CASE 1 CLINICAL PRESENTATION
A 37-year-old Malay woman with a history of hypertension, poorly controlled diabetes mellitus and polycystic ovarian syndrome presented with a one-week history of dry cough and rhinorrhoea. She had no family history of cardiac disease or sudden cardiac death. She was in close contact with a relative who had been recently diagnosed with coronavirus disease 2019 (COVID-19). On examination, her temperature was 37.0°C, blood pressure 132/90 mmHg, heart rate 82 beats/minute, respiratory rate 20 breaths/minute and saturation 98% on room air. Physical examination revealed dual heart sounds and clear lungs. What does her electrocardiogram (ECG) at presentation (Fig. 1a) and prior to discharge two weeks later (Fig. 1b) show?
Fig. 1.
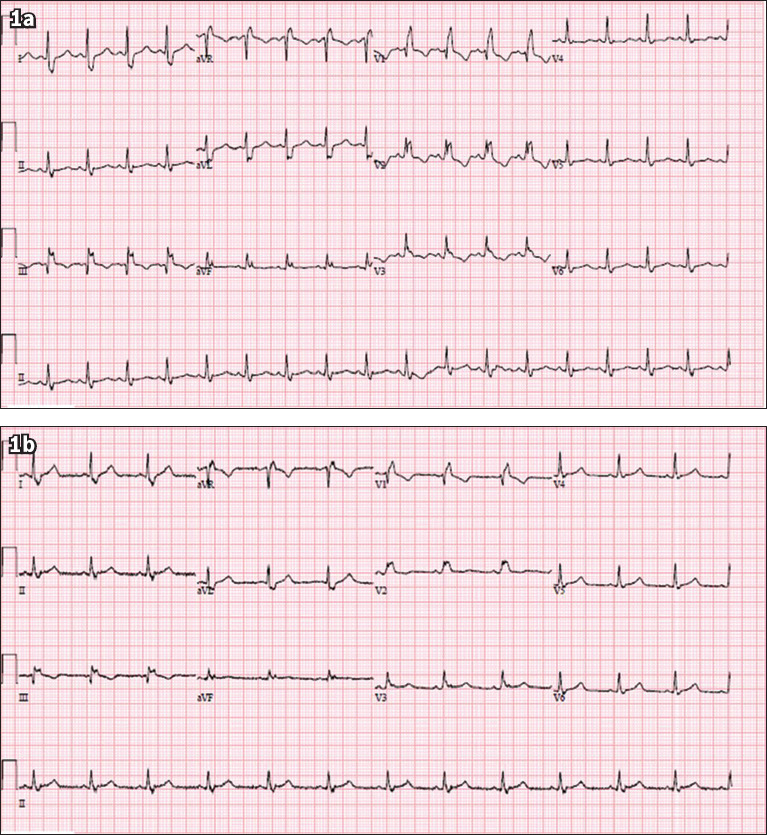
Case 1: ECG at (a) presentation and (b) prior to discharge two weeks later.
Her chest radiograph was normal and there was no cardiomegaly (Fig. 2). Laboratory investigations revealed a normal full blood count with no evidence of lymphopenia, and C-reactive protein, lactate dehydrogenase and ferritin levels were not elevated. Her creatinine level was normal, and there were no electrolyte derangements. The patient was managed with three days of oral lopinavir/ritonavir, but this medication was stopped due to gastrointestinal side effects.
Fig. 2.
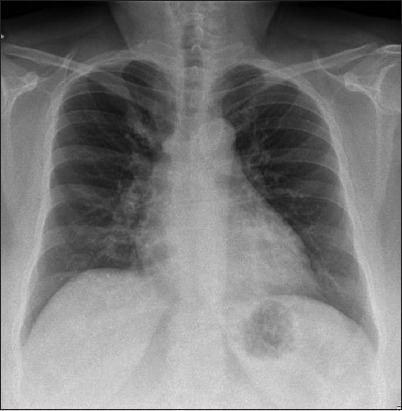
Case 1: Chest radiograph at presentation.
