Strain. Journal of Virology, 94, e02017–02019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch J, Barasona JA, Cadenas‐Fernández E, Jurado C, Pintore A, Denurra D, Cherchi M, Vicente J and Sánchez‐Vizcaíno JM, 2020. Retrospective spatial analysis for African swine fever in endemic areas to assess interactions between susceptible host populations. PLoS One, 15, e0233473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch J, Rodríguez A, Iglesias I, Muñoz MJ, Jurado C, Sánchez‐Vizcaíno JM and de la Torre A, 2016. Update on the risk of introduction of African swine fever by wild boar into disease‐free European Union countries. Transboundary and Emerging Diseases, 64, 1424–1432. 10.1111/tbed.12527 [DOI] [PubMed] [Google Scholar]
- Burmakina G, Malogolovkin A, Tulman ER, Zsak L, Delhon G, Diel DG, Shobogorov NM, Morgunov YP, Morgunov SY, Kutish GF, Kolbasov D and Rock DL, 2016. African swine fever virus serotype‐specific proteins are significant protective antigens for African swine fever. Journal of General Virology, 97, 1670. [DOI] [PubMed] [Google Scholar]
- Cadenas‐Fernandez E, Sanchez‐Vizcaino JM, Kosowska A, Rivera B, Mayoral‐Alegre F, Rodriguez‐Bertos A, Yao JX, Bray J, Lokhandwala S, Mwangi W and Barasona JA, 2020. Adenovirus‐vectored African Swine Fever Virus Antigens Cocktail Is Not Protective against Virulent Arm07 Isolate in Eurasian Wild Boar. Pathogens, 9, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappai S, Rolesu S, Coccollone A, Laddomada A and Loi F, 2018. Evaluation of biological and socio‐economic factors related to persistence of African swine fever in Sardinia. Preventive Veterinary Medicine, 152, 1–11. 10.1016/j.prevetmed.2018.01.004 [DOI] [PubMed] [Google Scholar]
- Cappai S, Rolesu S, Feliziani F, Desini P, Guberti V and Loi F, 2020. Standardized methodology for target surveillance against African swine fever. Vaccines, 8, 723. 10.3390/vaccines8040723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J, Fischer M, Zani L, Eschbaumer M, Fuchs W, Mettenleiter T, Beer M and Blome S, 2020. Stability of African swine fever virus in soil and options to mitigate the potential transmission risk. Authorea. 10.22541/au.159551305.54358820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco‐Garcia R, Barroso P, Perez‐Olivares J, Montoro V and Vicente J, 2018. Consumption of big game remains by scavengers: a potential risk as regards disease transmission in central Spain. Frontiers in Veterinary Science, 5, 4. 10.3389/fvets.2018.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho Ferreira HC, Weesendorp E, Elbers ARW, Bouma A, Quak S, Stegeman JA and Loeffen WLA, 2012. African swine fever virus excretion patterns in persistently infected animals: a quantitative approach. Veterinary Microbiology, 160, 327–340. 10.1016/j.vetmic.2012.06.025 [DOI] [PubMed] [Google Scholar]
- de Carvalho Ferreira H, Zúquete ST, Wijnveld M, Weesendorp E, Jongejan F, Stegeman A and Loeffen WLA, 2014. No evidence of African swine fever virus replication in hard ticks. Ticks and Tick‐borne Diseases, 5, 582–589. 10.1016/j.ttbdis.2013.12.012 [DOI] [PubMed] [Google Scholar]
- Chenais E, Depner K, Guberti V, Dietze K, Viltrop A and Ståhl K, 2019. Epidemiological considerations on African swine fever in Europe 2014–2018. Porcine Heal Management, 5, 6. 10.1186/s40813-018-0109-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini A, Cossu P and Firinu A, 1982. African swine fever in Sardinia. In: Wilkinson PJ (ed.). African swine fever. EUR 8466 EN, Pro CEC/FAO research seminar, Sardinia, September 1981, pp. 1–6. [Google Scholar]
- Croft S, Massei G, Smith GC, Fouracre D and Aegerter JN, 2020. Modelling spatial and temporal patterns of African swine fever in an isolated wild boar population to support decision‐making. Frontiers in Veterinary Science, 7, 154. 10.3389/fvets.2020.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukor J, Linda R, Václavek P, Mahlerová K, Šatrán P and Havránek F, 2020. Confirmed cannibalism in wild boar and its possible role in African swine fever transmission. Transbound Emerg Dis., 67, 1068–1073. 10.1111/tbed.13468 Epub 2020 Jan 16 PMID: 31886951. [DOI] [PubMed] [Google Scholar]
- Dellicour S, Desmecht D, Paternostre J, Malengreaux C, Licoppe A, Gilbert M and Linden A, 2020. Unravelling the dispersal dynamics and ecological drivers of the African swine fever outbreak in Belgium. Journal of Applied Ecology, 57, 1619–1629. 10.1111/1365-2664.13649 [DOI] [Google Scholar]
- Dhollander S, Belsham GJ, Lange M, Willgert K, Alexandrov T, Chondrokouki E, Depner K, Khomenko S, Özyörük F, Salman M, Thulke HH and Bøtner A, 2016. Assessing the potential spread and maintenance of foot‐and‐mouth disease virus infection in wild ungulates: general principles and application to a specific scenario in Thrace. Transbound Emerg Dis., 63, 165–174. 10.1111/tbed.12240 Epub 2014 Jun 6 PMID: 24903641. [DOI] [PubMed] [Google Scholar]
- Dixon LK, Stahl K, Jori F, Vial L and Pfeiffer DU, 2020. African swine fever epidemiology and control. Annual Review of Animal Biosciences, 8, 221–246. 10.1146/annurev-animal-021419-083741 [DOI] [PubMed] [Google Scholar]