ECG INTERPRETATION
Both the ECGs at presentation (Fig. 1a) and discharge (Fig. 1b) show normal sinus rhythm with a right bundle branch block. The initial ECG shows a prolonged corrected QT interval of 555 ms, while the ECG prior to discharge shows that the corrected QT interval had returned to 470 ms (by Bazett’s formula). The derivation of the corrected QT interval is shown in Fig. 3.
Fig. 3.
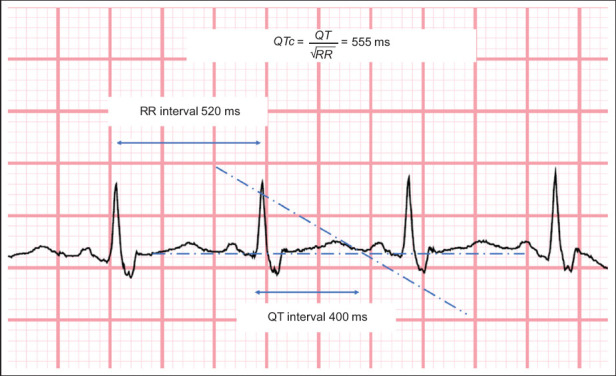
Figure shows calculation of the corrected QT interval (Bazett’s formula).
CLINICAL COURSE
The patient was admitted to the general ward for monitoring. Her nasopharyngeal swab confirmed the diagnosis of COVID-19. Her QT interval was prolonged prior to the administration of any medications. The patient was treated with oral lopinavir/ritonavir, which did not prolong the QT interval further. However, the medication was stopped due to gastrointestinal side effects. She was not switched to hydroxychloroquine because of the prolonged QT interval. Nevertheless, the patient improved clinically and did not require intensive care. She did not experience any lethal arrhythmias or palpitations while hospitalised. She recovered with no complications and tested negative for COVID-19 on two consecutive nasopharyngeal swabs. She was discharged after one month.
CASE 2 CLINICAL PRESENTATION
A 79-year-old Chinese man with a background of hypertension, hyperlipidaemia and ischaemic heart disease presented with a two-week history of dry cough and intermittent fever. He had undergone a coronary artery bypass graft surgery ten years ago. He had been regularly taking clopidogrel, simvastatin, bisoprolol, amlodipine and enalapril 2.5 mg every morning. He had no known history of atrial fibrillation and denied any chest pain, palpitation or shortness of breath on exertion. He did not have any positive contact with COVID-19 cases or travel history. On examination, his temperature was 37.2°C, blood pressure 112/61 mmHg, heart rate 84 beats/minute, respiratory rate 20 breaths/minute and saturation 100% on room air. Physical examination revealed dual heart sounds and crepitations in the right lung base. His laboratory findings were unremarkable apart from hyponatraemia (serum sodium 127 mmol/L; normal: 135–145 mmol/L) and lymphopenia (absolute lymphocyte count 0.53 × 109/L; normal: 0.91–3.52 × 109/L). His renal function, liver function, C-reactive protein level and ferritin level were normal. His chest radiograph showed right lower zone infiltrates (Fig. 4). The rest of the lungs were clear. His COVID-19 test was positive. What does the ECG show (Fig. 5)?
Fig. 4.
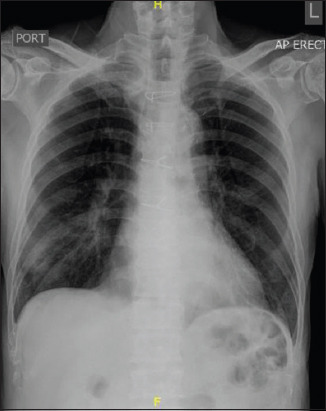
Case 2: Chest radiograph shows right lower zone infiltrates and the absence of cardiomegaly.