- Donnell VO, Risatti GR, Holinka LG, Krug PW, Carlson J, Velazquez‐Salinas L, Azzinaro PA, Gladue DP and Borca MV, 2017. Simultaneous deletion of the 9GL and UK genes from the African swine fever virus Georgia 2007 isolate offers increased safety and protection against homologous challenge. Journal of Virology, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnell VO, Holinka LG, Sanford B, Krug PW, Carlson J, Pacheco JM, Reese B, Risatti GR, Gladue DP and Borca MV, 2016. African swine fever virus Georgia isolate harboring deletions of 9GL and MGF360/505 genes is highly attenuated in swine but does not confer protection against parental virus challenge. Virus Research, 221, 8. [DOI] [PubMed] [Google Scholar]
- Dórea FC, Swanenburg M, van Roermund H, Horigan V, de Vos C, Gale P, Lilja T, Comin A, Bahuon C, Zientara S, Young B, Vial F, Kosmider R and Lindberg A, 2017. Data collection for risk assessments on animal health. EFSA supporting publication 2017;EN‐1171, 209 pp. 10.2903/sp.efsa.2017.en-1171 [DOI] [Google Scholar]
- Durrleman S and Simon R, 1989. Flexible regression models with cubic splines. Statistics in Medicine, 8, 551–561. [DOI] [PubMed] [Google Scholar]
- Eblé PL, Hagenaars TJ, Weesendorp E, Quak S, Moonen‐Leusen HW and Loeffen WLA, 2019. Transmission of African Swine Fever virus via carrier (survivor) pigs does occur. Veterinary Microbiology, 237, 108345. 10.1016/j.vetmic.2019.06.018 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. A framework to substantiate absence of disease: the risk based estimate of system sensitivity tool (RiBESS) using data collated according to the EFSA Standard Sample Description – an example on Echinococcus multilocularis . EFSA supporting publication 2012;EN‐366, 44 pp. 10.2903/sp.efsa.2012.en-366 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Evaluation of possible mitigation measures to prevent introduction and spread of African swine fever virus through wild boar. EFSA Journal 2014;12(3):3616, 23 pp. 10.2903/j.efsa.2014.3616 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Cortiñas Abrahantes J, Gogin A, Richardson J and Gervelmeyer A, 2017. Scientific report on epidemiological analyses on African swine fever in the Baltic countries and Poland. EFSA Journal 2017;15(3):4732, 73 pp. 10.2903/j.efsa.2017.4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Depner K, Gortazar C, Guberti V, Masiulis M, More S, Oļševskis E, Thulke H‐H, Viltrop A, Woźniakowski G, Cortiñas Abrahantes J, Gogin A, Verdonck F and Dhollander S, 2017. Scientific report on the epidemiological analyses of African swine fever in the Baltic States and Poland. EFSA Journal 2017;15(11):5068, 59 pp. 10.2903/j.efsa.2017.5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Boklund A, Cay B, Depner K, F€oldi Z, Guberti V, Masiulis M, Miteva A, More S, Olsevskis E, Satran P, Spiridon M, Stahl K, Thulke H‐H, Viltrop A, Woźniakowski G, Broglia A, Cortinas Abrahantes J, Dhollander S, Gogin A, Verdonck F, Amato L, Papanikolaou A and Gortazar C, 2018. Scientific report on the epidemiological analyses of African swine fever in the European Union (November 2017 until November 2018). EFSA Journal 2018;16(11):5494, 106 pp. 10.2903/j.efsa.2018.5494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Álvarez J, Bicout D, Boklund A, Bøtner A, Depner K, More SJ, Roberts H, Stahl K, Thulke HH, Viltrop A, Antoniou SE, Cortiñas Abrahantes J, Dhollander S, Gogin A, Papanikolaou A, Van der Stede Y, González Villeta LC and Gortázar Schmidt C, 2019. Research gap analysis on African swine fever. EFSA Journal 2019;17(8):e05811. 10.2903/j.efsa.2019.5811. PMID: 32626417; PMCID: PMC7009270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Miteva A, Papanikolaou A, Gogin A, Boklund A, Bøtner A, Linden A, Viltrop A, Schmidt CG, Ivanciu C, Desmecht D, Korytarova D, Olsevskis E, Helyes G, Wozniakowski G, Thulke H‐H, Roberts H, Abrahantes JC, Stahl K, Depner K, Gonzalez Villeta LC, Spiridon M, Ostojic S, More S, Vasile TC, Grigaliuniene V, Guberti V and Wallo R, 2020. Scientific report on the epidemiological analyses of African swine fever in the European Union (November 2018 to October 2019). EFSA Journal 2020;18(1):5996, 107 pp. 10.2903/j.efsa.2020.5996 [DOI] [Google Scholar]
- EFSA AHW Panel (EFSA Panel on Animal Health and Welfare), 2010a. Scientific Opinion on the role of tick vectors in the epidemiology of Crimean‐Congo hemorrhagic fever and African swine fever in Eurasia. EFSA Journal 2010;8(8):1703, 156 pp. 10.2903/j.efsa.2010.1703 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2010b. Scientific Opinion on African Swine Fever. EFSA Journal 2010;8(3):1556, 149 pp. 10.2903/j.efsa.2010.1556 [DOI] [Google Scholar]
- EFSA AHW Panel (EFSA Panel on Animal Health and Welfare), 2015. African swine fever. EFSA Journal 2015;13(7):4163, 101 pp. 10.2903/j.efsa.2015.4163 [DOI] [Google Scholar]
- EFSA AHW Panel (EFSA Panel on Animal Health and Welfare), More S, Miranda MA, Bicout D, Bøtner A, Butterworth A, Calistri P, Edwards S, Garin‐Bastuji B, Good M, Michel V, Raj M, Nielsen SS, Sihvonen L, Spoolder H, Stegeman JA, Velarde A, Willeberg P, Winckler C, Depner K, Guberti V, Masiulis M, Olsevskis E, Satran P, Spiridon M, Thulke H, Vilrop A, Wozniakowski G, Bau A, Broglia A, Abrahantes JC, Dhollander S, Gogin A, Gajardo IM, Verdonck F, Amato L and Schmidt CG, 2018. African swine fever in wild boar. EFSA Journal 2018;16(7):5344, 78 pp. 10.2903/j.efsa.2018.5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission (SANTE G3), 2015. Strategic approach to the management of African Swine Fever for the EU. SANTE/7113/2015 – Rev 12. Pp 27. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/ad_control-measures_asf_wrk-doc-sante-2015-7113.pdf
- FAO , 2013. African swine fever in the Russian Federation: risk factors for Europe and beyond. Empress Watch, Vol. 28, May 2013, Rome.
- Feliziani F, Rolesu S, Aloi D, Panichi G, Marongiu D and De Mia GM, 2010. Validazione dei criteri di analisi del rischio riguardo la diffusione e la persistenza della Peste Suina Africana (PSA) in Sardegna. [Validation analysis of risk factors conditioning the persistence and the diffusion of African Swine Fever (ASF) infection in Sardinia Region (Italy).] Sanità Pubblica Veterinaria (2010), n. 60. Available online: http://indice.spvet.it #472
- Fenati M, Armaroli E, Corrain R and Guberti V, 2009. Indirect estimation of porcine parvovirus maternal immunity decay in free‐living wild boar (Sus scrofa) piglets by capture‐recapture data. Vet J., 262–264. 10.1016/j.tvjl.2007.12.009 Epub 2008 Mar 4 PMID: 18295517. [DOI] [PubMed] [Google Scholar]
- Fila M and Woźniakowski G, 2020. African swine fever virus – the possible role of flies and other insects in virus transmission. Journal of Veterinary Research, 64, 1–7. 10.2478/jvetres-2020-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Hühr J, Blome S, Conraths FJ and Probst C, 2020. Stability of African Swine Fever Virus in carcasses of domestic pigs and wild boar experimentally infected with the ASFV “Estonia 2014” isolate. Viruses, 12, 1118. 10.3390/v12101118. PMID: 33019736; PMCID: PMC7600355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focardi S, Toso S and Pecchioli E, 1996. The population modelling of fallow deer and wild boar in a Mediterranean ecosystem. Forest Ecology and Management, 88, 7–14. [Google Scholar]
- Forth JH, Amendt J, Blome S, Depner K and Kampen H, 2018. Evaluation of blowfly larvae (Diptera: Calliphoridae) as possible reservoirs and mechanical vectors of African swine fever virus. Transbound Emerg Dis., 65, e210–e213. 10.1111/tbed.12688 Epub 2017 Aug 1 PMID: 28762629. [DOI] [PubMed] [Google Scholar]
- Frant M, Woźniakowski G and Pejsak Z, 2017. African swine fever (ASF) and ticks. No risk of tick‐mediated ASF spread in Poland and Baltic states. Journal of Veterinary Research, 61, 375–380. 10.1515/jvetres-2017-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoni G, Franzoni G, Dei Giudici S, Loi F, Sanna D, Floris M, Fiori M, Sanna ML, Madrau P, Scarpa F, Zinellu S, Giammarioli M, Cappai S, De Mia GM, Laddomada A, Rolesu S and Oggiano A, 2020. African swine fever circulation among free‐ranging pigs in Sardinia: data from the eradication program. Vaccines, 8, 549. 10.3390/vaccines8030549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederic RG, Megan ES, Erin LM, Michael TM and Mangkey AB, 2015. Detection of African swine fever, classical swine fever, and foot‐and‐mouth disease viruses in swine oral fluids by multiplex reverse transcription real‐time polymerase chain reaction. Journal of Veterinary Diagnostic Investigation, 27, 140–149. [DOI] [PubMed] [Google Scholar]
- Gabriel C, Blome S, Malogolovkin A, Parilov S, Kolbasov D, Teifke J and Beer M, 2011. Characterization of African Swine Fever Virus Caucasus Isolate in European Wild Boars. Emerging Infectious Disease journal, 17, 2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard JM, Vassant J and Klein F, 1987. Some characteristics of the population dynamics of wild boar (Sus scrofa scrofa) in a hunted environment. Gibier Faune Sauvage, 4, 31–47. [Google Scholar]
- Gallardo C, Nieto R, Soler A, Pelayo V, Fernández‐Pinero J, Markowska‐Daniel I, Pridotkas G, Nurmoja I, Granta R, Simón A, Pérez C, Martín E, Fernández‐Pacheco P and Arias M, 2015. Assessment of African swine fever diagnostic techniques as a response to the epidemic outbreaks in eastern European Union countries: how to improve surveillance and control programs. Journal of Clinical Microbiology, 53, 2555–2565. 10.1128/JCM.00857-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo C, Nurmoja I, Solar A, Delicado V, Simon A, Martin E, Perez C, Nieto R and Arias M, 2018. Evolution in Europe of African swine fever genotype II viruses from highly to moderately virulent. Veterinary Microbiology, 219, 70–79. 10.1016/j.vetmic.2018.04.001 [DOI] [PubMed] [Google Scholar]
- Gallardo C, Soler A, Nieto R, Cano C, Pelayo V, Sánchez MA, Pridotkas G, Fernandez‐Pinero J, Briones V and Arias M, 2017. Experimental Infection of Domestic Pigs with African Swine Fever Virus Lithuania 2014 Genotype II Field Isolate. Transboundary and Emerging Diseases, 64, 300. [DOI] [PubMed] [Google Scholar]
- Gallardo C, Soler A, Rodze I, Nieto R, Cano‐Gomez C, Fernandez‐Pinero J and Arias M, 2019. Attenuated and non‐haemadsorbing (non‐HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transboundary and Emerging Diseases, 66, 1399–1404. 10.1111/tbed.13132 [DOI] [PubMed] [Google Scholar]
- Gavier‐Widén D, Ståhl K and Dixon L, 2020. No hasty solutions for African swine fever. Science, 367, 622–624. 10.1126/science.aaz8590 [DOI] [PubMed] [Google Scholar]
- Gervasi V, Marcon A, Bellini S and Guberti V, 2020. Evaluation of the efficiency of active and passive surveillance in the detection of African swine fever in wild boar. Veterinary Science, 7, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giammarioli M, Gallardo C, Oggiano A, Iscaro C, Nieto R, Pellegrini C, Dei Giudici S, Arias M and De Mia GM, 2011. Genetic characterisation of African swine fever viruses from recent and historical outbreaks in Sardinia (1978–2009). Virus Genes, 42, 377–387. [DOI] [PubMed] [Google Scholar]
- Gogin A, Gerasimov V, Malogolovkin A and Kolbasov D, 2013. African swine fever in the North Caucasus region and the Russian Federation in years 2007–2012. Virus Research, 173, 198–203. 10.1016/j.virusres.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Grimm V, 2020. The ODD protocol: an update with guidance to support wider and more consistent use. Ecological Modelling, 428, 109105. [Google Scholar]