Fig. 5.

Case 2: ECG at presentation.
ECG INTERPRETATION
ECG shows an irregularly irregular rhythm at 60–70 beats/minute with a fibrillatory baseline. There is an incomplete right bundle branch block. The ST segment is normal, and there is no evidence of significant QT interval prolongation (corrected QT interval [QTc] 414 ms). These findings are consistent with atrial fibrillation.
CLINICAL COURSE
The patient was admitted to the general ward and his nasopharyngeal swab confirmed the diagnosis of COVID-19. He was given symptomatic relief for his upper respiratory tract symptoms. He remained afebrile in the ward and did not develop any exertional dyspnoea or desaturation. He did not require any antiviral therapy. His sodium levels returned to normal and lymphopenia improved on repeat testing. His calculated CHA2DS2-VASc score was 3. He was started on anticoagulation with a direct oral anticoagulant, apixaban. He recovered well and was subsequently discharged to a community care facility after two weeks.
CASE 3 CLINICAL PRESENTATION
A 65-year-old Indian man with a background of hypertension presented with a one-week history of dry cough and rhinorrhoea. He had no family history of cardiac disease or sudden cardiac death. He was in close contact with a relative who had been recently diagnosed with COVID-19. On examination, his temperature was 38.4°C, blood pressure 117/74 mmHg, heart rate 102 beats/minute, respiratory rate 20 breaths/minute and saturation 95% on room air. Physical examination revealed dual heart sounds and bilateral crepitations. His initial chest radiograph showed right middle and lower zone infiltrates (Fig. 6a). Laboratory investigations revealed a normal full blood count with no evidence of lymphopenia and normal renal function. C-reactive protein was elevated at 90 mg/dL (normal: 0–10 mg/dL), while lactate dehydrogenase was 921 U/L (normal: 250–580 U/L) and serum ferritin level was 950 μg/L (normal: 20–300 μg/L). His nasopharyngeal swab confirmed the diagnosis of COVID-19. The patient subsequently deteriorated with increasing shortness of breath and oxygen requirements. He was transferred to the intensive care unit (ICU) and intubated for mechanical ventilation. Repeat chest radiograph showed worsening bilateral infiltrates (Fig. 6b). The patient was treated with oral lopinavir/ritonavir for five days and interferon beta-1b for three days. On Day 3 of the ICU stay, the patient turned hypotensive. Troponin I level rose from 20.1 ng/L to 85.0 ng/L (normal: 0–17.4 ng/L). His ECG at presentation (Fig. 7a) and during the hypotensive episode in the ICU are shown (Fig. 7b). What do the ECGs show?
Fig. 6.

Case 3: Chest radiograph (a) at presentation and (b) after intubation.
Fig. 7.
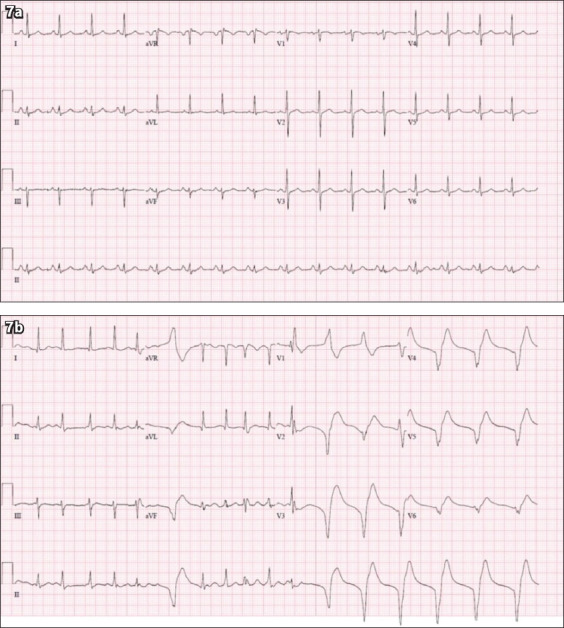
Case 3: ECG at (a) presentation and (b) during hypotensive episode in the intensive care unit.