- Grimm V, Berger U, Bastiansen F, Eliassen S, Ginot V, Giske J, Goss‐Custard J, Grand T, Heinz SK, Huse G, Huth A, Jepsen JU, Jørgensen C, Mooij WM, Müller B, Pe'er G, Piou C, Railsback S, Robbins AM, Robbins MM, Rossmanith E, Rüger N, Strand E, Souissi S, Stillman RA, Vabø R, Visser U and DeAngelis DL, 2006. A standard protocol for describing individual‐based and agent‐based models. Ecological Modelling, 198, 115–126. [Google Scholar]
- Grimm V, Berger U, DeAngelis DL, Polhill JG, Giske J and Railsback SF, 2010. The ODD protocol: a review and first update. Ecological Modelling, 221, 2760–2768. 10.1016/j.ecolmodel.2006.04.023 [DOI] [Google Scholar]
- Grimm V, Johnston ASA, Thulke H‐H, Forbes VE and Thorbek P, 2020. Three questions to ask before using model outputs for decision support. Nature Communication, 11, article 4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guberti V, Khomenko S, Masiulis M and Kerba S, 2018. Handbook on African swine fever in wild boar and biosecurity during hunting. Standing Group of Experts on African swine fever in Europe under the GF‐TADs umbrella. OIE, 2018. Available online: https://oiebulletin.com/?officiel=2019-1-asf-wildboar-en
- Guinat C, Gogin A, Blome S, Keil G, Pollin R, Pfeiffer DU and Dixon L, 2016a. Transmission routes of African swine fever virus to domestic pigs: current knowledge and future research directions. Veterinary Record, 178, 262–267. 10.1136/vr.103593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinat C, Gubbins S, Vergne T, Gonzales JL, Dixon L and Pfeiffer DU, 2016b. Experimental pig‐to‐pig transmission dynamics for African swine fever virus, Georgia 2007/1 strain. Epidemiology and Infection, 144, 25–34. 10.1017/S0950268815000862. Epub 2015 May 20. Erratum in: Epidemiology and Infection, 2016, 144(16), 3564–3566. PMID: 25989921; PMCID: PMC4697298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinat C, Porphyre T, Gogin A, Dixon L, Pfeiffer DU and Gubbins S, 2018. Inferring within‐herd transmission parameters for African swine fever virus using mortality data from outbreaks in the Russian Federation. Transboundary and Emerging Diseases, 65, e264–e271. 10.1111/tbed.12748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinat C, Reis AL, Netherton CL, Goatley L, Pfeiffer DU and Dixon L, 2014. Dynamics of African swine fever virus shedding and excretion in domestic pigs infected by intramuscular inoculation and contact transmission. Veterinary Research, 45, (26 September 2014)–(2026 September 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulenkin VM, Korennoy FI, Karaulov AK and Dudnikov SA, 2011. Cartographical analysis of African swine fever outbreaks in the territory of the Russian Federation and computer modeling of the basic reproduction ratio. Preventative Veterinary Medicine, 102, 167–174. 10.1016/j.prevetmed.2011.07.004 [DOI] [PubMed] [Google Scholar]
- Halasa T, Boklund A, Bøtner A, Mortensen S and Kjær LJ, 2019. Simulation of transmission and persistence of African swine fever in wild boar in Denmark. Prev Vet Med., 1, 68–79. 10.1016/j.prevetmed.2019.03.028 Epub 2019 Apr 1 PMID: 31027724. [DOI] [PubMed] [Google Scholar]
- Højsgaard S, Halekoh U and Yan J, 2006. The R Package geepack for generalized estimating equations. Journal of Statistical Software, 15, 1–11. [Google Scholar]
- Iglesias I, Muñoz MJ, Montes F, Perez A, Gogin A, Kolbasov D and de la Torre A, 2016. Reproductive ratio for the local spread of African Swine Fever in wild boars in the Russian Federation. Transboundary and Emerging Diseases, 63, e237–e245. 10.1111/tbed.12337 [DOI] [PubMed] [Google Scholar]
- Jedrzejewska B, Okarma H, Jedrzejewski W and Milkowski L, 1994. Effects of exploitation and protection on forest structure, ungulate density and wolf predation in Bialowieza Primeval Forest, Poland. Journal of Applied Ecology, 31, 664–676. [Google Scholar]
- Jordt AM, Lange M, Kramer‐Schadt S, Nielsen LH, Nielsen SS, Thulke HH, Vejre H and Alban L, 2016. Spatio‐temporal modeling of the invasive potential of wild boar–a conflict‐prone species‐using multi‐source citizen science data. Prev Vet Med., 124, 34–44. 10.1016/j.prevetmed.2015.12.017 Epub 2015 Dec 29 PMID: 26775815. [DOI] [PubMed] [Google Scholar]
- Kaden V and Lange E, 2004. Development of maternal antibodies after oral vaccination of young female wild boar against classical swine fever. Veterinary Microbiology, 103, 115–119. [DOI] [PubMed] [Google Scholar]
- Karalyan Z, Zakaryan H, Sargsyan K, Voskanyan H, Hakobyan L, Abroyan L, Avetisyan A, Arzumanyan H and Karalova E, 2012. Pathology of porcine peripheral white blood cells during infection with African swine fever virus. BMC Veterinary Research, 8, (28 February 2012)‐(2028 February 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalyan ZR, Ter‐Pogossyan ZR, Karalyan NY, Semerjyan ZB, Tatoyan MR, Karapetyan SA and Karalova EM, 2017. Hemophagocytic lymphohistiocytosis in acute African swine fever clinic. Veterinary Immunology and Immunopathology, 187, 64. [DOI] [PubMed] [Google Scholar]
- Keuling O, Baubet E, Duscher A, Ebert C, Fischer C, Monaco A, Podgórski T, Prévot C, Ronnenberg K, Sodeikat G, Stier N and Thurfjell H, 2013. Mortality rates of wild boar Sus scrofa L. in central Europe. European Journal of Wildlife Research, 59, 805–814. 10.1007/s10344-013-0733-8 [DOI] [Google Scholar]
- King K, Chapman D, Argilaguet JM, Fishbourne E, Hutet E, Cariolet R, Hutchings G, Oura CA, Netherton CL, Moffat K, Taylor G, Le Potier MF, Dixon LK and Takamatsu HH, 2011. Protection of European domestic pigs from virulent African isolates of African swine fever virus by experimental immunisation. Vaccine, 29, 4593–4600. 10.1016/j.vaccine.2011.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbasov D, Titov I, Tsybanov S, Gogin A and Malogolovkin A, 2018. African swine fever virus, Siberia, Russia, 2017. Emerging Infectious Diseases, 24, 796–798. 10.3201/cid2404.171238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalenko Y, Sidorov M and Burba L, 1964. Survival of African swine fever in the environment. Vestnik Selsk, Nauki. 62 pp. [Google Scholar]
- Kramer‐Schadt S, Fernandez N and Thulke H‐H, 2007. Potential ecological and epidemiological factors affecting the persistence of classical swine fever in wild boar Sus scrofa populations. Mammal Review, 37, 1–20. [Google Scholar]
- Kramer‐Schadt S, Fernández N, Eisinger D, Grimm V and Thulke H‐H, 2009. Individual variations in infectiousness explain long‐term disease persistence in wildlife populations. Oikos, 118, 199–208. [Google Scholar]
- Laddomada A, Rolesu S, Loi F, Cappai S, Oggiano A, Madrau MP, Sanna ML, Pilo G, Bandino E, Brundu D, Cherchi S, Masala S, Marongiu D, Bitti G, Desini P, Floris V, Mundula L, Carboni G, Pittau M, Feliziani F, Sanchez‐Vizcaino JM, Jurado C, Guberti V, Chessa M, Muzzeddu M, Sardo D, Borrello S, Mulas D, Salis G, Zinzula P, Piredda S, De Martini A and Sgarangella F, 2019. Surveillance and control of African Swine Fever in free‐ranging pigs in Sardinia. Transbound Emerg Dis, 66, 1114–1119. 10.1111/tbed.13138. Epub 2019 Feb 25. PMID: 30715791; PMCID: PMC6849606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M, 2015. Alternative control strategies against ASF in wild boar populations. EFSA Supporting Publication 2015;12(7):EN‐843, 29 pp. 10.2903/sp.efsa.2015.EN-843 [DOI] [Google Scholar]
- Lange M and Thulke H‐H, 2017. Elucidating transmission parameters of African swine fever through wild boar carcasses by combining spatio‐temporal notification data and agent‐based modelling. Stochastic Environmental Research and Risk Assessment, 31, 379–391. 10.1007/s00477-016-1358-8 [DOI] [Google Scholar]
- Lange M, Guberti V and Thulke H‐H, 2018. Understanding ASF spread and emergency control concepts in wild boar populations using individual‐based modelling and spatio‐temporal surveillance data. EFSA supporting publication 2018:15(11):EN‐1521, 46 pp. 10.2903/sp.efsa.2018.EN-1521 [DOI] [Google Scholar]
- Lange M, Reichold A and Thulke H‐H, 2021. Modelling advanced knowledge of African swine fever, resulting surveillance patterns on the population level and impact to reliable exit strategy definition. EFSA supporting publication 2021;6429, 33 pp. 10.2903/sp.efsa.2021.6429 [DOI] [Google Scholar]
- Licoppe A, Lievens J, Della Libera F, Herrin T, Malengreaux C, Boudart JL, De Waele V, Fichefet V, Linden A, Lesenfants C, Van Goethem A, Villers M, Scohy J‐P and Herman M, 2020. Use of boar trapping in the context of the management of African swine fever in Wallonia. Practical aspects, preliminary results and recommendations. Wallonia Public Service/SPW-ARNE/DEMNA. 10.13140/RG.2.2.19743.18085 [DOI] [Google Scholar]
- Loi F, Laddomada A, Coccollone A, Marrocu E, Piseddu T, Masala G, Bandino E, Cappai S and Rolesu S, 2019. Socio‐economic factors as indicators for various animal diseases in Sardinia. PLoS ONE, 14, e0217367. 10.1371/journal.pone.0217367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi F, Cappai S, Laddomada A, Oggiano A, Franzoni G, Feliziani F, Rolesu S and Guberti V, 2020. Mathematical approach to estimating the main epidemiological parameters of African swine fever in wild boar. Vaccines (Basel), 8, 521. 10.3390/vaccines8030521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokhandwala S, Petrovan V, Popescu L, Sangewar N, Elijah C, Stoian A, Olcha M, Ennen L, Bray J, Bishop RP, Waghela SD, Sheahan M, Rowland RRR and Mwangi W, 2019. Adenovirus‐vectored African Swine Fever Virus antigen cocktails are immunogenic but not protective against intranasal challenge with Georgia 2007/1 isolate. Veterinary Microbiology, 235, 10–20. 10.1016/j.vetmic.2019.06.006 Epub 2019 Jun 6 PMID: 31282366. [DOI] [PubMed] [Google Scholar]
- Marcon A, Linden A, Satran P, Gervasi V, Licoppe A and Guberti V, 2019. R0 estimation for the African swine fever epidemics in wild boar of Czech Republic and Belgium. Veterinary Science, 7, 2. 10.3390/vetsci7010002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur‐Panasiuk N and Woźniakowski G, 2020. Natural inactivation of African swine fever virus in tissues: influence of temperature and environmental conditions on virus survival. Veterinary Microbiology, 242, 108609. 10.1016/j.vetmic.2020.108609 Epub 2020 Feb 9 PMID: 32122613. [DOI] [PubMed] [Google Scholar]
- Mazur‐Panasiuk N, Żmudzki J and Woźniakowski G, 2019. African swine fever virus – persistence in different environmental conditions and the possibility of its indirect transmission. Journal of Veterinary Research, 63, 303–310. 10.2478/jvetres-2019-0058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebus CA and Dardiri AH, 1980. Western hemisphere isolates of African swine fever virus: asymptomatic carriers and resistance to challenge inoculation. American Journal of Veterinary Research, 41, 1867–1869 PMID: 7212418. [PubMed] [Google Scholar]
- Meier L, 2020. Ecological modelling of African swine fever transmission: upscaling experimental data to understand the role of virulence in population level persistence. PhD thesis, Carl von Ossietzky University of Oldenburg. [Google Scholar]
- Mellor PS, Kitching RP and Wilkinson PJ, 1987. Mechanical transmission of capripox virus and African swine fever virus by Stomoxys calcitrans. Research in Veterinary Science, 43, 109–112. PMID: 2820006. [PubMed] [Google Scholar]