ECG INTERPRETATION
The initial ECG (Fig. 7a) shows a normal sinus rhythm at a heart rate of 95 beats/minute. The subsequent ECG (Fig. 7b) shows the sinus rhythm deteriorating into an accelerated idioventricular rhythm of approximately 100 beats/minute. There were no discernible p waves in the latter half of the ECG, with a widened QRS complex.
CLINICAL COURSE
Within the next few hours, the patient collapsed and went into episodes of pulseless ventricular tachycardia requiring cardiopulmonary resuscitation and defibrillation. After return of spontaneous circulation, he was started on an amiodarone infusion. Transthoracic echocardiography demonstrated preserved left ventricular ejection fraction (55%), with normal chamber sizes and no regional wall motion abnormalities. He continued to improve clinically and was extubated after one week in the ICU. He had two consecutive nasopharyngeal swabs that tested negative for COVID-19 and was discharged from hospital after six weeks.
DISCUSSION
COVID-19 is an infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first described at the end of 2019. The first known cases were from a cluster of viral pneumonia in Wuhan, Hubei province, China.(1) Electrocardiographic manifestations at presentation are highly variable. Frequently, the ECG may reveal normal sinus rhythm with no significant abnormalities. In patients with existing heart disease, it is possible that COVID-19 may behave in a similar way to other acute systemic illness and precipitate common atrial arrhythmias. In patients with more severe cardiac involvement of COVID-19, more severe arrhythmias may be seen.
Other common electrocardiographic manifestations, in the form of cardiac and conduction system disease, have also been described in patients with COVID-19. This was in addition to other more direct cardiac manifestations such as acute coronary syndrome, myocarditis and heart failure.(2) These patients tend to be more severely ill, requiring intensive care and mechanical ventilation. Life-threatening arrhythmias may then present as cardiac involvement of the disease, or an overwhelming systemic inflammatory response induced by COVID-19 (as in Case 3).
The prevalence of arrhythmias and disease of the conduction system may be as high as 44% of patients in the intensive care unit and 17% of patients in the general cohort,(3) based on a single-centre study of 138 patients in Wuhan. Furthermore, it has been reported that 7.3% of patients presented with palpitations as part of their initial cluster of symptoms.(4) In patients that were more severely ill, the presence of acute arrhythmias and conduction system disease may have been attributed to hypoxia and the presence of electrolyte derangements. However, the aetiology that determines whether patients with COVID-19 may present with arrhythmias early in their disease or with mild disease remains to be elucidated.(5)
Patients with COVID-19 commonly present with fever and acute respiratory symptoms, such as cough, rhinorrhoea, sputum production and shortness of breath.(4) Indeed, most patients did not have symptoms of arrhythmias at presentation. Although palpitations were not an uncommon feature, it was rarely the dominant symptom reported by patients.(2)
Common electrocardiographic manifestations of COVID-19
There is no known electrocardiographic feature that is unique to COVID-19. The different electrocardiographic manifestations may be attributable to underlying acute infection or related to the unmasking of underlying cardiac disorders. The underlying mechanisms from which these electrocardiographic changes develop may remain unclear. Nevertheless, understanding the patient’s ECG would have important implications for therapy.