- Morelle K, Bubnicki J, Churski M, Gryz J, Podgórski T and Kuijper DPJ, 2020. Disease‐induced mortality outweighs hunting in causing wild boar population crash after African swine fever outbreak. Frontiers in Veterinary Science, 7, 378. 10.3389/fvets.2020.00378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T, Teuffert J, Staubach C, Selhorst T and Depner KR, 2005. Long‐term studies on maternal immunity for Aujeszky's disease and classical swine fever in wild boar piglets. Journal of Veterinary Medicine, Series B, 52, 432–436. [DOI] [PubMed] [Google Scholar]
- Mur L, Martínez‐López B, Costard S, de la Torre A, Jones BA, Martínez M, Sánchez‐Vizcaíno F, Muñoz MJ, Pfeiffer DU, Sánchez‐Vizcaíno JM and Wieland B, 2014. Modular framework to assess the risk of African swine fever virus entry into the European Union. BMC Veterinary Research, 10, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mur L, Boadella M, Martínez‐López B, Gallardo C, Gortazar C and Sánchez‐Vizcaíno JM, 2012. Monitoring of African Swine fever in the wild boar population of the most recent endemic area of Spain: ASF in a Spanish wild boar population. Transboundary and Emerging Diseases, 59, 526–531. 10.1111/j.1865-1682.2012.01308.x [DOI] [PubMed] [Google Scholar]
- Muzari MO, Skerratt LF, Jones RE and Duran TL, 2010. Alighting and feeding behaviour of tabanid flies on horses, kangaroos and pigs. Veterinary Parasitology, 170, 104–111. 10.1016/j.vetpar.2010.01.028. Epub 2010 Jan 28. PMID: 20153116. [DOI] [PubMed] [Google Scholar]
- Nielsen JP, Larsen TS, Halasa T and Christiansen LE, 2017. Estimation of the transmission dynamics of African swine fever virus within a swine house. Epidemiology and Infection, 145, 2787–2796. 10.1017/S0950268817001613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmoja I, Schulz K, Staubach C, Sauter‐Louis C, Depner K, Conraths FJ and Viltrop A, 2017a. Development of African swine fever epidemic among wild boar in Estonia – two different areas in the epidemiological focus. Scientific Reports, 7, 58. 10.1038/s41598-017-12952-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmoja I, Petrov A, Breidenstein C, Zani L, Forth JH, Beer M, Kristian M, Viltrop A and Blome S, 2017b. Biological characterization of African swine fever virus genotype II strains from north‐eastern Estonia in European wild boar. Transboundary and Emerging Diseases, 64, 2034–2041. 10.1111/tbed.12614 [DOI] [PubMed] [Google Scholar]
- Oganesyan AS, Petrova ON, Korennoy FI, Bardina NS, Gogin AE and Dudnikov SA, 2013. African swine fever in the Russian Federation: spatio‐temporal analysis and epidemiological overview. Virus Research, 173, 204–211. 10.1016/j.virusres.2012.12.009 [DOI] [PubMed] [Google Scholar]
- OIE (World Organisation for Animal Health), 2020. Self‐declaration by Estonia as a country free from African swine fever in domestic and captive wild pigs. Available online: https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Self-declarations/2018_09_Estonia_ASF_ENG.pdf/
- Olesen AS, Lohse L, Boklund A, Halasa T, Gallardo C, Pejsak Z, Belsham GJ, Rasmussen TB and Bøtner A, 2017. Transmission of African swine fever virus from infected pigs by direct contact and aerosol routes. Veterinary Microbiology, 211, 92–102. 10.1016/j.vetmic.2017.10.004 Epub 2017 Oct 4 PMID: 29102127. [DOI] [PubMed] [Google Scholar]
- Olesen AS, Lohse L, Boklund A, Halasa T, Belsham GJ, Rasmussen TB and Bøtner A, 2018. Short time window for transmissibility of African swine fever virus from a contaminated environment. Transbound Emerg Dis., 65, 1024–1032. 10.1111/tbed.12837. Epub 2018 Feb 19. PMID: 29457702. [DOI] [PubMed] [Google Scholar]
- Oļševskis E, Guberti V, Seržants M, Westergaard J, Gallardo C, Rodze I and Depner K, 2016. African swine fever virus introduction into the EU in 2014: experience of Latvia. Research in Veterinary Science, 105, 28–30. 10.1016/j.rvsc.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Oļševskis E, Schulz K, Staubach C, Seržants M, Lamberga K, Pūle D, Ozoliņš J, Conraths FJ and Sauter‐Louis C, 2020. African swine fever in Latvian wild boar – a step closer to elimination. Transboundary and Emerging Diseases, 67, 2615–2629. 10.1111/tbed.13611 [DOI] [PubMed] [Google Scholar]
- O'Neill X, White A, Ruiz‐Fons F and Gortázar C, 2020. Modelling the transmission and persistence of African swine fever in wild boar in contrasting European scenarios. Scientific Reports, 10, 5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, 2001. Akaike's information criterion in generalised estimating equations. Biometrics, 57, 120–125. [DOI] [PubMed] [Google Scholar]
- Pejsak Z, Truszczynski M, Niemczuk K, Kozak E and Markowska‐Daniel I, 2014. Epidemiology of African swine fever in Poland since the detection of the first case. Polish Journal of Veterinary Science, 17, 665–672. 10.2478/pjvs-2014-0097 [DOI] [PubMed] [Google Scholar]
- Pepin KM, Golnar AJ, Abdo Z and Podgórski T, 2020. Ecological drivers of African swine fever virus persistence in wild boar populations: insight for control. Ecology and Evolution, 10, 2846–2859. 10.1002/ece3.6100. PMID: 32211160; PMCID: PMC7083705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira de Oliveira R, Hutet E, Paboeuf F, Duhayon M, Boinas F, Perez de Leon A, Filatov S, Vial L and Le Potier M‐F, 2019. Comparative vector competence of the Afrotropical soft tick Ornithodoros moubata and Palearctic species, O. erraticus and O. verrucosus, for African swine fever virus strains circulating in Eurasia. PLoS ONE, 14, e0225657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira De Oliveira RP, Hutet E, Duhayon M, Guionnet JM, Paboeuf F, Vial L and Le Potier MF, 2020. Successful infection of domestic pigs by ingestion of the European soft tick O. erraticus that fed on African swine fever virus infected pig. Viruses, 12, 300. 10.3390/v12030300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov A, Forth JH, Zani L, Beer M and Blome S, 2018. No evidence for long‐term carrier status of pigs after African swine fever virus infection. Transboundary and Emerging Diseases, 14, 667. 10.1111/tbed.12881 [DOI] [PubMed] [Google Scholar]