Sinus tachycardia
Sinus tachycardia is the most common manifestation in patients with COVID-19. This may be seen in an inflammatory response or a response to fever. Patients who are acutely ill may also be volume depleted due to poor oral intake, and sinus tachycardia may be an expected compensatory response. Other rarer but potentially causative mechanisms for the development of sinus tachycardia include more sinister events such as myocarditis and pulmonary embolism.(6-8)
Prolonged QTc
A large New York study (n = 4,250) demonstrated that prolonged QTc > 500 ms was seen in 6.1% of patients at presentation.(9) The patient’s baseline QT interval may thus affect the choice of antiviral therapy used in COVID-19 (as in Case 1). Therefore, measuring the QT interval at baseline is clinically important, as patients may have been started on medications such as hydroxychloroquine and azithromycin as therapy for COVID-19.(10) These medications place patients at further risk of QTc prolongation and, consequently, lethal arrhythmias. Anti-retroviral medications such as boosted protease inhibitors (lopinavir and ritonavir) may also be associated with a possible risk of QTc prolongation, but the incidence of this is low.(11,12) To our knowledge, there have not been any reports of QTc prolongation with intravenous remdesivir, an experimental therapy for COVID-19. Furthermore, in critically ill patients, significant electrolyte derangements such as hypokalaemia may also contribute to prolongation of the QT interval.(13)
Atrial arrhythmias
Common atrial arrhythmias such as atrial fibrillation and atrial flutter have been reported in COVID-19 patients. We presented a case of new-onset atrial fibrillation in association with COVID-19 in an elderly male patient (Case 2). The development of atrial fibrillation in our patient may be due to his underlying hypertension and history of ischaemic heart disease, which was precipitated by the acute illness. Although Case 2 did not require mechanical ventilation, mechanically ventilated COVID-19 patients were found to have a nearly ten-fold higher prevalence of atrial arrhythmias compared to those who did not require ventilation.(14)
Unmasking underlying channelopathies
It has been reported that underlying Brugada syndrome and its characteristic electrocardiographic findings may be unmasked in the acute setting by COVID-19 infection.(15) Patients with underlying cardiac channelopathies such as Brugada syndrome and long QT syndrome may present with classic electrocardiographic changes in the context of acute febrile illness.(16)
Bradycardias
Bradycardias have not typically been reported in patients with COVID-19. However, severe bradycardia requiring pacemaker insertion was seen in up to 15% of patients with Middle East respiratory syndrome, another type of coronavirus.(17) Bradycardia has also been reported in patients with coronaviruses that cause the common cold, although this is usually seen in paediatric settings.(18,19)
Management considerations
Because of the risk of COVID-19 patients developing arrhythmias, conduction systemic disease and myocardial injury, all patients should receive a baseline ECG at presentation. This would allow for a recording of the patient’s QTc duration and baseline QRS-T morphology. If the patient’s condition deteriorates during the course of disease, repeat ECG testing may reveal dynamic changes, such as those that reflect myocardial injury.
Furthermore, patients on therapy may also need to have their ECG QTc intervals monitored to minimise the risk of lethal ventricular arrhythmias. Medications such as hydroxychloroquine may prolong a patient’s ECG QT interval by blocking the activity of potassium channels in the heart.(20) These effects are similar to other medications such as quinidine and chloroquine. It is important to recognise that hydroxychloroquine is metabolised by cytochrome P450 3A4 in the liver, and hence liver enzyme inhibitors could raise plasma levels of these medications and lead to cardiac toxicity.(21)
Despite these concerns, hydroxychloroquine has rarely been demonstrated to cause lethal arrhythmias such as Torsades de pointes (TdP). This drug has been used widely in rheumatology conditions and as an antimalarial, and was found to be relatively safe.(22) Hydroxychloroquine (with or without azithromycin) is often given for short durations (typically up to five days), which further lowers the risk of TdP in this population. In patients with COVID-19 who were on mechanical ventilation and treated with azithromycin and either high- or low-dose chloroquine, a higher proportion of patients with QTc prolongation (> 500 ms) was reported in the high-dose group (18.9% vs. 11.1%), although this difference did not reach statistical significance.(23) Two patients in the high-dose group experienced ventricular tachycardia, but no patient had TdP. The risk of QTc prolongation is lower with boosted protease inhibitors such as lopinavir and ritonavir, and it has not been reported in patients treated with intravenous remdesivir.