- Pietschmann J, Guinat C, Beer M, Pronin V, Tauscher K, Petrov A, Keil G and Blome S, 2015. Course and transmission characteristics of oral low‐dose infection of domestic pigs and European wild boar with a Caucasian African swine fever virus isolate. Archives of Virology, 160, 1657–1667. [DOI] [PubMed] [Google Scholar]
- Pietschmann J, Mur L, Blome S, Beer M, Pérez‐Sánchez R, Oleaga A and Sánchez‐Vizcaíno JM, 2016. African swine fever virus transmission cycles in Central Europe: evaluation of wild boar‐soft tick contacts through detection of antibodies against Ornithodoros erraticus saliva antigen. BMC Veterinary Research, 12, 1. 10.1186/s12917-015-0629-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgórski T and Śmietanka K, 2018. Do wild boar movements drive the spread of African swine fever? Transboundary and Emerging Diseases, 331, 296. 10.1111/tbed.12910 [DOI] [PubMed] [Google Scholar]
- Podgórski T, Scandura M and Jędrzejewska B, 2014. Next of kin next door – philopatry and socio‐genetic population structure in wild boar: dispersal and population structure in wild boar. Journal of Zoology, 294, 190–197. 10.1111/jzo.12167 [DOI] [Google Scholar]
- Podgórski T, Borowik T, Łyjak M and Woźniakowski G, 2019. Spatial epidemiology of African swine fever: host, landscape and anthropogenic drivers of disease occurrence in wild boar. Preventive Veterinary Medicine, 177, 104691. 10.1016/j.prevetmed.2019.104691 [DOI] [PubMed] [Google Scholar]
- Popescu L, Gaudreault NN, Whitworth KM, Murgia MV, Nietfeld JC, Mileham A, Samuel M, Wells KD, Prather RS and Rowland RRR, 2017. Genetically edited pigs lacking CD163 show no resistance following infection with the African swine fever virus isolate, Georgia 2007/1. Virology, 501, 102. [DOI] [PubMed] [Google Scholar]
- Probst C, Globig A, Knoll B, Conraths FJ and Depner K, 2017. Behaviour of free ranging wild boar towards their dead fellows: potential implications for the transmission of African swine fever. Royal Society Open Science, 4, 170054. 10.1098/rsos.170054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst C, Gethmann J, Amler S, Globig A, Knoll B and Conraths FJ, 2019. The potential role of scavengers in spreading African swine fever among wild boar. Scientific Reports, 9, 11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst C, Gethmann J, Amendt J, Lutz L, Teifke JP and Conraths FJ, 2020. Estimating the Postmortem Interval of Wild Boar Carcasses. Vet Sci., 7, 6. 10.3390/vetsci7010006. PMID: 31948042; PMCID: PMC7157510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Cordón PJ, Jabbar T, Chapman D, Dixon LK and Montoya M, 2020. Absence of long‐term protection in domestic pigs immunized with attenuated African swine fever virus isolate OURT88/3 or BeninΔMGF correlates with increased levels of regulatory T cells and interleukin‐10. Journal of Virology, 94, e00350‐20. 10.1128/JVI.00350-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford B, Holinka LG, Donnell VO, Krug PW, Carlson J, Alfano M, Carrillo C, Wu P, Lowe A, Risatti GR, Gladue DP and Borca MV, 2016. Deletion of the thymidine kinase gene induces complete attenuation of the Georgia isolate of African swine fever virus. Virus Research, 213, 165. [DOI] [PubMed] [Google Scholar]
- Schlafer DH and Mebus CA, 1987. Abortion in sows experimentally infected with African swine fever virus: pathogenesis studies. American Journal of Veterinary Research, 48, 246–254. [PubMed] [Google Scholar]
- Schlafer DH, McVicar JW and Mebus CA, 1984a. African swine fever convalescent sows: subsequent pregnancy and the effect of colostral antibody on challenge inoculation of their pigs. American Journal of Veterinary Research, 45, 1361–1366. [PubMed] [Google Scholar]
- Schulz K, Staubach C, Blome S, Viltrop A, Nurmoja I, Conraths FJ and Sauter‐Louis C, 2019. Analysis of Estonian surveillance in wild boar suggests a decline in the incidence of African swine fever. Scientific Reports, 9, 8490. 10.1038/s41598-019-44890-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K, Staubach C, Blome S, Nurmoja I, Viltrop A, Conraths FJ, Kristian M and Sauter‐Louis C, 2020. How to demonstrate freedom from African swine fever in wild boar – Estonia as an example. Vaccines (Basel), 8, 336. 10.3390/vaccines8020336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehl J, Pikalo J, Schäfer A, Franzke K, Pannhorst K, Elnagar A, Blohm U, Blome S and Breithaupt A, 2020. Comparative pathology of domestic pigs and wild boar infected with the moderately virulent African swine fever virus strain “Estonia 2014”. Pathogens, 9, 662. 10.3390/pathogens9080662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selva N, Jędrzejewska B, Jędrzejewski W and Wajrak A, 2005. Factors affecting carcass use by a guild of scavengers in European temperate woodland. Canadian Journal of Zoology, 83, 1590–1601. 10.1139/z05-158 [DOI] [Google Scholar]