CONCLUSION
Electrocardiographic manifestations of COVID-19 are highly variable and nonspecific. This paper illustrated three cases with different electrocardiographic features of COVID-19 and the subsequent implications for therapy. Other possible arrhythmias and conduction abnormalities were also discussed. In our institution, baseline ECGs are obtained at admission for all COVID-19 patients. Practitioners should take note of the electrocardiographic manifestations of COVID-19, as they may affect therapy and prognosis.
REFERENCES
- 1.World Health Organization. WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. [Accessed 30 April 2020]. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 .
- 2.Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75:2352–71. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133:1025–31. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakkireddy DR, Chung MK, Gopinathannair R, et al. Guidance for cardiac electrophysiology during the coronavirus (COVID-19) pandemic from the Heart Rhythm Society COVID-19 Task Force;Electrophysiology Section of the American College of Cardiology;and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. 2020 Apr 1; doi: 10.1016/j.hrthm.2020.03.028. https://doi.org/10.1016/j.hrthm.2020.03.028. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poissy J, Goutay J, Caplan M, et al. Pulmonary embolism in COVID-19 patients:awareness of an increased prevalence. Circulation. 2020;142:184–6. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 7.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1–6. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherian R, Poh KK. At the 'heart'of the COVID-19 outbreak:early cardiac implications and mitigating strategies. Singapore Med J. 2020;61:373–4. doi: 10.11622/smedj.2020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–9. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–9. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soliman EZ, Lundgren JD, Roediger MP, et al. Boosted protease inhibitors and the electrocardiographic measures of QT and PR durations. AIDS. 2011;25:367–77. doi: 10.1097/QAD.0b013e328341dcc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roden DM, Harrington RA, Poppas A, Russo AM. Considerations for drug interactions on QTc in exploratory COVID-19 treatment. J Am Coll Cardiol. 2020;75:2623–4. doi: 10.1016/j.jacc.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandael E, Vandenberk B, Vandenberghe J, Willems R, Foulon V. Risk factors for QTc-prolongation:systematic review of the evidence. Int J Clin Pharm. 2017;39:16–25. doi: 10.1007/s11096-016-0414-2. [DOI] [PubMed] [Google Scholar]
- 14.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–4. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang D, Saleh M, Garcia-Bengo Y, et al. COVID-19 infection unmasking Brugada syndrome. HeartRhythm Case Rep. 2020;6:237–40. doi: 10.1016/j.hrcr.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amin AS, Klemens CA, Verkerk AO, et al. Fever-triggered ventricular arrhythmias in Brugada syndrome and type 2 long-QT syndrome. Neth Heart J. 2010;18:165–9. doi: 10.1007/BF03091755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with Middle East Respiratory Syndrome coronavirus infection:a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–6. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sizun J, Soupre D, Giroux JD, et al. Nasal colonization with coronavirus and apnea of the premature newborn. Acta Paediatr. 1993;82:238. doi: 10.1111/j.1651-2227.1993.tb12650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sizun J, Soupre D, Legrand MC, et al. Neonatal nosocomial respiratory infection with coronavirus:a prospective study in a neonatal intensive care unit. Acta Paediatr. 1995;84:617–20. doi: 10.1111/j.1651-2227.1995.tb13710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu CI, Postema PG, Arbelo E, et al. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.03.024. S1547-5271(20)30285-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambhore A, Teo SG, Bin Omar AR, Poh KK. Importance of QT interval in clinical practice. Singapore Med J. 2014;55:607–12. doi: 10.11622/smedj.2014172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haeusler IL, Chan XHS, Guérin PJ, White NJ. The arrhythmogenic cardiotoxicity of the quinoline and structurally related antimalarial drugs:a systematic review. BMC Med. 2018;16:200. doi: 10.1186/s12916-018-1188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borba MGS, Val FFA, Sampaio VS, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection:a randomized clinical trial. JAMA Netw Open. 2020;3:e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