- Sereda AD, Balyshev VM, Kazakova AS, Imatdinov AR and Kolbasov DV, 2020. Protective properties of attenuated strains of African swine fever virus belonging to seroimmunotypes I‐VIII. Pathogens, 9, 274. 10.3390/pathogens9040274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhl K, Sternberg‐Lewerin S, Blome S, Viltrop A, Penrith M‐L and Chenais E, 2019. Lack of evidence for long term carriers of African swine fever virus – a systematic review. Virus Research, 272, 197725. 10.1016/j.virusres.2019.197725 [DOI] [PubMed] [Google Scholar]
- Stone SS, DeLay PD and Sharman EC, 1968. The antibody response in pigs inoculated with attenuated African swine fever virus. Canadian Journal of Comparative Medicine, 32, 455–460. [PMC free article] [PubMed] [Google Scholar]
- Taylor RA, Condoleo R, Simons RRL, Gale P, Kelly LA and Snary EL, 2020. The risk of infection by African swine fever virus in European swine through boar movement and legal trade of pigs and pig meat. Frontiers in Veterinary Science, 6, 486. 10.3389/fvets.2019.00486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulke H‐H, Tischendorf L, Staubach C, Selhorst T, Jeltsch F, Müller T, Schlüter H and Wissel C, 2000. The spatio‐temporal dynamics of a post‐vaccination resurgence of rabies in foxes and emergency vaccination planning. Preventive Veterinary Medicine, 47, 1–21. 10.1016/S0167-5877(00)00167-7 [DOI] [PubMed] [Google Scholar]
- Thurfjell H, Ball JP, Åhlén P‐A, Kornacher P, Dettki H and Sjöberg K, 2009. Habitat use and spatial patterns of wild boar Sus scrofa (L.): agricultural fields and edges. European Journal of Wildlife Research, 55, 517–523. 10.1007/s10344-009-0268-1 [DOI] [Google Scholar]
- Toïgo C, Servanty S, Gaillard JM, Brand S and Baubet É, 2010. Mortalité turelle et mortalité liée à la chasse: le cas du sanglier. Faune Sauvage, 288, 19–22. [Google Scholar]
- Torresi C, Fiori M, Bertolotti L, Floris M, Colitti B, Giammarioli M, Dei Giudici S, Oggiano A, Malmberg M, De Mia G, Belak S and Granberg F, 2020. The evolution of African swine fever virus in Sardinia (1978–2014) as revealed by whole‐genome sequencing and comparative analysis. Transboundary and Emerging Diseases, 67, 1971–1980. 10.1111/tbed.13540 [DOI] [PubMed] [Google Scholar]
- Vial L, 2009. Biological and ecological characteristics of soft ticks (Ixodida: Argasidae) and their impact for predicting tick and associated disease distribution. Parasite, 16, 191–202. 10.1051/parasite/2009163191 [DOI] [PubMed] [Google Scholar]
- Vilem A, Nurmoja I, Niine T, Riit T, Nieto R, Viltrop A and Gallardo C, 2020. Molecular characterization of African swine fever virus isolates in Estonia in 2014–2019. Pathogens, 9, 582. 10.3390/pathogens9070582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasova NN, Varentsova AA, Shevchenko IV, Zhukov IY, Remyga SG, Gavrilova VL, Puzankova OS, Shevtsov AA, Zinyakov NG and Gruzdev KN, 2015. Comparative analysis of clinical and biological characteristics of African swine fever virus isolates from 2013 year Russian Federation. British Microbiology Research Journal, 5, 203–215. [Google Scholar]
- Walczak M, Zmudzki J, Mazur‐Panasiuk N, Juszkiewicz M and Wozniakowski G, 2020. Analysis of the Clinical Course of Experimental Infection with Highly Pathogenic African Swine Fever Strain, Isolated from an Outbreak in Poland. Aspects Related to the Disease Suspicion at the Farm Level. Pathogens, 9, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JM, Datta S and Satten GA, 2003. Marginal analyses of clustered data when cluster size is informative. Biometrics, 59, 36–42. [DOI] [PubMed] [Google Scholar]
- Wilkinson PJ, 1984. The persistence of African swine fever in Africa and the Mediterranean. Preventive Veterinary Medicine, 2, 71–82. [Google Scholar]
- Yan J, 2002. Geepack: yet another package for generalised estimating equations. R‐News, 2/3, 12–14. [Google Scholar]
- Yan J and Fine JP, 2004. Estimating equations for association structures. Statistics in Medicine, 23, 859–880. [DOI] [PubMed] [Google Scholar]
- Zani L, Forth JH, Forth L, Nurmoja I, Leidenberger S, Henke J, Carlson J, Breidenstein C, Viltrop A, Höper D, Sauter‐Louis C, Beer M and Blome S, 2018. Deletion at the 5’‐end of Estonian ASFV strains associated with an attenuated phenotype. Scientific Reports, 8, 6510. 10.1038/s41598-018-24740-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zani L, Dietze K, Dimova Z, Forth JH, Denev D, Depner K and Alexandrov T, 2019. African swine fever in a Bulgarian backyard farm—a case report. Veterinary Science, 6, 94. 10.3390/vetsci6040094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zani L, Masiulis M, Bušauskas P, Dietze K, Pridotkas G, Globig A, Blome S, Mettenleiter T, Depner K and Karvelien≐ B, 2020. African swine fever virus survival in buried wild boar carcasses. Transboundary and Emerging Diseases, 10.1111/tbed.13554. [DOI] [PubMed] [Google Scholar]
- Zhao DM, Liu RQ, Zhang XF, Li F, Wang JF, Zhang JW, Liu X, Wang LL, Zhang JE, Wu XZ, Guan YT, Chen WY, Wang XJ, He XJ and Bu ZG, 2019. Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerging Microbes & Infections, 8, 438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow chart displaying terms of references, subquestions, data sources and assessment sections
Seroprevalence (seropositive proportion) in Estonia, from the last PCR‐positive sample in each LAU 1 region until 31 August 2020
Seroprevalence (seropositive proportion) in Latvia from the last PCR‐positive sample in each LAU 1 region until 31 August 2020
Estimated confidence achieved to detect ASF in LAU 1 regions assuming 1% prevalence for the first 52‐week surveillance period after the last PCR‐positive findings
Estimated confidence achieved to detect ASF in LAU 1 regions assuming 1% prevalence for the second 52‐week surveillance period after the last PCR‐positive findings


